- 1Department of Parasitology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
- 2Toxoplasmosis Research Center, Mazandaran University of Medical Sciences, Sari, Iran
- 3Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
- 4Fatemeh Zahra Hospital, Mazandaran University of Medical Sciences and Health Services, Sari, Iran
- 5Health Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran
Toxoplasmosis is one of the most prevalent infections in humans and animals caused by the intracellular protozoan parasite Toxoplasma gondii (T. gondii). Rodents, as intermediate and reservoir hosts, play a key role in the maintenance and transmission of T. gondii. They can be contaminated and maintain the parasite in the form of cysts in their bodies, demonstrating an infection source for their offsprings, predators (particularly felids), and other animals. Therefore, the present systematic review and meta-analysis study was carried out to evaluate the global seroprevalence of T. gondii in these mammals. For achieving the purpose of the current study, six English databases (PubMed, Science Direct, Web of Science, Scopus, ProQuest, and Google Scholar) were systematically searched for related studies from 1970 to 2018. Finally, a total of 52,372 records were screened, 105 records including 26,221 rodents were incorporated in the present study. By random effect models, the overall seroprevalence was calculated at 6% (95% CI = 6–7%), with the highest amount was observed in Africa (24%) and South America (18%), and the lowest amount in Europe (1%). The subgroup data analysis by gender manifested that the prevalence of Immunoglobulin G antibodies did not differ between genders (P > 0.05). Due to the significant heterogeneity, meta-regression models were applied based on serological techniques and continental regions; however, the obtained values were not statistically significant (P = 0.480 and P = 0.295, respectively). The present study revealed a relatively low level of T. gondii seroprevalence in rodents; however, if they were the main food source for their predators, they would cause high transmission of T. gondii.
Introduction
Toxoplasmosis is a highly prevalent zoonotic parasitic infection caused by Toxoplasma gondii (T. gondii), an obligate intracellular apicomplexan protozoan, that infects nearly 30% of the world human population (1, 2). This foodborne pathogen has complex life cycles, including the sylvatic transmission cycle in forest habitats, and domestic transmission cycle in human settlements, which might be hardly connected (3).
Felids as definitive hosts, excrete oocysts through feces (sexual stage) which infect intermediate hosts, a large range of homoeothermic animals (e.g., rodents and humans), resulting in the formation of tissue cysts (asexual stage) (4, 5). Transmission to intermediate hosts can also occur via two other main ways of congenitally or by eating undercooked meat containing tissue cysts (3, 6).
T. gondii in immunocompetent people is mostly asymptomatic, or with non-specific flu-like symptoms. However, this single-celled microorganism is medically important and causes serious consequences in immunocompromised people and pregnant women (7). The life-threatening encephalitis can occur in immunocompromised humans following the infection (5, 8). Primary infection during pregnancy can result in congenital toxoplasmosis with abortion, neonatal death, chorioretinitis, and neurological disorders in the unborn child (9, 10).
Rodents, the largest order of the class Mammalia with a number higher than the total number of other mammals, are characterized by upper and lower pairs of ever-growing incisors and a set of chewing teeth. They have short reproductive cycle and high compatibility for living in various habitats (11). They are responsible for the zoonotic transmission of several diseases to humans. Rodents play an important role in the maintenance of the T. gondii life cycle and epidemiology of toxoplasmosis because they are considered as reservoirs and carriers of the disease and the main source of infection for cats and their relatives (12, 13). This role is more important in species that live close to human habitats, because of the importance of its environment and human health. Establishing the infection transmission cycle by rodents causes releasing oocysts from infected felids and the spread of contamination in the environment, and thus increasing the infection risk of each of the parasite hosts in the environment, most importantly of humans in its habitats (14).
Direct transmission of toxoplasmosis from rodents to humans may occur when they are consumed as food by humans, as it is done by many human populations. For example, rodents such as rats and capybaras (Hydrochoerus hydrochaeris), one of the largest rodents in the world, are used by some nations and may be a source of T. gondii if their meat containing parasitic cysts is consumed undercooked (3, 15, 16). Therefore, it is necessary to pay attention to hygienic principles when preparing and cooking rodents in such populations. Furthermore, if rodents are accidentally eaten by livestock, they could mediate disease transmission to humans (11).
Considering the rodents' importance in the transmission of toxoplasmosis to felids and humans, as well as, abundance and distribution of rodents near the human settlements and in absence of a comprehensive study, we performed a global meta-analysis to assess the pooled seroprevalence of T. gondii in this mammals.
Methods
Design and Protocol Registration
This extensive research was conducted in accordance with the items reported in the PRISMA statement (www.prisma-statement.org). The details of the study protocol are available on the website of the International Prospective Register of Systematic Reviews with the identifier Central Registration Depository of 42018107622 (17).
Search Strategy
To elucidate the seroepidemiological status of T. gondii in rodents, an extensive and principled search was carried out on scientific publications from 1970 to 2018 using six English language databases of the following websites: (www.pubmed.gov), (www.sciencedirect.com), (www.webofknowledge.com), (www.scopus.com), (www.search.proquest.com), and (www.scholar.google.com).
The keywords were used based on medical subject heading terms: “Toxoplasma,” “Toxoplasmosis,” “T. gondii,” “Seroprevalence,” “Seroepidemiology,” “Prevalence,” and “Rodentia.” In addition, perusing the reference lists to retrieve additional related publications was conducted manually.
Study Selection
For the purpose of eligible screening, all the retrieved titles, abstracts, and full-texts if needed, were carefully perused and eligible studies were selected by two independent authors (TMG and MM). Disagreements, if any, were discussed and sorted out by consensus.
Finally, studies with full texts or abstracts available in English which examined the seroprevalence of antibodies against T. gondii in rodents with the total sample size larger than 20 were selected. The reviews, experimental, human-based, non-serological, repetitive manuscripts and those with inadequate data were excluded from the present study.
Data Extraction and Quality Assessment
The data extraction process was performed by two independent authors (MM and TMG) and disagreements were resolved by discussion and consensus. Using an information extraction sheet, the following data were recorded from the selected studies: first author, publication year, geographical region, sampling period, total sample size, gender and age distribution, number and percentage of seropositive rodents, and serological methods. The quality of included records was appraised using the Joanna Briggs Institute (JBI) Prevalence Critical Appraisal Tool (18).
Statistical Analysis
The present meta-analysis was carried out using Stata software (version 15; Stata Corp, College Station, TX, USA). Point estimations and 95% confidence intervals (CI) of anti-Toxoplasma Immunoglobulin G (IgG) seroprevalence were calculated for all the selected records. Chi-squared and I-squared tests were applied to evaluate the extent of variations among the independent studies. The I-squared values of lower than 25%, 25–50%, and higher than 50% were considered as low, moderate, and high heterogeneity, respectively.
To explore the causes of heterogeneity among the selected studies, meta-regression and subgroup analysis were performed based on serological techniques and continental regions. The subgroup analysis was also conducted according to the genders. The publication bias was examined by Egger's regression test and funnel plot asymmetry. According to the results of the heterogeneity test, a random effect model was used to pool the estimates and a forest plot was drawn to visualize the outcomes.
Furthermore, to evaluate the effect of each study on the overall effect size, a sensitivity analysis was performed by eliminating a single study at a time.
Results
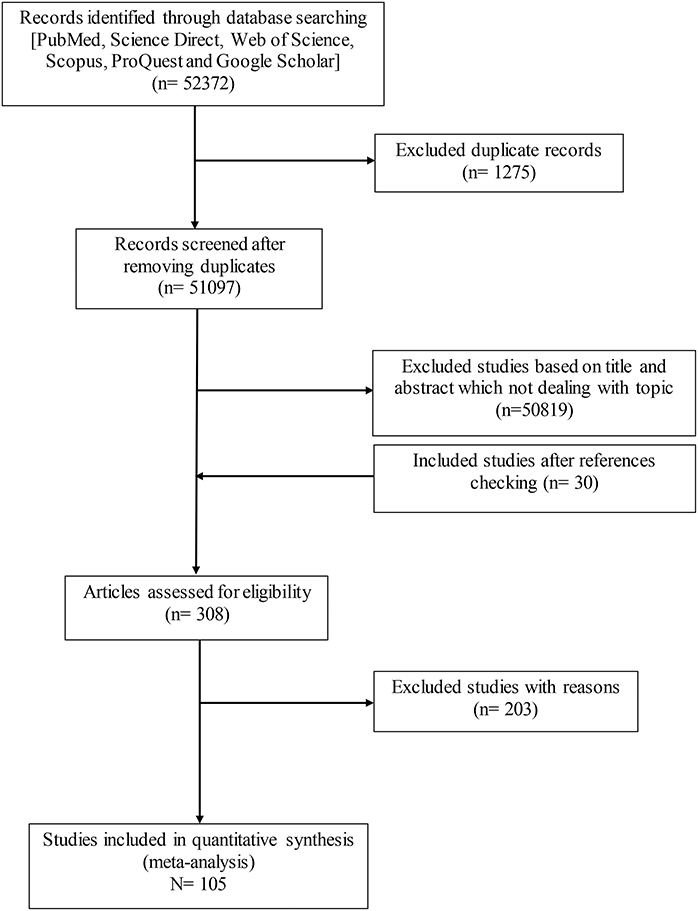
In this universal scientific research, initially 52,372 records were retrieved through principled search, 105 records from 44 countries were finally appraised appropriately to be entered into this global research. Totally, 26,221 rodents and 2,263 positive cases were analyzed for IgG antibodies against T. gondii.
Details of the search and study selection procedure are described in a PRISMA flow diagram (Figure 1). Table 1 lists the basic characteristics of the selected papers. A study conducted by Dabritz et al. (13), had two datasets (13) and only two studies were available for the continent of Australia (52, 53). The most performed serologic tests in the literature were, including modified agglutination test (MAT), Sabin-Feldman dye test (SFDT), indirect fluorescent antibody test (IFAT), latex agglutination test (LAT), direct agglutination test (DAT), enzyme-linked immunosorbent assay (ELISA), and indirect hemagglutination test (IHAT) in 39, 17, 14, 9, 7, 6, and 5 studies, respectively. The other serological tests were conducted in nine studies. The average score obtained from the JBI scale was six illustrating the moderate to the high quality of the selected records (Table 1).
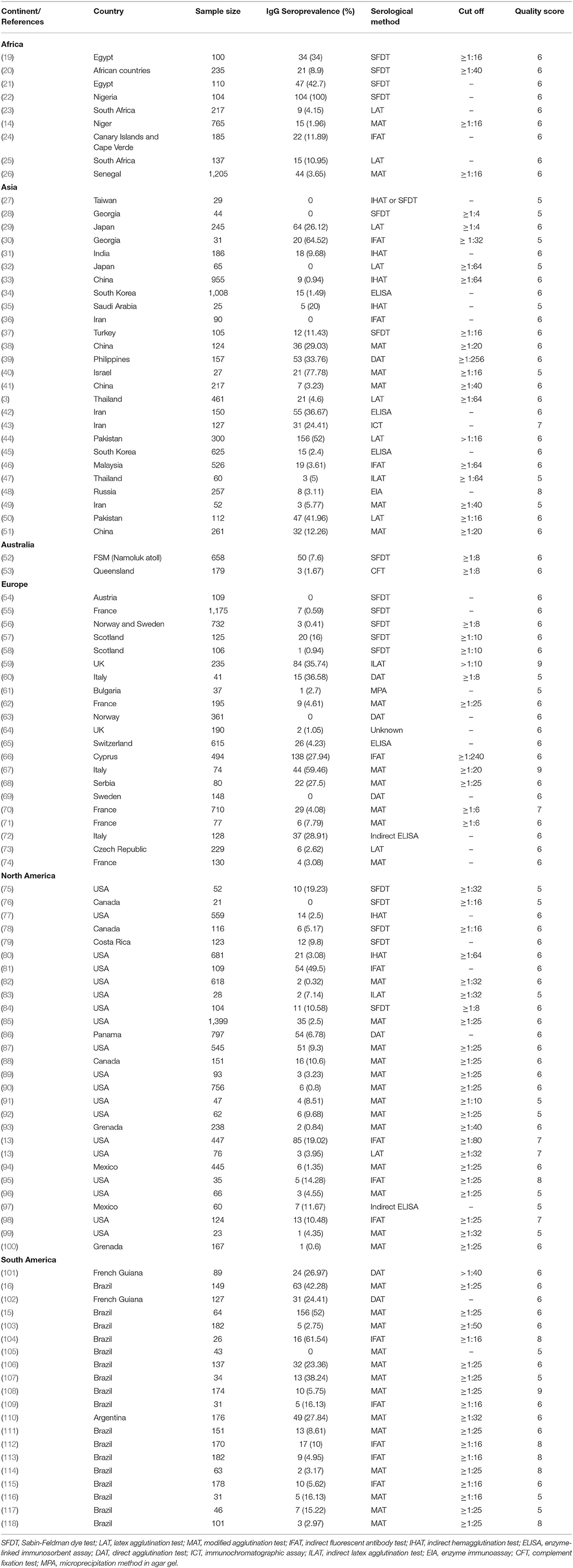
Table 1. Baseline characteristics of selected studies reporting seroprevalence of T. gondii in rodents.
The overall seroprevalence of anti-Toxoplasma IgG antibodies in rodents based on the random effect model was calculated at 6% (95%, CI = 6–7%). I-squared statistics indicated a high heterogeneity among the studies (I2 = 99.25%, P < 0.001). Figure 2 demonstrates a forest plot diagram of the current research. In the present analysis (by continental regions), the highest seroprevalence was evaluated in Africa and South America with the amounts of 24% (95% CI = 0–48%) and 18% (95% CI = 14–23%), respectively.
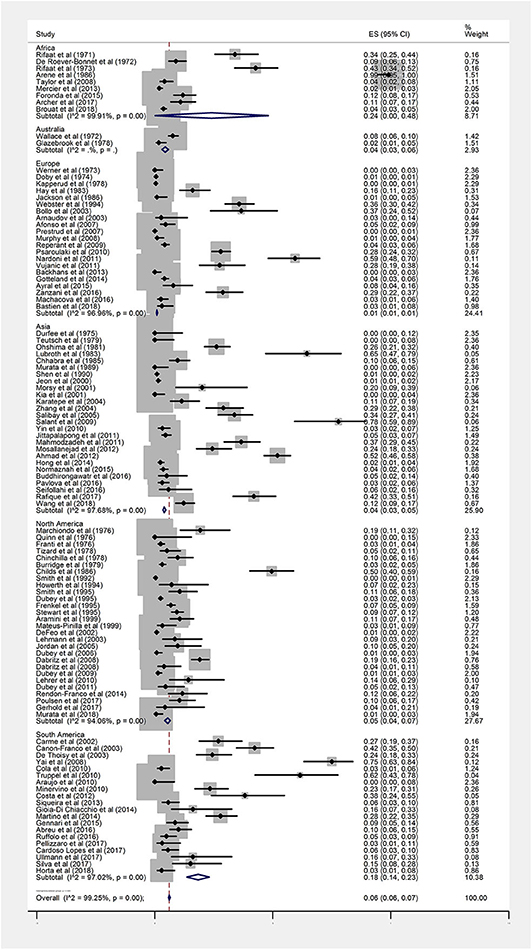
Figure 2. Forest plot diagram for seroprevalence of anti-Toxoplasma IgG antibodies in rodents (ES, effect size; CI, confidence interval).
The seroprevalence in North America, Australia, and Asia was measured at 5% (95% CI = 4–7%), 4% (95% CI = 3–6%), and 4% (95% CI = 3–5%), respectively. The Europe had the lowest seroprevalence with 1% (95% CI = 1–1%).
The subgroup data analysis of 16 documents describing values of the seroprevalence parasite by gender manifested that the pooled seropositivity value in male and female rodents was 4% (95% CI = 3–6%) and 2% (95% CI = 1–3%), respectively (Figures 3A,B). There was no statistically significant difference between these two groups due to the overlap of CI (P > 0.05).
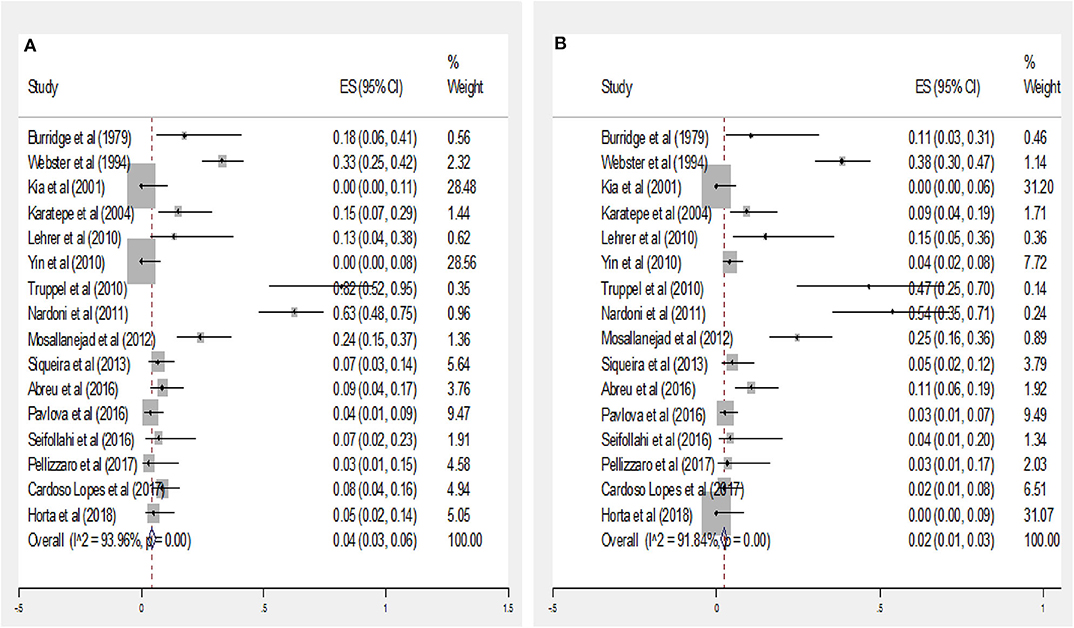
Figure 3. Forest plot diagram for seroprevalence of anti-Toxoplasma IgG antibodies in male (A) and Female (B) rodents (ES, effect size; CI, confidence interval).
In a subgroup analysis based on serological methods, the highest seroprevalence was found by IFAT 18% (95% CI = 13–23%), followed by LAT, SFDT, ELISA, MAT, IHAT, and DAT with the rate of 15% (95% CI = 9–22%), 14% (95% CI = 12–17%), 11% (95% CI = 7–15%), 8% (95% CI = 7–9%), 3% (95% CI = 1–6%), and 0% (95% CI = 0–1%), respectively. Other serological methods [e.g., indirect latex agglutination test (ILAT), immunochromatographic assay (ICT), enzyme immunoassay (EIA), complement fixation test (CFT), and microprecipitation method in agar gel (MPA)] showed the infection rate of 8% (95% CI = 4–11%; Table 2).
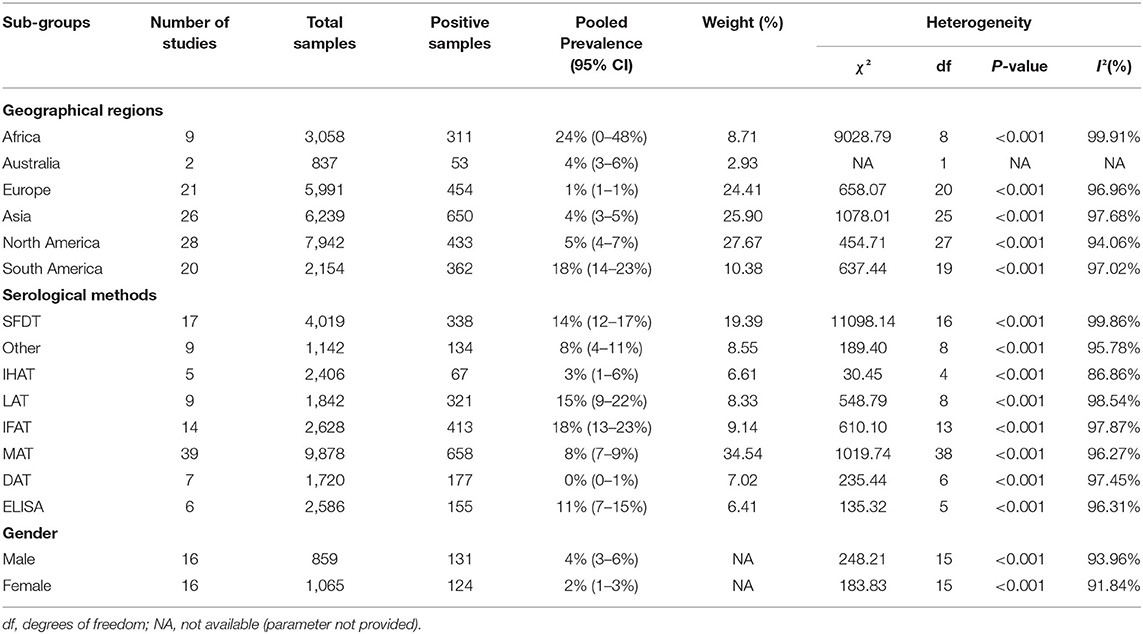
Table 2. Sub-group analysis of the seroprevalence of T. gondii based on geographical regions, serological methods and gender of rodents.
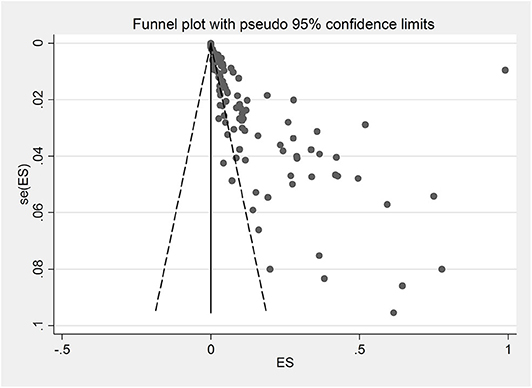
Due to the lack of adequate data on the rodents' age, subgroup analysis was not performed. The results of Egger's regression test indicated that publication bias was statistically significant (Egger bias: 5.650, P < 0.001). Figure 4 shows the Funnel plot for this purpose.
To detect the sources of heterogeneity among different studies, meta-regression analysis was applied based on serological methods and continental regions, the results showed that the illustrated values were not statistically significant (P = 0.480 and P = 0.295, respectively). The sensitivity analysis tool demonstrated that the effect of three studies on the overall effect size was significant (22, 63, 69).
Discussion
Toxoplasmosis, one of the most common infections in humans, is important both medically and economically. It causes many serious consequences in humans and animals with economic importance (e.g., livestock). It has been recorded that infection leads to abortions in many mammals (e.g., rodents, and livestock), could inhibit species recovery, and cause economic losses (5, 10, 119).
Rodents, as intermediate and reservoir hosts of this protozoan, can be contaminated and maintain the parasite in the form of cysts in their bodies, demonstrating an infection source for their offsprings, predators (particularly felids), and other animals (If rodents' bodies are accidentally eaten by them) (6, 11, 14). It has been shown animals such as livestock and pigs that are economically important, may accidentally or intentionally eat live small rodents or their carcasses and thus can get infection via digesting tissue cysts without the intervention of definitive hosts (11, 59).
By establishing the infection transmission cycle and consequently environment contamination by released oocysts from cats, rodents, especially species that live close to humans such as house mice, lead to increasing the risk of human exposure to the parasite (14). The rodent capybara that is used by humans in many countries of South and Central America, may be a potential source of infection for humans if its meat contains parasitic cysts and is consumed insufficiently cooked (15, 16).
Also, the consumption of rats as food by some populations may increase the risk of direct transmission from these rodents to humans, when eating, handling or preparing infected rats before cooking (3). Hence, people who consume rodents' meat should follow the principles of hygiene during meat preparation and cook the meat properly.
In the sylvatic transmission cycle in forest habitats, rodents as important wildlife intermediate and reservoir host of T. gondii, with maintaining parasite and its transmission cycle in these ecosystems may lead to increasing the probability of infection of wild felids and other animals, especially if they are the main prey (3).
Transmission of the parasite from wildlife to human habitats may rarely and accidentally occur by moving infected rodents and other parasite hosts. The accidental transportation of infected rodents from one region to another by human trade activities and other pathways causes strains to be transmitted internationally and sometimes new strains are introduced in the region (120).
Therefore, these mammals play a substantial role in the transmission of the infection to felids and most animals, as well as the dissemination of the infection in the environment and the risk of human infection. Consequently, comprehensive studies are required to reveal the status of toxoplasmosis in rodents and to better develop control measures and strategies. Hence, conducting further studies could help to reduce the infection rate in these mammals, decrease environmental contamination, and mitigate the risk of infection transmission. In order to achieve these goals, the current study was carried out to investigate the seroprevalence of T. gondii in rodents.
The present extensive study was the first systematic review to concentrate on the worldwide seroprevalence of toxoplasmosis in rodents, by screening scientific studies published from 1970 to 2018. In this attempt, the overall seroprevalence of anti-T. gondii IgG antibodies was calculated at 6% (95% CI = 6–7%), with the highest amount in Africa (24%), South America (18%), and the lowest amount in Europe (1%).
Our results illustrated a large variation in the seroprevalence of infection in various studies, ranging from 0 to 100%. In general, these variations were observed in different studies and geographical areas and were influenced by numerous factors, including abundance of definitive and intermediate hosts, distinct ecologic patterns, the sensitivity of used methods, variability in vertical transmission or susceptibility to infection between species, differences in climate conditions, and environmental factors (e.g., mud and water) affecting the sporulation and survival of oocysts (41, 70, 98). Depending on the situation, some of these factors had a more substantial role in the variation of the seroprevalence of infection than others. Therefore, it may be difficult to compare the results of different studies due to differences in important factors such as serological tests (with the variable sensitivity, specificity, and cut-off), rodent species, etc. The rodents resembling other mammals were contaminated with T. gondii through eating the infective oocysts residing in water, soil, and food. Also, ingestion of meat infected with parasite tissue cysts (via cannibalism), digesting earthworms as paratenic hosts of the pathogen, or congenital transmission lead to contamination of the rodents with T. gondii. The high level of congenital transmission recorded among some rodent species could affect the prevalence levels (64, 121). For example, congenital transmission occurs high in the wild rat populations, thus it can be an important route in parasite transmission and maintenance and lead to high prevalence levels in this species, regardless of environmental contamination (59).
The contamination level of the soil is different according to the region, depending on the densities of felids as definitive hosts, which excrete oocysts into the environment. In fact, rodents living in an environment with fewer cats, are less exposed to oocysts (62, 121). As high infection rates have been reported in rodents living in rural areas such as mice (59%) and rats (70%), because they are more in contact with cats and their feces (48).
The humid and warm climate is a suitable condition for the survival and dissemination of oocysts; therefore, areas with these climates show a high level of infection. In addition, water and damp soil can support the stability of oocysts for longer periods (43, 108). The oocysts are able to survive in moist soil for up to a year and low humidity and high temperatures can kill them (76, 118). Rodents such as muskrats that swim in water or semi-aquatic species (capybaras) show higher infection rates (ranging from 17 to 60%) than those that are less exposed to water environments (13). The seasonal variations of climate also affect the infection rate as it increases in the wet seasons than the dry seasons (98).
The estimation of seroprevalence infection may not reflect the actual amount of infected individuals, because, contrary to the resistance of some species to infection, others may be more susceptible and show more casualties. Therefore, the casualties are not included in the study and the reported amount will not be actual (121).
The antibody production is affected by various factors such as parasite genotype, infection persistence, and host age, etc., also the duration of immunity may vary and in some species, the antibody produced is reduced after a short time and becomes unrecognizable (76). On the other hand, some congenitally infected rats and mice do not develop antibodies while harboring parasites in their tissues (100). The used diagnostic techniques were very important, because of the low specificity and sensitivity of serological methods and usage of an improper cut-off value, may over/underestimate pathogen prevalence by false negative or positive results (121). Many researchers have shown that the prevalence of infection in rodents might be estimated less or more than the actual value when relying on serology, compared to other valid techniques, such as bioassay, as the gold standard for the diagnosis of T. gondii infection, or Polymerase chain reaction (PCR) (10, 14, 64, 69, 70). Regarding, the use of the serology technique along with bioassay and PCR could provide a more accurate estimation of the infection rate in rodents. According to the subgroup analysis of serological methods, the highest seroprevalence was detected by IFAT, followed by LAT, SFDT, ELISA, and MAT. Studies that used LAT, reported the lowest seroprevalence. These differences in the estimation of the prevalence may be due to the variable specificity, sensitivity, and cut-off of the used serological tests. Based on our findings, the pooled global seroprevalence of antibodies against T. gondii among rodents was relatively low. Although the infection levels in cats will be affected by the contamination levels in their consumed prey, a low infection rate among rodents may account for a high infection rate in cats since these animals may consume hundreds of rodents throughout their living (56). In fact, it is possible to relate the seroprevalence rates in felids and the number of rodents consumed, which varies according to the prey abundance, season, and local conditions (76, 122).
Considering the different prey availability according to the habitat and unequal effect of prey species on the infection risk for predators, examining both the predominant prey of felids and the prevalence of infection in them can help to better predict the T. gondii infection risk of felids in specific habitats (121).
Moreover, T. gondii has been demonstrated to be responsible for change of behavior patterns among rodents (e.g., increased attraction to felids urine, losing their innate fear of cats, and causing neurological impairment), which increases the risk of predation of the infected rodents and lead to infection transmission to the felids (70, 95, 98). Given the above, a low number of rodents infected with toxoplasmosis may lead to high transmission in felids and other predators depending on the situation.
Publication bias was statistically significant in the selected studies, probably for reasons, such as sample size, sampling procedure, and methodology.
In our study, it was concluded that the high seroprevalence of infection among rodents in Africa and South America was due to climate conditions and other aforementioned factors indicating an increased risk of infection transmission to felids, humans, and other animals. Therefore, effective control measures and strategies should be implemented in order to reduce the infection rates among rodents in these regions.
Data analysis of the few studies reporting infection rates by gender in rodents suggested that the prevalence of T. gondii antibodies did not differ between the genders with 4% in males and 2% in females, suggesting that both genders are almost equally exposed to this parasite. In some species of rodents such as rats, males have larger home ranges than females and thus a greater chance for acquiring infection (59).
Due to the lack of adequate data on rodents' age in the selected studies, subgroup analysis of age groups was not performed. In general, because of spending more time in the environment and the increased risk of exposure to parasites, the seropositive rate of T. gondii has been expected to be higher in aged animals than in younger ones, as shown in numerous animal species and humans (2, 123–126).
Cannibalism, one of the routes of infection transmission in rodents that is observed in some species, is more common in males and older animals than in females and younger ones, that can affect the burden of infection (59). Hence, specific feeding, foraging or social behaviors observed in rodents that vary from one species to another can determine the extent of exposure to the parasite and the differences in prevalences related to the sex and maturity in any species (3).
Conclusions
In conclusion, the present study revealed a relatively low level of T. gondii seroprevalence in rodents; however, if they were the main food source for their predators, they would cause high transmission and subsequently increase environmental contamination and the risk of infection transmission to humans and other animals.
Consequently, effective control measures and strategies are needed to reduce the infection rate in these mammals. Further studies are required to use the serology technique along with bioassay and PCR to provide a more accurate estimation of the infection rate in these animals.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AD and ShS contributed to the design of the study. TG and MMon conducted the systematic review of the literature and extracted data. MMoo performed all statistical analyses, data interpretation, and drafted the manuscript. TG contributed to the interpretation of data and writing of the first draft. AD supervised the study. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Student Research Committee of Mazandaran University of Medical Sciences for approving this research (No. 6348). The code of ethics of this plan is (IR.MAZUMS.REC.1398.6348).
Abbreviations
CI, confidence interval; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; JBI, Joanna Briggs Institute; IgG, Immunoglobulin G; MAT, modified agglutination test; SFDT, Sabin-Feldman dye test; IFAT, indirect fluorescent antibody test; LAT, latex agglutination test; DAT, direct agglutination test; ELISA, enzyme-linked immunosorbent assay; IHAT, indirect hemagglutination test; ILAT, indirect latex agglutination test; ICT, immunochromatographic assay; EIA, enzyme immunoassay; CFT, complement fixation test; MPA, microprecipitation method in agar gel; PCR, Polymerase chain reaction.
References
1. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
2. Mizani A, Alipour A, Sharif M, Sarvi S, Amouei A, Shokri A, et al. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: a systematic review and meta-analysis. Trop Med Health. (2017) 45:7. doi: 10.1186/s41182-017-0048-7
3. Jittapalapong S, Sarataphan N, Maruyama S, Hugot JP, Morand S, Herbreteau V. Toxoplasmosis in rodents: ecological survey and first evidences in Thailand. Vector Borne Zoonot Dis. (2011) 11:231–7. doi: 10.1089/vbz.2009.0238
4. Bodaghi B, Touitou V, Fardeau C, Paris L, LeHoang P. Toxoplasmosis: new challenges for an old disease. Eye. (2012) 26:241–4. doi: 10.1038/eye.2011.331
5. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/S0020-7519(00)00124-7
6. Khademvatan S, Foroutan M, Hazrati-Tappeh K, Dalvand S, Khalkhali H, Masoumifard S, et al. Toxoplasmosis in rodents: a systematic review and meta-analysis in Iran. J Infect Public Health. (2017) 10:487–93. doi: 10.1016/j.jiph.2017.01.021
7. Deng H, Devleesschauwer B, Liu M, Li J, Wu Y, van der Giessen JW, et al. Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of China: a systematic review and hierarchical meta-analysis. Sci Rep. (2018) 8:6218. doi: 10.1038/s41598-018-24361-8
8. Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E, Daryani A. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006–2016). Front Microbiol. (2017) 8:25. doi: 10.3389/fmicb.2017.00025
9. Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. (2013) 91:501–8. doi: 10.2471/BLT.12.111732
10. Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. (2012) 139:1375–424. doi: 10.1017/S0031182012000765
11. Rabiee MH, Mahmoudi A, Siahsarvie R, Kryštufek B, Mostafavi E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl Trop Dis. (2018) 12:6256. doi: 10.1371/journal.pntd.0006256
12. Kazemi-Moghaddam V, Dehghani R, Hadei M, Dehqan S, Sedaghat MM, Latifi M, et al. Rodent-borne and rodent-related diseases in Iran. Comp Clin Path. (2019) 28:893–905. doi: 10.1007/s00580-018-2690-9
13. Dabritz HA, Miller MA, Gardner IA, Packham AE, Atwill ER, Conrad PA. Risk factors for Toxoplasma gondii infection in wild rodents from central coastal California and a review of T. gondii prevalence in rodents. J Parasitol. (2008) 94:675–84. doi: 10.1645/GE-1342.1
14. Mercier A, Garba M, Bonnabau H, Kane M, Rossi JP, Dardé ML, et al. Toxoplasmosis seroprevalence in urban rodents: a survey in Niamey, Niger. Mem. Inst. Oswaldo Cruz. (2013) 108:399–407. doi: 10.1590/S0074-0276108042013002
15. Yai LE, Ragozo AM, Aguiar DM, Damaceno JT, Oliveira LN, Dubey JP, et al. Isolation of Toxoplasma gondii from capybaras (Hydrochaeris hydrochaeris) from São Paulo state, Brazil. J Parasitol. (2008) 94:1060–3. doi: 10.1645/GE-1548.1
16. Cañon-Franco WA, Yai LO, Joppert AM, Souza CE, D'Auria SN, Dubey JP, et al. Seroprevalence of Toxoplasma gondii antibodies in the rodent capybara (Hidrochoeris hidrochoeris) from Brazil. J Parasitol. (2003) 89:850. doi: 10.1645/GE-80R
17. Daryani A, Sarvi S, Sharif M, Moosazadeh M, Galeh TM, Nakhaei M, et al. Toxoplasmosis in rodents in the world: a systematic review and meta-analysis. PROSPERO (2018). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018107622
18. Munn Z, Moola S, Lisy K, Riitano D. The Joanna Briggs Institute Reviewers' Manual 2014: The Systematic Review of Prevalence and Incidence Data. Adelaide, SA: The Joanna Briggs Institute (2014).
19. Rifaat MA, Mahdi AH, Arafa MS, Nasr NT, Sadek MS. Isolation of Toxoplasma from Rattus norvegicus in Egypt. Trans R Soc Trop Med Hyg. (1971) 65:788–9. doi: 10.1016/0035-9203(71)90093-9
21. Rifaat MA, Nasr NT, Sadek MS, Arafa MS, Mahdi AH. The role of the domestic rat, Rattus alexandrinus as a reservoir host of Toxoplasma gondii in Egypt. J Trop Med Hyg. (1973) 76:257–8.
22. Arene FO. Prevalence of Toxoplasma gondii among West African rodent, Thryonomys swinderianus from the Niger Delta. J Hyg Epidemiol Microbiol Immunol. (1986) 30:215–7.
23. Taylor PJ, Arntzen L, Hayter M, Iles M, Frean J, Belmain S. Understanding and managing sanitary risks due to rodent zoonoses in an African city: beyond the Boston Model. Integr Zool. (2008) 3:38–50. doi: 10.1111/j.1749-4877.2008.00072.x
24. Foronda P, Plata-Luis J, del Castillo-Figueruelo B, Fernández-Álvarez Á, Martín-Alonso A, Feliu C, et al. Serological survey of antibodies to Toxoplasma gondii and Coxiella burnetii in rodents in north-western African islands (Canary Islands and Cape Verde). Onderstepoort J Vet Res. (2015) 82:1–4. doi: 10.4102/ojvr.v82i1.899
25. Archer CE, Appleton CC, Mukaratirwa S, Lamb J, Corrie Schoeman M. Endo-parasites of public-health importance recovered from rodents in the Durban metropolitan area, South Africa. S Afr J Infect Dis. (2017) 32:57–66. doi: 10.1080/23120053.2016.1262579
26. Brouat C, Diagne CA, Ismaïl K, Aroussi A, Dalecky A, Bâ K, et al. Seroprevalence of Toxoplasma gondii in commensal rodents sampled across Senegal, West Africa. Parasite. (2018) 25:32. doi: 10.1051/parasite/2018036
27. Durfee PT, Sung HT, Ma CH, Tsai CS, Cross JH. Serologic study of toxoplasmosis in Taiwan. Southeast Asian J Trop Med. Public Health. (1975) 6:170–4.
28. Teutsch SM, Juranek DD, Sulzer A, Dubey JP, Sikes RK. Epidemic toxoplasmosis associated with infected cats. N Engl J Med. (1979) 300:695–9. doi: 10.1056/NEJM197903293001302
29. Ohshima S, Tsubota N, Hiraoka K. Latex agglutination microtiter test for diagnosis of Toxoplasma infection in animals. Zentralbl Bakteriol Mikrobiol Hyg A. (1981) 250:376–82. doi: 10.1016/S0174-3031(81)80130-8
30. Lubroth JS, Dreesen DW, Ridenhour RA. The role of rodents and other wildlife in the epidemiology of swine toxoplasmosis. Prev Vet Med. (1983) 1:169–78. doi: 10.1016/0167-5877(83)90021-1
31. Chhabra MB, Gupta SL, Gautam OP. Toxoplasma seroprevalence in animals in northern India. Int J Zoonoses. (1985) 12:136–42.
32. Murata K. A serological survey of Toxoplasma gondii infection in zoo animals and other animals. Nihon Juigaku Zasshi. (1989) 51:935–40. doi: 10.1292/jvms1939.51.935
33. Shen L, Zhichung L, Biaucheng Z, Huayuan Y. Prevalence of Toxoplasma gondii infection in man and animals in Guangdong, People's Republic of China. Vet Parasitol. (1990) 34:357–60. doi: 10.1016/0304-4017(90)90082-M
34. Jeon SH, Yong TS. Serological observation of Toxoplasma gondii prevalence in Apodemus agrarius, a dominant species of field rodents in Korea. Yonsei Med J. (2000) 41:491–6. doi: 10.3349/ymj.2000.41.4.491
35. Morsy TA, El Bahrawy AF, El Dakhil MA. Ecto-and blood parasites affecting Meriones rex trapped in Najran, Saudi Arabia. J Egypt Soc Parasitol. (2001) 31:399–405.
36. Kia EB, Homayouni MM, Farahnak A, Mohebali M, Shojai S. Study of endoparasites of rodents and their zoonotic importance in Ahvaz, south west Iran. Iran J Public Health. (2001) 30:49–52.
37. Karatepe M, Babur C, Karatepe B, Kiliç S, Çakir M. Prevalence of Toxoplasma gondii antibodies in anatolian ground squirrels, Spermophilus xanthophrymnus (Rodentia: Sciuridae) from Nigde, Turkey. Rev Med Vet. (2004) 155:530–32.
38. Zhang SY, Jiang SF, He YY, Pan CE, Zhu M, Wei MX. Serologic prevalence of Toxoplasma gondii in field mice, Microtus fortis, from Yuanjiang, Hunan Province, People's Republic of China. J Parasitol. (2004) 90:437–9. doi: 10.1645/GE-168R
39. Salibay CC, Claveria FG. Serologic detection of Toxoplasma gondii infection in Rattus spp collected from three different sites in Dasmarinas, Cavite, Philippines. Southeast Asian J Trop Med Public Health. (2005) 36:46–9.
40. Salant H, Weingram T, Spira DT, Eizenberg T. An outbreak of Toxoplasmosis amongst squirrel monkeys in an Israeli monkey colony. Vet Parasitol. (2009) 159:24–9. doi: 10.1016/j.vetpar.2008.10.011
41. Yin CC, He Y, Zhou DH, Yan C, He XH, Wu SM, et al. Seroprevalence of Toxoplasma gondii in rats in southern China. J Parasitol. (2010) 96:1233–5. doi: 10.1645/GE-2610.1
42. Mahmodzadeh A. Survey of Toxoplasma gondii infection rate in Rattus by ELISA method in Tehran. Modares J Med Sci Pathobiol. (2011) 13:77-83.
43. Mosallanejad B, Avizeh R, Jalali MH, Hamidinejat H. Seroprevalence of Toxoplasma gondii among wild rats (Rattus rattus) in Ahvaz District, Southwestern Iran. Jundishapur J Microbiol. (2012) 5:332–5. doi: 10.5812/kowsar.20083645.2373
44. Ahmad MS, Maqbool A, Mahmood-ul-Hassan M, Mushtaq-ul-Hassan M, Anjum AA. Prevalence of Toxoplasma gondii antibodies in human beings and commensal rodents trapped from Lahore, Pakistan. J Anim Plant Sci. (2012) 22:51–53.
45. Hong SH, Lee SE, Jeong YI, Kim HC, Chong ST, Klein TA, et al. Prevalence and molecular characterizations of Toxoplasma gondii and Babesia microti from small mammals captured in Gyeonggi and Gangwon Provinces, Republic of Korea. Vet parasitol. (2014) 205:512–7. doi: 10.1016/j.vetpar.2014.07.032
46. Normaznah Y, Azizah MA, Azuan MI, Latifah I, Rahmat S, Nasir MA. Seroprevalence of Toxoplasma gondii in rodents from various locations in peninsular Malaysia. Southeast Asian J Trop Med Public Health. (2015) 46:388–95.
47. Buddhirongawatr R, Chaichoun K, Tungsudjai S, Udonsom R, Thompson A, Mahittikorn O, et al. Seroprevalence and phylogenetic analysis of Toxoplasma gondii from domestic cats, captive wild felids, free-range wild felids and rats in certain regions of Thailand. Thai J Vet Med. (2016) 46:209–18.
48. Pavlova EV, Kirilyuk EV, Naidenko SV. Occurrence pattern of influenza A virus, Coxiella burnetii, Toxoplasma gondii, and Trichinella sp. in the Pallas cat and domestic cat and their potential prey under arid climate conditions. Arid Ecosyst. (2016) 6:277–83. doi: 10.1134/S2079096116040089
49. Seifollahi Z, Sarkari B, Motazedian MH, Asgari Q, Ranjbar MJ, Abdolahi Khabisi S. Protozoan parasites of rodents and their zoonotic significance in Boyer-Ahmad District, Southwestern Iran. Vet Med Int. (2016) 2016:3263868. doi: 10.1155/2016/3263868
50. Rafique A, Iqbal F, Ashraf A, Jabeen F, Naz S, Mahmood MS. Seroprevalence of Toxoplasma gondii and its effect of hematological picture in commensal rodents in Faisalabad Pakistan. Pak J Agric Sci. (2017) 54:195–9. doi: 10.21162/PAKJAS/17.5550
51. Wang XL, Dong L, Zhang L, Lv Y, Li Q, Li HL. Seroprevalence and genetic characterization of Toxoplasma gondii in naturally infected synanthropic rodents in Yunnan Province, Southwestern China. J Parasitol. (2018) 104:383–8. doi: 10.1645/17-156
52. Wallace GD, Marshall L, Marshall MA. Cats, rats, and toxoplasmosis on a small Pacific island. Am J Epidemiol. (1972) 95:475–82. doi: 10.1093/oxfordjournals.aje.a121414
53. Glazebrook JS, Campbell RS, Hutchinson GW, Stallman ND. Rodent zoonoses in North Queensland: the occurrence and distribution of zoonotic infections in North Queensland rodents. Aust J Exp Biol Med Sci. (1978) 56:147–56. doi: 10.1038/icb.1978.16
54. Werner H, Aspöck H, Janitschke K. Serological studies on the occurrence of Toxoplasma gondii among wild living mammalia in eastern Austria. Zentbl Bakt I Orig Ser A. (1973) 224:257–63.
55. Doby JM, Desmonts G, Bealcourku JC, Akinchina GT. Systematic immunological investigation into toxoplasmosis in wild small mammals, in France. Folia Parasitol. (1974) 21:289–300.
56. Kapperud G. Survey for toxoplasmosis in wild and domestic animals from Norway and Sweden. J Wildl Dis. (1978) 14:157–62. doi: 10.7589/0090-3558-14.2.157
57. Hay J, Hutchison WM, Jackson MH, Siim JC. Prevalence of Toxoplasma infection in a wild rodent population from central Scotland. Ann Trop Med Parasit. (1983) 77:653–4. doi: 10.1080/00034983.1983.11811764
58. Jackson MH, Hutchison WM, Siim JC. Toxoplasmosis in a wild rodent population of central Scotland and a possible explanation of the mode of transmission. J Zool. (1986) 209:549–57. doi: 10.1111/j.1469-7998.1986.tb03610.x
59. Webster JP. Prevalence and transmission of Toxoplasma gondii in wild brown rats, Rattus norvegicus. Parasitology. (1994) 108:407–11. doi: 10.1017/S0031182000075958
60. Bollo E, Pregel P, Gennero S, Pizzoni E, Rosati S, Nebbia P, et al. Health status of a population of nutria (Myocastor coypus) living in a protected area in Italy. Res Vet Sci. (2003) 75:21–5. doi: 10.1016/S0034-5288(03)00035-3
61. Arnaudov DI, Arnaudov AT, Kirin DI. Study on the toxoplasmosis among wild animals. Exp Pathol Parasitol. (2003) 6:51–4.
62. Afonso E, Poulle ML, Lemoine M, Villena I, Aubert D, Gilot-Fromont E. Prevalence of Toxoplasma gondii in small mammals from the Ardennes region, France. Folia Parasitol. (2007) 54:313–4. doi: 10.14411/fp.2007.041
63. Prestrud KW, Åsbakk K, Fuglei E, Mørk T, Stien A, Ropstad E, et al. Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard. Vet Parasitol. (2007) 150:6–12. doi: 10.1016/j.vetpar.2007.09.006
64. Murphy RG, Williams RH, Hughes JM, Hide G, Ford NJ, Oldbury DJ. The urban house mouse (Mus domesticus) as a reservoir of infection for the human parasite Toxoplasma gondii: an unrecognised public health issue? Int J Environ Health Res. (2008) 18:177–85. doi: 10.1080/09603120701540856
65. Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. (2009) 136:329–37. doi: 10.1017/S0031182008005428
66. Psaroulaki A, Antoniou M, Toumazos P, Mazeris A, Ioannou I, Chochlakis D, et al. Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: a seroepidemiological study. Trans R Soc Trop Med Hyg. (2010) 104:733–9. doi: 10.1016/j.trstmh.2010.08.005
67. Nardoni S, Angelici MC, Mugnaini L, Mancianti F. Prevalence of Toxoplasma gondii infection in Myocastor coypus in a protected Italian wetland. Parasit Vectors. (2011) 4:240. doi: 10.1186/1756-3305-4-240
68. Vujanić M, Ivović V, Kataranovski M, Nikolić A, Bobić B, Klun I, et al. Toxoplasmosis in naturally infected rodents in Belgrade, Serbia. Vector Borne Zoonot Dis. (2011). 11:1209–11. doi: 10.1089/vbz.2010.0119
69. Backhans A, Jacobson M, Hansson I, Lebbad M, Lambertz ST, Gammelgård E, et al. Occurrence of pathogens in wild rodents caught on Swedish pig and chicken farms. Epidemiol Infect. (2013) 141:1885–91. doi: 10.1017/S0950268812002609
70. Gotteland C, Chaval Y, Villena I, Galan M, Geers R, Aubert D, et al. Species or local environment, what determines the infection of rodents by Toxoplasma gondii?. Parasitology. (2014) 141:259–68. doi: 10.1017/S0031182013001522
71. Ayral F, Artois J, Zilber AL, Widén F, Pounder KC, Aubert D, et al. The relationship between socioeconomic indices and potentially zoonotic pathogens carried by wild Norway rats: a survey in Rhône, France (2010–2012). Epidemiol Infect. (2015) 143:586–99. doi: 10.1017/S0950268814001137
72. Zanzani SA, Cerbo AD, Gazzonis AL, Epis S, Invernizzi A, Tagliabue S, et al. Parasitic and bacterial infections of Myocastor coypus in a metropolitan area of northwestern Italy. J Wildl Dis. (2016) 52:126–30. doi: 10.7589/2015-01-010
73. Machačová T, Ajzenberg D, Žákovská A, Sedlák K, Bártová E. Toxoplasma gondii and Neospora caninum in wild small mammals: seroprevalence, DNA detection and genotyping. Vet Parasitol. (2016) 223:88–90. doi: 10.1016/j.vetpar.2016.04.018
74. Bastien M, Vaniscotte A, Combes B, Umhang G, Germain E, Gouley V, et al. High density of fox and cat faeces in kitchen gardens and resulting rodent exposure to Echinococcus multilocularis and Toxoplasma gondii. Folia Parasitol. (2018) 65:1–9. doi: 10.14411/fp.2018.002
75. Marchiondo AA, Duszynski DW, Maupin GO. Prevalence of antibodies to Toxoplasma gondii in wild and domestic animals of New Mexico, Arizona and Colorado. J Wildl Dis. (1976) 12:226–32. doi: 10.7589/0090-3558-12.2.226
76. Quinn PJ, Ramsden RO, Johnston DH. Toxoplasmosis: a serological survey in Ontario wildlife. J Wildl Dis. (1976) 12:504–10. doi: 10.7589/0090-3558-12.4.504
77. Franti CE, Riemann HP, Behymer DE, Suther D, Howarth JA, Ruppanner R. Prevalence of Toxoplasma gondii antibodies in wild and domestic animals in northern California. J Am Vet Med Assoc. (1976) 169:901–6.
78. Tizard IR, Harmeson J, Lai CH. The prevalence of serum antibodies to Toxoplasma gondii in Ontario mammals. Can J Comp Med. (1978) 42:177–83.
79. Chinchilla M. Epidemiology of toxoplasmosis in Costa Rica: importance of domestic rodents. Rev Biol Trop. (1978) 26:113–24.
80. Burridge MJ, Bigler WJ, Forrester DJ, Hennemann JM. Serologic survey for Toxoplasma gondii in wild animals in Florida. J Am Vet Med Assoc. (1979) 175:964–7.
81. Childs JE, Seegar WS. Epidemiologic observations on infection with Toxoplasma gondii in three species of urban mammals from Baltimore, Maryland, USA. Int J Zoonoses. (1986) 13:249–61.
82. Smith KE, Zimmerman JJ, Patton S, Beran GW, Hill HT. The epidemiology of toxoplasmosis in Iowa swine farms with an emphasis on the roles of free-living mammals. Vet Parasitol. (1992) 42:199–211. doi: 10.1016/0304-4017(92)90062-E
83. Howerth EW, Reeves AJ, McElveen MR, Austin FW. Survey for selected diseases in nutria (Myocastor coypus) from Louisiana. J Wildl Dis. (1994) 30:450–3. doi: 10.7589/0090-3558-30.3.450
84. Smith DD, Frenkel JK. Prevalence of antibodies to Toxoplasma gondii in wild mammals of Missouri and east central Kansas: biologic and ecologic considerations of transmission. J Wildl Dis. (1995) 31:15–21. doi: 10.7589/0090-3558-31.1.15
85. Dubey JP, Weigel RM, Siegel AM, Thulliez P, Kitron UD, Mitchell MA, et al. Sources and reservoirs of Toxoplasma gondii infection on 47 swine farms in Illinois. J Parasitol. (1995) 81:723–9. doi: 10.2307/3283961
86. Frenkel JK, Hassanein KM, Hassanein RS, Brown E, Thulliez P, Quintero-Nunez R. Transmission of Toxoplasma gondii in Panama City, Panama: a five-year prospective cohort study of children, cats, rodents, birds, and soil. Am J Trop Med Hyg. (1995) 53:458–68. doi: 10.4269/ajtmh.1995.53.458
87. Stewart RL, Humphreys JG, Dubey JP. Toxoplasma gondii antibodies in woodchucks (Marmota monax) from Pennsylvania. J Parasitol. (1995) 81:126–7. doi: 10.2307/3284025
88. Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol Infect. (1999) 122:305–15. doi: 10.1017/S0950268899002113
89. Mateus-Pinilla NE, Dubey JP, Choromanski L, Weigel RM. A field trial of the effectiveness of a feline Toxoplasma gondii vaccine in reducing T. gondii exposure for swine J Parasitol. (1999) 85:855–60. doi: 10.2307/3285821
90. DeFeo ML, Dubey JP, Mather TN, Rhodes RC III. Epidemiologic investigation of seroprevalence of antibodies to Toxoplasma gondii in cats and rodents. Am J Vet Res. (2002) 63:1714–17. doi: 10.2460/ajvr.2002.63.1714
91. Lehmann T, Graham DH, Dahl E, Sreekumar C, Launer F, Corn JL, et al. Transmission dynamics of Toxoplasma gondii on a pig farm. Infect Genet Evol. (2003) 3:135–41. doi: 10.1016/S1567-1348(03)00067-4
92. Jordan CN, Kaur T, Koenen K, DeStefano S, Zajac AM, Lindsay DS. Prevalence of agglutinating antibodies to Toxoplasma gondii and Sarcocystis neurona in beavers (Castor canadensis) from Massachusetts. J Parasitol. (2005) 91:1228–30. doi: 10.1645/GE-543R.1
93. Dubey JP, Bhaiyat MI, Macpherson CL, de Allie C, Chikweto A, Kwok OH, et al. Prevalence of Toxoplasma gondii in rats (Rattus norvegicus) in Grenada, West Indies. J Parasitol. (2006) 92:1107–9. doi: 10.1645/GE-902R.1
94. Dubey JP, Velmurugan GV, Alvarado-Esquivel C, Alvarado-Esquivel D, Rodríguez-Peña S, Martínez-García S, et al. Isolation of Toxoplasma gondii from animals in Durango, Mexico. J Parasitol. (2009) 95:319–23. doi: 10.1645/GE-1874.1
95. Lehrer EW, Fredebaugh SL, Schooley RL, Mateus-Pinilla NE. Prevalence of antibodies to Toxoplasma gondii in woodchucks across an urban–rural gradient. J Wildl Dis. (2010) 46:977–80. doi: 10.7589/0090-3558-46.3.977
96. Dubey JP, Velmurugan GV, Rajendran C, Yabsley MJ, Thomas NJ, Beckmen KB, et al. Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. Int J Parasitol. (2011) 41:1139–47. doi: 10.1016/j.ijpara.2011.06.005
97. Rendón-Franco E, Xicoténcatl-García L, Rico-Torres CP, Muñoz-García CI, Caso-Aguilar A, Suzán G, et al. Toxoplasmosis seroprevalence in wild small rodents, potentially preys of ocelots in north-eastern Mexico. Parasite. (2014) 21:57. doi: 10.1051/parasite/2014058
98. Poulsen A, Fritz H, Clifford DL, Conrad P, Roy A, Glueckert E, et al. Prevalence and potential impact of Toxoplasma gondii on the endangered amargosa vole (Microtus californicus scirpensis), California, USA. J Wildl Dis. (2017) 53:62–72. doi: 10.7589/2015-12-349
99. Gerhold RW, Saraf P, Chapman A, Zou X, Hickling G, Stiver WH, et al. Toxoplasma gondii seroprevalence and genotype diversity in select wildlife species from the southeastern United States. Parasit Vectors. (2017) 10:508. doi: 10.1186/s13071-017-2456-2
100. Murata FH, Cerqueira-Cézar CK, Kwok OC, Tiwari K, Sharma RN, Su C, et al. Role of rats (Rattus norvegicus) in the epidemiology of Toxoplasma gondii infection in Grenada, West Indies. J Parasitol. (2018) 104:571–3. doi: 10.1645/18-58
101. Carme B, Aznar C, Motard A, Demar M, de Thoisy B. Serologic survey of Toxoplasma gondii in noncarnivorous free-ranging neotropical mammals in French Guiana. Vector Borne Zoonotic Dis. (2002) 2:11–7. doi: 10.1089/153036602760260733
102. de Thois B, Demar M, Aznar C, Carme B. Ecologic correlates of Toxoplasma gondii exposure in free-ranging neotropical mammals. J Wildl Dis. (2003) 39:456–9. doi: 10.7589/0090-3558-39.2.456
103. Cola GA Garcia JL da Costa L Ruffolo B Navarro IT Freire RL. Comparison of the indirect fluorescent antibody test and modified agglutination test for detection of anti-Toxoplasma gondii antibodies in rats. Semin Cienc Agrar. (2010) 31:717–22. doi: 10.5433/1679-0359.2010v31n3p717
104. Truppel JH, Reifur L, Montiani-Ferreira F, Lange RR, de RG, Gennari SM, et al. Toxoplasma gondii in Capybara (Hydrochaeris hydrochaeris) antibodies and DNA detected by IFAT and PCR. Parasitol Res. (2010) 107:141–6. doi: 10.1007/s00436-010-1848-4
105. Araújo JB, da Silva AV, Rosa RC, Mattei RJ, da Silva RC, Richini-Pereira VB, et al. Isolation and multilocus genotyping of Toxoplasma gondii in seronegative rodents in Brazil. Vet Parasitol. (2010) 174:328–31. doi: 10.1016/j.vetpar.2010.08.039
106. Minervino AH, Soares HS, Barrêto-Júnior RA, Neves KA, de Jesus Pena HF, Ortolani EL, et al. Seroprevalence of Toxoplasma gondii antibodies in captive wild mammals and birds in Brazil. J Zoo Wildl Med. (2010) 41:572–4. doi: 10.1638/2010-0046.1
107. Costa DG, Marvulo MF, Silva JS, Santana SC, Magalhães FJ, Lima Filho CD, et al. Seroprevalence of Toxoplasma gondii in domestic and wild animals from the Fernando de Noronha, Brazil. J Parasitol. (2012) 98:679–81. doi: 10.1645/GE-2910.1
108. Siqueira DB, Aléssio FM, Mauffrey JF, Marvulo MF, Ribeiro VO, Oliveira RL, et al. Seroprevalence of Toxoplasma gondii in wild marsupials and rodents from the Atlantic forest of Pernambuco state, northeastern region, Brazil. J Parasitol. (2013) 99:1140–4. doi: 10.1645/GE-2855.1
109. Chiacchio RG, Prioste FE, Vanstreels RE, Knöbl T, Kolber M, Miyashiro SI, et al. Health evaluation and survey of zoonotic pathogens in free-ranging capybaras (Hydrochoerus hydrochaeris). J Wildl Dis. (2014) 50:496–504. doi: 10.7589/2013-05-109
110. Martino PE, Stanchi NO, Silvestrini M, Brihuega B, Samartino L, Parrado E. Seroprevalence for selected pathogens of zoonotic importance in wild nutria (Myocastor coypus). Eur J Wildl Res. (2014) 60:551–4. doi: 10.1007/s10344-014-0805-4
111. Gennari SM, Ogrzewalska MH, Soares HS, Saraiva DG, Pinter A, Nieri-Bastos FA, et al. Toxoplasma gondii antibodies in wild rodents and marsupials from the Atlantic Forest, state of São Paulo, Brazil. Rev Bras Parasitol Vet. (2015) 24:379–82. doi: 10.1590/S1984-29612015045
112. Abreu JA, Krawczak FD, Nunes FP, Labruna MB, Pena HF. Anti-Toxoplasma gondii and anti-Neospora caninum antibodies in capybaras (Hydrochoerus hydrochaeris) from Itu Municipality, São Paulo. Rev Bras Parasitol Vet. (2016) 25:116–8. doi: 10.1590/S1984-29612016002
113. Ruffolo BB, Toledo RD, Martins FD, Bugni FM, Costa LD, Marana ER, et al. Isolation and genotyping of Toxoplasma gondii in seronegative urban rats and presence of antibodies in communicating dogs in Brazil. Rev Inst Med Trop Sao Paulo. (2016) 58:28. doi: 10.1590/s1678-9946201658028
114. Pellizzaro M, Conrado FD, Martins CM, Joaquim SF, Ferreira F, Langoni H, et al. Serosurvey of Leptospira spp. and Toxoplasma gondii in rats captured from two zoos in Southern Brazil. Rev Soc Bras Med Trop. (2017) 50:857–60. doi: 10.1590/0037-8682-0138-2017
115. Lopes KF, de Melo Germano R, Gerônimo E, Zago D, Dias EH, Chideroli RT, et al. A serological survey of agents causing leptospirosis and toxoplasmosis in Rattus rattus in the city of Umuarama, northwest Paraná, Brazila, Noroeste do Paraná, Brasil. Semin Cienc Agrar. (2017) 38:239–48. doi: 10.5433/1679-0359.2017v38n1p239
116. Ullmann LS, Gravinatti ML, Yamatogi RS, Santos LC, Moraes WD, Cubas ZS, et al. Serosurvey of anti-Leptospira sp. and anti-Toxoplasma gondii antibodies in capybaras and collared and white-lipped peccaries. Rev Soc Bras Med Trop. (2017) 50:248–50. doi: 10.1590/0037-8682-0315-2016
117. Silva JC, Ferreira F, Dias RA, Ajzenberg D, Marvulo MF, Magalhães FJ, et al. Cat-rodent Toxoplasma gondii type II-variant circulation and limited genetic diversity on the Island of Fernando de Noronha, Brazil. Parasit Vectors. (2017) 10:220. doi: 10.1186/s13071-017-2150-4
118. Horta MC, Guimarães MF, Arraes-Santos AI, Araujo AC, Dubey JP, Labruna MB, et al. Detection of anti-Toxoplasma gondii antibodies in small wild mammals from preserved and non-preserved areas in the Caatinga biome, a semi-arid region of Northeast Brazil. Vet Parasitol Reg Stud Rep. (2018) 14:75–8. doi: 10.1016/j.vprsr.2018.08.007
119. Sarvi S, Daryani A, Rahimi MT, Aarabi M, Shokri A, Ahmadpour E, et al. Cattle toxoplasmosis in Iran: a systematic review and meta–analysis. Asian Pac J Trop Med. (2015) 8:120–6. doi: 10.1016/S1995-7645(14)60301-1
120. Shwab EK, Zhu XQ, Majumdar D, Pena HF, Gennari SM, Dubey JP, et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. (2014) 141:453–61. doi: 10.1017/S0031182013001844
121. Afonso E, Thulliez P, Pontier D, Gilot-Fromont E. Toxoplasmosis in prey species and consequences for prevalence in feral cats: not all prey species are equal. Parasitology. (2007) 134:1963–71. doi: 10.1017/S0031182007003320
122. Tizard IR, Billett JB, Ramsden RO. The prevalence of antibodies against Toxoplasma gondii in some Ontario mammals. J Wildl Dis. (1976) 12:322–5. doi: 10.7589/0090-3558-12.3.322
123. Rostami A, Riahi SM, Fakhri Y, Saber V, Hanifehpour H, Valizadeh S, et al. The global seroprevalence of Toxoplasma gondii among wild boars: a systematic review and meta-analysis. Vet Parasitol. (2017) 244:12–20. doi: 10.1016/j.vetpar.2017.07.013
124. Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. (2014) 137:185–94. doi: 10.1016/j.actatropica.2014.05.015
125. Sharif M, Sarvi S, Shokri A, Teshnizi SH, Rahimi MT, Mizani A, et al. Toxoplasma gondii infection among sheep and goats in Iran: a systematic review and meta-analysis. Parasitol Res. (2015) 114:1–16. doi: 10.1007/s00436-014-4176-2
Keywords: toxoplasmosis, Toxoplasma gondii, seroprevalence, rodents, systematic review, meta-analysis
Citation: Galeh TM, Sarvi S, Montazeri M, Moosazadeh M, Nakhaei M, Shariatzadeh SA and Daryani A (2020) Global Status of Toxoplasma gondii Seroprevalence in Rodents: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 7:461. doi: 10.3389/fvets.2020.00461
Received: 16 January 2020; Accepted: 23 June 2020;
Published: 31 July 2020.
Edited by:
Anja Joachim, University of Veterinary Medicine Vienna, AustriaReviewed by:
Gunita Deksne, Institute of Food Safety, Animal Health and Environment “BIOR”, LatviaGuo-Hua Liu, Hunan Agricultural University, China
Copyright © 2020 Galeh, Sarvi, Montazeri, Moosazadeh, Nakhaei, Shariatzadeh and Daryani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Daryani, ZGFyeWFuaWlAeWFob28uY29t
 Tahereh Mikaeili Galeh1,2,3
Tahereh Mikaeili Galeh1,2,3 Shahabeddin Sarvi
Shahabeddin Sarvi Mahbobeh Montazeri
Mahbobeh Montazeri Ahmad Daryani
Ahmad Daryani