
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 13 August 2018
Sec. Veterinary Infectious Diseases
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00189
This article is part of the Research Topic Bovine Tuberculosis – International Perspectives on Epidemiology and Management View all 28 articles
 Jose A. Infantes-Lorenzo1,2
Jose A. Infantes-Lorenzo1,2 Claire E. Whitehead3
Claire E. Whitehead3 Inmaculada Moreno4
Inmaculada Moreno4 Javier Bezos1
Javier Bezos1 Alvaro Roy1
Alvaro Roy1 Lucas Domínguez1,5
Lucas Domínguez1,5 Mercedes Domínguez4†
Mercedes Domínguez4† Francisco J. Salguero2*†
Francisco J. Salguero2*†South American camelids are susceptible to tuberculosis, caused mainly by Mycobacterium bovis and M. microti. Despite the tuberculin skin test being the official test for tuberculosis, it has a very low sensitivity in these species (14–20%). Serological tests present the advantages of being rapid, easy to perform and facilitate analysis of large numbers of samples in a short period of time. Novel antigen discovery and evaluation would provide enhanced detection of specific antibodies against members of M. tuberculosis complex. Here, we describe the development and evaluation of an ELISA-type immunoassays to use in the diagnosis of tuberculosis in llamas and alpacas based on P22, a multiprotein complex obtained by affinity chromatography from bovine Purified Protein Derivative (bPPD), that showed high sensitivity and specificity in mice, cattle and goats. This work was performed in two stages. First, a preliminary panel of samples collected from tuberculosis-free (n = 396) and M. bovis-infected herds (n = 56) was assayed, obtaining high specificity (100%) and sensitivity ranging from 63 to 96%. Subsequently, the use of the serological assay was tested using samples from two herds suffering from clinical M. bovis (n = 88) and M. microti (n = 25) infection to evaluate the ability of the ELISA to detect infected animals. 11 out of 88 alpacas were positive to the ELISA in a M. bovis outbreak and 7 out of 25 in a M. microti outbreak. The P22 ELISA potentially provides a sensitive and specific platform for improved tuberculosis surveillance in camelids.
To date, tuberculosis (TB) is one of the most important diseases globally, both in animals and humans (1, 2). Animal TB has a broad range of domestic and wild mammal species hosts, including South American Camelids (SAC) that have become increasingly popular as production animals in recent years. Although llamas and alpacas are gaining more importance in fiber production (3, 4), these animals also have companion animal value and may have regular contact with humans and other susceptible animal species. SACs are a potential source of different pathogens that might be transmitted to humans and could pose a risk to human health (5). Among these diseases, alpacas and llamas are very susceptible to TB, caused by bacteria from the Mycobacterium tuberculosis complex (MTC), mainly by M. bovis and M. microti (6, 7).
The diagnosis of tuberculosis in SAC has been mainly based on the tuberculin skin test, both single and comparative intradermal tuberculin test (SIT and SCIT, respectively), but these show poor performance in general in these species (8, 9, 10) and low sensitivity between 14 and 20%. A sensitivity of only 14% was found in one llama herd outbreak for animals that presented with visible lesions at post-mortem examination within 3 months of the SCIT test (11). In another report, only one llama tested positive out of five that were subsequently found to have visible lesions from which M. bovis was cultured (12). The interferon gamma (IFN-γ) test, based on the stimulation of blood cells with Purified Protein Derivatives (PPDs) and subsequent detection of the IFN-γ released, has been also developed for the diagnosis of TB, but it has been difficult to standardize, is labor-intensive, and in SAC yields a low sensitivity and specificity (63.6 and 89.1%, respectively) (13). In addition, in-house and commercial serological assays for the detection of specific antibodies have been previously investigated with a wide range of results (8, 11–14), but have been tested in a low number of animals.
Serological tests have been able to detect infected animals before the onset of clinical disease (8). In addition, the booster effect on the antibody response caused after injection of tuberculin has been reported as a strategic option to increase the sensitivity of serological assays in some species (15, 16). In general terms, the specificity of the serological assays are moderate to high, ranged from 84.6 to 98%, depending on the study and serological test employed (13, 17, 18). However, they showed low to moderate sensitivity, ranging from 43 to 75%, even using sera samples collected after intradermal PPD injection (7, 13, 17, 18). More details of the serological test evaluated in SAC are provided in Table 1. For these reasons, it is necessary to develop and evaluate new assays in order to provide more sensitive and specific options for the serological diagnosis of TB in SACs.
The aim of the present study was to develop and evaluate a novel ELISA type assay for the detection of specific antibodies of MTC in alpacas and llamas based on P22 multiprotein complex (20), which is affinity-purified from the PPD of M. bovis, and has been shown to provide greater sensitivity in other host species (15, 16). The P22-based ELISA was tested in serum samples from alpacas naturally infected with M. bovis and M. microti from Spain and England and uninfected llamas and alpacas from Peru and England.
This work was performed in two stages: the first one included a preliminary panel of samples collected in TB-free and naturally M. bovis-infected herds to set the optimal cut-off point and calculate specificity and sensitivity of the ELISA; in a second stage, two farms suffering from clinical TB infection under different epidemiological situations were used to validate the test. Handling of the animals, testing and sampling were performed by accredited veterinarians. These were residual samples collected as part of routine surveillance or during breakdown sampling. All samples used in this study were serum samples. The animals used in this study were not experimental animals. All handling and sampling procedures were performed by veterinarians in accordance with the local legislation (Real Decreto 53/2013 in Spain, Ley de Protección y Bienestar animal N° 30407 in Peru, and the The Veterinary Surgeon Act 1966 in England).
The specificity of the serological tests was evaluated in two different TB-free herds of alpacas and llamas located in different regions in Peru (19). The first alpaca herd was located at 4,000 m of altitude in La Libertad (northwest) and the second llama herd was located at approximately 4,200 m of altitude in Puno (southeast). 120 alpacas (104 male and 16 female) and 40 llamas (all female) were tested. Both herds were considered TB-free (based on long history of TB-free infection, absence of compatible lesions and epidemiological investigations). No lesions consistent with TB were observed in any animal in the 5 years prior to the study during slaughterhouse surveillance and no TB outbreak was reported on farms near the herds of the study. In addition, one TB-free herd from southern England was also included. 236 samples were available from adult alpacas at this herd including 93 males and 143 females. The regulatory program for TB surveillance in SAC in England can be found in the Bovine TB Eradication Programme for England (http://apha.defra.gov.uk).
The sensitivity was evaluated using serum samples from animals (n = 56) from a herd located in central Spain where an M. bovis outbreak was detected. The herd was a mix of alpacas of Suri and Huacaya breed. No previous history of TBs was reported before this outbreak. In December 2011, field veterinarians detected clinical signs (anorexia, cachexia, respiratory distress) and/or sudden deaths in three alpacas. Compatible TB-like lesions were observed in the post-mortem examination of one of these alpacas and M. bovis infection was subsequently confirmed by bacterial culture (18). A total of 67 animals were slaughtered and subjected to post-mortem examination within 4 weeks after the ante-mortem tests. Animals with positive M. bovis cultures and/or presence of visible TB-like lesions compatible with TB (n = 56) were included in the study to assess sensitivity. Serum samples for detection of specific antibodies were collected prior to PPD inoculation and 15 days after.
The analysis was carried out in two herds with natural M. bovis or M. microti infection confirmed by the presence of lesions compatible with TB and/or microbiological culture. Herd A consisted of 88 animals of Huacaya breed in England. This farm was selected due to a TB outbreak commencing in November 2016. Two initial clinical cases were disclosed at necropsy with compatible TB lesions and M. bovis was isolated. Subsequently, a whole herd SCIT was performed and one alpaca was culled on the basis of a positive test. This alpaca was found to have lesions at necropsy. Serological testing took place 14 days later using Enferplex and cervid-DPP tests: two animals tested positive on the Enferplex test, were culled but found to have no visible lesions. All animals were skin-tested again 3 months later (using bovine tuberculin only) and also bled for further serological analysis (Enferplex only) 10 days following the skin test. Three animals were found positive on serology and were culled. At necropsy examination, two of these animals had no visible lesions while the third alpaca was found to have atypical lesions, comprising multiple small caseous lesions in a prescapular lymph node.
The herd B outbreak of TB was detected a herd of approximately 80 animals located in England in July 2017. The owner had performed surveillance serological testing (Enferplex) in May and identified a single animal that tested positive. At a retest 1 month later, the animal remained positive and was culled voluntarily on the basis of suspicion of disease. He was found to have lesions in the liver as well as bronchial and hepatic lymph nodes but no lesions in the lungs. At whole herd skin testing (SCIT), three further animals were disclosed and culled, although no visible lesions were found. At serological testing performed after the skin test, six animals were identified as positive on Enferplex and culled. Five of these animals had atypical lesions identified at post-mortem examination while the sixth had typical lesions in the lungs. A seventh alpaca was culled as a dangerous contact and also displayed atypical lesions at necropsy. 25 samples were available for analysis from 22 Suri alpacas (3 males and 19 females), one Huacaya male alpaca and two male llamas. M microti was never successfully cultured from these cases although PCR testing of lesion material was positive for M microti.
An in-house indirect ELISA that detects antibodies against a protein complex named P22, purified by affinity chromatography from bovine PPD [CZ Veterinaria (Porriño, Spain)] was developed. The indirect ELISA was performed as described previously with minor modifications (15). Briefly, plates were coated with P22 (10 μg/ml) and then blocked with 5% skimmed milk powder solution in phosphate buffered saline (PBS). After three washes with PBS plus 0.05%Tween 20 (PBST), sera were added in duplicate at 1:100 dilutions in skimmed milk and incubated for 60 min at 37°C. The optimal dilution of test serum was determined before by evaluating the reactivity of serum diluted from 1:10 to 1:640. 100 μl of detection antibody (Anti-llama IgG-HRP conjugate at 1:4,000 were added and the plates were incubated for 30 min at room temperature (RT). As before, the secondary antibody was titrated from 1:1,000 to 1:8,000 to choose the optimal dilution. The reaction was developed by adding 100 μl of o-phenylenediamine dihydrochloride substrate (FAST OPD, Sigma–Aldrich, St Louise, USA) incubated for 15 min in darkness and RT conditions. After that, the reaction was stopped with 50 μl of H2SO4 (3 N). The optical density (OD) was measured at 492 nm with an ELISA reader.
Negative control serum was obtained from TB-free llama previously described as M. bovis culture negative from TB-free areas and was included in every plate in quadruplicate. Positive controls were obtained from llamas previously described as M. bovis-infected confirmed by the presence of TB compatible lesions and M. bovis positive culture.
In order to reduce the cross-reactivity with non-tuberculous mycobacteria (NTM), a competitive ELISA was included. In this case the serum samples were diluted in skimmed milk supplemented with avian PPD [CZ Veterinaria (Porriño, Spain)] at 150 μg/ml. Only samples that yielded positive results to the indirect ELISA were analyzed by the competitive ELISA.
Sample results were expressed as an ELISA percentage (E%), calculated by the following formula: [sample E% = (mean sample OD/(2 × mean of negative control OD)) × 100]. Specificity was calculated in the TB-free population using the formula [Sp = true negatives/(true negatives + false positives) × 100]. Sensitivity was calculated in the TB-infected population by the formula [Se = true positives/(true positives + false negatives) × 100]. The cut-off value was calculated using a ROC analysis and was defined as the value at which the highest sum of Se plus Sp was obtained (21). Confidence intervals for Se and Sp were calculated using the 95% Wilson's confident interval (Epitools, Ausvet Pty Ltd., Canberra, Australia).
The ROC analysis evidenced the diagnostic value the P22 ELISA in SAC (Figure 1). The cut-off value was defined as the ratio of the mean sample OD to the double of the mean OD of the negative control. The P22 ELISA with a cut-off value set at 100 E% showed the best balance between sensitivity and specificity. Modifying the cut-off value (>100E%<) resulted in either a decreased specificity or a constant sensitivity and a cut-off value of 100 was, therefore, chosen for the P22 ELISA.
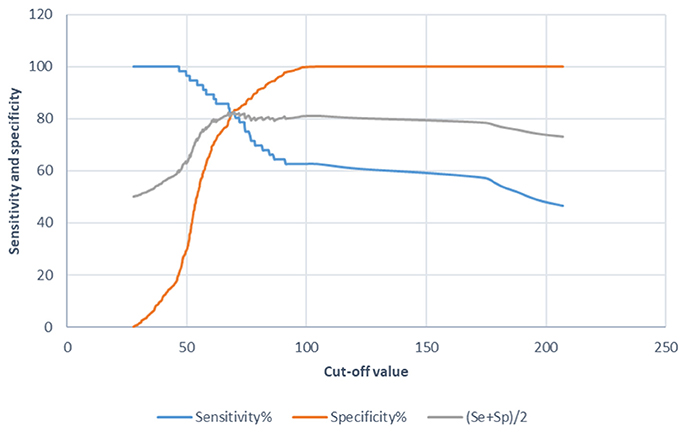
Figure 1. Diagnostic value graphics for the tuberculosis indirect ELISA in SACs when using the P22 as an antigen. Sensitivity (Se), specificity (Sp) and their semi-sum are the percentages on the Y-axis and the cut-off value on the X-axis.
The data including sensitivity, specificity, positive predictive value, negative predictive value and area under the curve (AUC), using confidence intervals of 95% (95% CI) for the ELISA with a chosen cut-off value of 100, are summarized in Table 2. Once the optimal cut-off was calculated, the specificity and sensitivity was studied in greater depth.
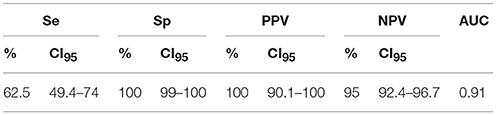
Table 2. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and area under the curve (AUC) with 95% confidence intervals (CI95) in the chosen cut-off value of 100 for P22 indirect ELISA in llamas and alpacas.
Specificity of the P22 ELISA in llama and alpaca herds is shown in Table 3. The 396 animals from TB-free herds were negative to the indirect ELISA. Thus, overall the specificity of P22 indirect ELISA was 100% (95% CI 99–100) in llamas and alpacas. In the absence of any positive animal, the competitive ELISA was not carried out.
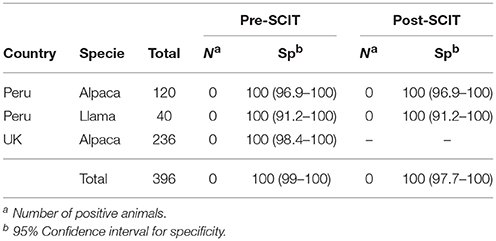
Table 3. Specificity and 95% Wilson's confident interval of the ELISA using serum samples from llama and alpacas taken before and 5 days after the SCIT test.
The sensitivity achieved with P22 indirect ELISA in the samples from Spain was 62.5% (35/56) (95% CI 49.4–74) before PPD inoculation, and 96.4% (54/56) (95% CI 87.9–99) 15 days after PPD inoculation (Table 4). The competitive ELISA showed similar sensitivity.
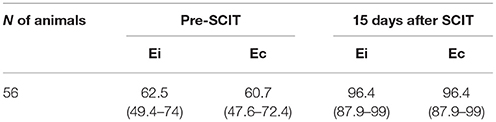
Table 4. Sensitivity and 95% Wilson's confident interval of indirect (Ei) and competitive ELISA (Ec) in TB-infected animals based on post-mortem examination (culture positive and/or presence of visible TB lesions).
In herd A, of the 88 animal analyzed, 11 were positive to indirect ELISA (Figure 2). However, three animals had E% over 150 and the remaining eight animals had values between 100 and 150%. The competitive ELISA showed similar results. The same three animals had over E% 150 again and only seven were between 100 and 150%, one less than using the indirect ELISA. This animal was negative in competitive ELISA and positive in indirect ELISA maybe due to cross reaction by NTM. Considering that four animals had visible lesions at necropsy, three were positive to both the indirect and competitive ELISA.
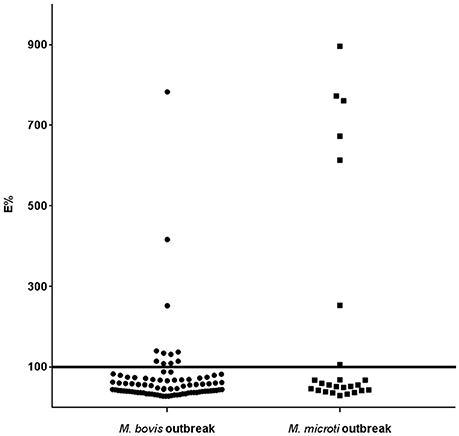
Figure 2. Histogram of the ELISA E% value of individual llama or alpaca tested by indirect ELISA using P22 as antigen in M. bovis and M. microti outbreaks. The horizontal line represents the chosen cut-off value.
In herd B, 25 serum samples were analyzed. Seven animals were positive in both indirect and competitive ELISA. Only one animal showed an E% value close to the cut-off point. The other six animals that had an E% over 300 (Figure 2), had visible lesions at post-mortem examination. Two animals with visible lesions at necropsy were negative to the ELISAs.
Since alpacas became an important animal in Europe and more tools for the diagnosis of TB in alpacas are needed, our results suggest that the indirect P22 ELISAs described here can provide better sensitivity and specificity than other TB antibody detection tests currently used in alpacas and could be used to detect both M. bovis and M. microti infection in SAC. As the indirect ELISA showed a specificity of 100%, and the purpose for the competitive ELISA was to remove antibodies against proteins shared between M. tuberculosis complex and non-tuberculous mycobacteria, the competitive ELISA is not useful in this case to improve the specificity of the diagnosis. Therefore, we focused on the indirect ELISA and propose this ELISA as a new tool for the diagnosis of TB in SACs.
Several serological tests for detection of antibodies against TB described previously showed specificity range from 84 to 98% (13, 17, 19). The P22 ELISA achieved an excellent specificity of 100%, higher than all serological test described up to date for diagnosis of TB in camelids. In addition, no effect of the injection of PPD was observed. The number of animal included in this study was large enough to have a reliable specificity data, including with samples 5 days after PPD injection. However, further studies with samples taken 15 days after the skin test are necessary to confirm this finding because 5 days post-PPD may be insufficient to observe optimal antibody boost.
Regarding sensitivity, our ELISA yielded a moderate average sensitivity of 62.5%, similar to those reported by other serological assays in SACs, which are between 43 and 74% (13, 17, 18). These results are similar to those obtained for TB in bovine using a P22-based ELISA test (15). Using samples obtained 15 days after skin test, the sensitivity of P22 ELISA increased to 96%. This result was higher than reported by all previous serological assays using samples 15 days post-skin test, which sensitivity was between 77 and 89% (18). This boosting effect has been reported in TB in goats, bovines and alpacas (16, 18, 22). Casal et al. (22) demonstrated that sensitivity was significantly higher in cattle using samples collected 15 days post-skin test (ranging from 66.7 to 85.2%). Our results obtained using samples 15 days after injection of PPD were promising and suggested that the P22 ELISA could be a useful TB diagnostic tool in SACs. Taking a blood sample at 15 days post-PPD injection would require an additional veterinary visit, with an associate cost. For this reason, it may not be suitable as a routine method. Despite the costly strategy, the increase of the sensitivity to almost 100% could justify its use in certain situations. The booster effect, including the P22 ELISA, has also been described as a useful approach in cases of explosive TB outbreaks in other species as goats (16).
Humoral response occurs primarily in advances stage of infection and its detection has been considered less effective in early stages of TB infection (23, 24). However, although the skin test is the official diagnostic test for TB in alpacas, SIT test showed poor performances in terms of sensitivity and our results showed a higher sensitivity than SIT. Similar results were obtained previously (11, 13, 18). The combination of the skin test and a serological assay could be an approach to maximize the detection of infected animal instead of IFN-γ because of low sensitivity and difficulties to perform (18). Therefore, implementation of serology in parallel with the skin test could reach sensitivity of 100% (18). Since serology represents a rapid and inexpensive assay, a previous study recommended testing the same samples using several serological assays for a better diagnosis of infected animals (13). In this sense, our P22 ELISA may serve as a preferred technique for the diagnosis of TB, together with other serological assays or skin test. In addition, previous published batches of P22 showed similar qualitative and quantitative composition (20) and, consequently make P22 a stable and reliable product.
TB in SACs is mainly caused by M. bovis and M. microti, and has been reported in several European countries including Spain, the Netherlands, Switzerland, Ireland and the UK (7, 9, 14, 25). The present study has demonstrated the potential of the ELISA in serodiagnosis of TB due to M. bovis and also M. microti. The high OD observed in six M. microti and three M. bovis infected animals suggest a new promising sensitive serological test. Moreover, out of four animals in M. bovis outbreak and eight animals in M. microti outbreak with visible lesions, three and six animals, respectively were positive to the ELISA, showing a good ability to detect animals in advance stages of diseases, which are considered to be the major excretors of bacterias (26, 27). In addition, the low rates of positive results found in the herd A also confirm the high specificity of the assays. Eight and one animals in herds A and B, respectively had an E% close to the cut-off. However, the specificity of the ELISA was 100% and, for this reason, the cross-reaction with other proteins in P22 shared with environmental mycobacteria was discarded. The level of antibodies in these animals was low and consequently the OD in the ELISA was also low.
In conclusion, the new multiprotein complex named P22 could be an alternative antigen for the detection of specific M. tuberculosis complex antibodies in SAC. Moreover, the P22-based indirect ELISA can be used as a cost effective, rapid and reliable tool for the large-scale screening and therefore, support the detection and management of tuberculosis in llamas and alpacas.
CW, JB, AR, and LD obtained the serum samples from the animals. JI-L, CW, IM, MD, and FS performed the laboratory techniques. JI-L, CW, MD, and FS wrote the manuscript that was edited, discussed and reviewed and accepted by all authors.
JI-L was supported by an FPU contract-fellowship (Formación de Profesorado Universitario) from the Ministerio de Educación, Cultura y Deporte of the Spanish Government (FPU2013/6000). AR is the recipient of an Industrial Doctorate contract (DI-15-08110) funded by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO) and the European Social Fund. This work was funded by the University of Surrey Innovation Voucher Scheme 2017-18.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors would like to thank Ana Belén Martinez and Soledad Crespo for their technical assistance and María Luisa de la Cruz for her support in statistical analysis.
1. Schiller I, Waters WR, Vordermeier HM, Jemmi T, Welsh M, Keck N, et al. Bovine tuberculosis in Europe from the perspective of an officially tuberculosis free country: trade, surveillance and diagnostics. Vet Microbiol. (2011) 151:153–9. doi: 10.1016/j.vetmic.2011.02.039
2. Pesciaroli M, Alvarez J, Boniotti MB, Cagiola M, Di Marco V, Marianelli C, et al. Tuberculosis in domestic animal species. Res Vet Sci. (2014) 97:S78–85. doi: 10.1016/j.rvsc.2014.05.015
3. Barlow AM, Mitchell KA, Visram KH. Bovine tuberculosis in llama (Lama glama) in the UK. Vet Rec. (1999) 145:639–40.
4. D'Alterio GL, Knowles TG, Eknaes EI, Loevland IE, Foster AP. Postal survey of the population of South American camelids in the United Kingdom in 2000/01. Vet Rec. (2006) 158:86–90. doi: 10.1136/vr.158.3.86
5. Halsby K, Twomey DF, Featherstone C, Foster A, Walsh A, Hewitt K, et al. Zoonotic diseases in South American camelids in England and Wales. Epidemiol Infect. (2017) 145:1037–43. doi: 10.1017/S0950268816003101
6. Twomey DF, Crawshaw TR, Anscombe JE, Farrant L, Evans LJ, McElligott WS, et al. TB in llamas caused by Mycobacterium bovis. Vet Rec. (2007) 160:170. doi: 10.1136/vr.160.5.170
7. Zanolari P, Robert N, Lyashchenko KP, Pfyffer GE, Greenwald R, Esfandiari J, et al. Tuberculosis caused by Mycobacterium microti in South American camelids. J Vet Intern Med. (2009) 23:1266–72. doi: 10.1111/j.1939-1676.2009.0377.x
8. Lyashchenko KP, Greenwald R, Esfandiari J, Meylan M, Burri IH, Zanolari P. Antibody responses in New World camelids with tuberculosis caused by Mycobacterium microti. Vet Microbiol. (2007) 125:265–73. doi: 10.1016/j.vetmic.2007.05.026
9. Ryan E, Dwyer P, Connolly D, Fagan J, Costello E, More S. Tuberculosis in alpaca (Lama pacos) on a farm in Ireland. 1. A clinical report. Ir Vet J. (2008) 61:527–31. doi: 10.1186/2046-0481-61-8-527
10. Garcia-Bocanegra I, Barranco I, Rodriguez-Gomez IM, Perez B, Gomez-Laguna J, Rodriguez S, et al. Tuberculosis in alpacas (Lama pacos) caused by Mycobacterium bovis. J Clin Microbiol. (2010) 48:1960–4. doi: 10.1128/JCM.02518-09
11. Dean GS, Crawshaw TR, de la Rua-Domenech R, Farrant L, Greenwald R, Higgins RJ, et al. Use of serological techniques for diagnosis of Mycobacterium bovis infection in a llama herd. Vet Rec. (2009) 165:323–4. doi: 10.1136/vr.165.11.323
12. Twomey DF, Collins R, Cranwell MP, Crawshaw TR, Higgins RJ, Dean GS, et al. Controlling tuberculosis in a llama (Lama glama) herd using clinical signs, tuberculin skin testing and serology. Vet J. (2012) 192:246–8. doi: 10.1016/j.tvjl.2011.05.014
13. Rhodes S, Holder T, Clifford D, Dexter I, Brewer J, Smith N, et al. Evaluation of gamma interferon and antibody tuberculosis tests in alpacas. Clin Vaccine Immunol. (2012) 19:1677–83. doi: 10.1128/CVI.00405-12
14. Alvarez J, Bezos J, Juan L, Vordermeier M, Rodriguez S, Fernandez-de-Mera IG, et al. Diagnosis of tuberculosis in camelids: old problems, current solutions and future challenges. Transbound Emerg Dis. (2012) 59:1–10. doi: 10.1111/j.1865-1682.2011.01233.x
15. Casal C, Infantes JA, Risalde MA, Diez-Guerrier A, Dominguez M, Moreno I, et al. Antibody detection tests improve the sensitivity of tuberculosis diagnosis in cattle. Res Vet Sci. (2017) 112:214–21. doi: 10.1016/j.rvsc.2017.05.012
16. Bezos J, Roy A, Infantes-Lorenzo JA, Gonzalez I, Venteo A, Romero B, et al. The use of serological tests in combination with the intradermal tuberculin test maximizes the detection of tuberculosis infected goats. Vet Immunol Immunopathol. (2018) 199:43–52. doi: 10.1016/j.vetimm.2018.03.006
17. Lyashchenko KP, Greenwald R, Esfandiari J, Rhodes S, Dean G, de la Rua-Domenech R, et al. Diagnostic value of animal-side antibody assays for rapid detection of Mycobacterium bovis or Mycobacterium microti infection in South American camelids. Clin Vaccine Immunol. (2011) 18:2143–47. doi: 10.1128/CVI.05386-11
18. Bezos J, Casal C, Alvarez J, Diez-Guerrier A, Rodriguez-Bertos A, Romero B, et al. Evaluation of the performance of cellular and serological diagnostic tests for the diagnosis of tuberculosis in an alpaca (Vicugna pacos) herd naturally infected with Mycobacterium bovis. Prev Vet Med. (2013) 111:304–13. doi: 10.1016/j.prevetmed.2013.05.013
19. Bezos J, Romero B, Delgado A, Alvarez J, Casal C, Venteo A, et al. Evaluation of the specificity of intradermal tuberculin and serological tests for diagnosis of tuberculosis in alpaca (Vicugna pacos) and llama (Lama glama) herds under field conditions in Peru. Vet Rec. (2014) 174:532. doi: 10.1136/vr.102463
20. Infantes-Lorenzo JA, Moreno I, Risalde MLA, Roy A, Villar M, Romero B, et al. Proteomic characterisation of bovine and avian purified protein derivatives and identification of specific antigens for serodiagnosis of bovine tuberculosis. Clin Proteomics (2017) 14:36. doi: 10.1186/s12014-017-9171-z
21. Aurtenetxe O, Barral M, Vicente J, de la Fuente J, Gortazar C, Juste RA. Development and validation of an enzyme-linked immunosorbent assay for antibodies against Mycobacterium bovis in European wild boar. BMC Vet Res. (2008) 4:43. doi: 10.1186/1746-6148-4-43
22. Casal C, Diez-Guerrier A, Alvarez J, Rodriguez-Campos S, Mateos A, Linscott R, et al. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol. (2014) 170:342–51. doi: 10.1016/j.vetmic.2014.02.036
23. Pollock JM, Neill SD. Mycobacterium bovis infection and tuberculosis in cattle. Vet J. (2002) 163:115–27. doi: 10.1053/tvjl.2001.0655
24. Welsh MD, Cunningham RT, Corbett DM, Girvin RM, McNair J, Skuce RA, et al. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology (2005) 114:101–11. doi: 10.1111/j.1365-2567.2004.02003.x
25. Twomey DF, Crawshaw TR, Anscombe JE, Barnett JE, Farrant L, Evans LJ, et al. Assessment of antemortem tests used in the control of an outbreak of tuberculosis in llamas (Lama glama). Vet Rec. (2010) 167:475–80. doi: 10.1136/vr.c4192
26. Waters WR, Maggioli MF, McGill JL, Lyashchenko KP, Palmer MV. Relevance of bovine tuberculosis research to the understanding of human disease: historical perspectives, approaches, and immunologic mechanisms. Vet Immunol Immunopathol. (2014) 159:113–32. doi: 10.1016/j.vetimm.2014.02.009
Keywords: South American camelids, diagnosis, ELISA, P22, tuberculosis
Citation: Infantes-Lorenzo JA, Whitehead CE, Moreno I, Bezos J, Roy A, Domínguez L, Domínguez M and Salguero FJ (2018) Development and Evaluation of a Serological Assay for the Diagnosis of Tuberculosis in Alpacas and Llamas. Front. Vet. Sci. 5:189. doi: 10.3389/fvets.2018.00189
Received: 07 May 2018; Accepted: 24 July 2018;
Published: 13 August 2018.
Edited by:
Daniel J. O'Brien, Michigan Department of Natural Resources, United StatesReviewed by:
Konstantin Lyashchenko, Chembio, United StatesCopyright © 2018 Infantes-Lorenzo, Whitehead, Moreno, Bezos, Roy, Domínguez, Domínguez and Salguero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco J. Salguero, Zi5zYWxndWVyb2JvZGVzQHN1cnJleS5hYy51aw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.