
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 25 June 2020
Sec. Digital Public Health
Volume 8 - 2020 | https://doi.org/10.3389/fpubh.2020.00260
This article is part of the Research Topic Creating Evidence from Real World Patient Digital Data View all 14 articles
 Richard L. Kravitz1†*
Richard L. Kravitz1†* Adrian Aguilera2
Adrian Aguilera2 Elaine J. Chen3
Elaine J. Chen3 Yong K. Choi4
Yong K. Choi4 Eric Hekler5
Eric Hekler5 Chris Karr6
Chris Karr6 Katherine K. Kim4
Katherine K. Kim4 Sayali Phatak7
Sayali Phatak7 Sayantani Sarkar4
Sayantani Sarkar4 Stephen M. Schueller8
Stephen M. Schueller8 Ida Sim9
Ida Sim9 Jiabei Yang10
Jiabei Yang10 Christopher H. Schmid10
Christopher H. Schmid10Although group-level evidence supports the use of behavioral interventions to enhance cognitive and emotional well-being, different interventions may be more acceptable or effective for different people. N-of-1 trials are single-patient crossover trials designed to estimate treatment effectiveness in a single patient. We designed a mobile health (mHealth) supported N-of-1 trial platform permitting US adult volunteers to conduct their own 30-day self-experiments testing a behavioral intervention of their choice (deep breathing/meditation, gratitude journaling, physical activity, or helpful acts) on daily measurements of stress, focus, and happiness. We assessed uptake of the study, perceived usability of the N-of-1 trial system, and influence of results (both reported and perceived) on enthusiasm for the chosen intervention (defined as perceived helpfulness of the chosen intervention and intent to continue performing the intervention in the future). Following a social media and public radio campaign, 447 adults enrolled in the study and 259 completed the post-study survey. Most were highly educated. Perceived system usability was high (mean scale score 4.35/5.0, SD 0.57). Enthusiasm for the chosen intervention was greater among those with higher pre-study expectations that the activity would be beneficial for them (p < 0.001), those who obtained more positive N-of-1 results (as directly reported to participants) (p < 0.001), and those who interpreted their N-of-1 study results more positively (p < 0.001). However, reported results did not significantly influence enthusiasm after controlling for participants' interpretations. The interaction between pre-study expectation of benefit and N-of-1 results interpretation was significant (p < 0.001), such that those with the lowest starting pre-study expectations reported greater intervention enthusiasm when provided with results they interpreted as positive. We conclude that N-of-1 behavioral trials can be appealing to a broad albeit highly educated and mostly female audience, that usability was acceptable, and that N-of-1 behavioral trials may have the greatest utility among those most skeptical of the intervention to begin with.
Accumulating evidence supports the adoption of various habits and behaviors to improve cognitive and emotional well-being. For example, Americans are urged to be more physically active, reduce stress, and connect socially (1–5). One problem with the plethora of recommendations is that individuals may be confused about which behaviors to adopt first. They can turn to trusted experts, but most of the evidence upon which those experts rely is based on studies that generate average effects. Evidence derived from groups may not necessarily apply to the individual because of heterogeneity in person-level and contextual factors (e.g., age, gender, personal preferences, community resources, and fit with a person's life or workflow) (6, 7). Furthermore, the impact of any behavior is likely to yield modest benefits, potentially accumulating over time. More precise information on the likelihood of benefit at the individual level could help motivate long term behavior change.
Certainly, many people can and do assess the personal value of behavioral interventions informally through trial and error. Some, however, may be interested in a more rigorous approach. N-of-1 trials are multiple crossover trials conducted in a single individual (8). While sharing some characteristics with informal “trials of therapy,” they lend rigor to the assessment and, along with parallel group randomized controlled trials, are ranked at the top of the so-called evidence hierarchy by experts (9, 10). They have been used extensively in clinical psychology and medicine (11–17). For fast-acting, short-lived behavioral interventions expected to influence near-term outcomes, N-of-1 trials are arguably the most direct method for inferring the effect of treatment on an individual. These trials may appeal to persons who wish to gain greater certainty that the behavioral intervention under consideration actually does (or does not) have benefit for them.
Despite their theoretical appeal, N-of-1 trials have gained limited traction among clinicians and the general public (18). Part of the reason may be that when implemented according to the highest scientific standards (which may include blinding, use of complex outcome measures, strict attention to adherence, etc.), many potential participants will decide that the likely benefits (in terms of insights and motivation) are simply not worth the trouble. However, we and others have demonstrated that the reach and feasibility of N-of-1 trials may be extended through use of mobile health (mHealth) technologies; in our own recent study of patients with chronic pain, 88% of patients starting an n-of-1 trial reported that the mobile app was “extremely or very helpful.”(19) Another barrier may be the absence of scalable tools that allow non-scientists to conduct systematic evaluations of behavioral interventions on themselves (20).
We conducted this study to determine whether an mHealth supported N-of-1 trial assessing simple behavioral interventions for improving short-term cognitive and emotional well-being was feasible and perceived as beneficial. Specifically, we asked:
• Will members of the general adult population participate in an mHealth-facilitated behavioral N-of-1 trial?
• How do participants rate the usability of the mHealth N-of-1 trial system?
• To what extent are participant's attitudes toward the intervention and intentions to persist with it influenced by trial participation? Specifically,
In addressing these questions, we sought to learn more about the utility of N-of-1 trials, the ways in which such trials affect participants' subsequent attitudes and behavioral intentions, and their prospects for broader adoption by the medical and behavioral community.
A national convenience sample of adult volunteers was recruited to engage in a 30-day single person (N-of-1) trial comparing the effects of one of four behavioral interventions on self-reported stress, focus, and happiness. Participants selected an intervention and were assigned for 30 days to randomly sequenced five-day periods performing the chosen activity and their “usual routine.” Outcome measures were collected via secure text messaging. This report focuses on the 259 subjects who completed a post-study survey. Ethics approval was granted through the UC Davis Institutional Review Board (IRB ID 1255435-4).
We promoted the study through social media and The Brian Lehrer Show (WNYC Public Radio). Potentially interested subjects were directed to the study website (studyofme.org), where they were given the opportunity to watch videos introducing the study and asked to select an activity of interest. Available activities included: (1) deep breathing meditation; (2) gratitude journaling; (3) physical activity; and 4) performing acts of kindness for strangers. Drawing on cognitive-behavioral techniques and positive psychology, activities were selected to be simple and easy to apply repeatedly (21–24). Volunteers were eligible if they were US adults > = 18 years, owned a smartphone or had regular access to the internet, and were interested in committing to a 30-day N-of-1 trial. In addition, subjects were encouraged to try an activity that they were not already doing, and if they were considering vigorous physical activity, they were advised to “first consult your doctor, especially if you have a chronic health problem, recurring injury, or are pregnant or nursing.”
After confirmation of eligibility and provision of online informed consent, participants completed a baseline questionnaire asking for contact information (mobile phone number and valid email, both of which were deleted from the dataset prior to analysis); time zone (so that study reminders would go out at the right time of day); N-of-1 trial start date within the next 7 days; demographic information (ethnicity, race, gender, education level, and household size); and several questions concerning experience with self-tracking and interest in the chosen activity.
All participants had the opportunity to read text and view a video providing detailed instructions on their chosen activity. Computer-generated N-of-1 trial sequences (e.g., UAUAAU, where U indicates 5 days performing usual routine and A indicates 5 days performing the chosen activity) were issued for each subject beginning on their chosen start date and continuing for 30 consecutive days. We used 5-day treatment periods as a compromise between the dictates of behavioral science (which would favor longer periods, to allow for adequate ramp-up and wash-out) (8) and statistical power (which would favor a greater number of switches between treatments). Participants received a text message through the HealthySMS system (25–27) each evening asking for ratings of stress, focus, and happiness for the day just finished and announcing tomorrow's activity.
Within 1 week of N-of-1 trial completion, HealthySMS sent participants a text message with a link to their personalized results (example provided in Figure 1).
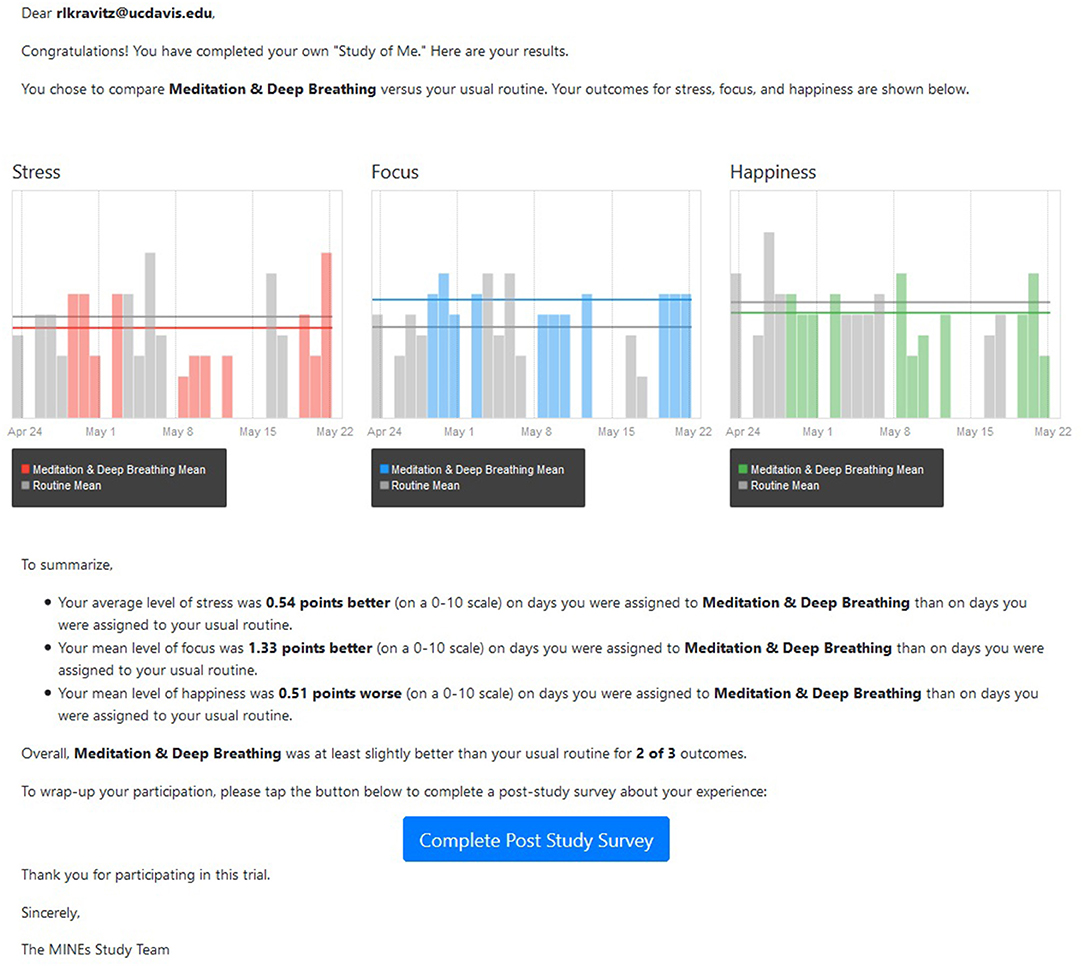
Figure 1. Sample participant results report. Participants in the study were provided with a both a graphic and a written summary depicting their gains (or losses) on days assigned to the intervention compared with days assigned to usual routine.
Daily stress, focus, and happiness were each assessed with a single-item question sent each evening by text messaging: (1) On average, how stressed were you today? Please select a value from 0 (not at all stressed) to 10 (extremely stressed); (2) On average, how well were you able to focus? Please select a value from 0 (not able to focus at all) to 10 (extremely focused throughout the day); (3) On average, how happy were you today? Please select a value from 0 (not happy at all) to 10 (extremely happy throughout the day). Single-item measures of these constructs are typically used in studies that require daily responses by participants to reduce participant burden (28), and have demonstrated good reliability and validity when compared to longer measures (29–31).
At the end of the 30-day period, participants were sent a post-study questionnaire requesting completion of the System Usability Scale (32) (Cronbach's alpha in the sample, 0.83), and four questions assessing: (1) pre-study expectations of benefit of the chosen activity (“Before you started your personalized experiment, how confident were you that ACTIVITY would be beneficial for you?” 1 = not-at-all confident…5 = extremely confident); (2) post-study interpretation of results (“What is your best guess about what the RESULTS of your personalized experiment mean? 4 = highly beneficial, 3 = somewhat beneficial, 2 = minimally beneficial, 1 = not beneficial); (3) post-study perceptions of activity helpfulness (“Now that you have completed your personalized experiment, how helpful do you think ACTIVITY was for you? (1 = not at all helpful…5 = extremely helpful); and (4) post-study behavioral intentions (“Based on your personalized experiment, how likely are you to continue doing ACTIVITY on a regular basis over the next six months? 1 = not-at-all likely…5 = extremely likely).
We created an Activity Enthusiasm Score as the mean of perceived helpfulness of the activity (1–5 scale) and likelihood of continuing activity on a regular basis (1–5 scale), both measured after N-of-1 completion on a 1–5 scale. Cronbach's alpha for this 2-item index was 0.77, indicating acceptable to good internal consistency (33).
We summarized the actual results of each subject's N-of-1 trial in two ways. First, we directly evaluated differences in means for focus, stress, and happiness (each reported on a 0–10 scale) by taking the difference between the mean value during activity days and the mean value during the participant's usual routine, reversing the sign for stress, then summing across the three outcomes. The theoretical range of this scale was −30 to +30 and the actual range was −7.4 to 9.3. Second, we counted the number of outcomes (stress, focus, happiness) in which the mean value of the participant's responses while performing the chosen activity was better (more positive or less negative) than the mean value of the participant's response while performing their usual routine. Possible values of this count variable ranged from 0 (no outcome better, even by a small amount, during activity days) to 3 (all outcomes better during activity days). The difference variable accounts for the magnitude of benefit but does not consider precision (i.e., the metric does not take into account the within-individual variance in reported outcomes nor the number of measures reported by each participant. The count variable focuses on the number of outcome dimensions that were “improved,” while ignoring the magnitude of the improvements. We chose to evaluate these metrics rather than more sophisticated alternatives because they more closely comport with the data actually supplied to participants as shown, for example, in Figure 1). Because the results using the two metrics were not materially different, we report only the difference measure.
Values were expressed as means with standard deviations for continuous variables and counts with proportions for categorical variables. For characteristics of the analytic sample, analysis of variance (ANOVA) was performed to test whether there were differences in the means of continuous variables across different chosen activity groups. Chi-square test was performed to test for whether there was association between categorical variables and chosen activity groups if the minimum expected cell count was greater than 1; otherwise, Fisher's exact test was performed. The same procedure was also applied when comparing those who completed the post-study survey with those who did not.
Multiple linear regression was used to assess the relationship between Activity Enthusiasm Score and expectations of benefit from the intervention, interpretation of n-of-1 results, and actual reported results as represented by the summated score along with their pairwise interactions. Goodness-of-fit was expressed by the coefficient of determination, R2. A significant relationship was determined by a p < 0.05. Stata software version 15 was used for regression modeling. R software version 3.6.1 was used to produce graphs.
Of 824 volunteers who accessed the online pre-study questionnaire (353 who selected deep breathing, 225 gratitude journaling, 191 physical activity, and 55 acts of kindness), 682 subjects completed the pre-study survey and were assessed for eligibility, 447 signed onto the HealthySMS platform, and 259 completed the post-study survey Figure 2. The mean proportion of daily assessments actually returned was 0.70 (SD 0.22).
Among the 259, the mean age was 51 and most were from the Eastern time zone, female, white, and very highly educated; a minority lived alone or had previously tried self-experimentation (Table 1) There were no significant associations between respondents' personal characteristics and the behavior intervention activity they chose to evaluate (Table 1). There were no meaningful or statistically significant demographic differences between the 259 participants included in the analytic sample and the remaining 188 participants who enrolled in the study but did not complete any part of the post-study questionnaire, except that completers were about 4 years older (Supplemental Material Table 1).
Most respondents strongly or somewhat agreed with positive statements about system usability and strongly or somewhat disagreed with negative statements (Table 2). The mean scale score was 4.35 (SD 0.57) (Table 2), corresponding to a percentage-based score of 4.35/5 × 100 = 87.1, well above the average of 68 previously reported and comparable to microwave ovens, which received a rating of 87 in a consumer survey of 1,058 participants (34, 35). System Usability Scale scores were lower, on average, among participants choosing Acts of Kindness compared to those choosing Deep Breathing or Physical Activity (p = 0.008, with pairwise comparisons between Acts of Kindness and both Deep Breathing and Physical Activity significant using the Bonferroni approach (p < 0.01 in each case, data not shown in tabular form). However, there were no significant differences in System Usability Scale scores by age, gender, race, or education (see Supplemental Material Table 2 for details).
On the post-study questionnaire, 32% of respondents recalled that prior to starting the N-of-1 trial they were very or extremely confident that the chosen activity would be beneficial. At the same time, 27 (10%) interpreted their reported N-of-1 results as indicating that the activity was not beneficial for them, 77 (30%) that the activity was minimally beneficial, 119 (46%) that the activity was somewhat beneficial, and 25 (10%) that the activity was highly beneficial. Participants with positive expectations for intervention benefit (i.e., those who reported being “very” or “extremely” confident that the chosen intervention would be beneficial) were more likely than their more skeptical peers to interpret their results as showing that the activity was “somewhat” or “highly” beneficial (68 vs. 53%, p = 0.036, data not shown in tabular form).
Table 3 examines the effects of pre-study expectations for benefit, the participant's interpretation of their own N-of-1 results, and actual reported results (as represented by the difference metric; see Methods) on Activity Enthusiasm Score. Model 1 shows that both expectations for intervention benefit (p < 0.001) and interpretation of own results (p < 0.001) were significantly associated with enthusiasm for the chosen activity, accounting for 33% of the variance. Substituting actual results for interpretation of results resulted in a regression (Model 2) that explained only 14% of the variance in enthusiasm. Finally, actual results were not significantly associated with enthusiasm after adjusting for pre-study confidence and results interpretation (Model 3).
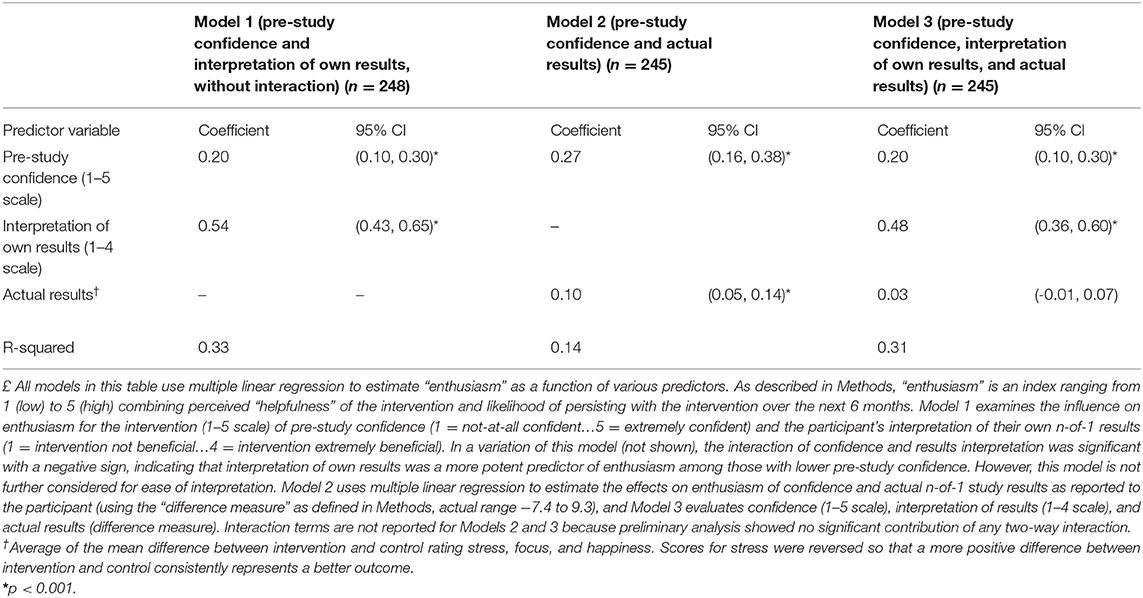
Table 3. Influence of pre-study confidence, interpretation of own results, and actual (reported) results on participant's “enthusiasm” for the behavioral intervention£.
The relationship of pre-study expectations for benefit, post-study interpretation of results, and Activity Enthusiasm Score is further illustrated in Figure 3. Essentially, if at the outset respondents were highly confident that their chosen activity was beneficial (top row), enthusiasm remained moderate to high regardless of actual study results (plenty of orange and red dots, and very few blue dots, even among participants who interpreted their own n-of-1 results as showing that the activity delivered little to no benefit). On the other hand, if initial confidence for benefit was low-to-moderate (bottom row), enthusiasm was more strongly related to the participant's interpretation of their own results (mostly blue dots in the “not beneficial” column, mostly red dots in the “highly beneficial” column). These results are replicated in tabular form in Supplemental Material Table 3.
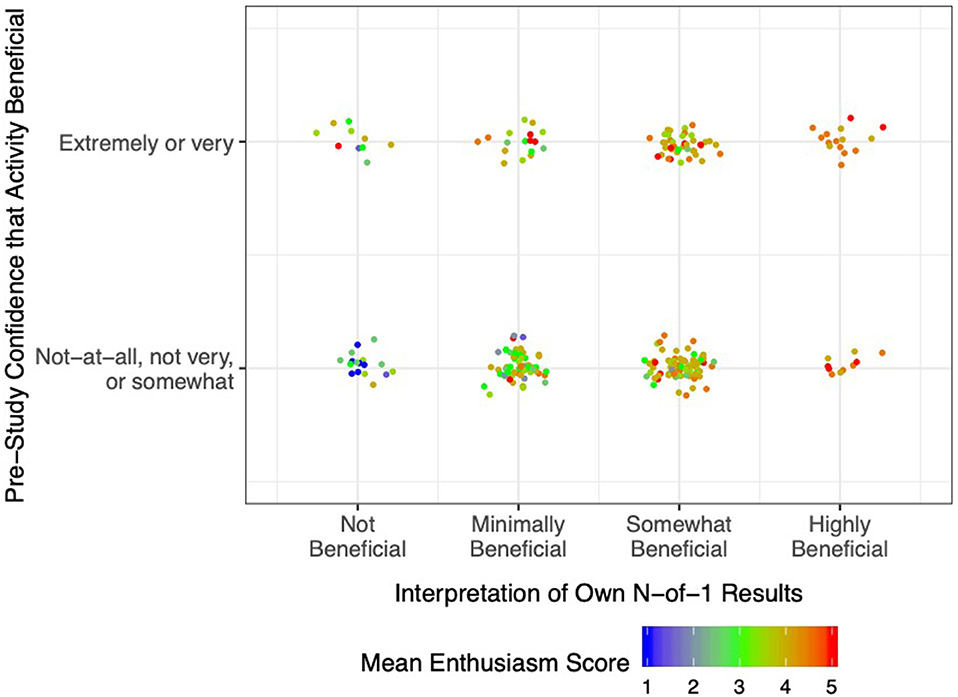
Figure 3. Post-Study Enthusiasm Scores (mean of perceived intervention helpfulness and likelihood of persisting with the activity in future) as a function of pre-study confidence in the activity (reported retrospectively) and interpretation of own n-of-1 trial results. Each dot represents a single participant, with warmer colors indicating greater enthusiasm.
As the most direct approach to estimating individual treatment effects, N-of-1 trials have been called the holy grail of clinical investigation (36). The method's appeal may also extend to selected lay audiences, such as the quantified-self movement (37). Broader uptake of N-of-1 trials could help people with and without chronic diseases to more quickly identify treatments or lifestyle interventions that are both appealing and effective. However, logistical barriers, technical concerns, and simple lack of awareness have impeded dissemination and uptake. The main contribution of the current study is to demonstrate that N-of-1 trials of behavioral interventions can attract substantial interest from a relatively broad cross-section of US adults. However, participants were highly educated and tilted strongly female. There are several possible explanations for limited participation among men and those without a college degree, including relative indifference to the topic of “wellness;” competing demands from other responsibilities; or a persistent “digital divide” curtailing access or limiting comfort with mobile devices (38). Nevertheless, our mHealth platform supporting these trials was rated highly usable by participants. Finally, participants' a priori expectations for benefit of their chosen behavioral intervention (as measured by confidence that the activity would be beneficial) as well as their a posteriori interpretation of their N-of-1 trial results were both significant independent predictors of enthusiasm for the intervention going forward.
We recruited participants using social media (principally Facebook) plus an on-air interview with WNYC Public Radio host Brian Lehrer. Over a brief recruitment period, 824 individuals demonstrated interest by visiting the study website, but unsurprisingly, there was significant attrition at every stage thereafter. Among the 259 subjects in the analytic cohort, the modal participant was a white, middle-aged, highly educated woman. However, less than one in five had prior experience with self-experimentation, indicating both that our sample was open to novel experiences and that N-of-1 trials may have appeal beyond the established self-tracking community.
Participants generally rated the HealthySMS platform as highly accessible and easy to use without technical support, despite modest misgivings about functional integration. These findings are especially remarkable in light of the native complexity of N-of-1 trials. For example, in our study, patients needed to become comfortable with a new behavioral intervention, switch off regularly between the intervention and their usual routine, and report daily ratings of stress, focus, and happiness.
Pre-study expectations for benefit from the chosen behavioral intervention was modestly associated with post-study enthusiasm for the intervention. At the same time, participants who interpreted their N-of-1 results as highly beneficial had much greater enthusiasm than those who interpreted their results as indicating that the intervention was not beneficial. However, the effect of participants' interpretations of their own results on enthusiasm for the intervention was greater among those with the least confidence in the intervention to begin with.
One interpretation of these findings is that N-of-1 trials had greater information value for participants who were more skeptical at the outset; in Bayesian terms, those with weak or negative priors relied more on the incoming data (39). Although considerable work in cognitive psychology indicates that humans are poor Bayesians (40), our results suggest that in the context of a self-experiment in which they are personally vested, participants may form conclusions based on a weighted average of pre-trial expectations and post-trial results. A plausible implication is that investigators should explicitly account for participants' prior beliefs in the context of N-of-1 experiments and, indeed, use them in constructing posterior probabilities that are returned to patients. Another possible explanation, drawing on expectation disconfirmation theory (41), is that participants who were pleasantly surprised by a positive result (despite expecting a negative outcome) were more likely to be enthused about the activity going forward.
Although participants' actual results (as conveyed by a graphical interface supported by text, as in Figure 1) were moderately correlated with their subjective interpretations, the former did not significantly predict intervention enthusiasm after adjusting for the latter, suggesting that actual results are mediated through participants' interpretations. Furthermore, participants' interpretations may not fully and accurately incorporate actual results—even among the highly educated. More work is needed on ways to accurately convey n-of-1 results to participants, especially in real-world, non-clinical settings where clinicians and investigators are unavailable to help.
The strengths of this study include attention to several novel questions and the use of innovative methods to attract participants and to support them in conducting their own single-patient trials. However, as with all studies, the findings must be evaluated in light of certain limitations. First, there was substantial attrition between expressing initial interest and completing a minimum number of study procedures. Second, the analytic sample was demographically narrow, limiting generalizability (42). This likely reflected some combination of our outreach methods (social media and public radio, which may appeal to a more socio-economically advantaged cohort); the “digital divide;” and the intrinsic appeal of “wellness” interventions and self-monitoring to certain demographic groups (e.g., women). Third, in measuring daily outcomes with single items, we likely sacrificed reliability in the interest of minimizing respondent burden. Fourth, measuring pre-study confidence in the benefits of the intervention after participants completed their N-of-1 trial could have introduced recall or “hindsight” bias. In retrospect, it would have been preferable to measure expectations prospectively, and future studies should do this. Hindsight bias would tend to narrow the gap between participants' expectations and post-hoc enthusiasm for the intervention. Had we measured expectations prospectively, we might have seen a more consistent gap in enthusiasm between those with high and low expectations. Finally, we made no attempt to measure either behavior change or psychological outcomes beyond 4–6 weeks after the start of each participant's N-of-1 trial.
In summary, this study demonstrates that N-of-1 trials can be disseminated to a broad, if demographically slanted, subset of the general US population in the interest of enhancing psychological well-being. Subjects appear to learn from their own N-of-1 experiences, although their learnings are tempered by prior beliefs. Our finding of increased influence of trial results among those with the lowest a priori expectations of benefit suggests that mHealth-supported, behavioral N-of-1 trials may have the greatest value for those with the lowest outcomes expectations; these individuals may be exactly those with more health problems and higher need. Further research is needed to clarify who can benefit from such trials, under what circumstances, and with respect to which medium and long-term outcomes.
The datasets generated and analyzed for this study can be accessed by writing to the corresponding author.
The studies involving human participants were reviewed and approved by the UC Davis Institutional Review Board (IRB ID 1255435-4). The patients/participants provided their written informed consent to participate in this study.
RK conceived of the study, obtained funding, performed selected analyses, and wrote the first draft of the manuscript. AA, CK, KK, and SS supported the technical implementation of the study, participated in data collection, and edited the manuscript. EC, EH, SP, SMS, and IS conceived of the study, contributed to design and implementation, and edited the manuscript. YC, JY, and CS performed and/or supervised the statistical analysis and edited the manuscript. All authors contributed to the article and approved the submitted version.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CK is the founder of Audacious Software LLC and provided software development, design, and support services for research efforts. AA is (together with University of California, Berkeley) the owner of the HealthySMS license. Fees are paid to UC Berkeley for its use. EH is scientific advisor to Fitabase. KK is an advisor to Bluenote Therapeutics. SS is a consultant with Otsuka Pharmaceutical. IS is a member of the Medical Advisory Board for 98point6; has stock options in Myia Labs, Inc.; is a scientific advisor and consultant for Myovant; and is a Board member and consultant at Vivli.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00260/full#supplementary-material
1. Clarke TC, Barnes PM, Black LI, Stussman BJ, Nahin RL. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. (2018) 1–8.
2. Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend? Can J Cardiol. (2016) 32:495–504. doi: 10.1016/j.cjca.2016.01.024
3. Holt-Lunstad J, Robles TF, Sbarra DA. Advancing social connection as a public health priority in the United States. Am Psychol. (2017) 72:517. doi: 10.1037/amp0000103
4. Christian H, Bauman A, Epping JN, Levine GN, McCormack G, Rhodes RE, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. (2018) 12:233–43. doi: 10.1177/1559827616643686
5. Oman D. Elephant in the room: why spirituality and religion matter for public health. In: Oman D, editor. Why Religion and Spirituality Matter for Public Health. Religion, Spirituality and Health: A Social Scientific Approach, Vol. 2. (Cham: Springer) (2018).
6. Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. (2004) 82:661–87. doi: 10.1111/j.0887-378X.2004.00327.x
7. Kent DM, Nelson J, Dahabreh IJ, Rothwell PM, Altman DG, Hayward RA. Risk and treatment effect heterogeneity: re-analysis of individual participant data from 32 large clinical trials. Int J Epidemiol. (2016) 45:2075–88. doi: 10.1093/ije/dyw118
8. Kravitz R, Duan N, Eslick I, Gabler N, Kaplan HC, Larson E, et al. Design and Implementation of N-of-1 Trials: A User's Guide. Rockville, MD: Agency for Healthcare Research and Quality. (2014).
9. Vohra S, Shamseer L, Sampson M, Bukutu C, Schmid CH, Tate R, et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015. Statement. J Clin Epidemiol. (2016) 76:9–17. doi: 10.1016/j.jclinepi.2015.05.004
10. OCEMB. The Oxford Levels of Evidence 2 Oxford, England: Oxford Center for Evidence Based Medicine. (2016). Available online at: https://www.cebm.net/index.aspx?o = 5653 (accessed May 1, 2020).
11. Gabler NB, Duan N, Vohra S, Kravitz RL. N-of-1 trials in the medical literature: a systematic review. Med Care. (2011) 49:761–8. doi: 10.1097/MLR.0b013e318215d90d
12. Jaeschke R, Adachi J, Guyatt G, Keller J, Wong B. Clinical usefulness of amitriptyline in fibromyalgia: the results of 23 N-of-1 randomized controlled trials. J Rheumatol. (1991) 18:447–51.
13. Li J, Gao W, Punja S, Ma B, Vohra S, Duan N, et al. Reporting quality of N-of-1 trials published between 1985 and 2013: a systematic review. J Clin Epidemiol. (2016) 76:57–64. doi: 10.1016/j.jclinepi.2015.11.016
14. Nikles CJ, Mitchell GK, Del Mar CB, Clavarino A, McNairn N. An n-of-1 trial service in clinical practice: testing the effectiveness of stimulants for attention-deficit/hyperactivity disorder. Pediatrics. (2006) 117:2040–6. doi: 10.1542/peds.2005-1328
15. Nikles CJ, Mitchell GK, Del Mar CB, McNairn N, Clavarino A. Long-term changes in management following n-of-1 trials of stimulants in attention-deficit/hyperactivity disorder. Eur J Clin Pharmacol. (2007) 63:985–9. doi: 10.1007/s00228-007-0361-x
16. Nikles CJ, Yelland M, Del Mar C, Wilkinson D. The role of paracetamol in chronic pain: an evidence-based approach. Am J Ther. (2005) 12:80–91. doi: 10.1097/00045391-200501000-00011
17. Zucker DR, Ruthazer R, Schmid CH, Feuer JM, Fischer PA, Kieval RI, et al. Lessons learned combining N-of-1 trials to assess fibromyalgia therapies. J Rheumatol. (2006) 33:2069–77.
18. Kravitz RL, Paterniti DA, Hay MC, Subramanian S, Dean DE, Weisner T, et al. Marketing therapeutic precision: potential facilitators and barriers to adoption of n-of-1 trials. Contemp Clin Trials. (2009) 30:436–45. doi: 10.1016/j.cct.2009.04.001
19. Kravitz RL, Schmid CH, Marois M, Wilsey B, Ward D, Hays RD, et al. Effect of mobile device-supported single-patient multi-crossover trials on treatment of chronic musculoskeletal pain: a randomized clinical trial. JAMA Int Med. (2018) 178:1368–77. doi: 10.1001/jamainternmed.2018.3981
20. Chen C, Haddad D, Selsky J, Hoffman JE, Kravitz RL, Estrin DE, et al. Making sense of mobile health data: an open architecture to improve individual- and population-level health. J Med Internet Res. (2012) 14:e112. doi: 10.2196/jmir.2152
21. Curry OS, Rowland LA, Van Lissa CJ, Zlotowitz S, McAlaney J, Whitehouse H. Happy to help? A systematic review and meta-analysis of the effects of performing acts of kindness on the well-being of the actor. J Exp Soc Psychol. (2018) 76:320–9. doi: 10.1016/j.jesp.2018.02.014
22. Davis DE, Choe E, Meyers J, Wade N, Varjas K, Gifford A, et al. Thankful for the little things: a meta-analysis of gratitude interventions. J Couns Psychol. (2016) 63:20–31. doi: 10.1037/cou0000107
23. Johnson S, Gur RM, David Z, Currier E. One-session mindfulness meditation: a randomized controlled study of effects on cognition and mood. Mindfulness. (2015) 6:88–98. doi: 10.1007/s12671-013-0234-6
24. Yeung RR. The acute effects of exercise on mood state. J Psychosom Res. (1996) 40:123–41. doi: 10.1016/0022-3999(95)00554-4
25. Avila-Garcia P, Hernandez-Ramos R, Nouri SS, Cemballi A, Sarkar U, Lyles CR, et al. Engaging users in the design of an mHealth, text message-based intervention to increase physical activity at a safety-net health care system. JAMIA Open. (2019) 2:489–97. doi: 10.1093/jamiaopen/ooz049
26. García Y, Ferrás C, Aguilera A, Ávila P. Usability and feasibility study of a remote cognitive behavioral therapy system with long-term unemployed women. J Technol Hum Serv. (2017) 35:219–30. doi: 10.1080/15228835.2017.1345672
27. Aguilera A, Bruehlman-Senecal E, Demasi O, Avila P. Automated text messaging as an adjunct to cognitive behavioral therapy for depression: a clinical trial. J Med Inter Res. (2017) 19:e148. doi: 10.2196/jmir.6914
28. May M, Junghaenel DU, Ono M, Stone AA, Schneider S. Ecological momentary assessment methodology in chronic pain research: a systematic review. J Pain. (2018) 19:699–716. doi: 10.1016/j.jpain.2018.01.006
29. Abdel-Khalek AM. Measuring happiness with a single-item scale. Soc Behav Personal. (2006) 34:139–49. doi: 10.2224/sbp.2006.34.2.139
30. Elo AL, Leppanen A, Jahkola A. Validity of a single-item measure of stress symptoms. Scand J Work Env Hea. (2003) 29:444–51. doi: 10.5271/sjweh.752
31. Gardner DG, Cummings LL. Single-item versus multiple-item measurement scales: an empirical comparison. Educ Psychol Meas. (1998) 58:898–915. doi: 10.1177/0013164498058006003
33. Gliem JA, Gliem RR (editors). Calculating, interpreting, and reporting Cronbach's alpha reliability coefficient for Likert-type scales. In: Midwest Research to Practice Conference in Adult, Continuing, and Community Education Voices on the Web. Columbus, OH (2003).
34. Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Intl J Hum Comput Inter. (2008) 24:574–94. doi: 10.1080/10447310802205776
35. Kortum PT, Bangor A. Usability ratings for everyday products measured with the system usability scale. Int J Hum Comput Inter. (2013) 29:67–76. doi: 10.1080/10447318.2012.681221
36. Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Personal Med. (2011) 8:161–73. doi: 10.2217/pme.11.7
37. Swan M. The quantified self: fundamental disruption in big data science and biological discovery. Big data. (2013) 1:85–99. doi: 10.1089/big.2012.0002
38. Gonzales A. The contemporary US digital divide: from initial access to technology maintenance. Inform Commun Soc. (2016) 19:234–48. doi: 10.1080/1369118X.2015.1050438
39. Duan NH, Kravitz RL, Schmid CH. Single-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J Clin Epidemiol. (2013) 66:S21–8. doi: 10.1016/j.jclinepi.2013.04.006
40. Kahneman D, Tversky A. On the psychology of prediction. Psychol Rev. (1973) 80:237. doi: 10.1037/h0034747
41. Serrano CI, Shah V, Abràmoff MD. Use of expectation disconfirmation theory to test patient satisfaction with asynchronous telemedicine for diabetic retinopathy detection. Int J Telemedicine Appl. (2018) 2018:7015272. doi: 10.1155/2018/7015272
Keywords: N-of-1 trial, single patient trial, mobile health, digital health, behavioral health, psychological well-being
Citation: Kravitz RL, Aguilera A, Chen EJ, Choi YK, Hekler E, Karr C, Kim KK, Phatak S, Sarkar S, Schueller SM, Sim I, Yang J and Schmid CH (2020) Feasibility, Acceptability, and Influence of mHealth-Supported N-of-1 Trials for Enhanced Cognitive and Emotional Well-Being in US Volunteers. Front. Public Health 8:260. doi: 10.3389/fpubh.2020.00260
Received: 24 December 2019; Accepted: 22 May 2020;
Published: 25 June 2020.
Edited by:
Giuseppe Fico, Polytechnic University of Madrid, SpainReviewed by:
Rebeca Isabel García-Betances, Polytechnic University of Madrid, SpainCopyright © 2020 Kravitz, Aguilera, Chen, Choi, Hekler, Karr, Kim, Phatak, Sarkar, Schueller, Sim, Yang and Schmid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard L. Kravitz, cmxrcmF2aXR6QHVjZGF2aXMuZWR1
†Beyond the first and last author, all authors are listed alphabetically
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.