
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health , 14 July 2020
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.00303
 Tiffany Yuh1*
Tiffany Yuh1* Murugi Micheni2
Murugi Micheni2 Stacy Selke3
Stacy Selke3 Lynda Oluoch2
Lynda Oluoch2 Catherine Kiptinness2
Catherine Kiptinness2 Amalia Magaret3
Amalia Magaret3 Bhavna Chohan4,5
Bhavna Chohan4,5 Kenneth Ngure6
Kenneth Ngure6 Anna Wald1,3,7
Anna Wald1,3,7 Nelly R. Mugo2,4
Nelly R. Mugo2,4 Alison C. Roxby1,4
Alison C. Roxby1,4Objectives: Globally, the highest rates of sexually transmitted infections (STIs) are among the 15-24 age group. Studying adolescent girls and young women (AGYW) pre-sexual debut could identify risk factors for STI acquisition.
Methods: We recruited a prospective cohort of low-risk AGYW aged 16-20 in Kenya. Participants were HIV and HSV-2 seronegative and reported no history of sexual intercourse or reported sex with one partner. Participants underwent genital exams, nucleic acid testing of vaginal swabs for Neisseria gonorrhea (NG), Chlamydia trachomatis (CT), Trichomonas vaginalis (TV), and vaginal gram stains for vaginal dysbiosis by Nugent score. STI correlates were described using χ2 test and t-test.
Results: We enrolled 400 AGYW, of which 322 (80.5%) reported never having had sex, while 78 (19.5%) reported prior sex with 1 partner. Among the 78 participants reporting prior sex, 20 (25.6%) reported contraception use in the last 3 months, with 60% using only emergency contraceptive pills. Despite self-reported history, of 373 subjects who underwent STI testing, 49 subjects (13.1%) tested positive for STIs, with 41 CT, 5 GC, and 3 TV cases. Of these 49 subjects, 33 (67.3%) reported no prior sexual intercourse. Bacterial vaginosis was rare and 90% of subjects had a normal Nugent score (0–3).
Conclusions: Upon baseline evaluation of a cohort of low risk AGYW, we found high numbers of STIs, especially CT, which is not routinely screened for in Kenyan settings. Interventions to address STIs and unintended pregnancy should target girls pre-sexual debut, including those who do not self-identify as at risk.
The World Health Organization estimates there were 376 million new cases of four curable sexually transmitted infections (STIs), chlamydia, gonorrhea, syphilis, and trichomoniasis, in 2016 (1). Globally, the highest reported rates of STIs are among youth ages 15-24 (2), especially in resource-poor countries (3). There are few large-scale studies about STI prevalence in low- and middle-income countries, but several studies of populations in sub-Saharan Africa note a higher prevalence of STIs in younger women than in older women, as well as in women over men (4, 5). The high prevalence of STIs observed among adolescent girls and young women (AGYW) compared to their male counterparts may be related to increased biological susceptibility, decreased educational, and economic opportunities, older sexual partners, increased risk for sexual coercion, and cultural norms and gender inequalities that reduce access to sexual health resources (6). The global STI epidemic will increase the burden of long-term health effects of undiagnosed and untreated disease, including pelvic inflammatory disease, infertility, ectopic pregnancy, and increased risk of HIV acquisition (1).
Few studies have followed adolescents longitudinally from pre-sexual debut. To understand risk factors for STI acquisition present from early adolescence, we enrolled a cohort of AGYW in Kenya to follow them as they transition from virginal to sexually active. We report baseline STI data from the study enrollment visit.
We recruited a cohort of AGYW aged 16-20 in Thika, Kenya between 2014 and 2016. Thika is located on hour from Nairobi and residents live in a suburban setting. A community advisory group composed of individuals with positions of influence within the community (including school principals, peer counselors, health care workers, district chiefs/administrators, religious leaders, women's groups) assisted with community engagement by introducing the study staff as health care workers conducting a research study with the aim of teaching young girls about reproductive health and preventing HSV-2. For those interested in the study, girls aged 16-17 were accompanied by their guardians to hear a health presentation, and if they desired participation, subsequently screened for the study. Those aged ≥18 years came independently for screening. We initially sought AGYW who reported no prior history of sexual intercourse. The protocol was subsequently amended to include those who reported only a single sex partner. Sex was defined as vaginal penetrative intercourse. The eligibility criteria were not divulged to potential study participants or to individuals outside of the study; parents/guardians did not learn whether their child met eligibility criteria. Potential participants needed to be willing to undergo external genital examinations and remain in follow-up for 3 years. Guardians who consented to study screening and participation for girls <18 years were informed that, after enrollment, all interactions between participants and study staff would remain confidential.
After consent was obtained, HIV and HSV-2 ELISA testing were performed, and demographic history was elicited. AGYW were excluded if HIV or HSV-2 positive. Qualifying participants returned within 3 weeks for an enrollment visit, at which time a female clinician performed an external genital exam, and obtained vaginal swab specimens for testing for Neisseria gonorrhea (GC), Chlamydia trachomatis (CT), Trichomonas vaginalis (TV), and bacterial vaginosis (BV). No testing was performed for syphilis because prior studies at the same site had found extremely low rates of syphilis, including among high risk populations, such that positive tests would be more likely to be false positives. Participants reporting prior sex completed a sexual history questionnaire. Information and samples were coded to protect confidentiality.
Participants were notified of any relevant study results directly and confidentially by study staff, and were treated for any identified STIs.
Study approval was obtained from the Kenya Medical Research Institute Scientific Ethics Review Unit, and the University of Washington Institutional Review Board. For participants under age 18, written informed assent was obtained, and written informed consent obtained from a parent/guardian. Assent was obtained separately and privately from parents/guardians, to allow participants to ask questions and to decide whether they wanted to participate free of parental influence. For participants age 18 or older, written informed consent was obtained. Participants received free, confidential medical services including contraception, HIV testing and condoms, and psychological counseling services. Participants also received transportation reimbursement for each study visit attended.
At the screening visit, a rapid HIV test was performed using whole blood from a finger prick, with the Determine HIV-1/2 Rapid test (Abbott, Chicago, Illinois). The Uni-Gold Recombigen HIV-1/2 (Trinity Biotech, Wicklow, Ireland) was used as a confirmatory test, performed at the Paediatrics Lab at Kenyatta National Hospital. At follow-up visits, blood was collected for HIV ELISA testing using Vironostika® HIV Uni-Form II Ag-Ab (Biomerieux, Marcy-l'Etoile, France). If the HIV test was positive, the participant was referred to the nearest HIV Comprehensive Care Clinic for follow-up. At the screening visit, a blood sample was sent to Kenyatta National Hospital for HSV-2 testing using the HerpeSelect HSV-2 ELISA test (Focus Diagnostics, Cypress, CA). For sample collection for GC, CT, and TV testing, a dacron swab was placed in the vagina (no speculum was used). The swab was shipped to the University of Washington Center for AIDS Research laboratory in Mombasa, Kenya for testing. Nucleic acid amplification testing was performed using the Gen-Probe APTIMA test (Hologic, Marlborough, MA). A vaginal swab was also collected for BV testing. The swab was used to create a Gram stain, and the slide was transported to Kenyatta National Hospital for evaluation by Nugent's criteria (7). A Nugent score ≥7 was defined as positive for BV.
An STI was defined as chlamydia, gonorrhea, or trichomoniasis by NAAT testing. The study was powered for HSV-2 acquisition. Based on HSV-2 prevalence data (8), projected incidence of 7.8% per year, and a median age of sexual debut of 18 (9), we assumed that enrolling 400 HSV-2 seronegative persons would result in 73 observed HSV-2 acquisitions, accounting for 13% dropout.
Data were entered into a password-secured REDCap database (10). Descriptive statistics were used to characterize the cohort. We examined whether STI positivity was associated with education, socioeconomic status, sexual history, and physical exam findings. χ2 test and Fisher's exact test were used to compare categorical variables, and t-test for independent samples for continuous variables. A multivariate logistic regression model was constructed, adjusting for possible cofactors of STI (age, BV, sexual activity, education level, rural residence, vaginal discharge). Each cofactor was added stepwise to the model and included in the final model if significantly associated (p <0.1). Data were analyzed using Stata version 15.1 (StataCorp, College Station, Texas); statistical significance was defined as 2-sided p value <0.05. Missing data was left out of the analysis.
We screened 610 AGYW, and 400 were enrolled. Two of the most common reasons for ineligibility were failure to return to clinic or report of more than one sexual partner (Figure 1). The median age of participants at enrollment was 18.6 years (IQR 17.6-19.4) and the median years of schooling was 12 (IQR 10-12) (Table 1). There were 155 (38.8%) AGYW residing in an urban setting. Within the home, 191 (47.8%) had running water, 290 (72.5%) had concrete floors, and 277 (69.3%) had electricity. Among enrolled participants, 322 (80.5%) reported no prior history of sex, while 78 (19.5%) reported one lifetime sexual partner.
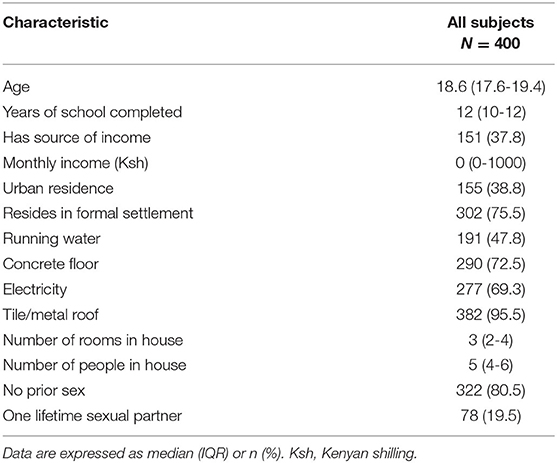
Table 1. Baseline characteristics of adolescent girls and young women enrolled in the prospective cohort.
Among the 78 participants who reported one lifetime sexual partner, the median age at first sexual intercourse was 18.7 years, and the median age of the sexual partner was 22 (IQR 21-24) (Table 2). Seventy (89.7%) of these participants endorsed sex with consent, and 20 (25.6%) reported receiving a reward for sex. Twenty (25.6%) AGYW reported contraception use in the last 3 months, with most (60%) reporting emergency pill use, and 35% reporting condom use.
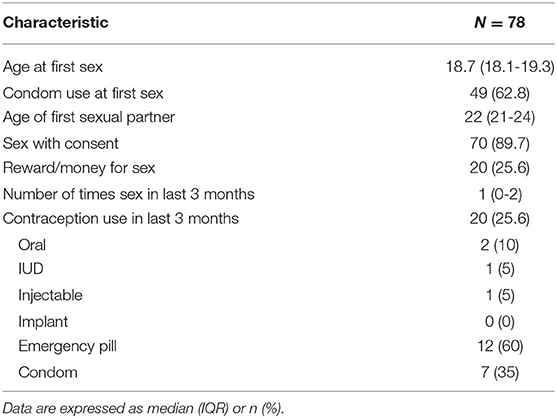
Table 2. Sexual history for adolescent girls and young women reporting prior sexual intercourse (N = 78).
Overall, 376 (94%) AGYW had external genital exams done, and 4 were circumcised. Eighteen (4.8%) of AGYW were noted to have an abnormal genital finding, with 11 participants (3%) having abnormal vaginal discharge. Of these 11 participants, 4 tested positive for an STI. Other abnormal genital exam findings included 6 participants with erythema, 4 with swelling, 2 with warts, 1 with a papular rash, and 3 with skin discoloration.
Of the 400 participants, 373 had a vaginal swab collected for GC, CT, or TV testing. Twenty participants did not have a swab collected due to menstruation; 7 did not have a swab collected for other reasons. Forty-nine (13.1%) tested positive for an STI, with CT detected in 41 (11%), GC in 5 (1.3%), and TV in 3 (0.8%) (Table 3). No persons had more than 1 STI. BV was found in 21 (5.6%) of the participants. Concurrent BV was detected among 5 participants with CT, and 2 with GC.
Testing positive for an STI did not correlate with age (p = 0.46) or whether the participant had a source of income (p = 0.29). Among the 297 AGYW who reported no prior sexual activity and had STI testing performed, 33 (11.1%) were diagnosed with either GC, CT, or TV, whereas 16 of 76 (21.1%) AGYW reporting prior sex tested positive (p = 0.022). BV positivity also was positively correlated with history of prior sex (p <0.001) and testing positive for an STI (p = 0.005).
Of the 76 AGYW who reported prior sex and underwent STI testing, 48 (63.2%) reported condom use the first time they had sex. Condom use trended toward an association with a lower STI detection, with 7 of 48 (14.6%) condom users testing positive for an STI, as compared to 9 of 28 (32.1%) AGYW reporting no condom use (p = 0.07). Of the 8 AGYW reporting coercion into sex, 7 had STI testing results available; 1 tested positive for CT and 1 for BV. Fifty-nine of 362 AGYW (16.3%) without abnormal vaginal discharge had GC, CT, TV, or BV detected, compared with 4 of 11 (36.4%) of AGYW with abnormal vaginal discharge (p = 0.08).
In a logistic regression model of STI predictors, BV, report of prior sex, and vaginal discharge were included in the adjusted model. BV continued to be associated with STI (adjusted OR 1.13, p = 0.04, 95% CI 1.01 – 1.27).
In our study of Kenyan AGYW with reported limited sexual experience, more than one in eight persons tested positive for GC, CT, or TV. The most common genital infection was Chlamydia trachomatis, which is comparable to results from several studies of adolescents in South Africa (4, 11–13). Prevalence of BV in this cohort, at 5.6%, is much lower than reported in most prior sub-Saharan African cohorts. The low prevalence of BV may be expected, given that our study is among very few reporting STI data from HSV-2 negative and sexually naïve AGYW. Nugent scores were normal in most of our cohort, whereas BV prevalence is markedly higher among other studies of sub-Saharan African women and girls (5). Our findings of extremely low prevalence of BV at study enrollment demonstrates that we may have been successful at enrolling AGYW at the beginning of sexual activity. Given that STIs were observed, our research may indicate that AGYW can go from sexual naivete to engaging in sexual intercourse to acquisition of STIs in a relatively short time frame, indicating a brief window to intervene to reduce risks.
We found that contraceptive use and condom use was low, with heavy reliance on over-the-counter emergency contraceptive pills (EC) as a primary method of family planning. This emphasizes the importance of counseling on and availability of other contraceptive methods, such as long-acting reversible contraceptives.
Many resource-limited countries rely on syndromic management of STIs as per WHO guidelines, in which signs and symptoms associated with an infection direct treatment for the likely causative organism (14). The high prevalence of chlamydia cases in our study underscores the inadequacy of relying on syndromic management, as most chlamydial infections are not symptomatic. The prevalence of CT among youth in the general population is likely even higher, given that our eligibility criteria required minimal prior sexual activity. Diagnostic testing to identify chlamydia and other STIs is urgently needed in this population. This may be difficult to implement, as laboratory-based testing requires expensive resources, facilities, and trained personnel. It can also result in loss-to-follow-up, given the delay in reporting of results. Point-of-care diagnostic testing for STIs is not currently available in Kenya, but should be an active topic of research as it could improve access to screening, provide rapid results, and facilitate timely treatment (15).
Our study was limited by reliance on self-report of sexual activity and risk behaviors, which can lead to recall and social desirability bias. In addition, the questionnaires were administered by clinicians. Participants in the study may have therefore felt pressured to report absence of sexual risk behaviors. The requirement of parental consent for minors may have also been a barrier to accurate reporting. We attempted to mitigate these effects by consenting the parent/guardian separately from the minor, as well as explaining that the data would remain confidential. Our results highlight the challenges of ascertaining behavioral risk factors for AGYW, as most STIs were detected among those who reported no prior sexual intercourse. This further emphasizes how important it is to study this unique and vulnerable population. The current landscape of HIV and STI prevention in sub-Saharan Africa relies on AGYW to self-identify as at risk of these infections to access prevention technology such as pre-exposure prophylaxis. Our research indicates that this may exclude large numbers of at-risk youth. An important next step in creating an AIDS-free generation will be interventions to reach AGYW who may not self-identify or be traditionally categorized as at risk for STI acquisition.
The datasets generated for this study are available on request to the corresponding author.
AR, AW, NM, and KN conceived and planned the study. LO, MM, CK, BC, SS, KN, NM, AW, and AR implemented the study and collected the data. TY, SS, AR, and AM analyzed the data. TY wrote the paper with input from all authors.
This research was funded by R01 HD091996-01 (AR) from NICHD, by P01 AI 030731 (AW), and by the Center for AIDS Research (CFAR) of the University of Washington/Fred Hutchinson Cancer Research Center AI027757. The funders had no role in study design, data collection, and analysis, or preparation of the manuscript. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Washington funded by UL1 TR002319, KL2 TR002317, and TL1 TR002318 from NCATS/NIH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the girls who participated in this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. World Health Organization. Report on Global Sexually Transmitted Infection Surveillance. (2018). Available online at: https://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691-eng.pdf?ua=1
2. Dehne KL, Riedner G. Sexually Transmitted Infections Among Adolescents: the Need for Adequate Health Services. Geneva: World Health Organization (2005).
3. Jespers V, Nostlinger C, van de Wijgert J. Adolescent sexual health: time to invest in a healthy future generation. Sex Transm Infect. (2016) 92:248–9. doi: 10.1136/sextrans-2015-052485
4. Francis SC, Mthiyane TN, Baisley K, Mchunu SL, Ferguson JB, Smit T, et al. Prevalence of sexually transmitted infections among young people in South Africa: A nested survey in a health and demographic surveillance site. PLoS Med. (2018) 15:e1002512. doi: 10.1371/journal.pmed.1002512
5. Torrone EA, Morrison CS, Chen PL, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. (2018) 15:e1002511. doi: 10.1371/journal.pmed.1002511
6. Gupta MD, Engelman R, Levy J, Luchsinger G, Merrick T, Rosen JE, et al. The Power of 1.8 Billion: Adolescents, Youth and the Transformation of the Future. New York, NY: United Nations Population Fund (2014).
7. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. (1991) 29:297–301. doi: 10.1128/JCM.29.2.297-301.1991
8. Mugo N, Dadabhai SS, Bunnell R, Williamson J, Bennett E, Baya I, et al. Prevalence of herpes simplex virus type 2 infection, human immunodeficiency virus/herpes simplex virus type 2 coinfection, and associated risk factors in a national, population-based survey in Kenya. Sex Transm Dis. (2011) 38:1059–66. doi: 10.1097/OLQ.0b013e31822e60b6
9. Ndayisaba G, Verwijs MC, van Eeckhoudt S, Gasarabwe A, Hardy L, Borgdorff H, et al. Feasibility and acceptability of a novel cervicovaginal lavage self-sampling device among women in Kigali, Rwanda. Sex Transm Dis. (2013) 40:552–5. doi: 10.1097/OLQ.0b013e31828e5aa5
10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81 doi: 10.1016/j.jbi.2008.08.010
11. Kaida A, Dietrich JJ, Laher F, Beksinska M, Jaggernath M, Bardsley M, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: implications for HIV prevention efforts. BMC Infect Dis. (2018) 18:499. doi: 10.1186/s12879-018-3380-6
12. Barnabas SL, Dabee S, Passmore JS, Jaspan HB, Lewis DA, Jaumdally SZ, et al. Converging epidemics of sexually transmitted infections and bacterial vaginosis in southern African female adolescents at risk of HIV. Int J STD AIDS. (2018) 29:531–9. doi: 10.1177/0956462417740487
13. Masese LN, Wanje G, Kabare E, Budambula V, Mutuku F, Omoni G, et al. Screening for sexually transmitted infections in adolescent girls and young women in mombasa, kenya: feasibility, prevalence, and correlates. Sex Transm Dis. (2017) 44:725–31. doi: 10.1097/OLQ.0000000000000674
14. World Health Organization. Sexually Transmitted and Other Reproductive Tract Infections. (2005). Available online at: https://www.who.int/reproductivehealth/publications/rtis/9241592656/en/
Keywords: adolescent, sexually transmitted infection, chlamydia, bacterial vaginosis, Africa
Citation: Yuh T, Micheni M, Selke S, Oluoch L, Kiptinness C, Magaret A, Chohan B, Ngure K, Wald A, Mugo NR and Roxby AC (2020) Sexually Transmitted Infections Among Kenyan Adolescent Girls and Young Women With Limited Sexual Experience. Front. Public Health 8:303. doi: 10.3389/fpubh.2020.00303
Received: 01 March 2020; Accepted: 04 June 2020;
Published: 14 July 2020.
Edited by:
Remco P. H. Peters, University of Pretoria, South AfricaReviewed by:
Admire Takuranenhamo Chikandiwa, Wits Health Consortium (WHC), South AfricaCopyright © 2020 Yuh, Micheni, Selke, Oluoch, Kiptinness, Magaret, Chohan, Ngure, Wald, Mugo and Roxby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiffany Yuh, dGlmZmFueS55dWhAcGVubm1lZGljaW5lLnVwZW5uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.