- 1Department of Veterinary Medicine and Animal Production, University of Naples Federico II, Naples, Italy
- 2CREMOPAR Campania Region, Naples, Italy
Canine vector-borne diseases (CVBDs) are a spectrum of diseases caused by different pathogens transmitted by blood-feeding arthropoda. The aim of this study was to investigate leishmaniosis, babesiosis, and filarial infections in dogs with three different lifestyles (hunting, stray, and sheep dogs) in Molise, the smallest region of southern Italy, where data available about these parasitic infections are very scant. A cross-sectional survey was conducted on 318 hunting, 180 stray, and 218 sheep dogs. Immunofluorescence antibody test, blood smear, molecular techniques and Knott's test were performed to detect Leishmania infantum, Babesia spp. and filarial nematodes. Association between positivity to CVBDs, age, sex, and living conditions was evaluated. An overall prevalence of 12.3% of CVBDs caused by L. infantum (10.2%), B. canis canis (0.3%) and filarial nematodes (2.1%) was detected. Three dogs showed co-infections of L. infantum and B. c. canis (0.1%) or Acanthocheilonema reconditum (0.3%). A significantly association was found only for filarial infection in hunting dogs. These parasites were reported also in dogs without clinical signs. It is very important to plan effective control programs for CVBDs to guarantee not only the health and welfare of pets, but also the public safety, because some of mentioned parasites are of zoonotic importance.
Introduction
Canine vector-borne diseases (CVBDs) are a spectrum of diseases caused by different infectious/parasitic pathogens transmitted by blood-feeding arthropoda such as fleas, lice, mosquitoes, phlebotomine sand flies and ticks (1). The most common CBVDs are anaplasmosis, babesiosis, bartonellosis, borreliosis, dirofilariosis, ehrlichiosis, leishmaniosis, rickettsiosis, dipylidosis, and thelaziosis (2). Most of these CVBDs are important not only for animal health and welfare, but also because they are of major zoonotic concern (e.g., Babesia venatorum, Babesia microti, Dirofilaria immitis, Dirofilaria repens, Leishmania spp.) (3). The interest on CVBDs has grown in the last two decades and therefore an increased number of studies have been published in the recent few years (4). The epidemiology of CVBDs (i.e., geographical distribution, prevalence, and pathogenicity) is changing due to several factors, especially climatic changes, ecosystem changes, increased mobility of dogs and humans and developing phenomena of chemoresistance to insecticides and acaricides (5). Consequently, CVBDs are spreading into areas considered non-endemic until recently (4).
Often, CVBDs cause chronic and asymptomatic infections and their diagnosis requires specific tests (6). Furthermore, co-infections are common, especially in areas suitable for many vector species, thus changing clinical manifestations and complicating diagnosis, therapy and prognosis (7–9). If dogs are not properly treated for CVBDs, they may act as reservoir of them, representing a zoonotic risk especially as the consequence of the increasing phenomenon of cohabitation with humans in urban and rural environments (10–13). Therefore, diagnosis and control of CVBDs are highly complex and challenging (5).
The epidemiological scenario of canine and feline vector-borne diseases in Italy has been recently reviewed (14). While most of regions have been investigated for CVBDs, a dearth of data are present for some regions of central-southern Italy. The aim of the present study was to investigate three parasitic CVBDs (leishmaniosis, babesiosis and filarial infections) in dogs with three different life styles (hunting, stray and sheep dogs) in Molise, the smallest region of southern Italy where data available about these parasitic infections are very scant.
Materials and Methods
Study Area and Collection of Samples
A cross-sectional survey was conducted between June 2017 and June 2018 in the Molise region of southern Italy (Latitude = 41°40′00″N; Longitude = 14°30′00″E) which extends over an area of 4,438 km2. The region is mainly mountainous and extends from 0 to 2,185 m above sea level. The climate is cold-temperate in the western part and Mediterranean in the eastern part of the region.
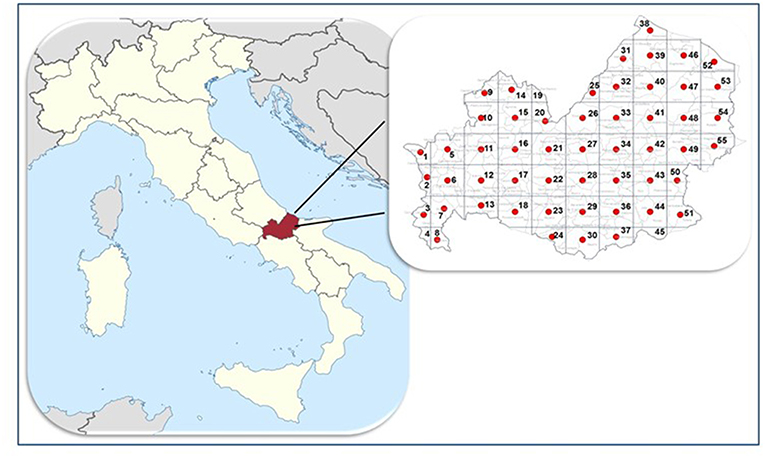
A grid-based approach within a Geographical Information System (GIS) was used in order to uniformly sample the dogs throughout the entire region (15). For this purpose, a grid representing quadrants of 10 × 10 km was overlaid on the regional map within the GIS. Thus, the Molise region was divided into 55 quadrants and the study was designed to sample 6 hunting, 6 stray and 6 sheep dogs in each quadrant (Figure 1) for a total of 990 dogs. From each dog, blood samples were collected as follows: 2 ml in tubes with EDTA and 2 ml in tubes with serum separator gel. Serum was separated by centrifugation at 360 g for 15 min and stored at −20°C until analysis.
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Data on age, sex and living conditions (hunting, stray, sheep dogs) were registered. Moreover, dogs were submitted for physical examination and a clinical form was completed.
Detection of Antibodies to Leishmania infantum
Serum samples were analyzed by an immunofluorescence antibody test (IFAT) provided by the National Reference Center for Leishmaniosis (CReNaL, Palermo, Italy) to detect anti-Leishmania antibodies (sensitivity = 96% and a specificity = 98%). Antigens used by CReNaL were promastigotes of strain MHOM/TN/80/IPT1. Samples were considered positive, if they showed a titer ≥1:160 (16). Reading was performed using a fluorescence microscope (Leica DM 2500, Germany) by three independent technicians.
Detection of Babesia
Blood smears were prepared using blood samples in EDTA and stained using Differential Quick Stain kit (Modified Giemsa) (Polysciences, Inc. Warrington, PA, USA), according to the manufacturer's instructions for detection of Babesia protozoa. Samples were analyzed by an expert examiner. Positive samples were then analyzed by molecular techniques. DNA was extracted by blood samples using the DNeasy Blood & Tissue Kit (QIAGEN, Germany). A semi-nested Polymerase chain reaction (PCR) was performed to amplify the 18S ribosomal RNA gene (17). The PCR products were detected on 2% ethidium bromide-stained low melting agarose gel (BIO-RAD, Spain). Positive amplicons were purified by QIAquick PCR purification kit (QIAGEN, Germany). The purified PCR products were sequenced in both forward and reverse directions and were analyzed by the Chromas version 2.1 software and finally compared with the NCBI/GenBank database using the Basic Local Alignment Search Tool (BLAST) and ClustalW software.
Detection of Microfilariae
Blood samples in EDTA were analyzed by the modified Knott's test to detect microfilariae of Dirofilaria immitis, D. repens and Acanthocheilonema reconditum (18, 19). The circulating microfilariae (mf) were identified based on their morphology and morphometry (19) and counted (mf/ml of blood) in 20 ul of blood. Morphometric analyses of the mf were then performed with a standard microscope equipped with calibrated measuring eyepieces at final magnification of 200–400×. Body length and diameter of 10 randomly selected mf were determined.
Statistical Analysis
Chi-squared test was performed using SPSS 23.0 software (IBM, Armonk, NY, USA) to study the association between positivity to CVBDs and dog's characteristics (age, sex, living conditions). Differences were considered significant at P < 0.05.
In addition, a spatial analysis was conducted to detect possible clusters of positive samples, using the average nearest neighbor (ANNI) index (20). If the index is < 1, the pattern exhibits clustering, while index of > 1 indicates a trend toward dispersion. A significance level of 99% was chosen in the analysis. Moreover, to confirm results obtained, the Moran's I test was used (21). A low negative z-score indicates a statistically significant spatial data outlier. A significance level of 95% was chosen for this test.
Results
Detection of CVBDs
A total of 716 blood and serum samples (72.3% of the expected sample) were collected: 318 from hunting, 180 from stray, and 218 from sheep dogs.
The age of animals ranged from 3 months to 17 years (median age = 3 years). Based on their age, dogs were divided into 5 classes: (a) 3–12 months; (b) 13–36 months; (c) 37–72 months; (d) 73–120 months; (e) 121–204 months. Regarding sex, 328/716 dogs were females (45.8, 95%, CI = 42.1–49.5%) and 388 males (54.2%, 95% CI = 50.5–57.9%).
An overall prevalence of 12.3% (95% CI = 10.0–15.0%) was detected for CVBDs caused by Leishmania infantum, Babesia spp. or filarial nematodes.
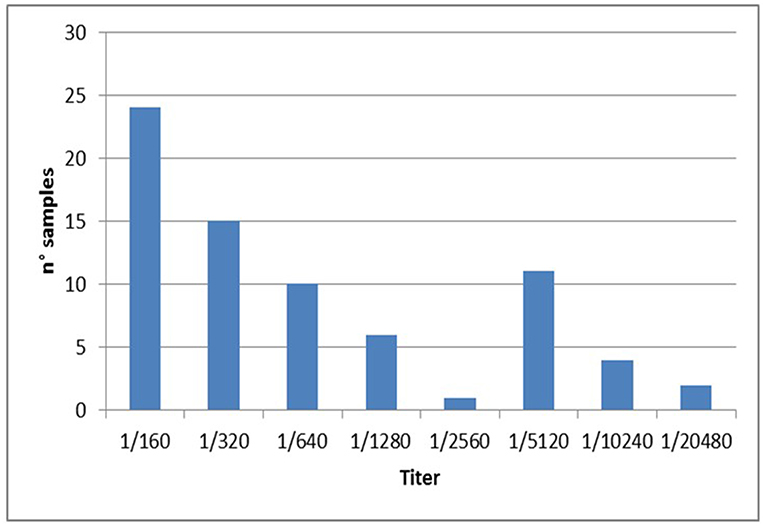
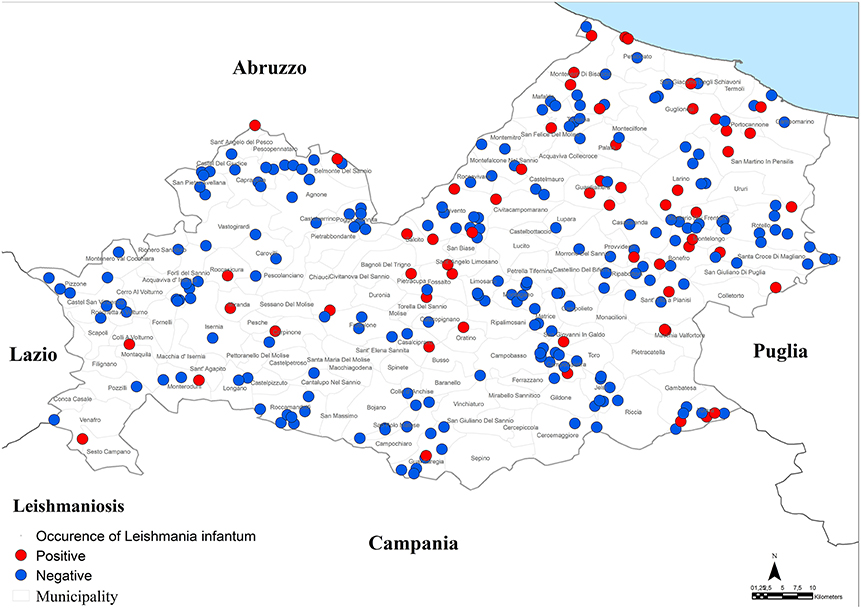
Specifically, a total of 73 dogs resulted positive for leishmaniosis (10.2%, 95% CI = 8.1–12.7%) with titers ≥ 1:160 up to 1:20480 (Figure 2; Tables 1–3). The positivity was not significantly associated with age, sex and living conditions (P > 0.05).
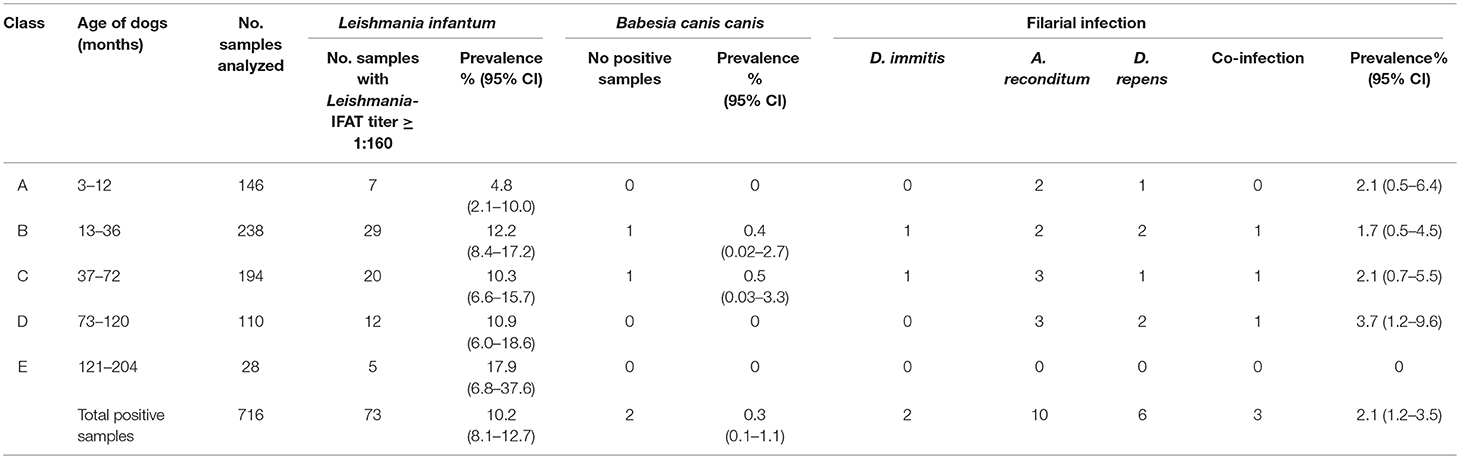
Table 1. Prevalence and 95% CI of positive samples to Leishmania infantum, Babesia canis canis and filarial infection for each class of age tested.
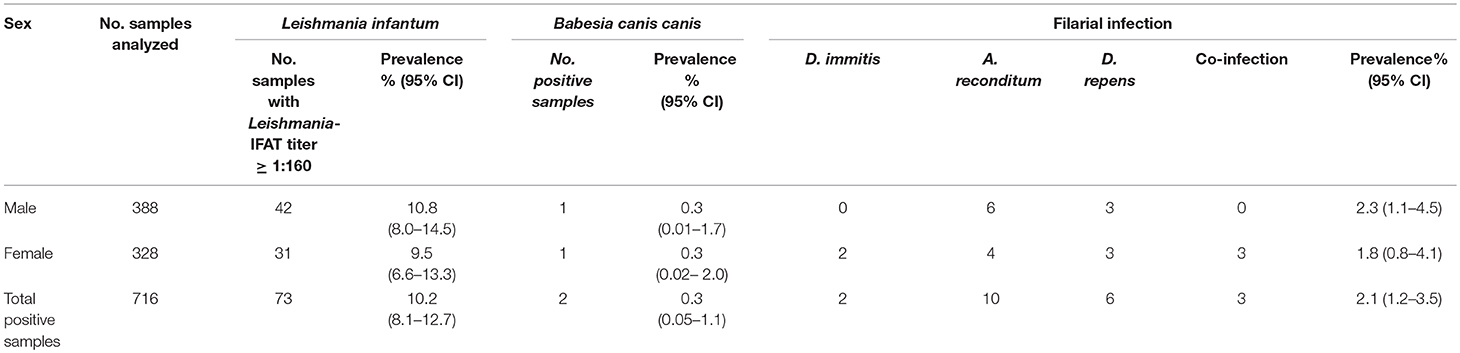
Table 2. Prevalence and 95% CI of positive samples to Leishmania infantum, Babesia canis canis and filarial infection for sex.
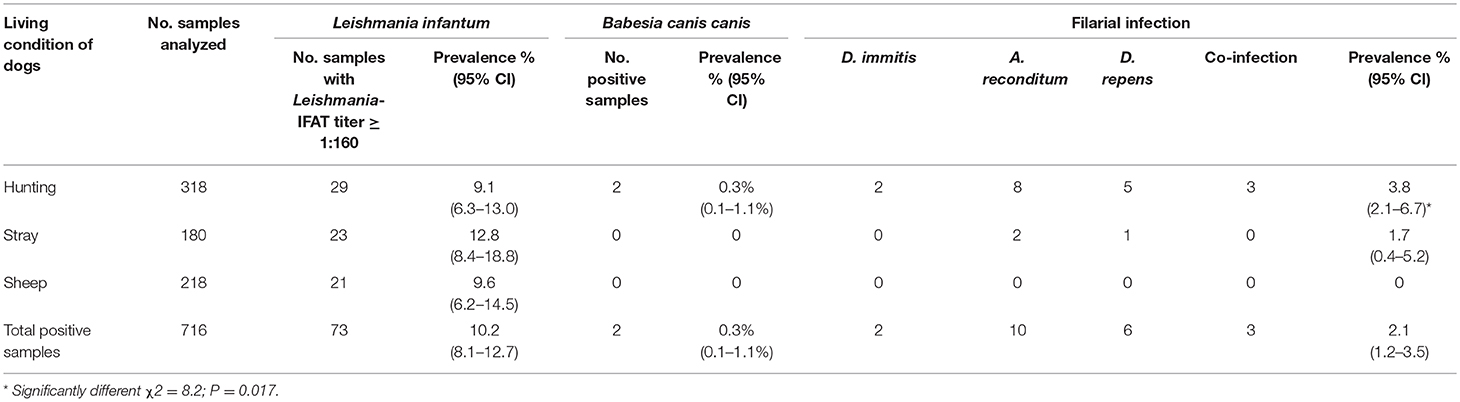
Table 3. Prevalence and 95% CI of positive samples to Leishmania infantum, Babesia canis canis and filarial infection for each living condition tested (hunting, stray and sheep dogs).
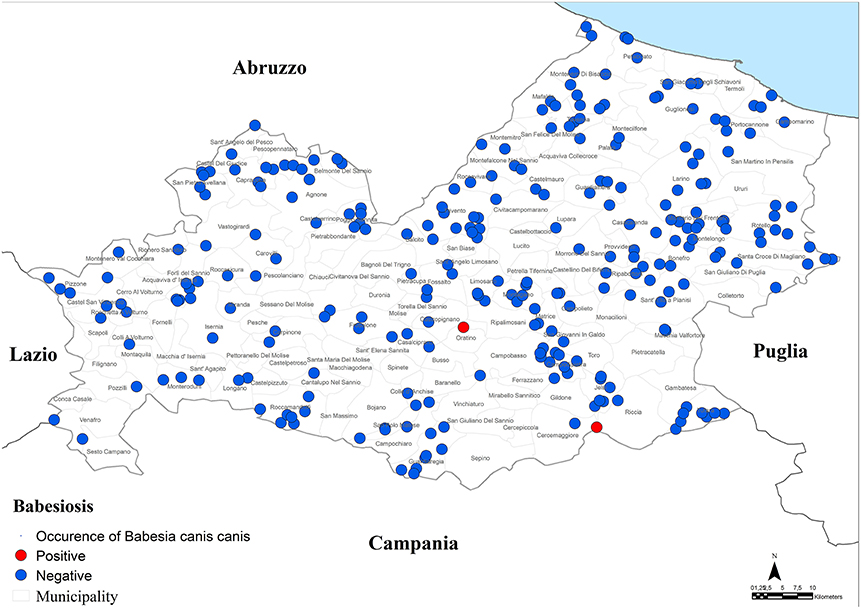
Results of microscopic analysis of blood smear for Babesia spp. showed that 2 dogs had large intraerythrocytic piroplasms compatible with a large Babesia. These samples were analyzed also by PCR and sequencing and an identity of 100% with B. canis canis sequence (GenBank accession number KX236456.1) was found. The two positive dogs for B. canis canis (0.3%, 95% CI = 0.1–1.1%) were both hunting dogs: 1 male of 40 months old and 1 female of 36 months old (Figure 4; Tables 1–3).
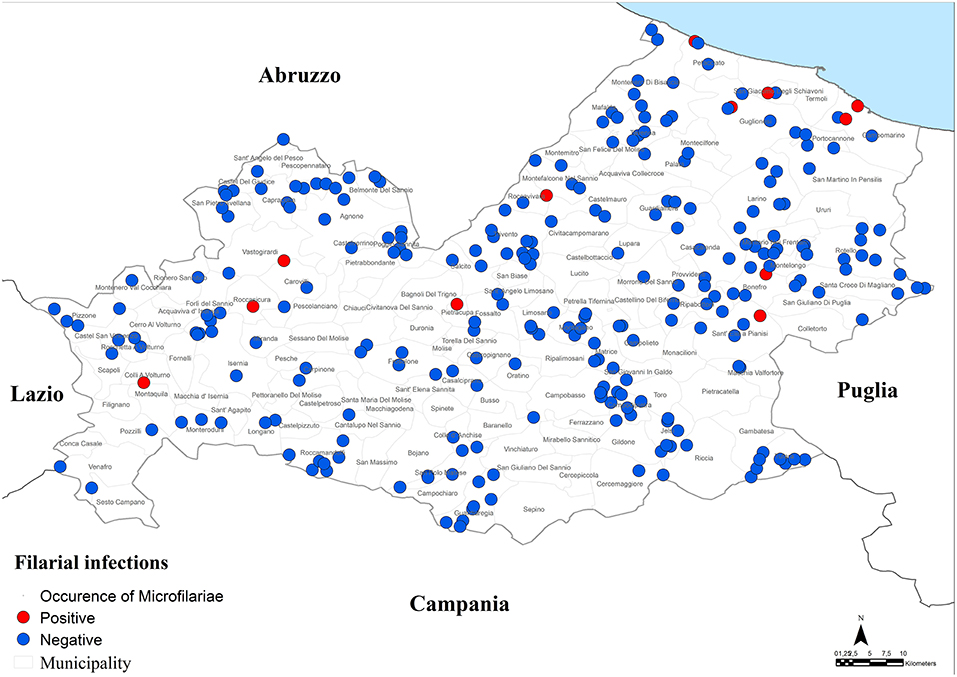
Moreover, a total of 15 dogs (2.1%, 95% CI = 1.2–3.5%) resulted positive for filarial infections (Figure 5). Specifically, 10 samples (1.3%; 95% CI = 0.7–2.5) were positive for A. reconditum, 6 (0.8%; 95% CI = 0.3–1.9) for D. repens and 2 (0.3%; 95% CI = 0.1–1.1) for D. immitis, with a higher significantly associated prevalence (P = 0.017) in hunting dogs (3.8%; 95% CI = 2.1–6.7), but positivity was not associated with age and sex (P > 0.05) (Tables 1–3). Two samples were co-infected with A. reconditum and D. immitis, while 1 sample with A. reconditum and D. repens. Finally, 2 samples showed co-infections of L. infantum and A. reconditum, whilst 1 sample showed a co-infection of L. infantum and B. canis canis.
Clinical Signs
Only 37% (95% CI = 26.2–49.1%) of positive dogs showed clinical signs for leishmaniosis and babesiosis. The most frequent clinical signs detected in positive dogs for these CVBDs were: loss of weight, depression, lymph-nodes and spleen enlargement, conjuntivitis, keratitis, blepharitis, alopecia, ulcers/nodules, and exfoliative dermatitis. No other clinical signs were recorded.
Only two positive dogs for filarial infections (13.3%; 95% CI = 2.3–41.6%) showed clinical signs, but unspecific for clinical diagnosis of this CVBD. Both dogs showed loss of weight, whilst only one of these, positive also for leishmaniosis, had blepharitis, and conjunctivitis.
At physical examination, thelaziosis was not found in any of the tested dogs.
It should be noted that the 716 dogs were analyzed also for intestinal nematodes (Trichuris, Toxocara, Toxascaris and Ancylostomidae), cardiopulmonary nematodes (Angiostrongylus and Capillaria) and cestoda (Dipylidium and other Taeniidae) (unpublished data), but no significantly association was found between them and vector borne diseases investigated in this study (P > 0.05).
Discussion
The limited extension of the Molise region territory, the small size of its dog population (56,729; www.lav.it) and the availability of an efficient veterinary system of active surveillance made it possible for us to have an accurate scenario of leishmaniosis and other CVBDs in dogs in this poorly investigated region.
Specifically, a prevalence of 10.2% of leishmaniosis, 2.1% for filarial infections and 0.3% for B. canis canis was found.
Canine leishmaniosis caused by L. infantum is endemic in all countries of the Mediterranean basin, but it is spreading northwards (20). In Italy, the median seroprevalence of leishmaniosis in 377 studies on dogs since 1971 to 2006 was 17.7% (range 10.7–21.1%), but prevalence of leishmaniosis is increasing over 40% in different zones not only of southern Italy, but also of northern Italy (12, 22, 23). In the present study, the prevalence recorded in Molise was 10.2%. Not many data are available about canine leishmaniosis in this region, but the more recent indicate an average prevalence of 18% in 2016 (unpublished data provided by CReNaL). The lower prevalence reported in our study can be correlated with enrolled dogs that were chosen at random, whilst the sera of dogs detected by CReNaL were all symptomatic for leishmaniosis.
This is the first time that B. canis canis is reported in the Molise region. PCR-positive prevalence (0.3%) for this parasite was higher than prevalence reported in hunting dogs from southern Italy (0.15%) (24) and from northern Italy (0%) (25, 26) but lower than a study conducted in central Italy with a prevalence of 2.3% (25). Higher prevalence values were reported in a study performed on 103 dogs from northern, 43 form central and 18 form southern Italy with PCR-positive prevalence of 29.1, 4.7, and 11.1% respectively, but all enrolled dogs in this study had clinicopathological findings compatible with tick-borne diseases (27).
Prevalence of filarial infections reported in this study (2.1%) was not very high. A. reconditum was the most prevalent (1.3%) filarial species and noteworthy was reported for the first time in the Molise region. The zoonotic D. repens was found in 0.8% of the tested dogs. Interestingly, a case of subcutaneous human filariosis caused by D. repens was described by Pampiglione et al. (28) in the arm of a children in Campobasso, one of the two provinces of the Molise region. In the same study the Knott's test was performed also on blood from 135 dogs, over 2 years old, confirming the presence of D. repens (3.0%) in dogs living few km from the clinical study (28). D. immitis was found in this study in 2 dogs (0.3%). For a long time D. immitis was recorded only in the Po River Valley, but more recently this parasite has spread to previously non-endemic areas of central and southern Italy (14, 29, 30). The two positive dogs were autochthonous hunting dogs that had never been abroad. A significantly higher association between hunting dogs and filarial infections was shown, according to literature (31, 32).
In this study, 3 dogs showed co-infections of L. infantum and B. canis canis (0.1%) or A. reconditum (0.3%). No associations were found between intestinal and cardio-pulmonary parasites and vector borne diseases investigated in this study. Recently, Baxiaras et al. (33) showed the severity of clinical signs of leishmaniosis is increased where a co-infection with other vector-borne pathogens is present. In some studies, a predisposition to leishmaniosis in dogs with other vector-borne pathogens has been described (34–36).
In conclusion, the detection of CVBDs in dogs with or without clinical signs (37) reinforces the importance of increasing the veterinary community, owners and public health authorities' awareness of the risk of infection, highlighting the need to plan effective control programs to guarantee the health and welfare of pets, and to enhance the safety of people (38).
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MG, VF, GC, GO, LR, and MPM contribute conception and design of the study. LC, MEM, and NE organized the database. MG, LR, and MPM wrote the manuscript. MEM and MPM performed the statistical analysis and GIS. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Regional Molise Health Authority funded this project. We thank the Regional Molise Health Authority responsible for this project, Dr. Nicola Rossi and Dr. Claudio di Ludovico and their team that collected samples: Dr. Annapaola del Giudice, Dr. Gabriella Giulietti, Dr. Giovanni Di Tella, and Dr. Teodoro Santilli.
References
1. Otranto D, Capelli G, Genchi C. Changing distribution patterns of canine vector borne diseases in Italy, leishmaniosis vs. dirofilariosis. Parasit Vectors. (2009) 2:S2. doi: 10.1186/1756-3305-2-S1-S2
2. Baneth G, Bourdeau P, Bourdoiseau G, Bowman D, Breitschwerdt E, Capelli G, et al. Vector-borne diseases–constant challenge for practicing veterinarians: recommendations from the CVBD World Forum. Parasit Vectors. (2012) 20:55. doi: 10.1186/1756-3305-5-55
3. Maia C, Almeida B, Coimbra M, Fernandes MC, Cristovao JM, Ramos C, et al. Bacterial and protozoal agents of canine vector-borne diseases in the blood of domestic and stray dogs from southern Portugal. Parasit Vectors. (2015) 8:138. doi: 10.1186/s13071-015-0759-8
4. Hofmann M, Hodžić A, Pouliou N, Joachim A. Vector-borne pathogens affecting shelter dogs in eastern Crete, Greece. Parasitol. Res. (2019) 118:1661–6. doi: 10.1007/s00436-019-06284-z
5. Miró G, Montoya A, Roura X, Galvez R, Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: a multicentre study. Parasit Vectors. (2013) 6:117. doi: 10.1186/1756-3305-6-117
6. Mencke N. Future challenges for parasitology: vector control and 'One health' in Europe: the veterinary medicinal view on CVBDs such as tick borreliosis, rickettsiosis and canine leishmaniosis. Vet Parasitol. (2013) 195:256–71. doi: 10.1016/j.vetpar.2013.04.007
7. Mylonakis ME, Koutinas AF, Baneth G, Polizopoulou Z, Fytianou A. Mixed Ehrlichia canis, Hepatozoon canis, and presumptive Anaplasma phagocytophilum infection in a dog. Vet Clin Pathol. (2004) 33:249–51. doi: 10.1111/j.1939-165X.2004.tb00382.x
8. Cortese L, Terrazzano G, Piantedosi D, Sica M, Prisco M, Ruggiero G, et al. Prevalence of anti-platelet antibodies in dogs naturally co-infected by Leishmania infantum and Ehrlichia canis. Vet J. (2001) 188:118–21. doi: 10.1016/j.tvjl.2010.03.015
9. De Tommasi AS, Otranto D, Dantas-Torres F, Capelli G, Breitschwerdt EB, de Cpraris D. Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors. (2013) 15:97. doi: 10.1186/1756-3305-6-97
10. Colwell DD, Dantas-Torres F, Otranto D. Vector-borne parasitic zoonoses: emerging scenarios and new perspectives. Vet Parasitol. (2011) 182:14–21. doi: 10.1016/j.vetpar.2011.07.012
11. Otranto D. Diagnostic challenges and the unwritten stories of dog and cat parasites. Vet Parasitol. (2015) 15:54–61. doi: 10.1016/j.vetpar.2015.06.002
12. Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Graco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: pathogen and vector circulation in a confined environment. Vet Parasitol. (2017) 236:144–51. doi: 10.1016/j.vetpar.2017.01.019
13. Otranto D. Arthropod-borne pathogens of dogs and cats: from pathways and times of transmission to disease control. Vet Parasitol. (2018) 15:68–77. doi: 10.1016/j.vetpar.2017.12.021
14. Otranto D, Dantas-Torres F. Canine and feline vector-borne diseases in Italy: current situation and perspectives. Parasit Vectors. (2010) 3:2. doi: 10.1186/1756-3305-3-2
15. Rinaldi L, Musella V, Biggeri A, Cringoli G. New insights into the application of geographical information systems and remote sensing in veterinary parasitology. Geospat Health. (2006) 1:33–47. doi: 10.4081/gh.2006.279
17. Zanet S, Bassano M, Trisciuoglio A, Taricco I, Ferroglio E. Horses infected by Piroplasms different from Babesia caballi and Theileria equi: species identification and risk factors analysis in Italy. Vet Parasitol. (2017) 236:38–41. doi: 10.1016/j.vetpar.2017.01.003
18. Knott J. A method for making microfilarial surveys on day blood. Trans RSoc Trop Med Hyg. (1939) 33:191–6. doi: 10.1016/S0035-9203(39)90101-X
19. Magnis J, Lorentz S, Guardone L, Grimm F, Magi M, Naucke TJ, et al. Morphometric analyses of canine blood microfilariae isolated by the Knott's test enables Dirofilaria immitis and D. repens species-specific and Acanthocheilonema (syn Dipetalonema) genus-specific diagnosis. Parasit Vectors. (2013) 6:48. doi: 10.1186/1756-3305-6-48
20. Gomez-Barroso D, Herrador Z, San Martin JV, Gherasim A, Aguado M, Romero-Mate A, et al. Spatial distribution and cluster analysis of a leishmaniasis outbreak in the south-western Madrid region, Spain, September 2009 to April 2013. Euro Surveill. (2015) 19:11–20. doi: 10.2807/1560-7917.ES2015.20.7.21037
21. Mollalo A, Mao L, Rashidi P, Galss GE. A GIS-based artificial neural network model for spatial distribution of tuberculosis across the continental United States. Int J Environ Res Public Health. (2019) 16:157. doi: 10.3390/ijerph16010157
22. Foglia Manzillo V, Gizzarelli M, Vitale F, Montagnaro S, Torina A, Sotera S, et al. Serological and entomological survey of canine leishmaniasis in Lampedusa island, Italy. BMC Vet Res. (2018) 14:286. doi: 10.1186/s12917-018-1606-x
23. Ferroglio E, Battisti E, Zanet S, Bolla C, Concialdi E, Triscuoglio A, et al. Epidemiological evaluation of Leishmania infantum zoonotic transmission risk in the recently established endemic area of Northwestern Italy. Zoonoses Public Health. (2018) 65:675–82. doi: 10.1111/zph.12477
24. Veneziano V, Piantedosi D, Ferrari N, Neola B, Santoro M, Pacifico L, et al. Distribution and risk factors associated with Babesia spp. infection in hunting dogs from Southern Italy. Ticks Tick Borne Dis. (2018) 9:1459–63. doi: 10.1016/j.ttbdis.2018.07.005
25. Cassini R, Zanutto S, Frangipane di Regalbono A, Gabrielli S, Calderini P, Moretti A, et al. Canine piroplasmosis in Italy: epidemiological aspects in vertebrate and invertebrate hosts. Vet. Parasitol. (2009) 165:30–5. doi: 10.1016/j.vetpar.2009.06.044
26. Vascellari M, Ravagnan S, Carminato A, Cazzin S, Carli E, Da Rold G, et al. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasit Vectors. (2016) 9:369. doi: 10.1186/s13071-016-1639-6
27. Solano-Gallego L, Trotta M, Carli E, Carcy B, Caldin M, Furlanello T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet Parasitol. (2008) 175:211–21. doi: 10.1016/j.vetpar.2008.07.024
28. Pampiglione S, Villani M, Fioravanti ML, Rivasi F. Human dirofilariasis in southern Italy. Molise Region Pathol. (1995) 87:139–41.
29. Pipia AP, Varcasia A, Tosciri G, Seu S, Manunta ML, Mura MM, et al. New insights onto cardiopulmonary nematodes of dogs in Sardinia, Italy. Parasitol Res. (2014) 113:1505–9. doi: 10.1007/s00436-014-3794-z
30. Del Prete L, Maurelli MP, Pennacchio S, Bosco A, Musella V, Ciuca L, et al. Dirofilaria immitis and Angiostrongylus vasorum: the contemporaneous detection in kennels. BMC Vet Res. (2015) 11:305. doi: 10.1186/s12917-015-0619-y
31. Mortarino M, Musella V, Costa V, Genchi C, Cringoli G, Rinaldi L. GIS modelling for canine dirofilariosis risk assessment in central Italy. Vet Parasitol. (2008) 158:177–82. doi: 10.4081/gh.2008.248
32. Lim S, Irwin PJ, Lee S, Oh M, Ahn K, Myung B, et al. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasit Vectors. (2010) 3:32. doi: 10.1186/1756-3305-3-32
33. Baxarias M, Álvarez-Fernández A, Martínez-Orellana P. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasit Vectors. (2018) 11:135. doi: 10.1186/s13071-018-2724-9
34. Cortese L, Pelagalli A, Piantedosi D, Cestaro A, Di Loia A, Lombardi P., et al. Effects of therapy on haemostasis in dogs infected with Leishmania infantum, Ehrlichia canis, or both combined. Vet Rec. (2009) 164:433–4. doi: 10.1136/vr.164.14.433
35. Cortes S, Vaz Y, Neves R, Maia C, Cardoso L, Ciampino L. Risk factors for canine leishmaniasis in an endemic Mediterranean region. Vet Parasitol. (2012) 189:189–96. doi: 10.1016/j.vetpar.2012.04.028
36. Vhrovec MG, Pantchev N, Failing K, Bauer C, Travers-Martin N, Zahner H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of Leishmaniosis. Parasitol Res. (2017) 116:131–44. doi: 10.1007/s00436-017-5499-6
37. Springer A, Montenegro VM, Schicht S, Globokar Vrohvec M, Pantchev N, Balzer J. Seroprevalence and current infections of canine vector-borne diseases in Costa Rica. Parasit Vectors. (2018) 11:585. doi: 10.1186/s13071-018-3173-1
Keywords: canine vector borne diseases (CVBDs), Leishmania infantum, Dirofilaria immitis, Dirofilaria repens, Acanthocheilonema reconditum, Babesia spp., dogs, Italy
Citation: Gizzarelli M, Foglia Manzillo V, Ciuca L, Morgoglione ME, El Houda Ben Fayala N, Cringoli G, Oliva G, Rinaldi L and Maurelli MP (2019) Simultaneous Detection of Parasitic Vector Borne Diseases: A Robust Cross-Sectional Survey in Hunting, Stray and Sheep Dogs in a Mediterranean Area. Front. Vet. Sci. 6:288. doi: 10.3389/fvets.2019.00288
Received: 10 June 2019; Accepted: 13 August 2019;
Published: 29 August 2019.
Edited by:
Maureen T. Long, University of Florida, United StatesReviewed by:
Carol Geralyn Chitko-McKown, United States Department of Agriculture, United StatesSemmannan Kalaiyarasu, ICAR-National Institute of High Security Animal Diseases (ICAR-NIHSAD), India
Copyright © 2019 Gizzarelli, Foglia Manzillo, Ciuca, Morgoglione, El Houda Ben Fayala, Cringoli, Oliva, Rinaldi and Maurelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Foglia Manzillo, dmFsZW50aW5hLmZvZ2xpYSYjeDAwMDQwO3VuaW5hLml0
 Manuela Gizzarelli
Manuela Gizzarelli Valentina Foglia Manzillo
Valentina Foglia Manzillo Lavinia Ciuca
Lavinia Ciuca Maria Elena Morgoglione
Maria Elena Morgoglione Nour El Houda Ben Fayala1
Nour El Houda Ben Fayala1 Giuseppe Cringoli
Giuseppe Cringoli Gaetano Oliva
Gaetano Oliva Laura Rinaldi
Laura Rinaldi Maria Paola Maurelli
Maria Paola Maurelli