- 1PEGASE, Agrocampus Ouest, INRA, Saint-Gilles, France
- 2USDA-ARS, Livestock Behavior Research Unit, West Lafayette, IN, United States
- 3Department of Animal Sciences, Purdue University, West Lafayette, IN, United States
- 4Department of Food Science, Purdue University, West Lafayette, IN, United States
Alternative feed supplements have shown promising effects in terms of performance, but their effects on welfare have had little evaluation. In the present study, we aimed at evaluating the effect of diet supplementation on welfare indicators. A total of 246 piglets were weaned and transported for 12 h. After transport, they were assigned to one of 3 diets for a 14-day period: A—an antibiotic diet including chlortetracycline and tiamulin, NA—a control diet without any antibiotic or feed supplement, GLN—a diet including 0.20% L-glutamine. After the 14-day period, all piglets were fed the same diet. Tear staining was measured 11 times post-weaning (from d0 to 147). Skin lesions were counted before and after weaning (d-2, 2, and 36). Novel object tests (NOT) were done in groups 4 times post-weaning (d17, 47, 85, 111). Samples for 16S rRNA gene composition were collected prior to transport (d0), following the 14-day period (d14) and at the conclusion of the nursery phase (d34). The NA pigs appeared less interested in novel objects. On d17, they avoided the object less than A pigs (P < 0.05). They spent less time exploring the object on d85 and took longer to interact with the object on d111 than A and GLN pigs (P < 0.05). NA pigs also appeared more sensitive to environment and management. They had larger tear stains than GLN pigs on d84 and 110 (P < 0.05). On d2, NA pigs had more lesions than A and GLN (P < 0.01). In terms of microbiota composition, GLN had higher α-diversity than A and NA (P < 0.001). Differences between dietary treatments were absent at d0, were demonstrated at d14 and disappeared at d34. Pearson correlations between aggression, stress and anxiety indicators and bacterial populations were medium to high from 0.31 to 0.69. The results demonstrate that short-term feeding strategy can have both short- and long-term effects on behavior and welfare, that may partly be explained by changes in gut microbiota composition. Supplementation with GLN appears to confer similar benefits to dietary antibiotics and thus could be a viable alternative.
Introduction
The routine use of antimicrobials in animal production has been identified as a potential factor in the development of antimicrobial resistance (1) and societal concern is driving increased stewardship, using the 5Rs model of responsibility, reduction, refinement, replacement, and review (2), especially in the use of medically-important antimicrobials. In the United States, increased stewardship has led to a 33% reduction in the domestic sale and distribution of all medically-important antimicrobials intended for use in food-producing animals, between 2016 and 2017 (3). However, certain swine production practices continue to impose increased risk of stress-induced disease susceptibility, and hence the use of antimicrobials is perceived to confer production advantages (4), although removal (5) or replacement with an alternative (6) may not be detrimental.
Stress can be defined as “an environmental effect on an individual which overtaxes its control systems and results in adverse consequences, eventually reduced fitness” (7). One of the most stressful events for pigs during production is that of weaning (8). At this time, they are subject to removal from the sow, an abrupt change in diet from milk to solid food and mixing into groups with unfamiliar pen-mates. In some countries, they may also be subjected to an additional stressor of transportation (9). These combined stressors often result in post-weaning diarrhea and compromised welfare post-weaning (10). The implication of various bacteria in post-weaning diarrhea has resulted in routine use of antimicrobials at this time-point, which impacts intestinal microbiota (11) but is seen to improve welfare, at least in the short term. The longer-term impact may be opposite. Reduced microbiome diversity and richness can reduce adaptability should the animal be challenged by dietary disturbances (12) or pathogens (13). This study used a combination of chlortetracycline, a medically-important antimicrobial aimed at prevention and treatment of gastrointestinal diseases, and tiamulin, a non-medically important antimicrobial aimed at prevention and treatment of respiratory diseases. This combination is the most commonly-prescribed antimicrobial mix used for 14d in post-weaning diets in the U.S., and has no adverse effects used at the concentrations prescribed. Alternatives to antimicrobials are being sought that will have the same health and welfare benefits, without the implications for antimicrobial resistance. These include phages, bacteriocins, peptides, probiotics, synthetic compounds, and nutraceuticals (14, 15). One potential nutraceutical is L-Glutamine, a conditionally-essential amino acid and immunomodulator that inhibits pro-inflammatory cytokines (16, 17). Dietary L-Glutamine supplementation can improve overall health and growth of newly weaned pigs (16–20) through a variety of mechanisms including: increased intestinal health and immune function, enhanced oxidative-defense capacity, prevention of intestinal atrophy, improved antibacterial activity, and greater nutrient absorption. It is well-known that L-Glutamine supplementation can improve piglet health and increase productivity (16–20), but little has been reported regarding its effects on swine welfare. In a study on simulated transport, weaned piglets fed diet with added L-Glutamine showed decreased intestinal damage, increased feed intake and growth, and decreased behaviors associated with illness compared to piglets provided diet without antibiotics (6). However, effects on other behaviors, and indicators of welfare and emotional state are not known.
Whereas, the potential effects of diet on behavior in pigs has been described (21), it is only in the last decade that the link between the gastrointestinal microbial population and brain function has begun to be elucidated (22, 23). We now know that the microbiome and the brain communicate bi-directionally via multiple suggested mechanisms, known collectively as the microbiome-gut-brain axis (24, 25). The existence of microbiome-gut-brain axis, and its relationship with behavior, affective state, cognition and stress response, is reported for numerous animal species, primarily rodents and humans. From human and other animal literature, the microbiome has been shown to play a role in anxiety and depression (26), fearfulness (27) and aggression (28) with shifts in microbiome linked to changes in these behaviors or affective states. As a route by which health and welfare of pigs can be manipulated, it is only recently receiving attention and is so far poorly understood (29, 30) but, theoretically, modification of the pig's gut microbiota offers a potential method to improve the pig's response to weaning and other lifetime stressors, and to improve overall health and welfare.
Since we know of the links between gut microbiome and fearfulness/anxiety and aggression, measures of interest will be behavioral responses during novel object tests carried out in the home pen so as to remove the confound of novel environment (31) and skin lesions post-mixing —a validated proxy measure of aggression (32). Tear staining will also be recorded, as the secretion of the Hardarian gland has been demonstrated as a potential tool for assessment of welfare in pigs on farm (33) and in a laboratory setting (34).
The objectives of the study were (1) To evaluate whether the administered diet can impact behavioral and welfare indicators during the administration period; (2) To determine if any diet effects can still be observed once the administration period is over; (3) To correlate behavioral indicators with specific microbiota composition.
Materials and Methods
Ethics Statement
All procedures involving animal use were approved by the Purdue University Animal Care and Use Committee (protocol #1603001385), and animal care and use standards were based upon the Guide for the Care and Use of Agricultural Animals in Research and Teaching (35).
Animals and Management
A total of 246 (138 barrows, 111 gilts) crossbred Duroc × (Landrace × Yorkshire) piglets from 32 L, free from any visible health issues, were used in the study. At weaning (means ± SD: age 18 ± 4.2 d; weight 3.2 kg < 5.4 ± 1.4 kg < 8.9 kg), piglets were removed from the sow and herded up an 11.0° incline ramp into a gooseneck livestock trailer (2.35 × 7.32 m; Wilson Trailer Company, Sioux City, IA) at a density of 0.07 m2/pig. In the trailer, the ambient temperature was 11.0 ± 0.2°C and the relative humidity was 63.1 ± 0.9%. Trailers were bedded with wood shavings and ventilation openings were adjusted based on the ambient temperature (36). Piglets were transported as a group in the trailer for approximately 12 h and 819 km without feed or water. Total transport time was determined by adding loading time, time spent in the trailer, unloading time, and the time it takes to be sorted into their respective pens in the nursery facility. The procedure of transportation is described in Duttlinger et al. (37). Immediately following transport, 6 of them were euthanized and dissected (see the detailed procedure below) whereas the others weaned piglets were herded out of the trailer, weighed, and sorted into 30 pens of 8 pigs/pen (10 pens per treatment), blocked weight and balanced for sex. Pens (1.22 × 1.37 m) initially provided approximately 0.21 m2 per pig. All pens contain one 5-hole dry self-feeder and a cup waterer to allow for ad libitum access to feed and water. Each pen was assigned to 1 of the 3 diets fed in 2 phases for a 14-day period post-transport: A—an antibiotic diet including a common commercially prophylactic antibiotic treatment (n = 80 piglets; 441 ppm of chlortetracycline + 38.6 ppm tiamulin), NA—a diet without any prophylactic antibiotics or feed supplement (n = 80 piglets), GLN—diet including a nutraceutical diet containing L-glutamine (n = 80 piglets; 0.20% L-glutamine as-fed). Base diets were similar for all diet treatments and were formulated to meet nutritional requirements based on piglet BW with identical ME, CP, SID Lys, Ca and P across the 3 treatment diets (for further details see (37)). On d15, dietary treatments were ceased and all piglets were provided the same standard diet for the remainder of the study both in the nursery, fed in 2 phases, and in the grow-finish barn, fed in 6 phases [for further details see (37)]. During the first week after weaning, 1 NA pig died. During the second week, 1 pig from each treatment died. Another NA pig died 5 weeks post-weaning and a GLN pig was removed from the study at 7 weeks because of a lameness.
On d0, 6 piglets (3 males and 3 females) were euthanized. On d15, 30 piglets (1 piglet from each treatment pen) were euthanized, and an additional 30 pigs (1 piglet from each treatment pen) were euthanized on d34, at the end of the nursery period. Piglet selection for euthanasia was balanced for sex, weight and absence of medical treatment required during the nursery phase. Euthanasia was carried out by CO2 exposure using a Euthanex® AgPro™ (Euthanex Corp, Easton, PA, USA) followed by exsanguination. All euthanized piglets were dissected for gut contents and gut mucus collection. The remaining 6 pigs/pen (n = 180 pigs) were moved to a grow-finish building where they remained in the same 30 groups until market. The grow-finish facility contained pens (1.68 m × 4.27 m) that provided ~1.19 m2 per pig. All pens contained one 2-hole dry self-feeder and a nipple waterer to allow for ad libitum access to feed and water.
Measurements
Tear Staining
Photographs of the left eye of each piglet were taken 11 times during the experiment to measure tear-staining: 24 to 48 h (d-2) before transport and on d2, 7, 15, 21, 28, 34, 47, 84, 110, and 146 after transport. Measurements were made on photographs by a single experienced person, blind to treatment, using the ImageJ software (38) to delimit the tear perimeter. The length of the iris was used as a scale to standardize the measurements. All the brownish areas on the direct periphery of the eye (bottom of the upper eyelid, top of the lower eyelid, internal and external corners) were recorded (34). The variable analyzed was the cumulative area covered by the stain.
Skin Lesions
Photographs of both sides of the body, as well as the head of each pig were taken 3 times during the experiment to measure skin body lesions: 24 to 48 h before transport (d-2) and on d2 and 39 after transport. Fresh skin lesions were counted on photographs by a single experienced person, blind to treatment, according to the Welfare Quality® Assessment Protocol in pigs (39) adapted for the first two stages as followed: all lesions above 1 cm were registered as 1 lesion; when at least 3 lesions below 1 cm were grouped within 2 cm, they were scored as 1 lesion. The variables analyzed were the total number of lesions in the front part of the body (as far back as the shoulder) and the cumulated number of skin lesions measured on the all body.
Novel Object Tests
Four 5-min novel object tests were carried out in the pigs' home pens. Four different objects, unfamiliar for the pigs, were used: a 20 cm swimming pool noodle, a 30 cm tall orange traffic cone, a 20 cm diameter PVC pipe wye socket and a 45 cm diameter aluminum trash can, respectively on d17, 47, 85, and 111 after transport. On the day prior to the test, all pigs were individually identified in each pen by a combination of 2 colors and 3 different locations (shoulder, back, hind-quarter) drawn on their body using livestock markers (All-Weather Paintstik™–La-Co Industries, Elk Grove Village, IL, USA). Immediately before introducing the object, the experimenter ensured all the pigs were standing and away from the feeder. However, some pigs did not see the object immediately when it was introduced in the pen. Therefore, variables regarding “interaction” with the object were corrected by the time needed for the pig to see the object for the first time (= head of the pig oriented toward the object with no contact with the object). The variables measured were: the number of withdrawals “Withdrawal” (sudden lateral or backward movements occurring with the head of the pig oriented toward the object), the latency to interact with the object for the first time “Interaction latency” (contact between the head of the pig and the object) which represents the interval between the first sight of the object and the first interaction with it, and the percentage of time spent interacting with the object “Interact duration” (total duration interacting with the object divided by the total duration from the first sight of the object to the end of the test). Video recordings were analyzed using the XP Observer 14 software (Noldus, The Netherlands) by a single experienced trained observer, blind to treatment.
Weighing Procedures
Once in the grow-finish barn, pigs were weighed every 3 weeks. Three of these handling procedures were videotaped on d55, 96, and 138. The gate of the pen was opened for 15 s with no human handling, and the number of pigs exiting the pen voluntarily was counted. After this period, a single experimenter, blind to treatment, entered the pen to bring out the remaining pigs and drive the group to the scale. The variables analyzed were: the number of pigs exiting the home pen voluntarily and the duration that it took the handler to remove all pigs from the pen ending with the rear feet of the last pig crossing the pen threshold “All out duration.” Video recordings were analyzed using the XP Observer 14 software (Noldus, The Netherlands) by a single experienced trained observer, blind to treatment.
Microbial Analyses of Gut Contents and Mucus
Samples for microbial analyses came from 6 piglets euthanized immediately following transport, 30 piglets (10 piglets per treatment) euthanized following the 14-d diet treatment period and from an additional 30 pigs (10 piglets per treatment) at the conclusion of the nursery phase (i.e., 20 d later).
We analyzed two kinds of samples for 16S rRNA gene composition: contents and mucus from three different locations in the gut (ileum, caecum and colon). The ileum part was a 10 cm section sampled 20 cm anterior to the ileal-cecal junction. The 10 cm colon section was collected on both sides of the top of the convolute of the ascending colon. The entire caecum was separated from the rest of the digestive tract. The tissue section was held on a board wrapped with autoclaved foil and cut through the longitudinal side. Gut contents samples were collected in sterile 2 ml tubes using sterilized spatulas, snap frozen with liquid nitrogen and stored at −80°C until DNA extraction. After collecting the gut content samples, the 10 cm intestinal tissue sections were washed with sterile PBS buffer until no remaining gut contents were visible. A sterile Cytosoft® cytology brush (Medical Packaging Corporation, Camarillo, CA, USA) was used to remove mucus and bacterial cells from the surface of the tissue. The brush was agitated in a 50 ml sterile Falcon tube containing 15 ml of PBS buffer and placed on ice. In the lab, the tubes were centrifuged at 5,000 g to pellet bacteria. The pellet was stored in 2 ml sterile tubes and frozen at −80°C until DNA extraction.
The DNA was extracted from 200 mg of each of the 6 frozen gut contents and mucus samples per pig by bead beating using the Fast DNA® SPIN kit for feces (MP Biomedicals Corporation, Irvine, CA, USA). Extracted DNA was then sent to the Argonne National Laboratory Environmental Sample Preparation and Sequencing Facility (Lemont, IL, USA) for PCR amplification of the V4 region of the 16S rRNA gene (515F-806R) (Forward: GTGYCAGCMGCCGCGGTAA; Reverse: GGACTACNVGGGTWTCTAAT) and sequenced using the MiSeq reagent kit V2 on an Illumina MiSeq (500 cycles) (Illumina Inc., San Diego, CA, USA). The sequencing library was generated using an integrated 12-base Golay barcode in the forward primer. Each 25 μL PCR reaction contains 9.5 μL of MO BIO PCR Water (Certified DNA-Free), 12.5 μL of QuantaBio's AccuStart II PCR ToughMix (2x concentration, 1x final), 1 μL Golay barcode tagged Forward Primer (5 μM concentration, 200 pM final), 1 μL Reverse Primer (5 μM concentration, 200 pM final), and 1 μL of template DNA. The conditions for PCR were as follows: 94°C for 3 min to denature the DNA, with 35 cycles at 94°C for 45 s, 50°C for 60 s, and 72°C for 90 s; with a final extension of 10 min at 72°C to ensure complete amplification.
Sequence Processing and Microbial Community Analyses
Briefly, 16S rRNA gene sequences were processed and clustered using the mothur v.1.39.3 standard operating procedure (SOP) designed for MiSeq data (40); the mothur MiSeq SOP was accessed in August 2018. The SILVA-based bacterial reference alignment (version 132) was used to identify the taxonomy of OTUs at a cluster cutoff of 97% sequence identity. Measures of richness, evenness and diversity were determined in mothur. Richness can be defined as the number of OTUs per sample, evenness represents the uniformity of the distribution of an individual amongst a community over different species, while α-diversity is a concept combining both richness and evenness (41). Richness and α-diversity were determined through the coverage, the number of OTUs observed, the Chao1, the ACE, the Shannon, the Simpson, the inverse Simpson estimators and β-diversity were calculated using the thetaYC distances as implemented in mothur. The OTU table was rarefied to 2,332 sequences per sample. A linear model with the treatment (A, GLN, NA), the day of sampling (14 or 34), the location (caecum, colon, ileum) and the type of sample (gut, mucus) was used for the richness, evenness and diversity indicator analyses. The effects of dietary treatments and days on the microbial community structure were tested using an analysis of molecular variance (AMOVA) of the thetaYC distance matrix in mothur. Results were adjusted by using the Bonferroni correction. The “metastats” command in mothur was then used to determine the OTUs responsible for the significant differences observed using AMOVA. The “corr.axes” and “otu.association” commands in mothur, specifying the default Pearson method, were then used in combination to estimate the significant Pearson correlations between behavioral indicators and bacterial populations.
Statistical Analysis
Statistical analyses were performed with the software R 3.4.3 (42). The variables of area of tear staining and the latency to interact with the novel object were normalized by logarithmic transformation before statistical analysis. Other variables were normal without transformation. In all the statistical analysis, P < 0.05 was considered statistically significant and 0.05 ≤ P < 0.1 as a trend.
Mixed effects model for repeated measures with the treatment, the day of sampling and the interaction between the two factors as fixed effects and the pen and the animal being included as random effects were used with the tear staining area, skin lesions and novel object test variables. The statistical unit for the previous traits was the animal. Regarding the weighing procedures variables, a second mixed effects model for repeated measures was used with the treatment, the day of sampling, the number of pigs per pen (5 or 6) and the interaction between the treatment and the day as fixed effects and the pen being included as random effects. The statistical unit for the weighing procedures variables was the pen. These analyses were done with the function lmer from the R package “lme4” 1.1-7. The emmeans function from the R package “emmeans” 1.2-2 was used to perform pairwise comparisons with the FDR correction when interactions were significant (P < 0.05) or were tendencies (P < 0.1).
Results
Ages and weights at different days are given as indications of performance in Table 1. Means, SEM, minimum and maximum of all untransformed data are reported in Table 1.
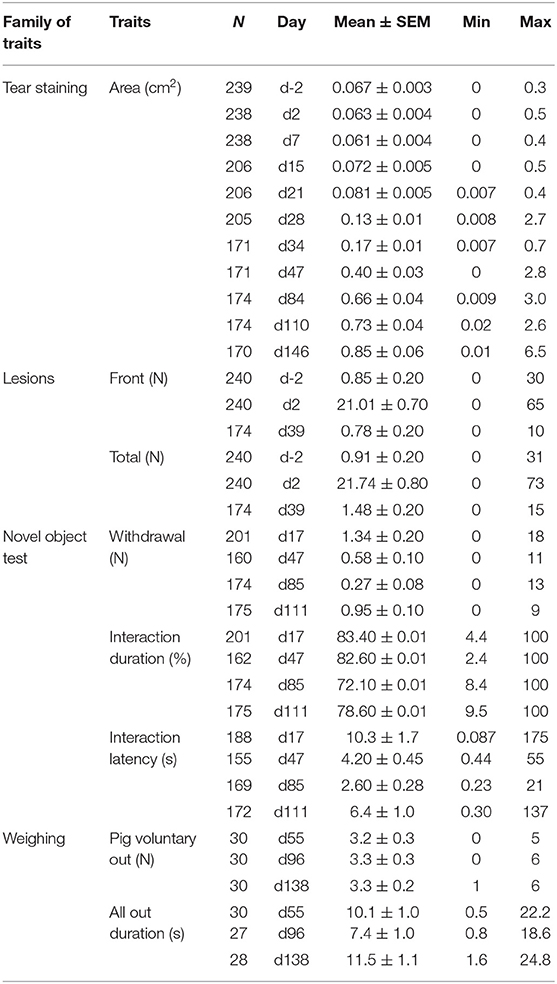
Table 1. Numbers (N), means, standard errors of the means (SEM), minimum (Min), maximum (Max) of welfare indicators or behaviors measured during tests, all dietary treatments mixed.
Tear Staining
The day of sampling was significant (P < 0.001) with higher values as the pigs aged (Table 2). On d84, tear staining areas of the NA pigs were larger than GLN pigs (P = 0.022) and tended to be higher than A pigs (P = 0.070). On d110, NA pigs had larger tear staining areas than GLN pigs (P = 0.003) and tended to have larger tear staining areas than A pigs (P = 0.080). No other significant effects were found (P > 0.1).
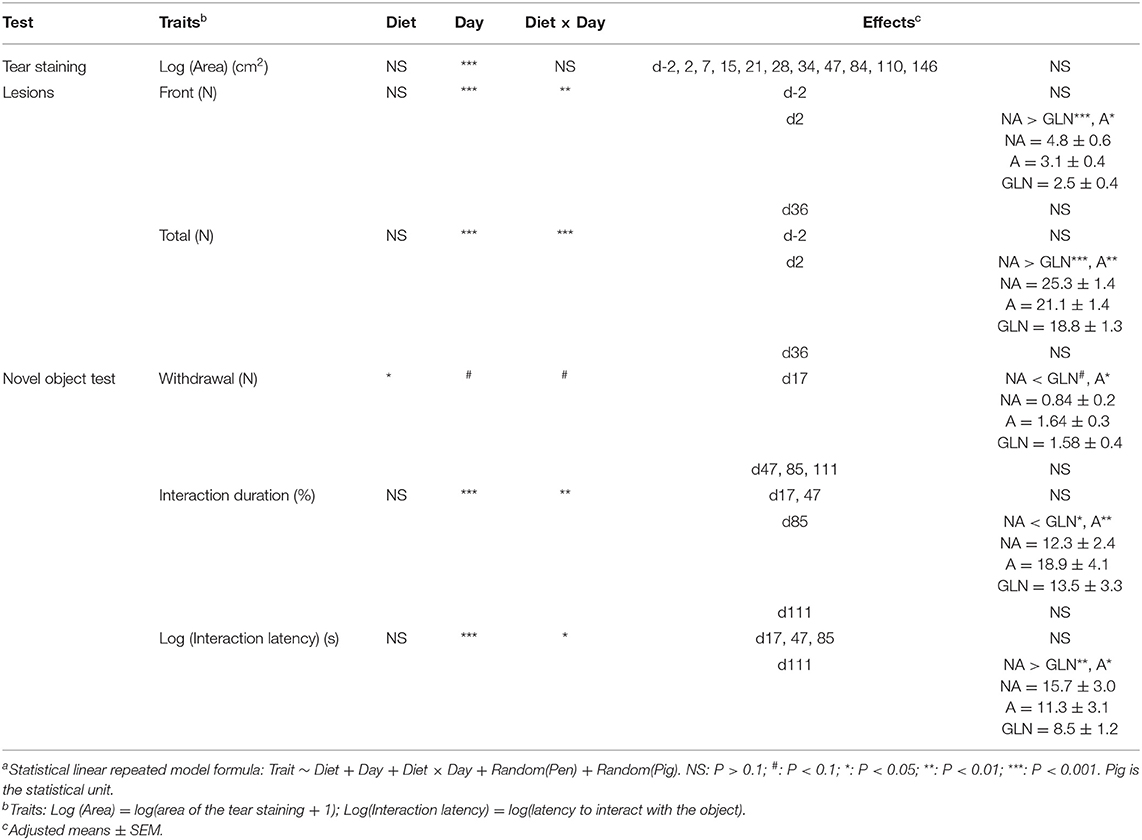
Table 2. Effect of the diets (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed) and the day (from 2 d before transport of weaned piglets to 147 d after) for repeated measures on tear staining area, numbers of skin lesions, and novel object test variables (155 < n < 240)a.
Skin Lesions
The interaction between the diet and the day of sampling was significant both for the front (P = 0.002) and the total (P < 0.001) lesions numbers (Table 2). No significant effect was found on d-2 and d39 (P > 0.1). On d2, NA piglets had more front skin lesions than GLN (P < 0.001) and A piglets (P = 0.011), as well as more total number of skin lesions than GLN (P < 0.001) and A piglets (P = 0.005).
Novel Object Tests
The interaction between the diet and the day of sampling was significant for the duration of interaction (P = 0.004) and the latency to interact with the object for the first time (P = 0.013) and there was a tendency for the number of withdrawal behaviors (P = 0.055) (Table 2). On d17, NA piglets avoided the object less than A piglets (P = 0.033) and tended to avoid it less than GLN piglets (P = 0.058). No significant effect was found on d47. On d85, NA pigs spent less time interacting with the object than GLN (P = 0.025) and A pigs (P = 0.008). On d111, NA pigs took a longer time to interact for the first time with the object than GLN (P = 0.002) and A pigs (P = 0.037).
Weighing Procedures
The interaction between the diet and the day of sampling was not significant, as well as the treatment and the day of sampling regarding both the number of pigs voluntarily exiting the pen or the duration needed for the handler to push out all the pigs from the pen (P > 0.1) (data not shown).
Microbiota Analyses
Richness, Diversity, and Composition of the Gut Microbiota
The richness, evenness and diversity indexes of the gut bacterial community varied with dietary treatment (A, NA, GLN), time (d0, d14 or d34), location (caecum, colon, ileum) and type (lumen content, mucus) (Table 3). GLN pigs had higher richness, evenness and diversity than A and NA pigs (P < 0.001). Richness, evenness and diversity changed over time (P < 0.001). Across both luminal and mucosal samples, the colon had the highest richness, evenness and diversity (P < 0.001). Mucosal samples were richer, more even and more diverse than gut content samples (P < 0.001). The average number of OTUs per sample was 150 ± 4. Good's coverage estimate was 97.5 ± 0.06% across all samples.
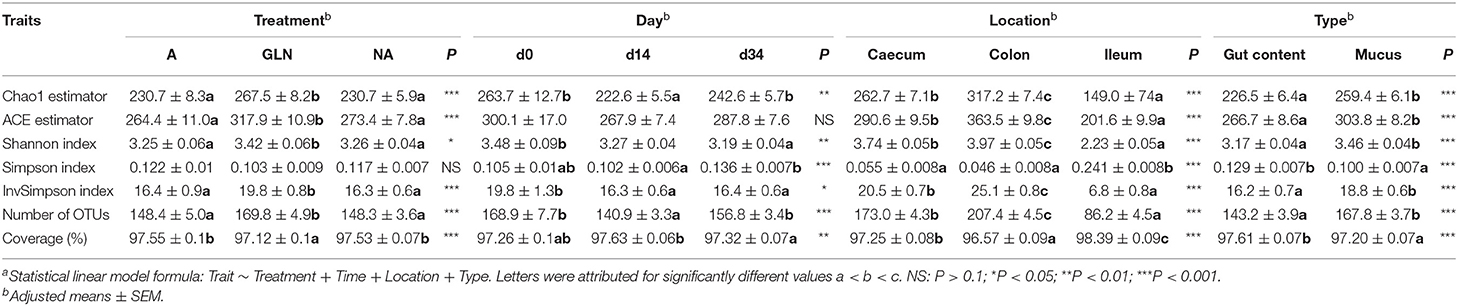
Table 3. α-diversity indicators of the microbiota in gut content and mucosal samples, at three different locations (Caecum, Colon, Ileum), at two different days (d14 and d34) after the beginning of three different two-week diets (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed)a.
Three different phyla were found across all samples: Firmicutes represented 77.5% of the total 16S rRNA gene sequences, Bacteroidetes 13.5% and Proteobacteria 4.9%. All the remaining phyla represented <2%. The major classes of the Firmicutes phylum were Clostridia (57.6% of the sequences), Bacilli (28.6%), Negativicutes (9.4%) and Erysipelotrichia (3.7%); of the Bacteroidetes phylum were Bacteroidia (95.4%) and Bacteroidetes unclassified (4.6%); of the Proteobacteria phylum were Gammaproteobacteria (49.7%), Proteobacteria unclassified (23.0%), Epsilonproteobacteria (19.7%), Betaproteobacteria (3.6%), and Deltaproteobacteria (3.4%).
Effects of Treatments, Time, Location, Type on Microbiota
The microbial community structure is graphically presented in Figures 1–3, respectively with a stacked bar chart, PCoA plots and a taxonomic LEfSe. The effects of dietary treatments, day of sampling, location of samples, type of samples and the interaction between dietary treatment and day on gut microbiota composition are presented in Table 4. Days 0, 14, and 34 varied in terms of microbiota composition in the caecum and colon for both lumen content and mucus samples (P < 0.05). For both lumen content and ileal mucosa samples, the microbiota composition was stable between d14 and 34 time, except for the GLN treatment. Within a day and a type of sample, ileum samples differed from caecum and colon samples (P < 0.001). Differences between dietary treatments were demonstrated on d14 but had disappeared by d34. GLN-fed piglets had higher percentages of Actinobacteria and Firmicutes (P < 0.001) but there were no differences in terms of percentages of Bacteroidetes and Proteobacteria (P > 0.1).
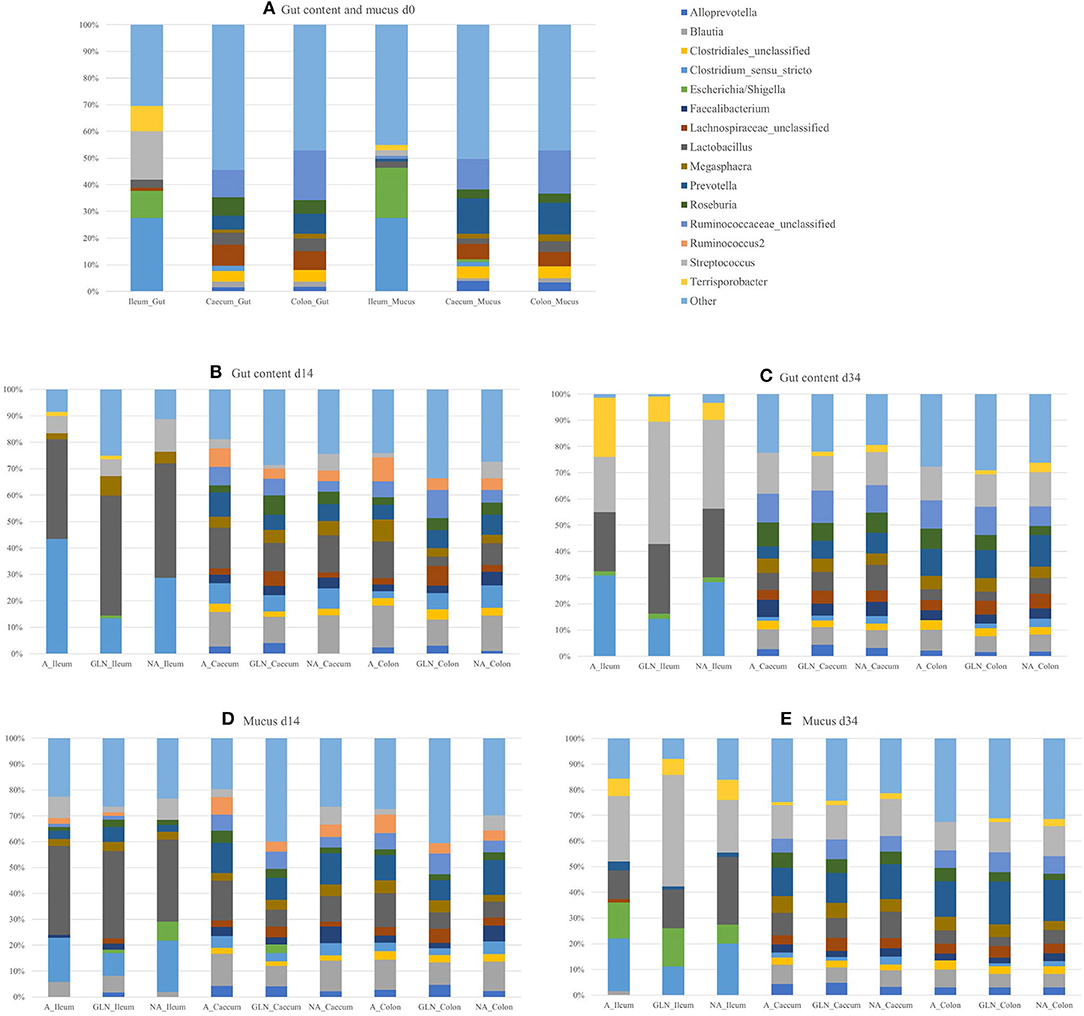
Figure 1. Relative abundances of genera for the three dietary treatments (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed), plotted by day of sampling (d0, d14, and d34), location of samples (Caecum, Colon, Ileum) and type of samples (gut content and mucus): (A) d0, (B) Gut d14, (C) Gut d34, (D) Mucus d14, and (E) Mucus d34. Genera outside the 15 most abundant are combined as “Other”.
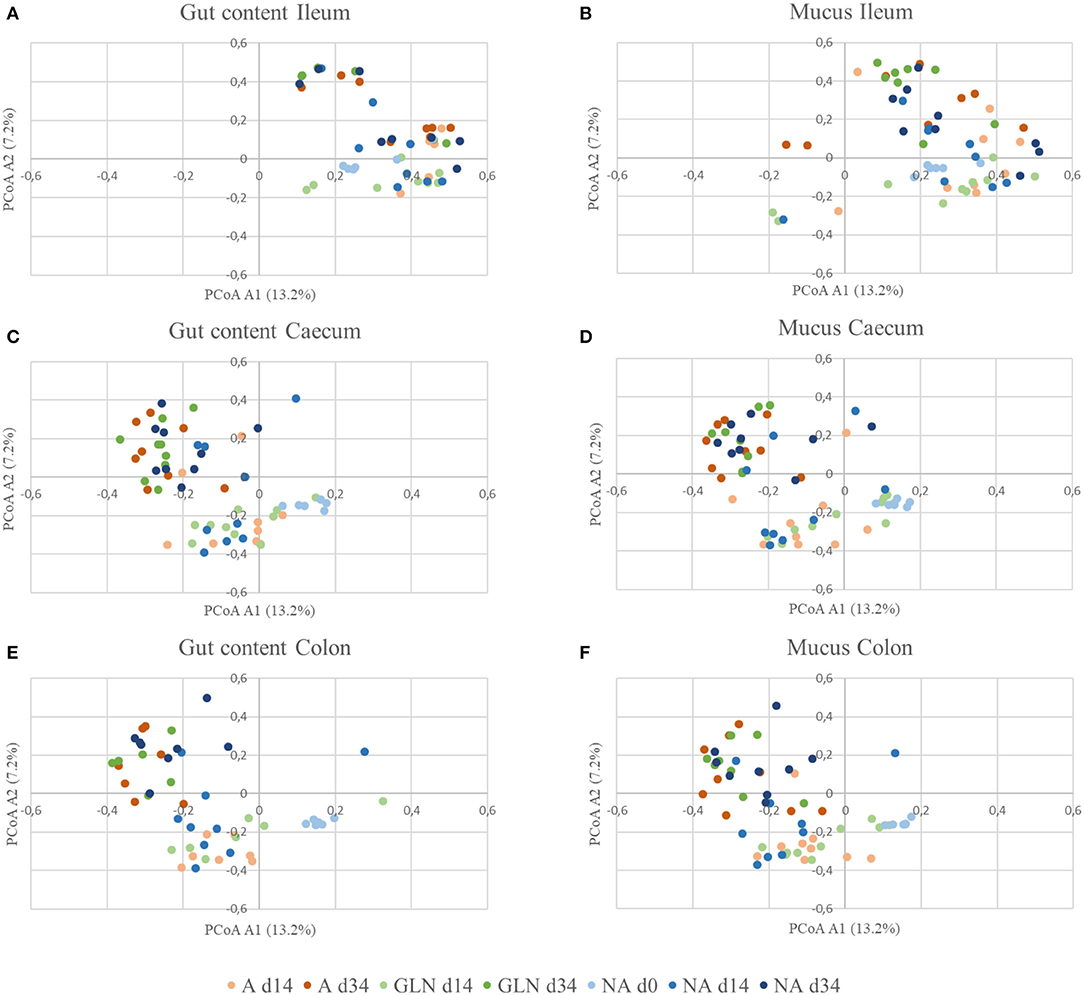
Figure 2. β-diversity of gut communities with respect to diet, location, and sample type. Distances were calculated using the Yue and Clayton theta metric and plotted using PCoA for the three dietary treatments (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed) on three day of sampling (d0, d14 and d34) plotted by location and type of samples: (A) Gut Ileum, (B) Mucus Ileum, (C) Gut Caecum, (D) Mucus Caecum, (E) Gut Colon and (F) Mucus Ileum.
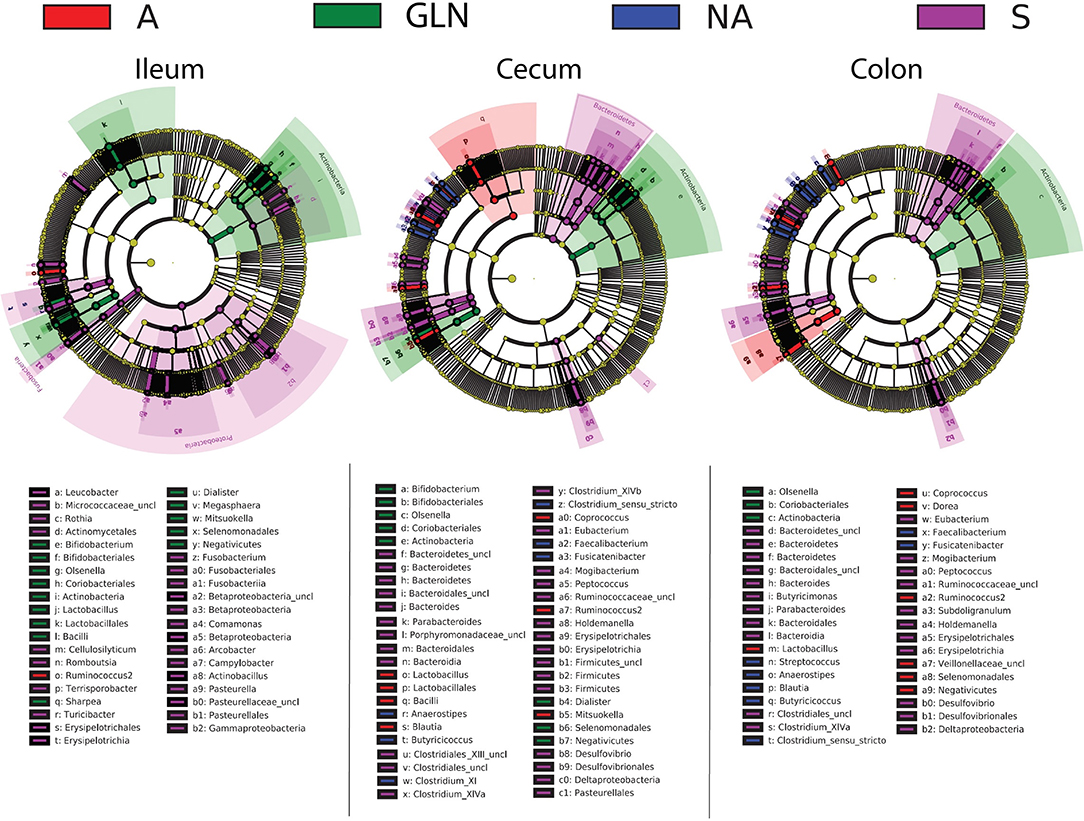
Figure 3. Linear discriminant analysis of taxa differentiating the pigs prior to dietary treatment (S: Sentinel pigs) and of the three dietary treatments at d14 (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed) by gut location (both lumen and mucus combined). Taxa with LDA scores > 3.5 as computed via LEfSe are plotted on the cladograms. Unclassified taxa are referenced as “uncl”.
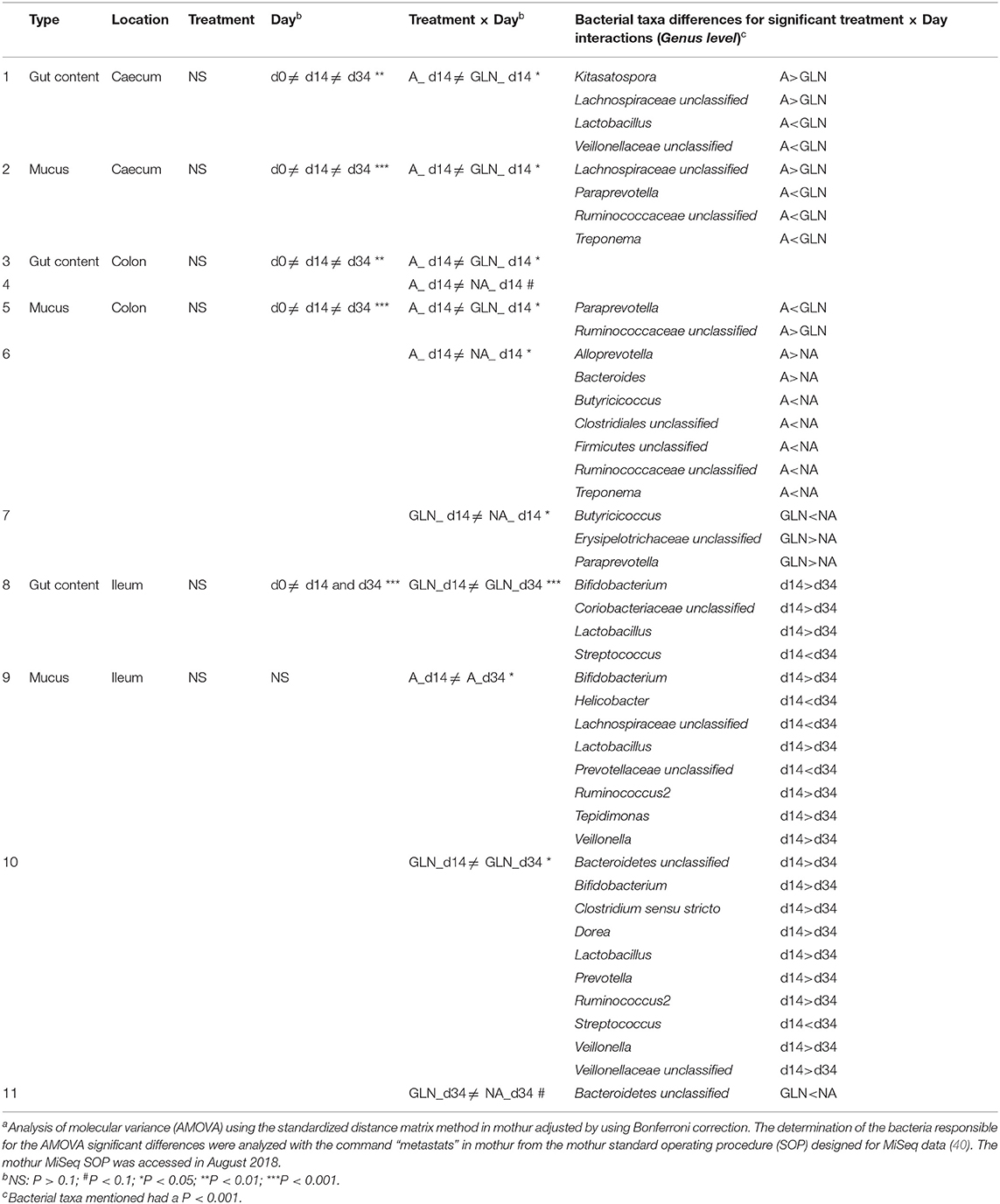
Table 4. Effects of dietary treatments (A: an antibiotic diet including 441 ppm of chlortetracycline and 38.6 ppm tiamulin; NA: a control diet without any prophylactic antibiotic or feed supplement; GLN: a diet including 0.20% L-glutamine as-fed), day of sampling (d0, d14, and d34), location of samples (Caecum, Colon, Ileum) and type of samples (gut content and mucus) on the gut microbiotaa.
Relation Between Bacterial Taxa and Behavioral Traits
Pearson correlations between aggression, stress and anxiety indicators and bacterial taxa were medium to high from 0.31 to 0.69 (Table 5). The number of total lesions 2 days before the start of the dietary treatment was correlated to the bacterial family Acidaminococcaceae (r = 0.65). Two days after the start of the dietary treatment, the number of total lesions was correlated with the Prevotellaceae family (r = 0.40) and the tear staining area was correlated with the order Clostridiales (r = 0.35). Significant correlations were found with variables up to 28 d after weaning. On d16, the number of withdrawals during the NOT was correlated with the Lachnospiraceae family (r = 0.69). The tear staining area was correlated with the family Porphyromonadaceae on d21 (r = 0.52) and with Acidaminococcaceae on d28 (r = 0.35).
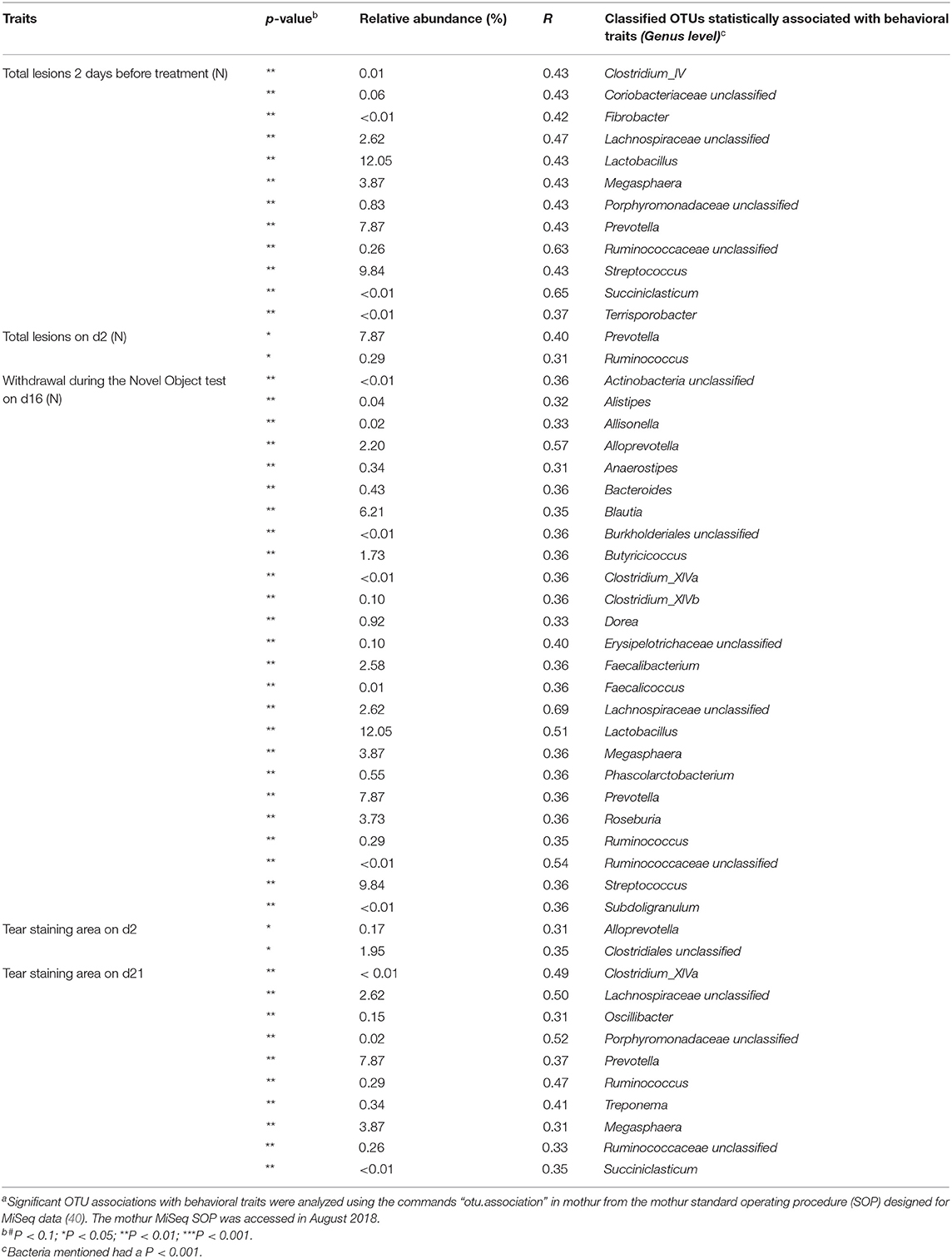
Table 5. Significant Pearson correlations between welfare and behavioral traits and bacterial taxaa.
Discussion
Specific feeding strategies for pigs to improve their ability to cope with stress or to alter behavior is a relatively unexplored area. In most studies dealing with feeding and stress and behavior in pigs, dietary changes have aimed to affect the time spent eating and the feeling of satiety to reduce aggressions, stress (43) and aberrant behaviors (44). However, there are a few studies that have emerged over the past 20 years that have revealed that dietary components per se can influence behavior.
Aggressiveness can be modulated via mineral intake, such as magnesium (45–47) and tryptophan supplementation (48–51). Besides preventing agonistic interactions from happening, an optimal ratio of fat, cholesterol and carbohydrate can even promote positive non-agonistic social interactions (52). Stress levels and fearful emotions can also be decreased with a large range of feed supplementation: vitamin E (46, 53), magnesium (47), tryptophan (48, 54–56), aromatic plant extracts (53, 57), chitosan (58), and the ratios of fat, cholesterol, carbohydrate (52), and linoleic acid in the diet (59). Finally, tryptophan has also demonstrated consistent effects in terms of reduction of aberrant behaviors, such as tail biting (49, 60), as well as changes in exploration in behavioral tests (61, 62). Exploration has also been increased by high linoleic acid ratio (59) or dietary cholesterol supplementation (63, 64). In the present study, pigs received three different diets: a diet including supplementary L-glutamine (GLN), a diet including an antibiotic treatment composed of chlortetracycline and tiamulin (A), and a diet without any prophylactic antibiotics or feed supplements (NA). The antibiotics and the L-glutamine were provided in feed. To the best of our knowledge, only one study recorded the behavior (excluding feeding or drinking) of pigs raised with either no supplementation in their feed, or orally supplemented with a common commercial prophylactic antibiotic or with L-glutamine (6). The authors demonstrated an increase in lying behavior and a decrease in standing behavior for NA pigs for the 48-h following a combined weaning and transport day. Those changes in behavior were correlated with reduced growth performance compared to GLN and A groups. They did not find any differences between the A and GLN groups. They suggested that these changes in behaviors for the NA group could reflect a higher degree of illness. Three welfare indicators—two of which are behavioral—have been recorded in the current study: tear staining, as a non-invasive indicator of stress (65); number of lesions, as an indirect indicator of aggressive behavior (32); and behavior during a novel object test, as an indicator or fear or anxiety (31). Similar to the study of Johnson and Lay (6), the NA treatment was the only treatment to differ from the other treatments. Oral antibiotics or L-glutamine provided similar beneficial effects regarding stress as minerals, amino-acids or plants extracts mentioned above. The NA piglets also had a higher number of lesions located in the front part of their body, as well as total lesions 48-h after mixing, representing more overall aggression in this treatment, with pigs likely more engaged in fighting (66). Results about aggression were only visible 48-h after mixing coinciding with the establishment of the hierarchy (67). Both A and GLN diets conferred similar positive effects in terms of aggression, as magnesium (45–47) and tryptophan supplementations (48–51). Finally, piglets supplemented with both A or GLN showed more withdrawals to a novel object dropped in their home pen at d16, but interacted longer with a novel object at d85 and interacted sooner with a novel object at d111. The three behaviors mentioned demonstrated a higher interest of the A and GLN treatment pigs for a novel object no matter their age and the time gap after the end of the feed supplementation. The higher number of withdrawals, in this particular situation, could also be interpreted as an increase in interest for the novel object as withdrawal appeared because of direct touching of the object that made it move. NA pigs adopted a strategy of flight in a corner of the pen. A better measure would have been the duration of cowering.
Feed supplements can be powerful tools as some studies have demonstrated effects on behavioral and welfare indicators only a few hours (62) or a few days after the beginning of the supplementation (46, 48–51, 54, 55). However, the majority of the studies have used a longer inclusion time of a few weeks (47, 52, 53, 56–61, 63, 64). Unfortunately, all the data collection is usually done only during the supplementation period or sometimes for a few days after. There is a critical knowledge gap about the longer-term effects of specific feed supplements over time. In the present study, the effects of short-term dietary treatments on stress and fear indicators were still significant up to 97 d after the end of the supplementation period. Inclusion of A or GLN treatments for 14 d induced similar short and long term effects on stress and behavioral indicators (tear staining, lesions and reactions during a novel object test). Those effects may come from changes in the microbiota composition. Indeed, GLN is a key regulator of microbiota composition as it participates in the nitrogen balance in the gut, which regulates the metabolism of bacteria (68). GLN promoted microbial richness and diversity in comparison to the A and NA treatments. Reduction of gut microbiota richness and diversity was associated with depression and anxiety-like behaviors in rodents (69, 70). In a study with obese humans, supplementation in 14 d with GLN induced a shift for the phyla Actinobacteria and Firmicutes, especially for the genera Dialister, Dorea, Pseudobutyrivibrio, and Veillonella (71). Shifts in the Firmicutes and Bacteroidetes phyla have also been observed in mice supplemented for 14 d with 1% L-glutamine (72). In the present study, pigs fed with the two dietary treatments A and GLN differed in terms of gut microbiota composition, while pigs from the NA group did not differ from the two other groups. Firmicutes was also the phylum mainly discriminating GLN and A groups after the 14-day dietary supplementation, followed by the phyla Actinobacteria, Bacteroidetes, Proteobacteria, and Spirochaetes. Shifts in bacterial populations were visible across all gut locations, ileum, caecum and colon, and in both gut content samples and mucus samples. Shifts over time and along the entire gut tract, observed in our study, is consistently reported in swine microbiota studies (73). The microbial composition is following a dynamic process around weaning, mainly depending on changes from liquid to solid feed (74, 75).
Antibiotics are known to strongly affect the microbiota composition by the depopulation of sensitive bacterial (58, 76). This reduction in diversity and bacterial abundances of the gut microbiota disrupts the gut ecosystem and weakens host immunity to pathogens (77, 78). In the current study, A treatment pigs had a richness and diversity comparable to NA pigs and significantly lower than GLN pigs. The lower richness and diversity of the gut microbiota in NA pigs could indicate intestinal disruptions caused by a high pathogenic load. Overall, α-diversity reported in the present study, matched results demonstrated in comparable pig studies. There was an ascending gradient of α-diversity, richness and evenness from the upper part (ileum) to the lower part (caecum and colon) of the gut tract (79–85). In term of microbial composition, ileum samples were drastically different from caecum and colonic samples which were highly similar (81, 83, 84, 86, 87). Moreover, in the present study we also confirmed that mucosa samples have higher richness than lumen samples regardless of the location in the gut tract (82, 85, 88). The three most abundant phyla determined in this study: Firmicutes, Bacteroidetes, and Proteobacteria also matched what is always reported for pigs at that age (73, 79, 82, 86, 87, 89). In terms of behavioral traits, GLN and A groups behaved similarly and were different from NA pigs, while in terms of microbiota composition the two groups were contrasted. L-glutamine conferred similar effects to antibiotics in terms of welfare and behavior, and should therefore be considered as a serious alternative to antibiotics, which may cause a decrease in microbiota diversity, select for antibiotic resistance, as well as increase the risk of colonization by pathogenic bacteria especially because of the creation of free ecological niches (90).
On d34, i.e., 20 d after the end of the dietary treatment, the differentiation of microbiota composition between dietary groups observed on d14 was not noticeable anymore. It could derive from two main causes: a cross contamination between groups, due to the fact that pigs in the neighboring pens had limited physical contact, leading to a convergence in gut microbiota composition; or an inevitable reversion to the commensal microbiota composition because of a powerful gut microbiota homeostasis. The first hypothesis is possible in the present study as pigs were penned separately by dietary treatment groups but were all located in the same room, which made cross contamination possible. Despite this loss of difference in microbiota composition, behavioral effects were still visible up to 97 d after the end of the dietary treatment. One possible explanation is related to the effect of microbiota on epigenetics. Indeed, it has already been demonstrated that microbial metabolism of diet can affect host gene expression via epigenetics pathways (91), including genes involved in the regulation of locomotor activity and anxiety-like behaviors, such as time spent in light in a light/dark box test compartment or open arms in an elevated plus maze test (92). During the supplementation period, substrates available for methylation can, for example, be altered or the activity of enzymes can be modulated by the presence of unusual compounds. Those alterations will then modify persistently the gene expression, as it will be transferred from one cell division to the next one enabling long term heritable changes. To promote long term gut microbiota differentiation, supplementation should also preferentially be given during the perinatal period, when the vulnerability of the entire organism is enhanced (92).
The gut microbiota plays a crucial role in the regulation of brain development, as well as in the emotional state of individuals (92–95). Relationships have been demonstrated between pathological emotional behaviors, such as anxiety and depression, and gastrointestinal disorders like irritable bowel syndrome (23). Challenges of rodents with pathogenic bacteria have provoked changes in behaviors within a few hours, resulting in an increase in anxiety and a decrease in exploratory behaviors (96, 97) via a direct action on the vagus nerve (98–100). However, Bercik et al. (101) also showed an effect of microbiota composition on emotional behavior of mice with a sectioned vagus nerve, revealing the existence of other modes of communication between the microbiota and the HPA axis (23, 102). Several studies using the rodent model demonstrated the importance of the microbiota on the emotional state of individuals, especially anxiety (97, 101–106). In humans, changes in gut microbiota through the supplementation of a probiotic reduced cortisol concentration, and therefore acting as an anxiolytic (107). Changes in gut microbiota composition affect the production of cytokines and tryptophan in the host, which are the main two pathways involved in the behaviors of depression and anxiety (108). Nowadays, the influence of gut microbiota on behaviors have been demonstrated in rodents (23). However, studies in livestock remain uncommon and detailed effects of specific bacterial populations on behavioral traits are unknown. Even if studies on livestock are still uncommon, they have all implicated the gut microbiota in host behavior (109–113). In hens, the aggressive damaging behavior of feather pecking was associated higher relative abundances of Clostridiales and a lower relative abundance of Lactobacillus in the luminal microbiota composition of ileum, caecum and colon sections combined (114, 115). In humans, Parashar and Udayabanu (94) have reviewed the relationships between specific bacteria populations and both stress and anxiety. They showed that a rise in stress coincides mostly with an increase in Bacteroidetes, a decrease in Actinobacteria, Firmicutes, and Proteobacteria, whereas a rise in anxiety coincides with either an increase or a decrease in Bacteroidetes and Firmicutes, an increase in Actinobacteria or a decrease in Proteobacteria. In the current study, we also revealed that some indicators of aggression, responses to stress and exploration have medium to strong correlations with specific bacterial populations. The phyla identified were Actinobacteria, Bacteroidetes, Fibrobacteres, Firmicutes, Proteobacteria, and Spirochaetes. That means that any feed supplements that can promote their development may affect the behavioral response of its individuals. Indeed, the brain is shaped both directly and indirectly by the gut microbiota through several pathways involving metabolites and neurochemicals' secretions. These molecules reach the brain via the blood stream or the nervous system and affect its development, neural and pain processes, and modulation of the HPA axis, therefore modulating the behaviors of individuals (116).
Conclusion
Comparisons between the three distinct 2-week diet strategies after transport on weaned pigs revealed that a short supplementation period can impact welfare indicators both during the administration period and after it. In terms of behaviors, GLN seemed to confer similar beneficial effects as antibiotic supplementation, and therefore should be considered as an alternative to the use of antibiotics. In terms of microbiota composition, supplementation produced significant shifts that disappeared over time. The effects of diet observed on behavioral indicators could be related to changes in microbiota composition as some specific bacterial taxa appeared to be associated with aggressiveness, stress and fear.
Data Availability Statement
The data analyzed in this study can be accessed at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA607434.
Author's Note
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Author Contributions
SP was responsible for study design, data collection, analysis and interpretation, and was the principle author of the manuscript. AD was responsible for study design, data collection and study coordination. BR was responsible for study conception and study coordination. SL was responsible for data analysis and interpretation. JJ was responsible for study design. JM-F was responsible for study design, data collection, analysis and interpretation, and was a major author of the manuscript. All authors contributed to manuscript revision and have read and approved the final manuscript.
Funding
This work was supported in part by the Pork Checkoff (grant no. 16–065), National Pork Board, Des Moines, IA, USA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the swine farm staff at Purdue University for daily animal care and the employees at the USDA-ARS Livestock Behavior Research Unit for assistance in animal care and data collection.
References
1. Schwarz S, Kehrenberg C, Walsh TR. Use of antimicrobial agents in veterinary medicine and food animal production. Int Antimicrob J Agents. (2001) 17:431–7. doi: 10.1016/S0924-8579(01)00297-7
2. Weese JS, Page SW, Prescott JF. Antimicrobial stewardship in animals. In: Giguère S, Prescott JF, Dowling PM, editors. Antimicrobial Therapy in Veterinary Medicine. Hoboken, NJ: John Wiley and Sons (2013). p. 117–32. doi: 10.1002/9781118675014.ch7
3. FDA (Food US and Drug Administration). 2017 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. (2018). Available online at: https://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM628538.pdf. (accessed February 12, 2019).
4. Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. (2002) 13:7–27. doi: 10.1081/ABIO-120005767
5. Diana A, Manzanilla EG, Diaz JAC, Leonard FC, Boyle LA. Do weaner pigs need in-feed antibiotics to ensure good health and welfare? PLoS ONE. (2017) 12:e0189434. doi: 10.1371/journal.pone.0189434
6. Johnson JS, Lay DC Jr. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with L-glutamine. J Anim Sci. (2017) 95:91–102. doi: 10.2527/jas.2016.1070
8. Campbell JM, Crenshaw JD, Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. (2013) 4:19. doi: 10.1186/2049-1891-4-19
9. Averos X, Herranz A, Sanchez R, Gosalvez LF. Effect of the duration of commercial journeys between rearing farms and growing-finishing farms on the physiological stress response of weaned piglets. Livest Sci. (2009) 122:339–44. doi: 10.1016/j.livsci.2008.09.019
10. Nabuurs MJ, van Zijderveld FG, de Leeuw PW. Clinical and microbiological field studies in the Netherlands of diarrhoea in pigs at weaning. Res Vet Sci. (1993) 55:70–7. doi: 10.1016/0034-5288(93)90037-G
11. Katouli M, Melin L, Jensen-Waern M, Wallgren P, Mollby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. (1999) 87:564–73. doi: 10.1046/j.1365-2672.1999.00853.x
12. Heiman ML, Greenway FL. A healthy gut microbiome is dependent on dietary diversity. Mol Metab. (2016) 5:317–20. doi: 10.1016/j.molmet.2016.02.005
13. Messori S, Trevisi P, Simongiovanni A, Priori D, Bosi P. Effect of susceptibility to enterotoxigenic Escherichia Coli F4 and of dietary tryptophan on gut microbiota diversity observed in healthy young pigs. Vet Microbiol. (2013) 162:173–9. doi: 10.1016/j.vetmic.2012.09.001
14. Ghosh C, Sarkar P, Issa R, Haldar J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. (2019) 27:323–38. doi: 10.1016/j.tim.2018.12.010
15. Sillankorva S, Pereira MO, Henriques M. Antibiotic alternatives and combinational therapies for bacterial infections. Front Microbiol. (2019) 9:335. doi: 10.3389/978-2-88945-789-2
16. Yi GF, Carroll JA, Allee GL, Gaines AM, Kendall DC, Usry JL, et al. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J Anim Sci. (2005) 83:634–43. doi: 10.2527/2005.833634x
17. Jiang ZY, Sun LH, Lin YC, Ma XY, Zheng CT, Zhou GL, et al. Effects of dietary glycyl-glutamine on growth performance, small intestine integrity, and immune responses of weaning piglets challenges with lipopolysaccharide. J Anim Sci. (2009) 87:4050–6. doi: 10.2527/jas.2008-1120
18. Wu G, Meier SA, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. Nutr J. (1996) 126:2578–84. doi: 10.1093/jn/126.10.2578
19. Domeneghini C, Di Giancamillo A, Bosi G, Arrighi S. Can nutraceuticals affect the structure of intestinal mucosa? Qualitative and quantitative microanatomy in L-Glutamine diet-supplemented weaning piglets. Vet Res Commun. (2006) 30:331–42. doi: 10.1007/s11259-006-3236-1
20. Wang J, Chen L, Li P, Li X, Zhou H, Wang F, et al. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. Nutr J. (2008) 138:1025–32. doi: 10.1093/jn/138.6.1025
21. Marchant-Forde JN, Parois SP, Johnson JS, Eicher SD. Dietary modification of behavior in pigs. In: Newberry RC, and Andersen IL, editors. Proceedings of 53rd Congress of the International Society for Applied Ethology. Wageningen: Wageningen Academic Press (2019).
22. Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. (2011) 12:453–66. doi: 10.1038/nrn3071
23. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
24. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol. (2009) 6:306–14. doi: 10.1038/nrgastro.2009.35
25. Bushby EV, Friel M, Goold C, Gray H, Smith L, Collins LM. Factors influencing individual variation in farm animal cognition and how to account for these statistically. Front Vet Sci. (2018) 5:193. doi: 10.3389/fvets.2018.00193
26. Foster JA, Neufeld K-AM. Gut–brain axis: how the microbiome influences anxiety and depression? Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
27. Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, et al. Microbes and neurodevelopment–absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. (2015) 50:209–20. doi: 10.1016/j.bbi.2015.07.009
28. Sylvia KE, Jewell CP, Rendon NM, St. John EA, Demas GE. Sex-specific modulation of the gut microbiome and behavior in siberian hamsters. Brain Behav Immun. (2016) 60:51–62. doi: 10.1016/j.bbi.2016.10.023
29. Brunberg EI, Rodenburg TB, Rydhmer L, Kjaer JB, Jensen P, Keeling LJ. Omnivores going astray: a review and new synthesis of abnormal behavior in pigs and laying hens. Front Vet Sci. (2016) 3:57. doi: 10.3389/fvets.2016.00057
30. Nowland TL, Plush KJ, Barton M, Kirkwood RN. Development and function of the intestinal microbiome and potential implications for pig production. Animals. (2019) 9:76. doi: 10.3390/ani9030076
31. Murphy E, Nordquist RE, van der Staay FJ. A review of behavioural methods to study emotion and mood in pigs, Sus scrofa. Appl Anim Behav Sci. (2014) 159:9–28. doi: 10.1016/j.applanim.2014.08.002
32. Turner SP, Farnworth MJ, White IMS, Brotherstone S, Mendl M, Knap P, et al. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Appl Anim Behav Sci. (2006) 96:245–59. doi: 10.1016/j.applanim.2005.06.009
33. Telkanranta H, Marchant-Forde JN, Valros AE. Tear staining in pigs: a potential tool for welfare assessment on commercial farms. Animal. (2016) 10:318–25. doi: 10.1017/S175173111500172X
34. DeBoer SP, Garner JP, McCain RR, Lay DC Jr, Eicher SD, Marchant-Forde JN. An initial investigation into the effects of social isolation and enrichment on the welfare of laboratory pigs housed in the pigturn® system assessed using tear staining, behaviour, physiology and haematology. Anim Welf . (2015) 24:15–27. doi: 10.7120/09627286.24.1.015
35. Federation of Animal Science Societies. Guide for the Careuse of Agricultural Animals in Research Teaching. 3rd ed. Champaign, IL: Federation of Animal Science Societies (2010).
36. National Pork Board. Transport Quality Assurance Handbook. 5th ed. Clive, IA: National Pork Board (2015).
37. Duttlinger AW, Kpodo KR, Lay DC Jr, Richert BT, Johnson JS. Replacing dietary antibiotics with 0.20% L-glutamine in swine nursery diets: impact on health and productivity of pigs following weaning and transport. J Anim Sci. (2019) 97:2035–52. doi: 10.1093/jas/skz098
38. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. (2012) 9:671–5. doi: 10.1038/nmeth.2089
39. Dalmau A, Velarde A, Scott K. Welfare Quality® Consortium. Assessment Protocol For Pigs (Sows and Piglets, Growing and Finishing Pigs). Lelystad: Welfare Quality® consortium (2009).
40. Kozich J, Westcott S, Baxter N, Highlander S, Schloss P. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. (2013) 141:599–609. doi: 10.1128/AEM.01043-13
42. R Core Team. R: a Language and Environment for Statistical Computing. R Foundation for statistical computing. Vienna (2015).
43. Meunier-Salaun MC, Edwards SA, Robert S. Effect of dietary fibre on the behaviour and health of the restricted fed sow. Anim Feed Sci Technol. (2001) 90:53–69. doi: 10.1016/S0377-8401(01)00196-1
44. de Leeuw JA, Bolhuis JE, Bosch G, Gerrits WJJ. Effects of dietary fibre on behaviour and satiety in pigs. Proc Nutr Soc. (2008) 67:334–42. doi: 10.1017/S002966510800863X
45. Caine WR, Schaefer AL, Aalhus JL, Dugan MER. Behaviour growth performancepork quality of pigs differing in porcine stress syndrome genotype receiving dietary magnesium aspartate hydrochloride. Can J Anim Sci. (2000) 80:175–82. doi: 10.4141/A99-068
46. Peeters E, Neyt A, Beckerst F, De Smet S, Aubert AE, Geers R. Influence of supplemental magnesium, tryptophan, vitamin C, and vitamin E on stress responses of pigs to vibration. J Anim Sci. (2005) 83:1568–80. doi: 10.2527/2005.8371568x
47. O'Driscoll K, Teixeira DL, O'Gorman D, Taylor S, Boyle LA. The influence of a magnesium rich marine supplement on behaviour, salivary cortisol levels, and skin lesions in growing pigs exposed to acute stressors. Appl Anim Behav Sci. (2013) 145:92–101. doi: 10.1016/j.applanim.2013.02.005
48. Li YZ, Kerr BJ, Kidd KT, Gonyou HW. Use of supplementary tryptophan to modify the behavior of pigs. J Anim Sci. (2006) 84:212–20. doi: 10.2527/2006.841212x
49. Martinez-Trejo G, Ortega-Cerrilla ME, Rodarte-Covarrubias LF, Herrera-Haro JG, Figueroa-Velasco JL, Galindo-Maldonado F, et al. Aggressiveness and productive performance of piglets supplemented with tryptophan. J Anim Vet Adv. (2009) 8:608–11.
50. Poletto R, Meisel RL, Richert BT, Cheng H-W, Marchant-Forde JN. Aggression in replacement grower and finisher gilts fed a short-term high-tryptophan diet and the effect of long-term human–animal interaction. Appl Anim Behav Sci. (2010) 122:98–110. doi: 10.1016/j.applanim.2009.11.015
51. Li YZ, Baidoo SK, Johnston LJ, Anderson JE. Effects of tryptophan supplementation on aggression among group-housed gestating sows. J Anim Sci. (2011) 89:1899–907. doi: 10.2527/jas.2010-3125
52. Haagensen AMJ, Sorensen DB, Sandoe P, Matthews LR, Birck MM, Fels JJ, et al. High fat, low carbohydrate diet limit fear and aggression in Gottingen minipigs. PLoS ONE. (2014) 9:e93821. doi: 10.1371/journal.pone.0093821
53. Zhang T, Zhou YF, Zou Y, Hu XM, Zheng LF, Wei HK, et al. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest Sci. (2015) 180:143–9. doi: 10.1016/j.livsci.2015.05.037
54. Koopmans SJ, Ruis M, Dekker R, van Diepen H, Korte M, Mroz Z. Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Physiol Behav. (2005) 85:469–78. doi: 10.1016/j.physbeh.2005.05.010
55. Koopmans SJ, Guzik AC, van der Meulen J, Dekker R, Kogut J, Kerr BJ, et al. Effects of supplemental L-tryptophan on serotonin, cortisol, intestinal integrity, and behavior in weanling piglets. J Anim Sci. (2006) 84:963–71. doi: 10.2527/2006.844963x
56. Koopmans SJ, Ruis M, Dekker R, Korte M. Surplus dietary tryptophan inhibits stress hormone kinetics and induces insulin resistance in pigs. Physiol Behav. (2009) 98:402–10. doi: 10.1016/j.physbeh.2009.07.001
57. Zou Y, Hu XM, Zhang T, Wei HK, Zhou YF, Zhou ZX, et al. Effects of dietary oregano essential oil and vitamin E supplementation on meat quality, stress response and intestinal morphology in pigs following transport stress. J Vet Med Sci. (2017) 79:328–35. doi: 10.1292/jvms.16-0576
58. Li JL, Shi BL, Yan SM, Jin L, Li TY, Xu YQ, et al. Effects of dietary supplementation of chitosan on stress hormones and antioxidative enzymes in weaned piglets. J Anim Vet Adv. (2013) 12:650–4. doi: 10.3923/javaa.2013.650.654
59. Clouard C, Gerrits WJJ, van Kerkhof I, Smink W, Bolhuis JE. Dietary linoleic and a-linolenic acids affect anxiety-related responses and exploratory activity in growing pigs. Nutr J. (2051) 145:358–64. doi: 10.3945/jn.114.199448
60. van der Meer Y, Gerrits WJJ, Jansman AJM, Kemp B, Bolhuis JE. A link between damaging behaviour in pigs, sanitary conditions, and dietary protein and amino acid supply. PLoS ONE. (2017) 12:e0174688. doi: 10.1371/journal.pone.0174688
61. Meunier-salaun M-C, Monnier M, Colleaux Y, Seve B, Henry Y. Impact of dietary trypyophan and behavioral type on behavior, plasma cortisol, and brain metabolites of young pigs. J Anim Sci. (1991) 69:3689–98. doi: 10.2527/1991.6993689x
62. Burrows MS, Moss BW, Beattie VE. The role of dietary manipulation in the control of aggression in pigs. Biochem Soc Trans. (1998) 26:S58. doi: 10.1042/bst026s058
63. Schoknecht PA, Ebner S, Pond WG, Zhang SD, McWhinney V, Wong WW, et al. Dietary cholesterol supplementation improves growth and behavioral response of pigs selected for genetically high and low serum cholesterol. Nutr J. (1994) 124:305–14. doi: 10.1093/jn/124.2.305
64. Pond WG, Mersmann HJ, Su DR, McGlone JJ, Wheeler MB, Smith EO. Neonatal dietary cholesterol and alleles of cholesterol 7-alpha hydroxylase affect piglet cerebrum weight, cholesterol concentration and behavior. Nutr J. (2008) 138:282–6. doi: 10.1093/jn/138.2.282
65. DeBoer SP, Marchant-Forde JN. Tear staining as a potential welfare indicator in pigs. In: The Proceedings of the 47th Congress of the International Society for Applied Ethology. Florianopolis (2013).
66. McGlone JJ. A quantitative ethogram of aggressive and submissive behaviours in recently regrouped pigs. J Anim Sci. (1985) 61:559–65. doi: 10.2527/jas1985.613556x
67. Meese GB, Ewbank R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim Behav. (1973) 21:326–34. doi: 10.1016/S0003-3472(73)80074-0
68. Forchhammer K. Glutamine signalling in bacteria. Front Biosci. (2007) 12:2069. doi: 10.2741/2069
69. Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney R, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. (2015) 48:165–73. doi: 10.1016/j.bbi.2015.04.004
70. Kelly JR, Borre Y, Brien CO, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
71. de Souza AZZ, Zambom AZ, Abboud KY, Reis SK, Tannihao F, Guadagnini D, et al. Oral supplementation with L-glutamine alters gut microbiota of obese and overweight adults: a pilot study. Nutrition. (2015) 31:884–9. doi: 10.1016/j.nut.2015.01.004
72. Ren WK, Duan JL, Yin J, Liu G, Cao Z, Xiong X, et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. (2014) 46:2403–13. doi: 10.1007/s00726-014-1793-0
73. Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot, et al. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
74. Holman DB, Chénier MR. Temporal changes and the effect of subtherapeutic concentrations of antibiotics in the gut microbiota of swine. FEMS Microbiol Ecol. (2014) 90:599–608. doi: 10.1111/1574-6941.12419
75. Guevarra RB, Lee JH, Lee SH, Seok MJ, Kim DW, Kang BN, et al. Piglet gut microbial shifts early in life: causes and effects. J Anim Sci Biotechnol. (2019) 10:1. doi: 10.1186/s40104-018-0308-3
76. Yu M, Zhang C, Yang Y, Mu C, Su Y, Yu K, et al. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. J Anim Sci Biotechnol. (2017) 8:60. doi: 10.1186/s40104-017-0192-2
77. Puiman P, Stoll B, Molbak L, de Bruijn A, Schierbeek H, Boye M, et al. Modulation of the gut microbiota with antibiotic treatment suppresses whole body urea production in neonatal pigs. Am J Physiol Gastrointest Liver Physiol. (2013) 304:G300–10. doi: 10.1152/ajpgi.00229.2011
78. Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. (2018) 59:4–12. doi: 10.3349/ymj.2018.59.1.4
79. Crespo-Piazuelo D, Estellé J, Revilla M, Criado-Mesas L, Ramayo-Caldas Y, Óvilo C. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci Rep. (2018) 8:12727. doi: 10.1038/s41598-018-30932-6
80. Fan P, Liu P, Song P, Chen X, Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep. (2017) 7:43412. doi: 10.1038/srep43412
81. Gresse R, Chaucheyras-Durand F, Dunière L, Blanquet-Diot S, Forano E. Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms. (2019) 7:343. doi: 10.3390/microorganisms7090343
82. Holman DB, Brunelle BW, Trachsel J, Allen HK. Meta-analysis to define a core microbiota in the swine gut. MSystems. (2017) 2:e00004–7. doi: 10.1128/mSystems.00004-17
83. Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, et al. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. (2014) 8:1566–76. doi: 10.1038/ismej.2014.12
84. Quan J, Cai G, Ye J, Yang M, Ding R, Wang X, et al. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci Rep. (2018) 8:4536. doi: 10.1038/s41598-018-22692-0
85. Zhang L, Wu W, Lee YK, Xie J, Zhang H. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. (2018) 9:48. doi: 10.3389/fmicb.2018.00048
86. Isaacson R, Kim HB. The intestinal microbiome of the pig. Anim Health Res Rev. (2012) 13:100–09. doi: 10.1017/S1466252312000084
87. Kelly J, Daly K, Moran AW, Ryan S, Bravo D, Shirazi-Beechey SP. Composition and diversity of mucosa-associated microbiota along the entire length of the pig gastrointestinal tract; dietary influences. Environ Microbiol. (2017) 19:1425–38. doi: 10.1111/1462-2920.13619
88. Mu C, Yang Y, Su Y, Zoetendal EG, Zhu W. Differences in microbiota membership along the gastrointestinal tract of piglets and their differential alterations following an early-life antibiotic intervention. Front Microbiol. (2017) 8:797. doi: 10.3389/fmicb.2017.00797
89. Looft T, Johnson TA, Allen HK, Bayles DO, Alt DP, Stedtfeld RD, et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc Natl Acad Sci USA. (2012) 109:1691–6. doi: 10.1073/pnas.1120238109
90. Barrow PA. Probiotics for chickens. In: R. Fuller, editor. Probiotics. Dordrecht: Springer (1992). p. 225–57. doi: 10.1007/978-94-011-2364-8_10
91. Hullar MA, Fu BC. Diet the gut microbiome, and epigenetics. Cancer J. (2014) 20:170–84. doi: 10.1097/PPO.0000000000000053
92. Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. (2011) 108:3047–52. doi: 10.1073/pnas.1010529108
93. Farmer AD, Randall HA, Aziz Q. It's a gut feeling: how the gut microbiota affects the state of mind. J Physiol. (2014) 592:2981–8. doi: 10.1113/jphysiol.2013.270389
94. Parashar A, Udayabanu M. Gut microbiota regulates key modulators of social behavior. Eur Neuropsychopharmacol. (2016) 26:78–91. doi: 10.1016/j.euroneuro.2015.11.002
95. Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, Ritvo P. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res. (2016) 36:889–98. doi: 10.1016/j.nutres.2016.06.009
96. Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. (2008) 22:354–66. doi: 10.1016/j.bbi.2007.08.009
97. Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. (2010) 139:2102–12. doi: 10.1053/j.gastro.2010.06.063
98. Lyte M, Varcoe JJ, Bailey MT. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol Behav. (1998) 65:63–8. doi: 10.1016/S0031-9384(98)00145-0
99. Wang X, Wang B-R, Zhang X-J, Xu Z, Ding Y-Q, Ju G. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World Gastroenterol J. (2002) 8:540–5. doi: 10.3748/wjg.v8.i3.540
100. Lyte M, Li W, Opitz N, Gaykema R, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. (2006) 89:350–7. doi: 10.1016/j.physbeh.2006.06.019
101. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. (2011) 141:599–609. doi: 10.1053/j.gastro.2011.04.052
102. Bendtsen KMB, Krych L, Sorensen DB, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS ONE. (2012) 7:1–13. doi: 10.1371/journal.pone.0046231
103. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. (2010) 170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005
104. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. (2011) 108:16050–55. doi: 10.1073/pnas.1102999108
105. Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut microbes. (2011) 2:256–61. doi: 10.4161/gmic.2.4.16108
106. Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. (2011) 23: 255–e119. doi: 10.1111/j.1365-2982.2010.01620.x
107. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation (Bifidobacterium longum R0175) in rats and human subjects. Br Nutr J. (2011) 105:755–64. doi: 10.1017/S0007114510004319
108. Rook GAW, Raison CL, Lowry CA. Can we vaccinate against depression? Drug Discov. (2012) 17:451–8. doi: 10.1016/j.drudis.2012.03.018
109. Duvaux-Ponter C, Rigalma K, Roussel-Huchette S, Schawlb Y, Ponter AA. Effect of a supplement rich in linolenic acid, added to the diet of gestating and lactating goats, on the sensitivity to stress and learning ability of their offspring. Appl Anim Behav Sci. (2008) 114:373–94. doi: 10.1016/j.applanim.2008.01.021
110. Liu HN, Radlowski EC, Conrad MS, Li Y, Dilger RN, Johnson RW. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. Nutr J. (2014) 144:1903–9. doi: 10.3945/jn.114.199828
111. Clouard C, Kemp B, Val-Laillet D, Gerrits WJJ, Bartels AC, Bolhuis JE. Prenatal but not early postnatal, exposure to a Western diet improves spatial memory of pigs later in life and is paired with changes in maternal prepartum blood lipid levels. FASEB J. (2016) 30:2466–75. doi: 10.1096/fj.201500208R
112. Parois SP, Calandreau L, Kraimi N, Gabriel I, Leterrier C. The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behav Brain Res. (2017) 331:47–53. doi: 10.1016/j.bbr.2017.05.022
113. Kraimi N, Calandreau L, Biesse M, Rabot S, Guitton E, Velge P, et al. Absence of gut microbiota reduces emotional reactivity in Japanese quails (Coturnix japonica). Front Physiol. (2018) 9:603. doi: 10.3389/fphys.2018.00603
114. Birkl P, Bharwani A, Kjaer JB, Kunze W, McBride P, Forsythe P, et al. Differences in cecal microbiome of selected high and low feather-pecking laying hens. Poult Sci. (2018) 97:3009–14. doi: 10.3382/ps/pey167
115. van der Eijk JA, de Vries H, Kjaer JB, Naguib M, Kemp B, Smidt H, et al. Differences in gut microbiota composition of laying hen lines divergently selected on feather pecking. Poult Sci. (2019) 98:7009–21. doi: 10.3382/ps/pez336
Keywords: alternative, L-glutamine, microbiota, pigs, stress, welfare
Citation: Parois SP, Duttlinger AW, Richert BT, Lindemann SR, Johnson JS and Marchant-Forde JN (2020) Effects of Three Distinct 2-Week Long Diet Strategies After Transport on Weaned Pigs' Short and Long-Term Welfare Markers, Behaviors, and Microbiota. Front. Vet. Sci. 7:140. doi: 10.3389/fvets.2020.00140
Received: 27 September 2019; Accepted: 24 February 2020;
Published: 17 March 2020.
Edited by:
Stephanie Torrey, University of Guelph, CanadaReviewed by:
Devin Holman, Agriculture and Agri-Food Canada, CanadaSophie Brajon, University of Porto, Portugal
Copyright © 2020 Parois, Duttlinger, Richert, Lindemann, Johnson and Marchant-Forde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy N. Marchant-Forde, amVyZW15Lm1hcmNoYW50LWZvcmRlJiN4MDAwNDA7dXNkYS5nb3Y=
 Severine P. Parois
Severine P. Parois Alan W. Duttlinger3
Alan W. Duttlinger3 Stephen R. Lindemann
Stephen R. Lindemann Jay S. Johnson
Jay S. Johnson Jeremy N. Marchant-Forde
Jeremy N. Marchant-Forde