
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 03 September 2020
Sec. Veterinary Neurology and Neurosurgery
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00610
This article is part of the Research Topic Canine Intervertebral Disc Disease: The Current State of Knowledge View all 11 articles
 Sarah A. Moore1*
Sarah A. Moore1* Andrea Tipold2
Andrea Tipold2 Natasha J. Olby3
Natasha J. Olby3 Veronica Stein4
Veronica Stein4 Nicolas Granger5,6 and Canine Spinal Cord Injury Consortium (CANSORT SCI)7
Nicolas Granger5,6 and Canine Spinal Cord Injury Consortium (CANSORT SCI)7Intervertebral disc extrusion (IVDE) is one of the most common neurologic problems encountered in veterinary clinical practice. The purpose of this manuscript is to provide an overview of the literature related to treatment of acute canine thoracolumbar IVDE to help construct a framework for standard care of acute canine thoracolumbar IVDE where sufficient evidence exists and to highlight opportunities for future prospective veterinary clinical research useful to strengthen care recommendations in areas where evidence is low or non-existent. While there exist a number of gaps in the veterinary literature with respect to standards of care for dogs with acute thoracolumbar IVDE, recommendations for standard care can be made in some areas, particularly with respect to surgical decompression where the currently available evidence supports that surgery should be recommended for dogs with nonambulatory paraparesis or worse. While additional information is needed about the influence on timing of decompression on outcome in dogs that are deep pain negative for longer than 48 h duration, there is no evidence to support treatment of the 48 h time point as a cut off beyond which it becomes impossible for dogs to achieve locomotor recovery. Surgical decompression is best accomplished by either hemilaminectomy or mini-hemilaminectomy and fenestration of, at a minimum, the acutely ruptured disc. Adjacent discs easily accessed by way of the same approach should be considered for fenestration given the evidence that this substantially reduces future herniation at fenestrated sites. Currently available neuroprotective strategies such as high does MPSS and PEG are not recommended due to lack of demonstrated treatment effect in randomized controlled trials, although the role of anti-inflammatory steroids as a protective strategy against progressive myelomalacia and the question of whether anti-inflammatory steroids or NSAIDs provide superior medical therapy require further evaluation.
Intervertebral disc extrusion (IVDE) is one of the most common neurologic problems encountered in veterinary clinical practice (1). Dogs with acute IVDE present with a spectrum of neurologic abnormalities caused by a combination of compression and contusion to the spinal cord via sudden extrusion of degenerated and calcified nucleus pulposus of the intervertebral disc through the annulus fibrosus and into the vertebral canal (2). Severity of clinical injury spans a continuum from paraspinal hyperesthesia up to paraplegia with loss of deep pain perception, where these patients with loss of deep pain perception are often termed “deep pain negative.” Injury grades are typically described as summarized in Table 1 (3, 4). Treatment recommendations for an individual dog with IVDE are based on a combination of factors, accounting for the aforementioned severity of neurologic signs presented, availability of specialty care in a geographical area, and preferences and financial limitations of the owner. Available treatment options include medical management (often termed “conservative therapy”), consisting of strict activity restriction, physiotherapy, analgesics and anti-inflammatory medications; or surgical decompression of the spinal cord to remove herniated material from the vertebral canal, followed by similar activity restriction, bladder management if needed, and pain management recommendations. The evidence available in the veterinary literature to guide practitioners in recommending one therapeutic approach over another in dogs with IVDE is relatively low as most published veterinary studies are either retrospective in nature or prospective case series. Some randomized clinical trials and two recent systematic review and meta-analyses are available to inform care recommendations; however, this small database of strong clinical evidence has resulted in a lack of uniform, science-based guidelines for the management of acute canine thoracolumbar IVDE.

Table 1. Modified Frankel scale used to describe the degree of neurologic impairment for dogs with intervertebral disc herniation.
The purpose of this manuscript is to build on previously published studies by incorporating broad historical and contemporaneous clinical data to construct a framework for standard care of acute canine thoracolumbar IVDE where sufficient evidence exists and to highlight opportunities for future prospective veterinary clinical research useful to strengthen care recommendations in areas where evidence is low or non-existent. While a number of distinct clinical presentations of intervertebral disc disease occur in dogs, this paper focuses specifically on acute thoracolumbar IVDE, from here on referred to as IVDE, for which the largest body of evidence exists to base treatment recommendations.
The first description of the clinical presentation of IVDE in dogs is credited to Dexler, who in the late 1800's described a condition of paralysis in dogs caused by compression of the spinal cord from abnormalities of the intervertebral disc that he termed “neoformations” (5). He attributed the neoformations to proliferation of the intervertebral disc, a hypothesis supported by subsequent works that used the term endochondrosis intervertebralis to describe the condition. It wasn't until the late 1930's and 1940's that authors began to suggest that these neoformations might in fact be herniation of the intervertebral disc similar to what had been observed in people (6, 7). In 1951 and 1952, Olsson and Hansen (respectively), published in-depth investigations of the syndrome; both supported the fact that these neoformations were in fact disc herniations (5, 8). Hansen's report on the condition further highlighted three breeds with an apparently high risk for the condition: the French bulldog, the dachshund, and the Pekingese (8).
Early diagnosis of IVDE was made by radiography, myelography and pathology. Improvement after conservative treatment was described in individual cases (7). Additional in-depth studies were performed by Hoerlein and published in 1956 and were summarized again after more than 30 years experience (9, 10). Diagnosis evolved over time to become based on physical and neurological examination, radiography, myelography and occasionally tomography. At that time, conservative therapy was recommended for dogs with spinal pain as the only clinical sign, for dogs with a first episode of IVDE and paresis, dogs who had IVDE in combination with other medical disorders, those with paralysis and no conscious perception of deep pain, and those with evidence of progressive myelomalacia (9). Conservative therapy consisted of general good nursing care including proper nutrition, cage rest, ensuring a clean environment, prevention of decubital sores, and bladder and bowel care. Various protocols for glucocorticoid administration were recommended and applied based on coincident clinical research, starting with dexamethasone, and then, in later years, methylprednisolone (11, 12). Physiotherapy, which included limb exercises and swimming, was recommended although a controlled study had not been undertaken at that time. Medications to control pain were recommended, but clinical texts emphasized that pain should not be completely relieved in the outpatient setting due to concern that a completely comfortable patient might undertake excessive movements and resist cage rest.
Hoerlein personally observed 1,184 dogs with IVDE between 1950 and 1975, finding good surgical results in 87% of paraplegic dogs and in 91% of paretic dogs (10). While a true comparative study was never carried out, the rate of functional recovery was described as much lower for cases managed conservatively, where only 22% of paraplegic dogs recovered the ability to ambulate without assistance (13). Additional cases of dogs who ranged from ambulatory paraparetic to paraplegic with deep pain sensation intact managed conservatively and published between 1950 and 1970 suggested that about half of cases recovered the ability to ambulate without surgery. Therefore, surgical treatment was recommended in cases with pain and/or paresis not responding to conservative care and in cases with substantial neurologic deficits where deep pain sensation was preserved with a reported success rate of 75–90% (9, 10, 14).
While historical evaluation of outcomes associated with surgical decompression in dogs with IVDE suggests that decompression offers improved recovery over conservative management for dogs with severe injuries, no studies have systematically compared outcomes between dogs managed medically and surgically for the condition. Beyond this, additional questions remain in the veterinary neurosurgical community regarding the importance of urgent decompression in deep pain negative dogs; the need for prophylactic fenestration to lessen recurrence of IVDE in dogs at high risk; and the value of neuroprotective strategies and post-operative interventions such as activity restriction and physiotherapy. The following sections discuss the relevant veterinary literature with respect to each area.
While only a few contemporary studies exist reporting medical management in dogs with severe neurologic deficits due to IVDE, and no randomized controlled studies compare these to surgical treatment, there is a substantial amount of historical literature from which to draw some basis for comparison (13, 15, 16). One difficulty in evaluating outcomes from early reports of canine IVDE is that patient assessment, diagnostic approach, terminology and description of neurologic deficits, and injury grading differ from more recent literature and make it challenging to draw strong conclusions about neurologic grade at presentation and its relationship to long-term outcome. A summary of reported outcomes for dogs with severe IVDE managed medically and published before 1983 is presented in Table 2, and a recent systematic review and meta-analysis compares results between medical and surgical management for cases published after 1983 (17). Reported outcomes for dogs managed medically after severe (non-ambulatory paraparetic or worse) neurologic injury due to IVDE described in more recent publications range from 50 to 100%, depending on the severity of injury and the study (17–24). Clinicians anecdotally suggest that while recovery of ambulation after surgical vs. medical management in dogs with paraparesis or paraplegia with intact deep pain sensation may ultimately be comparable, recovery is quicker and more complete for dogs that undergo surgical decompression (25). At present, most board-certified neurologists and orthopedic surgeons recommend surgical decompression for dogs who are non-ambulatory paraparetic or worse secondary to IVDE suggesting that this is the current standard of care (26).
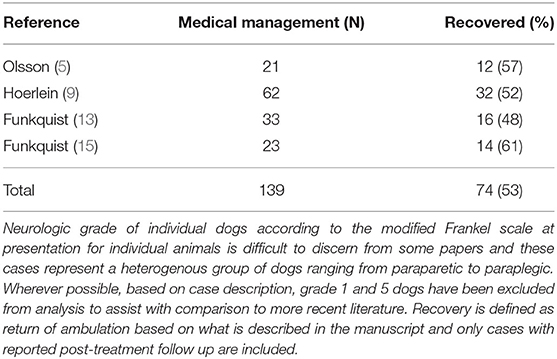
Table 2. Synthesis of outcome data from dogs managed medically for intervertebral disc herniation and published before 1983.
The question of whether or not surgical decompression should be considered standard for dogs with substantially compressive IVDE has been raised and revisited intermittently across the veterinary literature (16, 27, 28). Indeed, several notable studies report that a portion of dogs with substantially compressive IVDE can recover with conservative therapy alone, and that in some cases extruded disc material may even dissipate over time (29–31). Clinicians likely make their current recommendations for surgery based on both the historical literature and having absorbed implications from the field of experimental spinal cord injury suggesting that very early surgical decompression leads to enhanced recovery (32). The drive to perform surgical decompression in the most severe cases may also emanate, in part, from “modern” owner's expectations, our perception of animal welfare in recent decades, and the fact the demonstration of compression on cross-sectional imaging drives an impulse to decompress. Additional influence may also include fairly predictable outcomes with surgery, allowing dog owners to opt for surgery based on concrete numbers; however, no prospective randomized trial has evaluated conservative management vs. surgical decompression in dogs with severe IVDE. Currently, the likelihood of this study happening is low, given the lack of clinical equipoise across the veterinary community related to the currently available data indicating the value of decompression.
While no randomized prospective clinical trials have compared these two treatment options, we can draw some inferences from the existing literature to form a framework for discussing the value of decompressive surgery. In addition to the historical literature available for review, Langerhuus and Miles conducted a systematic review and meta-analysis of dogs with IVDE published between 1983 and 2012 and treated either medically or surgically (17). For dogs with severe injuries (non-ambulatory paraparetic or worse), a statistically greater proportion recovered independent ambulation following decompression via hemilaminectomy as compared to conservative treatment. Specifically, 93% of dogs who were non-ambulatory paraparetic or paraplegic with intact deep pain perception recovered ambulation after surgery whereas only 79% of dogs with non-ambulatory paraparesis and 62% of dogs who were paraplegic with intact deep pain perception recovered after conservative treatment alone. For dogs with the most severe injuries, those who were paraplegic and deep pain negative, the recovery rate was 61% after surgical decompression and 10% for those with conservative treatment (although of note here, data from only 25 deep pain negative dogs managed conservatively was available for inclusion). A summary of neurologic outcomes, as reported in this meta-analysis by injury grade is presented in Table 3. The influence of surgical decompression on time to return of independent ambulation (e.g., speed of recovery) is more difficult to assess via synthesis of previous studies. Fewer data points are available for inclusion in meta-analysis and preclude analysis of recovery by neurologic grade for all severities except paraplegia with intact deep pain perception; however, for dogs with paraplegia with intact deep pain perception, mean time to recovery of ambulation is significantly shorter after surgical decompression compared with conservative therapy (15 vs. 84 days, respectively) (17).
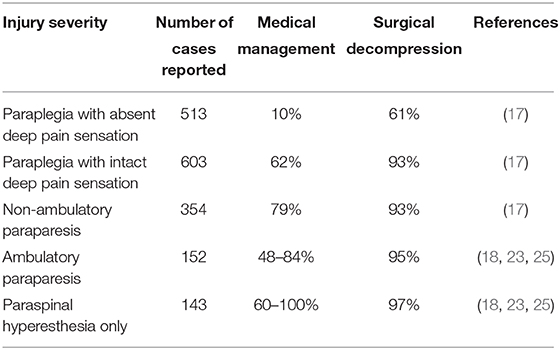
Table 3. Overall percent of dogs achieving neurologic recovery (defined as independent ambulation) based on presenting injury severity, as reported in the literature after 1983 by Langerhuus and others for medical and surgical management of canine thoracolumbar interverterbral disc herniation (IVDH).
Taken in its entirety, synthesis of the previously published veterinary literature supports the standard recommendation for surgical decompression in dogs that are non-ambulatory paraparetic or worse, and that surgery speeds recovery of ambulation and overall locomotor outcome in dogs with severe injuries. There are some limitations to the data used to come to this conclusion that must be acknowledged. Since no prospective, randomized studies are available, all synthetized reports comparing outcome incorporate data only from prospective case series and retrospective studies. Additionally, sufficiently detailed outcomes for dogs managed conservatively are available for a relatively small number of published cases, with only 113 cases described in the literature published since 1983 and a similarly small number described before that date. In comparison, >1,500 surgically treated cases and their associated outcomes are described over the same time period. The paucity of published medically managed cases with severe IVDE, while not surprising given current clinical standards, likely confounds comparison of outcomes. Limited publication of medically managed cases probably results from several factors that bias the entirety of the published literature on canine IVDE. First, there is an overall pre-existing clinical inclination toward recommending decompression for dogs that are non-ambulatory paraparetic or worse due to IVDE. In the face of severe clinical signs, clinicians are naturally driven to administer a treatment because they can. Second, most published case series originate from veterinary specialty referral centers where access to advanced care and recommendation for and compliance with decompressive surgery are likely to be higher. Few published cases originate from primary care facilities, where the rate of and approaches toward medical management are likely to be different. Third, data available and used for synthesis of the literature is largely of retrospective nature. Therefore, caution should be used in over interpretation.
As discussed above, while some population-level analysis of clinical data for dogs with severe injury from IVDE exists to guide treatment recommendations, there is a current gap in the veterinary literature with respect to medical and surgical outcomes for dogs with more mild injuries (those who have paraspinal hyperesthesia or ambulatory paraparesis). Because these dogs were not included in subgroup analysis of outcome in a recent meta-analysis, outcomes for those dogs are summarized in Table 3 from previous literature (18, 23, 25). While some clinicians steer owners toward conservative management in these more mildly affected cases, 12 and 59% of neurologists and orthopedic surgeons (respectively), report routinely recommending surgical decompression for dogs with a first episode of IVDE causing back pain or ambulatory paraparesis (26). The evidence to guide practitioners in these more mildly affected cases is currently lacking as few studies specifically examine outcome for dogs in this context. To the authors' knowledge, no large-scale published comparison exists for dogs these dogs with less severe injuries treated medically vs. surgically, although one study reported a 54.7% overall success rate for medical management of thoracolumbar IVDE in a cohort of dogs for which 83% were ambulatory on presentation, and several other studies report surgical outcome for a small number of dogs with mild injuries (23). Hurdles to designing randomized controlled studies for dogs with mild IVDE include the difficulty in obtaining a definitive diagnosis for dogs that are not managed surgically, and the fact that most mildly affected dogs are treated at primary care facilities without referral to a specialist. Albeit more challenging to quantify in dogs, and while recovery of locomotion is of considerable importance to owners of dogs with IVDE, relevant long-term outcomes for dogs with mild injuries might also focus on pain and quality of life measures. Given the fact that a substantial portion of dogs with IVDE present with only pain or mild neurologic deficits, large scale prospective studies could be ethically conducted in this area and represent an opportunity to improve our understanding of best practices in treating this substantial patient population (33–37). Well-powered and informative prospective studies in this area will likely require partnership between specialty referral hospitals and primary care facilities and may require the development of novel clinical assessment tools. In particular, it would be useful to conduct a longitudinal study—from puppy to end of life—recording the environment, health and behavior in breeds at risks of IVDE. Feasibility and utility of generating powerful epidemiological data to answer health questions spanning the life course has been previously demonstrated in dogs, cats, and people (38, 39).
A variety of descriptions of surgical approaches to address canine thoracolumbar IVDE have been previously published. These include hemilaminectomy, mini-hemilaminectomy/pediculectomy, dorsal laminectomy, partial corpectomy, and fenestration of the intervertebral disc with or without concurrent laminectomy for removal of herniated disc material (34, 40–42).
Early studies reported spinal decompression procedures for canine IVDE via either dorsal laminectomy or hemilaminectomy. Hoerlein and colleagues described a procedure for hemilaminectomy in detail, whereas Funkquist explored procedures for dorsal laminectomy (15, 43). A study published by Hoerlein in 1978, and using a questionnaire gathering information from 50 participating veterinary surgeons about the use of surgical approaches for treatment of IVDE, suggested that the best surgical results were obtained after hemilaminectomy and fenestration (44). These results were later confirmed in a second prospective but non-randomized study comparing hemilaminectomy to dorsal laminectomy for treatment of thoracolumbar IVDE, where hemilaminectomy was reported to significantly improved the surgeon's ability to retrieve herniated disc material and where this enhanced removal of herniated disc was associated with improved early locomotor recovery (45).
Today, hemilaminectomy with or without removal of the articular processes is most commonly described and represents the current decompressive procedure of choice for veterinary spinal surgeons. A recent survey indicated that 95% percent of veterinary neurologists and surgeons typically perform a hemilaminectomy or mini-hemilaminectomy in this scenario (26). While each disc herniation is different, and requires unique consideration for what constitutes the best surgical approach, efficiency in disc retrieval, reduced opportunity for laminectomy membrane formation, and reduced chance of postoperative neurologic decline likely form the relatively broad surgical consensus for hemilaminectomy in the surgical treatment of routine thoracolumbar IVDE (45–47). Table 4 summarizes the published literature relating to surgical approaches for canine IVDE, including benefits and unique challenges of each approach.
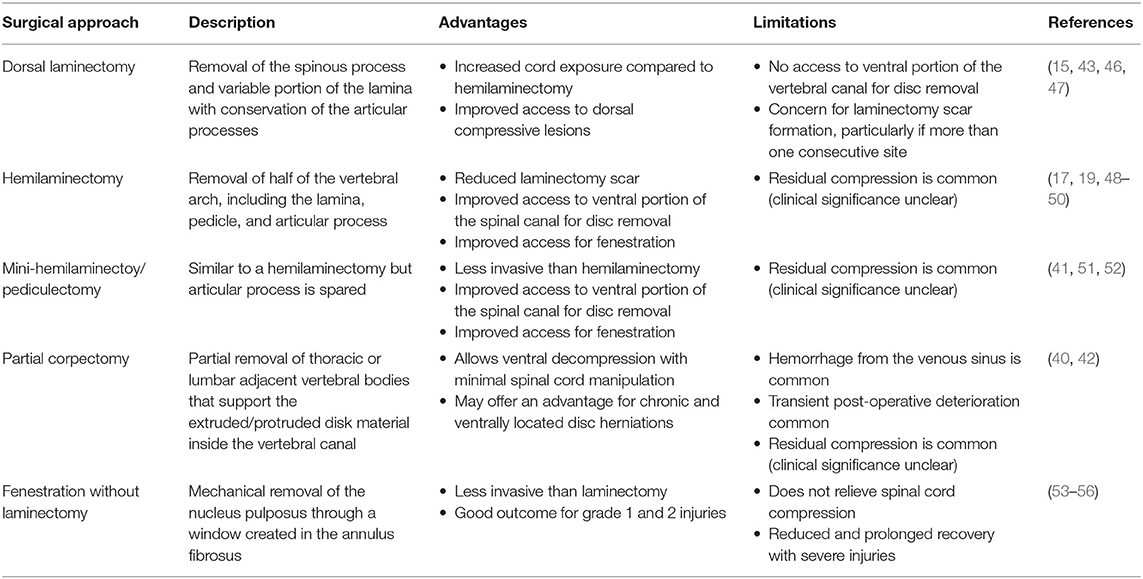
Table 4. A summary of published surgical approaches and reported outcomes for canine acute intervertebral disc herniation (IVDH) affecting the thoracolumbar spine.
Fenestration of the intervertebral disc without spinal cord decompression has also been historically proposed as a viable option for treatment of IVDE, with the idea re-introduced more recently into the veterinary literature by way of a systematic review presenting outcomes of previously published cases (27, 53, 54). While most articles on fenestration address its role in prophylaxis of recurrent disc extrusion (the arguments for and against that approach are presented below), some authors have suggested that fenestration alone may be useful to facilitate recovery of spinal cord function following IVDE. When first described as a therapeutic intervention for IVDE, the aim of fenestration was, in fact, to reduce intradiscal pressure with the goal of reducing a presumed dynamic lesion of the portion of the disc herniated into the epidural space (56). Dogs with IVDE ranging from pain-only to paraplegia with intact deep pain perception, treated only with lateral fenestration of the intervertebral disc were reported to experience a relatively high recovery rate; however, only a 33% recovery rate was reported for paraplegic deep pain negative dogs as compared to the 50–60% recovery rate typically observed for this group after hemilaminectomy (17, 20, 55). Similar to conservative management in deep pain negative dogs, the number of deep pain negative dogs managed with fenestration alone and published in the literature is quite small. Data also originates almost exclusively from retrospective studies, making outcome evaluation challenging. A recent systematic review suggests that outcome for dogs with mild injuries undergoing fenestration alone could be better than previously suggested; however, a retrospective study of 331 dogs undergoing percutaneous disc ablation without decompression (and thus a procedure similar to fenestration alone) demonstrated a recovery rate of only 38% for dogs with deep pain negative injuries, reinforcing the concept that patients with severe injuries benefit from decompression (27, 57). Currently, <10% of veterinary neurologists and orthopedic surgeons report routinely performing fenestration without concurrent decompression (26). This practice pattern may be influenced by previous work suggesting that 80% of dogs presented with back pain as their only clinical sign still have significant spinal cord compression, and thus logically might benefit from decompression (58). Of those surgeons who perform fenestration alone, most indicate they recommend this approach only for dogs with a presenting complaint of spinal pain alone or spinal pain with minimal neurologic deficits (26). At present, evidence to support fenestration alone as a viable surgical approach for canine IVDE is limited and historical literature supports that decompression of the spinal cord, with or without concurrent fenestration, provides improved recovery for dogs with severe (non-ambulatory paraparetic or worse) IVDE (Table 3). Lacking from the current literature is data on the incidence of postoperative chronic pain when fenestration is used without concurrent decompression.
When considering the evidence related to the timing of decompression, two clinically important questions arise. Those center on whether decompression should be performed urgently for dogs with severe injuries, and whether dogs that are deep pain negative for a prolonged duration have a reasonable potential for recovery after surgical decompression.
Early studies, and most veterinary neurosurgery texts, suggest that timing of decompression influences outcome, particularly for dogs who are deep pain negative secondary to IVDE. Early decompression is typically encouraged, with a recommended timeframe ranging from 12 to 48 h, beyond which prognosis is often suggested to worsen significantly. These recommendations originate from several retrospective studies, most of which include very few dogs with an injury duration of 48 h or greater. This sentiment seems to persist in spite of several studies that have shown the contrary, noting good functional recovery in some deep pain negative dogs with extended injury duration (72 h or more). A challenge in drawing firm conclusions on this topic is the small number of published deep pain negative cases with a duration of injury longer than 48 h and a confirmed postoperative outcome. Jeffery et al. recently evaluated the influence of a variety of clinical factors on outcome in 78 deep pain negative dogs using a prospective multicenter cohort study design (59). Similar to previous reports, his group was also unable to find an association between duration of deep pain negative status and outcome in this patient population; however, a relatively small number of dogs were available for inclusion where the duration of onset of locomotor dysfunction and initial evaluation at a referral center was >48 h. Currently, some clinicians treat this 48 h time point prior to referral as a “cliff” or abrupt point beyond which recovery in deep pain negative dogs cannot be achieved. There is certainly no literature to support this interpretation but the influence of prolonged deep pain negative status on recovery rate, and importantly on extent of recovery, is also not clear because the number of published cases in any one study is low. Designing a large-scale randomized controlled trial assessing the influence of duration of deep pain negative status on outcome after surgical decompression would be ethically challenging; however, a systematic review and meta-analysis of previously published cases could yield additional valuable information and may assist with guiding owners regarding prognosis for locomotor recovery and the overall utility of surgical decompression for more chronic cases. Larger scale, prospective longitudinal cohort studies could also be helpful and could leverage existing resources already in use across veterinary referral networks (60).
Recommendations for urgent decompression, particularly for dogs who are deep pain negative, likely stem from some of the previously mentioned studies on surgical outcomes as well as from the experimental and human spinal cord injury literature where some studies suggest that early decompression is associated with enhanced locomotor recovery; however, the human clinical literature is mixed with regard to the effect of timing of decompression on outcome. A more recent prospective, multi-institutional cohort study of 888 patients with acute spinal cord injury failed to demonstrate an influence of timing of decompression on outcome across the entire cohort and specifically in the group of ASIA Impairment Scale (AIS)- A individuals, those with a clinical injury severity somewhat analogous to deep pain negative status. Interestingly, this study did show an association between improved locomotor outcome and early decompression (<24 h) in groups of patients with incomplete (paresis or plegia with pain sensation intact) injuries (61). What becomes difficult in terms of comparison between dogs and people is that standard recovery curves differ substantially between the two species. Where the reported outcome for locomotor recovery in deep pain negative dogs ranges from 50 to 60%, the incidence of recovery in people with equivalent injuries is much lower; thus drawing strong direct parallels between recovery curves for the two can be challenging. It is likely that people with sensorimotor complete (deep pain negative) injuries represent a much more “complete” injury in many cases and therefore the ability to influence recovery may be more limited whereas those with less complete injuries may be more amenable to intervention. Additionally, most people presenting with spinal cord injury are polytrauma patients with acute concerns related to hypotension, internal injuries and other co-morbid conditions. Therefore, delay in decompression is often necessary in favor of stabilization of the patient for general anesthesia. In most dogs with IVDE, this is not the case and delay of anesthesia for medical reasons is rarely necessary.
As noted above, a recent study was not able to demonstrate improved neurologic outcome in deep pain negative dogs with early surgical intervention, although most patients included in that study were referred for decompressive surgery within 24 h, therefore potentially confounding the ability to demonstrate associations (59). Interestingly, while another recent retrospective study by Castel et al. also supported the lack of influence of timing of surgery on locomotor recovery, this study did find an association between delay of decompression beyond 12 h and increased risk of progressive myelomalacia, an uncommon but often fatal phenomenon observed almost exclusively in dogs who are deep pain negative secondary to IVDE (62).
Taken in total, the evidence in the veterinary literature supporting the need for emergent/immediate decompression in dogs that are deep pain negative secondary to IVDE is low and there likely exists a subset of dogs with severe spinal cord injury that, due to the severity of their injury, will not improve regardless of speed of intervention; however, the evidence to the contrary is also low and a threshold beyond which outcome may worsen has not been established. While warranting further investigation, an increased risk of myelomalacia with delayed decompression might support the recommendation to undertake decompression in severely injured dogs ideally within 12–24 h. The current literature lacks data specific to dogs that are paraplegic with intact deep pain, as these dogs are often grouped either with non-ambulatory paraparetic dogs, or with paraplegic deep pain negative dogs depending on study design. Specifically, it is not known how many dogs progress from paraplegic or paraplegic and deep pain negative when left briefly untreated, e.g., overnight.
Perhaps the most historically controversial issue in veterinary neurosurgery centers on the question of prophylactic fenestration of the intervertebral disc both at sites of current extrusion and at distant sites. The concept of intervertebral disc fenestration has been advanced by some veterinary spinal surgeons as a preventative measure which can be taken at the time of decompressive surgery to reduce future extrusion of disc material at sites adjacent to those affected at the time of the original procedure (34). Typically, fenestration of intervertebral discs between T11 and L4 are approached dorsolaterally or laterally at the time of surgery for a extruded disc, and a window is made into the annulus fibrosus with various means employed to evacuate any degenerated nucleus pulposus in situ (63–68). Fenestration of the L4-5 and L5-6 spaces is not typically performed due to concern for injury of the nerve roots essential for weight bearing at that location (69). In the context of acute canine IVDE, fenestration is performed “always” or “most of the time” by 69% of board-certified neurologists and 36% of board-certified surgeons (26). Clinicians who do not routinely fenestrate cite concerns including questionable efficacy; prolonged surgical time; complications such as hemorrhage, pneumothorax or nerve root injury; variable success in removal of in situ nucleus pulposus; potential for introduction of additional disc material into the vertebral canal; induction or worsening of degenerative changes to non-herniated discs, and the concern for adjacent segment disease (70, 71). Clinicians who do routinely fenestrate cite a recurrence rate as high as 40% for IVDE and the fact that dogs who present for a second bout of surgical IVDE have a rate of euthanasia as high as 44%, often due to financial concerns of the owner (34, 72).
A number of large-scale retrospectives, and two prospective studies have evaluated the effect of fenestration on recurrence of IVDE (34, 36, 66, 69, 72, 73). It is clear from these and other studies that the likelihood of recurrence increases with the number of calcified discs in situ present. All studies support the concept that prophylactic fenestration is generally successful in reducing future extrusion of disc material at fenestrated disc spaces and that second disc extrusions, when they occur in dogs that have undergone prophylactic fenestration in conjunction with a history of previous decompressive surgery, are more likely to happen at non-fenestrated sites (34, 73). Results of large-scale contemporary studies evaluating outcome and recurrence after hemilaminectomy with fenestration are detailed in Table 5. Of note, of the >1,100 cases of surgical fenestration and associated outcome reported in the studies, complications from fenestration were noted in only 15 cases (0.01%), suggesting that fenestration is a safe procedure and concern for surgical complication of various types may not be a valid reason for choosing not to fenestrate.
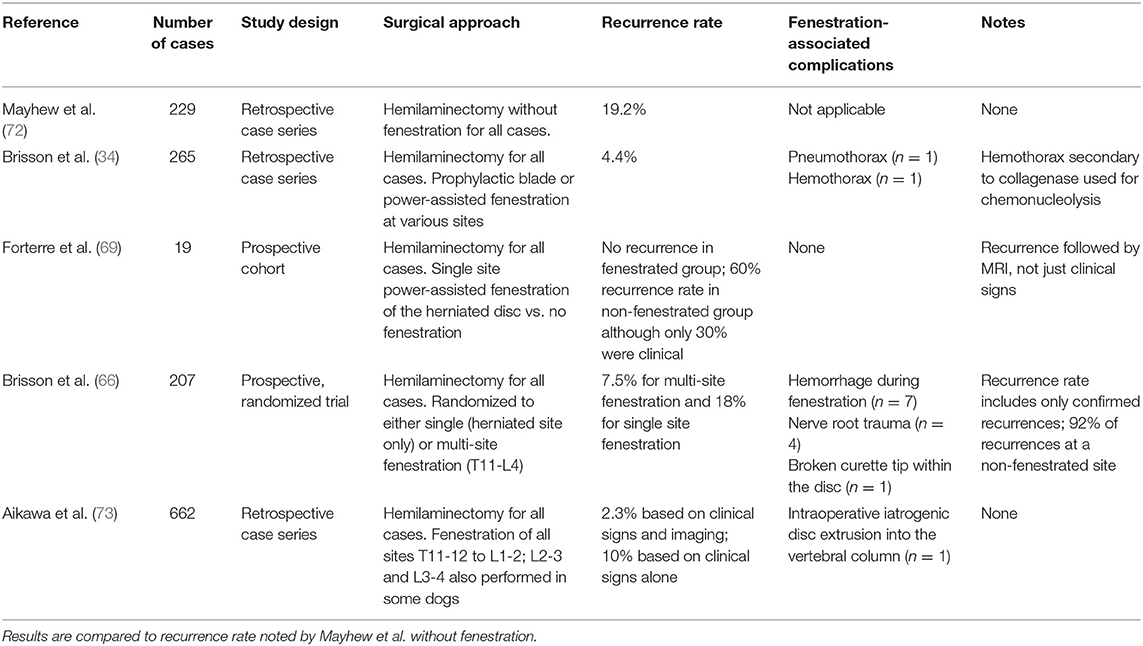
Table 5. Results of recent large-scale contemporaneous studies evaluating outcome and recurrence after hemilaminectomy with fenestration.
Several challenges exist in interpreting the existing literature on fenestration. First, there is no study that prospectively compares, in a randomized fashion, recurrence rate of IVDE in dogs that undergo hemilaminectomy with and without fenestration. As such, clinical demographic factors, surgeon preference and experience, fenestration technique, and other patient- and clinician- level factors likely bias the current literature. Developing a randomized trial to evaluate this question is difficult because veterinarians who fenestrate do so because they believe the literature supports that it is effective to reduce recurrence, and those who do not may not have experience or enthusiasm to do so if participating in a trial. Additionally, comparing recurrence rates between studies is difficult due to differences in outcomes monitoring and reporting. Some patients with a recurrence of IVDE may not return to a specialty care facility for a second incidence of clinical signs, or those with mild signs may be managed medically without confirmatory imaging. Thus, the true incidence and etiology of signs may be underestimated or unclear. To address this concern, longitudinal studies involving the owner could provide valuable data. Inclusion of only confirmed cases of IVDE recurrence in some studies, but not others, also limits comparison between techniques. Even so, the current literature supports prophylactic fenestration as a safe way to reduce future disc herniation at fenestrated sites. Published studies suggest that fenestration reduces the recurrence rate of IVDE, and surgeons should particularly consider fenestration of calcified discs. However, high quality evidence in support of this is not available and it is not known how many sites should be fenestrated to achieve the best outcome, nor is it known what is the effect of fenestration on development of IVDE in adjacent unfenestrated sites of substantial consequence (ex. L4-5 in the patient fenestrated from T11-L4).
Various interventions have been evaluated in the context of acute spinal cord injury, many targeting secondary injury processes such as ischemia and vasospasm, inflammation, free radical production, ion channel disturbances and glutamate excitotoxicity.
The use of high dose steroids, particularly methylprednisolone sodium succinate, in acute spinal cord injury takes its roots from the experimental spinal cord injury literature, where the proposed mechanism of therapy was prevention of lipid peroxidation and secondary free radical injury (74–77). This therapy was evaluated in several high-profile human spinal cord injury trials which showed a potential small treatment effect when administered within 8 h after injury, although the results of those studies, and their clinical implications, remain controversial (78–81). Some experimental evidence, including a study in dogs, had also suggested that this therapy might be less useful than originally anticipated (82). In a recent prospective randomized placebo controlled blinded clinical trial evaluating the effect of MPSS on outcome in paraplegic deep pain negative dogs with IVDE, no treatment effect was observed with respect to locomotor recovery (83).
Many veterinary clinicians continue to use corticosteroids such as prednisone or dexamethasone routinely at lower, anti-inflammatory doses for the management of canine IVDE (26). The question of whether treatment with non-steroidal anti-inflammatories (NSAIDs) or steroids is most appropriate represents a somewhat polarizing issue in veterinary medicine and is highly clinician-dependent. One retrospective study demonstrated decreased odds of successful outcome with conservative therapy, and lower owner-reported quality of life scores, with the use of corticosteroids, irrespective of dose, duration, or specific steroid administered (23). While no study provides a prospective comparison of NSAIDs vs. anti-inflammatory doses of steroids in the management of IVDE in dogs, a recent study retrospectively evaluated clinical risk factors for the development of progressive myelomalacia in deep pain negative dogs and suggested that administration of corticosteroids may have a protective effect (62). The use of steroids at anti-inflammatory doses or NSAIDs in management of canine IVDE is an area where clinical equipoise exists and while controlling for pre-treatment of dogs prior to referral would present a challenge, this question could lend itself to prospective randomized trials evaluating outcome, quality of life, and incidence of myelomalacia between the two treatments.
Polyethylene glycol (PEG) is a surfactant that gained popularity as a possible neuroprotective strategy for acute spinal cord injury. While not entirely understood, the proposed mechanism of PEG is that it acts as a fusogen to repair damaged neuronal cell membranes, prevent ion channel disturbances that lead to cytotoxic edema and secondary injury, and may also stimulate angiogenesis and promote axonal regeneration (84, 85). Studies in experimental models of SCI were encouraging for a positive treatment effect (86–88). Laverty et al. examined the therapy in a prospective open label canine clinical trial for dogs with IVDE (89). The canine study reported a positive treatment effect where a significantly higher number of deep pain negative dogs showed enhanced locomotor improvement but used a group of historical controls for which the recovery rate was less than typically reported for dogs who are deep pain negative secondary to IVDE. Subsequently, a prospective, placebo controlled, randomized, blinded trial evaluating the influence of PEG on outcome in deep pain negative dogs did not demonstrate a positive treatment effect (83).
A host of other neuroprotective strategies have been evaluated in both the laboratory and human clinical setting for treatment of acute spinal cord injury. Most promising based on positive findings in the experimental setting are minocycline, riluzole, and glybenclamide. At this time, there is no published efficacy data on any of these interventions for use in canine IVDE, although all three medications have known pharmacokinetics in the dog making them amenable to future veterinary clinical trials (90–92).
Several other adjunctive therapeutic strategies are not addressed in the present review but have been suggested throughout the veterinary literature. These interventions have only been evaluated in a small number of published studies, and include acupuncture, pulsed electromagnetic field therapy, chiropractic manipulation, and photobiomodulation (93–95).
With respect to the role of physical activity in the development of and recovery from IVDE, several clinical questions exist regarding ideal daily activity levels for prevention of disease in dogs “at risk,” what constitutes appropriate restriction of activity after decompressive surgery, and the role of physiotherapy in post-operative recovery.
The influence of daily activity level on disc degeneration and the development of IVDE has been previously explored by Packer et al. who evaluated the impact of lifestyle on IVDE risk. It was observed that dogs receiving >1 h of daily exercise were less likely to have IVDE compared to dogs receiving <30 min of daily exercise and not allowed to jump on and off furniture (96). This study suggests that, while activity modification is often recommended by clinicians as a preventative measure against IVDE in chondrodystrophic breeds, this recommendation might be counter-productive.
Veterinary neurologists and surgeons tend to view strict activity restriction (also termed “cage rest”) as a vital component of both conservative and post-operative management of IVDE; however, the impact of this recommendation is poorly studied in dogs (26). Cage rest is usually defined as confinement to a small run or cage at all times except when the animal needs to eliminate. Cited goals of cage rest include allowing healing of the annulus fibrosus to prevent further extrusion of nucleus pulposus, prevention of further traumatic injury in an ataxic animal, and reducing pain and inflammation associated with affected nerve roots and meninges (25, 97–99). The duration of recommended cage rest is variable; however, some authors recommend as much as 6–8 weeks (26, 97). Whereas, long-term bed rest has not been shown to be beneficial in people with lumbar disc herniations, the argument for cage rest in the management of canine IVDE might be more logical based on anatomy, underlying pathophysiology, and inherent inability to reason with veterinary patients as to why they should consciously self-limit activity (100, 101). The influence of cage rest on outcome has been examined in only one published retrospective veterinary study in which outcome in dogs managed conservatively for IVDE was not influenced by duration of cage rest (23).
An additional consideration with respect to activity modification is whether some forms of controlled activity, in the form of physiotherapy, might actually be beneficial to locomotor recovery in the postoperative setting, particularly in more severely affected dogs. This rationale stems from the experimental spinal cord injury literature, where a number of studies have demonstrated that intensive locomotor training can promote anatomic and physiologic changes within the injured spinal cord that might result in improved motor function (102–105). However, the experimental literature relating to physiotherapy is mixed, with persistent questions regarding timing and correct complement of activities to result in improved function vs. maladaptive neuroplasticity (106, 107). To a limited degree, some successful findings have translated to the human clinical setting where very small-scale open label studies have shown mild improvements in weight support, stepping, and spasticity with intensive locomotor training programs often coupled with cell-based therapies, or implantable epidural or nerve stimulation devices (108–112).
Various authors have advocated for the role of physiotherapy in canine IVDE, and physiotherapy is routinely recommended by many veterinary neurologists and orthopedic surgeons (26, 95, 113–115). Several studies have evaluated the role of physiotherapy in dogs with severe spinal cord injury caused by IVDE (93, 115, 116). Bennaim et al. conducted a prospective randomized controlled trial evaluating the influence of both physiotherapy and photobiomodulation on motor recovery in dogs with severe IVDE (non-ambulatory paraparesis or worse) (93). A positive treatment effect was not noted in that study, although the authors suggest that case numbers were not large enough to draw a firm conclusion. Conversely, a large-scale retrospective study of physiotherapy conducted by Jeong et al. evaluated neurologic outcome in dogs with IVDE causing injuries ranging from ambulatory paraparesis to paraplegic with absent nociception (115). The authors noted a significant improvement in locomotor outcome for dogs receiving surgical decompression coupled with physiotherapy when compared to those receiving surgical decompression alone. However, successful locomotor outcome for dogs with paraplegia with or without intact deep pain receiving decompressive surgery alone was only reported to be 17%, which is much lower than the typical outcomes reported in literature which range from 50 to 60%. Zidan et al. also conducted a prospective blinded trial where dogs with incomplete SCI caused by IVDE were randomized after surgical decompression to receive either a basic in-hospital physiotherapy program consisting only of passive range of motion and sling walking activities or a more intensive therapy program (116). No difference in locomotor outcome was observed between groups but this trial was designed to see if the rate of recovery of locomotion could be influenced in a population of non-ambulatory paraparetic and paraplegic deep pain positive dogs. It did not target dogs known to have less chance of recovery and suggests that a randomized controlled trial is indicated to investigate the influence of rehabilitation on recovery in dogs with more severe injuries that fail to show early improvement.
The current state of the veterinary literature does not support a role of routine physiotherapy to improve locomotor outcome in dogs with mild to moderate spinal cord injury caused by IVDE. A challenge in interpreting this literature is the fact that studies include dogs with all injury grades, many of which would assuredly recover with or without other intervention, making it difficult to demonstrate a treatment effect without a very large sample size. Additionally, there is not a standardized physiotherapy program followed across the field, making it is difficult to compare results between studies. Lastly, the type of physiotherapy shown to improve locomotor outcome in experimental injury models, and now employed in people with severe injuries, is a very intensive type of locomotor training. The types of activities described in these locomotor training protocols extend well-beyond the intensity of therapeutic activities implemented in veterinary medicine, which more classically includes under-water treadmill walking, passive range of motion exercises and assisted weight-supported walking. It should also be noted that the primary aim in the acute phase of IVDE is to regain movement, over-ground locomotion and balance but “under-water” treadmill is best suited to improve strength and muscle mass. Activities directed toward restoration of movement, such as the use of “over-ground” treadmill training (as shown in people to improve motor recovery and balance (e.g., using proprioceptive platforms) may actually be more rational early on in the recovery process for IVDE and should be encouraged first (117).
In people with severe spinal cord injury, more intensive protocols are often coupled with external or implantable assistive devices not used in veterinary medicine. For example, robotic assisted gait training clearly reduces spasticity and improves lower limbs motor function in people with severe spinal cord injury (118). Thus, findings from the human clinical setting may not reflect those reasonably expected in veterinary medicine using current approaches or previously evaluated patient populations. A recently published retrospective study by Gallucci et al. showed an increase in the development of “spinal walking” in deep pain negative dogs despite the fact that these dogs did not regain pain perception (119). Further prospective studies are needed to determine what impact intensive physiotherapy may have in deep pain negative dogs, or on parameters beyond locomotor recovery including important SCI comorbidities such as pressure sores, neuropathic pain, and spasticity. In that respect, consensus about retraining after spinal cord injury lags far behind what is applied in humans where the literature covering that topic is vast. In particular, recent reviews and meta-analysis have clearly shown the benefit of several physiotherapy interventions to improve voluntary muscle strength (120). This includes interventions such as resistance training, functional electrical stimulation or robotic gait training. Some of these are difficult to implement in dogs because of cost and the challenges of eliciting specific voluntary movements on command, but others such as functional electrical stimulation, “over-ground” treadmill training and proprioceptive platform training are more feasible.
There exist a number of gaps in the veterinary literature with respect to standards of care for dogs with acute thoracolumbar IVDE. Areas identified for future study include comparison of medical and surgical management for dogs with more mild signs associated with IVDE (those who are only painful or have ambulatory paraparesis), the effect of early decompression in locomotor recovery in dogs with non-ambulatory paraparesis or paraplegic with intact deep pain, and on the incidence of progressive myelomalacia in deep pain negative dogs, the effect of durotomy coupled with spinal decompression in dogs with severe injuries, and the influence of intensive physiotherapy in deep pain negative dogs. Recommendations for standard care can be made in some areas, particularly with respect to surgical decompression where the currently available evidence supports that surgery should be recommended for dogs with non-ambulatory paraparesis or worse. While additional information is needed about the influence on timing of decompression on outcome in dogs that are deep pain negative for longer than 48 h duration, there is no evidence to support treatment of the 48 h time point as a cut off beyond which it becomes impossible for dogs to achieve locomotor recovery. Surgical decompression is best accomplished by either hemilaminectomy or mini-hemilaminectomy and fenestration of, at a minimum, the acutely ruptured disc. Adjacent discs easily accessed by way of the same approach should be considered for fenestration given the evidence that this substantially reduces future herniation at fenestrated sites. Currently available neuroprotective strategies such as high does MPSS and PEG are not recommended due to lack of demonstrated treatment effect in randomized controlled trials. The role of anti-inflammatory steroids as a protective strategy against progressive myelomalacia and the question of whether anti-inflammatory steroids or NSAIDs provide superior medical therapy require further evaluation.
SM, AT, NO, VS, and NG conceived, wrote, and edited the manuscript. CANSORT-SCI edited the manuscript. All authors have approved the final submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation with one of the authors NG.
1. Bergknut N, Egenvall A, Hagman R, Gustås P, Hazewinkel HA, Meij BP, et al. Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. J Am Vet Med Assoc. (2012) 240:1300–9. doi: 10.2460/javma.240.11.1300
2. Hansen HJ. A pathologic-anatomical interpretation of disc degeneration in dogs. Acta Orthop Scand. (1951) 20:280–93. doi: 10.3109/17453675108991175
3. Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia I. Paraplegia. (1969) 7:179–92. doi: 10.1038/sc.1969.30
4. Levine GJ, Levine JM, Budke CM, Kerwin SC, Au J, Vinayak A, et al. Description and repeatability of a newly developed spinal cord injury scale for dogs. Prev Vet Med. (2009) 89:121–7. doi: 10.1016/j.prevetmed.2009.02.016
5. Olsson SE. On disc protrusion in dog (enchondrosis intervertebralis); a study with special reference to roentgen diagnosis and to the value of disc fenestration. Acta Orthop Scand Suppl. (1951) 8:1–95. doi: 10.3109/ort.1951.22.suppl-8.01
6. Tillmans S. Beiträge Zur Enchondrosis Intervertebral. und der Wirbelkanltumoren des Hundes (Dissertation). University of Giessen, Giessen, Germany (1939).
8. Hansen HJ. A pathologic-anatomical study on disc degeneration in dog, with special reference to the so-called enchondrosis intervertebralis. Acta Orthop Scand Suppl. (1952) 11:1–117. doi: 10.3109/ort.1952.23.suppl-11.01
9. Hoerlein BF. Further evaluation of the treatment of disc protrusion paraplegia in the dog. J Am Vet Med Assoc. (1956) 129:495–502.
10. Hoerlein BF. Intervertebral disk disease. In: Oliver JE, Hoerlein BF, Mayhew IG, editors. Veterinary Neurology. Philadelphia, PA: Saunders (1987). p. 321–42.
11. Hoerlein BF, Redding RW, Hoff EJ, McGuire JA. Evaluation of dexamethasone, DMSO, mannitol, and solcoseryl in acute spinal cord trauma. J Am Animal Hosp Assoc. (1983) 19:216–226.
12. Hoerlein BF, Redding RW, Hoff EJ Jr, McGuire JA. Evaluation of naloxone, crocetin, thyrotropin releasing hormone, methylprednisolone, partial myelotomy, and hemilaminectomy in the treatment of acute spinal cord trauma. J Am Animal Hosp Assoc. (1985) 21:67.
13. Funkquist B. Thoracolumbar disk protrusion with severe cord compression in the dog II. clinical observations with special reference to the prognosis in conservative treatment. Acta Vet Scand. (1962) 3:334.
14. Hoerlein BF. Canine Neurology: Diagnosis and Treatment. 3rd edn. Philadelphia, PA: Saunders Company (1978).
15. Funkquist B. Decompressive laminectomy in thoraco-lumbar disc protrusion with paraplegia in the dog. J Small Anim Pract. (1970) 11:445–51. doi: 10.1111/j.1748-5827.1970.tb05595.x
16. Knecht CD. The effect of delayed hemilaminectomy in the treatment of intervertebral disc protrusion in dogs. J Am Animal Hosp Assoc. (1970) 6:71–7.
17. Langerhuus L, Miles J. Proportion recovery and times to ambulation for non-ambulatory dogs with thoracolumbar disc extrusions treated with hemilaminectomy or conservative treatment: A systematic review and meta-analysis of case-series studies. Vet J. (2017) 220:7–16. doi: 10.1016/j.tvjl.2016.12.008
18. Davies JV, Sharp NJH. A comparison of conservative treatment and fenestration for thoracolumbar intervertebral disc disease in the dog. J Small Animal Pract. (1983) 24:721–9. doi: 10.1111/j.1748-5827.1983.tb00360.x
19. Scott HW, McKee WM. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J Small Anim Pract. (1999) 40:417–22. doi: 10.1111/j.1748-5827.1999.tb03114.x
20. Olby N, Levine J, Harris T, Muñana K, Skeen T, Sharp N. Long-term functional outcome of dogs with severe injuries of the thoracolumbar spinal cord: 87 cases (1996-2001). J Am Vet Med Assoc. (2003) 222:762–9. doi: 10.2460/javma.2003.222.762
21. Laitinen OM, Puerto DA. Surgical decompression in dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception: A retrospective study of 46 cases. Acta Vet Scand. (2005). 46:79–85. doi: 10.1186/1751-0147-46-79
22. Ruddle TL, Allen DA, Schertel ER, Barnhart MD, Wilson ER, Lineberger JA, et al. Outcome and prognostic factors in non-ambulatory hansen type I intervertebral disc extrusions: 308 cases. Vet Comp Orthop Traumatol. (2006) 19:29–34. doi: 10.1055/s-0038-1632970
23. Levine JM, Levine GJ, Johnson SI, Kerwin SC, Hettlich BF, Fosgate GT. Evaluation of the success of medical management for presumptive thoracolumbar intervertebral disk herniation in dogs. Vet Surg. (2007) 36:482–91. doi: 10.1111/j.1532-950X.2007.00295.x
24. Han HJ, Yoon HY, Kim JY, Jang HY, Lee B, Choi SH, et al. Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am J Chin Med. (2010) 38:1015–25. doi: 10.1142/S0192415X10008433
25. Sharp NJH, Wheeler SJ. Small Animal Spinal Disorders. 2nd edn. Philadelphia, PA: Elsevier (2005). p. 124–125.
26. Moore SA, Early PJ, Hettlich BF. Practice patterns in the management of acute intervertebral disc herniation in dogs. J Small Anim Pract. (2016) 57:409–15. doi: 10.1111/jsap.12496
27. Freeman P, Jeffery ND. Re-opening the window on fenestration as a treatment for acute thoracolumbar intervertebral disc herniation in dogs. J Small Anim Pract. (2017) 58:199–204. doi: 10.1111/jsap.12653
28. Jeffery ND, Freeman PM. The role of fenestration in management of Type I thoracolumbar disc degeneration. Vet Clin North Am Small Anim Pract. (2018) 48:187–200. doi: 10.1016/j.cvsm.2017.08.012
29. Prata R. Neurosurgical treatment of thoracolumbar disks - the rationale and value. J Am Anim Hosp Assoc. (1981) 17:17–26.
30. Steffen F, Kircher PR, Dennler M. Spontaneous regression of lumbar hansen type 1 disk extrusion detected with magnetic resonance imaging in a dog. J Am Vet Med Assoc. (2014) 244:715–8. doi: 10.2460/javma.244.6.715
31. Hong J, Ball PA. Images In Clinical Medicine. Resolution of lumbar disk herniation without surgery. N Engl J Med. (2016) 374:1564. doi: 10.1056/NEJMicm1511194
32. Dimar JR, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. (1999) 24:1623–33. doi: 10.1097/00007632-199908150-00002
33. Macias C, McKee WM, May C, Innes JF. Thoracolumbar disc disease in large dogs: A study of 99 cases. J Small Anim Pract. (2002) 43:439–46. doi: 10.1111/j.1748-5827.2002.tb00010.x
34. Brisson BA, Moffatt SL, Swayne SL, Parent JM. Recurrence of thoracolumbar intervertebral disk extrusion in chondrodystrophic dogs after surgical decompression with or without prophylactic fenestration: 265 cases (1995-1999). J Am Vet Med Assoc. (2004) 224:1808–14. doi: 10.2460/javma.2004.224.1808
35. Itoh H, Hara Y, Yoshimi N, Harada Y, Nezu Y, Yogo T, et al. A retrospective study of intervertebral disc herniation in dogs in japan: 297 cases. J Vet Med Sci. (2008) 70:701–6. doi: 10.1292/jvms.70.701
36. Aikawa T, Fujita H, Kanazono S, Shibata M, Yoshigae Y. Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000-2007). J Am Vet Med Assoc. (2012) 241:1617–26. doi: 10.2460/javma.241.12.1617
37. Granger N, Carwardine D. Acute spinal cord injury: Tetraplegia and paraplegia in small animals. Vet Clin North Am Small Anim Pract. (2014) 44:1131–56. doi: 10.1016/j.cvsm.2014.07.013
38. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the ‘children of the 90s’—the index offspring of the avon longitudinal study of parents and children. Int J Epidemiol. (2013) 42:111–27. doi: 10.1093/ije/dys064
39. The Bristol Cats Study (2020). Available online at: http://www.bristol.ac.uk/vet-school/research/projects/cats/ (accessed April 21, 2020).
40. Flegel T, Boettcher IC, Ludewig E, Kiefer I, Oechtering G, Böttcher P. Partial lateral corpectomy of the thoracolumbar spine in 51 dogs: Assessment of slot morphometry and spinal cord decompression. Vet Surg. (2011) 40:14–21. doi: 10.1111/j.1532-950X.2010.00747.x
41. Huska JL, Gaitero L, Brisson BA, Nykamp S, Thomason J, Sears WC. Comparison of the access window created by hemilaminectomy and mini-hemilaminectomy in the thoracolumbar vertebral canal using computed tomography. Can Vet J. (2014) 55:449–55.
42. Salger F, Ziegler L, Böttcher IC, Oechtering G, Böttcher P, Flegel T. Neurologic outcome after thoracolumbar partial lateral corpectomy for intervertebral disc disease in 72 dogs. Vet Surg. (2014) 43:581–8. doi: 10.1111/j.1532-950X.2014.12157.x
43. Gage ED, Hoerlein BF. Hemilaminectomy and dorsal laminectomy for relieving compressions of the spinal cord in the dog. J Am Vet Med Assoc. (1968) 152:351–9.
44. Hoerlein BF. The status of the various intervertebral disk surgeries for the dog. J Am Animal Hosp Assoc. (1978) 14:563.
45. Muir P, Johnson KA, Manley PA, Dueland RT. Comparison of hemilaminectomy and dorsal laminectomy for thoracolumbar intervertebral disc extrusion in dachshunds. J Small Anim Pract. (1995) 36:360–7. doi: 10.1111/j.1748-5827.1995.tb02950.x
46. Horne TR, Powers RD, Swaim SF. Dorsal laminectomy techniques in the dog. J Am Vet Med Assoc. (1977) 171:742–9.
47. Gambardella PC. Dorsal decompressive laminectomy for treatment of thoracolumbar disc disease in dogs: a retrospective study of 98 cases. Vet Surg. (1980) 9:24–6. doi: 10.1111/j.1532-950X.1980.tb01647.x
48. David T. Thoracolumbar hemilaminectomy in the dog using a power skull trephine (a photo essay). Vet Med Small Anim Clin. (1976) 71:477–9.
49. Anderson SM, Lippincott CL, Gill PJ. Hemilaminectomy in dogs without deep pain perception. Calif Vet. (1991) 45:24–8.
50. McKee WM. A comparison of hemilaminectomy (with concomitant disc fenestration) and dorsal laminectomy for the treatment of thoracolumbar disc protrusion in dogs. Vet Rec. (1992) 130:296–300. doi: 10.1136/vr.130.14.296
51. Jeffery ND. Treatment of acute and chronic thoracolumbar disc disease by “mini hemilaminectomy”. J Small Animal Pract. (1988) 29:611–6. doi: 10.1111/j.1748-5827.1988.tb02181.x
52. Svensson G, Simonsson US, Danielsson F, Schwarz T. Residual spinal cord compression following hemilaminectomy and mini-hemilaminectomy in dogs: a prospective randomized study. Front Vet Sci. (2017) 4:42. doi: 10.3389/fvets.2017.00042
53. Flo GL, Brinker WO. Lateral fenestration of thoracolumbar discs. J Am Animal Hosp Assoc. (1975) 11:619–26.
54. Denny HR. The lateral fenestration of canine thoracolumbar disc protrusions: A review of 30 cases. J Small Anim Pract. (1978) 19:259–66. doi: 10.1111/j.1748-5827.1978.tb05487.x
55. Butterworth SJ, Denny HR. Follow-up study of 100 cases with thoracolumbar disc protrusions treated by lateral fenestration. J Small Animal Pract. (1991) 32:443–7. doi: 10.1111/j.1748-5827.1991.tb00983.x
56. Olsson SE. Observations concerning disc fenestration in dogs. Acta Orthop Scand. (1951) 20:349–56. doi: 10.3109/17453675108991182
57. Kinzel S, Wolff M, Buecker A, Krombach GA, Stopinski T, Afify M, et al. Partial percutaneous discectomy for treatment of thoracolumbar disc protrusion: retrospective study of 331 dogs. J Small Anim Pract. (2005) 46:479–84. doi: 10.1111/j.1748-5827.2005.tb00276.x
58. Sukhiani HR, Parent JM, Atilola MA, Holmberg DL. Intervertebral disk disease in dogs with signs of back pain alone: 25 cases (1986-1993). J Am Vet Med Assoc. (1996) 209:1275–9.
59. Jeffery ND, Barker AK, Hu HZ, Alcott CJ, Kraus KH, Scanlin EM, et al. Factors associated with recovery from paraplegia in dogs with loss of pain perception in the pelvic limbs following intervertebral disk herniation. J Am Vet Med Assoc. (2016) 248:386–94. doi: 10.2460/javma.248.4.386
60. Moore SA, Zidan N, Spitzbarth I, Nout-Lomas YS, Granger N, da Costa RC, et al. Development of an international canine spinal cord injury (CSCI) observational registry: A collaborative data-sharing network to optimize translational studies of SCI. Spinal Cord. (2018) 56:656–65. doi: 10.1038/s41393-018-0145-4
61. Dvorak MF, Noonan VK, Fallah N, Fisher CG, Finkelstein J, Kwon BK, et al. The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: an observational canadian cohort study. J Neurotrauma. (2015) 32:645–54. doi: 10.1089/neu.2014.3632
62. Castel A, Olby NJ, Mariani CL, Muñana KR, Early PJ. Clinical characteristics of dogs with progressive myelomalacia following acute intervertebral disc extrusion. J Vet Intern Med. (2017) 31:1782–9. doi: 10.1111/jvim.14829
63. Holmberg DL, Palmer NC, VanPelt D, Willan AR. A comparison of manual and power-assisted thoracolumbar disc fenestration in dogs. Vet Surg. (1990) 19:323–7. doi: 10.1111/j.1532-950X.1990.tb01199.x
64. Takahashi T, Nakayama M, Chimura S, Nakahara K, Morozumi M, Horie K, et al. Treatment of canine intervertebral disc displacement with chondroitinase ABC. Spine. (1997) 22:1435–47. doi: 10.1097/00007632-199707010-00002
65. Morelius M, Bergadano A, Spreng D, Schawalder P, Doherr M, Forterre F. Influence of surgical approach on the efficacy of the intervertebral disk fenestration: a cadaveric study. J Small Anim Pract. (2007) 48:87–92. doi: 10.1111/j.1748-5827.2007.00269.x
66. Brisson BA, Holmberg DL, Parent J, Sears WC, Wick SE. Comparison of the effect of single-site and multiple-site disk fenestration on the rate of recurrence of thoracolumbar intervertebral disk herniation in dogs. J Am Vet Med Assoc. (2011) 238:1593–600. doi: 10.2460/javma.238.12.1593
67. Forterre F, Dickomeit M, Senn D, Gorgas D, Spreng D. Microfenestration using the CUSA excel ultrasonic aspiration system in chondrodystrophic dogs with thoracolumbar disk extrusion: a descriptive cadaveric and clinical study. Vet Surg. (2011) 40:34–9. doi: 10.1111/j.1532-950X.2010.00780.x
68. Thomovsky SA, Packer RA, Lambrechts NE, Moore GE. Canine intervertebral disc fenestration using a vacuum-assisted tissue resection device. Vet Surg. (2012) 41:1011–7. doi: 10.1111/j.1532-950X.2012.01060.x
69. Forterre F, Konar M, Spreng D, Jaggy A, Lang J. Influence of intervertebral disc fenestration at the herniation site in association with hemilaminectomy on recurrence in chondrodystrophic dogs with thoracolumbar disc disease: A prospective MRI study. Vet Surg. (2008) 37:399–405. doi: 10.1111/j.1532-950X.2008.00394.x
70. Grunert P, Moriguchi Y, Grossbard BP, Ricart Arbona RJ, Bonassar LJ, Härtl R. Degenerative changes of the canine cervical spine after discectomy procedures, an in vivo study. BMC Vet Res. (2017) 13:193. doi: 10.1186/s12917-017-1105-5
71. Harris G, Freeman P. Introduction of disc material into the vertebral canal by fenestration of thoracolumbar discs following decompressive surgery. Vet Comp Orthop Traumatol. (2020) 33:66–70. doi: 10.1055/s-0039-1700554
72. Mayhew PD, McLear RC, Ziemer LS, Culp WT, Russell KN, Shofer FS, et al. Risk factors for recurrence of clinical signs associated with thoracolumbar intervertebral disk herniation in dogs: 229 cases (1994-2000). J Am Vet Med Assoc. (2004) 225:1231–6. doi: 10.2460/javma.2004.225.1231
73. Aikawa T, Fujita H, Shibata M, Takahashi T. Recurrent thoracolumbar intervertebral disc extrusion after hemilaminectomy and concomitant prophylactic fenestration in 662 chondrodystrophic dogs. Vet Surg. (2012) 41:381–90. doi: 10.1111/j.1532-950X.2012.00970.x
74. Hall ED. Lipid antioxidants in acute central nervous system injury. Ann Emerg Med. (1993) 22:1022–7. doi: 10.1016/S0196-0644(05)82745-3
75. Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: Neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. (1994) 126:61–75. doi: 10.1006/exnr.1994.1042
76. Ildan F, Oner A, Polat S, Isbir T, Göcer AI, Kaya M, et al. Correlation of alterations on na(+)-K+/Mg+2 ATPase activity, lipid peroxidation and ultrastructural findings following experimental spinal cord injury with and without intravenous methylprednisolone treatment. Neurosurg Rev. (1995) 18:35–44. doi: 10.1007/BF00416476
77. Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G, Grijalva I, et al. Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. Neuroreport. (2000) 11:1765–7. doi: 10.1097/00001756-200006050-00033
78. Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, et al. A critical appraisal of the reporting of the national acute spinal cord injury studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. (2000) 13:185–99. doi: 10.1097/00002517-200006000-00001
79. Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. (2000) 93(Suppl 1):1–7. doi: 10.3171/spi.2000.93.1.0001
80. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. (2012) 1:CD001046. doi: 10.1002/14651858.CD001046.pub2
81. Fehlings MG, Wilson JR, Harrop JS, Kwon BK, Tetreault LA, Arnold PM, et al. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: A systematic review. Global Spine J. (2017) 7(Suppl. 3):116S−37. doi: 10.1177/2192568217706366
82. Coates JR, Sorjonen DC, Simpson ST, Cox NR, Wright JC, Hudson JA, et al. Clinicopathologic effects of a 21-aminosteroid compound (U74389G) and high-dose methylprednisolone on spinal cord function after simulated spinal cord trauma. Vet Surg. (1995) 24:128–39. doi: 10.1111/j.1532-950X.1995.tb01307.x
83. Olby NJ, Muguet-Chanoit AC, Lim JH, Davidian M, Mariani CL, Freeman AC, et al. A placebo-controlled, prospective, randomized clinical trial of polyethylene glycol and methylprednisolone sodium succinate in dogs with intervertebral disk herniation. J Vet Intern Med. (2016) 30:206–14. doi: 10.1111/jvim.13657
84. Shi R. Polyethylene glycol repairs membrane damage and enhances functional recovery: A tissue engineering approach to spinal cord injury. Neurosci Bull. (2013) 29:460–6. doi: 10.1007/s12264-013-1364-5
85. Lu X, Perera TH, Aria AB, Callahan LAS. Polyethylene glycol in spinal cord injury repair: A critical review. J Experi Pharmacol. (2018) 10:37–49. doi: 10.2147/JEP.S148944
86. Luo J, Borgens R, Shi R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J Neurochem. (2002) 83:471–80. doi: 10.1046/j.1471-4159.2002.01160.x
87. Luo J, Borgens R, Shi R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J Neurotrauma. (2004) 21:994–1007. doi: 10.1089/0897715041651097
88. Luo J, Shi R. Diffusive oxidative stress following acute spinal cord injury in guinea pigs and its inhibition by polyethylene glycol. Neurosci Lett. (2004) 359:167–70. doi: 10.1016/j.neulet.2004.02.027
89. Laverty PH, Leskovar A, Breur GJ, Coates JR, Bergman RL, Widmer WR, et al. A preliminary study of intravenous surfactants in paraplegic dogs: polymer therapy in canine clinical SCI. J Neurotrauma. (2004) 21:1767–77. doi: 10.1089/neu.2004.21.1767
90. Hnot ML, Cole LK, Lorch G, Rajala-Schultz PJ, Papich MG. Effect of feeding on the pharmacokinetics of oral minocycline in healthy research dogs. Vet Dermatol. (2015) 26:399–3. doi: 10.1111/vde.12246
91. Jeffery N, Boudreau CE, Konarik M, Mays T, Fajt V. Fajt Pharmacokinetics V, and safety of oral glyburide in dogs with acute spinal cord injury. PeerJ. (2018) 6:e4387. doi: 10.7717/peerj.4387
92. Perdigão APL, Antunes NJ, Juni LT, de Freitas NL, Rojas-Moscoso J, Corrãa SVM, et al. Pharmacokinetics of riluzole in beagle dogs. Drug Res. (2019) 69:40–5. doi: 10.1055/a-0645-1248
93. Bennaim M, Porato M, Jarleton A, Hamon M, Carroll JD, Gommeren K, et al. Preliminary evaluation of the effects of photobiomodulation therapy and physical rehabilitation on early postoperative recovery of dogs undergoing hemilaminectomy for treatment of thoracolumbar intervertebral disk disease. Am J Vet Res. (2017) 78:195–206. doi: 10.2460/ajvr.78.2.195
94. Zidan N, Fenn J, Griffith E, Early PJ, Mariani CL, Muñana KR, et al. The effect of electromagnetic fields on post-operative pain and locomotor recovery in dogs with acute, severe thoracolumbar intervertebral disc extrusion: A randomized placebo-controlled, prospective clinical trial. J Neurotrauma. (2018) 35:1726–36. doi: 10.1089/neu.2017.5485
95. Frank LR, Roynard PFP. Veterinary neurologic rehabilitation: The rationale for a comprehensive approach. Top Companion Anim Med. (2018) 33:49–57. doi: 10.1053/j.tcam.2018.04.002
96. Packer RM, Seath IJ, O'Neill DG, De Decker S, Volk HA. DachsLife 2015: An investigation of lifestyle associations with the risk of intervertebral disc disease in dachshunds. Canine Genet Epidemiol. (2016) 3:8. doi: 10.1186/s40575-016-0039-8
97. Coates JR. Intervertebral disk disease. Vet Clin North Am Small Anim Pract. (2000) 30:77–110. doi: 10.1016/S0195-5616(00)50004-7
98. Bagley R. Basics of treatment of important spinal cord diseases of dogs and cats. Fund Vet Clin Neurol. (2005) 14:323–49.
99. Dewey CW, Costa RC. Practical Guide to Canine and Feline Neurology. Ames, IA: John Wiley & Sons (2015).
100. Deyo RA, Diehl AK, Rosenthal M. How many days of bed rest for acute low back pain? A randomized clinical trial. N Engl J Med. (1986) 315:1064–70. doi: 10.1056/NEJM198610233151705 Available online at: https://www.wiley.com/en-us/Fundamentals+of+Veterinary+Clinical+Neurology-p-9780813828435
101. Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Lack of effectiveness of bed rest for sciatica. N Engl J Med. (1999) 340:418–23. doi: 10.1056/NEJM199902113400602
102. Van de Crommert HW, Mulder T, Duysens J. Neural control of locomotion: sensory control of the central pattern generator and its relation to treadmill training. Gait Posture. (1998) 7:251–63. doi: 10.1016/S0966-6362(98)00010-1
103. Côté MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. (2004) 24:11317–27. doi: 10.1523/JNEUROSCI.1486-04.2004
104. Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. (2014) 159:1626–39. doi: 10.1016/j.cell.2014.11.019
105. Rossignol S, Martinez M, Escalona M, Kundu A, Delivet-Mongrain H, Alluin O, et al. The “beneficial” effects of locomotor training after various types of spinal lesions in cats and rats. Prog Brain Res. (2015) 218:173–98. doi: 10.1016/bs.pbr.2014.12.009
106. Ferguson AR, Huie JR, Crown ED, Baumbauer KM, Hook MA, Garraway SM, et al. Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury. Front Physiol. (2012) 3:399. doi: 10.3389/fphys.2012.00399
107. Huie JR, Morioka K, Haefeli J, Ferguson AR. What is being trained? How divergent forms of plasticity compete to shape locomotor recovery after spinal cord injury. J Neurotrauma. (2017) 34:1831–40. doi: 10.1089/neu.2016.4562
108. Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. (2011) 377:1938–47. doi: 10.1016/S0140-6736(11)60547-3
109. Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. (2013) 22:1591–612. doi: 10.3727/096368912X663532
110. Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE. (2015) 10:e0133998. doi: 10.1371/journal.pone.0133998
111. Zewdie ET, Roy FD, Yang JF, Gorassini MA. Facilitation of descending excitatory and spinal inhibitory networks from training of endurance and precision walking in participants with incomplete spinal cord injury. Prog Brain Res. (2015) 218:127–55. doi: 10.1016/bs.pbr.2014.12.005
112. Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. (2014) 37:202–11. doi: 10.1179/2045772313Y.0000000149
113. Drum MG. Physical rehabilitation of the canine neurologic patient. Vet Clin North Am Small Anim Pract. (2010) 40:181–93. doi: 10.1016/j.cvsm.2009.09.009
114. Gordon-Evans WJ, Johnson AL, Knap KE, Griffon DJ. The effect of body condition on postoperative recovery of dachshunds with intervertebral disc disease treated with postoperative physical rehabilitation. Vet Surg. (2019) 48:159–63. doi: 10.1111/vsu.13142
115. Jeong IS, Piao Z, Rahman MM, Kim S, Kim NS. Canine thoracolumbar intervertebral disk herniation and rehabilitation therapy after surgical decompression: A retrospective study. J Adv Vet Anim Res. (2019) 6:394–402. doi: 10.5455/javar.2019.f359
116. Zidan N, Sims C, Fenn J, Williams K, Griffith E, Early PJ, et al. A randomized, blinded, prospective clinical trial of postoperative rehabilitation in dogs after surgical decompression of acute thoracolumbar intervertebral disc herniation. J Vet Intern Med. (2018) 32:1133–44. doi: 10.1111/jvim.15086
117. Yu P, Zhang W, Liu Y, Sheng C, So KF, Zhou L, et al. The effects and potential mechanisms of locomotor training on improvements of functional recovery after spinal cord injury. Int Rev Neurobiol. (2019) 147:199–217. doi: 10.1016/bs.irn.2019.08.003
118. Fang CY, Tsai JL, Li GS, Lien AS, Chang YJ. Effects of robot-assisted gait training in individuals with spinal cord injury: a meta-analysis. Biomed Res Int. (2020) 21:2102785. doi: 10.1155/2020/2102785
119. Gallucci A, Dragone L, Menchetti M, Gagliardo T, Pietra M, Cardinali M, et al. Acquisition of involuntary spinal locomotion (spinal walking) in dogs with irreversible thoracolumbar spinal cord lesion: 81 dogs. J Vet Intern Med. (2017) 31:492–7. doi: 10.1111/jvim.14651
120. Aravind N, Harvey LA, Glinsky JV. Physiotherapy interventions for increasing muscle strength in people with spinal cord injuries: A systematic review. Spinal Cord. (2019) 57:449–60. doi: 10.1038/s41393-019-0242-z
Sarah A. Moore DVM, DACVIM-Neurology
Associate Professor, Neurology and Neurosurgery
Department of Veterinary Clinical Sciences
The Ohio State University College of Veterinary Medicine
Columbus Ohio, 43026.
Natasha J. Olby Vet MB, PhD, DACVIM Neurology
Professor of Neurology/Neurosurgery
Dr. Kady M. Gjessing and Rhanna M. Davidson Distinguished
Chair of Gerontology
Department of Clinical Sciences
North Carolina State University College of Veterinary Medicine
Raleigh, NC 27607.
Jonathan M. Levine DVM, DACVIM-Neurology
Professor, Helen McWhorter Chair, and Head
Department of Small Animal Clinical Sciences
College of Veterinary Medicine and Biomedical Sciences
Texas A&M University
College Station, TX 77843-4474.
Melissa J. Lewis VMD, PhD, DAVCIM (Neurology)
Assistant Professor of Neurology
Purdue University College of Veterinary Medicine
Department of Veterinary Clinical Sciences
West Lafayette, IN 47907.
Nick D. Jeffery
Professor Neurology & Neurosurgery
Maureen E Mullins Professor in Small Animal Clinical Sciences
College of Veterinary Medicine
Texas A&M University
TX 77843.
Ronaldo Casimiro da Costa DMV, MSc, PhD, Dipl.
ACVIM - Neurology
Professor and Service Head, Neurology and Neurosurgery
Department of Veterinary Clinical Sciences
College of Veterinary Medicine
The Ohio State University
Columbus, OH 43210-1089.
Yvette S. Nout-Lomas DVM PhD
Department of Clinical Sciences
Colorado State University
Fort Collins, CO 80523-1678.
Joe Fenn BVet Med MVetMed FHEA MRCVS
Department of Clinical Science and Services
Royal Veterinary College, Hawkshead Lane
Hatfield, Hertfordshire, UK.
Dr. Nicolas Granger DVM PhD DECVN FHEA MRCVS
The Royal Veterinary College, University of London,
Hawkshead Lane, Hatfield, Hertfordshire,
United Kingdom & CVS Referrals, Bristol Veterinary
Specialists at Highcroft, Bristol, United Kingdom.
Dr. Ingo Spitzbarth Ph.D., Dipl. ECVP
Institute of Veterinary Pathology
Faculty of Veterinary Medicine
Leipzig University
D-04103 Leipzig
Germany.
Veronika M. Stein Prof. Dr. PhD DECVN
Division of Clinical Neurology
Department for Clinical Veterinary Medicine
Vetsuisse Faculty
University of Bern
CH-3012 Bern
Switzerland.
Prof. Dr. Andrea Tipold, Dipl ECVN
Dept Small Animal Medicine and Surgery
University of Veterinary Medicine Hannover
D-30559 HANNOVER
Germany/Europe.
Ji-Hey Lim
Neurology and Neurosurgery,
Department of Veterinary Medicine and Surgery
University of Missouri
MU Veterinary Health Center
University of Missouri,
Columbia, MO 65211.
Holger Volk PhD PGCAP Dipl ECVN
Dept of Small Animal Medicine and Surgery
University of Veterinary Medicine Hannover
D-30559 HANNOVER
Germany/Europe.
Keywords: dog, interveterbral disc, intervertebral disc disease, hemilaminectomy, spinal cord injury, hansen type I
Citation: Moore SA, Tipold A, Olby NJ, Stein V, Granger N and Canine Spinal Cord Injury Consortium (CANSORT SCI) (2020) Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Vet. Sci. 7:610. doi: 10.3389/fvets.2020.00610
Received: 11 June 2020; Accepted: 28 July 2020;
Published: 03 September 2020.
Edited by:
Steven De Decker, Royal Veterinary College (RVC), United KingdomReviewed by:
Takeshi Aikawa, Aikawa Vet Med Ctr, JapanCopyright © 2020 Moore, Tipold, Olby, Stein, Granger and Canine Spinal Cord Injury Consortium (CANSORT SCI). This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah A. Moore, bW9vcmUuMjIwNEBvc3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.