- 1Programa de Pós-Graduação em Medicina Veterinária, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil
- 2Laboratório de Imunomodulação e Protozoologia, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
- 3Departamento de Medicina e Cirurgia Veterinária, Instituto de Veterinária, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil
- 4Technical Services for Companion Animals, Zoetis, São Paulo, Brazil
- 5Programa de Pós-Graduação em Bioética, Ética Aplicada e Saúde Coletiva. Escola Nacional de Saúde Pública, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
Canine heartworm disease is a life-threatening disease caused by Dirofilaria immitis and is prevalent in Brazil. The standard drug for its treatment, melarsomine dihydrochloride, is a fast-killing organic arsenical chemotherapeutic agent not approved in Brazil. Therefore, an alternative strategy, such as macrocyclic lactone in combination with a tetracycline antibiotic, has to be used. The alternative method is a long-term therapy that could lead to compliance issues during treatment. The aim of this case report is to present a preliminary assessment on the efficacy and safety of an off-label biannual administration of slow-release moxidectin (0.5 mg/kg every 6 months), which is formulated for annual administration (0.5 mg/kg annually). This overdose was chosen to test if moxidectin serum levels could be maintained high enough to harm the worms. It was administered to a 4-year-old female dog in combination with a 30-day doxycycline course. The second dose of moxidectin was administered approximately a week before she gave birth to three healthy puppies. Microfilariae were not detected on day 180 of treatment. Serological tests showed that the worms were eliminated, as two negative antigen tests were obtained 6 months apart (at day 180 and day 360 of treatment). Therefore, the off-label biannual use of moxidectin in combination with doxycycline was effective in eliminating D. immitis in 360 days and was harmless for the pregnant dog and her offspring, suggesting that this strategy is promising. Although these results are encouraging, further studies are needed to confirm safety and efficacy issues.
Background
Canine heartworm disease is life threatening when left untreated (1, 2); therefore, treatment must be administered even under adverse conditions. The objective of the treatment must be to eliminate D. immitis in all stages as quickly as possible to improve the animal's clinical condition (2).
There are 2 distinct drug-based methods to eliminate the infection. Veterinarians must consider the animal's clinical conditions, stage of the disease, and treatment risk prior to deciding the treatment (2–4). Internationally recommended treatment is the fast-acting organic arsenical compound melarsomine dihydrochloride (2, 5–7), despite being unavailable in many countries (7), and the risk that it poses to severely ill dogs (2, 8). The reported advantage of this treatment is the speed at which it kills adult worms and prevents the heart and lungs disease from progressing (2, 7). The other method is the long-term use of macrocyclic lactone in combination with a tetracycline antibiotic. The antibiotic with best therapeutic results is doxycycline (9) and the macrocyclic lactones reported as efficacious are oral ivermectin (10–12), topical moxidectin (13, 14), and double annual dose of injectable moxidectin (15). This long-term treatment is also called “alternative treatment,” “slow kill,” “soft kill,” “doxy-moxi,” or “moxi-doxy” and is recommended to be used when the arsenical drug is unavailable or when fast kill is contraindicated (2, 7). The injectable moxidectin is an extended-release injectable formulation that can be used in breeding animals and a 5X safety margin has been shown (16).
Case Presentation
A 4-year-old (estimated) Pit Bull cross intact female (25 kg) was presented for routine evaluation after it was adopted from the streets. The results showed that she had asymptomatic D. immitis infection as indicated by the presence of microfilariae in Knott's modified test (17) and a positive D. immitis antigen test, although seronegative for Anaplasma spp., Ehrlichia spp., and Borrelia burgdorferi (SNAP®4DX Plus®, IDEXX Laboratories Inc., Westbrook, ME, USA). She was seronegative for Leishmania infantum (TR DPP® canine visceral leishmaniasis, Bio-Manguinhos, Brazil). The routine physical examination showed no clinical signs of the disease and the hematological and urine examination results were normal.
The heartworm pre-treatment tests included blood work (blood urea nitrogen test, creatinine, alanine transaminase, and alkaline phosphatase levels and complete blood count), Doppler echocardiogram and chest X-rays. The blood work and Doppler echocardiogram were normal; however, the X-rays showed mild right atrioventricular enlargement and mild increase in interstitial and bronchial pulmonary pattern (Figures 1, 2).
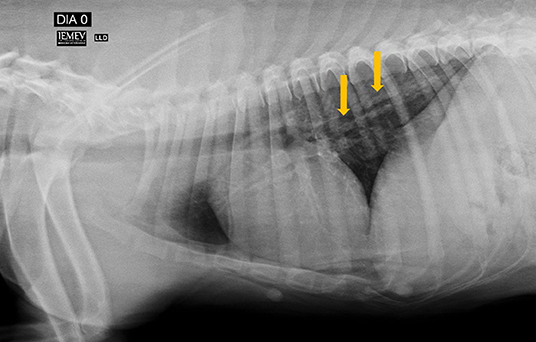
Figure 1. Right lateral position showing increased pulmonary radiodensity, especially characterized by a diffusely distributed bronchial pattern (arrows).
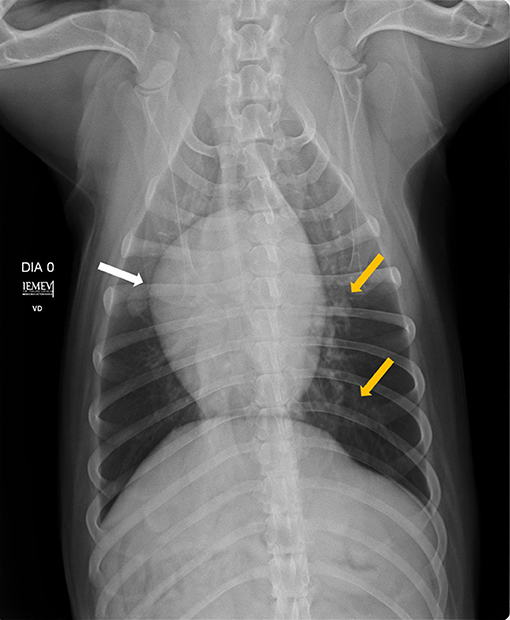
Figure 2. Ventrodorsal position showing right atrioventricular enlargement (white arrow) and diffusely distributed bronchial pattern (yellow arrows).
Since the organic arsenical drug is unavailable in Brazil, the treatment option was the use of slow-release injectable moxidectin (ProHeart® SR-12, Zoetis, Campinas, Brazil) biannually (0.5 mg/kg) instead of annually in combination with 30 days of doxycycline (Doxifin®, Ourofino, Cravinhos, Brazil) (10 mg/kg/BID). The owner was duly informed and clarified about the off-label alternative treatment and after her formal consent the administration of both drugs was initiated on the same day.
When the dog was presented for evaluation 6 months following the first moxidectin injection it was informed that she could be pregnant as she had been in heat approximately 30 days before the visit and she lived with an intact male dog. Abdominal ultrasound was performed, and pregnancy was confirmed. Therefore, chest X-rays for lungs evaluation were precluded. All the other pre-treatment examinations were repeated, and the results were within the reference range. No microfilariae were detected by Knott's modified test and D. immitis antigen test was negative. Since a D. immitis-infected dog receiving alternative treatment must present microfilariae and D. immitis antigen test negative results 6 months apart to confirm the elimination of the parasite, a second moxidectin injection (0.5 mg/kg) was administered 6 months after the first and the animal was kept under observation. Within 1 week following the second injection, the animal gave birth to three healthy puppies.
Blood work, Doppler echocardiogram, and chest X-rays were unchanged when she was presented 6 months after the second moxidectin dose and serological tests with the heat pre-treatment sample (18) confirmed that heartworm had been successfully eliminated. Since the animal was free of infection, the owner was advised to continue with the moxidectin injections annually.
Discussion
Although the exact time period the alternative method took to eliminate the infection is unknown, a negative D. immitis antigen test result was obtained at day 180 of treatment. When this is compared to the time needed by the recommended organic arsenical drug protocol to eliminate the infection (2), the alternative method took no more than 60 days longer. In addition, the elimination of microfilariae suggests that the alternative method is inoffensive to macrocyclic lactone resistance development. Therefore, alternative methods using moxidectin in combination with doxycycline are valid treatment options (9, 11, 13–15), particularly when melarsomine dihydrochloride is unavailable or contraindicated.
Even though this is a single case report, it adds evidence to show the safety of the off-label use of the annual slow-release injectable moxidectin formulation biannually as expected (19). Even more important is the fact that the use of a double dose of the slow release injectable moxidectin formulation (0.5 mg/kg every 6 months) caused no side effect whatsoever on the mother or her offspring.
The off-label biannual use of the slow-release injectable moxidectin (0.5 mg/kg every 6 months) in combination with doxycycline was efficacious in eliminating D. immitis infection. This treatment was clinically safe for the animal even during pregnancy and for her off-spring. In an overall view it can be suggested that veterinarians may use it for controlling heartworm disease at their discretion, although further studies are needed.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the owner of the animal for the treatment and for the publication of this case report.
Author Contributions
BA designed the treatment protocol, participated in the acquisition of data, interpretation of results, helped draft the manuscript, and was the primary veterinary practitioner for this case. CS performed the parasitological analysis and interpretation, and contributed to writing the manuscript. JF contributed to writing the manuscript. AM designed the treatment protocol and drafted the manuscript. NL designed the treatment protocol, assisted in interpretation of results, and helped draft the manuscript. All authors read and approved the final manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The treatment of the animal was financed by Zoetis and Ourofino Saúde Animal. The Instituto Oswaldo Cruz/Fundação Oswaldo Cruz financed the publication fees.
Conflict of Interest
BA received the medication from Zoetis Brazil and from Ourofino Saúde Animal. AM is a current employee of Zoetis in Brazil. NL is a consultant for Bayer Animal Health, Boehringer Ingelheim Animal Health, Idexx Laboratories, and Zoetis in Brazil.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge Cristiano von Simson for his always enlightening thoughts.
References
1. Labarthe N, Alves L, Serrão ML. Dirofilariose em pequenos animais domésticos e como zoonose. In: Almosny NRP, editor. Hemoparasitoses em Pequenos Animais Domésticos. Rio de Janeiro: LF livros (2002). p. 135.
2. Nelson CT, Jones S, Moorhead A. Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm Infection in Dogs. (2018). Available online at: https://heartwormsociety.org/images/pdf/2018-AHS-Canine-Guidelines.pdf (accessed July 29, 2019).
4. Atwell RB. Clinical signs and diagnosis of canine dirofilariasis. In: Boreham PFL, Atwell RB, editors. Dirofilariasis. Boca Raton, FL: CRC Press (1988). p. 61–81.
5. CAPC. Companion Animal Parasite Council. (2016). Available online at: https://capcvet.org/guidelines/heartworm (accessed July 29, 2019).
6. ESCCAP. European Scientific Consel companion animal parasite Guideline. 3rd ed. (2019). Available online at: https://www.esccap.org/uploads/docs/znkh6j1d_0775_ESCCAP_Guideline_GL5_v8_1p.pdf (accessed July 29, 2019).
7. TroCCAP. Tropical Council for Companion Animal Parasites. Guidelines for the Diagnosis, Treatment and Control of Canine Endoparasites in the Tropics. 2nd ed. (2019). Available online at: https://www.troccap.com/2017press/wp-content/uploads/2019/05/TroCCAP_Canine_Endo_Guidelines_English_Ver2.pdf (accessed July 31, 2019).
8. Bowman DD, Atkins CE. Heartworm biology, treatment, and control. Vet Clin North Am Small Anim Pract. (2009) 39:1127–58. doi: 10.1016/j.cvsm.2009.06.003
9. Savadelis MD, Day KM, Bradner JL, Wolstenholme AJ, Dzimianski MT, Moorhead AR. Efficacy and side effects of doxycycline versus minocycline in the three-dose melarsomine canine adulticidal heartworm treatment protocol. Parasit Vectors. (2018) 11:671. doi: 10.1186/s13071-018-3264-z
10. Mccall JW, Ryan WG, Roberts RE, Dzimianski MT. Heartworm adulticidal activity of prophy-lactic doses of ivermectin (6 mg/kg) plus pyrantel administered monthly to dogs. In: Recent Advances in Heartworm Disease. Tampa, FL: American Heartworm Society (1998). p. 209–15.
11. Bazzocchi C, Mortarino M, Grandi G, Kramer LH, Genchi C, Bandi C, et al. Combined ivermectin and doxycycline treatment has microfilaricidal and adulticidal activity against Dirofilaria immitis in experimentally infected dogs. Int J Parasitol. (2008). 38:1401–10. doi: 10.1016/j.ijpara.2008.03.002
12. Grandi G, Quintavalla C, Mavropoulou A, Genchi M, Gnudi G, Bertoni G, et al. A combination of doxycycline and ivermectin is adulticidal in dogs with naturally acquired heartworm disease (Dirofilaria immitis). Vet Parasitol. (2010). 169:347–51. doi: 10.1016/j.vetpar.2010.01.025
13. Bendas AJR. Avaliação da associação de moxidectina 2,5% e imidacloprida 10% tópica com doxiciclina no tratamento de cães (Canis familiaris linnaeus, 1758) naturalmente infectados por Dirofilaria immitis (Leidy, 1856) [Dissertation]. Universidade Federal Fluminense, Niterói, Brazil (2018).
14. Genchi M, Vismarra A, Lucchetti C, Viglietti A, Crosara S, Gnudi G, et al. Efficacy of imidacloprid 10%/moxidectin 2.5% spot on (Advocate®, Advantage Multi®) and doxycycline for the treatment of natural Dirofilaria immitis infections in dogs. Vet Parasitol. (2019) 273:11–6. doi: 10.1016/j.vetpar.2019.07.011
15. Alberigi B, Fernandes J, Paiva P, Mendes-de-Almeida F, Batalha F, Merlo A, et al. Canine heartworm treatment using a combination of ProHeart® SR 12 (Injectable Moxidectin) and oral doxycycline. In: 27th Conference of the World Association for the Advancements of Veterinary Parasitology. Madison, WI (2019). p. 322–3.
16. Krautmann MJ, Mahabir S, Fielder A, Collard W, Wolthuis TL, Esch K, et al. Safety of an extended-release injectable moxidectin suspension formulation (ProHeart®12) in dogs. Parasit Vectors. (2019) 12:433.
17. Newton WL, Wright WH. The occurrence of a dog filariid other than Dirofilaria immitis in the United States. J Parasitol. (1956) 42:246–58.
18. Little SE, Munzing C, Heise SR, Allen KE, Starkey LA, Johnson EM, et al. Pre-treatment with heat facilitates detection of antigen of Dirofilaria immitis in canine sample. Vet Parasitol. (2014) 203:250–2. doi: 10.1016/j.vetpar.2014.01.007
19. FDA (Food and Drug Administration). Freedom of Information Summary, NADA 141-519. Available online at: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/7307 (accessed August 3, 2019).
Keywords: heartworm, microfilariae, macrocyclic lactone, doxycycline, vector-borne disease
Citation: Alberigi B, Souza CSF, Fernandes JI, Merlo A and Labarthe N (2020) Use of Slow-Release Injectable Moxidectin for Treatment of Dirofilaria immitis Infection During Pregnancy. Front. Vet. Sci. 6:440. doi: 10.3389/fvets.2019.00440
Received: 19 August 2019; Accepted: 25 November 2019;
Published: 28 January 2020.
Edited by:
Meg M. Sleeper, University of Florida, United StatesReviewed by:
Susan Catherine Cork, University of Calgary, CanadaNathan Peterson, VCA West Los Angeles Animal Hospital, United States
Copyright © 2020 Alberigi, Souza, Fernandes, Merlo and Labarthe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celeste da Silva Freitas de Souza, Y3Nmc291emEmI3gwMDA0MDtpb2MuZmlvY3J1ei5icg==
 Bruno Alberigi
Bruno Alberigi Celeste da Silva Freitas de Souza
Celeste da Silva Freitas de Souza Julio Israel Fernandes
Julio Israel Fernandes Alexandre Merlo
Alexandre Merlo Norma Labarthe
Norma Labarthe