- 1EPIUnit – Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal
- 2Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
Background: The COVID-19 pandemic is an emerging concern regarding the potential adverse effects during pregnancy. This study reviews knowledge on the impact of COVID-19 on pregnancy and describes the outcome of published cases of pregnant women diagnosed with COVID-19.
Methods: Searches were conducted in PubMed®, Scopus®, Web of Science®, and MedRxiv® up to 26th June 2020, using PRISMA standards, to identify original published studies describing pregnant women at any gestational age diagnosed COVID-19. There were no date or language restrictions on the search. All identified studies were included irrespective of assumptions on study quality.
Results: We identified 161 original studies reporting 3,985 cases of pregnant women with COVID-19 (1,007 discharged while pregnant). The 2,059 published cases with pregnancy outcomes resulted in 42 abortions, 21 stillbirths, and 2,015 live births. Preterm birth occurred in 23% of cases. Around 6% of pregnant women required admission to an intensive care unit and 28 died. There were 10 neonatal deaths. From the 163 cases with amniotic fluid, placenta, and/or cord blood analyzed for the SARS-CoV-2 virus, 10 were positive. Sixty-one newborns were positive for SARS-CoV-2. Four breast milk samples from 92 cases showed evidence of SARS-CoV-2.
Conclusion: Emerging evidence suggests that vertical transmission is possible, however, there is still a limited number of reported cases with intrapartum samples. Information, counseling and adequate monitoring are essential to prevent and manage adverse effects of SARS-CoV-2 infection during pregnancy.
Introduction
The disease resulting from infection with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) and designated COVID-19 by the World Health Organization (WHO) was first identified in humans in December 2019, in the city of Wuhan, China (1), and can present from asymptomatic to a severe acute respiratory infection requiring intensive care (2, 3). The infection can occur at any age, but COVID-19 is proportionally uncommon in children (<1% of the total cases). The infection fatality rate is around 1% but much higher in older people or those with pre-existing medical conditions (such as heart disease, diabetes, chronic obstructive pulmonary disease) (2, 4).
Person-to-person transmission of COVID-19 is well-established and can occur when an infected person coughs, sneezes, or speaks and scattered droplets are inhaled or reach the mucous membranes of the mouth, nose, or eyes of susceptible. COVID-19 can also be transmitted through direct hand contact with surfaces or objects contaminated with SARS-CoV-2 followed by contact with the mouth, nose, or eyes (2).
Pregnant women and newborns receive special attention and there is an emerging concern with the potential risk of SARS-COV-2 vertical transmission (from mother to fetus) or associated malformations, and contagion during delivery and breastfeeding; likewise, it is important to determine the potential adverse effects of COVID-19 in pregnant women (5–7). Considering the rapidly evolving of the COVID-19 pandemic, which is reflected in the current lack of high-quality evidence, we aimed to review the published cases of pregnant women diagnosed with COVID-19.
Methods
The review follows the Preferred Reporting of Systematic Reviews and Meta-Analysis (PRISMA) guidelines (8, 9). This review was not registered with PROSPERO. We searched PubMed®, Scopus®, Web of Science®, and MedRxiv® electronic databases up to 26th June 2020 to identify original published studies describing pregnant women at any gestational age diagnosed with COVID-19 [confirmed by clinical/radiological evidence of pneumonia compatible with SARS-CoV-2 and/or by quantitative real-time polymerase chain reaction (PCR) or dual fluorescence PCR of SARS-CoV-2 infection]. The following search expression was used [(COVID-19 OR 2019-nCoV OR “novel coronavirus” OR SARS-CoV-2 OR “coronavirus 2”) AND (pregnancy OR delivery OR pregnant OR obstetric* OR maternal OR perinatal OR breastfeeding)]. Also, reference tracking was carried out to identify other potential studies to be included.
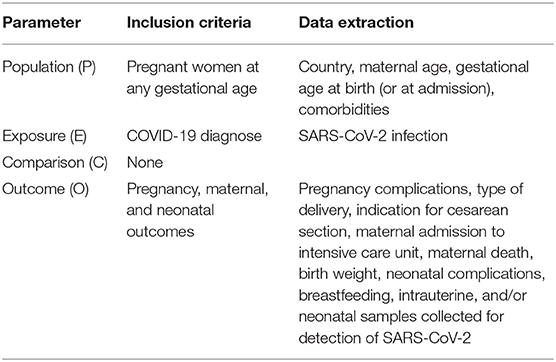
The PECO (Population, Exposure, Comparison, Outcome) structure design was used to define exposure, outcome, as well as inclusion and exclusion criteria for the review (Table 1). The question was “What are the main obstetric, maternal, and neonatal outcomes of SARS-CoV-2 infection during pregnancy and the potential risk of vertical transmission?”
Each reference retrieved was screened independently by two researchers following predefined criteria to determine eligibility for the systematic review. Studies were excluded if: (1) did not involve humans (e.g., in vitro or animal research); (2) non-original articles (e.g., book chapters, review articles, guidelines); (3) data not reporting pregnant women diagnosed with COVID-19; (4) only indicate prevalence estimations among pregnant women, with no description of perinatal outcomes; and (5) reporting breastfeeding after puerperium period, with no information about pregnancy. There were no date or language restrictions on the search.
Two researchers independently reviewed the included studies and extracted the following data: type of study, data collection period, maternal age, comorbidities and pregnancy complications, type of delivery, indication for cesarean section, gestational age at birth (or at admission or diagnosis), pregnancy outcome, maternal admission to intensive care unit (including mechanical ventilation), maternal death, neonatal outcomes (birth weight, neonatal complications, neonatal death, breastfeeding), intrauterine and/or neonatal samples collected for detection of SARS-CoV-2 (such as amniotic fluid, umbilical cord blood, placenta, breast milk, nasopharyngeal, and anal swabs), and their results (negative/positive and/or reactive/non-reactive).
All identified original observational studies reporting cases of pregnant women at any gestational age diagnosed with COVID-19 were included irrespective of study quality (preprints preliminary reports were also included). The research design of the studies was described based on the authors' classification, except for case reports which were considered when the manuscript described only one case.
Doubts on possible duplicates and/or differences in the data extraction were discussed and resolved by consensus, involving a third researcher whenever necessary.
Cases reported in more than one study, and for which it was possible to identify duplicates, were described only once, presenting the more detailed data. We identified duplicates based on author names and hospital location, publication date, participant admission date, maternal and neonatal characteristics, and outcomes. We did not contact the corresponding authors because of the time constraints and the importance to have immediate results.
Considering the heterogeneity observed across the studies, we decided to perform a narrative synthesis using the Synthesis Without Meta-analysis (SWiM) reporting guideline (intended to complement the PRISMA guidelines) (10). Descriptive statistics were presented (frequency and proportions) based on the total cases with available information.
Results
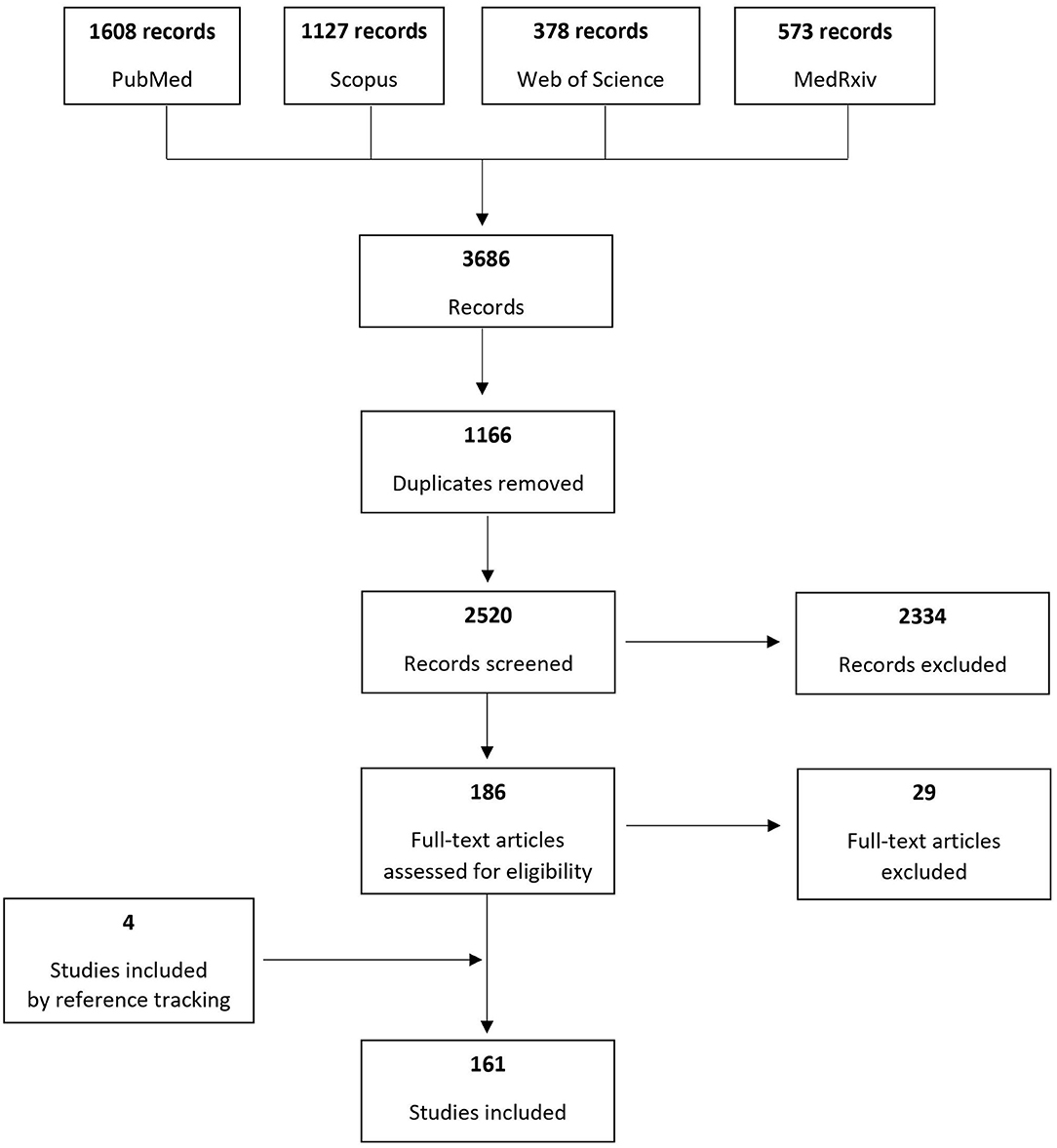
Based on the search of the four electronic databases, 3,686 records were identified and after removing duplicates 2,520 were screened to assess their eligibility for inclusion. One hundred sixty-one original studies published until June 26th, 2020 were included, reporting cases of pregnant women at any gestational age diagnosed with COVID-19. Figure 1 presents the process of study inclusion in the systematic review.
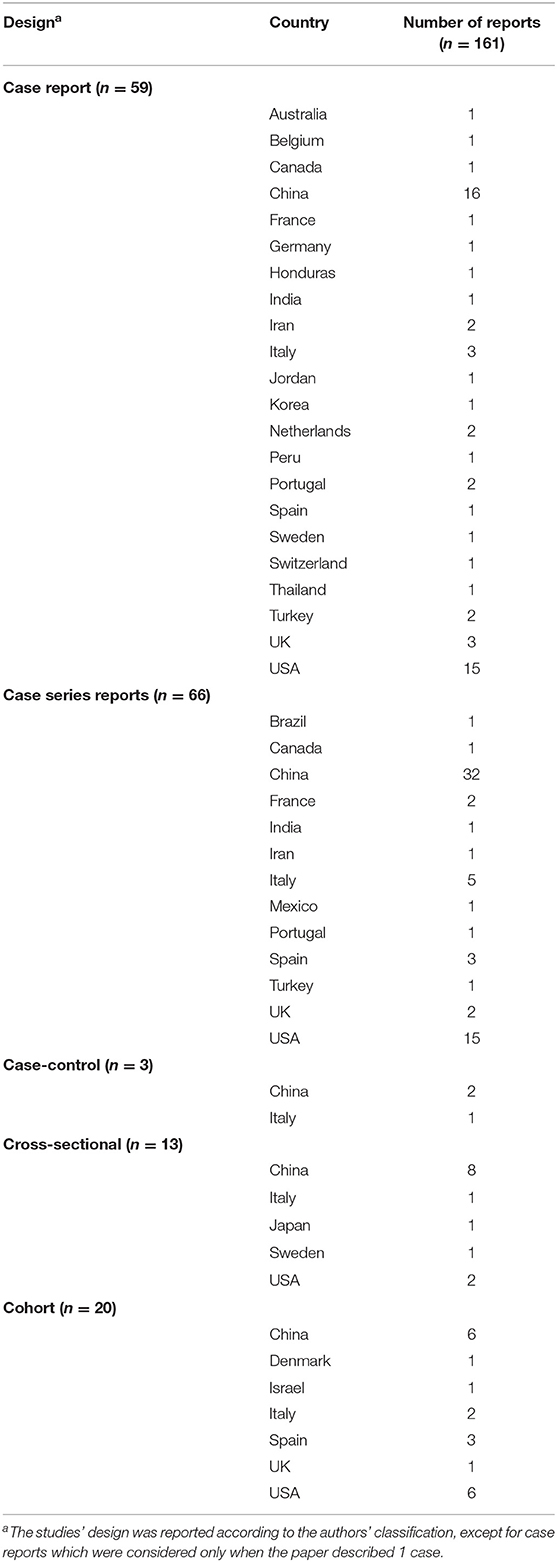
From the 161 studies included, 66 were case series reports (48% from China and 23% from USA) (11–76), 59 case reports (27% from China and 25% from USA) (77–135), 20 cohort studies (30% from China, 30% from USA, 15% from Spain) (136–155), 13 cross-sectional (62% China and 15% USA) (156–168), and 3 case-control studies (2 from China and 1 from Italy) (169–171). Table 2 shows the distribution of studies according to the study design.
The studies reported 3,985 pregnant women diagnosed with COVID-19 and the main characteristics are summarized in the Table 3. A detailed description of reported cases is presented in Supplementary Table 1.
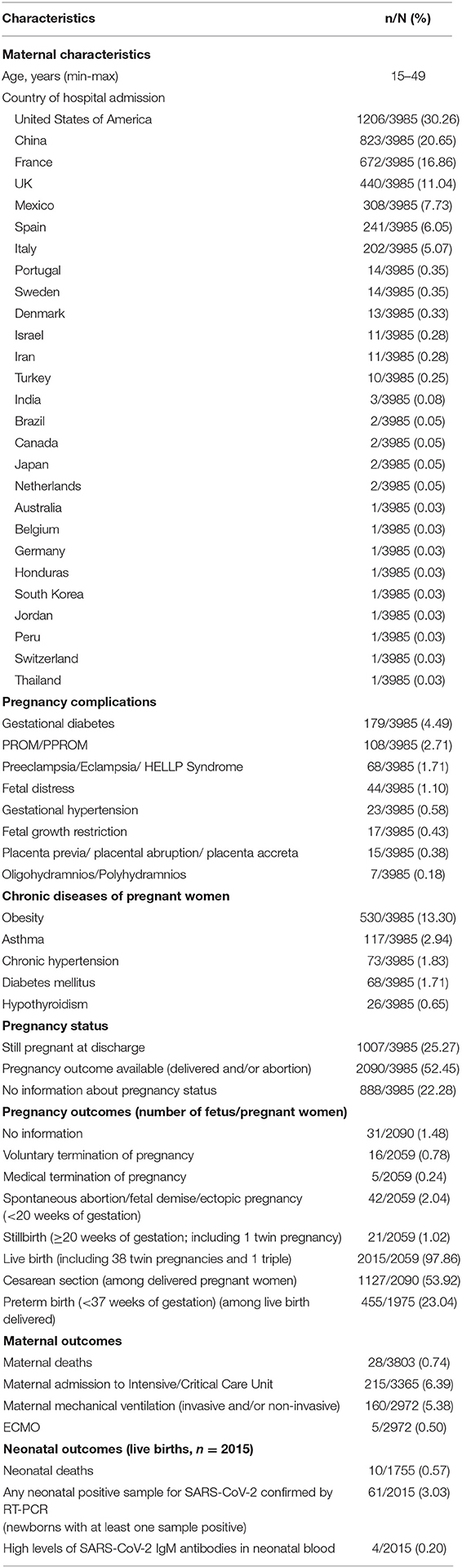
Table 3. Characteristics of pregnant women diagnosed with COVID-19 described in the literature (n = 3985).
The majority of cases occurred in the USA (n = 1,206, 30%), China (n = 823, 21%), France (n = 672, 17%), UK (n = 440, 11%), Mexico (n = 308, 8%), Spain (n = 241, 6%), and Italy (n = 202, 5%). Maternal age ranged from 15 to 49 years.
From the 3,985 pregnant women described, 2090 (52.4%) had a pregnancy outcome, 1007 (25.3%) were discharged during pregnancy (undelivered) and 888 (22.3%) had no information. From those with available information on gestational age (n = 1,896), 89% of women were in the third trimester of pregnancy (n = 1,685) and only 5% in the first trimester (n = 101).
Vertical Transmission of COVID-19
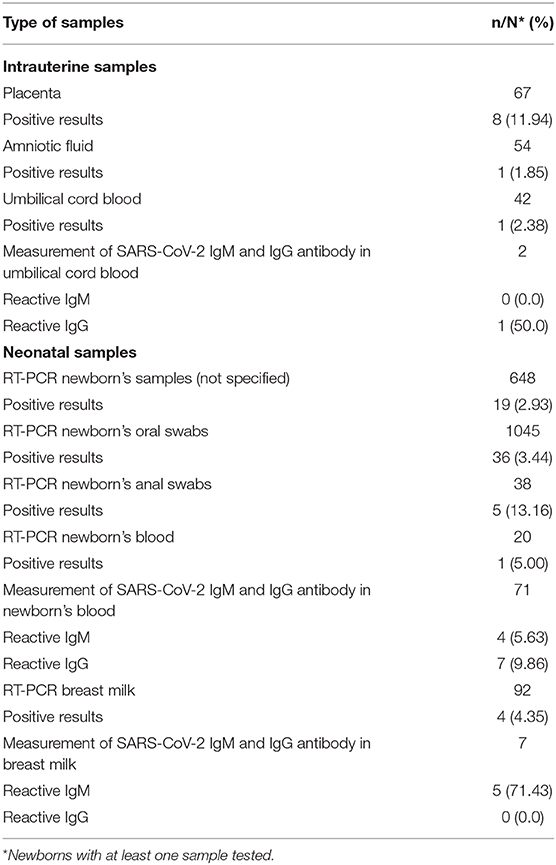
Among the intrauterine samples analyzed, 11.9% of placentas (n = 8/67), 1.8% of amniotic fluid (n = 1/54), and 2.4% umbilical cord blood (n = 1/42) were positive for SARS-CoV-2 virus (Table 4).
We identified 61 (3.0%) newborns with at least one positive sample for SARS-CoV-2 confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) (Table 3). Most studies detected the SARS-CoV-2 RNA by RT-PCR using samples from the newborn's nasopharyngeal or throat (n = 1,045), sample collection varying from immediately to 17 days after birth. Neonatal serum samples were tested in 71 newborns, being four reactive for IgM and seven for IgG (Table 4).
From the 92 newborns with at least one breast milk sample tested (sample collection varying from immediately after birth to 15 days after), 4 were positive for SARS-CoV-2 virus by RT-PCR and 5 reactive for IgM antibodies (Table 4). One study reported 3 out of 5 positive breast milk samples collected during the first 5 days after birth, neonatal nasopharyngeal samples were negative on day 1 and 5 and the newborn received expressed breast milk (15).
Maternal and Neonatal Outcomes
The obstetric conditions most frequently reported were gestational diabetes (4.5%), premature rupture of membranes (PROM/PPROM) (2.7%), pre-eclampsia/eclampsia/HELLP syndrome (1.7%), fetal distress (1.1%), gestational hypertension (0.6%), fetal growth restriction (0.4%), placenta previa/placental abruption/ placenta accreta (0.4%), and oligohydramnios/polyhydramnios (0.2%) (Table 3). The included studies reported 13.3% of obesity among pregnant women with COVID-19, 2.9% of asthma, 1.8% of chronic hypertension, and 1.7% of diabetes mellitus (Table 3).
Two hundred and fifteen pregnant women required admission to an intensive care unit (6.4%), 5.4% were mechanically ventilated (n = 160), and 0.5% required ECMO (n = 15). Twenty-eight maternal deaths with COVID-19 were reported (0.7%).
Among 2,059 pregnant women with pregnancy outcomes available, 16 (0.8%) resulted in termination of pregnancy due to maternal concerns regarding COVID-19 and 5 (0.2%) by medical reasons; 42 (2.0%) were spontaneous abortions/fetal demise (<20 weeks of gestation), 21 (1.0%) stillbirths (≥20 weeks of gestation, including one twin pregnancy); and 2015 (97.9%) live births (including 38 twin pregnancies and one triple pregnancy). Cesarean section was the most common type of delivery: 53.9% among 2,090 delivered pregnant women. Preterm birth occurred in 23.0% (455/1975) among live birth delivered and with available information on gestational age. Most of preterm births were iatrogenic for maternal and/or fetal compromise. There were 10 neonatal deaths (0.6%).
Although most of breast milk samples from COVID-19 infected mothers have tested negative for the SARS-CoV-2 virus, most infants did not receive any breast milk, considering those with available information (59.9%, n = 227/379).
Discussion
Worldwide, the incidence of infection in pregnant women at any gestational age is still unclear, as universal screening tests are not generally used, except in the presence of symptoms or at admission for delivery. In a New York's hospital that implemented SARS-CoV-2 testing in all pregnant women admitted for delivery, 15.4% of them were positive for SARS-CoV-2, but 87.9% were asymptomatic (172).
The clinical characteristics of COVID-19 were similar to those described in non-pregnant women, presenting mild or moderate symptoms (11, 19, 21, 32, 76, 160). A systematic review summarized the clinical manifestations of 108 pregnant women with confirmed COVID-19 and most of them presented fever (68%) and coughing (34%), and lymphocytopenia (59%) with elevated C-reactive protein (70%) (173).
Furthermore, pregnant women do not appear to be at increased risk of severe illness of SARS-CoV-2 infection compared with non-pregnant women in the general population (6, 145, 174). However, a study from Sweden suggests that the risk of being admitted to an intensive care unit may be higher in pregnant and postpartum women (n = 13) compared with non-pregnant women of similar age (n = 40) (156). Recently, the Centers for Disease Control and Prevention (CDC) COVID-19 surveillance indicates an increased risk of intensive care unit admissions (1 in 68 of pregnant vs. 1 in 110 non-pregnant women, crude risk ratio 1.6, 95% CI 1.3–1.9) and mechanical ventilation (1 in 195 of pregnant vs. 1 in 370 non-pregnant women, crude risk ratio 1.9, 95% CI 1.4–2.6), but no increase was observed in the rate of mortality (1 in 513 of pregnant vs. 1 in 400 of non-pregnant women, crude risk ratio 0.8, 95% CI 0.5–1.3) (175). However, a more severe presentation of COVID-19 is commonly described in pregnant women with chronic conditions, such as obesity, asthma, and diabetes (174).
Although most of the published cases confirm the absence of transmission of the SARS-CoV-2 virus antenatally or intrapartum, emerging evidence suggests that vertical transmission is possible (28, 30, 75, 77, 82, 151). However, this is still controversial due to a reduced number of reported cases with intrapartum samples (placenta, amniotic fluid, umbilical cord blood), large variability in the type of biological material analyzed and the time of its collection. Results from our review reveal that only 7.8% of women with pregnancy outcome had at least one intrauterine sample analyzed (n = 163/2090). If vertical transmission occurred in all the positive cases reported, the proportion of neonatal infection would be around 6.1% (n = 10/163). It is important to highlight that only two studies (one from Italy and another from Canada) reported three cases with positive RT-PCR for SARS-CoV-2 simultaneously on placenta and neonatal nasopharyngeal swabs samples (51, 97). Two of these neonates had positive samples collected at birth. Additionally, one termination of pregnancy and one miscarriage presented positive placental samples, without evidence of fetal SARS-CoV-2 infection (106, 121). Two studies also used maternal and neonatal serum samples to test for immunoglobulins M (IgM) and G (IgG) antibodies (30, 77). In a series of six cases that had blood collected after delivery evaluated, two of the newborns had high levels of IgG and IgM antibodies (>10 AU/mL) and three had high values of IgG antibodies with normal levels of IgM, but in none SARS-CoV-2 virus was detected by RT-PCR in the neonatal oropharyngeal exudate (30). A case study also reported high values of IgM and IgG antibodies in the newborn's blood at days 1 and 15 after delivery, but with RT-PCR for SARS-CoV-2 negative in five samples of nasopharyngeal exudates collected between the first 2 h and the 16th day of life (77).
Regarding the effect of the SARS-CoV-2 virus on the fetus, no congenital malformation has been reported so far associated to COVID-19. Higher risk of preterm birth has been reported as we observed 23% of preterm birth in our review compared to the estimated global preterm birth rate that is around 10–15% (176). In one study that compared groups of pregnant women with and without COVID-19, there were no significant differences in the occurrence of gestational diabetes, severe pre-eclampsia, PROM, fetal distress, meconium-stained amniotic fluid, premature delivery, neonatal asphyxia, and procedures for severe post-partum bleeding (31).
The maternal and neonatal outcomes observed so far are quite different from the two most serious coronavirus-related previous epidemics (6, 177–190). The first also appeared in China, in 2002-03, and was characterized by severe respiratory infections caused by the Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) . The second occurred in 2012, initially in the Middle East, the Middle East Respiratory Syndrome—Coronavirus (MERS-CoV) (6, 177). These epidemics have demonstrated the ability of coronavirus to cause serious complications during pregnancy (179, 190), with worse prognosis in pregnant women than non-pregnant women (181, 191).
In the 2002 epidemic, 12 pregnant women were infected with SARS-CoV, with a fatality rate of 25% (190). Among the seven pregnant women infected in the first trimester, four had a miscarriage (190). Two of the five pregnant women infected during the second or third trimester had fetal growth restriction and four had a preterm delivery (one spontaneous; three induced by the maternal condition) (190). In a review of the pregnancy outcomes of 11 women infected with MERS-CoV, seven pregnant women required admission to the intensive care unit and three died, of which only one had one comorbidity (asthma). Two fetal deaths occurred, and three of nine newborns were preterm (179).
However, considering that SARS-CoV-2 has genetic homology and some clinical similarities to SARS-CoV and MERS-CoV, and the immunological and physiological changes that occur during pregnancy, such as in cell-mediated immunity or lung function, that affect both the susceptibility and the clinical severity of pneumonia, it is important to pay particular attention to the monitoring of pregnant women with COVID-19, because maternal and perinatal adverse outcomes are potentially relevant (6, 177). One study reported two asymptomatic pregnant women at admission for delivery that rapidly evolved to severe COVID-19 disease requiring admission to an intensive care unit (14). Also, we identified 28 maternal deaths, with an infection fatality rate less than 1%, being similar to the other general populations (4). One article from Iran reported seven maternal deaths among nine pregnant women with severe COVID-19, of which three resulted in four stillbirths (1 twin pregnancy) and one in two neonatal deaths (1 twin pregnancy) (41). After concluding our search on the electronic databases, a paper from Brazil reporting 124 maternal deaths of pregnant or postpartum women was published, describing 22.6% admissions to the intensive care unit, of whom 64% had invasive ventilation (192). This raises awareness for questions related to access to healthcare services which may impact on the natural history of disease and reflect worldwide disparities on maternal outcomes.
Thus, it is essential to prevent the infection of COVID-19 and any other viral respiratory infection, as these infections represent an increased risk for the pregnant woman and for the pregnancy itself (6, 193, 194). It is therefore extremely important that pregnant women adopt preventive actions for COVID-19 with great intensity (174). For managing suspected or confirmed SARS-CoV-2 infection in pregnant women, recommendations for health professionals and services have already been published (174, 193, 195–197).
Most women in this review had a cesarean section, many of them without a clear medical indication. The decision on the type of delivery in pregnant women with suspected or confirmed infection with COVID-19 should consider maternal and fetal clinical characteristics, as in normal practice, and not the diagnosis of COVID-19 infection per se. Thus, there is no obstetric contraindication to any mode of delivery, unless the pregnant woman's clinical condition implies an emergent decision (174).
Regarding breast milk samples, the reviewed studies described four cases with evidence of SARS-CoV-2, from the 92 reported (15, 74, 97, 148). Despite that, there is not enough scientific evidence to unequivocally state that there is possibility that mothers with COVID-19 can transmit the virus through breast milk. Therefore, recommendations should be based on the available data and the analogy with past circumstances and predictable costs and benefits. Breastfeeding is recognized as the best form of child feeding due to the countless benefits for both the mother and the newborn, including the protection against gastrointestinal and respiratory infections (198). Thus, considering the benefits of breastfeeding and the fact that the transmission of other respiratory viruses is insignificant through breast milk, there is no indication to stop breastfeeding. According to the recommendations of WHO/UNICEF (199) and the Center for Disease Control and Prevention (CDC) of the United States (193), women with suspected or confirmed infection with COVID-19 can initiate or continue breastfeeding as long as clinical conditions permit. The CDC indicates that the decision to initiate or continue breastfeeding must be determined by the mother with COVID-19, together with family members and health professionals (193).
The major strength of this review is the inclusion of all study designs, including case reports and case series. To the best of our knowledge, this is the first review presenting a detailed description of clinical outcomes of each case identified (Supplementary Table 1). Limitations of this systematic review should be acknowledged. Considerable heterogeneity was observed across the studies, which did not allow us to conduct a meta-analysis. On the other hand, we cannot guarantee that we were able to identify all the cases of pregnant women described in the literature. Possibly there were additional cases currently presented in other types of publications, such as reports. Also, considering the importance of summarizing all existing cases, we did not assess the quality of the studies included in this review. Several studies had missing outcome data and selective reporting bias could not be excluded. Additionally, there may be some cases which could be duplicated, namely the studies which did not describe clinical characteristics case by case.
Conclusion
According to this review, preterm delivery seems to be more frequent among pregnant women with COVID-19. There is emerging evidence on possible vertical transmission (three positive results simultaneously in the placental samples and neonatal oral swabs for SARS-CoV-2 were reported), but the clinical relevance of the fetal infection is unclear. So far, there is not enough scientific evidence to unequivocally state that there is possibility that mothers with COVID-19 can transmit the virus through breast milk.
The maternal fatality rate was below 1% among the reported cases and hospitalizations in intensive care were less than 7%. Although the complications appear to be similar to those of non-pregnant women, services must be prepared to attend to complications, especially in pregnant women with comorbidities. Information, counseling and adequate monitoring are essential to prevent and manage adverse effects of SARS-CoV-2 infection during pregnancy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
CR and HB: conceptualization, methodology, data curation, and original draft preparation. IB and RD: methodology, data curation, writing-reviewing, and editing. All authors read and approved the final manuscript.
Funding
This study was funded by national funding from the Foundation for Science and Technology - FCT, under the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (UIDB/04750/2020); the PhD Grant SFRH/BD/111794/2015 (CR) was co-funded by the FCT and the POCH/FSE Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a preprint at MedRxiv Rodrigues et al. (200). The authors acknowledge the contribution of Ana Alfredo, Anzhela Sorokina, and Pedro Pimenta to data extraction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.558144/full#supplementary-material
References
1. Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. (2020) 91:264–6. doi: 10.1016/j.ijid.2020.01.009
2. Caldas J, Tavares M. Epidemiologia da COVID-19 [Online]. Available online at: http://asset.youoncdn.com/ab296ab30c207ac641882479782c6c34/070b44658f5569888804a14826ae273c.pdf (accessed April 13, 2020).
3. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
4. Russell TW, Hellewell J, Jarvis CI, van Zandvoort K, Abbott S, Ratnayake R, et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill. (2020) 25:2000256. doi: 10.2807/1560-7917.ES.2020.25.12.2000256
5. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. (2020) 395:760–2. doi: 10.1016/S0140-6736(20)30365-2
6. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. (2020) 222:415–26. doi: 10.1016/j.ajog.2020.02.017
7. Favre G, Pomar L, Musso D, Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. (2020) 395:e40. doi: 10.1016/S0140-6736(20)30311-1
8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
9. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
10. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. (2020) 368:l6890. doi: 10.1136/bmj.l6890
11. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. (2020) 395:809–15. doi: 10.1016/S0140-6736(20)30360-3
12. Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. (2020) 23:177–80. doi: 10.1177/1093526620925569
13. Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. (2020) 2:100118. doi: 10.1016/j.ajogmf.2020.100118
14. Breslin N, Baptiste C, Miller R, Fuchs K, Goffman D, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM. (2020) 2:100111. doi: 10.1016/j.ajogmf.2020.100111
15. Buonsenso D, Costa S, Sanguinetti M, Cattani P, Posteraro B, Marchetti S, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. (2020) 37:869–72. doi: 10.1055/s-0040-1710541
16. Campbell KH, Tornatore JM, Lawrence KE, Illuzzi JL, Sussman LS, Lipkind HS, et al. Prevalence of SARS-CoV-2 among patients admitted for childbirth in Southern Connecticut. JAMA. (2020) 323:2520–2. doi: 10.1001/jama.2020.8904
17. Cao D, Yin H, Chen J, Tang F, Peng M, Li R, et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: a retrospective study. Int J Infect Dis. (2020) 95:294–300. doi: 10.1016/j.ijid.2020.04.047
18. Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. (2020) 382:e100. doi: 10.1056/NEJMc2009226
19. Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. (2020) 67:655–63. doi: 10.1007/s12630-020-01630-7
20. Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, et al. [Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases]. Zhonghua Bing Li Xue Za Zhi. (2020) 49:418–23. doi: 10.3760/cma.j.cn112151-20200225-00138
21. Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. (2020). doi: 10.1002/jmv.25789. [Epub ahead of print].
22. Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. [Pregnant women complicated with COVID-19: a clinical analysis of 3 cases]. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2020) 49:240–4. doi: 10.3785/j.issn.1008-9292.2020.03.08
23. Cooke WR, Billett A, Gleeson S, Jacques A, Place K, Siddall J, et al. SARS-CoV-2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol. (2020) 250:259–60. doi: 10.1016/j.ejogrb.2020.05.025
24. Doria M, Peixinho C, Laranjo M, Mesquita Varejao A, Silva PT. Covid-19 during pregnancy: a case series from an universally tested population from the north of Portugal. Eur J Obstet Gynecol Reprod Biol. (2020) 250:261–2. doi: 10.1016/j.ejogrb.2020.05.029
25. Fan C, Lei D, Fang C, Li C, Wang M, Liu Y, et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa226. [Epub ahead of print].
26. Fox NS, Melka S. COVID-19 in pregnant women: case series from one large New York City obstetrical practice. Am J Perinatol. (2020) 37:1002–4. doi: 10.1055/s-0040-1712529
27. Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. (2020) 215:127–32. doi: 10.2214/AJR.20.23072
28. Liu W, Wang Q, Zhang Q, Chen L, Chen J, Zhang B, et al. Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprints. (2020) 2020020373. Available online at: https://www.preprints.org/manuscript/202002.0373/v1
29. Luo Y, Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. (2020) 20:513–4. doi: 10.1016/S1473-3099(20)30191-2
30. Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. (2020) 323:1848–9. doi: 10.1001/jama.2020.4861
31. Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province]. Zhonghua Fu Chan Ke Za Zhi. (2020) 55:166–71. doi: 10.3760/cma.j.cn112141-20200218-00111
32. Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. (2020) 9:51–60. doi: 10.21037/tp.2020.02.06
33. Amorim MMR, Soligo Takemoto ML, Fonseca EBD. Maternal deaths with coronavirus disease 2019: a different outcome from low- to middle-resource countries? Am J Obstet Gynecol. (2020) 223:298–9. doi: 10.1016/j.ajog.2020.04.023
34. Andrikopoulou M, Madden N, Wen T, Aubey JJ, Aziz A, Baptiste CD, et al. Symptoms and critical illness among obstetric patients with coronavirus disease 2019 (COVID-19) infection. Obstet Gynecol. (2020) 136:291–9. doi: 10.1097/AOG.0000000000003996
35. Blitz MJ, Rochelson B, Minkoff H, Meirowitz N, Prasannan L, London V, et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol. (2020) 223:595–9 e5. doi: 10.1016/j.ajog.2020.06.020
36. Buonsenso D, Raffaelli F, Tamburrini E, Biasucci DG, Salvi S, Smargiassi A, et al. Clinical role of lung ultrasound for diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. (2020) 56:106–9. doi: 10.1002/uog.22055
37. Chen Y, Peng H, Wang L, Zhao Y, Zeng L, Gao H, et al. Infants born to mothers with a new coronavirus (COVID-19). Front Pediatr. (2020) 8:104. doi: 10.3389/fped.2020.00104
38. Deng G, Zeng F, Zhang L, Chen H, Chen X, Yin M. Characteristics of pregnant patients with COVID-19 and liver injury. J Hepatol. (2020) 73:989–91. doi: 10.1016/j.jhep.2020.06.022
39. Giannini A, Mantovani A, Vezzoli C, Franchini D, Finazzi P. Lung ultrasound for pregnant women admitted to ICU for Covid-19 pneumonia. Minerva Anestesiol. (2020) doi: 10.23736/S0375-9393.20.14726-6. [Epub ahead of print].
40. Govind A, Essien S, Karthikeyan A, Fakokunde A, Janga D, Yoong W, et al. Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol. (2020) 251:272–4. doi: 10.1016/j.ejogrb.2020.05.004
41. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE, et al. Maternal death due to COVID-19. Am J Obstet Gynecol. (2020) 223:109.e1–e16. doi: 10.1016/j.ajog.2020.04.030
42. Hu X, Gao J, Luo X, Feng L, Liu W, Chen J, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol. (2020) 136:65–7. doi: 10.1097/AOG.0000000000003926
43. Huang W, Zhao Z, He Z, Liu S, Wu Q, Zhang X, et al. Unfavorable outcomes in pregnant patients with COVID-19. J Infect. (2020) 81:e99–101. doi: 10.1016/j.jinf.2020.05.014
44. Jain P, Thakur A, Kler N, Garg P. Manifestations in neonates born to COVID-19 positive mothers. Indian J Pediatr. (2020) 87:644. doi: 10.1007/s12098-020-03369-x
45. Kayem G, Lecarpentier E, Deruelle P, Bretelle F, Azria E, Blanc J, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. (2020) 49:101826. doi: 10.1016/j.jogoh.2020.101826
46. Khan S, Jun L, Nawsherwan, Siddique R, Li Y, Han G, et al. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect. (2020) 26:788–90. doi: 10.1016/j.cmi.2020.03.034
47. Khan S, Peng L, Siddique R, Nabi G, Nawsherwan, Xue M, et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. (2020) 41:748–50. doi: 10.1017/ice.2020.84
48. Li L, Liu D, Yang L. Follow-up information about the four pregnant patients with coronavirus disease (COVID-19) pneumonia who were still in the hospital at the end of our study. AJR Am J Roentgenol. (2020) 215:W17–W8. doi: 10.2214/AJR.20.23247
49. Lokken EM, Walker CL, Delaney S, Kachikis A, Kretzer NM, Erickson A, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.05.031. [Epub ahead of print].
50. Lumbreras-Marquez MI, Campos-Zamora M, Lizaola-Diaz de Leon H, Farber MK. Maternal mortality from COVID-19 in Mexico. Int J Gynaecol Obstet. (2020) 150:266–7. doi: 10.1002/ijgo.13250
51. Patane L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. (2020) 2:100145. doi: 10.1016/j.ajogmf.2020.100145
52. Perrone S, Deolmi M, Giordano M, D'Alvano T, Gambini L, Corradi M, et al. Report of a series of healthy term newborns from convalescent mothers with COVID-19. Acta Biomed. (2020) 91:251–5.
53. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. (2020) 154:23–32. doi: 10.1093/ajcp/aqaa089
54. Sun M, Xu G, Yang Y, Tao Y, Pian-Smith M, Madhavan V, et al. Evidence of mother-to-newborn infection with COVID-19. Br J Anaesth. (2020) 125:e245–e7. doi: 10.1016/j.bja.2020.04.066
55. Vlachodimitropoulou Koumoutsea E, Vivanti AJ, Shehata N, Benachi A, Le Gouez A, Desconclois C, et al. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. (2020) 18:1648–52. doi: 10.1111/jth.14856
56. Xu L, Yang Q, Shi H, Lei S, Liu X, Zhu Y, et al. Clinical presentations and outcomes of SARS-CoV-2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull. (2020) 65:1537–42. doi: 10.1016/j.scib.2020.04.040
57. Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. (2020) 223:111 e1–e14. doi: 10.1016/j.ajog.2020.04.014
58. Yang P, Wang X, Liu P, Wei C, He B, Zheng J, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. (2020) 127:104356. doi: 10.1016/j.jcv.2020.104356
59. Yu N, Li W, Kang Q, Zeng W, Feng L, Wu J. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect Dis. (2020). doi: 10.1016/S1473-3099(20)30320-0. [Epub ahead of print].
60. Hijona Elosegui JJ, Carballo Garcia AL, Fernandez Risquez AC. [New evidences that discard the possible vertical transmission of SARS-CoV-2 during pregnancy]. Med Clin. (2020) 155:313–4. doi: 10.1016/j.medcle.2020.05.020
61. Hijona Elosegui JJ, Carballo Garcia AL, Fernandez Risquez AC, Bermudez Quintana M, Exposito Montes JF. Does the maternal-fetal transmission of SARS-CoV-2 occur during pregnancy? Rev Clin Esp. (2020). doi: 10.1016/j.rce.2020.06.001. [Epub ahead of print].
62. Hirshberg A, Kern-Goldberger AR, Levine LD, Pierce-Williams R, Short WR, Parry S, et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. (2020) 223:286–90. doi: 10.1016/j.ajog.2020.04.029
63. Juusela A, Nazir M, Gimovsky M. Two cases of coronavirus 2019-related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. (2020) 2:100113. doi: 10.1016/j.ajogmf.2020.100113
64. Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. (2020) 14:193–8. doi: 10.1007/s11684-020-0772-y
65. Lucarelli E, Behn C, Lashley S, Smok D, Benito C, Oyelese Y. Mechanical ventilation in pregnancy due to COVID-19: a cohort of three cases. Am J Perinatol. (2020) 37:1066–9. doi: 10.1055/s-0040-1713664
66. Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. (2020) 46:151530. doi: 10.1016/j.anndiagpath.2020.151530
67. Nie R, Wang S-S, Yang Q, Fan C-F, Liu Y-L, He W-C, et al. Clinical features and the maternal and neonatal outcomes of pregnant women with coronavirus disease 2019. medRxiv. (2020). doi: 10.1101/2020.03.22.20041061. [Epub ahead of print].
68. Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. (2020) 2:100133. doi: 10.1016/j.ajogmf.2020.100133
69. Pereira A, Cruz-Melguizo S, Adrien M, Fuentes L, Marin E, Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. (2020) 99:839–47. doi: 10.1111/aogs.13921
70. Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. (2020). doi: 10.1016/j.ajog.2020.06.022. [Epub ahead of print].
71. Silverstein JS, Limaye MA, Brubaker SG, Roman AS, Bautista J, Chervenak J, et al. Acute respiratory decompensation requiring intubation in pregnant women with SARS-CoV-2 (COVID-19). AJP Rep. (2020) 10:e169–e75. doi: 10.1055/s-0040-1712925
72. Wu C, Yang W, Wu X, Zhang T, Zhao Y, Ren W, et al. Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women. Virol Sin. (2020) 35:305–10. doi: 10.1007/s12250-020-00227-0
73. Yassa M, Birol P, Mutlu AM, Tekin AB, Sandal K, Tug N. Lung ultrasound can influence the clinical treatment of pregnant women with COVID-19. J Ultrasound Med. (2020). doi: 10.1002/jum.15367. [Epub ahead of print].
74. Zhu C, Liu W, Su H, Li S, Shereen MA, Lv Z, et al. Breastfeeding risk from detectable severe acute respiratory syndrome coronavirus 2 in breastmilk. J Infect. (2020) 81:452–82. doi: 10.1016/j.jinf.2020.06.001
75. Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. (2020) 20:559–64. doi: 10.1016/S1473-3099(20)30176-6
76. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. (2020). doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print].
77. Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. (2020) 323:1846–8. doi: 10.1001/jama.2020.4621
78. Iqbal SN, Overcash R, Mokhtari N, Saeed H, Gold S, Auguste T, et al. An uncomplicated delivery in a patient with Covid-19 in the United States. N Engl J Med. (2020) 382:e34. doi: 10.1056/NEJMc2007605
79. Lee DH, Lee J, Kim E, Woo K, Park HY, An J. Emergency cesarean section performed in a patient with confirmed severe acute respiratory syndrome Coronavirus-2 -a case report. Korean J Anesthesiol. (2020) 73:347–51. doi: 10.4097/kja.20116
80. Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. (2020) 26:1335–6. doi: 10.3201/eid2606.200287
81. Liao X, Yang H, Kong J, Yang H. Chest CT Findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Med J. (2020) 37:226–8. doi: 10.4274/balkanmedj.galenos.2020.2020.3.89
82. Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, et al. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. (2020) 71:853–7. doi: 10.1093/cid/ciaa225
83. Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. (2020) 71:844–6. doi: 10.1093/cid/ciaa200
84. Wen R, Sun Y, Xing QS. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect. (2020) 53:499–500. doi: 10.1016/j.jmii.2020.03.004
85. Xia H, Zhao S, Wu Z, Luo H, Zhou C, Chen X. Emergency Caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth. (2020) 124:e216–8. doi: 10.1016/j.bja.2020.02.016
86. Zambrano LI, Fuentes-Barahona IC, Bejarano-Torres DA, Bustillo C, Gonzales G, Vallecillo-Chinchilla G, et al. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis. (2020) 36:101639. doi: 10.1016/j.tmaid.2020.101639
87. Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. (2020) 158:e9–13. doi: 10.1016/j.chest.2020.03.039
88. Ahmed I, Azhar A, Eltaweel N, Tan BK. First COVID-19 maternal mortality in the UK associated with thrombotic complications. Br J Haematol. (2020) 190:e37–8. doi: 10.1111/bjh.16849
89. Algarroba GN, Rekawek P, Vahanian SA, Khullar P, Palaia T, Peltier MR, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. (2020) 223:275–8. doi: 10.1016/j.ajog.2020.05.023
90. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. (2020) 37:861–5. doi: 10.1055/s-0040-1710050
91. Bani Hani DA, Alsharaydeh I, Bataineh AM, Al Athamneh M, Qamileh I, Al-Baik A, et al. Successful anesthetic management in cesarean section for pregnant woman with COVID-19. Am J Case Rep. (2020) 21:e925512. doi: 10.12659/AJCR.925512
92. Blauvelt CA, Chiu C, Donovan AL, Prahl M, Shimotake TK, George RB, et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19). Obstet Gynecol. (2020) 136:46–51. doi: 10.1097/AOG.0000000000003949
93. Du Y, Wang L, Wu G, Lei X, Li W, Lv J. Anesthesia and protection in an emergency cesarean section for pregnant woman infected with a novel coronavirus: case report and literature review. J Anesth. (2020) 34:613–8. doi: 10.1007/s00540-020-02796-6
94. Gidlof S, Savchenko J, Brune T, Josefsson H. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand. (2020) 99:948–9. doi: 10.1111/aogs.13862
95. Hong L, Smith N, Keerthy M, Lee-Griffith M, Garcia R, Shaman M, et al. Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Rep Womens Health. (2020) 27:e00217. doi: 10.1016/j.crwh.2020.e00217
96. Kalafat E, Yaprak E, Cinar G, Varli B, Ozisik S, Uzun C, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. (2020) 55:835–7. doi: 10.1002/uog.22034
97. Kirtsman M, Diambomba Y, Poutanen SM, Malinowski AK, Vlachodimitropoulou E, Parks WT, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. (2020) 192:E647–50. doi: 10.1503/cmaj.200821
98. Kuhrt K, McMicking J, Nanda S, Nelson-Piercy C, Shennan A. Placental abruption in a twin pregnancy at 32 weeks' gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM. (2020) 2:100135. doi: 10.1016/j.ajogmf.2020.100135
99. Li J, Wang Y, Zeng Y, Song T, Pan X, Jia M, et al. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynaecol Obstet. (2020) 150:126–8. doi: 10.1002/ijgo.13189
100. Lowe B, Bopp B. COVID-19 vaginal delivery - A case report. Aust N Z J Obstet Gynaecol. (2020) 60:465–6. doi: 10.1111/ajo.13173
101. Lu D, Sang L, Du S, Li T, Chang Y, Yang XA. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. (2020) 92:1660–4. doi: 10.1002/jmv.25927
102. Lyra J, Valente R, Rosario M, Guimaraes M. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port. (2020) 33:429–31. doi: 10.20344/amp.13883
103. Nesr G, Garnett C, Bailey C, Koshy R, Arami S. Immune thrombocytopenia flare with mild COVID-19 infection in pregnancy: a case report. Br J Haematol. (2020) 190:e146–8. doi: 10.1111/bjh.16928
104. Peng Z, Wang J, Mo Y, Duan W, Xiang G, Yi M, et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. (2020) 13:818–20. doi: 10.1016/j.jiph.2020.04.004
105. Piersigilli F, Carkeek K, Hocq C, van Grambezen B, Hubinont C, Chatzis O, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health. (2020) 4:476–8. doi: 10.1016/S2352-4642(20)30140-1
106. Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. (2020) 323:2198–200. doi: 10.1001/jama.2020.7233
107. Mehta H, Ivanovic S, Cronin A, VanBrunt L, Mistry N, Miller R, et al. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep Womens Health. (2020) 27:e00220. doi: 10.1016/j.crwh.2020.e00220
108. Polonia-Valente R, Moucho M, Tavares M, Vilan A, Montenegro N, Rodrigues T. Vaginal delivery in a woman infected with SARS-CoV-2 - The first case reported in Portugal. Eur J Obstet Gynecol Reprod Biol. (2020) 250:253–4. doi: 10.1016/j.ejogrb.2020.05.007
109. Schnettler WT, Al Ahwel Y, Suhag A. Severe acute respiratory distress syndrome in coronavirus disease 2019-infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM. (2020) 2:100120. doi: 10.1016/j.ajogmf.2020.100120
110. Sharma KA, Kumari R, Kachhawa G, Chhabra A, Agarwal R, Sharma A, et al. Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int J Gynaecol Obstet. (2020) 150:116–8. doi: 10.1002/ijgo.13179
111. Taghizadieh A, Mikaeili H, Ahmadi M, Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respir Med Case Rep. (2020) 30:101090. doi: 10.1016/j.rmcr.2020.101090
112. Vallejo V, Ilagan JG. A Postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet Gynecol. (2020) 136:52–5. doi: 10.1097/AOG.0000000000003950
113. Xiong X, Wei H, Zhang Z, Chang J, Ma X, Gao X, et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J Med Virol. (2020). doi: 10.1002/jmv.25857. [Epub ahead of print].
114. Yu Y, Fan C, Bian J, YinShen. Severe COVID-19 in a pregnant patient admitted to hospital in Wuhan. Int J Gynaecol Obstet. (2020) 150:262–3. doi: 10.1002/ijgo.13232
115. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID-19 infection. Prenat Diagn. (2020). doi: 10.1002/pd.5713. [Epub ahead of print].
116. Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. (2020) 27:e00221. doi: 10.1016/j.crwh.2020.e00221
117. Browne PC, Linfert JB, Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. Am J Perinatol. (2020) 37:866–8. doi: 10.1055/s-0040-1709993
118. Carosso A, Cosma S, Borella F, Marozio L, Coscia A, Ghisetti V, et al. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. (2020) 249:98–9. doi: 10.1016/j.ejogrb.2020.04.023
119. González Romero D, Ocampo Pérez J, González Bautista L, Santana-Cabrera L. Pronóstico perinatal y de la paciente embarazada con infección por COVID-19. Rev Clin Esp. (2020) 220:533–4. doi: 10.1016/j.rce.2020.04.006
120. Hansen KA, Stovall DW. Ectopic pregnancy during coronavirus disease 2019 (COVID-19): To operate, or not to operate. Obstet Gynecol. (2020) 136:288–90. doi: 10.1097/AOG.0000000000003995
121. Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. (2020) 130:4947–53. doi: 10.1101/2020.04.30.20083907
122. Inchingolo R, Smargiassi A, Moro F, Buonsenso D, Salvi S, Del Giacomo P, et al. The diagnosis of pneumonia in a pregnant woman with coronavirus disease 2019 using maternal lung ultrasound. Am J Obstet Gynecol. (2020) 223:9–11. doi: 10.1016/j.ajog.2020.04.020
123. Indraccolo U. A pregnant woman and the SARS-CoV-2 infection: how are barriers easily crossed? Recenti Prog Med. (2020) 111:259–60. doi: 10.1701/3347.33190
124. Joudi N, Henkel A, Lock WS, Lyell D. Preeclampsia treatment in severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol MFM. (2020) 2:100146. doi: 10.1016/j.ajogmf.2020.100146
125. Kang X, Zhang R, He H, Yao Y, Zheng Y, Wen X, et al. [Anesthesia management in cesarean section for a patient with coronavirus disease 2019]. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2020) 49:249–52. doi: 10.3785/j.issn.1008-9292.2020.03.04
126. Kelly JC, Dombrowksi M, O'Neil-Callahan M, Kernberg AS, Frolova AI, Stout MJ. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am J Obstet Gynecol MFM. (2020) 2:100130. doi: 10.1016/j.ajogmf.2020.100130
127. Kleinwechter H, Laubner K. Coronaviruserkrankung 2019 (COVID-19) und Schwangerschaft. Der Diabetologe. (2020) 16:242–6. doi: 10.1007/s11428-020-00611-0
128. Lang GJ, Zhao H. Can SARS-CoV-2-infected women breastfeed after viral clearance? J Zhejiang Univ Sci B. (2020) 21:405–7. doi: 10.1631/jzus.B2000095
129. Panichaya P, Thaweerat W, Uthaisan J. Prolonged viral persistence in COVID-19 second trimester pregnant patient. Eur J Obstet Gynecol Reprod Biol. (2020) 250:263. doi: 10.1016/j.ejogrb.2020.05.030
130. Rabice SR, Altshuler PC, Bovet C, Sullivan C, Gagnon AJ. COVID-19 infection presenting as pancreatitis in a pregnant woman: a case report. Case Rep Womens Health. (2020) 27:e00228. doi: 10.1016/j.crwh.2020.e00228
131. Rosen MH, Axelrad J, Hudesman D, Rubin DT, Chang S. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis. (2020) 26:971–3. doi: 10.1093/ibd/izaa109
132. Schoenmakers S, Snijder P, Verdijk R, Kuiken T, Kamphuis S, Koopman L, et al. SARS-CoV-2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. medRxiv. (2020). doi: 10.1101/2020.06.08.20110437. [Epub ahead of print].
133. Tang MW, Nur E, Biemond BJ. Immune thrombocytopenia due to COVID-19 during pregnancy. Am J Hematol. (2020) 95:E191–2. doi: 10.1002/ajh.25877
134. Vibert F, Kretz M, Thuet V, Barthel F, De Marcillac F, Deruelle P, et al. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. Eur J Obstet Gynecol Reprod Biol. (2020) 250:257–8. doi: 10.1016/j.ejogrb.2020.05.022
135. Yilmaz R, Kilic F, Arican S, Hacibeyoglu G, Suslu H, Koyuncu M, et al. Anesthetic management for cesarean birth in pregnancy with the novel coronavirus (COVID-19). J Clin Anesth. (2020) 66:109921. doi: 10.1016/j.jclinane.2020.109921
136. Ferrazzi E, Frigerio L, Savasi V, Vergani P, Prefumo F, Barresi S, et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. Bjog. (2020) 127:1116–21. doi: 10.1111/1471-0528.16278
137. Griffin I, Benarba F, Peters C, Oyelese Y, Murphy T, Contreras D, et al. The impact of COVID-19 infection on labor and delivery, newborn nursery, and neonatal intensive care unit: prospective observational data from a single hospital system. Am J Perinatol. (2020) 37:1022–30. doi: 10.1055/s-0040-1713416
138. Khoury R, Bernstein PS, Debolt C, Stone J, Sutton DM, Simpson LL, et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City medical centers. Obstet Gynecol. (2020) 136:273–82. doi: 10.1097/AOG.0000000000004025
139. Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. (2020) 369:m2107. doi: 10.1136/bmj.m2107
140. la Cour Freiesleben N, Egerup P, Hviid KVR, Severinsen ER, Kolte AM, Westergaard D, et al. SARS-CoV-2 in first trimester pregnancy - does it affect the fetus? medRxiv. (2020). doi: 10.1101/2020.06.08.20125195. [Epub ahead of print].
141. London V, McLaren R Jr, Atallah F, Cepeda C, McCalla S, Fisher N, et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. (2020) 37:991–4. doi: 10.1055/s-0040-1712164
142. Luo Q, Chen L, Yao D, Zhu J, Zeng X, Xia L, et al. Safety of breastfeeding in mothers with SARS-CoV-2 infection. medRxiv. (2020). doi: 10.1101/2020.05.30.20033407. [Epub ahead of print].
143. Martinez-Perez O, Vouga M, Cruz Melguizo S, Forcen Acebal L, Panchaud A, Munoz-Chapuli M, et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA. (2020) 324:296–9. doi: 10.1001/jama.2020.10125
144. Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. (2020) 127:1374–80. doi: 10.1111/1471-0528.16339
145. Mohr-Sasson A, Chayo J, Bart Y, Meyer R, Sivan E, Mazaki-Tovi S, et al. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet. (2020) 302:629–34. doi: 10.1007/s00404-020-05655-7
146. Pierce-Williams RAM, Burd J, Felder L, Khoury R, Bernstein PS, Avila K, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. (2020) 2:100134. doi: 10.1016/j.ajogmf.2020.100134
147. Savasi VM, Parisi F, Patane L, Ferrazzi E, Frigerio L, Pellegrino A, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol. (2020) 136:252–8. doi: 10.1097/AOG.0000000000003979
148. Wu Y, Liu C, Dong L, Zhang C, Chen Y, Liu J, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. (2020) 127:1109–15. doi: 10.1111/1471-0528.16276
149. Yang H, Hu B, Zhan S, Yang LY, Xiong G. Effects of severe acute respiratory syndrome coronavirus 2 infection on pregnant women and their infants. Arch Pathol Lab Med. (2020) 144:1217–22. doi: 10.5858/arpa.2020-0232-SA
150. Yin M, Zhang L, Deng G, Han C, Shen M, Sun H, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection during pregnancy in China: a retrospective cohort study. medRxiv. (2020). doi: 10.1101/2020.04.07.20053744. [Epub ahead of print].
151. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. (2020) 174:722–5. doi: 10.1001/jamapediatrics.2020.0878
152. Blitz MJ, Grunebaum A, Tekbali A, Bornstein E, Rochelson B, Nimaroff M, et al. Intensive care unit admissions for pregnant and nonpregnant women with coronavirus disease 2019. Am J Obstet Gynecol. (2020) 223:290–1. doi: 10.1016/j.ajog.2020.05.004
153. San-Juan R, Barbero P, Fernandez-Ruiz M, Lopez-Medrano F, Lizasoain M, Hernandez-Jimenez P, et al. Incidence and clinical profiles of COVID-19 pneumonia in pregnant women: a single-centre cohort study from Spain. EClinicalMedicine. (2020) 23:100407. doi: 10.1016/j.eclinm.2020.100407
154. Vintzileos WS, Muscat J, Hoffmann E, John NS, Vertichio R, Vintzileos AM, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol. (2020) 223:284–6. doi: 10.1016/j.ajog.2020.04.024
155. Zeng Y, Lin L, Yan Q, Wei W, Xiang Yang B, Huang R, et al. Update on clinical outcomes of women with COVID-19 during pregnancy. Int J Gynaecol Obstet. (2020) 150:264–6. doi: 10.1002/ijgo.13236
156. Collin J, Byström E, Carnahan A, Ahrne M. Public health agency of Sweden's brief report: pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. (2020) 99:819–22. doi: 10.1111/aogs.13901
157. Ferrazzi EM, Frigerio L, Cetin I, Vergani P, Spinillo A, Prefumo F, et al. COVID-19 Obstetrics Task Force, Lombardy, Italy: executive management summary and short report of outcome. Int J Gynaecol Obstet. (2020) 149:377–8. doi: 10.1002/ijgo.13162
158. Gulersen M, Blitz MJ, Rochelson B, Nimaroff M, Shan W, Bornstein E. Clinical implications of SARS-CoV-2 infection in the viable preterm period. Am J Perinatol. (2020) 37:1077–83. doi: 10.1055/s-0040-1713851
159. Liao J, He X, Gong Q, Yang L, Zhou C, Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet. (2020) 150:53–7. doi: 10.1002/ijgo.13188
160. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. (2020) 80:e7–13. doi: 10.1016/j.jinf.2020.03.007
161. Liu P, Zheng J, Yang P, Wang X, Wei C, Zhang S, et al. The immunologic status of newborns born to SARS-CoV-2-infected mothers in Wuhan, China. J Allergy Clin Immunol. (2020) 146:101–9.e1. doi: 10.1016/j.jaci.2020.04.038
162. Ochiai D, Kasuga Y, Iida M, Ikenoue S, Tanaka M. Universal screening for SARS-CoV-2 in asymptomatic obstetric patients in Tokyo, Japan. Int J Gynaecol Obstet. (2020) 150:268–9. doi: 10.1002/ijgo.13252
163. Qadri F, Mariona F. Pregnancy affected by SARS-CoV-2 infection: a flash report from Michigan. J Matern Fetal Neonatal Med. (2020). doi: 10.1080/14767058.2020.1765334. [Epub ahead of print].
164. Qiancheng X, Jian S, Lingling P, Lei H, Xiaogan J, Weihua L, et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. (2020) 95:376–83. doi: 10.1016/j.ijid.2020.04.065
165. Wang Z, Wang Z, Xiong G. Clinical characteristics and laboratory results of pregnant women with COVID-19 in Wuhan, China. Int J Gynaecol Obstet. (2020). doi: 10.1002/ijgo.13265. [Epub ahead of print].
166. Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet. (2020) 150:58–63. doi: 10.1002/ijgo.13165
167. Zeng QL, Li GM, Ji F, Ma SH, Zhang GF, Xu JH, et al. Clinical course and treatment efficacy of COVID-19 near Hubei Province, China: a multicentre, retrospective study. Transbound Emerg Dis. (2020). doi: 10.1111/tbed.13674. [Epub ahead of print].
168. Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, et al. Novel coronavirus infection in newborn babies aged <28 days in China. Eur Respir J. (2020) 55:2000697. doi: 10.1183/13993003.00697-2020
169. Cosma S, Carosso A, Cusato J, Borella F, Carosso M, Bovetti M, et al. COVID-19 and first trimester spontaneous abortion: a case-control study of 225 pregnant patients. medRxiv. (2020). doi: 10.1101/2020.06.19.20135749. [Epub ahead of print].
170. Li N, Han L, Peng M, Lv Y, Ouyang Y, Liu K, et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa352. [Epub ahead of print].
171. Yang H, Sun G, Tang F, Peng M, Gao Y, Peng J, et al. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J Infect. (2020) 81:e40–4. doi: 10.1016/j.jinf.2020.04.003
172. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. (2020) 382:2163–4. doi: 10.1056/NEJMc2009316
173. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. (2020) 99:823–9. doi: 10.1111/aogs.13867
174. Royal College of Obstetricians and Gynaecologists Royal College of Midwives Royal College of Paediatrics and Child Health Public Health England and Health Protection Scotland. Coronavirus (COVID-19) Infection in Pregnancy: Information for Healthcare Professionals (Version 11, July 24th 2020). [Online]. Available online at: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/ (accessed August 1, 2020).
175. Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:769–75. doi: 10.15585/mmwr.mm6925a1
176. Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
177. Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. (2020) 12:194. doi: 10.3390/v12020194
178. Stockman LJ, Lowther SA, Coy K, Saw J, Parashar UD. SARS during pregnancy, United States. Emerg Infect Dis. (2004) 10:1689–90. doi: 10.3201/eid1009.040244
179. Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. (2019) 52:501–3. doi: 10.1016/j.jmii.2018.04.005
180. Alserehi H, Wali G, Alshukairi A, Alraddadi B. Impact of Middle East Respiratory Syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect Dis. (2016) 16:105. doi: 10.1186/s12879-016-1437-y
181. Maxwell C, McGeer A, Tai KFY, Sermer M. No. 225-Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J Obstet Gynaecol Can. (2017) 39:e130–7. doi: 10.1016/j.jogc.2017.04.024
182. Assiri A, Abedi GR, Al Masri M, Bin Saeed A, Gerber SI, Watson JT. Middle east respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. (2016) 63:951–3. doi: 10.1093/cid/ciw412
183. Haines CJ, Chu YW, Chung TK. The effect of Severe Acute Respiratory Syndrome on a hospital obstetrics and gynaecology service. Bjog. (2003) 110:643–5. doi: 10.1046/j.1471-0528.2003.03007.x
184. Jeong SY, Sung SI, Sung JH, Ahn SY, Kang ES, Chang YS, et al. MERS-CoV infection in a pregnant woman in Korea. J Korean Med Sci. (2017) 32:1717–20. doi: 10.3346/jkms.2017.32.10.1717
185. Jiang X, Gao X, Zheng H, Yan M, Liang W, Shao Z, et al. Specific immunoglobulin g antibody detected in umbilical blood and amniotic fluid from a pregnant woman infected by the coronavirus associated with severe acute respiratory syndrome. Clin Diagn Lab Immunol. (2004) 11:1182–4. doi: 10.1128/CDLI.11.6.1182-1184.2004
186. Malik A, El Masry KM, Ravi M, Sayed F. Middle east respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg Infect Dis. (2016) 22:515–7. doi: 10.3201/eid2203.151049
187. Owolabi T, Kwolek S. Managing obstetrical patients during severe acute respiratory syndrome outbreak. J Obstet Gynaecol Can. (2004) 26:35–41. doi: 10.1016/S1701-2163(16)30694-6
188. Payne DC, Iblan I, Alqasrawi S, Al Nsour M, Rha B, Tohme RA, et al. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. (2014) 209:1870–2. doi: 10.1093/infdis/jiu068
189. Robertson CA, Lowther SA, Birch T, Tan C, Sorhage F, Stockman L, et al. SARS and pregnancy: a case report. Emerg Infect Dis. (2004) 10:345–8. doi: 10.3201/eid1002.030736
190. Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. (2004) 191:292–7. doi: 10.1016/j.ajog.2003.11.019
191. Lam CM, Wong SF, Leung TN, Chow KM, Yu WC, Wong TY, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. (2004) 111:771–4. doi: 10.1111/j.1471-0528.2004.00199.x
192. Takemoto MLS, Menezes MO, Andreucci CB, Nakamura-Pereira M, Amorim MMR, Katz L, et al. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int J Gynaecol Obstet. (2020). doi: 10.1002/ijgo.13300. [Epub ahead of print].
193. Centers for Disease Control and Prevention (CDC). If You Are Pregnant, Breastfeeding, or Caring for Young Children. [Online]. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html (accessed July 29, 2020).
194. Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. (2020) 55:435–7. doi: 10.1002/uog.22006
195. Favre G, Pomar L, Qi X, Nielsen-Saines K, Musso D, Baud D. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect Dis. (2020) 20:652–3. doi: 10.1016/S1473-3099(20)30157-2
196. Liang H, Acharya G. Novel corona virus disease (COVID-19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. (2020) 99:439–42. doi: 10.1111/aogs.13836
197. Poon LC, Yang H, Lee JCS, Copel JA, Leung TY, Zhang Y, et al. ISUOG Interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. (2020) 55:700–8. doi: 10.1002/uog.22013
198. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387:475–90. doi: 10.1016/S0140-6736(15)01024-7
199. UNICEF/World Health Organization. Coronavirus Disease (COVID-19): What Parents Should Know. [Online]. Available online at: https://www.unicef.org/stories/novel-coronavirus-outbreak-what-parents-should-know (accessed July 29, 2020).
Keywords: COVID-19, pregnancy, vertical transmission, perinatal outcomes, breastfeeding
Citation: Rodrigues C, Baía I, Domingues R and Barros H (2020) Pregnancy and Breastfeeding During COVID-19 Pandemic: A Systematic Review of Published Pregnancy Cases. Front. Public Health 8:558144. doi: 10.3389/fpubh.2020.558144
Received: 01 May 2020; Accepted: 29 October 2020;
Published: 23 November 2020.
Edited by:
Saralee Glasser, Gertner Institute for Epidemiology and Health Policy Research, IsraelReviewed by:
Sohinee Bhattacharya, University of Aberdeen, United KingdomVan Tong, Centers for Disease Control and Prevention (CDC), United States
Copyright © 2020 Rodrigues, Baía, Domingues and Barros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carina Rodrigues, Y2FyaW5hLnJvZHJpZ3Vlc0Bpc3B1cC51cC5wdA==
†These authors have contributed equally to this work
 Carina Rodrigues
Carina Rodrigues Inês Baía
Inês Baía Rosa Domingues
Rosa Domingues Henrique Barros
Henrique Barros