- 1College of Natural Sciences, Arba Minch University, Arba Minch, Ethiopia
- 2College of Animal Sciences and Veterinary Medicine, Henan Agricultural University, Zhengzhou, China
- 3Scientific Research Experiment Center & Laboratory Animal Center, Henan University of Chinese Medicine, Zhengzhou, China
- 4Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
Enterocytozoon bieneusi is the most frequently diagnosed microsporidian species in humans and occurs in a wide range of animals. This study was conducted in Central Ethiopia to determine the prevalence and genotypes of E. bieneusi in lambs in order to evaluate their public health significance. Three hundred eighty nine fecal samples were collected and screened using a nested PCR targeting the internal transcribed spacer (ITS) of the ribosomal RNA gene. All positive PCR products were sequenced to determine the genotypes. E. bieneusi was found in 39 (10.03%) of the lambs. Differences in the infection rates among sex and age groups were not significant (P > 0.05). Five ITS genotypes belonging to three known genotypes BEB6, COS-I, and COS-II, and two novel genotypes (ET-L1 and ET-L2) were identified in lambs. All five genotypes identified in the present study clustered within cattle-specific Group 2 in the ITS phylogenetic tree. This first molecular detection and characterization of E. bieneusi in lambs in Ethiopia has identified the need for further studies in humans and other domestic animals in order to determine the public health significance of E. bieneusi in Ethiopia.
Introduction
Microsporidia are obligate intracellular pathogens in humans and other animals worldwide (1, 2). Among the four microsporidian species infecting humans, Enterocytozoon bieneusi is the most frequently diagnosed species. It has also been reported in many mammals and birds (1). E. bieneusi primarily infects the intestinal absorptive cells and is mainly associated with chronic diarrhea and wasting syndrome (3).
Based on the internal transcribed spacer (ITS) nucleotide sequence, considerable genetic diversity has been found within E. bieneusi (1, 4). To date, more than 500 genotypes of E. bieneusi have been reported (5). The findings of previous studies suggest that both host-adapted genotypes with narrow host ranges and potentially zoonotic E. bieneusi genotypes with wide host ranges have been identified (1, 6).
The presence of 11 genetic groups (Group 1 to 11) has been described by phylogenetic analysis of E. bieneusi ITS sequences (5). Group 1 is the largest group containing 314 genotypes, which are commonly found both in humans and animals and considered to be zoonotic (5). The second largest group, Group 2, was considered to be adapted to ruminants (7). However, the frequent observation of some Group 2 genotypes, such as BEB4, BEB6, I, and J in other animals and humans suspends their host specificity and implies the zoonotic potential of some genotypes in this group. The genotypes in remaining genetic Groups 3–11 appear to be more host-specific and probably have no public health significance (5).
Aside from two studies in humans using microscopy and Polymerase Chain Reaction (PCR), E. bienusi has not been studied and characterized in Ethiopia (8, 9). The aim of this study was to determine the prevalence and the infecting genotypes of E. bieneusi in lambs in Oromia Special Zone, Central Ethiopia to extrapolate the public health importance.
Materials and Methods
Study Area
This study was conducted in Oromia Special Zone, Central Ethiopia. It is one of the zones in Oromia Region and bordered with Eastern Shewa zone in the east, North Shewa zone in the North East and South-west Shewa Zone in the South West. The mean annual temperature of the Zone is found between 20 and 25°C in the lowlands and 10–15°C in the central highlands. Based on the available meteorologically data, the mean annual rainfall varies from 700 to 1,400 mm in lowlands and highlands, respectively. Of the eight major towns of the Special Zone, Holeta, Sendafa, and Chancho towns were included in this study because of high sheep density (Figure 1).
Figure 1. Locations of the study area in Oromia Special Zone, Central Ethiopia [Adapted from Wegayehu et al. (10)].
Sample Animal and Specimen Collection
The fecal samples were collected from lambs younger than 3 months. The owners of the lambs who consent to provide fecal samples were included in this study. A total of 389 fresh fecal samples were collected from lambs in separate and labeled stool containers. The specimens were taken directly from the rectum of each animal or immediately after defecation using sterile disposable gloves. Identification number, animal species, age, and sex were recorded during sample collection. The specimens were preserved in 2.5% potassium dichromate solution, transported immediately to the laboratory on ice and stored at 4°C prior to deoxyribonucleic acid (DNA) extraction.
DNA Extraction
The preserved fecal specimens were washed with deionized water to remove the preservative. Genomic DNA was extracted from each fecal sample using the E.Z.N.A.® Stool DNA kit (Omega Biotek Inc., Norcross, USA). Briefly, about 200 mg of fecal specimen was added in a 2 ml centrifuge tube containing 200 mg of glass beads and placed on ice. Following, 300 μl buffer SP1 and proteinase K were added, and incubated at 70°C for 10 min. Subsequently, all the procedures outlined in product manual were performed according to the manufacturer's protocol. Finally DNA was eluted in 200 μl of elution buffer and the extract was stored at −20°C until used in PCR.
PCR and Sequence Analysis
Enterocytozoon bieneusi was detected using nested PCR targeting the ITS region of the rRNA gene (6). The first-round PCR was performed in a 25 μl reaction volume that includes 23 μl mixes (TaKaRa Bio, Otsu, Japan) and 2 μl DNA template. The primary PCR program was involved enzyme activation at 94°C for 5 min, followed by denaturation at 94°C for 30 s, annealing at 57°C for 30 s and primer extension at 72°C for 40 s for 35 cycles. A 7 min final extension at 72°C was done after 35 cycles followed by cooling at 4°C. The second-round PCR was conducted in a 25 μl reaction volume consisting of 23 μl mixes (TaKaRa Bio, Otsu, Japan) and 2 μl product of first PCR. The second PCR was run under the same conditions as the first except the annealing temperature which was reduced from 57 to 55°C for 30 s; and the cycle which was again reduced from 35 to 30 cycles. The amplificons were separated by electrophoresis on 1% agarose gel and visualized under a trans-illuminator after staining with ethidium bromide. To avoid contamination in PCR amplification, several precautions were taken, such as changing gloves prior to handling positive controls, using a separate set of pipettes and pipetting PCR reagents and amplified products in separate areas.
The secondary PCR products were purified using Montage PCR filters (Millipore, Bedford, MA) and sequenced using an ABI BigDye Terminator v. 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) on an ABI 3100 automated sequencer (Applied Biosystems). The nucleotide sequences obtained were aligned with reference E. bieneusi sequences using ClustalX software (ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/). The established nomenclature system was used in naming novel E. bieneusi ITS genotypes (4).
Bayesian inference (BI) and Monte Carlo Markov chain methods were used to construct the phylogenetic trees in MrBayes program (version 3.2.6). The posterior probability values were calculated by running 1,000,000 generations. A 50% majority rule consensus tree was constructed from the final 75% of the trees generated via BI. Analyses were run three times to ensure convergence and intensity to priors.
Data Analysis and Nucleotide Sequence Accession Numbers
Data were entered into computer using EpiData version 3.1 and transferred to STATA Software for analysis. Chi square test was used to verify association of E. bieneusi infections in different study groups. P-values were considered to be statistically significant when <0.05. The representative nucleotide sequences of the five E. bieneusi ITS genotypes obtained in the present study were deposited in the GenBank database under the accession numbers: KT948316, KT948317, and MN728943 to MN728945.
Results
E. bieneusi Infection in Lambs
Of the 389 fecal specimens screened by nested ITS-PCR (392 bp) of the rRNA gene, E. bieneusi was found in 39 (10.03%) lambs (Table 1). The prevalence of E. bieneusi varied across the study areas. Relatively higher prevalence (15.53%, 16/103) was found in Holeta followed by Chancho (9.38%, 12/128) and Sendafa (6.96%, 11/158). The prevalence difference was statistically not significant (χ2 = 5.1686, P = 0.075) among the three study areas.
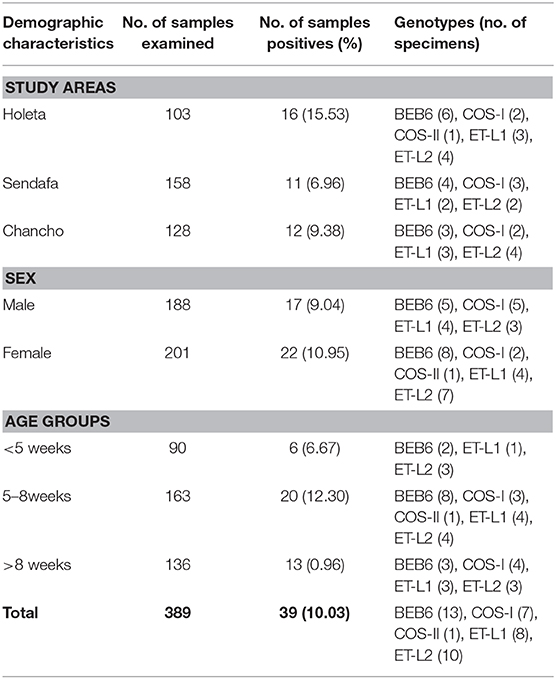
Table 1. Prevalence of E. bieneusi in lambs by study areas, sex, and age in Oromia Special Zone, central Ethiopia (January–June, 2014).
E. bieneusi Infection by Sex, Age, and Breed Groups
To investigate the distribution of E. bieneusi among lambs in relation to sex and age groups, data were arranged and summarized in Table 1. The prevalence of E. bieneusi was 9.04% (17/188) in male and 10.95% (22/201) in female lambs which was statistically not significant between the two groups. Although the age-associated difference in the occurrence of E. bieneusi infections was statistically not significant (χ2 = 0.3899, P = 0.532), lambs aged between 5 and 8 weeks had the highest infection rate with 12.30% (20/163) (χ2 = 2.029, P = 0.358).
Genotypes of E. bieneusi
The sequence analysis of 39 positive lamb specimens revealed the presence of five different genotypes in lambs. Three of them (BEB6, COS-I, and COS-II) were known genotypes and two (designated as ET-L1 andET-L2) were novel. The prevalent genotype was BEB6, being observed in 13 specimens followed by ET-L2, ET-L1, and COS-I observed in 10, 8, and 7 specimens, respectively. Genotype COS-II was found only in one specimen (Table 1).
Phylogenetic Analysis
Phylogenetic analysis revealed that the three known (BEB6, COS-I, and COS-II) and two novel (ET-L1 and ET-L2) genotypes identified herein all were clustered into the Group 2 (Figure 2).
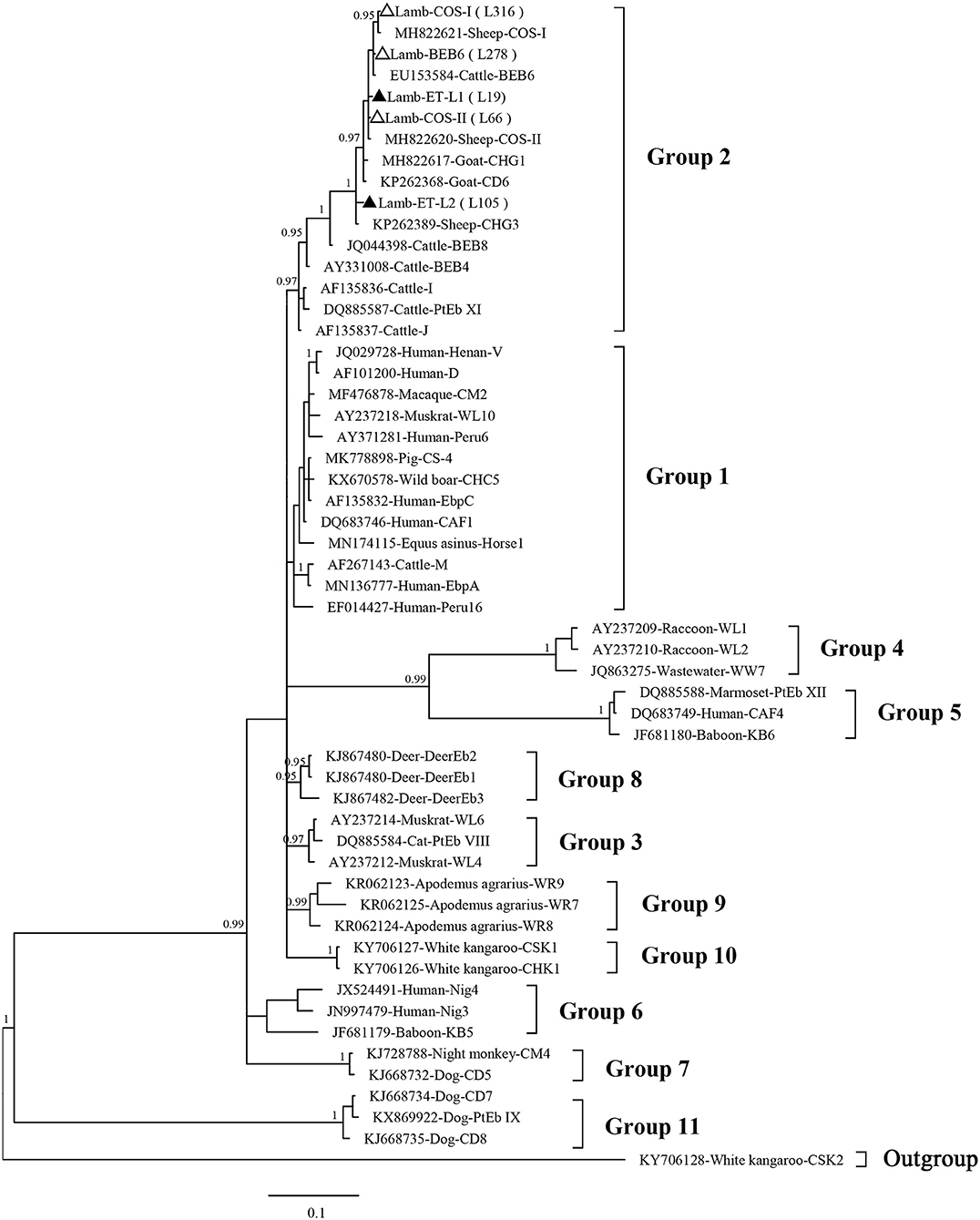
Figure 2. Phylogenetic tree based on Bayesian inference (BI) analysis of the Enterocytozoon bieneusi ITS sequences. Statistically significant posterior probabilities (0.95 and higher) are indicated on the branches. Known and novel E. bieneusi ITS genotypes identified in the present study are indicated by empty and filled triangles, respectively.
Discussion
Enterocytozoon bieneusi has been the focus of numerous epidemiological studies both in humans and animals because of its opportunistic and zoonotic potential. However, there is knowledge gap on E. bienusi epidemiology in Ethiopia. So far, this parasite has been studied and characterized in a few human samples in the country (8, 9). This is the first investigation of E. bieneusi in animals in Ethiopia.
In the present study, E. bieneusi was found in 10.03% of lambs in Central Ethiopia. Similar rate of infection was reported for E. bieneusi in sheep in Northeast China (13.9%, 68/489) (11), Southwest China (10.6%, 70/661) (12), and Egypt (11.2%, 10/89) (13); while lower infection rates were detected in sheep in East-central China (3.4%, 28/832) (14) and Slovakia (0/33) (15). However, a higher prevalence of E. bieneusi was found in Inner Mongolian (China) lambs (77.8%, 126/162) (16), Swedish lambs (68%, 49/72) (17), Brazilian sheep (19.2%, 24/125) (18), and in sheep from some other studies in China (19–22). The differences in the prevalence are likely due to differences in the geographic and ecological setup.
Although not significant, E. bieneusi infection was slightly higher in female lambs than males. This is in accordance with report from China (19), who showed somewhat higher prevalence in female sheep. Together, these findings suggest that infection occurs regardless of the sex of the lambs.
Many studies showed the variation in infection rates of E. bieneusi in different age group of sheep, as well as in lambs (12, 14, 17, 21). In present study, only lambs younger than 3 months were assessed. Although, statistically not significant, lambs aged between 5 and 8 weeks had the highest infection rate (12.3%).
The genotype BEB6 identified in cattle in the United States (23), was also identified as the dominant E. bieneusi genotype (13/39, 33.3%) in lambs in the present study. Many other studies demonstrated that the genotype BEB6 was the dominant E. Bieneusi genotype in sheep in Sweden (17), Brazil (18), and China (16, 21). Although this genotype was considered to be cattle-specific, it is now recognized as a dominant genotype in humans, animals and birds in a wide geographic distribution, and has zoonotic potential (11, 17, 19, 24, 25). Genotypes COS-I and COS-II, commonly reported in cattle, sheep, deer, and non-human primates in previous studies (2, 14, 26, 27), were recognized as the known E. bieneusi genotypes. In contrast, the novel genotypes (ET-L1 and ET-L2) were first identified in lambs in this study. The sequence of new genotype ET-L1 (KT948316) had one nucleotide difference (G-to-T substitution) from that of genotype COS-II (KJ850433) at position 265, whereas sequence of new genotype ET-L2 (KT948317) had one nucleotide difference (G-to-A substitution) from that of genotype CM21 (KU604931) at position 174.
The phylogenetic analysis revealed that all five genotypes identified herein are clustered into the so-called cattle-specific Group 2. On other hand, no genotype was clustered under the zoonotic Group 1. However, some genotypes in the Group 2, such as BEB6, I, and J, have been detected in humans in recent years and are a potential source of zoonotic transmission between sheep or other animals and humans (2, 28).
The previous studies conducted in patients infected with the human immunodeficiency virus-1 in Ethiopia simply showed the prevalence of E. bieneusi infection using PCR and Uvitex-2B stain (8, 9). As ITS-genotyping was not performed it is not clear which genotype was infecting humans. Further molecular studies are needed to understand the patterns of transmission.
Conclusions
This is first molecular investigation of E. bieneusi in Ethiopia, revealed that this species is the most prevalent microsporidia in lambs. Five E. bieneusi genotypes (three known and two novel) were identified in lambs in present study, and genotype BEB6 was the dominant genotype. Further studies in humans and other domestic animals are necessary to assess the public health significance of E. bieneusi in Ethiopia.
Data Availability Statement
The datasets generated for this study can be found in the Nucleotide sequences of the ITS of the two novel E. bieneusi genotypes obtained in the present study (ET-L1 and ET-L2) were deposited in the GenBank database under accession numbers KT948316 and KT948317.
Ethics Statement
This animal study was reviewed and approved by National Health Research Ethics Review Committee, Ministry of Science, and Technology. Support letters were obtained from concerned health and agricultural offices and administrative authorities at community level. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
TW and LZ conceived the idea. TW, JL, and MK conducted the experiments. TW, JL, MK, and LZ wrote and revised the manuscript.
Funding
This study was supported by the State Key Program of National Natural Science Foundation of China (No. 31330079), and Innovation Scientists and Technicians Troop Construction Projects of Henan Province (No. 134200510012). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Santín M, Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. (2011) 90:363–71. doi: 10.1016/j.rvsc.2010.07.014
2. Wang SS, Wang RJ, Fan XC, Liu TL, Zhang LX, Zhao GH. Prevalence and genotypes of Enterocytozoon bieneusi in China. Acta Trop. (2018) 183:142–52. doi: 10.1016/j.actatropica.2018.04.017
3. Desportes I, Le Charpentier Y, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, et al. Occurrence of a new microsporidan: Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. J Protozool. (1985) 32:250–4. doi: 10.1111/j.1550-7408.1985.tb03046.x
4. Santín M, Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J Eukaryot Microbiol. (2009) 56:34–8. doi: 10.1111/j.1550-7408.2008.00380.x
5. Li W, Feng Y, Santin M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. (2019) 35:436–51. doi: 10.1016/j.pt.2019.04.004
6. Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW III, Xiao L. Molecular characterization of microsporidia indicates that wild mammals Harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. (2003) 69:4495–501. doi: 10.1128/AEM.69.8.4495-4501.2003
7. Thellier M, Breton J. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite. (2008) 15:349–58. doi: 10.1051/parasite/2008153349
8. Endeshaw T, Kebede A, Verweij JJ, Wolday D, Zewide A, Tsige K, et al. Detection of intestinal microsporidiosis in diarrhoeal patients infected with the human immunideficiency virus (HIV-1) using PCR and Uvitex-2B stain. Ethiop Med J. (2005) 43:97–101.
9. Endeshaw T, Kebede A, Verweij JJ, Zewide A, Tsige K, Abraham Y, et al. Intestinal microsporidiosis in diarrheal patients infected with human immunodeficiency virus-1 in Addis Ababa, Ethiopia. Jpn J Infect Dis. (2006) 59:306–10.
10. Wegayehu T, Karim MR, Erko B, Zhang L, Tilahun G. Multilocus genotyping of Giardia duodenalis isolates from calves in Oromia Special Zone, Central Ethiopia. Infect Genet Evol. (2016) 43:281–8. doi: 10.1016/j.meegid.2016.06.005
11. Jiang Y, Tao W, Wan Q, Li Q, Yang Y, Lin Y, et al. Zoonotic and potentially host-adapted Enterocytozoon bieneusi genotypes in sheep and cattle in Northeast China and an increasing concern about the zoonotic importance of previously considered ruminant-adapted genotypes. Appl Environ Microbiol. (2015) 81:3326–235. doi: 10.1128/AEM.00328-15
12. Chen D, Wang SS, Zou Y, Li Z, Xie SC, Shi LQ, et al. Prevalence and multi-locus genotypes of Enterocytozoon bieneusi in black-boned sheep and goats in Yunnan Province, Southwestern China. Infect Genet Evol. (2018) 65:385–91. doi: 10.1016/j.meegid.2018.08.022
13. Al-Herrawy AZ, Gad MA. Microsporidial spores in fecal samples of some domesticated animals living in Giza, Egypt. Iran J Parasitol. (2016) 11:195–203.
14. Li WC, Wang K, Gu YF. Detection and genotyping study of Enterocytozoon bieneusi in sheep and goats in East-central China. Acta Parasitol. (2019) 64:44–50. doi: 10.2478/s11686-018-00006-8
15. Valenčáková A, Danišová O. Molecular characterization of new genotypes Enterocytozoon bieneusi in Slovakia. Acta Trop. (2019) 191:217–20. doi: 10.1016/j.actatropica.2018.12.031
16. Ye J, Xiao L, Wang Y, Guo Y, Roellig DM, Feng Y. Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Vet Parasitol. (2015) 210:235–9. doi: 10.1016/j.vetpar.2015.04.011
17. Stensvold CR, Beser J, Ljungström B, Troell K, Lebbad M. Low host-specific Enterocytozoon bieneusi genotype BEB6 is common in Swedish lambs. Vet Parasitol. (2014) 205:371–4. doi: 10.1016/j.vetpar.2014.06.010
18. Fiuza VRDS, Lopes CWG, Cosendey RIJ, de Oliveira FCR, Fayer R, Santín M. Zoonotic Enterocytozoon bieneusi genotypes found in Brazilian sheep. Res Vet Sci. (2016) 107:196–201. doi: 10.1016/j.rvsc.2016.06.006
19. Shi K, Li M, Wang X, Li J, Karim MR, Wang R, et al. Molecular survey of Enterocytozoon bieneusi in sheep and goats in China. Parasit Vectors. (2016) 9:23. doi: 10.1186/s13071-016-1304-0
20. Wu Y, Chang Y, Chen Y, Zhang X, Li D, Zheng S, et al. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect Genet Evol. (2018) 64:46–51. doi: 10.1016/j.meegid.2018.06.012
21. Yang H, Mi R, Cheng L, Huang Y, An R, Zhang Y, et al. Prevalence and genetic diversity of Enterocytozoon bieneusi in sheep in China. Parasit Vectors. (2018) 11:587. doi: 10.1186/s13071-018-3178-9
22. Zhang Q, Cai J, Li P, Wang L, Guo Y, Li C, et al. Enterocytozoon bieneusi genotypes in Tibetan sheep and yaks. Parasitol Res. (2018) 117:721–7. doi: 10.1007/s00436-017-5742-1
23. Fayer R, Santín M, Trout JM. Enterocytozoon bieneusi in mature dairy cattle on farms in the eastern United States. Parasitol Res. (2007) 102:15–20. doi: 10.1007/s00436-007-0746-x
24. Zhao W, Yu S, Yang Z, Zhang Y, Zhang L, Wang R, et al. Genotyping of Enterocytozoon bieneusi (Microsporidia) isolated from various birds in China. Infect Genet Evol. (2016) 40:151–4. doi: 10.1016/j.meegid.2016.02.037
25. Deng L, Yue CJ, Chai YJ, Wang WY, Su XY, Zhou ZY, et al. New genotypes and molecular characterization of Enterocytozoon bieneusi in pet birds in Southwestern China. Int J Parasitol Parasites Wildl. (2019) 10:164–9. doi: 10.1016/j.ijppaw.2019.08.001
26. Huang J, Zhang Z, Yang Y, Wang R, Zhao J, Jian F, et al. New genotypes of Enterocytozoon bieneusi isolated from Sika deer and Red deer in China. Front Microbiol. (2017) 8:879. doi: 10.3389/fmicb.2017.00879
27. Wang HY, Qi M, Sun MF, Li DF, Wang RJ, Zhang SM, et al. Prevalence and population genetics analysis of Enterocytozoon bieneusi in dairy cattle in China. Front Microbiol. (2019) 10:1399. doi: 10.3389/fmicb.2019.01399
Keywords: Enterocytozoon bieneusi, genotypes, internal transcribed spacer, lambs, Ethiopia
Citation: Wegayehu T, Li J, Karim MR and Zhang L (2020) Molecular Characterization and Phylogenetic Analysis of Enterocytozoon bieneusi in Lambs in Oromia Special Zone, Central Ethiopia. Front. Vet. Sci. 7:6. doi: 10.3389/fvets.2020.00006
Received: 01 October 2019; Accepted: 07 January 2020;
Published: 29 January 2020.
Edited by:
Armanda Bastos, University of Pretoria, South AfricaReviewed by:
Hamed Mirjalali, Shahid Beheshti University of Medical Sciences, IranLei Deng, Sichuan Agricultural University, China
Copyright © 2020 Wegayehu, Li, Karim and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teklu Wegayehu, dGVrbHV3ZWcyMDA3QHlhaG9vLmNvbQ==; Longxian Zhang, emhhbmdsb25neGlhbjg5OTlAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
 Teklu Wegayehu
Teklu Wegayehu Junqiang Li
Junqiang Li Md. Robiul Karim
Md. Robiul Karim Longxian Zhang2*
Longxian Zhang2*