- 1Institute of Milk Hygiene, University of Veterinary Medicine Vienna, Vienna, Austria
- 2Bioactive Microbial Metabolites (BiMM), Bio-Resources & Technologies Tulln, Tulln, Austria
- 3Veterinary Health Service Laboratory, Ried im Innkreis, Austria
Bovine mastitis is a worldwide disease of dairy cattle associated with significant economic losses for the dairy industry. One of the most common pathogens responsible for mastitis is Staphylococcus (S.) aureus. Due to the development and spreading of antibiotic resistance, the search for novel antimicrobial substances against S. aureus is of great importance. The aim of this study was to evaluate two dihydroxybenzaldehydes for the prevention of bovine mastitis. Therefore we determined the minimal inhibitory concentration (MICs) of gentisaldehyde (2,5-dihydroxybenzaldehyde) and 2,3-dihydroxybenzaldehyde of a diverse set of 172 bovine mastitis S. aureus isolates using an automated robot-based microdilution method. To characterize the bovine isolates we determined the genotype by spa-typing, the antimicrobial resistance to eight antibiotic classes using the disk diffusion method and the MICs of three commonly used antiseptics (benzalkonium chloride, chlorhexidine, and iodine). Further we investigated the cytotoxicity of gentisaldehyde and 2,3-dihydroxybenzaldehyde in bovine mammary epithelial MAC-T cells using the XTT assay. The S. aureus strains showed a high genetic diversity with 52 different spa-types, including five novel types. Antibiotic susceptibility testing revealed that 24% of isolates were resistant to one antimicrobial agent and 3% of isolates were multi-resistant. The occurrence of antibiotic resistance strongly correlated with the spa-type. Both dihydroxybenzaldehydes showed antimicrobial activities with a MIC50 of 500 mg/L. The MIC of gentisaldehyde significantly correlated with that of 2,3-dihydroxybenzaldehyde, whereas no correlation was observed with the MIC of the three antiseptics. Cytotoxicity testing using bovine mammary epithelial MAC-T cells revealed that gentisaldehyde and 2,3-dihydroxybenzaldehyde show low toxicity at MIC50 and MIC90 concentrations. In conclusion, gentisaldehyde and 2,3-dihydroxybenzaldehyde exhibited antimicrobial activities against a diverse range of bovine mastitis S. aureus strains at low-cytotoxic concentrations. Therefore, both compounds are potential candidates as antiseptics to prevent bovine mastitis and to reduce the use of antibiotics in dairy cows.
Introduction
Mastitis is an inflammatory disease of the mammary gland that is usually caused by bacterial infection (1). Bovine mastitis is today a worldwide problem in dairy cows leading to significant economic losses due to decreased milk yield. Although the lethality rate of cows suffering from mastitis is low, they have a higher risk for premature culling (2). Additionally, in the case of severe clinical mastitis, animals experience pain and discomfort leading to depressed appearance, weight loss, and abnormal postures (3). The most common mastitis pathogens include Gram-negative bacteria such as Escherichia coli, Pseudomonas spp. and Klebsiella spp. and the Gram-positive species Staphylococcus (S.) aureus, Streptococcus agalactiae, Streptococcus dysgalactiae, and Streptococcus uberis (1). S. aureus is known to be the primary agent responsible for bovine mastitis, including clinical as well as subclinical mastitis. Its special abilities to form biofilms or hide within host phagocytes and epithelial cells of the mammary gland might account for its ability to evade antibiotics, leading to prolonged therapy or even persistence (4).
Antimicrobial drug classes commonly used in mastitis therapy are beta-lactams (including the penicillins and cephalosporins), aminoglycosides, lincosamides, tetracyclines, macrolides, quinolones, polypeptide antibiotics, and combinations of trimethoprim and sulfonamides (5, 6). The first choice antimicrobial agent for the treatment of S. aureus mastitis is penicillin G and in the case of beta-lactamase-producing and therefore penicillin-resistant strains, macrolides such as tylosin or isoxazolylpenicillins such as cloxacillin (5, 6). The growing prevalence of antibiotic resistant bacteria leading to treatment failures is a major health problem (7, 8). Therefore there is an increasing need for alternative methods such as hygiene optimization, the improvement of animal health using feed additives and the use of vaccines to manage infectious diseases in livestock (7). On dairy farms proper disinfection of milking equipment before and after the milking procedure remains crucial to avoid the spreading of mastitis pathogens from cow to cow. Such biocides must have broad-spectra activity against bacteria, but they should not adversely affect the milking equipment (9). Examples of antimicrobial agents used for these purposes include iodine-based products, which are most often used as an antiseptic for teat disinfection (10), but also various products containing phenolics, alcohol or chlorhexidine as germicides (11). However, it has been reported that in addition to antibiotic resistance, bacteria are less susceptible to commonly used antiseptics, e.g., chlorhexidine, benzalkonium chloride or povidone-iodine (12). Additionally, a high prevalence of genes responsible for benzalkonium chloride- and chlorhexidine-resistances has also been discovered in human S. aureus isolates (13). Thus, novel antimicrobial substances are urgently required (8). Historically natural sources such as soil microorganisms (bacteria, fungi) have been of special importance for the development of novel antimicrobials (14–17). However in recent years an increasing number of studies have been reporting the discovery of plant-derived antimicrobials (18). Recently benzaldehydes, isolated from essential oils from plants (e.g., almond oil), have shown antimicrobial activity against bacterial pathogens (16, 19–21). From all the tested benzaldehydesthe phenolic derivatives 2,3-dihydroxybenzaldehyde and 2,5-dihydroxybenzaldehyde (gentisaldehyde) have shown the highest antimicrobial activities (16).
In this study we investigated the antimicrobial activities of these two phenolic benzaldehydes using 172 bovine mastitis S. aureus isolates. These strains were characterized by spa-typing, antibiotic susceptibility testing and determination of minimum inhibitory concentrations (MICs) for three commonly used antiseptics (benzalkonium chloride, chlorhexidine and iodine solution). In addition to MIC determinations, we investigated the cytotoxicity of these phenolic benzaldehydes using bovine mammary epithelial MAC-T cells to evaluate their potential for use in the prevention of bovine mastitis.
Materials and Methods
S. aureus Isolates and Cultivation
S. aureus strains (n = 172) from quarter milk samples of Austrian dairy cows with clinical or subclinical mastitis were isolated by the Upper Austrian Veterinary Health Service laboratory in Ried im Innkreis, Austria. Bacterial cultures were stored using cryogenic vials (Cryobank, CRYO/M, MAST GROUP Ltd., Bootle, United Kingdom). Staphylococcus aureus subsp. aureus ATCC® 29213™ (LGC Standards GmbH - Germany Office, Wesel, Germany) was used as a reference strain.
Bacteria were cultivated overnight on COS agar (Columbia-Agar supplemented with 5% sheep blood, Biomerieux, Marcy-l'Étoile, France) at 37°C. One colony was selected for all further investigations.
Species Identification and Genotyping (spa-Typing)
DNA isolation was performed using the Nucleospin® DNA-Extraction Kit (NucleoSpin® Tissue, Macherey-Nagel, Düren, Germany), according to the manufacturer's instructions. For species confirmation we used a modified species-specific PCR based on amplification of the nuc-gene, according to Brakstad et al. (22). The final reaction volume of 25 μl contained the following: 0.2 pmol/μl of each primer, 1X PCR buffer, 1.5 mM MgCl2, 2 U Platinum Taq DNA polymerase (Invitrogen™ Platinum™ Taq DNA Polymerase (kit), Carlsbad, USA), 0.2 mM dNTP-mix (Thermo Fisher Scientific, Waltham, USA), 5 μl of isolated genomic DNA and DEPC-treated water (Thermo Fisher Scientific). PCR cycling conditions were as follows: initial denaturation for 2 min at 94°C; followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s and elongation at 72°C for 30 s; and final elongation at 72°C for 3.5 min. A negative (no DNA added) and a positive control (genomic DNA of S. aureus ATCC® 29213™) were included in each experiment. To detect genotype diversity, spa-typing was performed using the primers spa-1113F and spa-1514R (23). The final reaction volume of 50 μl included 0.18 pmol/μl of each primer, 1X PCR buffer, 3 mM MgCl2, 1.5 U Platinum Taq DNA polymerase, 0.4 mM dNTP-mix, 2 μl of isolated genomic DNA and DEPC-treated water. PCR cycling conditions were as follows: initial denaturation for 15 min at 94°C; followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s and elongation at 72°C for 1 min; and a final elongation at 72°C for 10 min. Negative and positive controls were included similar to the nuc-PCR. The PCR products were sequenced (LGC Genomics GmbH, Berlin, Germany; Sanger sequencing) and spa-types determined using the STspaTYPER website1 The sequence of novel spa-types was submitted to the RidomSpaServer2 for registration.
Antibiotic Susceptibilities
Antibiotic susceptibilities to eight antimicrobial agents (benzylpenicillin, cefoxitin, clindamycin, erythromycin, gentamicin, norfloxacin, tetracycline, and trimethoprim/sulfamethoxazole; Oxoid™ antibiotic discs, Thermo Fisher Scientific) belonging to eight different antimicrobial classes were determined using the disk diffusion method, according to EUCAST (24, 25). Antibiotic multi-resistance was defined as resistance to three or more antibiotic classes.
MIC Determinations
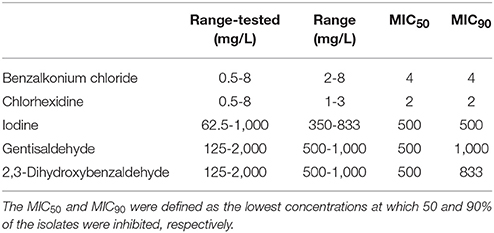
For MIC determination we used benzalkonium chloride (alkyl distribution from C8H17 to C16H33, Acros Organics, Geel, Belgium), chlorhexidine diacetate hydrate (Acros Organics), Lugol's solution [containing 5% (w/w) iodine and 10% (w/w) potassium iodide (both Merck, Darmstadt, Germany)], gentisaldehyde (2,5-dihydroxybenzaldehyde, Sigma-Aldrich, St. Louis, USA) and 2,3-dihydroxybenzaldehyde (Acros Organics). Stock solutions were freshly prepared in double-distilled water and sterile filtered using a 0.2 μm syringe filter before each experiment at the following concentrations: 3.2 × 104 mg/L for benzalkonium chloride, 1.6 × 104 mg/L for chlorhexidine, 5 × 104 mg/L for iodine, 2 × 104 mg/L for gentisaldehyde, and 5 × 103 mg/L for 2,3-dihydroxybenzaldehyde. For MIC determination we used the following concentrations: 0.5, 1, 2, 4, and 8 mg/L for benzalkonium chloride and chlorhexidine; 62.5, 125, 250, 500, and 1,000 mg/L for iodine and 125, 250, 500, 1,000, and 2,000 mg/L for gentisaldehyde and 2,3-dihydroxybenzaldehyde.
For the MIC determination we used an automated robot-based microdilution method using the MicroLab Star Let pipetting robot (Hamilton Robotics, Reno, USA) and the automated Cytomat system, which included the Cytomat (Thermo Fisher Scientific), the Rack Runner (Hamilton Robotics) and the BioTek Reader (Synergy H1 Microplate Reader, BioTek Instruments, Inc., Winooski, USA).
Bacteria were initially cultivated in 1 ml of TSB (Tryptone Soya Broth, OXOID LTD., Basingstoke, England) for 6 h at 37°C. Afterwards 20 μl of bacterial culture was spotted onto tryptone soya agar (TSA, Trypto-Casein Soy Agar, Biokar Diagnostics, Beauvais, France) supplemented with 0.6% yeast extract (Biokar Diagnostics) prepared in a 96-well plate and incubated overnight at 37°C. Thereafter bacteria were inoculated in 200 μl TSB and incubated at 37°C for 8 h (stationary growth phase). Twenty microliter of bacterial culture was then inoculated into 1,380 μl of Mueller-Hinton broth (OXOID LTD, deep well plate) and incubated at 37°C for 18 h (stationary growth phase).The 96-well microtiter plates were prefilled with 100 μl Mueller-Hinton broth containing the antimicrobial compound, 80 μl Mueller-Hinton broth and 20 μl of the liquid bacterial cultures per well using the MicroLab Star Let pipetting robot. Each plate included eight wells of Mueller-Hinton broth with the antimicrobial compound at the specific concentration without inoculum. Additionally, bacteria were grown in Mueller-Hinton broth without any antimicrobial compound. Plates were sealed with sterile tape (NUNC, Thermo Fisher Scientific) and incubated in the automated Cytomat system at 37°C for 24 h. Absorbance was determined at 600 nm at 1 h intervals for 24 h. In total, three biological replicates were performed. The minimal concentration at which bacterial growth was inhibited for 24 h was defined as the MIC. The mean MIC was calculated from three biological replicates using Microsoft Excel. The MIC50 and MIC90 values were defined as the lowest concentration of the agents at which 50 and 90% of the isolates were inhibited, respectively.
Cytotoxicity
The cytotoxicity of gentisaldehyde and 2,3-dihydroxybenzaldehyde was investigated by determining the percentage of metabolically active cells using the XTT assay. The XTT assay is based on the activity of the mitochondrial dehydrogenase enzyme at reducing XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] to an azure formazan. The quantity of formazan produced is proportional to the number of living cells and can be measured optically at 450 nm (26–28). Bovine mammary epithelial MAC-T cells were cultivated in Minimum Essential Medium with Earle's Balanced Salts (MEM/EBSS, GE Healthcare Life Sciences, HyClone Laboratories, South Logan, Utah, USA) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific), penicillin G (100 U/ml; AppliChem GmbH, Darmstadt, Germany), streptomycin sulfate (100 μg/ml, Fisher BioReagents™, Thermo Fisher Scientific), 5 μg/ml insulin (10 mg/mL in 25 mM HEPES, pH 8.2; Sigma-Aldrich), and 5 μg/ml hydrocortisone (Sigma-Aldrich) at 37°C in a humidified atmosphere (95% relative humidity) containing 5% CO2. For the cytotoxicity assay, 3 × 104 cells per well were seeded in a 96-well microtiter plate and incubated overnight at 37°C. Cells were incubated for 24 h at 37°C in 200 μl MEM/EBSS with gentisaldehyde and 2,3-dihydroxybenzaldehyde at the following concentrations: 125, 250, 500, 1,000, and 2,000 mg/L. Cells incubated in media alone were used as a control and defined as 100% metabolically active cells. The cell supernatant was removed and cells washed four times with 100 μl Dulbecco's Phosphate-Buffered Saline (Gibco™, Thermo Fisher Scientific). Cells were incubated for 2 h at 37°C in 100 μl of MEM/EBBS and 25 μl 1 mg/ml XTT (Molecular Probes™ XTT, Invitrogen) solution prepared in MEM/EBBS supplemented with 1 μM phenanzinemethosulfate (PMS, Fisher Scientific). Absorbance was measured at 450 nm using the Tecan Instruments® reader before and shortly after the addition of the XTT solution and after two h of incubation at 37°C (Tecan Group Ltd., Männedorf, Switzerland). The mean values and standard deviation of four biological replicates performed in triplicate were calculated.
Statistics
Microsoft® Excel 2007 and SPSS.20 software (SPSS Inc., Chicago USA) were used for statistical analysis. To determine significant associations between the MICs of the different antimicrobials, spa-type and antibiotic resistance (non-parametric values) we calculated the Phi-coefficient (p < 0.05). Phi-coefficient values (mathematical quantity) from 0.7 to 1 were defined as strong association, from 0.3 to 0.7 as weak and < 0.3 as little or no association. Only spa-types and antibiotic resistance shown by at least three strains were included. To determine correlations between the MIC of the different antimicrobial compounds, we used the Pearson correlation (p < 0.05). Pearson correlation values (mathematical amount) > 0.5 were defined as strong correlations, from 0.3 to 0.5 as moderate and from 0.1 to 0.3 as weak correlations. To analyze the cell viability data, we first used the Welch test to confirm variance homogeneity. Since data showed variance inhomogeneity, we used the post-hoc Games-Howell test to determine significant differences between cell viabilities (percentages of metabolically active cells) under different conditions (control, different concentrations of the test compounds); p < 0.05 were considered to be statistically significant.
Results
Genetic Diversity and Antibiotic Resistance of Bovine Mastitis S. aureus Strains
The 172 S. aureus strains belonged to 52 different spa-types, including five novel spa-genotypes (t16183, t16193, t16194, t16195, and t16196). The most prevalent spa-types were t529 (22% of isolates), t524 (11%), and t2953 (10%). Thirty-Eight spa-types (73%) were represented by less than three strains.
Antibiotic susceptibility testing revealed that 24% of isolates were resistant to one, 2% to two antibiotic agents and 3% of isolates were multi-resistant (resistant to antibiotics belonging to three different classes, Figure 1A). Benzylpenicillin-resistance was the most common resistance (29% of isolates) followed by tetracycline-resistance (5% of isolates, Figure 1B). Less than 3% of strains were resistant to other antibiotic agents. None of the S. aureus strains were resistant to trimethoprim/sulfamethoxazole.
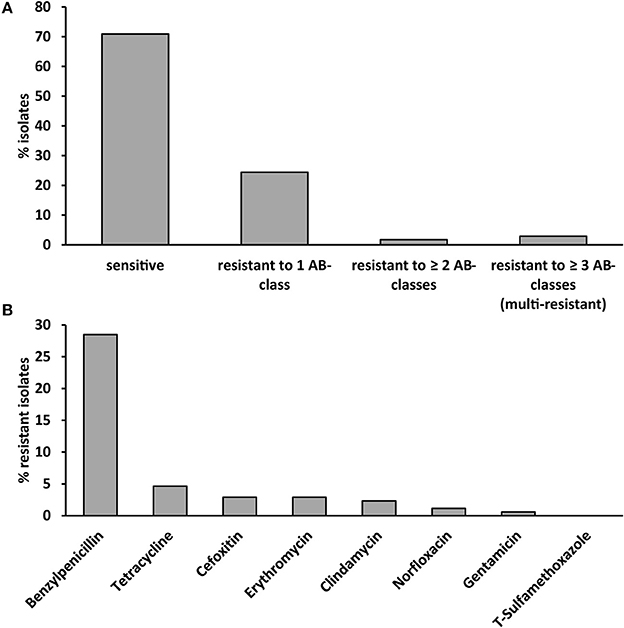
Figure 1. Antibiotic susceptibility of the 172 investigated S. aureus strains isolated from cases of bovine mastitis. (A) Antibiotic susceptibility, resistance and multi-resistance (resistance to ≥ 3 antibiotic classes). (B) Resistance to eight different antibiotics belonging to eight different antibiotic classes. T-sulfamethoxazole: trimethoprim/sulfamethoxazole.
The occurrence of any type of antibiotic resistance correlated with the spa-type (phi coefficient 0.860, p < 0.001, Table S1). We observed a strong association between the spa type and antibiotic resistance for all antibiotic classes (phi coefficient 0.738–1, p < 0.001) except gentamycin (phi coefficient 0.573, p < 0.001, Table S1). All strains of t024 (n = 6) and t091 (n = 3), 72% of t2953 strains (n = 18) and 60% of t008 (n = 5) strains were resistant to benzylpenicillin (Table S2). All three t001 strains were resistant to benzylpenicillin, cefoxitin, and tetracycline.
Benzalkonium Chloride, Chlorhexidine, and Iodine Susceptibilities
Benzalkonium chloride inhibited the growth of 92% of S. aureus isolates at a concentration of 4 mg/L and chlorhexidine inhibited the growth of 88% of isolates at 2 mg/L (Figures 2A,B). The growth of 95% of isolates was inhibited by 500 mg/L iodine (Figure 2C). No difference between the MIC50 and MIC90 was observed for all three compounds (Table 1).
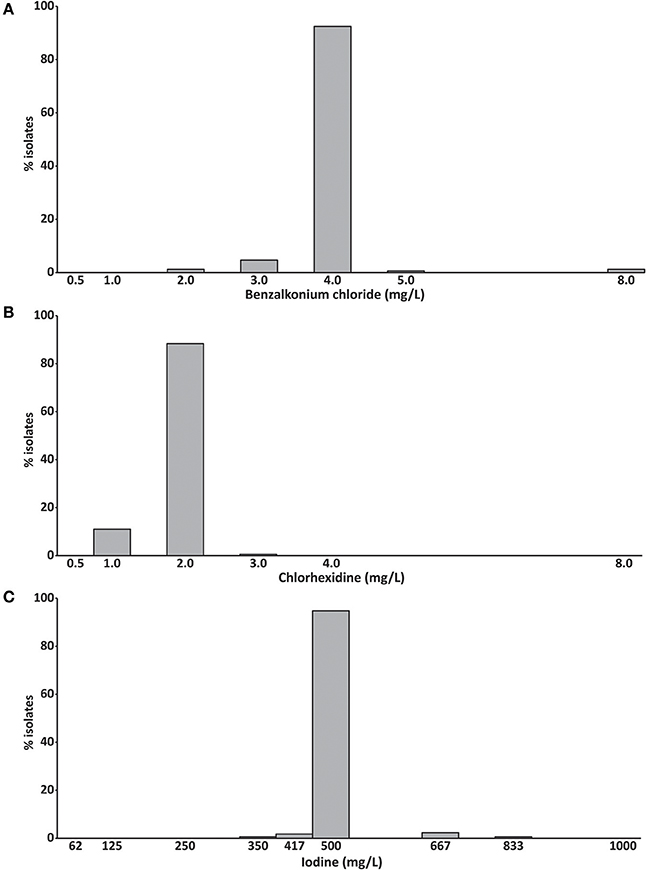
Figure 2. Minimum inhibitory concentrations of (A) benzalkonium chloride, (B) chlorhexidine and (C) iodine (mg/L) of 172 bovine mastitis S. aureus isolates. Data are presented as mean MICs of three independent replicates.
We observed a weak correlation between the MICs of benzalkonium chloride and chlorhexidine (Pearson correlation R = 0.191, p = 0.012, Table S3), the spa-type and the MIC of benzalkonium chloride (phi coefficient 0.633, p = 0.002), and the spa-type and the MIC of chlorhexidine (phi coefficient, 0.579, p < 0.001, Table S4). The benzalkonium chloride MIC also correlated also with the occurrences of cefoxitin and tetracycline resistance (phi coefficient 0.306 and 0.251, p < 0.03). Interestingly, the chlorhexidine MIC correlated significantly with the occurrences of all different antibiotic resistances except benzylpenicillin (phi coefficients: 0.217–0.536, p < 0.02). No correlation between the iodine MIC, the spa-type and any antibiotic resistance was detected.
Antimicrobial Activities of Gentisaldehyde and 2,3-Dihydroxybenzaldehyde
The MIC50 of both compounds was 500 mg/L (Table 1). Sixty-Seven percent of S. aureus isolates showed a MIC of 500 mg/L for gentisaldehyde and 69% for 2,3-dihydroxybenzaldehyde, respectively (Figure 3). No S. aureus strains showed a MIC lower than 500 mg/L and higher than 1,000 mg/L for both compounds. The MIC90 was 1,000 mg/L for gentisaldehyde and 833 mg/L for 2,3-dihydroxybenzaldehyde (Table 1).
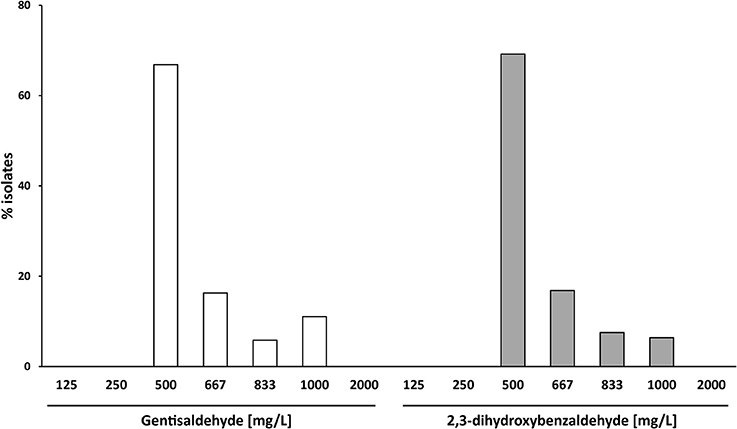
Figure 3. Minimum inhibitory concentrations of gentisaldehyde and 2,3-dihydroxybenzaldehyde (mg/L) of 172 bovine mastitis S. aureus isolates. Data are presented as mean MICs of three independent replicates.
We observed a moderate correlation between the MIC of gentisaldehyde and 2,3-dihydroxybenzaldehyde (Pearson correlation R = 0.497, p < 0.0001, Table S3). The gentisaldehyde MIC correlated weakly with the occurrences of gentamicin and erythromycin resistances (phi coefficient 0.308 and 0.262, p < 0.01), whereas the MIC of 2,3-dihydroxybenzaldehyde was associated with the spa-type (phi-coefficient 0.695, p = 0.013) and the occurrence of resistance to all antibiotic classes except tetracycline (phi-coefficient 0.222–0.376, p < 0.05, Table S4).
Cytotoxicity
Cytotoxicity of both compounds at all tested concentrations in bovine mammary epithelial MAC-T cells was low. The percentage of metabolically active cells was higher than 64% for all tested gentisaldehyde and 2,3-dihydroxybenzaldehyde concentrations (Figure 4). However, cell viability was significant lower at all concentrations compared to the control. No significant difference between cell viabilities at MIC50 and MIC90 was observed for both compounds.
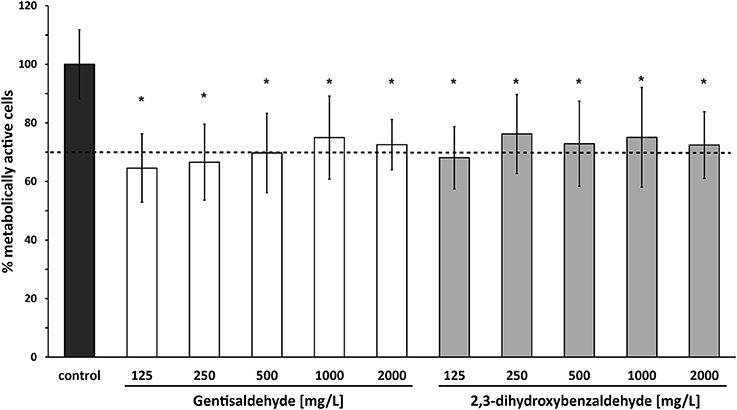
Figure 4. Cytotoxicity of gentisaldehyde and 2,3-dihydroxybenzaldehyde. Metabolically active bovine mammary epithelial MAC-T cells (%) were measured after 24 h of treatment with gentisaldehyde and 2,3-dihydroxybenzaldehyde (125–2,000 mg/L). Control: cells incubated in media without any compound. Data represent mean values ± SD of four biological replicates performed in triplicate. *p < 0.05 indicates a statistically significant difference compared to the control. Dashed line indicates threshold of 70% metabolically active cells, defined as non-toxic.
Discussion
The increasing rate of bacteria resistant to antibiotics and antiseptics poses a major problem to human and veterinary health. Consequently, there is a desperate demand for novel antimicrobial agents active against resistant bacteria (8, 12, 29). In recognition of this requirement, this study investigated alternative antimicrobial compounds that might be suitable for the prevention of bovine mastitis. We examined two promising phenolic benzaldehydes reported to have antibacterial activities (16, 21).
The bovine S. aureus mastitis strain set used in this study showed a high number of different spa-types, indicating high genotype diversity. The most prevalent spa-types have also been found with a similar occurrence in other studies that have investigated bovine S. aureus isolates (30–32). The strain set comprised 24% of isolates resistant to one antibiotic class and a low number of multi-resistant strains. Most commonly observed was resistance to benzylpenicillin (29%) and tetracycline (5% of isolates), whereas there were no isolates resistant to the trimethoprim/sulfamethoxazole combination. A recent study showed a similar prevalence of penicillin resistant S. aureus strains isolated in Switzerland and France (33). Further, low or even no resistance to the sulfatrimethoprim combination has also been reported in other countries (34). Multi-resistance of S. aureus in bovine mastitis has been reported for 1.4–17% of isolates. However, mono- and multi-resistance of S. aureus mastitis isolates varies highly across different countries (34). Further, as the term “multi-resistance” is often not clearly defined, it is difficult to make direct comparison between different studies and countries.
The occurrence and the type of antibiotic resistance strongly correlated with the spa-type. Recently, this has also been reported between clindamycin and erythromycin resistances and the spa-type; although there were no correlations between the occurrences of cefoxitin, gentamicin, tetracycline, and trimethoprim/sulfamethoxazole resistances (35). However, in this study the spa-types have been identified by PCR and not by sequencing. In respect of bovine S. aureus isolates, spa-types t2953 and t524 have been reported to be penicillin resistant (33). Similarly, 72% of S. aureus strains belonging to genotype t2953 showed penicillin resistance in our study, whereas only one isolate of spa-type t524 was penicillin resistant. Several spa-types, such as t011, which was also found in this study, are known to be methicillin- and multi-resistant (36).
Data on the susceptibility of S. aureus mastitis strains to antiseptics are rare. In the present study we found susceptibility to three antiseptics with MIC50 (and MIC90) values of 4 mg/L for benzalkonium chloride, 2 mg/L for chlorhexidine and 500 mg/L for iodine. A recent study including 37 S. aureus mastitis strains reported higher MIC50 and MIC90 values for iodine compared to our study, but a similar MIC for chlorhexidine (37).
A weak correlation between the MICs of benzalkonium chloride and chlorhexidine was found, which suggests a similar mode of action. This mode of action appears to be primarily linked to modification and impairment of cell membrane permeability (38, 39). Consequently, the presence of efflux pumps in bacteria can especially confer higher tolerance to both compounds (40, 41). Since the occurrence of multidrug efflux pumps such as NorA, NorB or MepA results in multiple antimicrobial resistance (41), we expected a strong correlation between benzalkonium chloride and chlorhexidine MICs and antibiotic resistance. However, we detected only a weak association, mainly between the chlorhexidine MIC and the occurrence of antibiotic resistance (except to benzylpenicillin). The reason could be the low number of resistant strains included in this study. Alternatively, the MIC for iodine did not correlate with any other parameter. This is in line with other studies showing almost no cross-resistance between iodine and other antibiotics in bovine mastitis S. aureus (37, 42). The exact mode of action of iodine is still not clear. However, iodine is known to affect rapidly key groups of proteins, nucleotides and fatty acids (43).
Both test compounds—gentisaldehyde as well as its structural derivate 2,3-dihydroxybenzaldehyde—revealed antimicrobial activities at low-toxic concentrations. The MIC50 values of both compounds were high (500 mg/L), equivalent to that of iodine. Friedman et al. revealed similar antimicrobial activities for the two dihydroxybenzaldehydes against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica using only one strain of each species (16). Their measured BA50 (bacterial activity) values for all four pathogens were 1,460 mg/L for gentisaldehyde and 670 mg/L for 2,3-dihydroxybenzaldehyde.
We revealed a moderate correlation between the MIC values for both compounds. The MIC value of both compounds also correlated with the spa-type at similar levels, but this correlation was only significant for 2,3-dihydroxybenzaldehyde. We also detected differences between the two compounds in respect of correlations between their MICs and the occurrence of antibiotic resistance. However, since the correlations were weak and only a limited number of resistant isolates were included in this study, it is difficult to speculate whether gentisaldehyde has higher antimicrobial activity against antibiotic resistant strains than 2,3-dihydroxybenzaldehyde. Since we observed no correlation between the MIC of the antiseptics and the two new compounds, we can conclude that S. aureus strains resistant to common antiseptics are likely susceptible to gentisaldehyde and 2,3-dihydroxybenzaldehyde. Furthermore, this suggests a different mode of action and resistance mechanism. Nevertheless, the mode of action of gentisaldehyde and 2,3-dihydroxybenzaldehyde has still to be elucidated.
Results of the cytotoxicity testing of the two benzaldehydes underscores their potential use as antiseptics, since more than 64% of cells were viable after incubation with the compounds at concentrations ranging from 125 to 2,000 mg/L. A threshold of more than 70% viable cells is conventionally defined as non-toxic (27, 28, 44), and therefore gentisaldehyde and 2,3-dihydroxybenzaldehyde show low toxicity to bovine mammary epithelial cells. The cytotoxicity of these compounds has not been tested before. Since bovine mammary cells are not the ideal model for teat disinfectants further cytotoxicity testing e.g. using primary bovine skin cells are needed.
In conclusion, we show here that gentisaldehyde and its structural derivate 2,3-dihydroxybenzaldehyde have antimicrobial activities on a diverse set of bovine S. aureus mastitis isolates, including (multi-) resistant strains. As both compounds show low toxicity to bovine mammary cells they are potential candidates for further investigation as alternative antimicrobials in the prevention of bovine mastitis. Further studies to examine their antimicrobial activities against other common mastitis pathogens (e.g., streptococci and Gram-negative species) and elucidate their mode of action are warranted.
Data Availability Statement
Data generated during the presented study are available from the corresponding author (KR) upon reasonable request.
Author Contributions
Isolates were collected and provided by BL. Methods were developed by AS, CZ, and KR. All experiments were performed by AS. Raw data were evaluated by AS. Statistical analyses were performed by KR. Results were interpreted by AS and KR. KR and MW coordinated the study. All authors read and approved the final manuscript.
Funding
This work was supported by the FFG-funded project Advancement of Dairying in Austria (ADDA, grant number 843543).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Georg Mößlacher and Gottfried Schoder (Upper Austrian Animal Health Service) for providing the isolates and Desislava Yankova (BiMM Bioactive Microbial Metabolites, Tulln, Austria) for technical support. We also thank Joseph Strauss (Research Platform Bioactive Microbial Metabolites-BiMM and Department of Applied Genetics and Cell Biology, BOKU University of Natural Resources and Life Sciences Vienna, Tulln/Donau, Austria) for providing expertise and for critical discussion of the results, and Dr. Cameron McCulloch for language editing of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2018.00148/full#supplementary-material
Footnotes
1. ^ST spaTYPER, URL: http://spatyper.fortinbras.us/
2. ^RidomSpaServer of the Ridom GmbH, URL: http://spa.ridom.de/
References
1. Hillerton JE, Berry EA. Treating mastitis in the cow – a tradition or an archaism. J Appl Microbiol. (2005) 98:1250–5. doi: 10.1111/j.1365-2672.2005.02649.x
2. Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. (2003) 34:475–91. doi: 10.1051/vetres:2003027
3. Leslie KE, Petersson-wolfe CS. Assessment and management of pain in dairy cows with clinical mastitis. Vet Clin North Am Food Anim Pract. (2012) 28:289–305. doi: 10.1016/j.cvfa.2012.04.002
4. Kamaruzzaman NF, Chong SQY, Edmondson-brown KM. Bactericidal and anti-biofilm effects of polyhexamethylene biguanide in models of intracellular and biofilm of Staphylococcus aureus isolated from bovine mastitis. Front Microbiol. (2017) 8:1518. doi: 10.3389/fmicb.2017.01518
5. Wendt K, Bostedt H, Mielke H, Fuchs HW editors. Euter- und Gesäugekrankheiten. Jena: Fischer (1994).
6. Winter PM editor. Praktischer Leitfaden Mastitis: Vorgehen beim Einzeltier und im Bestand. Stuttgart: Parey (2008).
7. Lee C, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health (2013) 10:4274–305. doi: 10.3390/ijerph10094274
8. Lin S, Koh J, Aung TT, Ling W, Sin W, Lim F et al. Semisynthetic flavone-derived antimicrobials with therapeutic potential against methicillin-resistant Staphylococcus aureus (MRSA). J Med Chem. (2017) 60:6152–65. doi: 10.1021/acs.jmedchem.7b00380
9. Fröhling A, Wienke M, Rose-meierhöfer S, Schlüter O. Improved method for mastitis detection and evaluation of disinfectant efficiency during milking process. Food Bioprocess Technol. (2010) 3:892–900. doi: 10.1007/s11947-010-0366-9
10. Martins CMMR, Pinheiro ESC, Gentilini M, Benavides ML, Santos MV. Efficacy of a high free iodine barrier teat disinfectant for the prevention of naturally occurring new intramammary infections and clinical mastitis in dairy cows. J Dairy Sci. (2017) 100:3930–9. doi: 10.3168/jds.2016-11193
11. Gleeson D, Brien OB, Flynn J, Callaghan OE, Galli F, Gleeson D. Effect of pre-milking teat preparation procedures on the microbial count on teats prior to cluster application. Ir Vet J. (2009) 62:461–7. doi: 10.1186/2046-0481-62-7-461
12. He X, Zhang H, Cao J, Liu F. A novel method to detect bacterial resistance to disinfectants. Genes Dis. (2017) 4:163–9. doi: 10.1016/j.gendis.2017.07.001
13. Mayer S, Boos M, Beyer A, Fluit AC, Schmitz F-J. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible European isolates of Staphylococcus aureus J. J Antimicrob Chemother. (2001) 47:893–905. doi: 10.1093/jac/47.6.896
14. Keller NP, Turner G, Bennett JW. Fungal secondary metabolism-from biochemistry to genomics. Nat Rev Microbiol. (2005) 3:937–47. doi: 10.1038/nrmicro1286
15. Zutz C, Bacher M, Parich A, Kluger B, Gacek-matthews A, Schuhmacher R et al. Valproic acid induces antimicrobial compound production in Doratomyces microspores. Front Microbiol (2016) 7:510. doi: 10.3389/fmicb.2016.00510
16. Friedman M, Henika PR, Mandrell RE. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. (2003) 66:1811–21. doi: 10.4315/0362-028X-66.10.1811
17. Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP et al. A new antibiotic kills pathogens without detectable resistance. Nature (2015) 517:455–9. doi: 10.1038/nature14098
18. Subramani R, Narayanasamy M, Feussner K-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech (2017) 7:172. doi: 10.1007/s13205-017-0848-9
19. Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot. (2002) 65:1545–60. doi: 10.4315/0362-028X-65.10.1545
20. Geng H, Yu X, Lu A, Cao H, Zhou B, Zhou L. Extraction, chemical composition, and antifungal activity of essential oil of bitter almond. Int J Mol Sci. (2016) 17:1421. doi: 10.3390/ijms17091421
21. Kim JH, Faria NCG, Martins MDL, Chan KL, Campbell BC. Enhancement of antimycotic activity of amphotericin B by targeting the oxidative stress response of Candida and Cryptococcus with natural dihydroxybenzaldehydes. Front Microbiol. (2012) 3:261. doi: 10.3389/fmicb.2012.00261
22. Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. (1992) 30:1654–60.
23. Stegger M, Andersen PS, Kearns A, Pichon B, Holmes MA, Edwards G et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA LGA251. Clin Microbiol Infect. (2012) 18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x
24. European Committee on Antimicrobial Susceptibility Testing. Antimicrobial Susceptibility Testing. EUCAST Disk Diffusion Method. Version 5.0. January 2015. Available online at: www.eucast.org
25. European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.1, valid from 2017-03-10. Available online at: http://www.eucast.org
26. Scudiero DA, Shoemaker RH, Paul KD, Monks A, Tierney S, Nofziger TH et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. (1988) 48:4827–33.
27. International Standard. ISO 10993-5:2009. Biological Evaluation of Medical Devices - Part 5: Tests for in vitro Cytotoxicity. 3rd ed. 2009-06.
28. Tyliszczak B, Drabczyk A, Kudłacik-Kramarczyk S, Bialik-Was K, Kijkowska R, Sobczak-Kupiec A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surfaces B Biointerfaces (2017) 160:325–30. doi: 10.1016/j.colsurfb.2017.09.044
29. Mitchell G, Lafrance M, Boulanger S, Séguin DL, Guay I, Gattuso M et al. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant Staphylococcus aureus and prevents virulence gene expression. J Antimicrob Chemother. (2012) 67:559–68. doi: 10.1093/jac/dkr510
30. Sakwinska O, Giddey M, Moreillon M, Morisset D, Waldvogel A, Moreillon P. Staphylococcus aureus host range and human-bovine host shift. Appl Environ Microbiol. (2011) 77:5908–15. doi: 10.1128/AEM.00238-11
31. Stutz K, Stephan R, Tasara T. spa, clfA, and fnbA genetic variations lead to Staphaurex test-negative phenotypes in bovine mastitis Staphylococcus aureus isolates. J Clin Microbiol. (2011) 49:638–46. doi: 10.1128/JCM.01148-10
32. Kümmel J, Stessl B, Gonano M, Walcher G, Bereuter O, Fricker M et al. Staphylococcus aureus entrance into the dairy chain: tracking S. aureus from dairy cow to cheese. Front Microbiol. (2016) 7:1603. doi: 10.3389/fmicb.2016.01603
33. Sakwinska O, Morisset D, Madec JY, Waldvogel A, Moreillon P, Haenni M. Link between genotype and antimicrobial resistance in bovine mastitis-related Staphylococcus aureus strains, determined by comparing swiss and French isolates from the Rhone Valley. Appl Environ Microbiol. (2011) 77:3428–32. doi: 10.1128/AEM.02468-10
34. Oliver SP, Murinda SE. Antimicrobial resistance of mastitis pathogens. Vet Clin North Am Food Anim Pract. (2012) 28:165–85. doi: 10.1016/j.cvfa.2012.03.005
35. Hosseini SM, Karami P, Kazemian H, Karimitabar Z, Mohamadi A, Khaledi A. Relationship between antibiotic resistance with spa gene polymorphism coding protein A and its typing with PCR-RFLP technique in S. aureus isolated from foodstuffs. J Clin Microbiol Infect. (2017) 4:1–5. doi: 10.5812/ajcmi.12105
36. Nemeghaire S, Argudín M, Haesebrouck F, Butaye P. Epidemiology and molecular characterization of methicillin-resistant Staphylococcus aureus nasal carriage isolates from bovines. BMC Vet Res. (2014) 10:153. doi: 10.1186/1746-6148-10-153
37. Azizoglu RO, Lyman R, Anderson KL. Bovine Staphylococcus aureus: dose response to iodine and chlorhexidine and effect of iodine challenge on antibiotic susceptibility. J Dairy Sci. (2013) 96:993–9. doi: 10.3168/jds.2012-5857
38. Maris P. Modes of action of disinfectants. Rev Sci Tech. (1995) 14:47–55. doi: 10.20506/rst.14.1.829
39. Wand ME, Bock LJ, Bonney LC, Sutton JM. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother. (2017) 61:1–12. doi: 10.1128/AAC.01162-16
40. Wessels S, Ingmer H. Modes of action of three disinfectant active substances: a review. Regul Toxicol Pharmacol. (2013) 67:456–67. doi: 10.1016/j.yrtph.2013.09.006
41. Costa SS. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J. (2013) 7:59–71. doi: 10.2174/1874285801307010059
42. Behiry A, El Schlenker G, Istvan Szabo, Roesler U. In vitro susceptibility of Staphylococcus aureus strains isolated from cows with subclinical mastitis to different antimicrobial agents. J Vet Sci. (2012) 13:153–61. doi: 10.4142/jvs.2012.13.2.153
43. Donnell GMC, Russell AD. Antiseptics and disinfectants : activity, action, and resistance. Clin Microbiol Rev. (1999) 12:147–79.
Keywords: mastitis, 2, 3-dihydroxybenzaldehyde, 2, 5-dihydroxybenzaldehyde, gentisaldehyde, Staphylococcus aureus, antiseptic, antibiotic resistance
Citation: Schabauer A, Zutz C, Lung B, Wagner M and Rychli K (2018) Gentisaldehyde and Its Derivative 2,3-Dihydroxybenzaldehyde Show Antimicrobial Activities Against Bovine Mastitis Staphylococcus aureus. Front. Vet. Sci. 5:148. doi: 10.3389/fvets.2018.00148
Received: 12 March 2018; Accepted: 13 June 2018;
Published: 11 July 2018.
Edited by:
Rodrigo Guabiraba, INRA Centre Val de Loire, FranceReviewed by:
Bo Han, China Agricultural University, ChinaPierre Germon, Institut National de la Recherche Agronomique (INRA), France
Copyright © 2018 Schabauer, Zutz, Lung, Wagner and Rychli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathrin Rychli, a2F0aHJpbi5yeWNobGlAdmV0bWVkdW5pLmFjLmF0
†Present Address: Christoph Zutz, Environment Agency Austria (Umweltbundesamt), Department for Chemicals and Biocides, Vienna, Austria
 Andrea Schabauer1
Andrea Schabauer1 Martin Wagner
Martin Wagner Kathrin Rychli
Kathrin Rychli