
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL STUDY PROTOCOL article
Front. Public Health, 17 September 2020
Sec. Planetary Health
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.00500
 Christopher D. Golden1,2,3,4*†
Christopher D. Golden1,2,3,4*† Benjamin L. Rice4,5†
Benjamin L. Rice4,5† Hervet J. Randriamady4
Hervet J. Randriamady4 Arisoa Miadana Vonona4
Arisoa Miadana Vonona4 Jean Frederick Randrianasolo4
Jean Frederick Randrianasolo4 Ambinintsoa Nirina Tafangy4
Ambinintsoa Nirina Tafangy4 Mamy Yves Andrianantenaina4
Mamy Yves Andrianantenaina4 Nicholas J. Arisco3
Nicholas J. Arisco3 Gauthier N. Emile4
Gauthier N. Emile4 Faustin Lainandrasana4
Faustin Lainandrasana4 Robuste Fenoarison Faraniaina Mahonjolaza4
Robuste Fenoarison Faraniaina Mahonjolaza4 Hermann Paratoaly Raelson4
Hermann Paratoaly Raelson4 Vololoniaina Ravo Rakotoarilalao4
Vololoniaina Ravo Rakotoarilalao4 Anjaharinony Andry Ny Aina Rakotomalala4
Anjaharinony Andry Ny Aina Rakotomalala4 Alex Dominique Rasamison4
Alex Dominique Rasamison4 Rebaliha Mahery4
Rebaliha Mahery4 M. Luciano Tantely6
M. Luciano Tantely6 Romain Girod6
Romain Girod6 Akshaya Annapragada7
Akshaya Annapragada7 Amy Wesolowski8
Amy Wesolowski8 Amy Winter8
Amy Winter8 Daniel L. Hartl9
Daniel L. Hartl9 James Hazen10
James Hazen10 C. Jessica E. Metcalf5,11
C. Jessica E. Metcalf5,11Madagascar has experienced significant environmental change since 1960, particularly through forest clearing for agricultural expansion. Climatic patterns are undergoing change in Madagascar as well, with increasing temperatures, droughts, and cyclonic activity. The impact of these environmental and climatic changes will pose threats to food availability, income generation, and local ecosystems, with significant potential effects on the spatial and temporal distribution of disease burden. This study seeks to describe the health status of a large sample of geographically and socially diverse Malagasy communities through multiple clinical measurements, detailed social surveys, and paired data on regional variation in local ecologies. With an increased understanding of the current patterns of variation in human health and nutrition, future studies will be better able to identify associations with climate and anticipate and mitigate the burdens expected from larger, longer-term changes. Our mixed-method approach included an observational cross-sectional study. Research subjects were men, women, and children from 1,125 households evenly distributed across 24 communities in four ecologically and socio-demographically distinct regions of Madagascar. For these 1,125 households, all persons of both sexes and all ages therein (for a total of 6,292 individuals) were recruited into the research study and a total of 5,882 individuals were enrolled. Through repeated social survey recalls and focus group meetings, we obtained social and demographic data, including broad categories of seasonal movements, and characterized the fluctuation of income generation, food production and dietary consumption. Through collection of clinical and biological samples for both point-of-care diagnoses and laboratory analyses, we obtained detailed occurrence (and importantly co-occurrence) data on micronutrient nutritional, infectious disease, and non-communicable disease status. Our research highlights the highly variable social, cultural, and environmental contexts of health conditions in Madagascar, and the tremendous inter-regional, inter-community, and intra-community variation in nutritional and disease status. More than 30% of the surveyed population was afflicted by anemia and 14% of the population had a current malaria infection. This type of rich metadata associated with a suite of biological samples and nutritional and disease outcome data should allow disentangling some of the underlying drivers of ill health across the changing landscapes of Madagascar.
Madagascar is characterized by high social, cultural, and ecological variation across its geography (1). This spatial variation is extreme, as rainfall varies 10-fold between regions of Madagascar, and some regions experience some of the highest levels of inter-annual variation in rainfall seen globally (2). There is also temporal variation, as seasonal cycles in food availability, wealth, human movement, and local ecologies drive shifting patterns in health through effects on nutrition, infectious diseases, and non-communicable diseases. Environmental and socio-demographic change will therefore alter the underlying determinants of nutritional status and infectious and non-communicable disease risk. The purpose of this study was to characterize variation in nutrition and disease risk across social and ecological settings in Madagascar.
Madagascar has experienced significant environmental change since 1960, particularly through forest clearing for agricultural expansion (3, 4). The pace of deforestation varies across time and geographies within Madagascar, and has been shown to increase during periods of political upheaval (5). The process of regular forest clearing and burning alters local ecologies, with downstream effects on water and soil. The adverse consequences of these ecosystem changes lead to challenges in conserving Madagascar's exceptional biodiversity [e.g., (6)], and they also significantly affect risks of malnutrition (7, 8) and disease (1, 9).
Climatic patterns are undergoing change in Madagascar as well. Historical records from 1961 to 2005 demonstrate an increasing temperature in 67% of locations across the island nation, with mean temperature expected to increase by 2.0–6.5°C by 2100 (10). Long-term trends indicate that temperature will increase, while rainfall is expected to increase in variability even further (1). Moreover, four general circulation models agree that the destructive potential of cyclones is expected to increase by 2–17% between 2060 and 2100 (10). Over the past 30 years, there have been more than thirty severe floods and five major droughts that have crippled the agricultural sector, killed hundreds of people and indirectly affected thousands to millions (1).
The impact of these environmental and climatic changes will pose threats to food availability, income generation, and local ecosystems, significantly affecting disease burden and the timing and magnitude of epidemics. Furthermore, the severity of impact will vary, in part, dependent on the baseline environmental, nutritional, and disease dynamics local to a region. However, few previous studies have characterized inter-regional variation in health in Madagascar. Therefore, this study seeks to describe the health status of a large sample of geographically and socially diverse Malagasy people through multiple clinical measurements and detailed social surveys. In addition, data were collected on regional variation in local ecologies, with a particular focus on mosquito vector habitats. Future studies of associations among climate, environment, and health can benefit from these new estimates and improve our ability to better anticipate and mitigate the burdens expected from larger, longer-term environmental and societal changes.
In this study protocol we describe the methods used to (i) quantify variation in disease risk within and between rural communities in Madagascar, (ii) characterize potential socio-economic determinants of disease and nutrition status including food availability and human movement patterns, and (iii) estimate regional differences in the diversity and abundances of habitats used by mosquito vectors of disease. Previous studies of linkages between disease, nutrition, and environments in rural Madagascar, such as cohort studies in northeastern Madagascar (11, 12), were largely confined to a single ecological setting. This study aimed at collecting data across distinct ecological regions, using standardized methodology in a cross-sectional sample to allow the direct comparisons between regions. We first describe the study regions and the recruitment and enrollment procedures, then the methods relevant to collecting each data type, followed by a brief summary of the interim results.
Through focus group meetings (Supplementary Data Sheet 1) and social (Supplementary Data Sheets 2–4) and health (Supplementary Data Sheet 5) survey instruments, we obtained social and demographic data, including broad categories of seasonal movements, and characterized the fluctuation of income generation, food production and dietary consumption. Through collection of clinical and biological samples for both point-of-care diagnoses and laboratory analyses, we obtained detailed occurrence (and importantly co-occurrence) data on micronutrient nutritional, infectious disease, and non-communicable disease status.
Within the context of larger research programs, these data provide a much-needed first step toward future studies that will incorporate additional environmental variables (e.g., associating health outcomes with remotely sensed data including household proximity to forest and landscape composition).
Understanding the interconnections among environmental and climatic attributes, varying socio-cultural contexts, and the prevalence of malnutrition and disease is important to projecting future burdens, and planning interventions. The data collected over the course of this survey provide foundations to linking environmental and climatic data to nutritional and disease data in Madagascar, allowing development of integrated models of disease dynamics and their underlying risk factors.
Our mixed-method approach included an observational cross-sectional study. The regions sampled were selected to represent the major ecological regions within Madagascar. In Madagascar, gradients in elevation and varying patterns of precipitation (see Figure 1) create a hypervariable natural landscape across the 587,000 km2 island (2). The continuous variation in environmental variables across the county has been delimited into broad ecological regions with similar climatic and natural vegetation profiles. Generally, Madagascar can be divided into the higher elevation central plateau (wooded grassland-bushland mosaic and terraced agriculture), the high rainfall east coast (humid rainforest, secondary forest and flooded lowland rice paddy agriculture), the seasonally dry west coast (dry deciduous forest and secondary grasslands), and the more arid south and southwest (dry spiny forest-thicket and secondary grasslands) (11, 12). These ecological regions align with those used by government departments to separate the country into areas for aggregating health data or interventions [for example, the national Malaria Indicator Surveys; (13)].
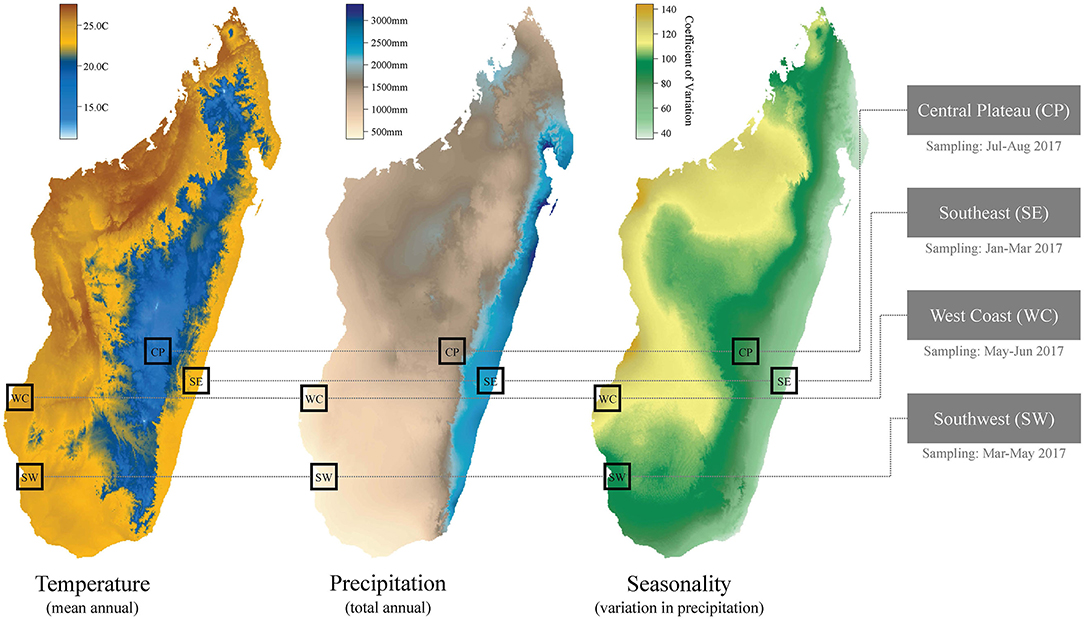
Figure 1. Climatic variation and regional focus for the cross-sectional study. Temperature and precipitation data sourced from WorldClim (14). Months of sampling listed below the four study regions.
Research subjects were men, women, and their children from 1,125 households evenly distributed across 24 communities in four ecologically and socio-demographically distinct regions of Madagascar (the southeast, southwest, west coast, and central plateau) (see Figure 1). These communities had already been enrolled in USAID-funded Catholic Relief Services (CRS) and Adventist Development and Relief Agency (ADRA) interventions aimed at reducing food insecurity through multiple pathways. CRS interventions include community-based nutrition and health activities, agriculture and livelihoods support, natural resource management, and disaster risk reduction and response activities. CRS and ADRA worked in every community in selected districts. Regional priorities were selected by the USAID mission as areas of food security challenges. Our team then used a stratified approach to randomizing communities in selected districts, after classifying communities by their proximity to the nearest town (greater than or less than 20 km) with an equal number of sites in each study region in each category (within 20 km of the city or more than 20 km from the city). Particular environmental features (e.g., forests, protected areas, rivers, etc.) were not used in selecting communities. Variation in community population size was not recorded but all communities were small, rural, agricultural settlements (tanana) that are typical of these areas and had ~50 to 250 households.
We refer to the four regions sampled in this study by their geography (see Table 1 for their corresponding administrative regions, sample sizes, and climatic variables): (i) the southeast (SE), (ii) the southwest (SW), (iii) the west coast (WC), and (iv) the central plateau (CP). Note that the southwest and west coast regions we distinguish in our study are located within the same, large, administrative region of Madagascar (Atsimo Andrefana), but we consider them as separate ecological regions in this study due to their geographic distance (~200 km) and marked differences in climate and vegetation. For example, total annual rainfall in the WC is approximately double that of the SW (Table 1), due to a large seasonal swing in rainfall in the WC (Figure 1).
Within the 24 sampled communities, we randomly sampled ~50 households in each community for a total of 200–300 households per region, and a total household enrollment of 1,125 across all regions. For these 1,125 households, all persons of both sexes and all ages therein (for a total of 6,292 individuals) were recruited into the research study and 5,882 individuals were enrolled. There was no screening based on race or ethnicity. Households were defined as regularly cohabitating groups of individuals that included at least one reproductive aged woman and a child. This definition of households was used as it aligns with the typical household groupings in these areas and reproductive aged women (women 13–45 years of age) and young children (children 5 years of age and younger) are most sensitive to malnutrition (15, 16) and certain types of infectious and communicable disease risk [e.g, malaria; (17)].
Subjects were offered no compensation for participating in interviews or providing clinical samples at the primary sampling time, but were offered 1,000 Malagasy ariary (~$0.28USD) at the follow-up interview performed the following year. This amount of money was compensation for time lost from labor activities due to participation in the follow-up survey and was deemed to not be coercive.
We recruited individuals with a two-stage opt-out procedure; individuals could opt-out prior to enrollment that preceded the questionnaire portion of the study or prior to the subsequent biological sampling portion of the study. Initially, one or more local authorities, typically local community leaders, chiefs (president-fokontany), or community elders, accompanied the field research manager (BLR) to conduct a community meeting where speeches were given to describe the work. This was the only culturally appropriate way to describe our research and allow for questions and answers to be heard by all community members. Following this meeting, we used the community census provided by the local authority to randomize households for participation. Households without reproductive aged women and/or children under 5 years of age were excluded. Randomization occurred by assigning numbers to households in the community and then using a random number generator to select households to enroll. In some cases, community censuses were out of date or inaccurate. For these cases, community censuses were updated to include all households by conferring with community leaders and heads of local family groups.
Selected households were then visited by the investigator and the local community authority to invite participation. After a verbal summary of the study was presented by a member of the study team, individuals within selected households expressing interest in participation were invited to review a more detailed explanation of the study. For adults that were not literate, verbal explanations were provided. Informed consent was obtained from adults, verbal assent was obtained from children over 12 years of age, and permission was obtained from parents or guardians of younger children (see below for ethical approval declarations). Interviews and clinical measures were performed in private and lasted no more than 1–2 hours in total. The local authority was not present during the interviews or clinical assessments. Not showing up for the interview was viewed by our team as the subject declining to be interviewed.
In addition to household surveys, our team also conducted community-level focus groups where we asked 4–6 adults of each gender to participate in providing some basic information about the community. These focus groups covered topics such as distance to infrastructure (roads, hospitals, etc.), general comments on local practices relevant to nutrition and disease (how people cope with food insecurity, etc.), and characterizing local markets (determining prices and access to different goods, etc.).
Our team also conducted biological sampling. A blood sample was obtained from all enrolled individuals who were willing to provide a blood sample. Not showing up for the blood sampling portion of the study was viewed by our team as the subject declining to participate in this portion of the study. Individuals were sampled in the early morning, prior to their first meal, so that micronutrient concentrations in plasma would not be affected by recent eating and to not disrupt individuals' labor during the day. Blood samples were used for point-of-care malaria diagnoses and hemoglobin testing for anemia, as well as plasma collection and dried blood spot preservation for other analyses of disease and nutritional markers (Table 2). Individuals testing positive for malaria by point-of-care rapid diagnostic test (RDT) or hemoglobin testing results indicative of anemia were offered treatment and a consultation with a physician. Individuals deemed by physicians to require additional treatment after point-of-care testing were referred to a hospital.
Blood samples were obtained through venous blood draw by trained, Malagasy medical staff (details on blood sample processing methodology are discussed below). However, we did not collect venous blood draws from the following: children under 2 years of age, whom the physician deemed too small for a blood draw and men and women 55 years of age and older. Only finger pricks were collected from these groups. Additionally, for individuals where the venous blood draw failed to obtain a sample, individuals wanting to receive point-of-care diagnoses were offered the choice to provide a finger prick sample.
Primary sampling took place between January and August 2017. Questionnaires, biological sampling, and focus groups were conducted in unison for a community such that all data collection for all households (and the individuals therein) in a community was completed over a maximum interval of seven days. Communities were sampled consecutively such that all communities in a region were sampled within a 6-week interval. Follow-up questionnaires were performed in each region from January to December 2018, every 2–3 months, in order to estimate intra-annual variation.
To understand the importance of dietary intake to human nutritional outcomes, we utilized a mixed-methods approach to quantify both household and individual levels of consumption (Table 2). Heads of household were asked to complete an adapted version of the Coping Strategies Index and the FAO's Household Food Insecurity Access Scale (18). Information was also gathered on crops grown and sold, and from a focused module on natural resource extraction including fishing and hunting. Dietary recalls (both 24 h and 1 week) were completed for every individual enrolled in the study at the time of the clinical sample, and then three or more additional times at 2 to 3-month intervals following the initial survey to capture seasonal differences in food availability and consumption [following protocols of (19)].
The members of each household were invited to participate in the clinical and diagnostic aspects of the health research. A local healthcare professional recorded the following anthropometric measurements for each child: height/length, weight, and mid-upper arm circumference for children 5 years of age and under, and cranial circumference for children 2 years of age and under (Table 2). Height and weight were also recorded for adult women and men. Blood pressure was measured for all individuals over 16 years of age using an OMRON 10 Series (Model BP786N) monitor and temperatures were recorded using infra-red thermometers for all subjects. Subjects traveled (always less than a 30-min walk) to a private room where the health assessments were conducted. Lidocaine was applied to the arm's surface to dull the pain of needle insertion when individuals feared the pain of the blood draw. Venous blood draws were collected in one Sarstedt S-Monovette tube (5–7 mL) with lithium heparin and then processed using previously established protocols (19, 20). This whole blood was used to source blood for: (i) rapid diagnostic tests (RDTs) for malaria (a fingerprick was used if no venous blood draw was taken) (SD Bioline Malaria Ag P.f/Pan RDT), (ii) hemoglobin status through the use of a HemoCue 201+, (iii) a thin blood smear for parasitemia counts for malaria and cell counts (iv) dried blood spots on Whatman filter paper FTA cards (two spots per individual), and (v) one drop on OmegaQuant filter paper treated with HUFASaveTM. The remaining whole blood was then separated by centrifuge into plasma and a blood pellet, with the plasma being aliquoted into 1 or 2 (depending on quantity of plasma obtained) 1.8 mL cryotubes that were then preserved in liquid nitrogen prior to freezing at −80C.
One aliquot of frozen plasma was shipped to the Western Human Nutrition Research Center (USDA) for nutritional analyses while the remaining plasma was stored at the Harvard T.H. Chan School of Public Health for future disease (e.g., serology for viral disease) tests. For nutritional analyses we analyzed for content of ferritin, transferrin receptor, zinc, vitamin A (retinol), folate, and vitamin B12 and will continue to be analyzed for other potential nutritional targets. Inflammation markers such as alpha glycolic protein (AGP) and C-reactive protein (CRP) were also measured here to control for the role of inflammation in affecting nutritional status. Dried blood from OmegaQuant filter paper was used to characterize fatty acid profiles for each sampled individual following established protocols (21). We were unable to prospectively conduct power calculations for nutritional outcomes for our study population because we did not have any baseline estimates of deficiency.
Processing of dried blood spots on Whatman FTA cards involved the extraction of nucleic acid material from the dried blood spot and genetic analysis will be performed to determine the presence or absence, as well as genotypes, of Plasmodium malaria parasites. Plasmodium genotype data will be used to characterize malaria population genetic diversity to infer estimates of transmission histories of communities. For stratification purposes only, genotyping of specific human loci known to impact the likelihood of malaria infection [e.g. hemoglobin type; (22)], Duffy-binding protein detection (23), etc. will be performed.
In addition, we collected a fecal sample from individuals enrolled in the study by providing fecal sample tubes (with spoons) and allowing individuals to collect this themselves by defecating and then spooning a sample into a tube. Approximately 83% of individuals returned a fecal sample. These fecal samples were stored in 90% ethanol and shipped to the Harvard T.H. Chan School of Public Health for microscopic analysis for intestinal parasite detection.
Plasma samples stored in cryotubes at −80C will be tested for antibodies to viral pathogens in order to evaluate the force of infection associated with a set of target pathogens, including interactions between nutritional and immune status. This will allow us to characterize the landscape of immunity for these focal pathogens [sensu (24)].
For each enrolled household, one surveyor from the four surveyors on the research team first asked the head of household basic demographic questions about all members of the household (Supplementary Data Sheet 2). From this, a household census was created and each individual in the household was assigned an anonymous, unique individual ID code. All participants in a household were then asked to respond to a series of demographic, socio-economic, health, and nutrition questions (Supplementary Data Sheets 2–5). Additional subsets of questions relevant to only specific members of the household were asked, including questions particular to heads of households, all adults, or reproductive aged women (e.g., questions about pregnancy and breastfeeding). Prior to participation in the blood draw portion of the study, all participants were asked a set of questions about current health status, medication history, vaccination history, and disease risk associated behaviors (e.g., mosquito bednet usage). For younger children, typically under the age of nine, surrogate responses from a parent or responsible family member were used.
Interview length depended on the age and sex of the individual participant, with interviews of heads of households being the longest (~30–45 min in total) due to the inclusion of questions regarding general household characteristics in addition to the base set of questions asked to all individuals. Interviews of children were briefer, averaging ~10–15 min in total.
Supplementary Data Sheets contain the full set of questions asked to heads of households, adults, and all individuals (English translations of the Malagasy question prompts are shown). Questionnaires were administered verbally, in local dialects in Malagasy, and responses were recorded on a tablet (Samsung Galaxy Tab A) using KoBoCollect software. Surveyors were trained to adapt the text of questions to local dialects, though in some cases French language words were the most commonly used locally and so the question prompts occasionally contain a mixture of Malagasy and French wording.
To investigate the ecology of disease vectors, our team (led by NJA) characterized the peri-domicile larval habitat of mosquitoes in each of the 24 study communities using two methods: (1) a grid-based system to search for sources of mosquito vector larvae within 25 m of households; and (2) stratified transects. In sites where households were tightly clustered, we conducted a systematic habitat search of the clustered area and 25 m beyond the perimeter of households on the edges of the cluster. In sites with less concentrated households, we conducted habitat searches in a circular area of radius 25 m around each household. All mosquito larval habitats were mapped and geocoded using a Garmin Oregon 550t. In addition to mapping the presence/absence of larval habitats, we also conducted transect surveys to identify the larval habitats and species composition of anopheline mosquitoes endemic to each local ecology of each research site. Two 100 m transects were mapped within one undisturbed area and one area that represented the dominant human-altered land-use type in each study community. All mosquito larval habitats and distinct changes in land-use along the transect and 5 m to each side of the transect line were geocoded.
In both protocols, all habitats containing water were sampled for larvae using dippers and pipettes in a standardized method (1). Larvae were collected and stored in 95% ethanol. Information on the presence of water, the presence of other animals, the habitat's dimensions, the type of habitat, and the presence and number of eggs and pupae at the time of sampling was recorded. Additionally, all early and late instar larvae were enumerated. All larvae were sorted by genus and instar prior to identification. All 3rd and 4th instar Anopheles larvae were identified morphologically to the lowest possible taxonomic level at the Institut Pasteur de Madagascar (led by MLT). These vector data were combined with ecological and environmental data to characterize the distribution of vectors of infectious disease in these regions.
A total of 6,292 individuals (both sexes, all ages) within 1,125 households were recruited in the study (see Figure 2). Demographics and preliminary anemia and malaria data are shown in Table 3. Of the 6,292 enrolled individuals, 5,601 individuals (89.0% of the total enrollees) also consented/assented to and were present to participate in the blood draw portion of the study.
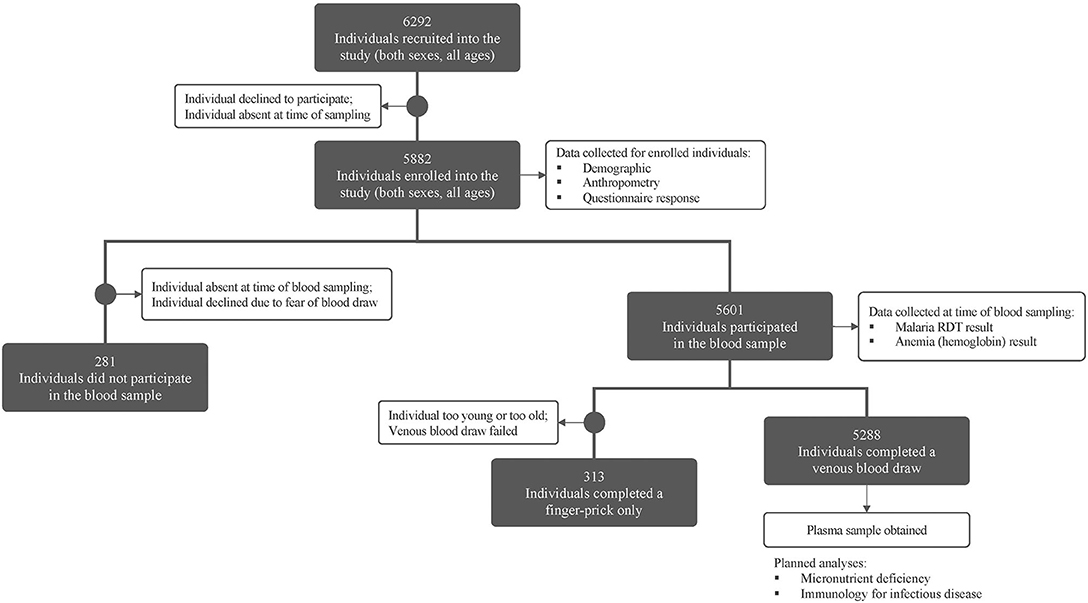
Figure 2. Consort figure for study enrollment and sampling. Enrollment and blood sampling in the MAHERY cross-sectional study. See methods for a full description of all sample types and data collected.
The most common reasons individuals did not participate in the blood draw portion of the study were: (i) absence from the community during the time blood draws were performed, often due to fishing, hunting, or other resource-extraction activities; or (ii) declining participation due to a fear of blood draws; or (iii) cultural taboos. Among all enrolled individuals, 52.8% were female, however females contributed only 35.6% of the individuals who did not perform a blood draw. Of the males who missed the blood draw, a vast majority (71.0%) were adults, indicating that men were more likely to be absent from or decline participation in the blood draw.
Of the 5,601 individuals who participated in the blood draw portion of the study, a venous blood draw was performed for 5,288 individuals (94.4%) while a finger prick blood sample only was obtained for the remaining individuals (due to their being too young, too old, or due to failed venous blood draws, see above for blood draw methodology).
This study aimed to collect data on the distribution of nutritional and disease outcomes among men, women, and children of all ages in rural communities across varying ecological settings in Madagascar. Successful recruitment, enrollment, and sampling of between 1,308 and 1,482 individuals per region for four ecologically distinct regions of Madagascar provides an opportunity to characterize pathways that drive disease risk and analyze associations between risk factors and health outcomes at the individual, household, community, and regional scale.
One potential limitation of the study is the existence of a subset of individuals (11.0%) within enrolled households that did not participate in the full set of data collection procedures employed in the complete study. The observation that a disproportionate percentage of the individuals that did not participate in the blood draw portion of the study were male (64.4%), for example, indicates that these missed individuals were not a random subset of the study population. However, participation rates for individuals within selected households were high (nearly 9 in 10 individuals completed a blood draw), and participation rates were highest among the groups (women and children) known to be most vulnerable to nutritional and disease risk. This suggests that, while future studies may benefit from additional emphasis on the recruitment and retention of adult men, our sample is unlikely to be significantly biased when assessing trends in disease in nutrition.
Further study of the sample material and data collected during this study is ongoing to characterize the highly variable contexts of health conditions in Madagascar and the vulnerability of rural communities therein. Indeed, preliminary data from this study indicates that more than 30 and 14% of the surveyed population had anemia or malaria infection, respectively. Future analyses will include: (1) the role of malaria and intestinal parasites in modulating nutritional status; (2) the role of proximity of forest or proximity to the sea in affecting micronutrient nutritional status; (3) the role of landscape diversity in affecting the diversity and abundance of larval mosquito vectors of disease and several others (see Supplementary Table 1). This study developed rich metadata associated with a suite of biological samples and nutritional and disease outcome data to allow examination of the underlying drivers of ill health across the diverse landscapes of Madagascar.
All households were recruited and enrolled, and each individual consented or assented, following our IRB approved study (Protocol #16-0166, Committee on the Use of Human Subjects, Office of Human Research Administration at the Harvard T.H. Chan School of Public Health). The study was also reviewed and approved by the Malagasy Ministry of Health and the ethical review board at the Institut National de Santé Publique et Communautaire (INSPC) No 03/MSANP/SG/INSPC/DG/DFR. Informed consent was obtained from adults, verbal assent was obtained from children over 12 years of age, and permission was obtained from parents or guardians of younger children. Both the HSPH IRB and the INSPC review board waived the requirement for written informed consent for this study, and approved the study's consent procedures described previously because requiring signatures was deemed culturally inappropriate for the targeted populations.
CG, BR, and CM led overall study design and protocol development for the research. BR and HJR led the field research team in data collection. HJR supervised the surveyor research team and managed survey data collection. BR, AV, JR, AT, VR, AANAR, and RM performed the venous blood draws, blood sample processing, and clinical measurement data collection. MA, FL, RFFM, HPR, and ADR assisted design of the questionnaire and collected the questionnaire response data. NA, BR, and MT developed the study protocol on mosquito larval transects. NA and GE led the field data collection on mosquito larval transects and MT and RG identified mosquito specimens. BR, AA, AWe, AWi, CM, and DH led the infectious disease data analysis portion of the study. CG, JH, and CM sourced funding for the project. CG and BR drafted the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
We are grateful for the support from the United States Agency for International Development (Grant No. AID-FFP-A-14-00008) implemented by Catholic Relief Services (CRS) in consortium with four local implementing partners in Madagascar. The views and opinions expressed in this paper are those of the authors and not necessarily the views and opinions of the United States Agency for International Development. We also thank the Wellcome Trust Our Planet, Our Health program (grant 106866/Z/15/Z) for providing funding to CM for this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the many people across Madagascar who welcomed us to conduct research within their communities. We would like to thank some key individuals of Ministère de de la Santé Publique (MSANP) who deeply supported this project: Dr. Herlyne Ramihantaniarivo (former Directeur Général de la MSANP), Pr. Jean de Dieu Marie Rakotomanga (Directeur Général de l'INSPC), Dr. Marie Rolland Ratsimbazafy (Responsable de l'INSPC), Celestin Tara (Assistant Technique de l'INSPC), Dr. Harinelina Randriamasiarijaona (Chef Service de la Nutrition), Dr. Orieux Tovotsimihefo (Médecin Inspecteur Morombe), Dr. Raphael Hotahiene (Directeur de Lutte contre les Maladies Transmissibles), Dr. Maurice Randriarsion (Médecin Inspecteur Mananjary), Dr. Georges Rakotondranaivo (Médecin Inspecteur Ambatofinandrahana), Dr. Noromalala Sylvie Tidahy (Directeur de la Santé Familiale), Hery Rakotoniary (Médecin Inspecteur Fandriana). We express our thanks to the mayors and clinic chiefs of the rural communes of Mananjary (Tsaravary, Mahatsara Atsimo, Ankatafana and Antsenavolo), Toliara II (Tsianisiha, Maromiandra, and Belalanda), Morombe (Morombe and Basibasy), and Amoron'i Mania (Alakamisy Ambohimahazo, Ambondromisotra, and Soavina). We would like to thank Bureau de Développement ECAR de Mananjary (BDEM), Caritas Morombe (CMB), and Conseil Diocésain de Développement de Toliara (CDD), the CRS team, including Jocelyn Ranaivosoa, and the ADRA ASOTRY team in Amoron'i Mania that have facilitated our work with the local communities.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00500/full#supplementary-material
ADRA, Adventist Development and Relief Agency; CRS, Catholic Relief Services; HSPH, Harvard T.H. Chan School of Public Health; MAHERY, Madagascar Health and Environmental Research; RDT, rapid diagnostic test; USAID, United States Agency for International Development; USD, United States Dollar.
1. Bouley T, Midgley A, Shumake-Guillemot J, Golden CD, Ebi KL. Climate Change and Health Diagnostic: Madagascar. Risks and Opportunities for Climate-Smart Health and Nutrition Investment. Investing in Climate Change and Health Series. Washington, DC: World Bank. Report number: 121945. (2018)
2. Dewar RE, Richard AF. Evolution in the hypervariable environment of Madagascar. Proc Natl Acad Sci USA. (2007) 104:13723–7. doi: 10.1073/pnas.0704346104
3. Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F. Fifty years of deforestation and forest fragmentation in Madagascar. Environ Conserv. (2007) 34:325–33. doi: 10.1017/S0376892907004262
4. Vieilledent G, Grinand C, Rakotomalala FA, Ranaivosoa R, Rakotoarijaona JR, Allnutt TF, et al. Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biol Conserv. (2018). 222:189–97. doi: 10.1016/j.biocon.2018.04.008
5. Allnut TF, Asner GP, Golden CD, Powell GV. Mapping recent deforestation and forest disturbance in northeastern Madagascar. Trop Conserv Sci. (2013) 6:1–15. doi: 10.1177/194008291300600101
6. Morelli TL, Smith AB, Mancini AN, Balko EA, Borgerson C, Dolch R, et al. The fate of Madagascar's rainforest habitat. Nat Clim Chang. (2020) 10:89–96. doi: 10.1038/s41558-019-0647-x
7. Golden CD, Fernald LC, Brashares JS, Rasolofoniaina BR, Kremen C. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. Proc Natl Acad Sci USA. (2011) 108:19653–6. doi: 10.1073/pnas.1112586108
8. Rasolofoson RA, Ricketts TH, Jacob A, Johnson KB, Pappinen A, Fisher B. Forest conservation: a potential nutrition-sensitive intervention in low-and middle-income developing countries. Front Sustain Food Syst. (2020) 4:20. doi: 10.3389/fsufs.2020.00020
9. Burkett-Caden ND, Vittor AY. Deforestation and vector-borne disease: forest conversion favors important mosquito vectors of human pathogens. Basic Appl Ecol. (2018) 26:101–10. doi: 10.1016/j.baae.2017.09.012
10. Tadross M, Randriamarolaza L, Rabefitia Z, Zheng KY. Climate Change in Madagascar; Recent Past and Future. Antananarivo: Climate Systems Analysis Group, University of Cape Town, South Africa and National Meteorological Office. (2008)
12. Goodman SM, Raherilalao MJ, Wohlhauser S, (eds.). The Terrestrial Protected Areas of Madagascar: Their History, Description, and Biota. Antananarivo: Association Vahatra (2018).
13. Institut National de la Statistique (INSTAT), Programme National de Lutte contre le Paludisme (PNLP), Institut Pasteur de Madagascar (IPM), and ICF International. Madagascar Malaria Indicator Survey 2016. [Enquête sur les Indicateurs du Paludisme (EIPM)]. Calverton: INSTAT. PNLP, IPM and ICF International (2016).
14. WorldClim. WorldClim - Global Climate and Weather Data. (2020). Available online at: http://www.worldclim.org (accessed March 16, 2020).
15. Lartey A. Maternal and child nutrition in Sub-Saharan Africa: challenges and interventions. Proc Nutr Soc. (2008) 67:105–8. doi: 10.1017/S0029665108006083
16. Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. (2012). 61(Suppl. 1):8–17. doi: 10.1159/000345165
17. Clouston SA, Yukich J, Anglewicz P. Social inequalities in malaria knowledge, prevention and prevalence among children under 5 years old and women aged 15–49 in Madagascar. Malar J. (2015) 14:499. doi: 10.1186/s12936-015-1010-y
18. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3). Food and Nutrition Technical Assistance Project, Academy for Educational Development. (2007). doi: 10.1037/e576842013-001
19. Golden CD, Borgerson C, Rice BL, Allen LH, Anjaranirina EJG, Barrett CB, et al. Cohort description of the Madagascar Health and Environmental Research–Antongil (MAHERY–Antongil) study in Madagascar. Front Nutr. (2019) 6:109. doi: 10.3389/fnut.2019.00109
20. Golden CD, Anjaranirina EJG, Fernald LC, Hartl DL, Kremen C, Milner JDA, et al. Cohort profile: the Madagascar Health and Environmental Research (MAHERY) study in north-eastern Madagascar. Int J Epidemiol. (2017) 46:1747–8. doi: 10.1093/ije/dyx071
21. Harris WS, Del GL, Tintle NL. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis. (2017) 262:51–4. doi: 10.1016/j.atherosclerosis.2017.05.007
22. Edwards RL, Creese AJ, Baumer M, Griffiths P, Bunch J, Cooper HJ. Hemoglobin variant analysis via direct surface sampling of dried blood spots coupled with high-resolution mass spectrometry. Anal Chem. (2011) 83:2265–70. doi: 10.1021/ac1030804
23. Gunalan K, Lo E, Hostetler JB, Yewhalaw D, Mu J, Neafsey DE, et al. Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci USA. (2016) 113:6271–6. doi: 10.1073/pnas.1606113113
24. Metcalf CJE, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. (2016) 388:728–30. doi: 10.1016/S0140-6736(16)30164-7
25. Banky Foiben'i Madagasikara. Marché de Change. (2017). Available online at: http://www.banky-foibe.mg (accessed April 01, 2019).
26. World Health Organization. Haemoglobin concentration for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System (2011). Available online at: https://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed January 27, 2020).
Keywords: food security, micronutrient nutrition, planetary health, seasonality, migration, malaria, infectious disease, disease ecology
Citation: Golden CD, Rice BL, Randriamady HJ, Vonona AM, Randrianasolo JF, Tafangy AN, Andrianantenaina MY, Arisco NJ, Emile GN, Lainandrasana F, Mahonjolaza RFF, Raelson HP, Rakotoarilalao VR, Rakotomalala AANA, Rasamison AD, Mahery R, Tantely ML, Girod R, Annapragada A, Wesolowski A, Winter A, Hartl DL, Hazen J and Metcalf CJE (2020) Study Protocol: A Cross-Sectional Examination of Socio-Demographic and Ecological Determinants of Nutrition and Disease Across Madagascar. Front. Public Health 8:500. doi: 10.3389/fpubh.2020.00500
Received: 22 April 2020; Accepted: 04 August 2020;
Published: 17 September 2020.
Edited by:
Susanne Sokolow, Stanford University, United StatesReviewed by:
Cyril Caminade, University of Liverpool, United KingdomCopyright © 2020 Golden, Rice, Randriamady, Vonona, Randrianasolo, Tafangy, Andrianantenaina, Arisco, Emile, Lainandrasana, Mahonjolaza, Raelson, Rakotoarilalao, Rakotomalala, Rasamison, Mahery, Tantely, Girod, Annapragada, Wesolowski, Winter, Hartl, Hazen and Metcalf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher D. Golden, Z29sZGVuQGhzcGguaGFydmFyZC5lZHU=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.