- Cancer Research Program-6, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, India
Human breast cancers (HBCs) are one of the leading causes of global cancer death among women. Domesticated canines are the most affected domestic species with a prevalence rate of breast cancer more than three times in women. While the human cancer patients receive substantial diagnostic and treatment facilities, inadequacy in canine cancer care, calls for greater attention. Fine Needle Aspiration Cytology (FNAC) is comparatively simple, quick, and easily reproducible technique, which aids in pre-surgical diagnosis. In humans, FNAC has a standard protocol, the Robinson's grading system, which has high correlation with the established histological grading system of Scarff Bloom- Richardson. However, Canine Mammary Tumors (CMTs), which are known to be similar to HBCs in biological behavior and gene expressions, still bank on the histopathological methods for diagnostic purposes. This review sheds light on various factors that could be considered for developing a standard FNAC technique for CMT grading and analyzes its future perspectives.
Introduction
In humans, breast cancer is the most common form of cancer that entrains the highest mortality rate in women (1). Diagnosis and prognosis of breast cancers rely on the triple wedge, mammography, clinical and laboratory examination of tumors. In general, histological type, tumor size, lymph node status, nuclear grade, proliferative index, and hormonal status are the key factors, which play a pivotal role in dictating prognosis. The National Institute of Health Consensus Conference on Adjuvant Therapy for Breast Cancers held in Bethesda, Maryland has concluded that all the above parameters are mandatory for the histopathological reports of Human breast cancers (HBCs) (2). In addition to these prominent factors, cytological grading plays a pivotal role in cancer diagnosis and it has also proved to be an important prognostic factor in predicting the metastasis-free and overall survival of the patients (3). Though histological analysis continues to be the gold standard for tumor grading, a pre-surgical cytological grading could be a better strategy as it is simple and easily reproducible. The Conference on the uniform approach to breast fine-needle aspiration biopsy at the National Cancer Institute, Bethesda has also suggested the inclusion of cytological grading of tumors in the histological reports (4).
As the constantly aggravating risk and augmenting deaths caused by HBCs continues to be a grave concern, the past few decades of cancer research has propelled toward the search for a model organism that can mimic and provide a better understanding of the underlying molecular pathology behind HBCs. The strikingly similar nature of Canine Mammary Tumors (CMTs) and HBCs in their biological behavior and molecular characteristics (5) along with the recurrence post-surgery and metastasis to distant organs like lungs and liver, make dogs an ideal model for studies involving HBCs. In canines, mammary tumors account for nearly 50% of the neoplastic cases in female dogs. The mammary tumors are mostly common in aged, intact female dogs, with a probable incidence as much as thrice that of women (6). While the promulgated rate of CMT being 42% across dogs of all breeds (7), about 50–70% of these tumors progress to malignancy (8, 9). This is most likely as a result of delayed diagnosis and poor prognosis of CMTs. The prognosis and treatment of the CMTs rely mainly on tumor staging and grading. Currently, the grading of CMTs are mostly carried out by histopathological analysis of tumor tissues, as there is no established system for Fine Needle Aspiration Cytology (FNAC) based grading.
The FNAC has been widely practiced in canines which uses Fine needle aspirates (FNA) collected via a less-invasive method to differentiate the benign tumors from the malignant ones. However, this doesn't involve any cytological grading. Even though there are numerous reports on the diagnosis of CMTs using cytology as a tool (10–12), their grading utilizing the cytological techniques has not been widely attempted. This review discusses and evaluates the possibility of recommending cytological grading system in canines, taking cues from the histopathological grading of canines and the well-established cytological grading system of human tumors.
Cytological Grading for Human Mammary Carcinomas
In human breast cancer patients, FNAC is the prominent technique used for cytological grading of the breast cancers for pre-surgical diagnosis. FNAC was first used by Hayes Martin and Edward Ellis in 1930. This technique was lying dormant for nearly two decades until researchers from Scandinavian Karolinska University started exploring its utility for diagnosis of palpable breast masses. Until the 1990s, FNAC was predominantly used for the pre-surgical diagnosis of tumors, after which the Core Needle Biopsy (CNB) came into practice (13). In recent years, FNAC has been overshadowed in the developed countries by CNB (1).
Though CNB has the advantage of confirming calcifications seen during ultrasonography, thereby avoiding unnecessary surgical interventions for benign lesions (14), it is cumbersome as its sampling needs local anesthesia and expertise, since advertent sampling might result in either a non-representative sample, the hematomas or infections. However, in many countries, FNAC is still preferred over CNB, as the former is a simple, less invasive, and cost-effective method for breast cancer diagnosis (13).
Apart from differentiating the benign and malignant tumors, FNAC can be used as a powerful tool for cytological tumor grading too. Also, cytological grading has been reported to hold a statistical concordance of 66–76% with the histological grading (15). Thus, cytological grading in HBCs tends to be highly reproducible and at many instances, act as a substitute for histological grading to facilitate diagnosis and prognosis of HBCs. Neoadjuvant therapy based on cytological grading has gained impetus, as it has aided in the appropriate selection of suitable therapeutic strategies (16, 17).
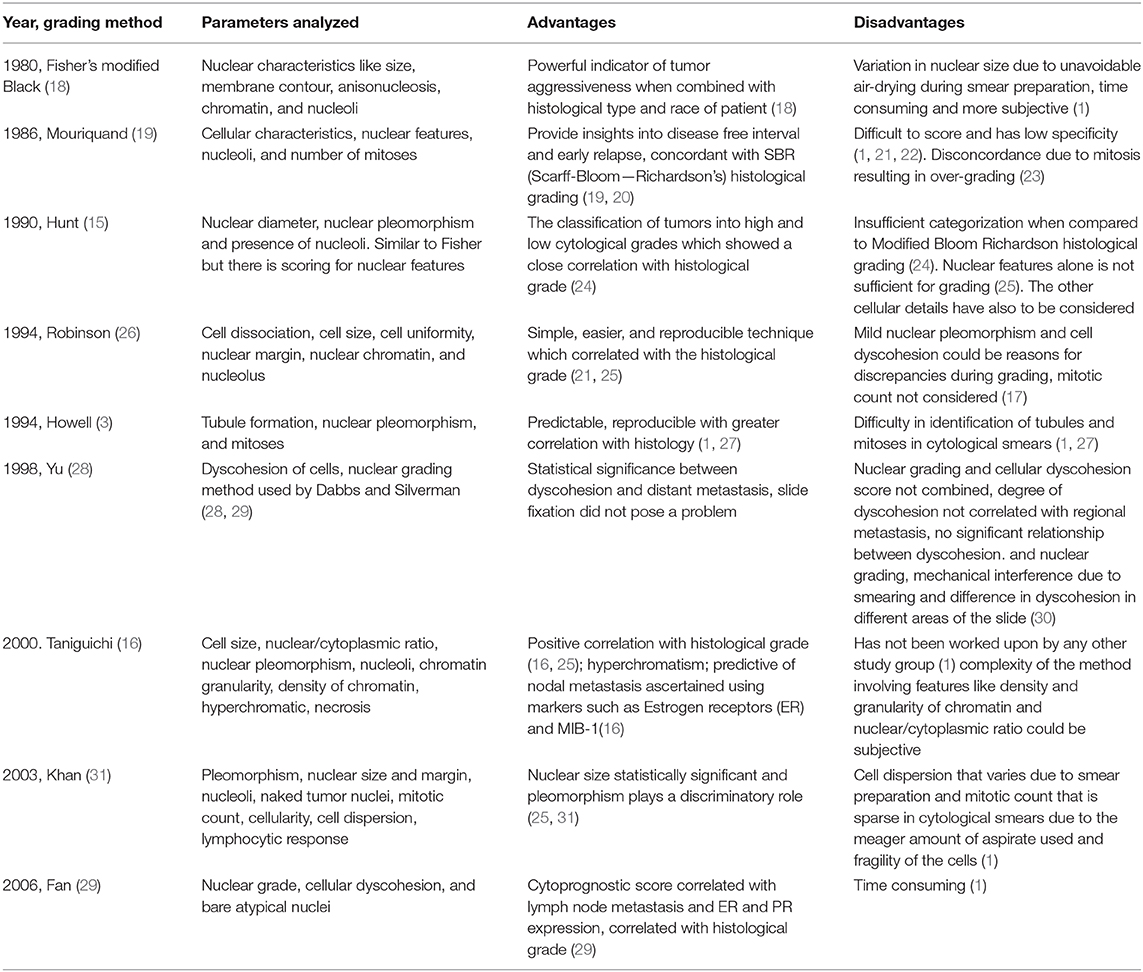
The details about various three-tier cytological grading methods for human breast invasive ductal carcinoma are depicted in Table 1.
Each one of the cytological grading systems in HBCs mentioned in Table 1 has adopted a three-tier scoring; hence, all the parameters are scored at three levels depending on the cytomorphological characteristics. Thus, the final score obtained from the analysis aids in the grading of the tumors represented as Grade 1, 2, and 3.
An example of the widely used Robinson's cytological grading system is depicted in Table 2.
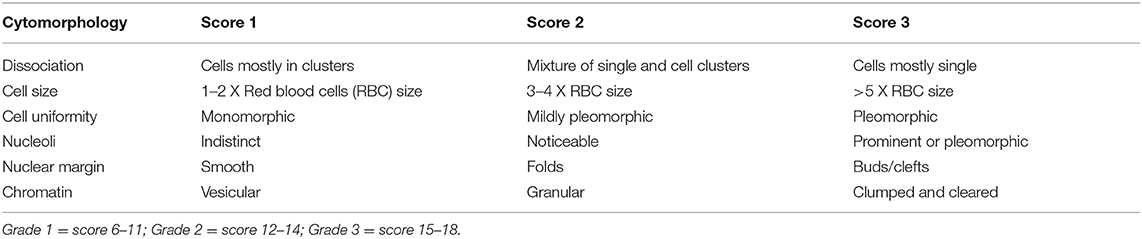
Table 2. Robinson's cytological grading of HBCs (26).
However, there are certain controversies over the application of cytological grading for the predictive analysis. Two schools of thought exist about the prediction of lymph node metastasis utilizing cytological grading, wherein some pathologists claim that there is a possibility of assessing the lymph node metastasis from the cytological grading of the HBCs (32, 33), while the others rule out any such correlation (14, 34).
Mammary Tumors and Cytology in Canines
Mammary tumors in intact female dogs is a very common disease. The CMT reports state that complex carcinomas are the most commonly represented tumor type, followed by simple, solid, mixed, anaplastic carcinomas, and fibrosarcomas (35). The CMTs recorded most frequently are the malignant infiltrating ductal type tumors (36).
FNAC can be used for the pre-surgical evaluation of the CMTs similar to that of HBCs (37, 38). However, it is utilized only for differentiating the benign tumors from the malignant ones, and not for cytological grading. The numerous cytological parameters evaluated for differentiation includes cellularity, variability in the size and shape of nucleus and cytoplasm, nuclear to cytoplasmic ratio, size and number of nucleoli, chromatin clumping and clearing, background components such as mucosecretory material, extracellular matrix, necrotic debris, inflammatory cells, and erythrocytes as opined by National Cancer Institute Fine-Needle Aspiration of Breast Workshop Subcommittees (4). The illustration of the cytological features used to differentiate the tumor types in canines is given in Figure 1.
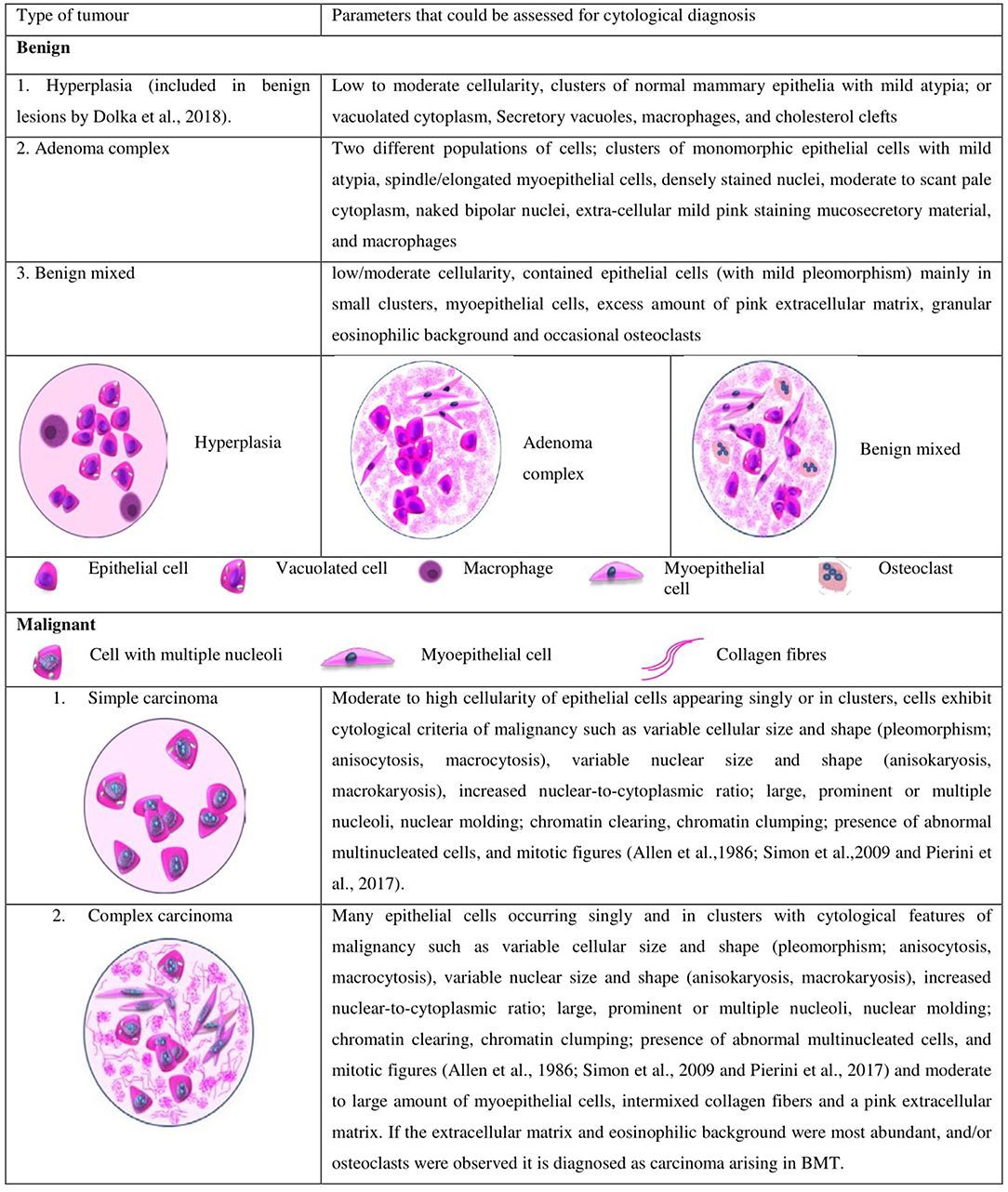
Figure 1. Different types of tumors and the cytological parameters currently used in canines for differentiating malignant and benign tumors.
Cytological Grading of Canine Mammary Tumors: A Technique Less Explored
Cytological grading using FNAC has not been in the limelight as far as CMTs are concerned. Though the use of FNAC for cytological differentiation of CMTs and for analysis of their different cellular origins has been well-established (37, 39–41), there lies a lacuna on its application with regard to cytological grading. The cytological evaluation for the diagnosis of CMTs accounts for a sensitivity of 65–88% and specificity of 94–96% (37, 39) in comparison to the gold standard method of histopathological grading. The cytological grading system which is being utilized for the HBCs can also be applied to the CMTs for their diagnosis and prognosis; however, in spite of having an accuracy of about 88.5% (38), this technique has been less explored for the grading of CMTs.
The Current Cytological Grading System in Canines
The literature review reveals that there are not any reports regarding the cytological grading system in canines using FNAC, except for a single publication (42). The numerous features analyzed in that study and the resultant score card adapted by them is depicted in Table 3.
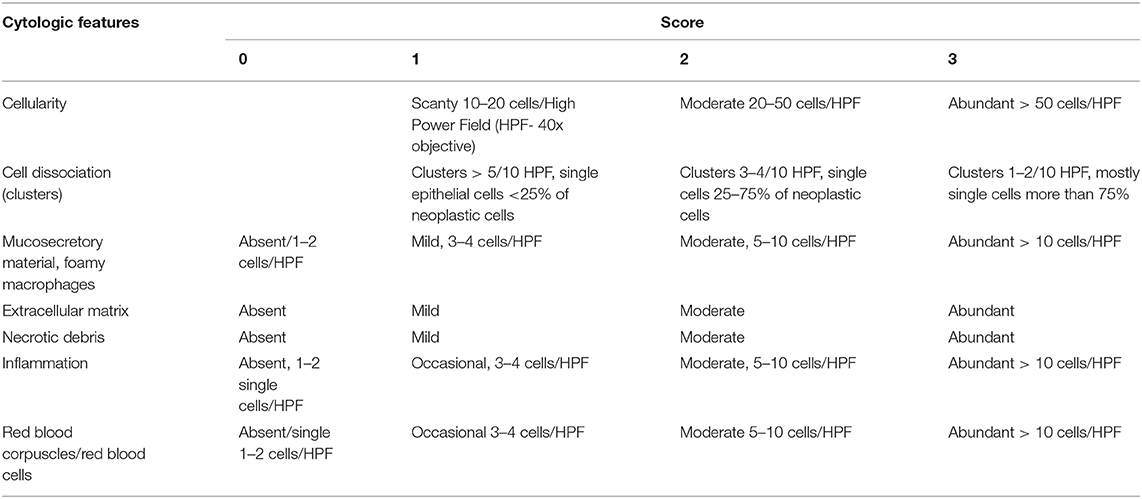
Table 3. Scoring system for the cytological samples of CMT (42).
Their scheme was a modified version of the most acclaimed Robinson's grading system in HBCs. They reported that despite the similarities of CMTs with HBCs, mammary mixed tumors are more common in dogs than humans. This observation necessitates for an appropriate sampling technique and an experienced cytopathologist in aiding the diagnosis of CMTs. Previously, Dolka's group had already observed that cellularity and cytological background did not influence the overall survival period; however, Grade 2 and Grade 3 CMTs had a lower survival period and later begat to cancer associated mortality. Nevertheless, their study failed to prove that the cellular dissociation in cytological specimen could be a predictive factor for nodal metastasis in CMTs, in contrast to the report in HBCs (30). As there is an acute shortage of information regarding cytological grading in CMTs, establishment of a well-elucidated standard protocol requires cumulative effort from researchers all over to characterize and evaluate the grading system for canines.
Discussion
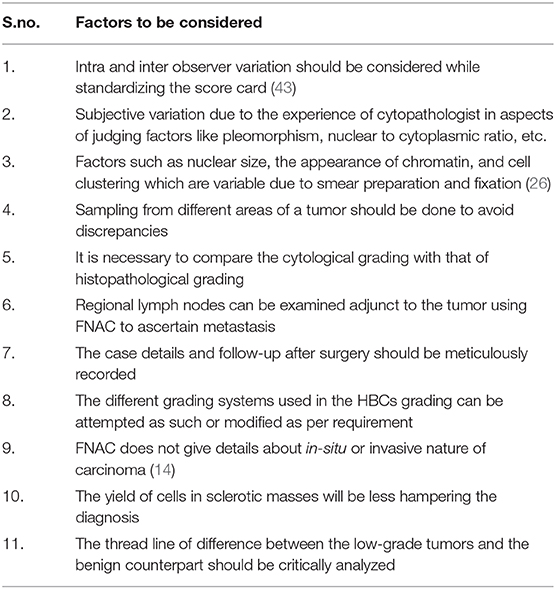
Cytology has gained the status of a tool for pre-operative diagnosis of CMT with satisfactory sensitivity and specificity to differentiate benign and malignant lesions (37, 38). However, there are few impediments to resolve. The practical implications like the heterogenous nature of CMT, extensive necrosis, inflammation, and the challenge posed by mixed and complex tumors (10, 40) are noteworthy. These aspects have always made an ambiguous mark on the cytological diagnosis of CMT with the possibility of false-positives and false-negatives. There are always one or two snags like the cytological diagnosis of in-situ carcinoma, which gives a rather menacing image than real. Hence, cytological diagnosis and grading had setbacks. These issues however, can be addressed to a possible extent with consistent and regular practice by which cytology can get a better status in the realm of diagnosis. A keen methodical strategy that has to be carried out to improvise the cytological grading into an efficient diagnostic tool for CMTs. Several key factors have been enlisted in Table 4 that could be considered while standardizing an efficient protocol for the cytological grading of CMTs. The obstacles arising during the standardization procedures can be tackled by practical expertise and cross-references with the literature on HBCs.
Though, the FNAC based grading systems has its advantages that would play an exquisite role in the diagnosis of CMTs, histopathological grading will continue to hold its eminence in the final diagnosis of the CMTs due to the paucity of work done in this realm (42). Several cytology-based systems which are used in the grading of invasive carcinomas of HBCs can be explored in CMTs in its naïve or modified forms, which would help to elucidate the most appropriate system of cytological grading for CMTs. The cytological grading system for canines as recommended in this review, given in Table 5, is a cumulative convergence of numerous factors discussed in different reports (3, 16, 26, 44).
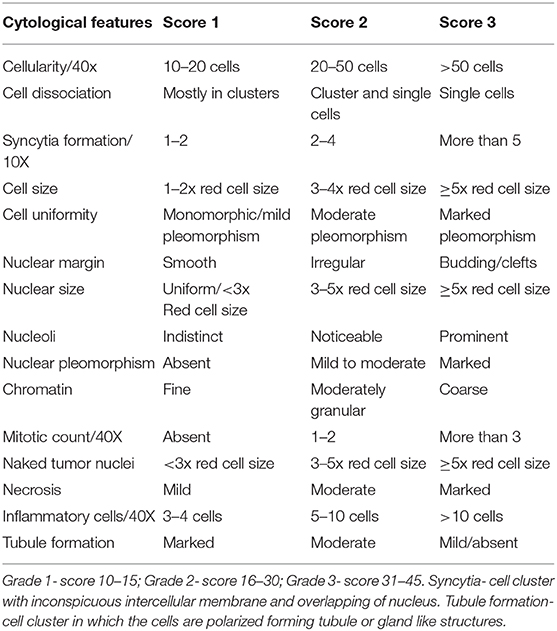
Table 5. Suggested score card for grading canine mammary tumors with inputs from Robinson's, Khan's, Taniguchi's, Howell's grading systems and Bonzanini et al. (44).
An illustration depicting the cells characterizing the numerous cytological grades has been represented in Figure 2, which will aid not only in the pre-surgical diagnosis, but also in the planning of neoadjuvant therapy. Further studies, involving molecular markers along with cytological grading, will help in authenticating the similarities between HBCs and CMTs, thus, making canines the unambiguous models for analyzing HBCs; as it has already been reported and established that canines can act as models for better understanding of HBCs and aiding the cancer research. Canines also develop cancers with an intact immune system similar to that of humans. Factors affecting the disease outcome, including tumor size, stage and lymph node invasion, are synonymous in HBCs and CMTs (5). A noteworthy feature in dogs and humans is the magnitude of genetic parity between them. For example, the breast cancer type I susceptibility gene (BRCA1), a prominent tumor suppressor gene whose mutation predisposes women to hereditary breast-ovarian cancers, has been reported to bear 84% sequence homology in dogs and humans. The higher magnitude of genetic similarity makes the dogs a better comparative genomics model over other species that are currently aiding human disease studies (45). Thereby, current times have seen a surge in canine cancer research as more and more number of clinical trial facilities are upcoming that would involve canine models. The University of Pennsylvania, Philadelphia, is housing a canine cancer clinical trial facility and recently, The Tallwood Canine Cancer Research Initiative at Jackson Laboratory (JAX) has made an initiative to bank and sequence canine tumors. The cytological grading in case of human subjects is well-established and has helped in the pre-surgical evaluation of the patients. The technique is less-invasive and reproducible and aids the diagnosis of the state of differentiation from benign to malignant one, thus serving as a pre-surgical pathological aid (1). An adequate grading system and resultant therapeutic regimes would help the substantial reduction in the financial loss and emotional stress of the pet owners.
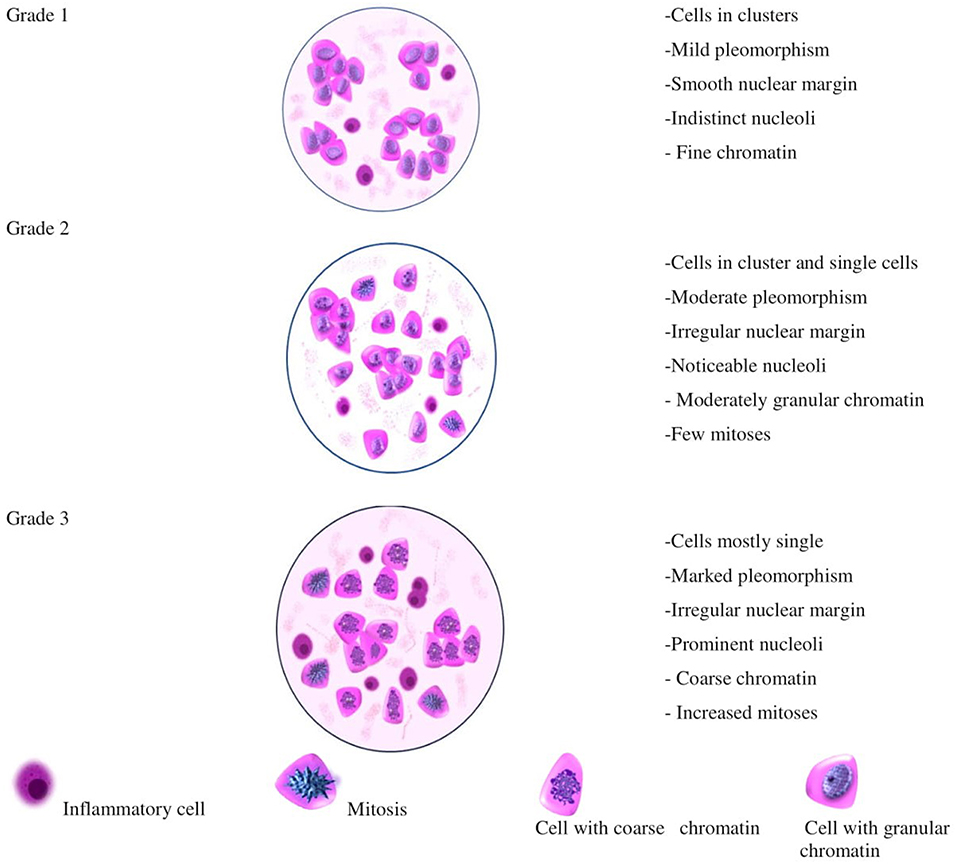
Figure 2. Illustration depicting the appearance of cells that could be considered for different cytological grades in CMT.
This review provides insights into unleashing the possibilities of developing an efficient cytological grading system for CMTs, which would assist veterinary cytopathologists in diagnostic and prognostic purposes and serve our companion animal with the same diagnostic facility as that of the humans.
Author Contributions
KK identified the lacunae and prepared the draft. AR helped in creating the illustrations. AW, RN, and DP helped in editing the review. PS conceived the concept of the review and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Rajiv Gandhi Centre for Biotechnology (intramural grant), University of Kerala, Department of Science and Technology (Inspire Fellowship to AR), University Grants Commission (DP) and Science and Engineering Research Board (No. EMR/2017/002222) for funding. The authors also thank Dr. N. Divakaran Nair, Professor (Retd), Department of Veterinary Pathology, College of Veterinary and Animal Sciences, Mannuthy, Kerala, for critical reading and correcting the manuscript.
References
1. Bansal C, Pujani M, Sharma KL, Srivastava AN, Singh US. Grading systems in the cytological diagnosis of breast cancer: a review. J Cancer Res Ther. (2014) 10:839–45. doi: 10.4103/0973-1482.140979
2. Eifel P, Axelson JA, Costa J, Crowley J, Curran W, Deshler A, et al. National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. (2001) 93:979–89. doi: 10.1093/oxfordjournals.jncimonographs.a003460
3. Howell LP, Gandour-Edwards R, O'Sullivan D. Application of the Scarff-Bloom-Richardson tumor grading system to fine-needle aspirates of the breast. Am J Clin Pathol. (1994) 101:262–5. doi: 10.1093/ajcp/101.3.262
4. (1997). The uniform approach to breast fine-needle aspiration biopsy. National Cancer Institute Fine-Needle Aspiration of Breast Workshop Subcommittees. Diagn Cytopathol. 16:295–311. doi: 10.1002/(SICI)1097-0339(1997)16:4<295::AID-DC1>3.0.CO;2-D
5. Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. (2018) 15:8195–205. doi: 10.3892/ol.2018.8411
6. Owen LN. A comparative study of canine and human breast cancer. Invest Cell Pathol. (1979) 2:257–75.
7. Moe L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl. (2001) 57:439–43.
8. Salas Y, Marquez A, Diaz D, Romero L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002-2012: a growing animal health problem. PLoS ONE. (2015) 10:e0127381. doi: 10.1371/journal.pone.0127381
9. Vascellari M, Capello K, Carminato A, Zanardello C, Baioni E, Mutinelli F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): risk factors and similarities to human breast cancer. Prev Vet Med. (2016) 126:183–9. doi: 10.1016/j.prevetmed.2016.02.008
10. Cassali GD, Gobbi H, Malm C, Schmitt FC. Evaluation of accuracy of fine needle aspiration cytology for diagnosis of canine mammary tumours: comparative features with human tumours. Cytopathology. (2007) 18:191–6. doi: 10.1111/j.1365-2303.2007.00412.x
11. Sontas BH, Yuzbasioglu Ozturk G, Toydemir TF, Arun SS, Ekici H. Fine-needle aspiration biopsy of canine mammary gland tumours: a comparison between cytology and histopathology. Reprod Domest Anim. (2012) 47:125–30. doi: 10.1111/j.1439-0531.2011.01810.x
12. Pierini A, Millanta F, Zanforlin R, Vannozzi I, Marchetti V. Usefulness of cytologic criteria in ultrasound-guided fine-needle aspirates from subcentimeter canine mammary tumors. J Vet Diagn Invest. (2017) 29:869–73. doi: 10.1177/1040638717718886
13. Lukasiewicz E, Ziemiecka A, Jakubowski W, Vojinovic J, Bogucevska M, Dobruch-Sobczak K. Fine-needle versus core-needle biopsy - which one to choose in preoperative assessment of focal lesions in the breasts? Literature review. J Ultrason. (2017) 17:267–74. doi: 10.15557/JoU.2017.0039
14. Denley H, Pinder SE, Elston CW, Lee AH, Ellis IO. Preoperative assessment of prognostic factors in breast cancer. J Clin Pathol. (2001) 54:20–4. doi: 10.1136/jcp.54.1.20
15. Hunt CM, Ellis IO, Elston CW, Locker A, Pearson D, Blamey RW. Cytological grading of breast carcinoma–a feasible proposition? Cytopathology. (1990) 1:287–95. doi: 10.1111/j.1365-2303.1990.tb00362.x
16. Taniguchi E, Yang Q, Tang W, Nakamura Y, Shan L, Nakamura M, et al. Cytologic grading of invasive breast carcinoma. Correlation with clinicopathologic variables and predictive value of nodal metastasis. Acta Cytol. (2000) 44:587–91. doi: 10.1159/000328533
17. Robles-Frias A, Gonzalez-Campora R, Martinez-Parra D, Robles-Frias MJ, Vazquez-Cerezuela T, Otal-Salaverri C, et al. Robinson cytologic grading of invasive ductal breast carcinoma: correlation with histologic grading and regional lymph node metastasis. Acta Cytol. (2005) 49:149–53. doi: 10.1159/000326123
18. Fisher ER, Redmond C, Fisher B. Histologic grading of breast cancer. Pathol Annu. (1980) 15(Pt 1):239–51.
19. Mouriquand J, Gozlan-Fior M, Villemain D, Bouchet Y, Sage JC, Mermet MA, et al. Value of cytoprognostic classification in breast carcinomas. J Clin Pathol. (1986) 39:489–96. doi: 10.1136/jcp.39.5.489
20. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. (1957) 11:359–77. doi: 10.1038/bjc.1957.43
21. Das AK, Kapila K, Dinda AK, Verma K. Comparative evaluation of grading of breast carcinomas in fine needle aspirates by two methods. Indian J Med Res. (2003) 118:247–50.
22. Pandey P, Dixit A, Chandra S, Kaur S. A comparative and evaluative study of two cytological grading systems in breast carcinoma with histological grading: an important prognostic factor. Anal Cell Pathol. (2014) 2014:767215. doi: 10.1155/2014/767215
23. Wani FA, Bhardwaj S, Kumar D, Katoch P. Cytological grading of breast cancers and comparative evaluation of two grading systems. J Cytol. (2010) 27:55–8. doi: 10.4103/0970-9371.70738
24. Rekha TS, Nandini NM, Dhar M. Validity of different cytological grading systems of breast carcinoma–a hospital-based study in South India. Asian Pac J Cancer Prev. (2011) 12:3013–6.
25. Chandanwale S, Mishra N, Kaur S, Paranjape S, Pandey A, Jha M. Comparative analysis of six cytological grading systems in breast carcinoma. Clin Cancer Invest J. (2016) 5:409–15. doi: 10.4103/2278-0513.197858
26. Robinson IA, McKee G, Nicholson A, D'Arcy J, Jackson PA, Cook MG, et al. Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. (1994) 343:947–9. doi: 10.1016/S0140-6736(94)90066-3
27. Walke VA, Gunjkar G. Comparative evaluation of six parametric Robinson and three parametric Howell's modification of Scarf-BloomRichardson grading method on breast aspirates with histopathology: a prospective study. Cytojournal. (2017) 14:31. doi: 10.4103/cytojournal.cytojournal_31_17
28. Yu GH, Cajulis RS, De Frias DV. Tumor cell (dys)cohesion as a prognostic factor in aspirate smears of breast carcinoma. Am J Clin Pathol. (1998) 109:315–9. doi: 10.1093/ajcp/109.3.315
29. Fan F, Namiq AL, Tawfik OW, Thomas PA. Proposed prognostic score for breast carcinoma on fine needle aspiration based on nuclear grade, cellular dyscohesion and bare atypical nuclei. Diagn Cytopathol. (2006) 34:542–6. doi: 10.1002/dc.20529
30. Schiller AB, Tadros TS, Birdsong GG, Grossl NA. Cellular dyscohesion in fine-needle aspiration of breast carcinoma. Prognostic indicator for axillary lymph node metastases? Am J Clin Pathol. (2001) 115:219–23. doi: 10.1309/PR6K-7RXQ-NJUD-443Q
31. Khan MZ, Haleem A, Al Hassani H, Kfoury H. Cytopathological grading, as a predictor of histopathological grade, in ductal carcinoma (NOS) of breast, on air-dried Diff-Quik smears. Diagn Cytopathol. (2003) 29:185–93. doi: 10.1002/dc.10285
32. Vasudev V, Rangaswamy R, Geethamani V. The cytological grading of malignant neoplasms of the breast and its correlation with the histological grading. J Clin Diagn Res. (2013) 7:1035–9. doi: 10.7860/JCDR/2013/5906.3093
33. Pal S, Gupta ML. Correlation between cytological and histological grading of breast cancer and its role in prognosis. J Cytol. (2016) 33:182–6. doi: 10.4103/0970-9371.190449
34. Ravikumar G, Rout P. Comparison of cytological versus histopathological grading of invasive ductal carcinoma of the breast with correlation of lymph node status. Middle East J Cancer. (2015) 6:91–6.
35. Tavasoly A, Golshahi H, Rezaie A, Farhadi M. Classification and grading of canine malignant mammary tumors. Vet Res Forum. (2013) 4:25–30.
36. Al-Mansour MA, Kubba MAG, Al-Azreg SA, Dribika SA. Comparative histopathology and immunohistochemistry of human and canine mammary tumors. Open Vet J. (2018) 8:243–9. doi: 10.4314/ovj.v8i3.3
37. Simon D, Schoenrock D, Nolte I, Baumgartner W, Barron R, Mischke R. Cytologic examination of fine-needle aspirates from mammary gland tumors in the dog: diagnostic accuracy with comparison to histopathology and association with postoperative outcome. Vet Clin Pathol. (2009) 38:521–8. doi: 10.1111/j.1939-165X.2009.00150.x
38. Haziroglu R, Yardimci B, Aslan S, Yildirim MZ, Yumusak N, Beceriklisoy H, et al. Cytological evaluation of canine mammary tumours with fine needle aspiration biopsy technique. Rev Med Vet. (2010) 161:212–8.
39. Hellmen E, Lindgren A. The accuracy of cytology in diagnosis and DNA analysis of canine mammary tumours. J Comp Pathol. (1989) 101:443–50. doi: 10.1016/0021-9975(89)90027-3
40. Allen SW, Prasse KW, Mahaffey EA. Cytologic differentiation of benign from malignant canine mammary tumors. Vet Pathol. (1986) 23:649–55. doi: 10.1177/030098588602300601
41. Simeonov R, Stoikov D. Study on the correlation between the cytological and histological tests in the diagnostics of canine spontaneous mammary neoplams. Bulg J Vet Med. (2006) 9:211–9.
42. Dolka I, Czopowicz M, Gruk-Jurka A, Wojtkowska A, Sapierzynski R, Jurka P. Diagnostic efficacy of smear cytology and Robinson's cytological grading of canine mammary tumors with respect to histopathology, cytomorphometry, metastases and overall survival. PLoS ONE. (2018) 13:e0191595. doi: 10.1371/journal.pone.0191595
43. Moroz K, Lipscomb J, Vial LJ Jr, Dhurandhar N. Cytologic nuclear grade of malignant breast aspirates as a predictor of histologic grade. Light microscopy and image analysis characteristics. Acta Cytol. (1997) 41:1107–11. doi: 10.1159/000332796
44. Bonzanini M, Morelli L, Bonandini EM, Leonardi E, Pertile R, Dalla Palma P. Cytologic features of triple-negative breast carcinoma. Cancer Cytopathol. (2012) 120:401–9. doi: 10.1002/cncy.21207
Keywords: breast, canine, cytology, grading, human, tumor
Citation: Kuppusamy K, Rajan A, Warrier A, Nadhan R, Patra D and Srinivas P (2019) Cytological Grading of Breast Tumors—The Human and Canine Perspective. Front. Vet. Sci. 6:283. doi: 10.3389/fvets.2019.00283
Received: 16 May 2019; Accepted: 08 August 2019;
Published: 27 August 2019.
Edited by:
Alessandra Sfacteria, University of Messina, ItalyReviewed by:
Felisbina Luisa Queiroga, University of Trás-os-Montes and Alto Douro, PortugalFrederique Nguyen, INRA UMR703 Ecole Nationale Vétérinaire, Agroalimentaire et de l'alimentation de Nantes-Atlantique, France
Copyright © 2019 Kuppusamy, Rajan, Warrier, Nadhan, Patra and Srinivas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Priya Srinivas, cHJpeWFzcmluaXZhc0ByZ2NiLnJlcy5pbg==
 Krithiga Kuppusamy
Krithiga Kuppusamy Aarathi Rajan
Aarathi Rajan Priya Srinivas
Priya Srinivas