
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron., 27 May 2020
Sec. Pest Management
Volume 2 - 2020 | https://doi.org/10.3389/fagro.2020.00004
This article is part of the Research TopicExploring GxExM Synergies in World-Wide Wheat Production and the Opportunities for International CollaborationView all 19 articles
The wheat stem sawfly (Cephus cinctus Norton) has plagued wheat production for over a century in North America. Host plant resistance in the form of wheat cultivars with a solid pith is a key management strategy. In this study we assessed the interaction of plant density, stem thickness and sawfly sex allocation using the most recent bread wheat cultivar with the resistant trait registered in Canada. The resistant cultivar with the solid stem trait was Lillian and it was compared to triticale and Go wheat, both of which lacked this trait. We confined 1 meter square crop plots using cages and half of the area in some cages was thinned manually to obtain thicker stems. We hypothesized that plant densities would affect stem diameters and solid pith expression, and these would affect host choices by the sawfly and sex ratio allocations. Our data showed that stems with a thicker diameter consistently produced more females compared to thinner stems that were more likely to produce males regardless of wheat cultivar. Shifting the plant population to lower average stem diameters in the resistant cultivar Lillian resulted in a male biased sex ratio, but not consistently. Further field studies are needed to test the hypothesis that at low plant densities of a resistant cultivar, the sex ratio would be more even due to higher female mortality in thicker stems.
Two species of grass stem mining sawflies (Hymenoptera: Cephidae) are significant pests of wheat in temperate regions. Cephus pygmaeus L., the European wheat stem sawfly from Eurasia, is an intermittent pest in that region (Özberk et al., 2005). In the Northern Great Plains of North America, the endemic Cephus cinctus Norton, the wheat stem sawfly (hereafter WSS), if by far a more serious pest and has been researched intensively for over a century (Beres et al., 2011d). Historically, WSS has threatened wheat production in the southern prairies of Canada, and Montana and North Dakota in the USA. Recent molecular studies (Lesieur et al., 2016) have revealed three population clusters of wheat stem sawfly: northern (on spring wheat, Canadian prairies, and adjacent northern USA), mountain (winter and spring wheat in western Montana) and southern (spring and winter wheat, north USA, and as far south as Colorado) (Lestina et al., 2016). Specific phenology of each cluster varies depending on the region and the host. In all cases, larvae mine inside the stem feeding on parenchymous tissue and near the end of the summer migrate to the base of the stem where they notch it to construct an overwintering chamber (Criddle, 1923). Mature larvae overwinter below the crown zone and complete pupation the following spring. Adults chew a hole through the plug on top of the stub and emerge ready to mate and with a full complement of eggs (Holmes, 1979). Like all hymenoptera, WSS are haplodiploid so that unfertilized eggs produce males (Mackay, 1955). Adults only live around a week and emerge intermittently, thus making management with chemicals ineffective (Holmes, 1979).
Yield losses to WSS can be substantial during outbreak cycles and require mitigation strategies. Losses stem from larval mining and unrecovered toppled stems and can reach 30% (Holmes, 1977; Beres et al., 2007). Both the European and the North American WSS can reduce grain yield by about 2 kg/ha with every increase in percent of stems cut (Özberk et al., 2005; Beres et al., 2007). Beres et al. (2011d) estimated that annual losses caused by WSS in North America can reach $350 Million. These authors recommended an integrated pest management approach (IPM) centered on host plant resistance in the form of wheat cultivars with solid stem pith (Farstad, 1940). Other key components of the IPM package (Beres et al., 2011b) include crop rotations to diversify agricultural landscapes (Rand et al., 2014), conservation biological control (Shanower and Hoelmer, 2004), trap crops with semiochemicals (Weaver et al., 2009; Buteler et al., 2010), and cultural methods such as residue management (Beres et al., 2011a) and planting cultivar blends (Cárcamo et al., 2016). Despite considerable progress toward IPM of this pest, in some regions of the USA it remains a major threat to wheat production and its resurgence still constitutes a biotic threat for cereals in Canada.
Host plant resistance is a key strategy in insect pest management. For WSS, several lines of durum and bread wheat with some level of WSS resistance have been developed in North America. All rely on the development of solid pith in the lumen that reduces egg laying (Varella et al., 2017), survivorship of immatures (Holmes and Peterson, 1958) or reduces adult fitness (Morrill et al., 2000; Cárcamo et al., 2005). Local environmental conditions and agronomic practices can interact with genetic expression of the solid pith so that in some cases it is poorly expressed and plants are damaged (Holmes, 1984). At high plant density (over 350 plants/m2), cultivars with the solid pith trait can have weak expression and incur economic damage (Beres et al., 2011c); this may also happen under rainy or cloudy conditions (Platt, 1941). Thus, there is a need for more case studies under a variety of environments to better understand genotype by environment interactions relevant for WSS pest management.
Hymenopteran insects such as WSS can alter the sex ratio of their progeny in relation to host quality. For example, parasitoids lay more female than male eggs in better quality hosts, such as larger ones (Wang et al., 2008). This phenomenon has also been documented for some herbivores, including WSS, although the effects of cultivars on sex ratios are inconclusive (McGinnis, 1950; Holmes and Peterson, 1963). Within cultivars, however, some authors have noted that more females than males emerge from wheat stems that have higher average diameters (Wall, 1952; Cárcamo et al., 2005). Stem diameter (= stem thickness) is expected to increase with decreasing plant density, which can also influence pith expression (Beres et al., 2011c). Holmes and Peterson (1963) noted more male emergence from the solid stem cultivar Rescue than from hollow stem cultivars. They explained the difference as follows: (1) early in the flight period there are more mated females than later in the season when males have died, (2) these mated females lay female eggs fertilized with sperm, but some are killed by the solid pith as eggs or larvae, (3) scarcity of males later in the flight period results in unfertilized eggs that yield more males which go on to survive because some of the earlier cohort dies in solid pith cultivars. The authors dismissed stem thickness effects or cultivar preferences. However, an earlier study by McGinnis (1950) noted clear cultivar differences in the damage of two hollow stemmed cultivars (Red Bobs and Thatcher) and some evidence for sex ratio effects. Furthermore, recent studies by Weaver et al. have clearly demonstrated that plant volatile differences have differential attractiveness to WSS (Weaver et al., 2009). It is quite likely that resulting sex ratios are driven my multiple factors that include differential attraction to the host through plant volatiles as shown by Weaver et al. (2009), varying survival of the males and females in relation to host quality (Cárcamo et al., 2005), and the mating biology of WSS (Cossé et al., 2002), which limits the availability of sperm throughout its short adult life.
The goal of the current study was to continue to improve our understanding of sawfly-plant interactions that influence the pest population dynamics, particularly sex ratios. To this end, we confined WSS with plants in cages and manipulated host plant densities with and without the solid pith trait. Our objectives were to determine if stem thickness would interact with the solid pith trait to influence wheat stem sawfly sex ratio. More specifically we wanted to test the following hypotheses: (1) female sawflies choose thicker stems over thin stems to lay female eggs, therefore, the sex ratio from stands dominated by thicker stems in our cages should be female biased in a hollow stem cultivar; (2) stands dominated by thicker stems of a resistant cultivar with more solid pith would have a less female biased sex ratio because of potential higher mortality of female larvae in these stems. Finally we were interested in corroborating the effect of sawfly damage on individual wheat stem yield.
This study was conducted in southern Alberta, Canada in 2009 and 2010. In 2009 it was located 1 km east of Lethbridge (49°41′ N, 112°44′ W) on research plot land of Agriculture and Agri-Food Canada (AAFC). In 2010, it was located 10 km west of Lethbridge at the wheat stem sawfly nursery (49°44′ N, 112°57′ W) near Coalhurst established by AAFC researchers (Peterson et al., 1968; Beres et al., 2005). Both sites are in the Moist Mixed Grassland Ecoregion within the Prairies Ecozone. This semiarid region has a long term mean annual air temperature around 5°C and 350–400 mm precipitation with soils classified predominantly as Orthic Dark Brown Chernozem clay loams. Annual precipitation in the Lethbridge area was 208 mm in 2009 and 367 mm in 2010.
The cereal hosts included wheat and triticale. In 2009, triticale (x Triticosecale, cultivar Pronghorn) and red spring wheat (Triticum aestivum, cultivar Lillian) were used. In 2010 triticale was replaced with the hollow stemmed wheat cultivar Go. Triticale was included because of the potential to develop this crop for industrial purposes and the need to understand its risk of damage by sawflies. Pronghorn triticale (Salmon et al., 1997) is an early maturing cultivar well-adapted to the southern Prairies; it is high yielding and the check standard to measure new cultivars, but a known host of WSS (Beres et al., 2013). Go was used in 2010 because it was one of the most widespread red spring cultivars in the region at the time and a well-documented host of WSS (Cárcamo et al., 2016). Lillian is a solid-stemmed cultivar with sufficient resistance to WSS and high yield (DePauw et al., 2005) with a high degree of adoption by growers in years with high sawfly pressure. In some regions, depending on weather conditions the solid pith expression can be inconsistent, therefore, this cultivar could be considered semi-resistant.
A cage assay was conducted both years to manipulate stand densities to obtain thicker stems and assess impact on wheat stem sawfly sex ratio, stem infestation, and interactions with yield. In 2009 triticale and Lillian wheat were planted in adjacent strips on 21 May at a rate of 200 seeds per meter square. Four, one meter square areas were designated within each of the strips and half of each area was thinned manually to remove tillers, by clipping with scissors, two times prior to the stem elongation stage (Zadok 32). Cages (1 m square and 1.2 m tall, clear nylon screen with 12 threads/cm) were deployed on 30 June 2009 and kept until crop maturity. Therefore, each cage in 2009 contained both a sparse (thinned) stand and a high density plant stand each about 0.5 m2.
In 2010, 4 wheat strips were planted at the Coalhurst site to include a high and a low seeding rate for two cultivars (Figure 1). Two adjacent strips of Go wheat were planted at 450 and 150 seeds per m square, and two similar strips of Lillian were planted immediately west at the same two seeding rates. For the high seeding rate strips, the same treatment performed in 2009 was executed: 4 cages in each of the two strips had half of the area inside each cage thinned manually by clipping the tillers with scissors. This treatment was intended to provide a choice of thin vs. thick stems for the sawfly to lay eggs within a cage. An additional 4 cages were set up in these same strips (alternating pattern) and left intact at high plant densities. Finally, at each of the wheat strips planted at low densities, four cages were set up, and all tillers were removed as described above in the entire 1 m square (Figure 1). These last two treatments were intended to deny an oviposition choice to WSS so that they would encounter mostly thin or mostly thick stems. For both years of the study each treatment was replicated 4 times and represented by 4 cages that were deployed on a certain cultivar strip and received a particular plant density manipulation.
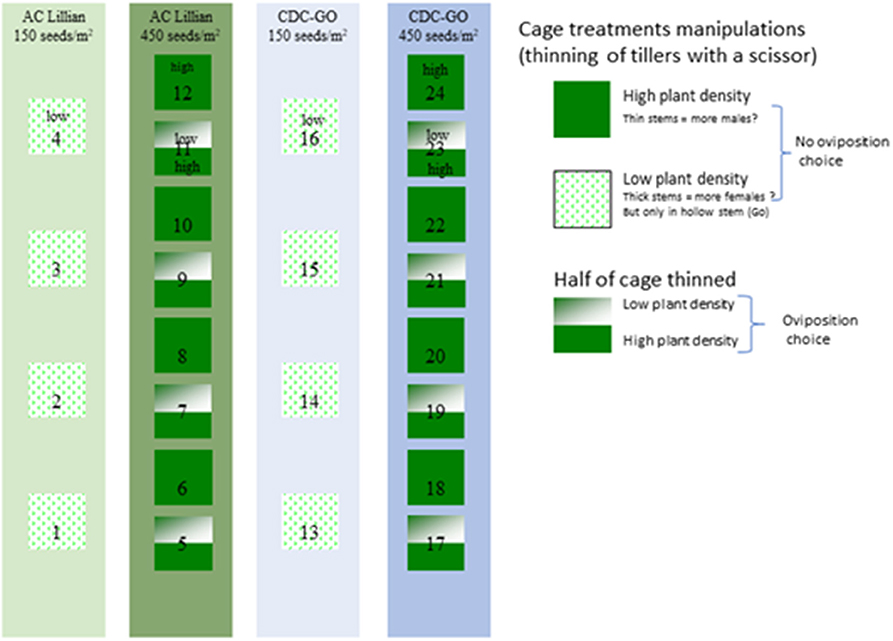
Figure 1. Layout of the cages within the adjacent strips of CDC Go and Lillian wheat planted at 150 or 450 plants/m2 near Coalhurst, Alberta Canada. Some cages were thinned manually with scissors completely or only half to obtain thicker stems and assess effects on sawfly sex ratio.
Cages were deployed on 29 June 2010 and similar to 2009, they were left until plant maturity. To inoculate the cages with WSS, plant stubs (damaged plants with overwintered sawfly larvae) were collected from a field near Taber (about 50 km east of Lethbridge) on 24 June 2009 and from the Coalhurst nursery on 30 June 2010. The plant remains were sorted in the laboratory and 50 wheat stubs were “planted” in the middle of each caged area. Parasitoid wasps (Bracon cephi) were aspirated from the cages as much as possible to minimize sawfly mortality. A subsample of 100 stubs were set up for emergence in the laboratory to estimate population size and sex ratios introduced into the cages. Survivorship from this subsample showed about 90% emergence and over 70% were females.
Several response variables were measured each year. Prior to crop senescence, cages were removed and all plants were dug out carefully, and placed in large paper bags. A random subsample of at least 20 stems from each cage or manipulated half was taken to measure the stem diameter at 3 angles using a digital caliper. Cut stems were measured 1 cm below the cut area and for uncut stems 1 cm below the second node. In 2010, a similar sample size of uncut stems were dissected longitudinally and each undamaged internode was given a rank where zero was a completely hollow lumen and 5 was completely solid. Both years, cut stubs expected to have mature larvae were placed to overwinter in a room at 10°C and 8:16 h, L:D regime from late September until late March. At this time, each stub was placed in a plastic vial with moistened sand and moved to room temperature (22°C) and long light:dark photoperiod regime (16:8 h). The sex of each adult that emerged was recorded. A similar sample size of mature plants were collected from an uncaged area in each of the strips to estimate effects of the cage on stem diameter (both years) and pith expression (2010 only) and potential interactions with WSS.
Stem diameter, pith and grain weight were analyzed using the GLIMMIX procedure in SAS (2013). The mixed models included cage as the random factor and location, variety, and plant density as fixed factors. An additional analysis was conducted using type of sawfly damage as a fixed factor nested within plant density and cages. Fixed factors were included where they were warranted based on the p < 0.05 of the F-test (fixed factors and their interactions). The inverse Gaussian or the Gamma distribution were selected to model the stem diameter data based on the model fit statistics, i.e., the Bayesian information criterion (BIC). For pith and grain weight, the beta-binomial and Gaussian (normal) distribution were selected, respectively. The relationship between female proportion and stem diameter for each cereal crop or cultivar was modeled using a modified Weibull function (SAS PROC NLIN):
where F indicates the proportion of females and d indicates the stem diameter. The number of males vs. females in each of the plant density and cultivar treatments were integer counts and as such they were assumed to be Poisson distributed for comparison using a t-test:
The corresponding P-values were calculated in R using the pt function with df = Inf and lower.tail = FALSE (R Core Team, 2019).
In all cases, Bonferroni's adjustment for multiple comparisons was used when there were fewer than seven categorical levels, and Scheffe's adjustment was used when there were seven or more levels.
Stem diameter for the plant population sampled inside the cage or at an adjacent open patch was affected by some of the treatments in 2009 (Figure 2), but not in 2010 (Figure 3). In 2009, Lillian stems were over 2 mm thick in the low plant density stand and under 2 mm in the area with higher plant density, outside, or inside the cage [F(1,458) = 45.59, P < 0.0001]. Triticale stems were thicker than those of Lillian [F(1,458) = 18.54, P < 0.0001]. In 2010 there were no significant differences with respect to stem diameters for any of the treatments inside or outside the cages. Most stems were between 1.5 and 3.0 mm in diameter below the second internode.
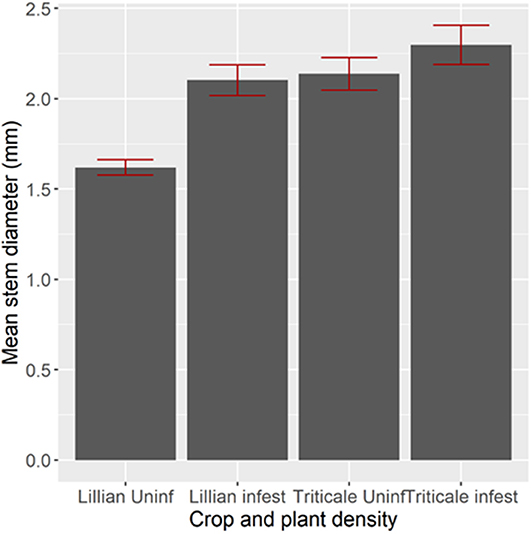
Figure 2. Stem diameters (mm) of wheat and triticale in stands with tillers removed (Low) or left at high density (High) in 2009 inside cages. Four cages were used to replicate the plant density manipulation.
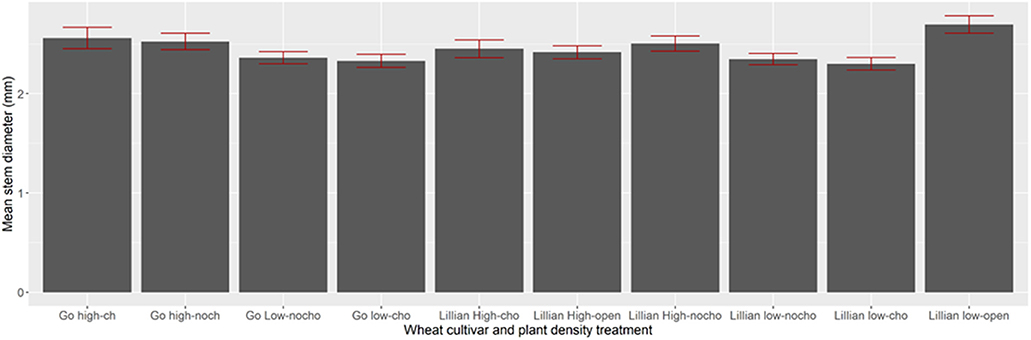
Figure 3. Stem diameters of two wheat cultivars outside (0) or inside (1) cages near Coalhurst, Alberta in 2010. Density Choice: refers to the stand density, high or low, in half the cage (choice) or the entire stand in the cage (no choice). HighChoi, high with choice; LowChoic, low with choice; HighNoch, high with no choice; LowNoCho, Low no choice. Four cages were used to replicate the plant density manipulation for each cultivar.
Pith solidness was assessed only in 2010 and it was affected by the cages and cultivar [F(8,614) = 41.08, P < 0.0001], but not by plant density treatments inside cages (Figure 4). The highest pith rating was around 3 out of 5 for Lillian grown in the open and higher than Lillian plants confined with cages. Inside the cages, Lillian had significantly higher solid pith at 2.2 than Go at 1.6 [F(8,614) = 40.23, P < 0.0001].
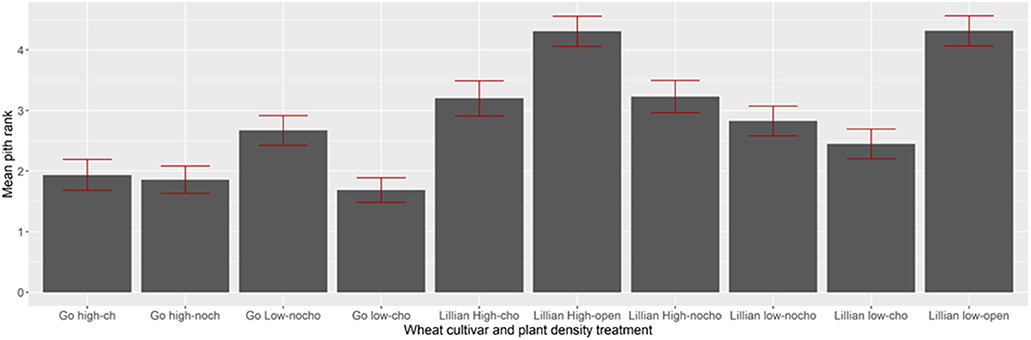
Figure 4. Pith of the stem lumen (solidness rank) of wheat cultivars in 2010 inside and outside cages. Entries are means and standard errors. Refer to Figure 3 for abbreviations. Four cages were used to replicate the plant density manipulation for each cultivar.
Stem diameter influenced host acceptance for wheat, but not for triticale. In 2009, Lillian infested stems were significantly thicker than un-infested stems (Figure 5, Bonferroni adjusted comparison, t1, 458 = 2.69, P = 0.0442). In 2010 (Figure 6), infested stems (cut or not cut by sawfly), were significantly thicker than un-infested stems in both wheat cultivars [F(2,724) = 84.15, p < 0.0001]. Grain weight followed the same pattern as the stem diameters: sawfly-infested, thicker stems, had significantly higher seed weights than un-infested thinner stems [p < 0.001, Figure 7, F(2,1494) = 67.97, p < 0.0001].
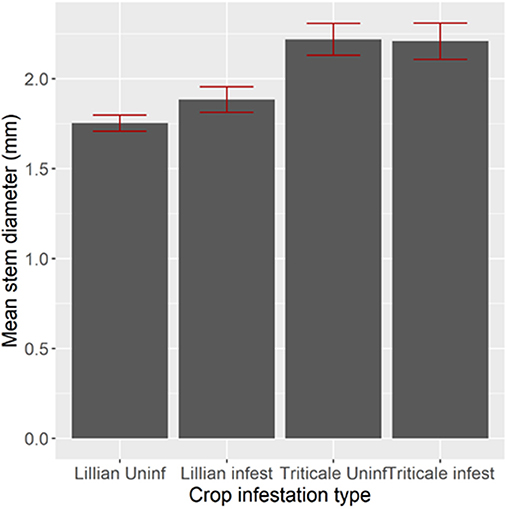
Figure 5. Stem diameter of Lillian wheat and triticale infested or un-infested by wheat stem sawfly confined with cages in 2009 near Lethbridge, Alberta. Four cages were used to replicate the plant density manipulation for each cultivar.
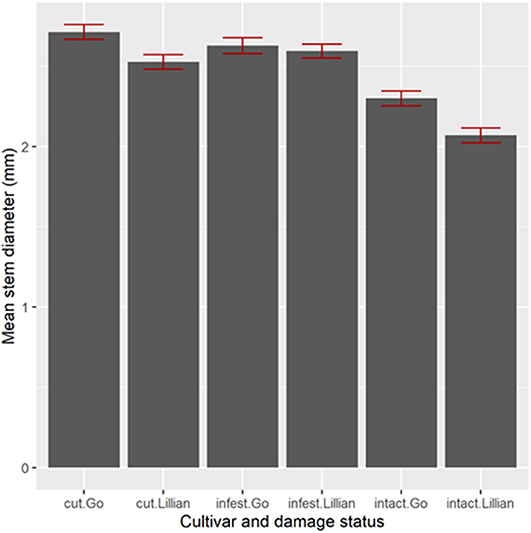
Figure 6. Stem diameters of wheat infested, cut or un-infested by wheat stem sawfly inside cages in 2010. Four cages were used to replicate the plant density manipulation for each cultivar.
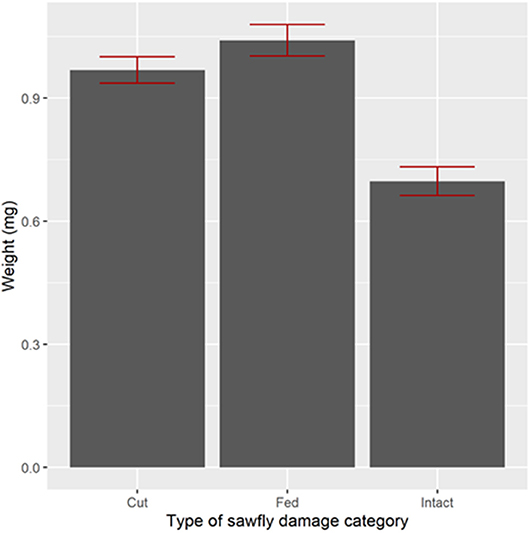
Figure 7. Average weights (mg) of wheat seed weights in relation to wheat stem sawfly infestation in a cage study in 2010 near Coalhurst, Alberta. Entries are arithmetic means and ± 1 standard error. Four cages were used to replicate the plant density manipulation for each cultivar.
Female and male counts differed significantly in the plant density treatments within cultivars in 2009 (Table 1, t-test, p < 0.05). In 2009, from the 130 adults reared, there were more females than males from the low plant density stands in the cages of Lillian wheat, but this pattern was reversed from the high density stand for this cultivar, which had a lower average stem diameter than the former. For triticale, both areas within the cages, with high or low plant densities, produced more females than males, but the overall number of sawfly adults was lower than those from wheat. In 2010, 232 adults emerged from the Go and Lillian wheat cultivars combined. With one exception, the sex ratios were female biased and ranged from about 0.6 to 0.7. The number of females was significantly higher than males only from the high plant density treatment of Lillian without the stand choice (p = 0.0092). The only treatment with an even sex ratio was for the low plant density treatment for Lillian with no stand choice, but only 4 adults emerged from these cages (Table 1).
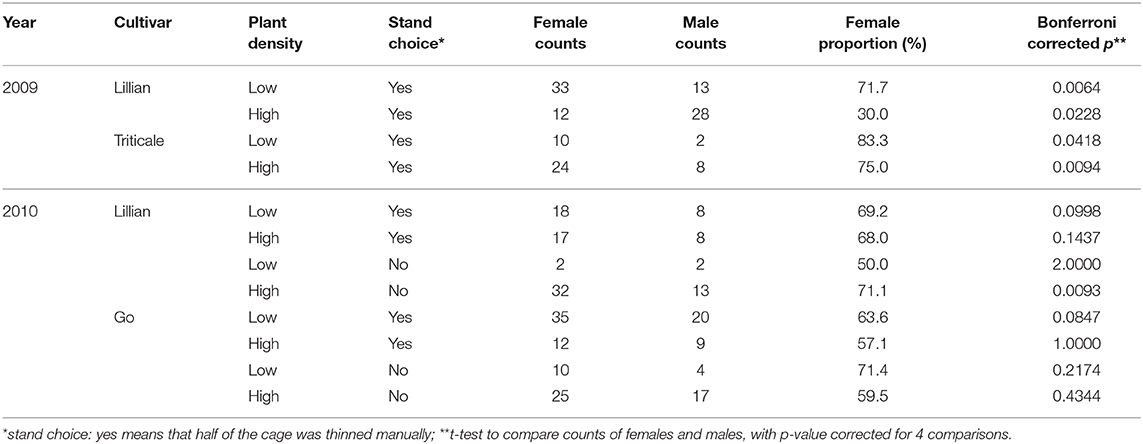
Table 1. Sex ratio of wheat stem sawfly that emerged from wheat or triticale stubs in 2009 and 2010.
The relationship between stem diameter and female proportion (weather a female or male emerged from a given stem) was further explored using non-linear functions. A Weibull non-linear function (Figure 8A) explained the relationship and showed different responses for Lillian () and triticale (); in this equation, is the female proportion and d is the stem diameter. A stem diameter near 2.0 mm and about 2.2 mm were required for a near even sex ratio of sawfly emerging from Lillian and triticale, respectively. To achieve a female dominance close to 80%, only an increase of 0.3 mm in stem diameter would be needed in Lillian, but almost a full mm was required to reach this proportion of females in triticale. In 2010, a similar non-linear relationship between sex ratio and stem diameter was observed for the two wheat cultivars Lillian and Go (Figure 8B), but it approached linearity for Go. For both cultivars, slightly thicker stems over 2.2 mm were required to produce an even sex ratio. Stems around 3 mm in thickness resulted in a highly biased female sex ratio near 80% in Lillian similar to 2009; for the cultivar Go, stems around 3 mm had a female proportion under 70%.
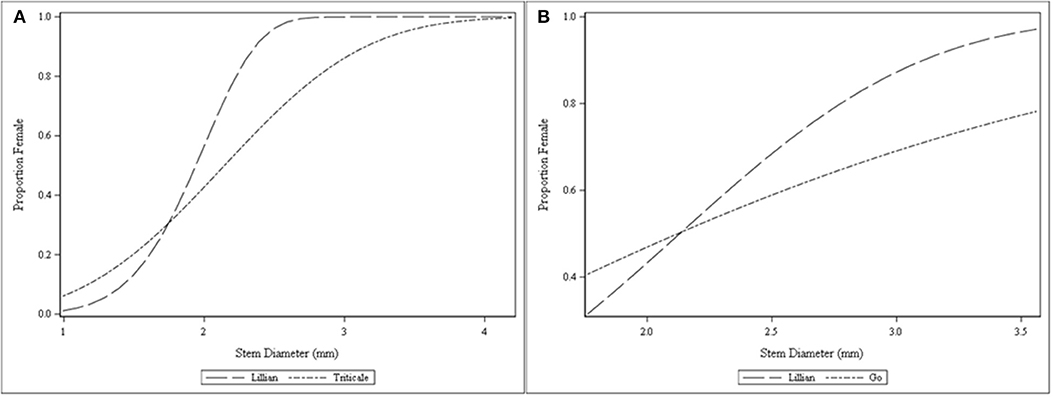
Figure 8. Modified Weibull functions explaining the relationship of cereal host stem diameter and wheat stem sawfly sex ratio (female proportion) from the cage study near Lethbridge in 2009 (A) and 2010 (B). Four cages were used to replicate the plant density manipulation for each cultivar.
Host quality can influence host selection and sex ratios of haplo-diploid Hymenoptera such as wheat stem sawfly. For this sex determination system, unmated females lay male eggs, and those mated can allocate gender depending on host quality. This trait may be exploited for management of herbivorous insect pests such as the wheat stem sawfly. In this study we manipulated plant densities by varying seeding rates and also inside cages through manual thinning. We used the latest bread wheat solid stem cultivar registered in Canada, Lillian, and compared it to a hollow stemmed host, triticale in 2009 and Go wheat in 2010. We expected that at lower plant densities, average stem diameter should be greater than at higher densities. A number of studies have shown that more females emerge from stem stubs with higher diameter than from thinner stems (Wall, 1952; Morrill and Weaver, 2000; Morrill et al., 2000; Cárcamo et al., 2005). In general, over the 2 years of the study, sex ratios were female biased in most treatments with only two exceptions. In 2009, Lillian stems from the high plant density stand inside cages had a significantly male biased sex ratio compared to the low plant density stand that produced significantly more females than males. Clipping tillers to reduce plant density inside cages may have changed the volatile profile (Weaver et al., 2009) and induce stronger defenses in the main stems of these plants that could affect larval survivorship (Karban et al., 2000). However, the fact that more females than males emerged from the main stems suggests that this was not the case because it is known that females are more sensitive than males (Morrill et al., 2000) to reductions in host quality presumably associated with higher defenses.
We suggest that four hypothesis may explain the pattern of male biased sex ratios in stands with more thin stems. (1) Our data lend support to the hypothesis that in a stand composed of mainly thin stems, females lay mostly male eggs rather than allocating the gender on a relative stem thickness basis (Cárcamo et al., 2005). (2) Alternatively, female larvae may not have survived if their nutritional requirements were not met in stems that were not thick enough to meet their higher nutritional requirements. This hypothesis is supported by the studies of Morrill et al. (2000) and Morrill and Weaver (2000) who showed that females are more sensitive to host quality than males. (3) Solid pith may result in higher female mortality if WSS laid more female eggs in stems that are slightly thicker, which under ideal environmental conditions should have more pith than thinner stems. The latter hypothesis is unlikely because the sex ratios were female biased in the low plant density stands of Lillian that had stem diameters over 2 mm in 2010. (4) A fourth explanation cannot be ruled out from our design: higher plant densities may have reduced light intensity inside the cages and influenced WSS mating behavior and confounded progeny sex ratio. WSS may have mated less frequently inside cages with a higher stand density due to poor light (McGinnis, 1950). If this was the case then they would have laid more unfertilized male eggs, thus biasing the sex ratio. A field experiment under natural light conditions is needed to overcome this confounding factor. The only other instance of non-female biased sex ratio was in 2010 in the Lillian treatment with low plant density that had 2 males and 2 females in total. It is not known why survivorship was so poor in this treatment, but it could not be related to solid pith because this trait was poorly expressed inside the cages regardless of plant density. The poor development of solid pith may be explained by the shading inside the cage because it is known that environmental conditions can limit expression of this trait (Platt, 1941). Teasing apart these hypotheses presents considerable logistical challenges.
Local mate competition and mating status can be a strong determinant of sex ratio in Hymenoptera (Henter, 2004). We did not control the founding sex ratios in our study, but expect that there were enough males to fertilize females. However, it is possible that some did not mate and may show less discrimination between thick and thin stems. The effect of mating status on sex ratio allocation in relation to host quality has not been studied extensively. However, Gerling et al. (1987) showed that unmated females of Encarsia deserti (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Homoptera: Aleyrodidae) failed to discriminate between parasitized and un-parasitized hosts, unlike those that had mated and could lay female eggs. A challenging controlled study that provides very high light intensity in cages with various ranges of stem diameters with treatments including mated and unmated females as well as varying proportions of founding sex ratios would help answer this question for WSS.
Cereal species and cultivar may interact with stem diameter to influence sex ratios. Triticale has relatively larger stem diameters averaging 2.9 mm compared to Lillian wheat with an average of around 2.2 mm in our low density treatment. These differences translated to corresponding differences in sex ratios: 80 and 50% females, respectively, when the plant density treatments were combined. This suggests that sawfly may lay more female eggs in crops with thicker stems as supported by the studies by Morrill et al. (2000); even at the high plant densities, average stem diameters of triticale were likely high enough to entice females to lay fertilized female eggs. This hypothesis is further supported by the extensive behavioral observations by Buteler et al. (2009) showing that female WSS assess host quality prior to oviposition. In the case of solid stem cultivars, attractive thick stems may present a dead end trap for sawfly immatures, and reduce female dominated sex ratio (Holmes and Peterson, 1963; Buteler et al., 2010). Furthermore, Varella et al. (2017) demonstrated that quantitative trait loci associated with the solid stem phenotype influence oviposition behavior of WSS. Also, Beres et al. (2011c) showed that Lillian maximizes solid pith in the stem lumen at densities below 250 plants per square meter. One of our objectives was to test the idea that for solid stemmed wheat cultivars at low plant densities, thicker stems of Lillian would have more solid stems that would kill more females thus reducing the female dominated population. We were unable to test this hypothesis because of the poor expression of solidity of the lumen in this cultivar during our study years. A field study without cages remains to be done to assess cereal crop and variety interaction effects on sex ratio. Such a test should include representative cultivars with alternative source of solid pith found in durum wheats such as Golden Ball (Triticum durum var Golden Ball), which have relatively thick stems and solid lumens, yet seem to produce female biased sex ratios (Farstad et al., 1949). Unknown germplasm factors not related to pith or stem thickness, likely affect WSS sex ratio as suggested by the studies by McGinnis (1950) with two hollow stemmed bread wheat cultivars, Red Bobs and Thatcher.
Our analysis of stem diameter and female emergence relationships suggested that the response can be non-linear and varies with the crop species. Both type of responses, linear or non-linear, showed that a female-dominated sex ratio is ensured even when the crop species or cultivar has an overall lower stem diameter. Our results corroborate those reported by Morrill and Weaver (2000) where they also showed clear effects of stem diameter on WSS sex ratio. Triticale and Go wheat have thicker stems than Lillian and it seems that a female dominated sex ratio would occur at a lower stem diameter for the cultivar that had the thinner stems. For example, 70% female emergence occurred around a stem diameter of 2.5 mm for Lillian but over 3 mm for the other two cultivars. This ensures that even if a wheat stand is dominated by thin stems a sawfly may still lay a large number of female eggs to maximize its fitness. A similar non-linear relationship and similar levels of stem thickness to achieve 70% female dominance was noted by Morrill et al. (2000) at a Montana (USA) site. This relationship is similar to the pattern of sex ratio in relation to host quality in some parasitoid wasps. For example Tetrastichus julis (Eulophidae), consistently lays a female dominated clutch regardless of cereal leaf beetle instar host size, but similar to our case study, the sex ratio becomes even more female dominated with the size of its host (Kher, Dosdall and Carcamo unpublished data). Thus, it appears that at some level, insects that control progeny gender follow a relative sex allocation rule to ensure female dominance. It would be of interest to test for a lower limit and force females to lay eggs on hosts that are far below the usual host size to see if there is a point where only males are laid. Further study of the host germplasm in terms of pith expression and stem diameter are still warranted, particularly in environments that maximize solid pith.
Plant traits such as stem diameter and height affect the initial host selection by wheat stem sawfly (Buteler et al., 2009) and confounds individual stem comparisons of plant yield. Regardless of cereal species or cultivar, it was clear in our study that sawfly preferentially attacked stems with larger stem diameter compared to thinner stems. Furthermore, seed weights were consistently higher in infested stems than in those not attacked by the sawfly. This is expected to result from the inherently higher yield potential of larger stems than smaller stems. Detailed studies of photosynthesis in infested stems have shown clear reductions of kernel weight attributed to larval feeding (Macedo et al., 2007; Delaney et al., 2010). Delaney et al. (2010) reported a reduction in yield loss for a solid stem cultivar compared to a hollow stem cultivar and speculated for potential compensation in such cultivars. The pattern of higher yield in infested than un-infested stems has been observed in previous studies. Wu et al. (2011) used stem diameter as a covariate to attempt to standardize effects of a parasitoid attack to wheat stem sawfly on grain yield in main stems and tillers in several cultivars. They still found that for most comparisons, un-infested stems had lower seed weights than those that were infested by sawfly or where the immature sawfly had been killed by the parasitoid. A similar pattern of lower yield potential from un-infested stems than those infested had been reported in earlier studies (Holmes, 1977). Yet, the sawfly is a serious pest of wheat and at the plant population level, there are well-documented yield reductions both from larval stem mining [around 10% according to Holmes (1977)] and unrecovered grain from lodging (14% as per Beres et al., 2007). These losses have been documented when susceptible cultivars with hollow stem lumen are compared side by side with more resistant solid stem lumen cultivars (Beres et al., 2007). These authors and Özberk et al. (2005) demonstrated a very strong negative relationship between C. cinctus or C. pygmaeus damage and yield, which was equivalent to about 2 kg/ha of loss yield for every incremental percentage of stems cut by sawfly. Clearly, despite the confounding effect of stem diameter on yield when comparing individual stems, the sawfly is a destructive economical insect pest.
Our objectives were to continue elucidating complex insect-plant interactions between cereal crops and wheat stem sawfly. We hypothesized that plant densities would affect stem diameters and solid pith expression, and these would affect host choices by the sawfly, and sex ratio allocations. Our data showed that stems with a thicker diameter consistently produced more females compared to thinner stems that were more likely to produce males regardless of wheat cultivar. Shifting the plant population to lower average stem diameters in the resistant cultivar Lillian resulted in a male biased sex ratio, but not consistently. In this study solid pith expression in cages was poor and we were unable to test the hypothesis that at low plant densities of the resistant cultivar, the sex ratio would be more even due to higher female mortality in thicker stems. A field test needs to be conducted at several sites with sufficient natural sawfly populations to elucidate this interaction.
All datasets generated for this study are included in the article/supplementary material.
HC, BB, and XW conceptualized, designed the study and participated in its execution and data collection. TL and XW did most of the data collection. TS did the statistical analysis. HC wrote the first draft and BB, XW, TL, and TS edited it.
This study was funded through Agriculture and Agri-Food Canada's Matching Investment Initiative with leveraging funds provided by the Western Grains Research Foundation's Producer Checkoff to BB.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful for the excellent laboratory and field technical support at AAFC's Lethbridge Research and Development Center provided by R. Dyck, S. Simmill, S. Daniels, and C. Herle.
Beres, B. L., Byers, J. R., and Cárcamo, H. A. (2005). The wheat stem sawfly: a nursery tale from the short grass prairies. Biol. Surv. Can. 1, 1–6. Available online at: https://biologicalsurvey.ca/acg/wheatstemsawfly.pdf
Beres, B. L., Cárcamo, H. A., and Byers, J. R. (2007). Effect of wheat stem sawfly damage on yield and quality of selected Canadian spring wheat. J. Econ. Entomol. 100, 79–87. doi: 10.1093/jee/100.1.79
Beres, B. L., Cárcamo, H. A., Byers, J. R., Clarke, F. R., Pozniak, C. J., Basu, S. K., et al. (2013). Host plant interactions between wheat germplasm source and wheat stem sawfly Cephus cinctus Norton (Hymenoptera: Cephidae) I. Commercial cultivars. Can. J. Plant Sci. 93, 607–617. doi: 10.4141/cjps2012-088
Beres, B. L., Cárcamo, H. A., Dosdall, L. D., Yang, R.-C., Evenden, M. L., and Spaner, D. M. (2011a). Do interactions between residue management and direct seeding system affect wheat stem sawfly and grain yield? Agron. J. 103, 1635–1644. doi: 10.2134/agronj2011.0055
Beres, B. L., Cárcamo, H. A., Weaver, D. K., Dosdall, L. M., Evenden, M. L., Hill, B. D., et al. (2011b). Integrating the building blocks of agronomy and biocontrol into an IPM strategy for wheat stem sawfly. Prairie Soils Crops 4, 54–65. Available online at: https://prairiesoilsandcrops.ca/articles/volume-4-7-print.pdf
Beres, B. L., Cárcamo, H. A., Yang, R. C., and Spaner, D. M. (2011c). Integrating spring wheat sowing density with variety selection to manage wheat stem sawfly. Agron. J. 103, 1755–1764. doi: 10.2134/agronj2011.0187
Beres, B. L., Dosdall, L. M., Weaver, D. K., Cárcamo, H. A., and Spaner, D. M. (2011d). Biology and integrated management of wheat stem sawfly and the need for continuing research. Can. Entomol. 143, 105–125. doi: 10.4039/n10-056
Buteler, M., Weaver, D. K., Bruckner, P. L., Carlson, G. R., Berg, J. E., and Lamb, P. F. (2010). Using agronomic traits and semiochemical production in winter wheat cultivars to identify suitable trap crops for the wheat stem sawfly. Can. Entomol. 142, 222–233. doi: 10.4039/n09-072
Buteler, M., Weaver, D. K., and Peterson, R. K. D. (2009). Oviposition behavior of the wheat stem sawfly when encountering plants infested with cryptic conspecifics. Environ. Entomol. 38, 1707–1715. doi: 10.1603/022.038.0624
Cárcamo, H. A., Beres, B. L., Clarke, F., Byers, R. J., Mündel, H. H., May, K., et al. (2005). Influence of plant host quality on fitness and sex ratio of the wheat stem sawfly (Hymenoptera: Cephidae). Environ. Entomol. 34, 1579–1592. doi: 10.1603/0046-225X-34.6.1579
Cárcamo, H. A., Beres, B. L., Larson, T. R., Klima, C. L., and Wu, X.-H. (2016). Effect of wheat cultivars and blends on the oviposition and larval mortality of Cephus cinctus (Hymenoptera: Cephidae) and parasitism by Bracon cephi (Hymenoptera: Braconidae). Environ. Entomol. 45, 397–403. doi: 10.1093/ee/nvv231
Cossé, A. A., Bartelt, R. J., Weaver, D. K., and Zilkowski, B. W. (2002). Pheromone components of the wheat stem sawfly: identification, electrophysiology, and field bioassay. J. Chem. Ecol. 28, 407–423. doi: 10.1023/A:1017946527376
Criddle, N. (1923). The life habits of Cephus cinctus Nort. in Manitoba. Can. Entomol. 55, 1–4. doi: 10.4039/Ent551-1
Delaney, K., Weaver, D., and Peterson, R. (2010). Photosynthesis and yield reductions from wheat stem sawfly (Hymenoptera: Cephidae): interactions with wheat solidness, water stress, and phosphorus deficiency. J. Econ. Entomol. 103, 516–524. doi: 10.1603/EC09229
DePauw, R. M., Townley-Smith, T. F., Humphreys, G., Knox, R. E., Clarke, F. R., and Clarke, J. M. (2005). Lillian hard red spring wheat. Can. J. Plant Sci. 85, 397–401. doi: 10.4141/P04-137
Farstad, C. W. (1940). The development of Western wheat stem sawfly (Cephus cinctus Nort) in various host plants as an index of resistance. Ph.D. dissertation, Iowa State University, Retrieved from https://lib.dr.iastate.edu/rtd/13540 (accessed May 5, 2020).
Farstad, C. W., Platt, A. W., and McGinnis, A. J. (1949). “Influence of wheat varieties on the sex ratio of the wheat stem sawfly, Cephus cinctus (Hymenoptera: Cephidae),” in 80th annual report of the Entomological Society of Ontario, ed W. E. Heming (Toronto, ON: Printer to the King), 27–28.
Gerling, D., Spivak, D., and Vinson, S. B. (1987). Life history and host discrimination of Encarsia deserti (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Homoptera: Aleyrodidae). Ann. Entomol. Soc. Am. 80, 224–229. doi: 10.1093/aesa/80.2.224
Henter, H. J. (2004). Constrained sex allocation in a parasitoid due to variation in male quality. J. Evol. Biol. 17, 886–896. doi: 10.1111/j.1420-9101.2004.00746.x
Holmes, N. D. (1977). The effect of the wheat stem sawfly, Cephus cinctus (Hymenoptera: Cephidae), on the yield and quality of wheat. Can. Entomol. 109, 1591–1598. doi: 10.4039/Ent1091591-12
Holmes, N. D. (1984). The effect of light on the resistance of hard red spring wheats to the wheat stem sawfly, Cephus cinctus (Hymenoptera:Cephidae). Can. Entomol. 116, 677–684. doi: 10.4039/Ent116677-5
Holmes, N. D., and Peterson, L. K. (1958). “Oviposition and survival of the wheat stem sawfly, Cephus cinctus Nort. (Hymenoptera: Cephidae), in various hosts,” in Proceeding of the 10th International Congress of Entomology (Montreal). 3:459.
Holmes, N. D., and Peterson, L. K. (1963). Effects of variety and date of seeding spring wheats and location in the field on sex ratio of the wheat stem sawfly, Cephus cinctus Nort. (Hymenoptera: Cephidae). Can. J. Zool. 41, 1217–1222. doi: 10.1139/z63-101
Karban, R., Baldwin, I. T., Baxter, K. J., Laue, G., and Felton, G. W. (2000). Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125, 66–71. doi: 10.1007/PL00008892
Lesieur, V., Martin, J.-F., Weaver, D. K., Hoelmer, K. A., Smith, D. R., Morrill, W. L., et al. (2016). Phylogeography of the wheat stem sawfly, Cephus cinctus Norton (Hymenoptera: Cephidae): implications for pest management. PLoS ONE 11:e0168370. doi: 10.1371/journal.pone.0168370
Lestina, J., Cook, M., Kumar, S., Morisette, J., Ode, P. J., and Peairs, F. (2016). MODIS imagery improves pest risk assessment: a case study of wheat stem sawfly (Cephus cinctus, Hymenoptera: Cephidae) in Colorado, USA. Environ. Entomol. 45, 1343–1351. doi: 10.1093/ee/nvw095
Macedo, T. B., Weaver, D. K., and Peterson, R. K. D. (2007). Photosynthesis in wheat at the grain filling stage is altered by larval wheat stem sawfly (Hymenoptera: Cephidae) injury and reduced water availability. J. Entomol. Sci. 42, 228–238. doi: 10.18474/0749-8004-42.2.228
Mackay, M. R. (1955). Cytology and parthenogenesis of the wheat stem sawfly, Cephus cinctus, Norton (Hymenoptera: Cephidae). Can. J. Zool. 33, 161–174. doi: 10.1139/z55-011
McGinnis, A. J. (1950). Sex ratio studies on the wheat stem sawfly, Cephus cinctus Nort. Master Thesis, Montana State University, 63.
Morrill, W. L., Gabor, J. W., Weaver, D. K., Kushnak, G. D., and Irish, N. J. (2000). Effect of host plant quality on the sex ratio and fitness of female wheat stem sawflies (Hymenoptera: Cephidae). Environ. Entomol. 29, 195–199. doi: 10.1093/ee/29.2.195
Morrill, W. L., and Weaver, D. K. (2000). Host plant quality and male wheat stem sawfly (Hymenoptera: Cephidae) fitness. J. Entomol. Sci. 35, 478–482. doi: 10.18474/0749-8004-35.4.478
Özberk, I., Atl, A., Yucel, A., Ozberk, F., and Coskun, Y. (2005). Wheat stem sawfly (Cephus pygmaeus L.) damage; impacts on grain yield, quality and marketing prices in Anatolia. Crop Protection 24, 1054–1060. doi: 10.1016/j.cropro.2005.03.006
Peterson, L. K., McKenzie, H., and Holmes, N. D. (1968). Establishment of a nursery for evaluation of resistance of strains of wheat to the wheat stem sawfly. J. Econ. Entomol. 61, 836–838. doi: 10.1093/jee/61.3.836
Platt, A. W. (1941). The influence of some environmental factors on the expression of the solid stem character in certain wheat varieties. Sci. Agric. 23, 139–151.
R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. (Vienna). Available online at: https://www.R-project.org/
Rand, T. A., Waters, D. K., Blodgett, S. L., Knodel, J. J., and Harris, M. O. (2014). Increased area of a highly suitable host crop increases herbivore pressure in intensified agricultural landscapes. Agric. Ecosyst. Environ. 186, 135–143. doi: 10.1016/j.agee.2014.01.022
Salmon, D. F., Cortez, M. J., Helm, J. H., Jedel, P. E., and Duggan, T. R. (1997). Registration of 'Pronghorn' triticale. Crop Sci. 37, 1392–1393. doi: 10.2135/cropsci1997.0011183X003700040082x
Shanower, T. G., and Hoelmer, K. A. (2004). Biological control of wheat stem sawflies: Past and future. J. Agric. Urban Entomol. 21, 197–221. Available online at: https://www.researchgate.net/profile/Kim_Hoelmer/publication/260364047_Biological_control_of_wheat_stem_sawflies_Past_and_future/links/02e7e530e35202f778000000/Biological-control-of-wheat-stem-sawflies-Past-and-future.pdf
Varella, A. C., Weaver, D. K., Peterson, R. K. D., Sherman, J. D., Hofland, M. L., Blake, N. K., et al. (2017). Host plant quantitative locie affect specific behavioral sequences in oviposition by a stem-mining insect. Therorethical Appl. Genet. 130, 187–197. doi: 10.1007/s00122-016-2805-0
Wall, A. (1952). The diameter of the wheat stem in relation to the length and sex of emerging sawfly (Cephus cinctus Nort.). Sci. Agric. 32, 272–277.
Wang, X.-Y., Yang, Z.-Q., Wu, H., and Gould, J. R. (2008). Effects of host size on the sex ratio, clutch size, and size of adult Spathius agrili, an ectoparasitoid of emerald ash borer. Biol. Control 44, 7–12. doi: 10.1016/j.biocontrol.2007.10.011
Weaver, D. K., Buteler, M., Hofland, M. L., Runyon, J. B., Nansen, C., Talbert, L. E., et al. (2009). Cultivar preferences of ovipositing wheat stem sawflies as influenced by the amount of volatile attractant. J. Econ. Entomol. 102, 1009–1017. doi: 10.1603/029.102.0320
Wu, X.-H., Cárcamo, H. A., Beres, B. L., and Pang, B.-P. (2011). Parasitoid (Bracon cephi) effects on grain yield of selected genotypes of wheat infested by Cephus cinctus. Aust. J. Crop Sci. 5, 1102–1107. Available online at: https://www.cropj.com/carcamo_5_9_2011_1102_1107.pdf
Keywords: Cephus cinctus, sex ratio, fitness, yield, solid stem
Citation: Cárcamo H, Beres B, Wu X, Larson T and Schwinghamer T (2020) Effect of Plant Density on Wheat Stem Sawfly Sex Ratio. Front. Agron. 2:4. doi: 10.3389/fagro.2020.00004
Received: 23 January 2020; Accepted: 17 April 2020;
Published: 27 May 2020.
Edited by:
Jeremy Dean Allison, Canadian Forest Service, CanadaReviewed by:
David Keith Weaver, Montana State University, United StatesCopyright © 2020 Cárcamo, Beres, Wu, Larson and Schwinghamer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Héctor Cárcamo, aGVjdG9yLmNhcmNhbW9AY2FuYWRhLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.