
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 09 January 2020
Sec. Genitourinary Surgery
Volume 6 - 2019 | https://doi.org/10.3389/fsurg.2019.00074
 Ohad Kott1,2*
Ohad Kott1,2* Borivoj Golijanin1,3
Borivoj Golijanin1,3 Jorge F. Pereira4
Jorge F. Pereira4 Alison Chambers5
Alison Chambers5 Alison Knasin6
Alison Knasin6 Christopher Tucci1,2
Christopher Tucci1,2 Dragan Golijanin1,2
Dragan Golijanin1,2Introduction: Partial nephrectomy (PN), has become the gold standard for the surgical management of small renal masses, due to excellent oncologic control with concomitant preservation of nephron units. However, data regarding the association of obesity with perioperative outcomes following PN are mixed. Therefore, the association between obesity (using BMI) and post-operative complications (POC) rate following Robotic assisted laparoscopic PN (RPNx) was tested.
Methods: Two hundred and fifty-one adult patients who underwent RPNx from 1/2011 to 5/2017 at a single institution, with at least 90 days follow-up were identified and included. No patients were excluded. Electronic medical records were reviewed to record all POC within 90 days of surgery. A piecewise generalized linear model for binary outcomes (logistic) was used to model the proportion of subjects with POC by their BMI. The slope of the line is adjusted to a BMI of 30 Kg/m2.
Results: BMI is significantly associated with POC rate. POC rate decreased with increasing BMI below the inflection point of 30 Kg/m2 (0.848[0.756, 0.952]) (OR [95% CI], p = 0.005). POC rate was found to increase with increasing BMI above the BMI inflection of 30 Kg/m2 (1.102 [1.027, 1.182], p = 0.0071).
Conclusions: In this cohort study, BMI showed an association with PC. It may be important to take BMI into account in surgical and clinical management considerations of RPNx, since higher rates of POC are associated with patients who are underweight, morbidly obese, and even with normal BMI. Further research is required on larger cohorts of RPNx patients to provide better description of this phenomenon and elucidate the role of BMI in development of POC.
Partial nephrectomy (PN), has become the gold standard for the surgical management of small renal masses, due to excellent oncologic outcomes with concomitant preservation of nephron units (1–3). Furthermore, when compared to open partial nephrectomy (OPN) minimally invasive partial nephrectomy (MIPN) has been associated with improved perioperative outcomes (4–8), including fewer post-operative complications (POC), shorter operative time, and a decreased length of stay (9, 10). Robotic assisted laparoscopic partial nephrectomy (RPNx) has become the most common method of MIPN due to its additional benefits over laparoscopic partial nephrectomy (LPN) such as shorter warm ischemia time (WIT) (11), lower rates of positive surgical margins, lower complication rates and enhanced nephron sparing (12). In addition to improving surgical techniques, it is also important to identify modifiable patient factors that may improve postoperative outcomes such as body mass index (BMI).
Elevated BMI is associated with several comorbidities that are associated with poor surgical outcomes (13), and has been shown to be an independent predictor of increased perioperative morbidity (14–17). Specifically, increased BMI is associated with a higher rates of surgical site infections, medication dosage errors, difficult ventilation, positioning related injuries, and postoperative mortality rates (18–21). While urologic data has focused on the effect of obesity on perioperative morbidity, literature from general surgery demonstrates a paradoxical relation between BMI and surgical outcomes (22–24). Recent studies have demonstrated a BMI paradox whereby lower mortality rates were noted among the overweight and the mildly obese patients, while increased mortality rates were seen in the underweight and extremely obese populations (23, 24). Evaluation of the BMI paradox in urologic surgery is lacking, and as such we seek to test the association between BMI and POC (POC) following RPNx.
After institutional review board approval, we conducted a retrospective chart review and identified 251 adult patients who consecutively underwent RPNx for the treatment of a renal mass at our medical center between January 2011 and May 2017. All procedures were performed using the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA) by a single surgeon experienced in robot-assisted laparoscopic urological surgery. All procedures were done using similar surgical techniques as previously described (25).
Electronic medical records were reviewed to identify all POC within 90 days of surgery, graded according to the Clavien-Dindo system (26). Original analysis analyzed major and minor complications separately; however, only six major complications were found in our patient population so major and minor complications were grouped together as POC for all subsequent analysis. Patients' baseline characteristics recorded included: age, gender, smoking history, BMI, anticoagulants use, and medical comorbidities (diabetes, Hypertension, cardiac arrhythmia, coronary artery disease, COPD, GERD). Tumor characteristics recorded included: tumor size and RENAL nephrometry scores (27). Perioperative variables recorded included: operative time, estimated blood loss (EBL), abdominal insufflation volume, post-operative ambulation time, and length of stay (LOS). Patient information was collected and managed using REDCap electronic data capture tools hosted at Lifespan. No patients were excluded from this cohort or the data analysis.
Medians and interquartile range (IQR) were used to report continuous variables. Frequencies and proportions were used to report categorical variables.
A generalized linear model for binary outcomes (logistic, proc glimmix) was used to model the proportion of subjects with POC by their BMI. A piecewise approach allowed the slope of the line to adjust at the BMI of 30. BMI 30 was selected as an adjustment point as it is accepted by the World Health Organization as lower range of obesity. From the model the odds ratios (slopes) were estimated below 30 BMI and above 30 BMI. Additionally, the probabilities of POC were estimated at BMIs of 20, 30, and 40 Kg/m2.
A receiver-operator characteristic (ROC) curve was generated from the multivariate model and AUC was used to assess ability of BMI to discriminate between presence and absence of POC. Optimal cutoffs (Sensitivity-specificity) were also determined from the model both below and above BMI of 30. The model was also assessed with an interaction term (multi-variate analysis) to understand the influence of confounders (operation time, diabetes, hypertension, and age) on the relationship between BMI and POC. Statistical analysis and hypothesis testing were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC).
Our study cohort included 251 patients. Patient baseline characteristics are reported in Tables 1, 2. With the multivariate model, the odds of having POC were found to be significantly below 1 for BMIs under 30 Kg/m2 (0.85[0.76, 0.95]) (odds ratio [95% CI], p = 0.005). The odds were found to be significantly above 1 over the BMI inflection of 30 Kg/m2 (01.10[1.03, 1.18], p = 0.007) (Figure 1). Odds below 1 indicate a decrease in probability of POC, while odds above 1 indicate an increase in probability of POC. Probabilities of a POC at 20, 30, and 40 BMI were 0.39[0.22, 0.60], 0.11[0.07, 0.18], and 0.24[0.15, 0.36], respectively.
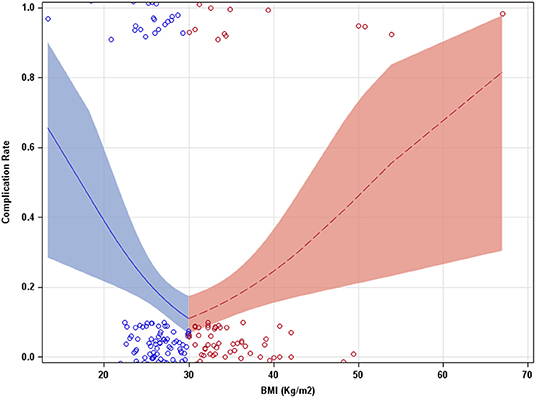
Figure 1. Complication rate as a function of BMI (Kg/m2). A piecewise approach allowed the slope of the model to adjust at a BMI of 30. Bands indicate 95% confidence intervals.
ROC analysis showed an AUC of 0.5998 (accuracy of 59.98% for BMI to discriminate between presence and absence of POC) (Figure 2). Perfect accuracy is defined as an AUC of 1. The optimal BMI cut offs (determined using sensitivity-specificity) for the lower part and upper part of the curve were 26.7 and 35.7 Kg/m2, respectively. When the model was also assessed with interaction terms to test the separate influence of confounders (operation time, diabetes, hypertension, and age) on the relationship between BMI and PC. Confounders where not found to significantly contribute to the model (all p ≥ 0.4983, Table 2), while BMI was still found to be significantly related to POC in all models (Table 2).
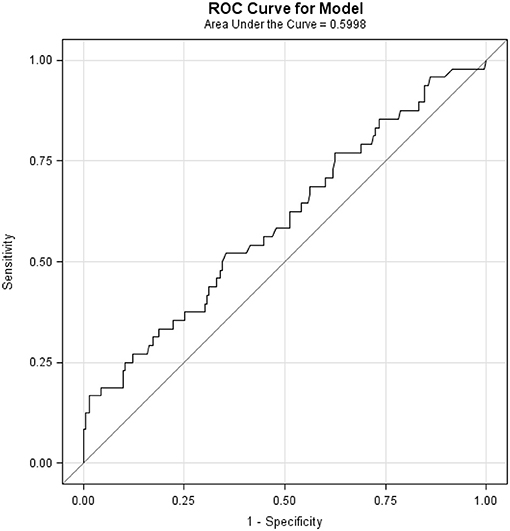
Figure 2. Receiver-Operator Characteristic (ROC) Curve. Area under the curve (AUC) of 0.5998 shows the ability of BMI to discriminate between presence and absence of POC. Perfect accuracy is defined as an AUC of 1.0.
In this retrospective single institution study, we have shown that BMI was associated with POC rate in patients undergoing RPNx. The rate of POC (odds) was found to be lower with increasing BMI up until the BMI inflection point (30 Kg/m2). Above the BMI of 30 the POC rate (odds) was found to be higher. This suggests that underweight and morbidly obese patients have the greatest risk of developing POC. This may indicate a paradoxically lower risk for POC after RPNx with overweight and mildly obese patients compared with patients at a normal weight. Our result are consistent with several studies examining large patient populations undergoing non-bariatric surgical procedures that demonstrated a paradoxical association between BMI and POC (23, 28, 29). These previous studies found the association function to have a function curve with a nadir of lowest complications rate at BMI between 25 and 35 Kg/m2. This is similar to our study where we found, the estimated probabilities (complication rate) at a BMI of 20 or 40 had a higher rate of POC than those with a BMI of 30. Additionally, our study saw cutoff values for BMI at 26.7 and 35.7 Kg/m2, with patients in the range between these two values having the lowest probability of PC. Although higher rates of POC are observed in underweight and obese patients, these results do not prove causality and interdependence between BMI and PC.
Our study and the aforementioned general surgery literature, stands in contrast to studies that found no association between BMI and surgical outcomes (30) or described a direct linear relationship showing that obesity is associated with increased risk of morbidity and mortality including cancer (31, 32) and higher renal mass complexity (33). One such study specifically examining LPN concluded that high BMI was associated with increased risk of major complications in patients who underwent LPN (34). Others found BMI to be a predictor of poor surgical outcomes in open partial nephrectomies but not in LPN (35). This may be explained by the excess of perinephric fat that requires longer dissection during PN. Perinephric fat was found to be significantly correlated with operative time in renal procedures while BMI had no correlation with operative time (36). However, other studies found no association between obesity (either measured by visceral and perinephric fat or by BMI) and surgical outcomes in RPNx (37, 38). When looking at a large study population, such as the NSQIP data base, there was no association between BMI and POC after OPN and MIPN (39). This study concluded that obese patients undergoing MIPN had lower POC rate than those undergoing OPN. However, these studies may have overlooked a more complex relationship between BMI and PC, as they did not allow for changes in POC rate with increasing BMI. Not allowing for POC rate changes with increasing BMI could underestimate probabilities of low BMI individuals and overestimate probabilities of moderate BMI individuals.
The accuracy of BMI in predicting POC was 59.98%, which indicates that there may be other factors that could help explain the remaining uncertainty in the prediction of PC. The confounders of operation time, patient age, anticoagulant use comorbidities (e.g., diabetes, hypertension) were evaluated for their ability to help define the remaining uncertainty in the multivariate model. Each confounder term was added to the model as an interaction term to test to see if they provided an alternative prediction of POC or if they modified BMI's relationship to PC. The influence of the confounders did not significantly contribute to the model (all interaction terms p ≥ 0.4983). This suggests that while these confounders may have an independent relationship with POC, they do not appreciably modify the association of BMI with POC and do not help explain the remaining uncertainty in the model.
Additionally, the number of POC may be too small to detect a more subtle effect of these modifiers on the outcome. Alternatively, the adverse effects of low BMI may be mediated by metabolic and inflammatory responses that occur after major surgical procedures like partial nephrectomy. It was previously suggested (23) that patients who are overweight or mildly obese may experience a protective effect against the inflammatory response and increased metabolic demands associated with the physical insult of surgery due to larger nutritional reserves and a more efficient metabolic state than underweight patients and even normal weight patients. If this hypothesis is correct, mildly obese patients may have better response to the metabolic and inflammatory stress of surgery, engage faster tissue repair even in the setting of low caloric and protein intake.
The change in inflammatory response amongst overweight and mildly obese patients is thought to be modulated via the immune system. One theory proposes that moderate amounts of adipose tissue may protect against inflammation via the secretion adipokines such as adiponectin, IL-1, IL-6, and soluble TNF-α receptors which work to neutralize endotoxins (40). As such, obese patients may normally live in constant state of mild inflammation, from metabolic reasons, and are adapted to it. Therefore, after surgery they may quickly respond to surgical injury, stress, and inflammation and engage in immune response and tissue repair.
This study is not without limitations. To limit possible confounding factors, the study population was limited to surgical patients operated by a single, highly specialized surgeon in a tertiary referral center. As such, there may be some level of selection bias in the type of patients that present to the medical center. Our findings may not reflect other surgeons in other clinical settings. Furthermore, while this study's cohort size is larger than several recently published studies evaluating partial nephrectomy outcomes (33–36), this study size is still too small to allow for a full detailed analysis of the complex relationship between BMI, tumor characteristics, and POC.
In conclusion, patient BMI may be associated with increased risk of POC and should be considered during preoperative planning and patient counseling. Surgeons evaluating patients that are underweight, morbidly obese, and even with normal BMI should take that into consideration. Further research is required to further characterize and precisely quantify and delineate the exact effects of obesity over surgical outcomes of RPNx using a larger cohort of patients.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Lifespan—The Miriam Hospital IRB, Providence, RI. Ref# 214214 45CFR 46.110(5). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
OK: project development, data collection and management, data analysis, manuscript writing, and editing. BG: data collection, data analysis, and manuscript editing. JP: project development, data analysis, and manuscript editing. AC: data analysis and manuscript editing. AK: project development, data collection, and manuscript editing. CT: project development and manuscript editing. DG: project conception, project development, and manuscript editing.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PN, Partial nephrectomy; BMI, Body mass index; POC, Post-operative complications; RPNx, Robotic assisted laparoscopic partial nephrectomy; OPN, Open partial nephrectomy; MIPN, Minimally invasive partial nephrectomy; LPN, Laparoscopic partial nephrectomy; WIT, Warm ischemia time; COPD, Chronic obstructive pulmonary disease; GERD, Gastroesophageal reflux disease; EBL, Estimated blood loss; LOS, Length of stay; IQR, Interquartile range; ROC, Receiver-operator characteristic; AUC, Area under the curve (in receiver operated probability curve); CI, Confidence interval; NSQIP, National Surgical Quality Improvement Program (of American College of Surgeons).
1. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. (2017) 198:520–9. doi: 10.1016/j.juro.2017.04.100
2. Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2017) 15:804–34. doi: 10.6004/jnccn.2017.0100
3. Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. (2012) 88:51–7. doi: 10.1016/j.juro.2012.03.006
4. Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. (2003) 170:64–8. doi: 10.1097/01.ju.0000072272.02322.ff
5. Lucas SM, Mellon MJ, Erntsberger L, Sundaram CP. A comparison of robotic, laparoscopic and open partial nephrectomy. JSLS. (2012) 16:581–7. doi: 10.4293/108680812X13462882737177
6. Becker A, Pradel L, Kluth L, Schmid M, Eichelberg C, Ahyai S, et al. Laparoscopic versus open partial nephrectomy for clinical T1 renal masses: no impact of surgical approach on perioperative complications and long-term postoperative quality of life. World J Urol. (2015) 33:421–6. doi: 10.1007/s00345-014-1318-1
7. Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh QD, Menon M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. J Urol. (2014) 191:907–12. doi: 10.1016/j.juro.2013.10.099
8. Tan HJ, Wolf JS Jr, Ye Z, Hafez KS, Miller DC. Population level assessment of hospital based outcomes following laparoscopic versus open partial nephrectomy during the adoption of minimally invasive surgery. J Urol. (2014) 191:1231–7. doi: 10.1016/j.juro.2013.11.002
9. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. (2007) 178:41–6. doi: 10.1016/j.juro.2007.03.038
10. Banegas MP, Harlan LC, Mann B, Yabroff KR. Toward greater adoption of minimally invasive and nephron-sparing surgical techniques for renal cell cancer in the United States. Urol Oncol. (2016) 34:433.e9–17. doi: 10.1016/j.urolonc.2016.05.021
11. Mullins JK, Feng T, Pierorazio PM, Patel HD, Hyams ES, Allaf ME. Comparative analysis of minimally invasive partial nephrectomy techniques in the treatment of localized renal tumors. Urology. (2012) 80:316–22. doi: 10.1016/j.urology.2012.03.043
12. Curtiss KM, Ball MW, Gorin MA, Harris KT, Pierorazio PM, Allaf ME. Perioperative outcomes of robotic partial nephrectomy for intrarenal tumors. J Endourol. (2015) 29:293–6. doi: 10.1089/end.2014.0348
14. Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. (2007) 167:1720–8. doi: 10.1001/archinte.167.16.1720
15. Hrabe JE, Sherman SK, Charlton ME, Cromwell JW, Byrn JC. Effect of BMI on outcomes in proctectomy. Dis Colon Rectum. (2014) 57:608–15. doi: 10.1097/DCR.0000000000000051
16. Kazaure HS, Roman SA, Sosa JA. Obesity is a predictor of morbidity in 1,629 patients who underwent adrenalectomy. World J Surg. (2011) 35:1287–95. doi: 10.1007/s00268-011-1070-2
17. Watanabe J, Tatsumi K, Ota M, Suwa Y, Suzuki S, Watanabe A, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. Int J Colorectal Dis. (2014) 29:343–51. doi: 10.1007/s00384-013-1803-9
18. Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. (2003) 361:2032–5. doi: 10.1016/S0140-6736(03)13640-9
19. Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Siracuse JJ, Schermerhorn ML. Body mass index: surgical site infections and mortality after lower extremity bypass from the National Surgical Quality Improvement Program 2005-2007. Ann Vasc Surg. (2010) 24:48–56. doi: 10.1016/j.avsg.2009.05.003
20. Bale E, Berrecloth R. The obese patient. Anaesthetic issues: airway and positioning. J Perioper Pract. (2010) 20:294–9. doi: 10.1177/175045891002000805
21. Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. (2011) 25:27–36. doi: 10.1016/j.bpa.2010.12.002
22. Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity. (2008) 16:442–50. doi: 10.1038/oby.2007.36
23. Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg. (2009) 250:166–72. doi: 10.1097/SLA.0b013e3181ad8935
24. Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg. (2013) 37:2561–8. doi: 10.1007/s00268-013-2162-y
25. Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. (2009) 55:592–99. doi: 10.1016/j.eururo.2008.12.028
26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
27. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. (2009) 182:844–53. doi: 10.1016/j.juro.2009.05.035
28. Valentijn TM, Galal W, Tjeertes EK, Hoeks SE, Verhagen HJ, Stolker RJ. The obesity paradox in the surgical population. Surg. (2013) 11:169–76. doi: 10.1016/j.surge.2013.02.003
29. Nafiu OO, Kheterpal S, Moulding R, Picton P, Tremper KK, Campbell DA, et al. The association of body mass index to postoperative outcomes in elderly vascular surgery patients. Anesth Analg. (2011) 112:23–9. doi: 10.1213/ANE.0b013e3181fcc51a
30. Abdullah N, Dalela D, Barod R, Larson J, Johnson M, Mass A, et al. Robotic partial nephrectomy for renal tumours in obese patients: perioperative outcomes in a multi-institutional analysis. Can Urol Assoc J. (2015) 9:859. doi: 10.5489/cuaj.3197
31. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. (2006) 355:763–78. doi: 10.1056/NEJMoa055643
32. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. (1999) 341:1097–105. doi: 10.1056/NEJM199910073411501
33. Bertrand LA, Thomas LJ, Li P, Buchta CM, Boi SK, Orlandella RM, et al. Obesity as defined by waist circumference but not body mass index is associated with higher renal mass complexity. Urol Oncol. (2017) 35:661.e1–e6. doi: 10.1016/j.urolonc.2017.06.058
34. Aboumarzouk OM, Stein RJ, Haber GP, Kaouk J, Chlosta PL, Somani BK. Laparoscopic partial nephrectomy in obese patients: a systematic review and meta-analysis. BJU Int. (2012) 110:1244–50. doi: 10.1111/j.1464-410X.2012.11094.x
35. Kaneko G, Miyajima A, Kikuchi E, Nakagawa K, Oya M. The benefit of laparoscopic partial nephrectomy in high body mass index patients. Jpn J Clin Oncol. (2012) 42:619–24. doi: 10.1093/jjco/hys061
36. Anderson KM, Lindler TU, Lamberton GR, Baron PW, Ojogho OK, Baldwin DD. Laparoscopic donor nephrectomy: effect of perirenal fat upon donor operative time. J Endourol. (2008) 22:2269–74. doi: 10.1089/end.2008.9725
37. Ioffe E, Hakimi AA, Oh SK, Agalliu I, Ginzburg N, Williams SK, et al. Effect of visceral obesity on minimally invasive partial nephrectomy. Urology. (2013) 82:612–8. doi: 10.1016/j.urology.2013.04.058
38. Wiens EJ, Pruthi DK, Chhibba R, McGregor TB. Feasibility of laparoscopic partial nephrectomy in the obese patient and assessment of predictors of perioperative outcomes. Urol Ann. (2017) 9:27–31. doi: 10.4103/0974-7796.198888
39. Sharma V, Aggarwal A, McGuire BB, Rambachan A, Matulewicz RS, Kim JY, et al. Open vs minimally invasive partial nephrectomy: assessing the impact of bmi on postoperative outcomes in 3685 cases from national data. J Endourol. (2015) 29:561–7. doi: 10.1089/end.2014.0608
Keywords: partial nephrectomy, minimally invasive, obesity paradox, BMI, complications
Citation: Kott O, Golijanin B, Pereira JF, Chambers A, Knasin A, Tucci C and Golijanin D (2020) The BMI Paradox and Robotic Assisted Partial Nephrectomy. Front. Surg. 6:74. doi: 10.3389/fsurg.2019.00074
Received: 16 July 2019; Accepted: 09 December 2019;
Published: 09 January 2020.
Edited by:
Andreas Becker, Goethe University Frankfurt, GermanyReviewed by:
Christian Paul Meyer, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2020 Kott, Golijanin, Pereira, Chambers, Knasin, Tucci and Golijanin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ohad Kott, b2hhZGtvdHRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.