
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health , 12 October 2018
Sec. Health Economics
Volume 6 - 2018 | https://doi.org/10.3389/fpubh.2018.00291
 Monica Capozzi1
Monica Capozzi1 Chiara De Divitiis1
Chiara De Divitiis1 Alessandro Ottaiano1
Alessandro Ottaiano1 Tramontano Teresa2
Tramontano Teresa2 Maurizio Capuozzo3
Maurizio Capuozzo3 Piera Maiolino2
Piera Maiolino2 Gerardo Botti4
Gerardo Botti4 Salvatore Tafuto1*
Salvatore Tafuto1* Antonio Avallone1
Antonio Avallone1 The Abdominal Oncology Group†
The Abdominal Oncology Group†Introduction: The therapeutic scenario of Oncology is enriching of innovative agents which are determining an increase in public expenditure because of their high cost. In Italy, a web-based government Registry is used to monitor the clinical use of these drugs and, in later phases, to obtain funds reimbursement according to specific economic agreements with companies.
Methods: A health policy expert Pharmacist was included in the multidisciplinary team of the Department of Abdominal Oncology of the National Cancer Institute (NCI) of Naples “G. Pascale Foundation” in order to improve the management of the Registry for oncologic drugs monitoring. Pharmacist activities were: basal data registration, prescription appropriateness, drug request, response monitoring, toxicity reporting, follow-up, reimbursement request. These activities were conducted in strict interrelation with clinicians. The source of data were medical records and a web-based national reimbursement platform. The analysis of the economic impact of this strategy was descriptive and it was indicated as resources recovery comparing 2 years: 2015 vs. 2016. The currency reference used was the Euro (€).
Results: A total of 932 patients were followed-up and registered, 365 treatments are ongoing at the Department of Abdominal Oncology (NCI of Naples, Italy). The most prescribed biologic drug in advanced gastrointestinal cancers was bevacizumab. Compared to the year 2015, in 2016 we recorded a strong increase of reimbursements: EUR 881.712,42 vs. EUR 214.554,98.
Conclusions: We suggest that the reimbursement process can be improved when a health policy reimbursement professional Pharmacist is integrated in the multidisciplinary team along with clinicians.
The therapeutic scenario of Oncology is enriching of innovative agents which are determining an increase in public expenditure because of their high cost (1). Biologic drugs in oncology are the fastest-growing pharmacological category worldwide; the increase in their use is paralleled by the progressive increase in the comprehension of the cancer biology. In the future, biologic target-based drugs will substitute “conventional” chemotherapies because of higher specificity against cancer cells (more on target interactions) and lower toxicity profile (less off target interactions). To date, in gastrointestinal oncology, some monoclonal antibodies (cetuximab, panitumumab, bevacizumab, ramucirumab, trastuzumab), a recombinant fusion protein (aflibercept) and a small molecule (regorafenib) are available to treat patients with metastatic disease. However, new biologics continue to emerge particularly in the field of cancer immunology. The steep prices of biologics concern researchers, clinicians, patients, and it is due to many factors (i.e. patents, intellectual property, competition, production costs, etc.) whose description and discussion are beyond the scope of the present report. In last years, many efforts have been pursued by the Healthcare Systems of the European countries to optimize the national pharmaceutical expenditure ensuring the access to these innovative and highly expensive treatments to patients. The National Health Authorities are called upon to determine the price of the new drugs in relation to their effectiveness and innovativeness; however, the data at the time of authorization of medicinal products for marketing by European Medicines Agency (EMA) are often insufficient to accurately estimate the effects (efficacy and toxicity) in real practice (2). Managed Entry Agreements (MEA) are specific economic instruments in order to facilitate the access to these high-cost innovative agents (“access policy”) (3–9). MEA can be divided into two main groups: financial-based and performance-based. The first consists on price-volume discount (manufacturers pay-back the excess of public established threshold expenditure) and dose-capping scheme (manufacturers refund the overdoses of established doses required per year per drug). The second, “performance-based” consists on outcome-based evaluations: the efficacy data are collected and the cost is reduced or reimbursed according to the outcome obtained in real practice. However, details about MEA (entity of reimbursements, timings, outcomes, etc.) cannot be revealed because of their private nature.
In Italy, the Agenzia Italiana del Farmaco (AIFA, Italian governative agency for pharmaceutical products) negotiated different agreements with pharmaceutical industry. The contractual arrangements include both financial- and performance-based agreements (10). The new authorized drugs are immediately included in a specific “registry” of AIFA: a government web-based tool in order to monitor appropriateness, use, toxicity and efficacy of pharmaceuticals. One hundred thirty innovative drugs are currently monitored on Italian Registry.
In this report, we show a real practice experience of reimbursement at the Department of Abdominal Oncology of the National Cancer Institute of Naples.
An expert Pharmacist (MC) was involved in a project at the Department of Abdominal Oncology of the National Cancer Institute of Naples “ G. Pascale Foundation” in order to evaluate the economic impact of improving the management of AIFA Registry for high-cost innovative drugs (including monoclonal antibodies or small molecules inhibiting the proliferative signals or with antiangiogenic properties or differentiating agents or inhibitors of metastasis/invasiveness or drugs with multiple mechanisms of action). The selected drugs were: bevacizumab, cetuximab, panitumumab, trastuzumab. Reasons for excluding nab-paclitaxel, ramucirumab, aflibercept and regorafenib are described in results. During the entire 2016, management of AIFA registry was done in strict collaboration with a health policy reimbursement expert Pharmacist.
The Pharmacist was involved in following specific data entry activities: (i) patients' and disease characteristics registration (basal data), (ii) evaluation of the eligibility criteria (prescription appropriateness), (iii) registration of code, type, number and quantity of drug vials dispensed (drug request), (iv) disease status monitoring (response monitoring), (v) adverse reactions reporting (toxicity reporting), (vi) “end of treatment” module (follow-up). Finally, the Pharmacist managed the request forms for reimbursement (reimbursement request) interacting with companies and following that process from the submission to the approval of refunding. These activities were conducted in strict interrelation with clinicians.
The source of data of this report were medical records and the web-based national reimbursement platform. The analysis was descriptive and the outcome of this study was indicated as cumulative recovery of resources comparing 2 years: 2015 vs. 2016. The currency reference used was the Euro (€). Statistical inference was not applied given the high differences observed.
The Department of Abdominal Oncology of the National Cancer Institute of Naples is particularly devoted to the diagnosis and treatment of cancers of the colon, rectum, stomach and pancreas. The medical division is actively involved in data entry and updating of the AIFA Registry in accordance with national legislation (Decree Law 6 July 2012 n. 95, “Spending review Law”). Table 1 shows the most important innovative drugs used. For the medicinal product drugs Cetuximab and Panitumumab the monitoring activity has been stopped since October 2th, 2016 and February 27 th, 2017, respectively.
Actually, a total of 932 patients are followed-up and 365 treatments are ongoing for gastrointestinal oncology specialties MEA-repayable. The overall picture of the cumulative number of treatments from January 2013 to December 2016 is shown in Table 2. The most prescribed biologic drug in advanced disease was bevacizumab. There was an increase in the use of panitumumab and of new authorized drugs (nab-paclitaxel and ramucirumab). By contrast, a significant decrease was registered for cetuximab. However, at the date of presentation of this report, MEA were not yet activated for Nab-paclitaxel and Ramucirumab so that these drugs have been excluded from the comparative analysis (2015 vs. 2016). Furthermore, also aflibercept was excluded because MEA reimbursement started in the late 2015. Data cannot be extracted for regorafenib because the web-based registration and monitoring started in January 2017.
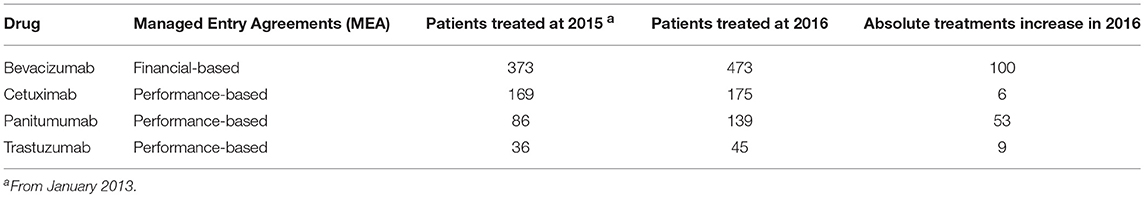
Table 2. Enrolment of patients in the AIFA Registry according to specific drugs and years (from 2013 to 2016).
Compared to the year 2015, in 2016 a strong increase of funds reimbursement was observed. In twelve months of project activity (from March 2016 to February 2017) EUR 881.712,42 have been reimbursed against the EUR 214.554,98 of 2015 (Figure 1). In particular, in 2016, 54 Bevacizumab, 26 Cetuximab, 10 Trastuzumab, 18 Panitumumab treatments were closed and successfully submitted for reimbursement. In Figure 1 we show also the comparison between 2015 vs. 2016 in terms of reimbursements for trastuzumab (advanced HER2+ gastric cancer), panitumumab, cetuximab (advanced RAS wild type colorectal cancer), and bevacizumab (advanced colorectal cancer). The increase in reimbursement was not attributable to increase of treated patients in 2016 compared to previous years (Table 2). In fact, the use of cetuximab and trastuzumab was reduced in 2016; the reduction of cetuximab was paralleled by a significant increase of panitumumab.
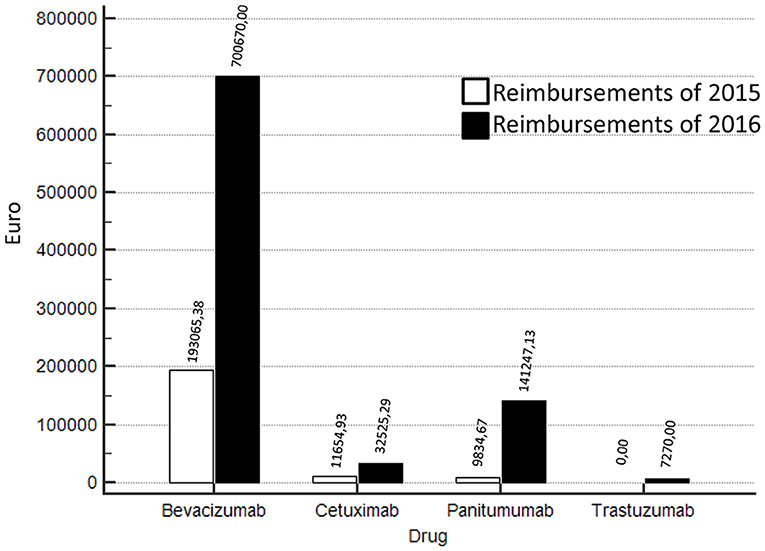
Figure 1. Reimbursements comparisons 2016 vs 2015 according to different biologics (bevacizumab, cetuximab, panitumumab, trastuzumab).
Most of reimbursements was attributable to bevacizumab which was stable over the observed years. Additionally, in 2015, the procedures of reimbursement were already active. Thus, the differences in results are attributable to a more careful monitoring activity and more timely submission of requests for reimbursement to pharmaceutical companies.
Our study suggest that the AIFA registry is a real chance of founding if managed by qualified professionals.
The Healthcare System has to satisfy the challenge of optimizing the national public pharmaceutical expenditure ensuring to patients the access to innovative treatments. In Italy, the AIFA Registry assess the patient's eligibility for treatment, collects epidemiological data, drug safety and efficacy profile. This should ensure the appropriate use of medicines as recommend by guidelines and provide data about the “real world” efficacy of the drugs. The AIFA Registry was established in 2005 and completely renewed in 2013 and it belongs to the Information System of the National Health Service that estimates, through the data collected, the benefit/risk and cost/effectiveness ratios of pharmaceuticals. The AIFA Registry is also part of a broader European program of Health Technology Assessment (HTA) representing the multidisciplinary approach to analyze the effects of therapeutic innovation in clinical practice in order to reduce public expenditure (11). However, the application of MEA requires the correct use of monitoring, in accordance with very specific requirements and deadlines regarding the restaging of the disease, the number of therapy cycles, the monitoring and reporting of therapy response, the timely communication of adverse events, and correct follow-up information.
In the present study, a Pharmacist was involved in the multidisciplinary team of the Abdominal Oncology Department of the National Cancer Institute in order to implement specific activities related to the AIFA registry for the patients with advanced gastrointestinal cancer going to start innovative therapy. In particular the Pharmacist was committed to entry, manage and discuss with clinicians the basal data, prescription appropriateness, drug requests, response monitoring, toxicity reporting, “end of treatment” module. Finally, the Pharmacist managed all the process of reimbursement request from the submission to the approval of refunding. Interestingly, after including this professional figure in the multidisciplinary team, from March 2016 to February 2017, there was an increase in reimbursements at the Department of Abdominal Oncology of the National Cancer Institute of Naples; in fact, EUR 881.712,42 were reimbursed against the EUR 214.554,98 of 2015 (Figure 1) and that that increase was not attributable to the number of patients (Table 2).This was related to improvements of all activities related to AIFA registry management and reimbursement requests. Optimization of costs along with clinical efficacy is a goal of sanitary systems and many experiences in literature have already indicated strategies both in oncology and other therapeutic areas in order to achieve this objective: patients' selection (12–14), drug-days (15–17), monitoring of prescription appropriateness (18–20) and MEA (10).
Uncertainty of clinical outcomes and high costs are among the main challenges of innovative drugs; the first issue needs to be faced with intensive clinical and translational research, the second one has prompted the adoption of refunding systems including MEA. The results of this report indicate that MEA are an important source of reimbursement for innovative drugs but this system requires highly skilled and dedicated personnel. In the new era of high-cost innovative biologic drugs, professionals figures, beside clinicians, should be involved in the management of economic-related issues of anti-neoplastic agents.
In the present study we show that the reimbursement process of biologics in gastrointestinal oncology can be improved when a health policy reimbursement professional Pharmacist is integrated in the multidisciplinary team along with clinicians. The present study can be considered as an exploratory report lacking in literature data on the integration of Pharmacists into the clinical multidisciplinary teams. Improving the management of MEA-related issues could represent a successful strategy in the “real world” to improve reimbursements and finally reduce the costs of biologic drugs.
CD, AO, GB, ST, and AA for enlistment and treatment of patients and prescription of drugs. MoC, TT, MaC, and PM for drug delivery and processing of pharmaceutical spending data. Abdominal Oncology Group were involved in treatment of patients and writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Prof. A. Gallipoli D'Errico and the association Lega Italiana Per La Lotta Contro i Tumori (LILT) of Naples in Italy for the collaboration. We thank Associazione Viva of Palma Campania for its helpful contribution.
1. Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country pricecomparison study. Lancet Oncol. (2016) 17:39–47. doi: 10.1016/S1470-2045(15)00449-0
2. Fojo T, Lo AW. Price, value, and the cost of cancer drugs. Lancet Oncol. (2016) 17:3–5. doi: 10.1016/S1470-2045(15)00564-1
3. Pauwels K, Huys I, Vogler S, Casteels M, Simoens S. Managed entry agreements for oncology drugs: lessons from the European experience to inform the future. Front Pharmacol. (2017) 8:171. doi: 10.3389/fphar.2017.00171
4. Adamski J, Godman B, Ofierska-Sujkowska G, Osinska B, Herholz H, Wendykowska K, et al. Risk sharing arrangements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. (2010) 10:153. doi: 10.1186/1472-6963-10-153
5. National Institute for Health and Care Excellance (NICE) List of Technologies With Approved Patient Access Scheme, Recommended by Nice for Use in NHS. Available online at: https://www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit/list-of-technologies-with-approved-patient-access-schemes
6. Tandvårds- & läkemedelsförmånsverket TLV. The Swedish Pharmaceutical Reimbursment System (2017). Available online at: https://www.tlv.se/Upload/English/ENG-swe-pharma-reimbursement-system.pdf
7. Espin J, Rovira J, Garcia L. Experiences and Impact of European Risk-Sharing Schemes Focusing on Oncology Medicines. Brussels: European Commission (2011).
8. Mueller S, Brandt S, Wilke T. The German among drug reimbursement process: factors associated with Gba-decisions about the additional benefit. Value Health (2015) 18:A546. doi: 10.1016/j.jval.2015.09.1737
9. European Federation of Pharmaceutical Industries and Associations. Available online at: https://www.efpia.eu/publications/data-center/the-pharma-industry-in-figures-economy
10. Ferrario A, Kanavos P. Dealing with uncertainty and high prices of new medicines: a comparative analysis of the use of managed entry agreements in Belgium, England, the Netherlands and Sweden. Soc Sci Med. (2015) 124:39–47. doi: 10.1016/j.socscimed.2014.11.003
11. Montilla S, Xoxi E, Russo P, Cicchetti A, Pani L. Monitoring registries at Italian Medicines Agency: fostering access, guaranteeing sustainability. Int J Technol Assess Health Care (2015) 31:210–213. doi: 10.1017/S0266462315000446
12. Kim D, Kim SY, Lee JS, Hong YS, Kim JE, Kim KP, et al. Primary tumor location predicts poor clinical outcome with cetuximab in RAS wild-type metastatic colorectal cancer. BMC Gastroenterol. (2017) 17:121. doi: 10.1186/s12876-017-0694-6
13. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
14. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. (2018) 379:122–37. doi: 10.1056/NEJMoa1803164
15. Damuzzo V, Russi A, Chiumente M, Masini C, Rebesco B, Gregis F, et al. Optimization of resources by drug management: a multicentred web-administered study on the use of ipilimumab in Italy. J Oncol Pharm Pract. (2018). doi: 10.1177/1078155218755867. [Epub ahead of print].
16. Fasola G, Aprile G, Marini L, Follador A, Mansutti M, Miscoria M. Drug waste minimization as an effective strategy of cost-containment in oncology. BMC Health Serv Res. (2014) 14:57. doi: 10.1186/1472-6963-14-57
17. Winger BJ, Clements EA, DeYoung JL, O'Rourke TJ, Claypool DL, Vachon S, et al. Cost savings from dose rounding of biologic anticancer agents in adults. J Oncol Pharm Pract. (2011) 17:246–51. doi: 10.1177/1078155210366171
18. Pacey S, Warner J, Li Wan Po A. A multidisciplinary approach to hospital-based drug cost containment. J Clin Pharm Ther. (1998) 23:203–11. doi: 10.1046/j.1365-2710.1998.00153.x
19. De Rijdt T, Willems L, Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am J Health Syst Pharm. (2008) 65:1161–72. doi: 10.2146/ajhp070506
Keywords: targeted drugs, reimbursement, public health, managed entry agreements, gastro-intestinal cancers, multidisciplinary team
Citation: Capozzi M, De Divitiis C, Ottaiano A, Teresa T, Capuozzo M, Maiolino P, Botti G, Tafuto S, Avallone A and The Abdominal Oncology Group (2018) Funds Reimbursement of High-Cost Drugs in Gastrointestinal Oncology: An Italian Real Practice 1 Year Experience at the National Cancer Institute of Naples. Front. Public Health 6:291. doi: 10.3389/fpubh.2018.00291
Received: 23 June 2018; Accepted: 24 September 2018;
Published: 12 October 2018.
Edited by:
Nemanja Rancic, Military Medical Academy, SerbiaReviewed by:
Ana Sabo, University of Novi Sad, SerbiaCopyright © 2018 Capozzi, De Divitiis, Ottaiano, Teresa, Capuozzo, Maiolino, Botti, Tafuto, Avallone and The Abdominal Oncology Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salvatore Tafuto, cy50YWZ1dG9AaXN0aXR1dG90dW1vcmkubmEuaXQ=
†Abdominal Oncology Group: Rossana Casaretti, Antonino Cassata, Anna Nappi, Guglielmo Nasti, Carmela Romano, Lucrezia Silvestro
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.