
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 14 July 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.00381
This article is part of the Research Topic Coronavirus Disease (COVID-19): Pathophysiology, Epidemiology, Clinical Management and Public Health Response View all 400 articles
 Harapan Harapan1,2,3*
Harapan Harapan1,2,3* Abram L. Wagner4
Abram L. Wagner4 Amanda Yufika5
Amanda Yufika5 Wira Winardi6
Wira Winardi6 Samsul Anwar7
Samsul Anwar7 Alex Kurniawan Gan1
Alex Kurniawan Gan1 Abdul Malik Setiawan8
Abdul Malik Setiawan8 Yogambigai Rajamoorthy9
Yogambigai Rajamoorthy9 Hizir Sofyan7
Hizir Sofyan7 Mudatsir Mudatsir1,2,3
Mudatsir Mudatsir1,2,3Introduction: Several vaccine candidates are being clinically tested in response to the 2019 coronavirus disease (COVID-19) pandemic. This study was conducted to assess the acceptance of a 50 or 95% effective COVID-19 vaccine, when it becomes available in southeast Asia, among the general population in Indonesia.
Methods: A cross-sectional online survey was conducted between March 25 and April 6, 2020. Participants were asked if they would accept a free vaccine which was 95 or 50% effective. Using a logistic regression model, we assessed the associations between sociodemographic characteristics, exposure to COVID-19 information, or perceived risk of infection with acceptance of a hypothetical COVID-19 vaccine.
Results: Among 1,359 respondents, 93.3% of respondents (1,268/1,359) would like to be vaccinated for a 95% effective vaccine, but this acceptance decreased to 67.0% (911/1,359) for a vaccine with 50% effectiveness. For a 95% effective vaccine, being a healthcare worker and having a higher perceived risk of COVID-19 infection were associated with higher acceptance, adjusted odds ratio (aOR): 2.01; 95%CI: 1.01, 4.00 and aOR: 2.21; 95%CI: 1.07, 4.59, respectively; compared to civil servants, being retired was associated with less acceptance (aOR: 0.15; 95%CI: 0.04, 0.63). For a 50% effective vaccine, being a healthcare worker was also associated with greater acceptance, aOR: 1.57; 95%CI: 1.12, 2.20.
Conclusion: Acceptance of a COVID-19 vaccine was highly influenced by the baseline effectiveness of the vaccine. Preparing the general population to accept a vaccine with relatively low effectiveness may be difficult.
The current 2019 coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major threat worldwide and especially to countries in southeast Asia (1–3). A systematic review of 53,000 hospitalized patients indicated that 20.2% of COVID-19 cases developed severe disease with a mortality rate of ~3.1% (4). In the elderly and among those with comorbidities, such as cardiovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease, mortality increases significantly (5–8). Although some drugs have been used to treat severe COVID-19 patients (9–12), no specific therapies have been approved by the US Food and Drug Administration. Development and deployment of a vaccine is therefore one of the most promising strategies in this crisis.
Vaccine development began in several research centers and pharmaceutical companies as soon as SARS-CoV-2 was identified as the causative agent and the first genome sequence was published. On March 16, 2020, the first COVID-19 vaccine candidate, an mRNA-based vaccine developed by Moderna Inc, entered a Phase 1 clinical trial (NCT04283461) in the US and later a non-replicating vector-based vaccine, developed by China's CanSino Biologics was also tested in China (ChiCTR2000030906) (13). Other vaccine candidates, including DNA-based vaccines, inactivated, live attenuated, sub-unit, and replicating viral vector-based vaccines are also being developed (13). It is unclear how effective these vaccines will be. If the COVID-19 vaccine resembles an influenza vaccine, effectiveness could be 50% or lower (14). People may have strong preferences for a vaccine to be highly effective (15), and a vaccine with a low effectiveness estimate could impact people's willingness to be vaccinated. It is also possible that individuals will perceive a pandemic vaccine to be less safe based on its newness or perceived lack of testing (15). Safety perceptions could also influence vaccine acceptance (16).
High vaccination coverage globally may be required to stop the COVID-19 pandemic. However, vaccine demand in low- and middle-income countries (LMICs) is less well-studied and there may be different considerations from the population compared to high income countries (17). LMICs may have less capacity to introduce new vaccines and may need to deal with citizenry who have hesitant beliefs (18). Indonesia is a middle-income country with relatively low vaccine coverage and high vaccine hesitancy (18–20). Some studies have been conducted to assess acceptance on new vaccines against emerging and re-emerging infectious diseases in southeast Asia, such as for dengue (21–25), Zika (26), and Ebola (27). No study has been conducted on COVID-19 vaccine acceptance in the region. This study sought to assess the acceptance of a hypothetical COVID-19 vaccine among the general population in Indonesia. The results of this study might be important for the government to formulate the best approach to implement mass vaccination programs for COVID-19 in Indonesia, as well as other countries in southeast Asia region, in the future.
Currently no COVID-19 vaccine is available and therefore we framed the study questions around a hypothetical vaccine, in an approach that was similar to previous studies (21, 26, 28–30). Due to limitations in doing face-to-face research during the current active COVID-19 outbreak in Indonesia, we did an online cross-sectional study between March 25 and April 6, 2020. The target population was the adult population of Indonesia. The samples were recruited from seven provinces (Aceh, West Sumatra, Jambi, DKI Jakarta, Yogyakarta, and Bali) and all adults who were able to read and understand Bahasa Indonesia were considered eligible. Invitations to participate in the study, hosted by Google Forms, were distributed on the WhatsApp communication platform. This media and communication platform was chosen since 64% of the Indonesian population currently use this platform and the users are relatively varied across age groups and other sociodemographic characteristics. The participants were recruited using a simplified-snowball sampling technique where invited candidate participants were requested to pass the invitations to their WhatsApp contacts. The minimum sample size was 1,068, based on the conservative assumption that the acceptability rate was 50 with a 3% margin of error and a confidence interval of 95%. To recruit the samples, participants were purposefully selected to include both urban and suburban areas.
The survey was estimated to take ~10 min to complete. To collect the information, a set of questions were constructed and developed. The questionnaire included sections on sociodemographic data, exposure to COVID-19 information, perceived risk of being infected with COVID-19, and acceptance of a vaccine. The questions were first pre-tested and were revised and finalized based on feedback from pre-testers.
The response variable was acceptance of a hypothetical COVID-19 vaccine in Indonesian population. To assess the acceptance, the respondents were provided with the following information: (a) a vaccine is currently not available for COVID-19, but we want study participants to think about a hypothetical vaccine; (b) the hypothetical COVID-19 vaccine would be developed and tested clinically in humans; (c) clinical trials would show that the vaccine had a 5% chance of producing side effects like fever, skin rash and pain; and (d) the government would offer it as a free and optional vaccine. To assess the acceptance rate of the vaccine, the respondents were given two scenarios with different vaccine efficacies (95 and 50%). Participants were asked to respond to the question of whether they would be vaccinated with a new COVID-19 vaccine for each scenario (i.e., for 95 and 50%). The possible responses were “yes” or “no.”
Some explanatory variables were collected. Sociodemographic characteristics included age, gender, educational attainment, occupation, religion, marital status, monthly income, and type of urbanicity. Age was grouped into five categories (< 20, 21–30, 31–40, 41–50, and >51 years old); educational attainment was grouped into junior/senior school graduates, diploma graduates, and university graduates/post-graduates; and type of job was divided into five groups (civil servant, private sector employee, entrepreneur, student, and retired). Individual monthly income was grouped into < 2.5 million Indonesian Rupiah (IDR), 2.5–5 million, 6–10 million, and more than 10 million (< US$ 154.7, US$ 154.7–US$ 309.4, US$ 371.2–$ 618.8, and >US$ 618.8 using an April 4, 2020 exchange rate). Urbanicity of respondents was divided into rural and urban. Respondents were also asked whether they were working as a healthcare worker (HCW) or not and whether they had heard about COVID-19 prior to the survey. Their perceived risk of being infected with COVID-19 within the next month was assessed on a scale of 0 to 100% using a question based off previous studies (31, 32), where 0% indicates the lowest while 100% was the highest perceived risk. For statistical analysis the score was classified into five groups: 0, 10–20, 30–40, 50–60, and more than 60%.
A logistic regression model was employed to identify determinants of participants' acceptance of a COVID-19 vaccine. The analysis was conducted for both vaccine efficacies (i.e., 95 and 50%). In the first step, associations between explanatory variables and response acceptance were analyzed separately. In the second step, all variables with p ≤ 0.25 in the first step were included in the adjusted analysis. The significance of crude odds ratio (OR) from univariate analyses and adjusted OR (aOR) in multivariate analyses were assessed at α = 0.05. All analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA).
The protocol of this study was approved by the Institutional Review Board of the School of Medicine, Universitas Syiah Kuala, Banda Aceh (041/EA/FK-RSUDZA/2020) and the National Health Research and Development Ethics Commission (KEPPKN) of the Ministry of Health of the Republic of Indonesia (#1171012P).
We received 1,402 responses during the survey period; 43 of them were excluded due to incomplete data. More than half of the respondents (698/1,359; 51.4%) were among those aged 21–30 years old and 66.1% of them (898/1,359) graduated from a university (Table 1). Overall, 27.6% of respondents (375/1,359) worked in the private sector, 47.5% (645/1,359) earned < 2.5 million (equal to US$ 154.7) each month, and more than 75% (1041/1,359) lived in cities. Almost 40% (533/1,359) of the survey participants believed that they had a 0% risk of being infected with SARS-CoV-2.
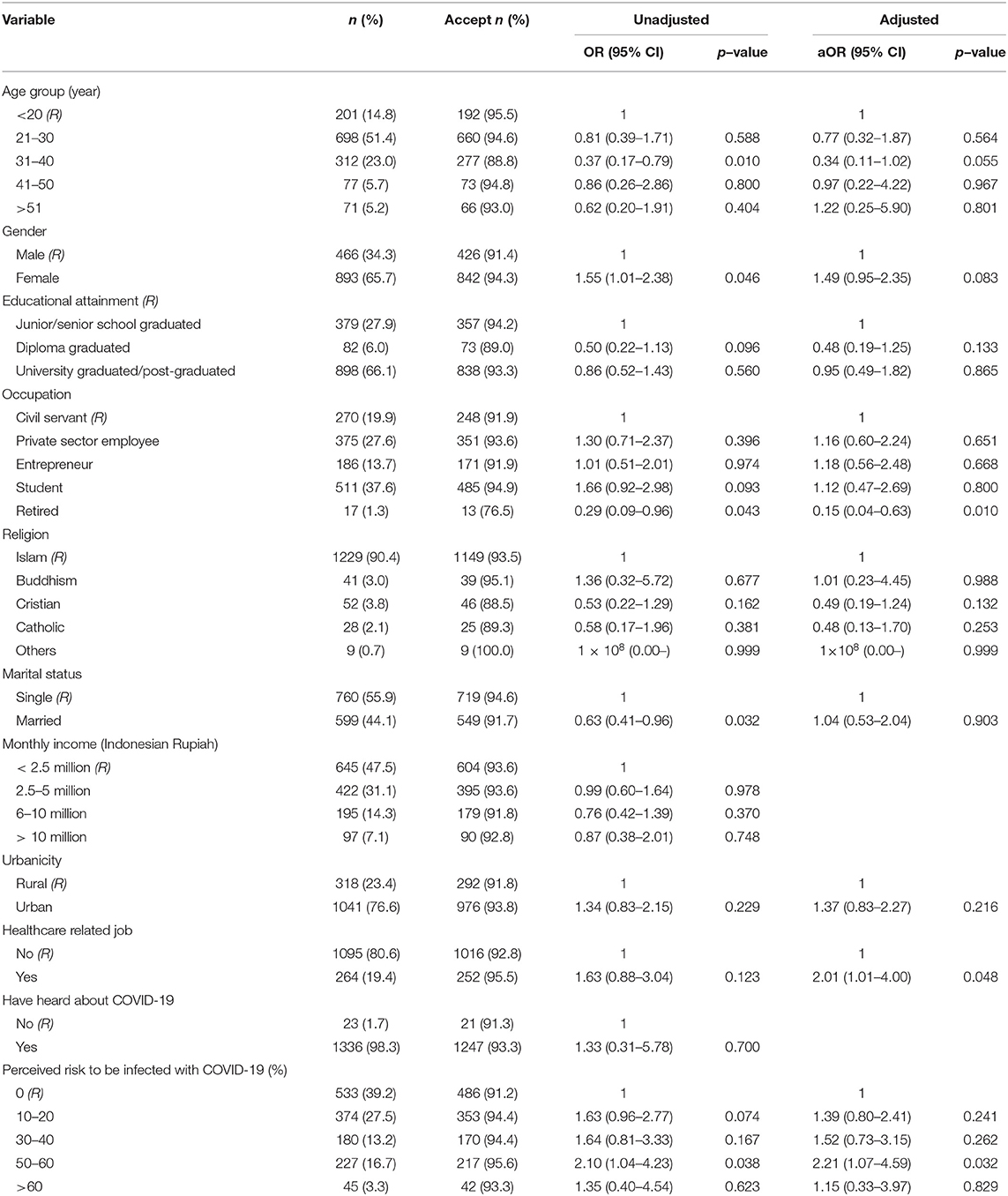
Table 1. Unadjusted and adjusted logistic regression analyses showing factors associated with acceptance of a COVID-19 vaccine in Indonesia, 95% effectiveness (n = 1,359).
If the vaccine was 95% effective, 93.3% participants (1,268/1,359) would like to be vaccinated when it is provided freely by government. However, this percentage decreased to 67.0% (911/1,359) if vaccine efficacy was 50%.
In the first scenario, 95% effectiveness, an adjusted analysis found that being a HCW and having a higher perceived risk were associated with higher acceptance; being retired was associated with less acceptance compared to civil servants (Table 1). Those who were working as HCWs were twice as likely to accept a COVID-19 vaccine, aOR: 2.01; 95%CI: 1.01, 4.00, p = 0.048. In addition, those with a high score of perceived risk to be infected (50–60%) had twice the odds of vaccine acceptance compared to those with no perceived risk to be infected in the next month (aOR: 2.21; 95%CI:1.07, 4.59, p = 0.032). Those who were retired were less likely to accept the vaccine compared to those who were working as a civil servant, with the aOR: 0.15 (95%CI: 0.04, 0.63).
With a lower vaccine efficacy (50%), being a HCW was the only characteristic associated with vaccine acceptance. Those who were working as a HCW had 1.57 times greater odds of accepting the vaccine compared to those who were working in non-medical sectors, aOR: 1.57; 95%CI: 1.12, 2.20, p = 0.009 (Table 2).
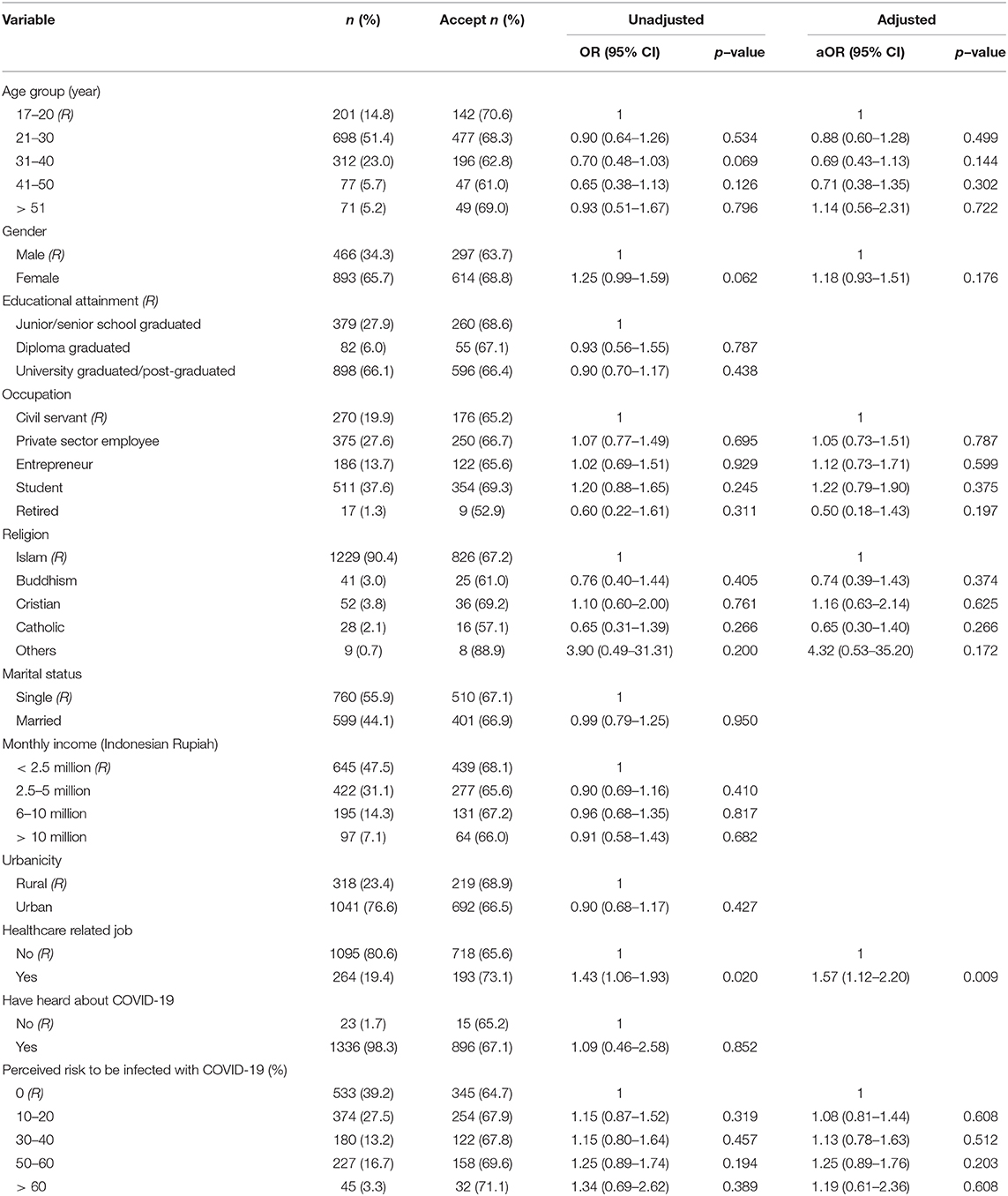
Table 2. Unadjusted and adjusted logistic regression analyses showing factors associated with acceptance of a COVID-19 vaccine in Indonesia, 50% effectiveness (n = 1,359).
Vaccines are a key strategy to stop the escalation of the COVID-19 pandemic. As of April 8, 2020, there were more than 100 COVID-19 vaccine candidates being developed (33). This vaccine development is proceeding at a fast pace; prior to March 30, 2020, two vaccine candidates had entered Phase 1 clinical trials (13) while on April 9, five vaccine candidates in total were in Phase 1 clinical trials (33). In the region of southeast Asia, studies have been conducted to assess the acceptance of a vaccine against infectious diseases (21–26, 34). This present study was conducted to understand how the COVID-19 vaccine, when available, will be accepted by the general population in Indonesia, by asking individuals about a hypothetical vaccine—an approach used in many past studies (21, 26, 28–30). Understanding vaccine acceptance in Indonesia is important, given the large population and because the country has relatively high vaccine hesitancy for existing vaccines and relatively low vaccination coverage (18, 19). Characterizing how vaccine efficacy could impact acceptance is also important, given that actual or perceived vaccine efficacy could be relatively low.
Our findings indicated that when the vaccine is provided freely, 93.3 and 67.0% of participants would like to be vaccinated if the vaccine had 95% and 50% effectiveness, respectively. The acceptance rate for the first model (i.e., 95% efficacy) is far higher compared to acceptance of other new vaccines in southeast Asia (21, 22, 25, 34). This indicates that a majority of the general population in the country are supportive of the COVID-19 vaccine. This is not surprising because this study was started on March 25, 2020, when the number of COVID-19 cases started to sharply increase in Indonesia; 790 confirmed cases have been reported (35). It should be noted that the acceptance rate was measured under the presumption that the vaccine was provided freely by the government. Therefore, in the case that the vaccine needs to be purchased, or if it is not fully subsided by government, analyses assessing the acceptance at certain vaccine prices (i.e., willingness to pay) will need to be conducted not only in Indonesia but also in other countries in the southeast Asia region. We also note that it is unclear what the herd immunity threshold for COVID-19 is (36), and 67.0% vaccination coverage may be lower than what is required to stop the spread of disease.
Our study indicated that HCWs were more supportive of a COVID-19 vaccine than non-HCWs. Self-protection and desire to protect family, friends, and patients have been the drivers of HCWs' decision to get vaccinated in previous studies (37, 38). Since HCWs have more comprehensive knowledge about COVID-19, their relatively high awareness may lead them to protect themselves and not to transmit the virus to their family members. This might lead them to be more willing to accept the vaccine compared to those who working in non-medical sectors. In addition, our further analysis also suggested that the perceived risk of HCWs was higher compared to non-HCWs.
One important finding is that those who had a higher perceived risk to be infected with COVID-19 were more likely to accept the vaccine, but only for the 95% effective vaccine. Previous studies in Asia have found that perceived risk or perceived susceptibility to an infection is associated with positive support for vaccination (29, 30, 39). Another study also found that high perceived risk was associated with COVID-19 vaccine acceptance among general community members in Saudi Arabia (40) and among HCWs in China (41). Therefore, it is important to increase the perceived risk among communities since our study found that almost 40% of the respondents had a perceived risk of 0%. Low perceived risk may not only be correlated with vaccine acceptance, but also adherence to social distancing measures and other public health countermeasures. These relationships may be complicated—for example, an individual highly compliant with social distancing measures may perceive their risk to be low but still want to obtain a vaccine.
We also found that being retired had low acceptance compared to those who were working as civil servant. Lower vaccine acceptance among the retired population might be influenced by lower perceived risk. Although the elderly are more vulnerable to COVID-19, most of the retired population in Indonesia and indeed in southeast Asian countries have low mobility and spend more time at home with less travel. These behaviors may lead them to having a lower perceived risk of being infected with SARS-CoV-2, and eventually may lead to lower acceptance of a vaccine. Moreover, their acceptance might also be influenced by knowledge about the disease. Much of the information about COVID-19 is spread through social media or online media, which is less frequently accessed by older adults. Therefore, older adults might have less exposure to information about COVID-19 that could contribute to framing their risk perception. In addition, less social media use might also be associated with less knowledge among the elderly and this could affect their perceived risk and vaccine acceptance. However, this study did not measure respondents' knowledge of COVID-19 and we were unable to elucidate these relationships.
The study has several limitations. Generalizability of the survey results may be impacted by how we distributed the questionnaire. We used the WhatsApp platform, and so it may miss people from lower socioeconomic classes such as farmers, those with lower educational attainment, and those who were illiterate. According to UNESCO Indonesia, the literacy rate of adults (aged 15 and above) was 95.98% (42) and previous studies using community samples found that at least 96% of the community graduated from primary school (26, 43). As reported in other online studies in Indonesia (44–47), selection bias could also be related to the sampling technique and differential access to internet infrastructure across the country, as some regions have better internet access than others. Finally, acceptance was assessed using a hypothetical vaccine, which may differ from the respondents' revealed preferences in a real-life situation.
Acceptance of the COVID-19 vaccine in Indonesia is influenced by the effectiveness of the vaccine. Acceptance is relatively high when the vaccine has a very high effectiveness, but it reduced to only 67.0% when the vaccine efficacy is 50%. If the COVID-19 vaccine has lower efficacy, governments will have to introduce more strategies to persuade their population to become vaccinated. In addition, since acceptance is associated with perceived risk for COVID-19, it is also important to increase the perceived risk in communities.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: http://doi.org/10.6084/m9.figshare.12477143.
The studies involving human participants were reviewed and approved by Institutional Review Board of the School of Medicine, Universitas Syiah Kuala, Banda Aceh (041/EA/FK-RSUDZA/2020) and National Health Research and Development Ethics Commission (KEPPKN) of the Ministry of Health of the Republic of Indonesia (#1171012P). The ethics committee waived the requirement of written informed consent for participation.
HH: conceptualization, methodology, software, data curation, formal analysis, resources, writing—original draft, writing—review and editing, project administration. AW: conceptualization, methodology, writing—review and editing. AY: methodology, writing—original draft, investigation, data curation, writing—review and editing, project administration. WW: methodology, investigation, project administration, data curation. SA: software, formal analysis, writing—review and editing. AG: investigation, project administration. AS: investigation. YR: writing—review and editing. HS: software, writing—review and editing. MM: project administration. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Dr. Zinatul Hayati who facilitated the ethical application for the study.
1. Lim WS, Liang CK, Assantachai P, Auyeung TW, Kang L, Lee WJ, et al. COVID-19 and older people in Asia: AWGS calls to actions. Geriatr Gerontol Int. (2020) 20:547–58. doi: 10.1111/ggi.13939
2. Chhetri JK, Chan P, Arai H, Chul Park S, Sriyani Gunaratne P, Setiati S, et al. Prevention of COVID-19 in older adults: a brief guidance from the international association for gerontology and geriatrics (IAGG) Asia/Oceania region. J Nutr Health Aging. (2020) 24:471–2. doi: 10.1007/s12603-020-1359-7
3. Bhutta ZA, Basnyat B, Saha S, Laxminarayan R. Covid-19 risks and response in South Asia. BMJ. (2020) 368:m1190. doi: 10.1136/bmj.m1190
4. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
5. Verity R, Okell L, Dorigatti I, Winskill P, Whittaker C, Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. (2020) 20:669–77. doi: 10.1016/S1473-3099(20)30243-7
6. Zhao X, Zhang B, Li P, Ma C, Gu J, Hou P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.17.20037572
7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
8. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. (2020) 323:1775–6. doi: 10.1001/jama.2020.4683
9. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
10. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1101/2020.02.06.20020974
11. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
12. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382:1564–7. doi: 10.1056/NEJMc2004973
13. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. (2020) 382:1969–73. doi: 10.1056/NEJMp2005630
14. CDC: Innluenza - Past Seasons Vaccine Effectiveness Estimates. (2020). Available online at: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed May 01, 2020).
15. Sun X, Wagner AL, Ji J, Huang Z, Zikmund-Fisher BJ, Boulton ML, et al. A conjoint analysis of stated vaccine preferences in Shanghai, China. Vaccine. (2020) 38:1520–5. doi: 10.1016/j.vaccine.2019.11.062
16. Wagner AL, Boulton ML, Sun X, Mukherjee B, Huang Z, Harmsen IA, et al. Perceptions of measles, pneumonia, and meningitis vaccines among caregivers in Shanghai, China, and the health belief model: a cross-sectional study. BMC Pediatr. (2017) 17:143. doi: 10.1186/s12887-017-0900-2
17. Nichter M. Vaccinations in the Third World: a consideration of community demand. Soc Sci Med. (1995) 41:617–32. doi: 10.1016/0277-9536(95)00034-5
18. Yufika A, Wagner AL, Nawawi Y, Wahyuniati N, Anwar S, Yusri F, et al. Parents' hesitancy towards vaccination in Indonesia: a cross-sectional study in Indonesia. Vaccine. (2020) 38:2592–9. doi: 10.1016/j.vaccine.2020.01.072
19. Harapan H, Anwar S, Dimiati H, Hayati Z, Mudatsir M. Diphtheria outbreak in Indonesia, 2017: an outbreak of an ancient and vaccine-preventable disease in the third millennium. Clin Epidemiol Global Health. (2019) 7:261–2. doi: 10.1016/j.cegh.2018.03.007
20. Syiroj ATR, Pardosi JF, Heywood, AE. Exploring parents' reasons for incomplete childhood immunisation in Indonesia. Vaccine. (2019) 37:6486–93. doi: 10.1016/j.vaccine.2019.08.081
21. Harapan H, Anwar S, Setiawan AM, Sasmono RT, Aceh Dengue S. Dengue vaccine acceptance and associated factors in Indonesia: a community-based cross-sectional survey in Aceh. Vaccine. (2016) 34:3670–5. doi: 10.1016/j.vaccine.2016.05.026
22. Hadisoemarto PF, Castro MC. Public acceptance and willingness-to-pay for a future dengue vaccine: a community-based survey in Bandung, Indonesia. PLoS Negl Trop Dis. (2013) 7:e2427. doi: 10.1371/journal.pntd.0002427
23. Lee JS, Mogasale V, Lim JK, Carabali M, Sirivichayakul C, Anh DD, et al. A multi-country study of the household willingness-to-pay for dengue vaccines: household surveys in Vietnam, Thailand, and Colombia. PLoS Negl Trop Dis. (2015) 9:e0003810. doi: 10.1371/journal.pntd.0003810
24. Vo TQ, Tran QV, Vo NX. Customers' preferences and willingness to pay for a future dengue vaccination: a study of the empirical evidence in Vietnam. Patient Prefer Adherence. (2018) 12:2507–15. doi: 10.2147/PPA.S188581
25. Yeo HY, Shafie AA. The acceptance and willingness to pay (WTP) for hypothetical dengue vaccine in Penang, Malaysia: a contingent valuation study. Cost Eff Resour Alloc. (2018) 16:60. doi: 10.1186/s12962-018-0163-2
26. Harapan H, Mudatsir M, Yufika A, Nawawi Y, Wahyuniati N, Anwar S, et al. Community acceptance and willingness-to-pay for a hypothetical Zika vaccine: a cross-sectional study in Indonesia. Vaccine. (2019) 37:1398–406. doi: 10.1016/j.vaccine.2019.01.062
27. Mudatsir M, Anwar S, Fajar JK, Yufika A, Ferdian MN, Salwiyadi S, et al. Willingness-to-pay for a hypothetical Ebola vaccine in Indonesia: a cross-sectional study in Aceh. F1000Res. (2019) 8:1441. doi: 10.12688/f1000research.20144.1
28. Harapan H, Anwar S, Bustamam A, Radiansyah A, Angraini P, Fasli R, et al. Willingness to pay for a dengue vaccine and its associated determinants in Indonesia: a community-based, cross-sectional survey in Aceh. Acta Trop. (2017) 166:249–56. doi: 10.1016/j.actatropica.2016.11.035
29. Rajamoorthy Y, Radam A, Taib NM, Rahim KA, Wagner AL, Mudatsir M, et al. The relationship between perceptions and self-paid hepatitis B vaccination: a structural equation modeling approach. PLoS One. (2018) 13:e0208402. doi: 10.1371/journal.pone.0208402
30. Rajamoorthy Y, Radam A, Taib NM, Rahim KA, Munusamy S, Wagner AL, et al. Willingness to pay for hepatitis B vaccination in Selangor, Malaysia: a cross-sectional household survey. PLoS One. (2019) 14:e0215125. doi: 10.1371/journal.pone.0215125
31. Klonoff EA, Landrine H, Lang DL. Women Health: Research on Gender, Behaviour and Policy, Vol.3. Lawrence Elbaum Associates (1997).
32. Gidengil CA, Parker AM, Zikmund-Fisher BJ. Trends in risk perceptions and vaccination intentions: a longitudinal study of the first year of the H1N1 pandemic. Am J Public Health. (2012) 102:672–9. doi: 10.2105/AJPH.2011.300407
33. Thanh Le T, Andreadakis Z, Kumar A, Gomez Roman R, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. (2020) 19:305–6. doi: 10.1038/d41573-020-00073-5
34. Harapan H, Anwar S, Ferdian M, Salwiyadi S, Imanda A, Azhars R, et al. Public acceptance of a hypothetical Ebola virus vaccine in Aceh, Indonesia: a hospital-based survey. Asian Pac J Trop Dis. (2017) 7:193–8. doi: 10.12980/apjtd.7.2017D6-386
35. Ministry of Health: Info Khusus COVID-19. (2020). Available online at: https://infeksiemerging.kemkes.go.id (accessed:18, April 2020).
36. D'souza G, Dowdy D. Early Herd Immunity Against COVID-19: A Dangerous Misconception. Available online at: https://coronavirus.jhu.edu/from-our-experts/early-herd-immunity-against-covid-19-a-dangerous-misconception (accessed May 1, 2020).
37. Vasilevska M, Ku J, Fisman DN. Factors associated with healthcare worker acceptance of vaccination: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. (2014) 35:699–708. doi: 10.1086/676427
38. Nguyen TTM, Lafond KE, Nguyen TX, Tran PD, Nguyen HM, Ha VTC, et al. Acceptability of seasonal influenza vaccines among health care workers in Vietnam in 2017. Vaccine. (2020) 38:2045–50. doi: 10.1016/j.vaccine.2019.12.047
39. Sundaram N, Purohit V, Schaetti C, Kudale A, Joseph S, Weiss MG. Community awareness, use and preference for pandemic influenza vaccines in Pune, India. Hum Vaccin Immunother. (2015) 11:2376–88. doi: 10.1080/21645515.2015.1062956
40. Padhi BK, Almohaithef MA. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. medRxiv [Preprint]. (2020). doi: 10.1101/2020.05.27.20114413
41. Fu C, Wei Z, Pei S, Li S, Sun X, Liu P. Acceptance and preference for COVID-19 vaccination in health-care workers (HCWs). medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.09.20060103
42. Indonesia UNESCO: Country Programing Document 2014–2017. Jakarta: UNESCO Office Jakarta and Regional Bureau for Science in Asia and the Pacific (2014).
43. Harapan H, Rajamoorthy Y, Anwar S, Bustamam A, Radiansyah A, Angraini P, et al. Knowledge, attitude, and practice regarding dengue virus infection among inhabitants of Aceh, Indonesia: a cross-sectional study. BMC Infect Dis. (2018) 18:96. doi: 10.1186/s12879-018-3006-z
44. Harapan H, Setiawan AM, Yufika A, Anwar S, Wahyuni S, Asrizal FW, et al. Confidence in managing human monkeypox cases in Asia: a cross-sectional survey among general practitioners in Indonesia. Acta tropica. (2020) 206:105450. doi: 10.1016/j.actatropica.2020.105450
45. Harapan H, Setiawan AM, Yufika A, Anwar S, Wahyuni S, Asrizal FW, et al. Knowledge of human monkeypox viral infection among general practitioners: a cross-sectional study in Indonesia. Pathogens and Global Health. (2020) 114:68–75. doi: 10.1080/20477724.2020.1743037
46. Harapan H, Rajamoorthy Y, Utomo PS, Anwar S, Setiawan AM, Alleta A, et al. Knowledge and attitude towards pregnancy-related issues of Zika virus infection among general practitioners in Indonesia. BMC Infect Dis. (2019) 19:693. doi: 10.1186/s12879-019-4297-4
Keywords: COVID-19, SARS-CoV-2, vaccine, vaccination, acceptance
Citation: Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, Setiawan AM, Rajamoorthy Y, Sofyan H and Mudatsir M (2020) Acceptance of a COVID-19 Vaccine in Southeast Asia: A Cross-Sectional Study in Indonesia. Front. Public Health 8:381. doi: 10.3389/fpubh.2020.00381
Received: 12 May 2020; Accepted: 30 June 2020;
Published: 14 July 2020.
Edited by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceReviewed by:
Abhay Machindra Kudale, Savitribai Phule Pune University, IndiaCopyright © 2020 Harapan, Wagner, Yufika, Winardi, Anwar, Gan, Setiawan, Rajamoorthy, Sofyan and Mudatsir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harapan Harapan, aGFyYXBhbkB1bnN5aWFoLmFjLmlk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.