- 1Department of Pharmacoeconomics, The Institute of Mother and Child, Warsaw, Poland
- 2Department of Pharmacoeconomics, Faculty of Pharmacy, Medical University of Warsaw, Warsaw, Poland
- 3Division of Pharmacoepidemiology and Clinical Pharmacology, Faculty of Science, Utrecht Institute for Pharmaceutical Sciences, Utrecht, Netherlands
- 4Unit of Health Technology Assessments, Turkish Ministry of Health, Turkish Medicines and Medical Devices Agency, Ankara, Turkey
- 5Ascent Global Market Solutions (Non-profit), Walnut Creek, CA, United States
- 6Center for Economics and Health Technology Assessment, Republican Center for Health Development, Ministry of Health, Nur-Sultan, Kazakhstan
- 7State Budgetary Institution Research Institute for Healthcare Organization and Medical Management of Moscow Healthcare Department, Moscow, Russia
- 8Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Warsaw, Poland
- 9Department of Pharmacoeconomics, Faculty of Pharmacy, University of Medicine and Pharmacy of Craiova, Craiova, Romania
- 10Independent Researcher, Warsaw, Poland
- 11Department of Management and Economy of Pharmacy, Medicine Technology and Pharmacoeconomics, Postgraduate Faculty, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
- 12Republican Center of Medical Genetics, Yerevan State Medical University, Yerevan, Armenia
- 13Department of Inborn Errors of Metabolism and Paediatrics, The Institute of Mother and Child, Warsaw, Poland
Background: Despite international initiatives on collaboration within the field of rare diseases, patient access to orphan medicinal products (OMPs) and healthcare services differ greatly between countries. This study aimed to create a comprehensive and in-depth overview of rare diseases policies and reimbursement of OMPs in a selection of 12 countries in the Western Eurasian region: Armenia, France, Germany, Kazakhstan, Latvia, The Netherlands, Poland, Romania, Russia, Turkey, Ukraine, and the United Kingdom.
Methods: A systematic literature review was performed and an analysis of publicly available legislative and rare disease health policy data was undertaken in five focus areas: rare disease definition, newborn screening, registries, national plans, access to/reimbursement of OMPs.
Results: Screening programs are broadly implemented but the number of screened diseases differs significantly (2–35 diseases), either between EU and non-EU countries, between EU member states and sometimes even within a single country. In most countries rare disease registries are operating with regional, national, European or worldwide coverage. The number of rare disease registries is growing, as a result of the National Plans (EU) and increased international scientific cooperation. France, Russia, and Poland have a centrally acting registry. National plans are present in all EU countries but implementation varies and is ongoing. The number of reimbursed OMPs in the selected countries ranges from nearly all available OMPs in the Netherlands, Germany, and France to zero in Armenia. Reimbursement rules differ considerably regionally and a trend is observed of reimbursement conditions getting stricter for expensive (orphan) drugs.
Discussion: Inequality in patient access to new OMPs still exists due to variations in national policies, healthcare budgets, health insurance, and reimbursement systems. The observed differences are challenging for rare disease patients, health authorities and manufacturers alike. Progress can be seen, however, and international cooperation and harmonization is slowly but steadily expanding in the rare disease arena.
Introduction
Between 6,000 and 8,000 rare diseases have been identified, most of genetic origin and with severe clinical manifestations. Due to insufficient knowledge on disease pathology, diagnosis is frequently delayed, often resulting in early and irreversible complications. Thirty percent of rare disease patients die before the age of five1. Pharmacotherapy, known as orphan drugs or Orphan Medicinal Products (OMPs), exists for <3% of rare diseases2 (1–3). Registration and reimbursement are the two main policy hurdles before a drug can reach a patient. Regulatory legislation for OMPs has been harmonized across the European Union (EU), with simultaneous regulatory approval for OMPs across 28 member states (4). However, differences remain in reimbursement and pricing systems in member states, based on factors such as healthcare budget (related to a country's GDP), type of healthcare and health insurance system, patient co-payment rules, reimbursement timelines and evidence requirements (i.e., type, level, and presentation). Consequently, patient access is often unpredictable and restricted while reimbursement strategies for manufacturers are fragmented and complex. The high prices of many orphan drugs, often combined with a limited amount of clinical evidence (mainly due to small patient populations), can lead to Incremental Cost-Effectiveness Ratios (ICER) that exceed “willingness to pay” levels (5). Budget restriction measures, especially around “expensive drugs” (which OMPs often are), are increasingly common. Reference pricing methods (i.e., HTA agencies comparing and referencing to drug prices in other countries or regions) can influence manufacturers to postpone or even avoid entering certain markets due to a possible cascading price-drop effect elsewhere (6). These are a few of the factors that can cause inequality in patient access to new medical technologies and treatments (7). A 2017 survey by EURORDIS confirmed that 24% of rare disease patients did not receive treatment because of no drug availability in their country (vs. 7% of the general population) and 15% due to inability to pay for treatment (vs. 6%) (8).
A recent step toward HTA harmonization between EU member states is the official proposal of the EU Health Technology Assessment (HTA) Regulation in 2018, which has been planned to be adopted in 2019. A pivotal component of this regulation is a centralized Joint Clinical Assessment (JCA) at the European level, which is aimed at establishing the (clinical) value of the treatment for HTA purposes (9). Such a central assessment would reduce HTA workload in the individual member states, promote the sharing of knowledge and leverage the expertise of rare disease experts and patient representatives in the EU. In essence, the JCA resembles the shared regulatory assessment done by the European Medicines Agency (EMA) in the Centralized Procedure (9). The JCA could improve the quality and speed of HTA for OMPs at the national level and promote further HTA harmonization. However, details on implementation, member state representation and how the joint clinical assessment will be legally binding (for national HTA purposes) is still under discussion and some concerns are already being voiced (10). The final HTA decision making, which depends on country specific factors such as the structure of the healthcare system, reimbursement factors and budgeting aspects, will likely remain at the national level.
Rare disease policies are a high focus area, given the medical need surrounding rare diseases and the relatively large impact these diseases and their treatment potentially have on healthcare budgets. The reimbursement status of orphan drugs in Eastern Europe has been described by several authors recently (11–15). There have been multiple publications describing OMP policies in Central and Eastern Europe in single countries (16–18) or covering a larger number of countries in Europe (5, 19–22). Pejcic et al. focused on HTA and pricing as well as rare disease policies in 14 Eastern European countries (23). In 2015 Gammie et al. presented a comprehensive review of legislations, regulations and policies in 35 countries describing in detail the national orphan drug policies, orphan drug marketing authorization processes (and accelerated procedures), incentives, marketing exclusivity, pricing, and reimbursement (2015) (24). Dharssi et al. evaluated key patient-needs across five dimensions: improving coordination of care, diagnostic resources, access to treatment, patient awareness and support, and promoting innovative research in 11 EU and non-EU countries (25).
However, there is still little comprehensive and in-depth information available in the English literature on orphan drug policies and HTA processes within the European Commonwealth of Independent States (CIS), such as in Russia, Armenia, and Kazakhstan in comparison to European Union countries. This field is rapidly evolving due to implementation of national plans for rare diseases in some European countries and HTA developments. Therefore, the aim of this article is to bridge the identified gaps by presenting an overview and comparison of current rare disease policies, HTA and reimbursement processes for orphan drugs in a broader range of Eurasian countries.
Materials and Methods
For this publication an analysis of rare disease policies was undertaken, focused on the following topics, including several “core areas” as defined by the EU (Council Recommendations of 2009) (26):
- Rare disease definition,
- Newborn screening (NBS) for rare diseases,
- National plans (NP) for rare diseases,
- Rare disease registries (central vs. disease-specific),
- Reimbursement and HTA approaches for orphan drugs, including access to orphan drugs (measured by the number of reimbursed OMPs) and availability of early access methods (e.g., compassionate use, named patient-programs, conditional reimbursement).
Other aspects mentioned in the 2009 EU Council Recommendation such as research on rare diseases empowerment of patient organizations, and sustainability were not researched as they are difficult to quantify and assess in an objective manner. Codification and inventorying of rare diseases were excluded as well in this paper, as these have little direct impact on treatment. In addition, the authors decided to include newborn screening, reimbursement (incl. early access programmes) and HTA processes, in order to present a more holistic overview of rare disease policies in each country.
The 12 countries included in this study were selected to be diverse from a geographical and socio-economical viewpoint and represent a wide range of rare disease policy development across the western Eurasian region: Armenia (AM), France (FR), Germany (DE), Kazakhstan (KZ), Latvia (LV), The Netherlands (NL), Poland (PL), Romania (RO), Russia (RU), Turkey (TR), Ukraine (UA), and the United Kingdom (UK).
A systematic literature review was performed to identify previous research and relevant publications, using the following keywords: rare disease, rare disorder, orphan drug, orphan medicinal product, health policy, reimbursement, HTA, health technology assessment, newborn screening, patient registry, national plan, legislation, access, Poland, Germany, Netherlands, Holland, Kazakhstan, Russia, Ukraine, Turkey, Armenia, France, UK, United Kingdom, England, Scotland, Northern Ireland, Wales, Romania, Latvia. Articles published from 2017 to 2019 were included. The review resulted in 681 publications that were screened by title/abstract, 610 publications were excluded due to insufficient relevance to the selected focus areas, 71 full-text articles were assessed for eligibility, of which 10 were included. All steps of the literature review (identification, screening, eligibility, inclusion, and data extraction) were performed by two independent researchers, according to PRISMA methodology (please refer to Figure 1).
Figure 1. Reproduced with permission from PRISMA 2009 flow diagram (27).
In order to gather further information in the scope of the article, an explorative internet search (gray literature review) was done of publicly listed policies, legislations, guidelines, governmental publications and other sources of relevant orphan drug HTA information. This was done by searching the websites of local Health Authorities, e.g., the Ministry of Health and HTA agency. The most up-to-date data the authors could find was included. Experts from all countries were interviewed to confirm the obtained information or in case public information was insufficient, unclear, contradictory or lacking. The authors' intention was to select a fair representation of different types of stakeholders involved in market access processes of orphan drugs. Public institution representatives, payers, scientists, clinicians, and commercial entity representatives from the countries were interviewed. Their number was dependent on the quality of information available from public sources and the willingness of stakeholders to provide additional data as well as a degree of involvement in the study. A list of questions was sent to the experts by email and followed up by phone interviews. Approval by an ethics committee was not required for this research.
Definition of Rare Disorders, Orphan Drugs and Epidemiology
The EU has officially defined rare diseases as being rare when they affect fewer than 1 in 2000 (i.e., a prevalence of 5 or less per 10,000) (28) and in most of the selected countries this definition is used [FR, DE, LV, NL, PL, RO, UK, and UA (29, 30)]. In Russia the maximum prevalence for a rare disease is defined as 1 in 10,000 (31). There is no data available on the maximum prevalence for a rare disease in Kazakhstan (32). Some countries use additional definitions in situations where a condition is not officially defined as rare, such as in the UK, where the National Health Service (NHS) classifies all conditions that require specialized medical care also as rare if they occur in <500 citizens yearly (29)3. Turkey defines a rare disease when they affect no more than 1 in 100,000, which is 50 times less frequent than the European Union definition (33, 34). There is no specific definition for “rare disease” in Armenian legislation, only “levels of disability” which define whether the patient will receive the necessary medicines for free or not4.
The Netherlands defines the classification “orphan drug” as either having an official EU orphan designation or if it targets a disease with a prevalence of <1 in 150,000 and shows a clinically proven therapeutic benefit and no other registered medicine exists5.
France introduced an extra definition of “rare cancer” if the cancer occurs in <6 in 100,000 per year or requires specialized treatment due to untypical tumor location or complex disease characteristics (29, 35). Effective from October 2018, Scotland has introduced a new definition for ultra-orphan drugs: “medicines that are used to treat a condition with a prevalence of 1 in 50,000 or less or around 100 people in Scotland,” which will mostly be used to facilitate early access programs and reimbursement processes6.
Newborn Screening
Newborn screening (NBS) is used to identify and effectively treat certain rare disorders at an early stage and to prevent irreversible damage. NBS is performed in all countries selected for this review. There is, however, a lack of uniformity between screening programs, mainly in the number of screened disorders, ranging from 2 to 35 (see Table 1). Poland currently screens for 28 rare diseases (36), The Netherlands 20 (37, 38), Germany 157,8 (39), France and Russia 5 and (N.B.: 35 in Moscow) (29)9, Ukraine 4 (41), Turkey 6 (40, 42)10. Armenia4, Kazakhstan11 (43), Latvia and Romania12 (44, 45), only screen for phenylketonuria and congenital hypothyroidism. England, Scotland, and Wales screen for nine diseases, whereas Northern Ireland (part of the UK as well) screens only for 5 (29)13,14. In several countries the number of screened diseases is being expanded or planned to be expanded, notably in Turkey (going from 6 to 10 screened diseases)11 and the Netherlands (from 20 to 32) (37), but without specific timelines.
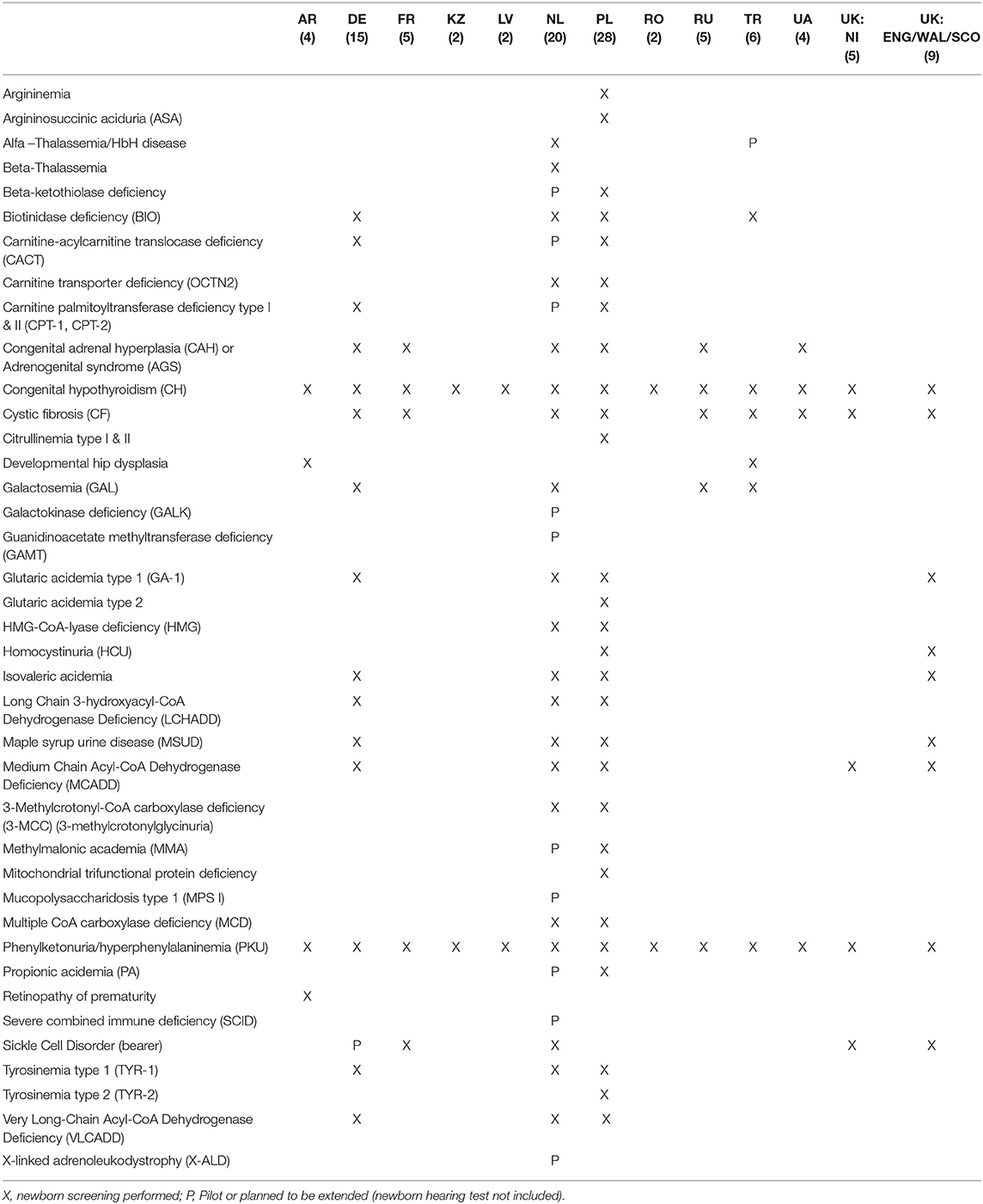
Table 1. New born screening of rare diseases per country (29)4, (36–38)7,8, (39)9, (40–42)10,11, (43)12, (44, 45)13,14.
National Plans for Rare Diseases
In 2009 the European Council issued the recommendation for EU member states to create and adopt a plan focused on rare disorders by the end of 2013, with the goal to have an overall Community strategy for “ensuring effective and efficient recognition, prevention, diagnosis, treatment, care, and research for rare diseases in Europe” (46). For this purpose, the European Project for Rare Diseases National Plans Development (EUROPLAN) was introduced to promote and help EU members with the construction and implementation of their national plans15,16.
NL, DE, UK, LV have created a national plan within the timelines defined by European Commission but in most of these EU countries the implementation is in progress (16, 29)17,18,19,20. In Poland and Romania, a National Plan for RDs was developed but has never been implemented21,22. The most recent version of Polish Plan for RDs for 2017–2019, was written under the auspices of the Polish MoH and was planned to be approved in the 3rd quarter of 201922,23. France was a forerunner in introducing a National Plan in 2004, with an assigned budget of €100 M for implementation over 2005–2008 (29). The 3rd French national plan has been created for 2018–2022. Rare disorder patients in France can also receive support from the so-called Cancer Plan (latest version 2014–2019, in case of rare oncological diseases, and the National Plan for Rare Handicaps (2014–2018), addressing rare physical disabilities (29, 47–49).
In Russia a special program exists (on the federal level) for financing 12 high-cost diseases: hemophilia, cystic fibrosis, pituitary dwarfism, Gaucher disease, lymphoid malignant neoplasms, hematopoietic and related tissues, multiple sclerosis, hemolytic-uremic syndrome, juvenile arthritis with systemic onset, mucopolysaccharidosis type I, II, and VI24 (50).
Both Kazakhstan and Turkey have national programmes for rare diseases, but they are undergoing implementation. In the non-EU countries in this review (KZ, TR) (40, 51–53) a national strategy targeting rare diseases was not adopted and, in some cases there is even a complete lack of legislation that addresses the needs of rare disease patients and orphan drug topics (e.g., AM)4. Table 2 describes the most important characteristics of the national plans and their status at the time of writing.

Table 2. Description of National Plan for Rare Diseases per country (16, 29)4, (54–64)25,26, (65)27, (66)28,29.
Disease Registries
A limited number of registries for rare disorders exist in most of the selected countries, even though it is a focus topic in many of the national plans. The first outcomes of implementing rare disease registries are already visible and resulted in scientific collaboration such as the Network dedicated to Rare Adult Cancer (RAC), through which knowledge on epidemiology, survival prognosis, prevalence, burden of rare cancers is shared. Registries are either public or private non-profit or for profit (54–56)25.
France implemented a central registry (fr. Banque Nationale de Données Maladies Rares, BNDMR) that collects data for all rare disorders, next to 12 other rare disorder registries. The central registry gathers epidemiological data in order to optimize clinical practice and healthcare policies. It also serves to facilitate patients to therapeutic programs and clinical trials. Rather uniquely, data on patients' family members is also collected. The epidemiological data is aggregated within the centers for rare diseases (Centres des maladies rares) CEMARA program (replaced by the BaMaRa application in 2017) which has identified more than 380,000 patients and 4,200 rare disorders (29, 57–59). Since 2017 109 CRMRs (multi-site reference centers) were created, 387 reference centers and 1,757 competence centers identified, as well as 83 resource and competence centers (CRCs) (67). Up to May 2019 there were 143 RD registries in France (60). Poland has a Central Registry for Inherited Disorders which is obligatory to report birth defects to since 2014 as well as 10 disease specific registries26.
Germany has acted on the NP recommendation to create disease registries such as the Open Source Registry System for Rare Diseases (Open-Source-Registersystem für Seltene Erkrankungen) (29, 61)25. Currently in Germany there are 149 RD registries (13 regional, 94 national, 18 European, and 24 global)25 and a central portal (61, 62).
The UK has 74 functioning registries under control (incl. 12 global, 13 European), also by public or private institutions (29)25.
Latvia has one registry for multiple diseases, called the “Registry for Certain Diseases,” which include rare cancers, hereditary disorders, managed by the Centre for Disease Prevention and Control. There are plans to implement a central registry for rare disorders within the national plan (16, 29)25.
Until May 2019 32 RD registries in The Netherlands existed, however, the national plan led to appointing around 350 reference centers that are able to comply with the EU standards, including 5 of the 24 new European Reference Networks (ERN) (65)27. The large number of centers will be working together in clusters, to prevent fragmentation (66).
Romania has two disease registries (biliary atresia and cystic fibrosis), both contributing to European registries28.
Turkey has five working registries, one for oral ulcers in Behcet disease, cystic fibrosis (contributing to EUROCARE cystic fibrosis registry), Duchenne, Becker, and spinal muscular dystrophy (contributing to TREAT-NMD), pediatric atypical hemolytic uremic syndrome, severe chronic neutropenia (contributing the SCN international registry) (40, 63). A registry for rare pediatric metabolic disorders is financed by Hacettepe University Hospital and the Metabolic Disease Foundation (METVAK). Turkey participates in European registries E-IMD (40, 64).
Russia is the only non-EU country in this review having a central rare disorder registry (31). There are no official rare disease registries in Kazakhstan, but work is underway to establish a national rare disease registry to help identify common genetic mutations within the Kazakh population, which is intended to collaborate internationally (51)29. Armenia has no registries4 and is also the only country that does not have patient organizations gathering data. Disease registries are under development in Ukraine, which currently has one, for spinal muscular atrophy24.
Rare Disease Policies and Access to Orphan Drugs
Although the European Commission has granted 2121 “Orphan Designations” from 2000 until 2019, “only” 164 orphan drug marketing applications were approved via EMA's centralized procedure in this period (1–3).
In contrast to the regulatory process, which is performed centrally and leads to a simultaneous drug approval for all 28 EU members, health technology assessment, pricing, and reimbursement are still executed on the national level. This can lead to differences in patient access, as illustrated below. Data from 2015 shows that the Netherlands reimbursed all OMPs registered in the EU except 3 (Ceplene®, Mepact®, and Bronchitol®) (68). In Germany the total number of reimbursed OMPs was 13330. Since the 2011 introduction of legislation aiming at controlling prices of patented pharmaceuticals and to curb spending (Act to Reorganize the Pharmaceuticals' Market in the Statutory Health Insurance System, AMNOG) until March 1st 2017, 51 orphan drug reimbursement procedures have been finalized by Germany's Federal Joint Committee (Gemeinsame Bundesausschuss, G-BA)31. OMPs are most widely accessible in Germany and France (69).
France reimburses 116 orphan drugs, England 68, Scotland 55, and Wales 47 (65). England, <50% of centrally authorized OMPs are routinely funded by the NHS, with one-third of these recommended by NICE (69).
Latvia reimburses 25 orphan drugs, 21 via three reimbursement pathways (the reimbursement list, individual reimbursement and the CCUH program “Medicinal treatment for children with rare diseases”) and 4 through multiple reimbursement mechanisms (15).
Poland reimburses 48, the vast majority of which within so-called “Drug Programs” (DPs), introduced by the MoH in 2012 for expensive medical technologies (replacing previous “therapeutical programs”) (11, 70–72). DPs are mainly designed to control consumption of the most expensive drugs22.
Romania has 70 reimbursed OMPs21,32. Russia has been reimbursing 27 high-cost drugs for orphan diseases on the federal level and 43 in the Moscow region (73–75)33, which is an example of regional differences in patient access. Ukraine reimburses 23 active substances for 7 diseases approved for state procurement based on the national drug program inclusion criteria (76, 77), 12 diseases for children and adults, covering 65 INNSs.
In Turkey currently 43 orphan drugs are reimbursed but 22 of them are not currently marketed in Turkey, for this reason, Social Security Institutions use direct importation for those products (78, 79).
Kazakhstan has 42 reimbursed OMPs at the country level and 2 reimbursed rare disease funds. However, according to the Kazakh definition of orphan drugs/rare diseases there are 150 orphan drugs for 50 disease classes (80, 81).
In Armenia there is no reimbursement as seen in the other countries: many medicines are given via donations4. Medicines are distributed free of charge from the MoH warehouse to polyclinics and hospitals nationwide. All medicines are obtained through tenders posted by the Armenian MoH. When a rare disease does not cause physical or mental disability, all costs for required medicines or medical nutrition are borne by the patient4.
Early Access (Compassionate Use, Named Patient Programme, Conditional Reimbursement)
According to Balasubramanian et al., 20 out of 28 EU member states had an established compassionate use programme (CUP) (82). A CUP exists in all EU countries selected for this review, except Poland (work on implementation of a national CUP is ongoing)23 (82).
In the EU it is also possible to request a CUP centrally via the EMA Committee for Medicinal Products for Human Use (CHMP) when adequate clinical evidence exists on safety and efficacy, but most CUPs are executed on the country level via the local regulatory authority. Only 5 CUPs have been granted through the CHMP so far34.
Early access programs are not offered in Kazakhstan, Armenia, Russia, and Ukraine (51)4,24,35.
France makes extensive use of CUPs for rare diseases, with 70% of the currently reimbursed orphan drugs having had early access before the marketing authorization (59). France is also unique in the fact that it has a legal framework for “early access” for already registered drugs for which a new (medical need) indication is still under assessment, called RTU (Recommendation for Temporary Use) (83, 84). Sixteen products have received an RTU in France so far (83). RTU allows for a more flexible access approach than many other countries, such as the Netherlands, that only allows non-registered drugs for a CUP, regardless of whether the (orphan) indication is approved or not36.
In Turkey exist three well-established processes to get access to unapproved drugs, e.g., approved off label-use of registered drugs (e.g., different indication/dosage, or non-approved patient subgroups), Named Patient Imports and CUPs (33, 34). A CUP is acceptable for products that have entered a phase-III clinical program and in case of serious or life-threatening conditions, but only if patients cannot enroll in a clinical trial in Turkey. The Medicines and Medical Devices Agency supervises these programs (33, 34). Scotland has a two-tier program for access to non-routine drugs (i.e., drugs normally not available in the Scottish healthcare system) called PACS, with tier 1 reserved for ultra-orphan drugs and tier 2 for other non-routine drugs (not approved by the Scottish Medicine Consortium). Cost-effectiveness is explicitly excluded from any argumentation for access37.
HTA and Reimbursement Processes for Orphan Drugs
Rare disease populations are small and often show large disease heterogeneity, which leads to difficulties in generating well-powered and controlled randomized clinical trials and useful outcomes. This makes the generation of (high quality) evidence on clinical efficacy and cost-effectiveness troublesome. In turn, HTA assessment processes are usually not tailored to deal with these rare diseases and orphan drugs characteristics. Many countries still reimburse OMP's despite a lower quality of evidence and accept higher prices, often because of societal/compassion-related arguments and the limited total budget impact of the rare disease treatment. Some countries have reduced requirements for evidence and other waivers for rare disease treatments. For example, in France a cost-effectiveness analysis is not required. The Haute Autorité de Santé (HAS) assesses therapeutic benefit, calculated as Service Medical Rendu (SMR), which takes into account: clinical effectiveness, safety of alternatives, clinical relevance in overall treatment strategy, disease severity, population size, and indication (for chronic and preventable diseases) (29).
Similarly, in Turkey orphan drugs are exempt from submitting pharmacoeconomic analyses, which allows OMPs to enter the market faster (if budget impact is within limits) (33, 34). In Romania OMPs receive additional value points (55) during the HTA process, which increases chances for reimbursement (29)12.
Since 2012 a conditional reimbursement has been possible in the Netherlands, in cases of discussion/doubt over a therapeutic benefit, cost-effectiveness, or the predicted budget impact of a medical intervention (only available for outpatient drugs)38. These conditional approvals were intended to ensure patients could get early access to innovative medicines while maintaining budget control. This program came with the requirement to provide additional scientific data within 4 or 7 years (in exceptional cases), for which a subsidy could be requested with a maximum of 400,000 €. However, the number of products that applied for conditional reimbursement up to 2017 turned out to be low. Therefore, the conditional reimbursement program has been replaced by a more general subsidy program, focused at supporting small and medium manufacturers38.
Romania also has a conditional reimbursement program, which aims to allow patient access to new drugs quickly, while still keeping a focus on evidence-based medicine and budget control39.
In the UK, NICE performs an HTA assessment using incremental cost-effectiveness ratios which are usually implemented by the regions, although a re-assessment or a purely regional HTA can also be done in Scotland, Wales, and Northern-Ireland. The NICE HTA process is based on a threshold level per ICER, with increasing evidence requirements if certain ICER levels are exceeded. Orphan and ultra-orphan drugs can get higher limits40,41,42,43,44,45,46.
The Act to Reorganize the Pharmaceuticals' Market in the Statutory Health Insurance System (AMNOG), introduced in Germany in 2011, changed reimbursement of new innovative drugs considerably31. Manufacturers are allowed to set prices freely during the first year after marketing authorization, with a mandatory 7% discount to statutory health insurances. An “early benefit assessment” is done after 12 months, after which reimbursement will be recalculated, taking into account the perceived additional benefit of the medicine47 (85). Lower evidence thresholds for OMPs were applied within the process and an automatic “additional benefit” for OMPs was assumed, with no necessary comparison against alternative therapies. This streamlined and simplified the reimbursement process for OMPs considerably. The “Legislation for more safety in the supply of pharmaceuticals” (GSAV) introduced in 2019 changed several parameters for OMP reimbursement in Germany, by removing several benefits for OMPs and increasing the likelihood of price reductions for OMPs (67, 86). Under GSAV, OMP manufacturers are more likely to have to invest in data collection activities (e.g., patient registries) and perform comparative analyses. The automatic added benefit clause is removed for OMPs with an annual revenue >€50 M. In this case, a comparative analysis will have to be provided. GSAV now includes both hospital as outpatient costs in the revenue calculations, increasing the likelihood of exceeding the threshold. G-BA will be authorized to perform periodic re-evaluation of the drug's benefits (and conduct price negotiations if deemed necessary). The actual impact of GSAV on orphan drugs, i.e., availability/patient access, pricing, time to market, and disease/drug understanding, remains to be seen. GSAV legislation might lead to more structured, approach toward Real World Evidence (RWE) creation in the rare disease/orphan drug field. It is possible that Germany will push these topics onto the EU agenda during its co-presidency in 2020/2021 (67, 86).
Some countries are looking at novel and alternative methods of assessing orphan drugs, such as Poland who is considering to use MCDA (Multi-criteria decision analysis) in its HTA policies48.
A detailed overview of HTA and reimbursement processes is presented in Table 3.

Table 3. Comparison of reimbursement systems of orphan drugs and rare diseases policies (15, 16, 29, 70–72)4,24, (68)30,31, (11, 69)32, (73–75)33, (76–84)34,35,36,37,38,39,40,41,42,42,43,44,45,46,47, (67, 85, 86)1,48, (87)2,3, (88, 89)4,5, (90, 91)6,7,8,9, (92, 93).
Discussion
Limitations of the Study
In order to get a complete overview of the Eurasian region, many more countries would have to be included, however, this went beyond the scope of this article and would overly enlarge it. This overview presents the most recent information that was possible to retrieve at the time of writing, but policies and regulations are continuously changing. Sometimes new information is difficult to find and only available in local languages. The politicization of the (orphan) drug price debate results in shifting political viewpoints highly dynamic healthcare policies. Not all country data is comparable, i.e., mismatches exist in definitions, different aspects of rare disease policies that are covered and the level of detail, on top of structural differences in healthcare systems. To keep this information relevant and up-to-date, research should be done periodically to expand and include the latest information. The German GSAV shows that new and extensive policies can be introduced quickly, especially in an era of rising cost-awareness. Sharing scientific progress and relevant policy developments in a collaborative manner is very relevant in the orphan drug arena, where knowledge and experience are often scarce. A publicly accessible “policy repository” could be a useful tool for researchers and policy makers to share best practices and combine efforts, but which would require continuous input and resources.
This study shows that large differences exist between selected countries with regard to orphan drug policies, solutions, available healthcare budgets, and the level of patient access. This applies to EU vs. non-EU countries, EU member states, and even within a single country. Despite these variations that make it difficult to create a comprehensive overview of policies or generate a clear-cut conclusion, the authors have attempted to capture a representative picture.
Newborn Screening
Good examples of intra-country differences are newborn screening and orphan drug reimbursement between the regions of the UK (i.e., Northern Ireland vs. Scotland, England, and Wales) and in Moscow vs. the rest of Russia. Newborn children are screened for the highest number of rare disorders in Poland (28), followed by The Netherlands (20). On the lower end of the scale, Kazakhstan, Latvia, Romania screen for only two diseases. Russia has the region with the broadest newborn screening in this review (35 RD's in Moscow), although large parts of the country have a much smaller program9. Aggregation of data concerning newborn screening is not always straightforward, since many rare metabolic disorders have different names or subtypes which can be considered either as one disease or as separate rare conditions, depending on publications and local guidelines. Disease carriership is sometimes counted as a separate condition (e.g., sickle cell disease and sickle cell carriership in the Netherlands). Overall though, the national plans have led to expansion of the amount of screened diseases. Implementing a new screened disease requires testing and validation of new technology, so the implementation status is sometimes not clear to the public.
Despite the wide international consensus on the efficiency of NBS for phenylketonuria in terms of costs and effectiveness, this consensus is challenged as new disorders are proposed to be included in a NBS program (93). NBS programs might be relatively inexpensive, even when the confirmatory diagnostic tests for both the true and false positives and the follow-up and treatment costs of affected children are included. However, the high heterogeneity of the disorders potentially detected by screening, and the lack of robust and long-term scientific evidence on the effectiveness of the treatments and the natural history of the disorders, pose a number of methodological difficulties that limit the applicability of standard pharmacoeconomic evaluation methods to prove its cost-effectiveness.
Disease Registries, National Plans for Rare Diseases
The national plans have stimulated the creation of registries as scientific centers, but implementation varies per region. Government publications have been reviewed to assess the availability of patient/disease registries, but whether the mentioned registries are operational, being implemented or merely announced is sometimes not transparent. Reorganization, grouping, and renaming of registries is common. Other institutions, such as universities or patient organizations are often involved in gathering this data but they were not included in this review. The ongoing implementation of national plans in the EU since 2013 has reinforced the international recognition of rare disorders in governmental programs substantially, leading to alteration and implementation of various policies. The newly approved European Reference Networks are a good example. The results of this increased data gathering will hopefully lead to better understanding, diagnosis and treatment of rare diseases, but this will take time.
Access to Treatment
The main effect of the fragmentation of reimbursement policies is unequal access to treatment. The number of reimbursed OMPs in the selected countries ranges from 100+ OMPs in The Netherlands, Germany, France to zero in Armenia. The EU countries are leading in access to OMPs but positive developments for patients are also seen outside the EU, e.g., in Russia and Kazakhstan. Like France, Turkey also has implemented regulatory flexibility, by allowing the use and importation of drugs for non-registered orphan indications (i.e., managed off-label use).
In some countries legislation is completely lacking, leaving patients without many options to get access to any (expensive) medication, such as in Armenia. Early access programs can temporarily alleviate an urgent medical need for OMP with a low burden for society and patients, and since these are relatively easy to implement they should be introduced in all countries.
HTA and Reimbursement
No specifically tailored HTA approaches were identified for orphan drugs, although waivers and reduced data requirements are often present in some form or another. Many countries use standard HTA processes but do reimburse OMP's despite lacking evidence.
Rare diseases commonly place a large burden on family and caregivers, the impact of which is usually not taken into consideration in standard cost-effectiveness analyses (94–96). In light of the lack of appropriate HTA tools that can incorporate benefits and costs specific to rare disease treatments beyond the standard cost per QALY, e.g., socio-economic aspects, Multi-Criteria Decision Analysis (MCDA) is an approach that could be considered. MCDA can support decision-making processes by capturing and weighting a range of factors of a certain intervention, the result of which is one composite outcome score. This outcome can be used for comparison between technologies (97, 98). MCDA has been implemented in legislation in Lombardia (for diagnostics, medical devices, interventional procedures, and medicinal products including OMPs) and also in Hungary for new hospital medical technologies (99, 100). Poland is currently considering the use of MCDA for this purpose.
Researchers in the rare disease area are also looking into the use of MCDA, which has resulted in a list of scientific publications and MCDA model designs, but full consensus on MCDA is still lacking and further research is needed to support implementation in (rare disease) HTA (94, 101–116).
Reimbursement rules are harder to unify than regulatory legislation, due to regional economic and political differences, also in the EU. However, signs of international cooperation are visible, as the European Parliament Committee on Environment, Public Health and Food Safety (ENVI) is investigating shared HTA and pricing projects in the EU49. The European Mechanism of Coordinated Access to Orphan Medicinal Products (MoCA) project is a step toward international harmonization and improvement of patient access to OMPs. This platform aims to facilitate an early dialogue on pricing and reimbursement already during the development phase of OMPs between pharmaceutical companies and competent authorities50 (117). The Transparent Value Framework (TVF) which is an MCDA-like method developed by Hughes-Wilson, was also tested within this project in order to develop a coordinated mechanism between the 12 participating Member States and orphan drug developers to evaluate the value of OMPs51.
The EU HTA Regulation that was announced builds on these earlier initiatives, centered around the concept of a centrally performed Joint Clinical Assessment (JCA) that can be used by national HTA agencies (8). Economic factors will probably still be evaluated nationally, but a central “clinical value” assessment would avoid duplication efforts, reduce workload and make the HTA process more transparent and predictable for all stakeholders. Given the pressure on costs, especially in the area of expensive medicines, sharing, and implementation of new cost-reduction policies is to be expected.
New Scientific Methodology
Several new scientific and methodological approaches are being developed to improve evidence generation and analysis for small population groups, including new trial designs and clinical endpoints such as was done in the EU FP7 framework recently and its subprograms IDEAL (Integrated DEsign and AnaLysis of small population group trials), InSPiRe (Innovative methodology for Small Populations Research) and ASTERIX (Advances in Small Trials dEsign for Regulatory Innovation and eXcellence)52. Goal Attainment Scaling (GAS) came out as an example of a “rediscovered” endpoint that can capture individual and heterogeneous symptoms via personalized outcome parameters (118, 119). N = 1 trial methodology (single-subject design) allows to perform a double-blind randomized placebo-controlled trial with one single patient, via randomized treatment cycles of both drug and control. Although limitations exist (e.g., suitable for chronic conditions only), the method seems appropriate for ultra-rare diseases (120). Drug manufacturers can benefit from all these developments, e.g., with improved clinical methodology for rare diseases and clear, predictable and transparent orphan drug legislation and HTA processes that are adapted for orphan drugs. In turn, this can support regulators and payers when assessing the value and benefits of OMPs. It is not clear, however, if and how fast these new developments will result in actual benefits for rare disease patients, i.e., improved access to a wider range of drugs. International medical and scientific collaboration for rare diseases already exists for a while (e.g., Orphanet, Eurordis), but cooperation on HTA issues and patient access is still lagging behind. Unified international approaches to tackle common issues surrounding orphan drugs are being developed slowly.
This article has looked at a broad range of initiatives over a wider region, and it can be concluded that no single country in this review can be marked as having the “most optimal” rare disease solutions. A broad national newborn screening program can be accompanied by a relatively small reimbursement program in the same country. Learnings should be taken from the respective national experiences and by sharing of policy related information, which was also the aim of this publication. In order to create additional momentum, initiatives that can effectively support orphan drug access should be prioritized and placed on the public agenda, preferably supported by strong political entities. The rarity and complexity of the rare disease/orphan drug arena makes collaboration and harmonization essential. Only in this way the 350 million people suffering from rare disorders around the world can hope to expect fair and equal access to treatments in the future53. Continuous research and sharing of information is highly recommended to identify and promote best practices in the rare disease policy field.
Author Contributions
MC coordinated the group of researchers in gathering and unifying information and provided information about Poland. AB-K performed data analysis, data gathering, wrote the publication, and provided information about Poland, Latvia, France, United Kingdom. KA provided information about Turkey. MD editorial changes and provided information about Armenia. KG provided information about Kazakhstan. MH-V provided information about Russia. AT-S provided information about Romania. CK proofread and provided information about The Netherlands and Germany. OP and OZ provided information about Ukraine. NK provided information about Armenia. JS-C provided information about Poland.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^https://www.eurordis.org/sites/default/files/publications/Fact_Sheet_RD.pdf (accessed September 18, 2019).
2. ^http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/12/WC500240710.pdf (accessed September 18, 2019).
3. ^https://www.england.nhs.uk/commissioning/spec-services/highly-spec-services/ (accessed September 18, 2019).
4. ^Interview with Armenian key opinion leader (accessed September 5, 2019).
5. ^https://www.nza.nl/regelgeving/beleidsregels/BR_CU_2018__Weesgeneesmiddelen (accessed September 18, 2019).
6. ^https://news.gov.scot/news/treatments-for-rare-conditions (accessed September 18, 2019).
7. ^https://www.g-ba.de/informationen/richtlinien/15/ (accessed September 18, 2019).
8. ^https://muko.info/ueber-mukoviszidose/neugeborenen-screening.html (accessed September 18, 2019).
9. ^https://mosgorzdrav.ru/ru-RU/news/default/card-print/1802.html (accessed September 18, 2019).
10. ^https://dosyaism.saglik.gov.tr/Eklenti/11173,259822214447pdf.pdf?0 (accessed September 18, 2019).
11. ^https://newjournal.ssmu.kz/publication/249/realizatsiya-skriningovykh-programm-vkazakhstane-na-sovremennom-etape/ (accessed September 18, 2019).
12. ^Order of Romanian MoH no. 387/2015 regarding the change and completion of the Order of the MoH no. 861/2014 for approving the criteria and methodology for health technology assessment, Ordinul nr. 387/2015 privind modificarea şi completarea Ordinului ministrului sănătăţii nr. 861/2014 pentru aprobarea criteriilor şi metodologiei de evaluare a tehnologiilor medicale.
13. ^http://www.nhs.uk/Conditions/pregnancy-and-baby/Pages/newborn-blood-spot-test.aspx (accessed September 18, 2019).
14. ^http://www.gov.scot/Topics/Health/Services/Screening/Newborn (accessed September 18, 2019).
15. ^https://ec.europa.eu/health/rare_diseases/national_plans/detailed_en (accessed September 18, 2019).
16. ^http://www.europlanproject.eu/NationalPlans?idMap=1 (accessed September 18, 2019).
17. ^Dutch National Plan Rare Diseases. http://www.nfu.nl/img/pdf/nationaal-plan-zeldzame-ziekten.pdf (accessed September 18, 2019).
18. ^https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/N/NAMSE/National_Plan_of_Action.pdf (accessed September 18, 2019).
19. ^https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/684461/Rare_Disease_Policy_Board_-_Second_Progress_Report_2016-2018.pdf (accessed September 18, 2019).
20. ^https://www.england.nhs.uk/wp-content/uploads/2018/01/implementation-plan-uk-strategy-for-rare-diseases.pdf (accessed September 18, 2019).
21. ^Interview with Romanian key opinion leader (accessed August 11, 2019).
22. ^Interview with Polish and Romanian key opinion leader (accessed August 13, 2019).
23. ^https://bip.kprm.gov.pl/kpr/bip-rady-ministrow/prace-legislacyjne-rm-i/prace-legislacyjne-rady/wykaz-prac-legislacyjny/r32597703041012,Narodowy-Plan-dla-Chorob-Rzadkich.html (accessed September 18, 2019).
24. ^Interview with Russian key opinion leader (accessed September 1, 2019).
25. ^https://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf (accessed September 18, 2019).
26. ^http://www.rejestrwad.pl/o-rejestrze/historia-prwwr (accessed September 18, 2019).
27. ^European Reference Networks. https://ec.europa.eu/health/ern/networks_en (accessed September 18, 2019).
28. ^http://www.anm.ro/_/ORDINE/ORDIN%20%20%20Nr%20387_2015_modif%20si%20complet%20OMS%20861_2014.pdf (accessed September 18, 2019).
29. ^https://www.zakon.kz/4777791-nacionalnyjj-reestr-redkikh.html (accessed September 18, 2019).
30. ^https://www.slideshare.net/OHENews/access-to-orphan-drugs-in-the-uk-and-other-european-countries (accessed September 18, 2019).
31. ^http://skc-beratung.de/wp-content/uploads/2017/03/White_Paper_SKC.pdf (accessed September 18, 2019).
32. ^https://www.cnas.ro/page/listamedicamentelor-2019.html (accessed September 18, 2019).
33. ^http://www.iokpb1.ru/perechen-7-nozologii-2019.pdf (accessed September 18, 2019).
34. ^http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000293.jsp&mid=WC0b01ac05809f843c (accessed September 18, 2019).
35. ^Interview with Ukrainian key opinion leader (accessed September 5, 2019).
36. ^https://www.cbg-meb.nl/mensen/voor-handelsvergunninghouders/inhoud/voor-aanvraag-handelsvergunning/compassionate-use-programma (accessed September 18, 2019).
37. ^https://www.gov.scot/news/reforming-access-to-new-medicines/ (accessed September 18, 2019).
38. ^Letter of the Healthcare Minister on restructuring of the conditional reimbursement ruling https://www.rijksoverheid.nl/documenten/kamerstukken/2017/02/21/kamerbrief-over-herinrichting-van-de-regeling-voor-voorwaardelijke-pakkettoelating (accessed September 18, 2019).
39. ^http://www.cnas.ro/casbr/page/contracte-cost-volum-cost-volum-rezultat.html (accessed September 18, 2019).
40. ^https://www.england.nhs.uk/wp-content/uploads/2013/04/cdf-sop.pdf (accessed September 18, 2019).
41. ^http://gov.wales/newsroom/health-and-social-services/2017/170110fund/?lang=en (accessed September 18, 2019)
42. ^https://www.scottishmedicines.org.uk/About_SMC/Policy_statements/A_Guide_to_Quality_Adjusted_Life_Years (accessed September 18, 2019).
43. ^http://www.bbc.com/news/uk-scotland-scotland-politics-32761132 (accessed September 18, 2019).
44. ^https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-highly-specialised-technologies-guidance/HST-interim-methods-process-guide-may-17.pdf (accessed September 18, 2019).
45. ^https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making (accessed September 18, 2019).
46. ^The interview with British key opinion leader (accessed July 14, 2019).
47. ^The interview with German key opinion leader (July 5, 2019).
48. ^https://www.gov.pl/web/zdrowie/rada-ministrow-przyjela-dokument-polityka-lekowa-panstwa-20182022 (accessed September 18, 2019).
49. ^http://www.europarl.europa.eu/RegData/etudes/STUD/2015/542219/IPOL_STU(2015)542219_EN.pdf (accessed September 30, 2019).
50. ^https://www.eurordis.org/content/moca (accessed September 30, 2019).
51. ^Hughes-Wilson W. MoCA Concept and Pilot Project, Feedback From the Process Around the First Pilot Project. Berlin: ECRD. Available online at: http://download2.eurordis.org.s3.amazonaws.com/moca/presentations/PRES-2014-05%20MoCA%20Concept%20and%20Pilot%20Project%20(Hughes-Wilson).pdf (accessed September 30, 2019).
52. ^https://ec.europa.eu/research/fp7/index_en.cfm (accessed September 30, 2019).
53. ^https://globalgenes.org/rare-diseases-facts-statistics/ (accessed September 30, 2019).
References
1. European Medicines Agency: Annual Report on the Use of the Special Contribution for Orphan Medicinal Products. (2018). Available online at: https://www.ema.europa.eu/en/documents/report/annual-report-use-special-contribution-orphan-medicinal-products-2018_en.pdf (accessed September 18, 2019).
2. Orpha.net. Orphanet Report Series, Lists of Medicinal Products for Rare Diseases in Europe. (2018). Available online at: http://www.orpha.net/orphacom/cahiers/docs/GB/list_of_orphan_drugs_in_europe.pdf (accessed September 18, 2019).
3. European Medicines Agency. Orphan Medicines Figures 2000-2018. (2018). Available online at: https://www.ema.europa.eu/en/documents/other/orphan-medicines-figures-2000-2018_en.pdf (accessed September 18, 2019).
4. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 Laying Down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary use and Establishing a European Medicines Agency. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0726 (accessed September 18, 2019).
5. Medic G, Korchagina D, Young KE, Mondher Toumi M, Postma MJ, Wille M, et al. Do payers value rarity? An analysis of the relationship between disease rarity and orphan drug prices in Europe. J Mark Access Health Policy. (2017) 5:1299665. doi: 10.1080/20016689.2017.1299665
6. Dylst P, Vulto A, Simoens S. The impact of reference-pricing systems in Europe: a literature review and case studies. Expert Rev Pharmacoecon Outcomes Res. (2011) 11:729–37. doi: 10.1586/erp.11.70
7. Rémuzat C, Urbinati D, Mzoughi O, El Hammi E, Belgaied W, Mondher Toumi M. Overview of external reference pricing systems in Europe. J Mark Access Health Policy. (2015) 3: 27675. doi: 10.3402/jmahp.v3.27675
8. EURORDIS. Access to Treatment: Unequal Care for European Rare Disease Patients. A Rare Barometer Survey. (2017). Available online at: https://www.eurordis.org/sites/default/files/2017_02_17_Access%20to%20treatment_Analysis_Final.pdf (accessed September 18, 2019).
9. European Commission. Proposal for a Regulation of the European Parliament and of the Council on health technology assessment and amending Directive 2011/24/EU. (2018) Available online at: https://ec.europa.eu/health/sites/health/files/technology_assessment/docs/ev_20180209_co01_en.pdf (accessed September 18, 2019).
10. Vella Bonanno PV, Bucsics A, Simoens S, Martin AP, Oortwijn W, Gulbinovič J, et al. Proposal for a Regulation on Health Technology Assessment in Europe – opinions of policy makers, payers and academics from the field of HTA. Expert Rev Pharmacoecon Outcomes Res. 19:251–61. doi: 10.1080/14737167.2019.1575730
11. Malinowski KP, Kawalec P, Trabka W, Czech M, Petrova G, Manova M, et al., Reimbursement legislations and decision making for orphan drugs in central and Eastern European Countries. Front. Pharmacol. (2019) 10:487. doi: 10.3389/fphar.2019.00487
12. Malinowski KP, Kawalec P, Trabka W, Sowada C, Pilc A. Reimbursement of orphan drugs in europe in relation to the type of authorization by the European medicines agency and the decision making based on health technology assessment. Front. Pharmacol. (2018) 9:1263. doi: 10.3389/fphar.2018.01263
13. Kamusheva M, Manova M, Savova AT, Petrova GI, Mitov K, Harsányi A, et al. Comparative analysis of legislative requirements about patients' access to biotechnological drugs for rare diseases in Central and Eastern European Countries. Front. Pharmacol. (2018) 9:795. doi: 10.3389/fphar.2018.00795
14. Detiček A, Locatelli I, Kos M. Patient access to medicines for rare diseases in European Countries. Value Health. (2018) 21:553–60. doi: 10.1016/j.jval.2018.01.007
15. Logviss K, Krievins D, Purvina S: Impact of orphan drugs on Latvian budget. Orphanet J Rare Dis. (2016) 11:59. doi: 10.1186/s13023-016-0434-y
16. Logviss K, Krievins D, Purvina S. Rare diseases and orphan drugs: Latvian story. Orphanet J Rare Dis. (2014) 9:147. doi: 10.1186/s13023-014-0147-z
17. Iskrov G, Miteva-Katrandzhieva T, Stefanov R. Challenges to orphan drugs access in Eastern Europe: the case of Bulgaria. Health Policy. (2012) 108:10–8. doi: 10.1016/j.healthpol.2012.08.013
18. Tordrup D, Tzouma V, Kanavos P. Orphan drug considerations in Health Technology Assessment in eight European countries. Rare Dis Orphan Drugs. (2014) 1:86–97. Available online at: http://eprints.lse.ac.uk/59402/1/Tordrup_Tzouma_Kanavos_Orphan-drug-considerations-in-HTA_2014.pdf (accessed September 18, 2019).
19. Zelei T, Molnár MJ, Szegedi M, Kaló Z. Systematic review on the evaluation criteria of orphan medicines in Central and Eastern European countries. Orphanet J Rare Dis. (2016) 11:72. doi: 10.1186/s13023-016-0455-6
20. Young KE, Soussi I, Hemels M, Toumi M. A comparative study of orphan drug prices in Europe. J Mark Access Health Policy. (2017) 5:1297886. doi: 10.1080/20016689.2017.1297886
21. Young KE, Soussi I, Toumi M. The perverse impact of external reference pricing (ERP): a comparison of orphan drugs affordability in 12 European countries. A call for policy change. J Mark Access Health Policy. (2017) 5:136981. doi: 10.1080/20016689.2017.1369817
22. Czech M, Baran-Kooiker A, Holownia M, Kooiker C, Sykut-Cegielska J. Bridging East with West of Europe – a comparison of orphan drug policies in Poland, Russia and the Netherlands. Acta Poloniae Pharmaceut Drug Res. (2018) 75:1409–22. doi: 10.32383/appdr/90995
23. Pejcic AV, Iskrov G, Raycheva R, Stefanov R, Jakovljevic M. Transposition and implementation of EU rare disease policy in Eastern Europe. Expert Rev Pharmacoecon Outcomes Res. (2017) 17:557–66. doi: 10.1080/14737167.2017.1388741
24. Gammie T, Lu CY, Babar ZU-D. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS ONE. (2015) 10:e0140002. doi: 10.1371/journal.pone.0140002
25. Dharssi S, Wong-Rieger D, Harold M, Terry S. Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet J Rare Dis. (2017) 12:63. doi: 10.1186/s13023-017-0618-0
26. Official Journal of the European Union. COUNCIL RECOMMENDATION of 8 June 2009 on an Action in the Field of Rare Diseases (2009/C 151/02). Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF (accessed September 18, 2019).
27. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
28. Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000R0141 (accessed September 18, 2019).
29. Libura M, Władysiuk M, Małowicka M, Grabowska E, Gałazka-Sobotka M, Gryglewicz J. Rare Disease in Poland, Current Status and Perspectives [Choroby rzadkie w Polsce, stan obecny i perspektywy]. Warszawa: Uczelnia Łazarskiego (2016). Available online at: https://www.lazarski.pl/fileadmin/user_upload/dokumenty/instytuty/Choroby_rzadkie_w_Polsce_Stan_obecny_i_perspektywy.pdf (accessed September 18, 2019).
30. The Law of Ukraine, On Amendments to the Fundamentals of the Ukrainian Legislation on Health Care for the Provision of Prevention and Treatment of Rare (Orphan) Diseases. Sevastopol: Bulletin of the Verkhovna Rada (2014). 894. Available online at: http://zakon4.rada.gov.ua/laws/show/1213-18 (accessed September 18, 2019).
31. Federal Regulation dated 21 November 2011, No 323-F3. Base of Healthcare for Russian Federation Citizenship. Available online at: http://www.consultant.ru/document/cons_doc_LAW_121895/ (accessed September 18, 2019).
32. Ministry of Health and Social Development of the Republic of Kazakhstan RSE. “Republican Center for Health Development” Medicinal Information and Analytical Center: Orthopedics and Rare Diseases Methodical recommendations. ASTANA (2015). Available online at: http://www.druginfo.kz/docs/metod/orfan-ru.pdf (accessed September 18, 2019).
33. Belgin G, Macarthur D. Access to Orphan Drugs in Turkey. PharmExec.com. (2016, Jan 04) (accessed September 18, 2019).
34. Kiliç P, Koçkaya G, Yemşen Ö, Tan C, Handan Öztunca F, Aksungur P, et al. Orphan drug regulation in Turkey. JPHSR. (2013) 4:151–3. doi: 10.1111/jphs.12018
35. French National Cancer Institute. French National Networks for Rare Cancers in Adults, Support for the Decision. INCa (2015). Available online at: http://www.e-cancer.fr/content/download/119846/1431699/file/Cancers-rares-adultes-english-2015.pdf (accessed September 18, 2019).
36. Minister of Health. The Newborn Screening Program in Poland for 2015-2018 of the Minister of Health, Legal Basis: Art. 48 of the Act of 27 August 2004 on Healthcare Services Financed from Public Funds. Minister Zdrowia (2017). Available online at: https://www.gov.pl/documents/292343/416494/tre%C5%9B%C4%87$+$programu.doc/05ac14cd-3f1b-574b-55c2-29d4eccf2c3c (accessed September 18, 2019).
37. RIVM List of Diseases Screened via the Heelprick. Available online at: https://www.rivm.nl/Onderwerpen/H/Hielprik/De_ziektes_die_de_hielprik_opspoort (accessed September 18, 2019).
38. RIVM. Extension of Heelprick Screening. Available online at: https://www.rivm.nl/Onderwerpen/H/Hielprik/Uitbreiding_van_de_hielprikscreening
39. Beschluss 1 des Gemeinsamen Bundesausschusses über eine Neufassung der Richtlinien über die Früherkennung von Krankheiten bei Kindern bis zur Vollendung des 6. Lebensjahres (Kinder-Richtlinien): Formale und inhaltliche Überarbeitung (Neustrukturierung). Available online at: https://www.g-ba.de/informationen/beschluesse/2287/ (accessed September 18, 2019).
41. Cabinet of Ministers of Ukraine. Decision of March 13, 2019 No. 255 On Approving the List of Medicines and Medical Devices Purchased under Purchase Agreements with Specialized Organizations Performing Procurement by Budget Funds in the Year 2019 Individual State Programs and Complex Programmatic Activities. Available online at: https://zakon.rada.gov.ua/laws/show/255-2019-%D0%BF (accessed September 18, 2019).
42. Tezel B, Dilli D, Bolat H, Sahman H, Ozbaş S, Acican D, et al. The development and organization of newborn screening programs in Turkey. J Clin Lab Anal. (2014) 28:63–9. doi: 10.1002/jcla.21645
43. Order of the Minister of Health of the Republic of Kazakhstan from September 9, 2010 No. 704 “On Approval of the Rules of Screening Organization”. Available online at: http://adilet.zan.kz/rus/docs/V1000006490 (accessed September 18, 2019).
44. Burgard P, Cornel M, Di Filippo F, Haege G, Hoffmann GF, Lindner M, et al. EU Tender “Evaluation of population newborn screening practices for rare disorders in Member States of the European Union”, Short Executive Summary of the Report on the practices of newborn screening for rare disorders implemented in Member States of the European Union, Candidate, Potential Candidate and EFTA Countries. (2011). Available online at: http://www.isns-neoscreening.org/wp-content/uploads/2016/06/Summary20111018.pdf (accessed September 18, 2019).
45. Groselj U, Zerjav Tansek M, Smon A, Angelkova N, Anton D, Baric I, et al. Newborn screening in southeastern Europe. Mol Genet Metabol. (2014) 113:42–5. doi: 10.1016/j.ymgme.2014.07.020
46. Commission of the European Communities. Communication From the Commission to the European Parliament, The Council, The European Economics and Social Committee and the Committee of the Regions on Rare Diseases: Europe's Challenges. (2008). Available online at: http://www.europlanproject.eu/Resources/docs/ECCommunication_COM-2008-679final.pdf (accessed September 18, 2019).
47. https://www.cnsa.fr/grands-chantiers/strategie-et-plans-nationaux/les-schemas-et-plans-handicaps-rares-et-maladies-rares (accessed September 18, 2019).
48. https://www.sciencesetavenir.fr/sante/marisol-touraine-annonce-un-3e-plan-des-maladies-rares_30686 (accessed September 18, 2019).
49. http://en.e-cancer.fr/The-Cancer-Plan-2014-2019 (accessed September 18, 2019).
50. Regulation of The Russian Federation Government dated November 26, 2018, No. 1416. About the Order of the Organization of Providing Medicines Persons With Hemophilia, Cystic Fibrosis, Pituitary Nanism, Gaucher Disease, Malignant Lymphoid Tumors, Hematopoietic and Related Tissues, Multiple Sclerosis, Hemolytic-Uremic Syndrome, Juvenile Arthritis With Systemic Onset, Mucopolysaccharidosis Types I, II and VI, Persons After Organ and (or) Tissue Transplantation. (2018). Available online at: https://minzdrav.midural.ru/uploads/document/4564/pp-1416-26-11-18.pdf (accessed September 30, 2019).
52. http://www.resmigazete.gov.tr/eskiler/2015/08/20150809-16-1.pdf (accessed September 18, 2019).
53. http://zakon4.rada.gov.ua/laws/show/590-2016-%D1%80#n11 (accessed September 18, 2019).
54. Ray-Coquard I, Pujade Lauraine E, Le Cesne A, Pautier P, Vacher Lavenue MC, Trama A, et al. Improving treatment results with reference centres for rare cancers: where do we stand? Euro J Cancer. (2017) 77, 90–98. doi: 10.1016/j.ejca.2017.02.006
55. Trama, A, Marcos-Gragera, R, Sánchez Pérez MJ, van der Zwan JM, Ardanaz E, Bouchardy C, et al. Data quality in rare cancers registration: the report of the RARECARE data quality study. Tumori J. (2017) 103:22–32. doi: 10.5301/tj.5000559
56. Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. RARECARE working group. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. (2011) 47:2493–511. doi: 10.1016/j.ejca.2011.08.008
57. http://www.eucerd.eu/?page_id=540 (accessed September 18, 2019).
58. http://www.bndmr.fr (accessed September 18, 2019).
59. http://www.bndmr.fr/espace-documentaire/publications-scientifiques/ (accessed September 18, 2019).
60. https://solidarites-sante.gouv.fr/IMG/pdf/pnmr3_-_en.pdf (accessed September 18, 2019).
61. https://www.osse-register.de/OSSE_summary_en.pdf. (accessed September 18, 2019).
62. https://www.cherh.de/zipse110.html?&L=1. (accessed September 18, 2019).
63. https://www.kistikfibrozisturkiye.org/ (accessed September 18, 2019).
64. http://www.hasta.saglik.gov.tr/TR,12541/uluslararasi-nadir-hastaliklar-panel-ve-calistayi.html (accessed September 18, 2019).
65. Slotadvies afstemmingsoverleg zeldzame ziekten. ZonMW. (2017). Available online at: https://www.rijksoverheid.nl/binaries/rijksoverheid/documenten/rapporten/2017/04/03/slotadvies-afstemmingsoverleg-zeldzame-ziekten/slotadvies-afstemmingsoverleg-zeldzame-ziekten.pdf (accessed September 18, 2019).
66. Letter to Parliament on Rare Diseases. (2018) Available online at: https://www.rijksoverheid.nl/binaries/rijksoverheid/documenten/kamerstukken/2018/06/18/kamerbrief-over-zeldzame-aandoeningen/kamerbrief-over-zeldzame-aandoeningen.pdf. (accessed September 18, 2019).
67. Federal Law Gazette Year 2019 Part I No. 30 issued to Bonn on August 15, 2019. 1202-1220/Bundesgesetzblatt Jahrgang 2019 Teil I Nr. 30, ausgegeben zu Bonn am 15. (2019). 1202–20. Available online at: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/G/GSAV_bgbl119_S.1202_150819.pdf (accessed September 18, 2019).
68. Package Management Orphan Drugs. (2017). Available online at: https://www.zorginstituutnederland.nl/werkagenda/publicaties/publicatie/2017/12/21/monitor-weesgeneesmiddelen-2017 (accessed September 18, 2019).
69. Zamora B, Maignen F, O'Neill P, Mestre-Ferrandiz J, Garau M. (2019). Comparing access to orphan medicinal products in Europe. Orphanet J Rare Dis. 14:95. doi: 10.1186/s13023-019-1078-5
70. https://www.gov.pl/zdrowie/programy-lekowe (accessed September 18, 2019).
71. http://receptariusz.pl/lek-na-chorobe-Program-lekowy-leczenieatypowego-zespo\T1\lu-hemolityczno-mocznicowego-aHUS$-$37650216.html (accessed September 18, 2019).
72. http://www.bip.mz.gov.pl/legislacja/akty-prawne/obwieszczenie-ministra-zdrowia-z-dnia-26-lutego-2018-r-w-sprawie-wykazu-refundowanych-lekow-srodkow-spozywczych-specjalnego-przeznaczenia-zywieniowego-oraz-wyrobow-medycznych-na-1-marca-2018/ (accessed September 18, 2019).
73. Pejcic AV, Iskrov G, Jakovljevic MM, Stefanov R. Access to orphan drugs - comparison across Balkan countries. Health Policy. (2018) 122:583–9. doi: 10.1016/j.healthpol.2018.04.009
74. Decree of the Government of the Russian Federation No. 1155 of December 26, 2011. On the Purchase of Medicines Intended to Provide Persons With Hemophilia, Cystic Fibrosis, Pituitary Nasal Disease, Gaucher's Disease, Malignant Neoplasms of Lymphoid, Haematopoietic and Related Tissues, Disseminated Sclerosis, Persons After Organ and / or Tissue Transplantation “(Together With the” Regulation on The Procurement of Medicines Intended to Provide Persons With Hemophilia, Cystic Fibrosis, Pituitary Abscess, Gaucher Disease, Malignant Governmental Neoplasms of Lymphoid, Haematopoietic and Related Tissue, Multiple Sclerosis, Persons After Transplantation of Organs and (or) Tissues. (2017). Available online at: http://www.consultant.ru/document/cons_doc_LAW_124285/ (accessed September 18, 2019).
75. Resolution of the Government of the Russian Federation No. 403 of April 26, 2012. On the Procedure for Maintaining the Federal Register of Persons Suffering Life-threatening and Chronic Progressing Rare (Orphan) Diseases, Leading to a Reduction in the Lifetime of Citizens or Their Disabilities, and its Regional Segment. (2012). Available online at: http://base.garant.ru/70168888/ (accessed September 18, 2019).
76. http://www.apteka.ua/article/383101 (accessed September 18, 2019).
77. https://zakon.rada.gov.ua/laws/show/255-2019-%D0%BF (accessed September 18, 2019).
78. Social Security Institution. Appendix 4/A. (2018). Available online at: http://www.sgk.gov.tr/wps/wcm/connect/8803e7a1-92c1-456e-a5de-31e3d4e8c5ca/ek_19012018_03.xlsx?MOD=AJPERES&CONVERT_TO=url&CACHEID=8803e7a1-92c1-456e-a5de-31e3d4e8c5ca (accessed September 18, 2019).
79. Social Security Institution. Appendix 4/C. (2018). Available online at: http://www.sgk.gov.tr/wps/wcm/connect/c664e6b6-741a-4603-89a0-5096176916c9/ek_19042018_00.xlsx?MOD=AJPERES&CONVERT_TO=url&CACHEID=c664e6b6-741a-4603-89a0-5096176916c9 (accessed September 18, 2019).
80. Order of the Minister of Health and Social Development of the Republic of Kazakhstan of 22 May 2015 No. 370. On the Approval of the List of Orphan (Rare) Diseases. (2015). Available online at: http://adilet.zan.kz/rus/docs/V1500011511 (accessed September 18, 2019).
81. Order of the Minister of Health and Social Development of the Republic of Kazakhstan dated May 29, 2015 No. 432. On approval of the list of orphan drugs. (2015) Available online at: http://adilet.zan.kz/rus/docs/V1500011494 (accessed September 18, 2019).
82. Balasubramanian G, Morampudi S, Chhabra P, Gowda A, Zomorodi B. An overview of Compassionate Use Programs in the European Union member states. Intractable Rare Dis Res. (2016) 5:244–54. doi: 10.5582/irdr.2016.01054
83. ANSM Liste des spécialités faisant l'objet d'une RTU. http://ansm.sante.fr/Activites/Recommandations-Temporaires-d-Utilisation-RTU/Liste-des-specialites-faisant-actuellement-l-objet-d-une-RTU/(offset)/1 (accessed September 18, 2019).
84. The French Agency for the Safety of Health Products/Agence Nationale de Sécurité du Médicament et des produits de santé: Temporary Recommendation for Use (RTUs) Principles and information on the methods used by the ANSM for establishment and implementation October 2012 (accessed September 18, 2019).
85. Bill of the Federal Government. Draft of a Law to Strengthen the Supply of Drugs in Statutory Health Insurance; Gesetzentwurf der Bundesregierung: Entwurf eines Gesetzes zur Stärkung der Arzneimittelversorgung in der GKV (GKV-Arzneimittelversorgungsstärkungsgesetz – AMVSG). Available online at: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/Gesetze_und_Verordnungen/GuV/A/AMVSG_Kabinettvorlage.pdf (accessed September 18, 2019).
86. Draft Law for More Safety in the Supply of Pharmaceuticals From 05.06.2019. /Beschlussempfehlung und Bericht. Ausschuss für Gesundheit. Entwurf eines Gesetzes für mehr Sicherheit in der Arzneimittelversorgung vom 05.06.2019. Drucksache 19/10681. Available online at: http://dip21.bundestag.de/dip21/btd/19/106/1910681.pdf (accessed September 18, 2019).
87. Fischer KE, Tom Stargardt T. Early benefit assessment of pharmaceuticals in Germany: manufacturers' expectations versus the federal joint committee's decisions. Med Decis Making. (2014) 34:1030–47. doi: 10.1177/0272989X14546377
88. The Code of the Republic of Kazakhstan of September 18, 2009. On the Health of the People and the Health Care System. Available online at: https://online.zakon.kz/document/?doc_id=30479065 (accessed September 18, 2019).
89. ZIN. Coverage Management of Specialized Medicines. (2014) Available online at: https://www.zorginstituutnederland.nl/publicaties/rapport/2013/12/03/pakketbeheer-specialistische-geneesmiddelen (accessed September 18, 2019).
90. Kawalec P, Tesar T, Vostalova L, Draganic P, Manova M, Savova A. Pharmaceutical regulation in Central and Eastern European countries: a current review. Front Pharmacol. (2017) 8:892. doi: 10.3389/fphar.2017.00892
91. Koçkaya G, Wertheimer A, Kilic P, Tanyeri, Vural M, Akbulat A, et al. An overview of the orphan medicines market in Turkey. Value Health Reg Issues. (2014) 4:47–52. doi: 10.1016/j.vhri.2014.06.009
92. Morrell L, Wordsworth S, Fu H, Rees S, Barker R. Cancer drug funding decisions in Scotland: impact of new end-of-life, orphan and ultra-orphan processes. BMC Health Services Res. (2017) 17:613. doi: 10.1186/s12913-017-2561-0
93. Castilla-Rodríguez I, Vallejo-Torres L, Couce ML, Valcárcel-Nazco C, Mar J, Serrano-Aguilar P. Cost-effectiveness methods and newborn screening assessment. rare diseases epidemiology: update and overview. Adv Exp Med Biol. (2017) 1031:267–81. doi: 10.1007/978-3-319-67144-4_16
94. Paulden M, Stafinski T, Menon D, McCabe C. Value-based reimbursement decisions for orphan drugs: a scoping review and decision framework. Pharmacoeconomics. (2015) 33:255–69. doi: 10.1007/s40273-014-0235-x
95. Kodra Y, Cavazza M, Schieppati A, De Santis M, Armeni P, Arcieri R, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. (2014) 12 (Suppl. 3):s567–75. doi: 10.2450/2014.0042-14s
96. Angelis A, Kanavos P, López-Bastida J, Linertová R, Juan Oliva-Moreno, Pedro Serrano-Aguilar P, et al. and BURQOL-RD Research Network: social/economic costs and health-related quality of life in patients with epidermolysis bullosa in Europe. Eur J Health Econ. (2016) 17 (Suppl. 1):31–42. doi: 10.1007/s10198-016-0783-4
97. Thokala P, Devlin N, Marsh K, Baltussen R, Boysen M, Kalo Z, et al. Multiple criteria decision analysis for health care decision making—an introduction: report 1 of the ISPOR MCDA emerging good practices task force. Value Health. (2016) 19:1–13. doi: 10.1016/j.jval.2015.12.003
98. Marsh K, IJzerman M, Thokala P, Baltussen R, Boysen M, Kaló Z, et al. Multiple criteria decision analysis for health care decision making—emerging good practices: report 2 of the ISPOR MCDA emerging good practices task force. Value Health. (2016) 19:125–37. doi: 10.1016/j.jval.2015.12.016
99. Endrei D, Molics B, Ágoston I. Multicriteria decision analysis in the reimbursement of new medical technologies: real-world experiences from Hungary, Letter to the editor. Value Health. (2014) 19:123–4. doi: 10.1016/j.jval.2014.01.011
100. Radaelli G, Lettieri E, Masella C, Merlino L, Strada A, Tringali M. Implementation of eunethta core ModelR in Lombardia: the VTS framework. Int J Technol Assess Health Care. (2014) 30:105–12. doi: 10.1017/S0266462313000639
101. Wagner M, Khoury H, Willet J, Rindress D, Goetghebeur M. Can the EVIDEM framework tackle issues raised by evaluating treatments for rare diseases: analysis of issues and policies, and context-specific adaptation. Pharmacoeconomics. (2016) 34:285–301. doi: 10.1007/s40273-015-0340-5
102. Hughes-Wilson W, Palma A, Schuurman A, Simoens S. Paying for the orphan drug system: break or bend? is it time for a new evaluation system for payers in Europe to take account of new rare disease treatments? Orphanet J Rare Dis. (2012) 7:74. doi: 10.1186/1750-1172-7-74
103. Iskrov G, Miteva-Katrandzhieva T, Stefanov R: Multi-criteria decision analysis for assessment and appraisal of Orphan Drugs. Front. Public Health. 4:214. doi: 10.3389/fpubh.2016.00214
104. Tsiachristas A, Koenders JM, Kanters TA. Multi-Criteria decision analysis for reimbursing orphan drugs: a dutch demonstration study using the analytic hierarchy process method. Value Health. (2014) 17:A541–2. doi: 10.1016/j.jval.2014.08.1744
105. Sussex J, Rollet P, Garau M, Schmitt C, Kent A, Hutchings A. A pilot study of multicriteria decision analysis for valuing orphan medicines. Value Health. (2013) 16:1163–9. doi: 10.1016/j.jval.2013.10.002
106. Fedyaeva VK, Omelyanovsky VV, Rebrova O, Khan N, Petrovskaya EV. MCDA approach to ranking rare diseases in Russia: preliminary. Value Health. (2014) 17:A539. doi: 10.1016/j.jval.2014.08.1729
107. Schey C, Irwin J, Teneishvili M, Krabbe PFM, Connolly M. Assessing the relationship between individual attributes identified in review of multi-criteria decision analysis (MCDA) of rare diseases and annual treatment costs in rare endocrine disorders. Value Health. (2014) 17:A562. doi: 10.1016/j.jval.2014.08.1860
108. Wagner M, Khoury H, Bennetts L, Berto P, Ehreth J, Badia X, et al. Appraising the holistic value of Lenvatinib for radio-iodine refractory differentiated thyroid cancer: a multi-country study applying pragmatic. BMC Cancer. (2017) 17:272. doi: 10.1186/s12885-017-3258-9
109. Gilabert-Perramon A, Torrent-Farnell J, Catalan A, Prat A, Fontanet M, Puig-Peiró R, et al. Drug evaluation and decision Making in Catalonia: development and validation of a methodological framework based on multi-criteria decision analysis (MCDA) for orphan drugs. Int J Technol Assess Health Care. (2017) 33:111–20. doi: 10.1017/S0266462317000149
110. Garau M, Marsden G, Devlin N, Amedeo Mazzanti N, Profico A. Applying a Multi-criteria Decision Analysis (MCDA) Approach to Elicit Stakeholders' Preferences in Italy. The Case of Obinutuzumab for Rituximab-Refractory Indolent Non-Hodgkin Lymphoma (iNHL). Research Paper 16/08. Office of Health Economics Research (2016). Available online at: https://www.ohe.org/publications/applying-multi-criteria-decision-analysis-mcda-approach-elicit-stakeholders%E2%80%99 (accessed September 30, 2019).
111. Schey C, Krabbe PFM, Postma MJ, Connolly MP. Multi-criteria decision analysis (MCDA):testing a proposed MCDA framework for orphan drugs. Orphanet J Rare Dis. (2017) 12:10. doi: 10.1186/s13023-016-0555-3
112. Gutierrez L, Patris J, Hutchings A, Cowell W. Principles for consistent value assessment and sustainable funding of orphan drugs in Europe. Orphanet J Rare Dis. (2015) 10:53. doi: 10.1186/s13023-015-0269-y
113. Simoens S. Health technologies for rare diseases: does conventional HTA still apply? Expert Rev Pharmacoecon Outcomes Res. (2014) 14:315–7. doi: 10.1586/14737167.2014.906903
114. Kanters TA, Hakkaart L, Rutten van Molken MPMH, Redekop WK. Access to orphan drugs in western Europe: can more systematic policy making really help to avoid different decisions about the same drug? Expert Rev Pharmacoecon Outcomes Res. (2015) 15:557–9. doi: 10.1586/14737167.2015.1045882
115. Serpik VG, Yagudina RI. Pathways of implementation of multi-criteria decision analysis into orphan drug approval procedure for drug supply programs in Russian Federation. Value Health. (2014) 17:A323–686. doi: 10.1016/j.jval.2014.08.2001
116. Baran-Kooiker A, Czech M and Kooiker C. Multi-criteria decision analysis (MCDA) models in health technology assessment of orphan drugs—a systematic literature review. Next steps in methodology development? Front Public Health. (2018) 6:287. doi: 10.3389/fpubh.2018.00287
117. Nicod E, Annemans L, Bucsics A, Lee A, Upadhyaya S, Facey K. HTA programme response to the challenges of dealing with orphan medicinal products: process evaluation in selected European countries. Health Policy. (2017) 123:140–51. doi: 10.1016/j.healthpol.2017.03.009
118. Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs community. Ment Health J. (1968) 4:443. doi: 10.1007/BF01530764
119. Gaasterland CM, Jansen-van der Weide MC, Weinreich SS, van der Lee JH. A systematic review to investigate the measurement properties of goal attainment scaling, towards use in drug trials. BMC Med Res Methodol. (2016) 16:99. doi: 10.1186/s12874-016-0205-4
Keywords: rare diseases, newborn screening, national plan, patient registries, reimbursement, policy
Citation: Czech M, Baran-Kooiker A, Atikeler K, Demirtshyan M, Gaitova K, Holownia-Voloskova M, Turcu-Stiolica A, Kooiker C, Piniazhko O, Konstandyan N, Zalis'ka O and Sykut-Cegielska J (2020) A Review of Rare Disease Policies and Orphan Drug Reimbursement Systems in 12 Eurasian Countries. Front. Public Health 7:416. doi: 10.3389/fpubh.2019.00416
Received: 14 October 2019; Accepted: 24 December 2019;
Published: 28 January 2020.
Edited by:
Piotr Romaniuk, Medical University of Silesia, PolandReviewed by:
Stephen Groft, National Center for Advancing Translational Sciences (NCATS), United StatesAgnieszka Zimmermann, Medical University of Gdansk, Poland
Copyright © 2020 Czech, Baran-Kooiker, Atikeler, Demirtshyan, Gaitova, Holownia-Voloskova, Turcu-Stiolica, Kooiker, Piniazhko, Konstandyan, Zalis'ka and Sykut-Cegielska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Baran-Kooiker, YWxla3NhbmRyYS5iYXJhbkBnbWFpbC5jb20=
 Marcin Czech1
Marcin Czech1 Aleksandra Baran-Kooiker
Aleksandra Baran-Kooiker Adina Turcu-Stiolica
Adina Turcu-Stiolica Oresta Piniazhko
Oresta Piniazhko