- 1SAMRC Microbial Water Quality Monitoring Centre, University of Fort Hare, Alice, South Africa
- 2Applied and Environmental Microbiology Research Group, Department Of Biochemistry and Microbiology, University of Fort Hare, Alice, South Africa
- 3Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
Limpet (Scutellastra cochlear) serves as seafood recipe and an important member of the aquatic food chain. It is an abundant mollusc in some aquatic environment in South Africa. In this study, we investigated the potential of the molluscs harvested from the Buffalo, Swartkops, and Kowie estuaries in the Eastern Cape Province, South Africa to serve as transient or maintenance reservoir of Vibrio species. The mollusc and source water samples were collected monthly from the rivers between December 2016 and November 2017. The reservoir category of the limpet samples recovered was determined by employing the combination of MPN-PCR method and statistical analysis (comparison of mean and proportion tests). The densities of Vibrio spp. in limpet and their source water samples were determined using MPN-PCR methods. Presumptive isolates were recovered by processing the samples with thiosulfate-citrate-bile salts-sucrose agar and where necessary, samples were enriched with alkaline peptone water. The presumptive isolates were identified using PCR methods with emphasis on six Vibrio species of public health importance. Vibrio spp. were detected in all the limpet samples but not in all the water samples. The densities of Vibrio spp. in the limpet samples were more than the densities of Vibrio species in their source water and these were significant at P < 0.05. In like manner, five out of the six key Vibrio pathogens targeted in this study were more prevalent in limpet samples than in source water samples. Based on our findings, we concluded that our method though could be improved on, is efficient for the determination of reservoir types of bacterial-carrying organisms. We also concluded that the limpet found in the estuaries are not just a transient but a maintenance reservoir of Vibrio spp. which could cause vibrio-related infections.
Introduction
The factors responsible for the persistence of cholera and cholera-like infections in the world despite the effort put in place by international public health stakeholders such as The Centre for Disease Control and Prevention (CDC) and World Health Organization (WHO) have been debated over the years. One of the factors that is on for debate by public health stakeholders is the possibility of the presence of reservoirs for Vibrio cholerae and other pathogenic vibrios in the environment. A year (2012–2013) survey on toxigenic Vibrio cholera O1 in Haiti by Alam et al. (1) suggested that the persistence of cholera is due to the existence of environmental reservoir. This finding was challenged by CDC (2) in a letter addressed to the authors of the study on the basis that the epidemiological data supplied were not explicit enough. It was pointed out that the work of Alam et al. (1) was carried out during an ongoing cholera epidemic and heavy rainfall which proposed the possibility of contamination of waterbody by open-air defecation especially by individuals with the disease. Thus, Stanislas and Renaud (2) pointed out the need to carry out this kind of investigation when there is no outbreak in other to confirm if V. cholerae reservoirs actually exist in the environment or if the so-called reservoirs are just transient vectors. The outbreak of vibrio infections such as cholera is believed to be preceded by environmental bloom and subsequent spilling of the pathogens into human population via vehicles and vectors of infections such as water and aquatic animals (1, 3). The ability of these pathogens to bloom in the environment could be as a result of the existence of reservoirs which concentrate and shield them against unfavorable conditions. There are several studies that have confirmed the presence of pathogenic Vibrio spp. in abiotic and biotic components of the aquatic environments. The notable studies are those that reported foodborne infections outbreaks caused by seafood. The seafood in this category can be broadly classified into three and these are fishes, molluscs, and crustaceans. Some of the molluscs that have been linked to vibrio infections include bivalves such as mussels, oysters, scallops, and clams while some of the crustaceans that have been implicated in vibrio infections are crabs, shrimps, and prawns. Likewise, the presences of the pathogens in both freshwater and saltwater have been reported. Studies on the isolation of these pathogenic vibrios from molluscs and crustaceans have been reported from different geographical locations of the world. These reports include those from Korea, Spain, Poland, Morroco, Egypt (4–8). The various studies mentioned earlier have been able to prove that aquatic animals have the ability to harbor pathogenic Vibrio species but none have been able to experimentally proven if these seafood are just transient or concentrating carriers. Thus this study statistically compared the densities of total halophiles and Vibrio species in limpet (Scutellastra cochlear) samples and their source water and the prevalence of the targeted six key Vibrio pathogens in limpet and their source water in an attempt to put forward a method that could be used to confirm if an organism is a transient or a concentrating reservoir of bacteria of interest which in this case is Vibrio spp. The Vibrio species isolated were further delineated into the key six vibrio pathogens (Vibrio cholerae, Vibrio mimicus, Vibrio fluvialis, Vibrio alginolyticus, Vibrio vulnificus, and Vibrio parahaemolyticus) to substantiate the findings of this study.
Limpet (Scutellastra cochlear), a native of South Africa (9, 10) which serves as seafood recipe and an important member of the aquatic food chain is an abundant mollusc in some aquatic environment in South Africa. It has been reported that limpet should be prepared like other gastropods e.g., abalone to achieve tasty recipe. The cooking is to be done at high temperature for say 30 s or at low temperature for a longer period (11). Southern Africa has been recognized as the world's foremost biodiversity and biomass hotspot for this mollusc. The density of the organism per square land mass of some of the aquatic environment in Southern Africa is as high as 2,600 (12). Limpet serves an important ecological role in the aquatic food chain and as earlier mentioned a food recipe for human (13). Although the prevalence of Vibrio spp. in several molluscs that are important to humans have been investigated, unfortunately, there is a dearth of information as regards the relationship between Vibrio spp. and Limpet. Thus, we assessed the potential of the limpets recovered from the Buffalo, Swartkops, and Kowie estuaries in the Eastern Cape Province, South Africa as a reservoir of Vibrio species with emphasis on six key potential pathogenic ones. The reservoir type of the mollusc was also determined.
Materials and Methods
Sampling Sites and Sample Collections
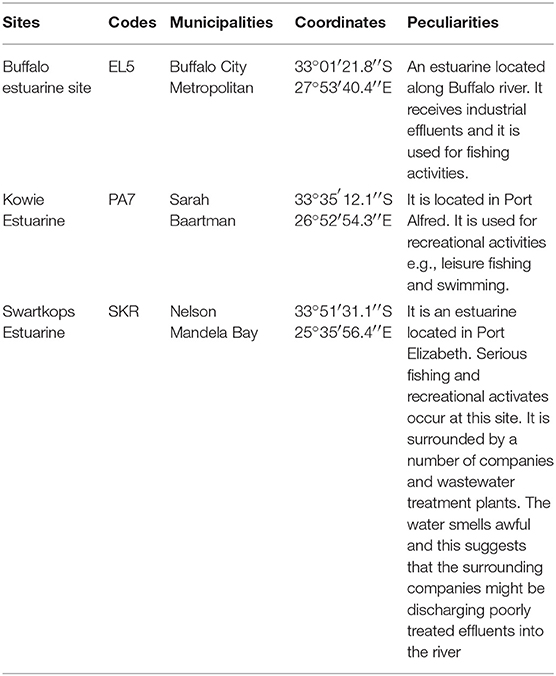
The coordinates of the sampling sites and their peculiar features that qualified them for inclusion in this study are as articulated in Table 1 below.
All the Limpet samples used in this study were purchased from fishermen, transferred into a sterile stomacher bags and transported on ice to the laboratory of the Applied and Environmental Microbiology Research Group (AEMREG), University of Fort Hare, Alice, South Africa. In like manner, water resources from where the limpet samples were recovered were also sampled and all samples were processed within 6 h of collection. Limpet samples were identified using limpet atlas (9, 14–16) and samples (water and limpet) were collected per month based on availability of limpet between December 2016 and November 2017.
Determination of Total Vibrio spp. Density in Limpet (Scutellastra cochlear) and Water Samples
Total vibrio density in the limpet and water samples were determined using 3-tubes by 5-dilutions end-point MPN-PCR techniques as described by Noorlis et al. (17), Copin et al. (18), and Ramos et al. (19). The MPN-PCR method is as reported by Tunung et al. (20). Briefly, 5–12 pieces each of molluscs limpet, were aseptically pooled together in a sterile stomacher bags and pummeled using stomacher machine (BagMixer 400, Interscience). A 10 g portion of the pummeled samples was afterwards added to a separate conical flask containing 90 ml sterile 3% NaClTryptic Soy broth to form initial homogenate which is the first dilution (101). The remaining four dilutions were prepared from the initial homogenate using alkaline peptone water (APW) as the diluent and 1 ml aliquot from each of the dilutions was inoculated into a tube containing fresh sterile 9 ml of APW. The setups were done in triplicates and resulting inoculated tubes were incubated at 37°C for 24 h. The water samples were analyzed in a similar manner except that the first dilution was prepared using double strength APW. After the incubation period, turbid tubes were recorded and corresponding MPN values were extrapolated from BAM-MPN excel spreadsheet. The extrapolated values were taken as the total halophile density. Afterwards, DNA extracted from 1 ml aliquot from each turbid tube was subjected to PCR following the procedure stated in sections Genomic DNA Extraction and Molecular Confirmation of Presumptive Vibrio isolates below, for the detection of 16S rRNA gene region specific for Vibrio spp. Turbid tubes that were positive for Vibrio spp. were noted and corresponding MPN values were extrapolated as earlier stated. The MPN value at this point was taken as total vibrio density. Total halophile's density and Vibrio species density of water samples from which limpet were recovered where also determined using MPN-PCR method as described earlier. This was done in other to statistically compare the total Vibrio species load in aquatic animals and their source water without introducing bias.
Enrichment of Samples
In other to achieve an optimum isolation of the targeted Vibrio spp. 10 g sample each of the remaining pummeled limpet samples in section Determination of Total Vibrio spp. Density in Limpet (Scutellastra cochlear) and Water Samples above and 10 mL of source water samples were also inoculated into 90 ml sterile APW. The setups were agitated gently for 1–2 min and incubated at 37°C for 24 h (21). After incubation period, a loop-full was carefully taken from the pellicle formed on the surface of the APW and streaked on fresh sterile TCBS plates.
Isolation of Presumptive Vibrio spp. From Limpet Samples
About 5 to 10 distinct yellow and green colonies from section Enrichment of Samples were carefully picked and purified on fresh sterile TCBS followed by nutrient agar plates that contained 3% NaCl. The recovered isolates were taken as presumptive Vibrio spp. The purified presumptive Vibrio spp. were grown overnight at 37°C on sterile nutrient agar plates and 20% glycerol stock of the pure isolates were then prepared and stored in −80°C for further analysis.
Molecular Identification of Presumptive Vibrio Isolates
Genomic DNA Extraction
The DNA extraction was carried out by following the boiling method procedure described by Maugeri et al. (22). An 18 h old culture incubated at 37°C was prepared on sterile nutrient agar plate from the stock culture of presumptive Vibrio isolates stored at −80°C and genomic DNA was extracted using boiling method. Single colonies were picked from the nutrient agar plates, suspended in a microfuge tube containing 200 μl of sterile distilled water and vortexed for even distribution of cells in the diluent. The cells were then lysed using AccuBlock (Digital dry bath, Labnet) for a period of 15 min at 100°C. The resulting solution was afterwards centrifuged at 110,000x g for 2 min using a MiniSpin microcentrifuge to remove the cell debris. The supernatant which is the cell lysate was then used as DNA templates in PCR assay.
Molecular Confirmation of Presumptive Vibrio Isolates
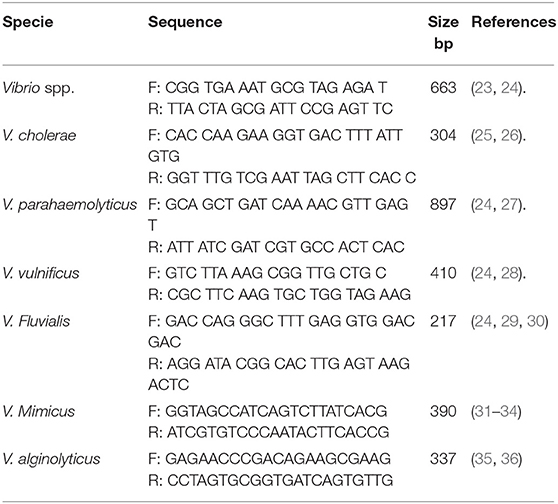
The confirmation of presumptive Vibrio isolates was done using Vibrio genus-specific primers that target the variable regions around position 700 and 1325 within 16S rRNA gene in a PCR assay. The cell lysate (5 μL) prepared in section Genomic DNA Extraction above was used in a 25 μl reaction containing 12.5 μl of one Taq 2X Master Mix Standard Buffer (BioLabs, UK), 1 μl of 10 μM of forward and reverse primer and 5.5 μl of nuclease-free water. The thermal cycler profile was a single enzyme activation for 15 min at 93°C followed by 35 cycles at 92°C for 40 s, 57°C for 1 min and 72°C for 1.5 min and a final extension at 75°C for 7 min. V. alginolyticus DSM 2171 was used as the positive control. The primer sequences, source of primer and expected amplicon size is presented in Table 2.
Speciation of Confirmed Vibrio Species
The confirmed Vibrio species were delineated into the six Vibrio spp. targeted in this study using species-specific primers. The primer sets that target conserved region of fla E, GroEl, ToxR, OmpW, vhm, and GyrB genes were used in PCR reactions to confirm Vibrio spp. as V. parahaemolyticus, V. vulnificus, V. fluvialis, V. cholerae, V. mimicus, and V. alginolyticus respectively. Duplex PCR protocol was developed for simultaneous identification of V. cholerae and V. mimicus while triplex PCR protocol was developed for simultaneous identification of V. vulnificus, V. fluvialis, and V. alginolyticus following the multiplex PCR protocol guide of Sint et al. (37) Briefly, DNA templates were extracted as stated in section Genomic DNA Extraction from positive controls. The duplex PCR reaction was made up of 12.5 μl Taq 2X Master Mix Standard Buffer (BioLabs, UK), 1 μl (0.2 μM) of forward and reverse primer set specific for each of the targeted species, 2.5 μl each of their DNA templates and 3.5 μl of nuclease-free water. The reaction mixture for the triplex PCR was made up of 12.5 μl Taq 2X Master Mix Standard Buffer (BioLabs, UK), 0.5 μl (0.2 μM) per forward and reverse primer set for each targeted species, 2.5 μl each of their DNA templates and 2 μl of nuclease-free water. The multiplex system was then tested in a gradient PCR with single extracts and a mix of all targeted species to determine the optimal annealing temperature using positive controls. The procedure was repeated severally to ascertain the reproducibility of the result. The multiplex PCR amplification efficiency, sensitivity, and primer specificity were tested following (37) procedure. Molecular Identification of V. parahaemolyticus was carried out using simplex PCR protocol in section Molecular Confirmation of Presumptive Vibrio isolates except that the annealing temperature employed was 64°C. The positive controls used were V. parahaemolyticus (DSM 10027), V. vulnificus (DSM 10143), V. fluvialis (DSM 19283), V. mimicus (DSM 19130), and V. alginolyticus (DSM 19130) and one locally isolated V. cholerae. The annealing temperatures selected for duplex and triplex PCR protocols, based on the gradient PCR outcomes, were 54.5°C and 66.3°C, respectively. These optimized conditions were used for the speciation of the confirmed Vibrio spp. into the six Vibrio species we target in this study. E. coli ATCC 35150 was used as the internal control for all PCR reactions. The primer sequences, the source of primer and expected amplicons sizes are presented in Table 2.
Statistical Analysis
The mean halophile and vibrio densities in limpet and source water samples were statistically compared using One-Way Analysis of Variance (ANOVA). Fisher's Least Significant Difference (LSD) post-hoc test was used to further compare and explore the significance of mean differences of the halophiles and Vibrio spp. densities in limpet and source water samples. The Statistical Package for Social Sciences (SPSS) version 20 was employed for the analysis. Also, the significant differences between the prevalence of each of the vibrio species we considered in this study amidst the population of confirmed Vibrio species isolated from limpet and source water samples were computed using 5000 Monte Carlo simulations tool of the XLSTAT evaluation 2018.1 software. The critical P value was set at 0.05.
Result
Total Halophiles and Total Vibrio Species Densities in Limpet and Source Water Samples
The mean total halophile and total Vibrio species densities observed for limpet samples and source water are presented in Figure 1 below while the significant levels (P values) for the statistical comparison of the mean halophiles and Vibrio species densities in the limpet samples and source water are presented in Table 3.
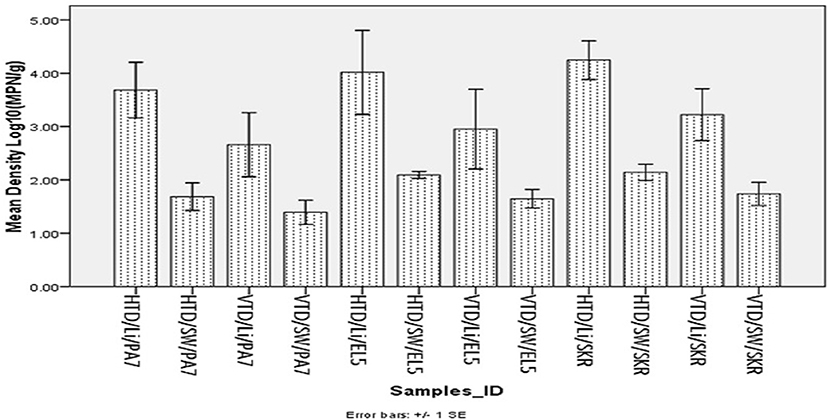
Figure 1. Mean density of halophile and Vibrio species in limpet and source water. Key: HTD, halophile total density; VTD, Vibrio spp. total density; Li, limpet sample; SW, limpet source water samples; PA7, Kowie estuarine; EL5, Buffalo estuarine; SKR, swartkops estuarine.
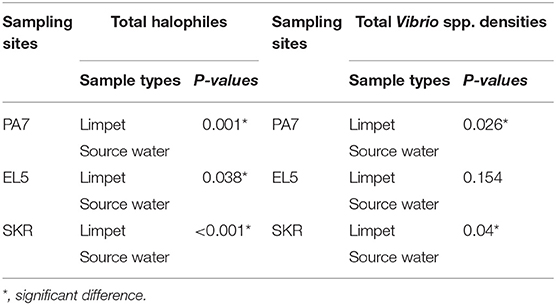
Table 3. Significant levels of the statistical comparison of halophiles and Vibrio spp. densities in limpet samples and source water samples.
The mean of total halophile density in limpet samples ranges from 3.69 ± 1.48 Log10 (MPN/g) in samples from PA7 to 4.25 ± 1.15 Log10 (MPN/g) in samples from SKR. In the limpet source water samples, the mean halophile density ranged between 1.69 ± 0.73 Log10 (MPN/g) in samples from PA7 and 2.14 ± 0.48 Log10 (MPN/g) in samples from SKR. The ranges of the mean of the total Vibrio species density in limpet and source water samples were 2.66 ± 1.7 Log10 (MPN/g) (limpet samples from PA7) to 3.22 ± 1.54 Log10 (MPN/g) (limpet samples from SKR) and 1.39 ± 0.64 Log10 (MPN/g) (source water samples from PA7) to 1.74 ± 0.69 Log10 (MPN/g) (source water samples from SKR) respectively. Of the samples from the three sites studied, lowest mean of the total halophile densities and mean of the total Vibrio species densities were observed in PA7 samples while the highest were observed in the samples collected at site SKR. The relatively high halophile and Vibrio species densities observed at site SKR could be as a result of high pollution observed at this site throughout the sampling period.
The densities of both halophiles and Vibrio species in limpet samples were consistently higher than that observed in sources water. This observation was statistically significant at P < 0.05 for all comparisons except for the densities of Vibrio species in limpet and source water samples from site EL5.
Pooled Prevalence of the Six Targeted Vibrio spp. in Limpet and Sources Water Samples From Site PA7, EL5, and Skr
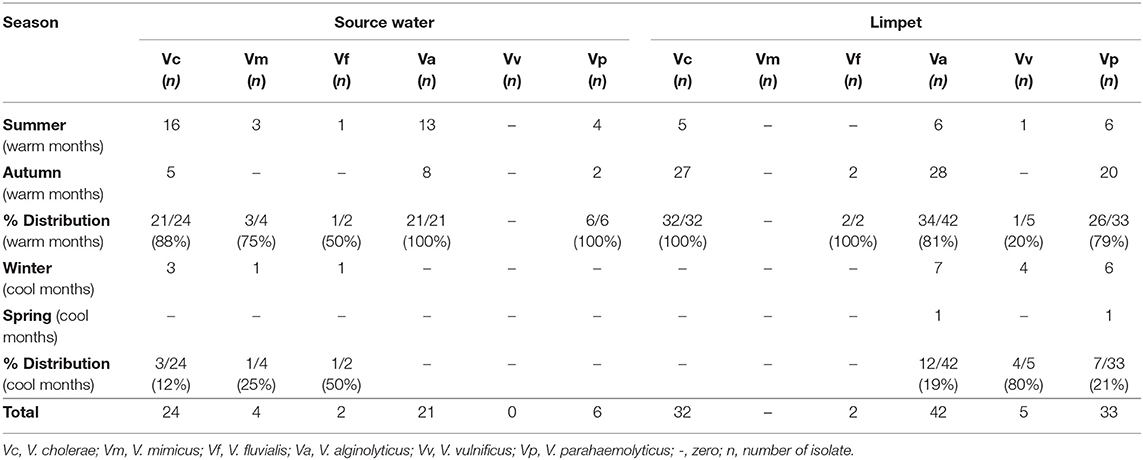
The prevalence of Vibrio spp. and each of the six targeted species in the presumptive Vibrio isolates recovered from all limpet samples (n = 197) and water samples (n = 193) is presented in Figure 2. The overall prevalence of Vibrio spp. was 84% (166/197) and 81% (156/193) in limpet and source water samples, respectively. The prevalence of each of the targeted species amidst the confirmed Vibrio spp. (n = 166) isolated from limpet samples was 19% (32/166), 0% (0/166), 25% (42/166), 1% (2/166), 3% (5/166), and 20% (33/166) for V. cholerae, V. mimicus, V. alginolyticus, V. fluvialis, V. vulnificus, and V. parahaemolyticus while the prevalence of each of the targeted species amidst the confirmed Vibrio spp. (n = 156) isolated from source water samples was 15% (24/156), 3% (4/156), 13% (21/156), 1% (2/156), 0% (0/161), and 4% (6/161) for V. cholerae, V. mimicus, V. alginolyticus, V. fluvialis, V. vulnificus, and V. parahaemolyticus respectively. The prevalence of V. alginolyticus, and V. parahaemolyticus in limpet and source water samples were significantly different (Figure 2) but the prevalence of the remaining four of the six targeted Vibrio species in the two samples were not significantly different at a critical P value of 0.05 (Figure 2). The distribution of the six Vibrio species targeted in this study per season is as presented in Table 4. The table showed that greater percentage of the Vibrio species were isolated during the warm period of our sampling regime.
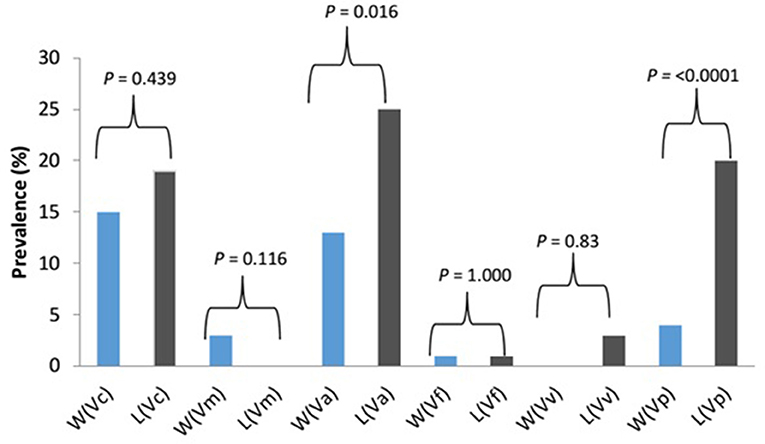
Figure 2. Prevalence and significant differences of six key vibrio pathogens in Vibrio species isolated from limpet and water samples. Key: W, water; L, limbet; Vc, V. cholerae; Vm, V. mimicus; Vf, V. fluvialis; Va, V. alginolyticus; Vv, V. vulnificus; Vp, V. parahaemolyticus; blue bars, prevalence in source water samples; black bars, prevalence in limbet samples.
The representative samples of electrophoresis gel pictures of PCR product of the amplification of Vibrio genus-specific region of the 16S rRNA gene is as shown in Figure 3 while electrophoresis gel pictures shown in Figures 4, 5 are for duplex PCR amplicons for the confirmation of V. cholerae, and V. mimicus; triplex PCR for the confirmation of V. alginolyticus, V. fluvialis, and V. vulnificus, respectively. An electrophoresis gel picture of the singleplex PCR product for the confirmation of V. parahaemolyticus is given in Figure 6.
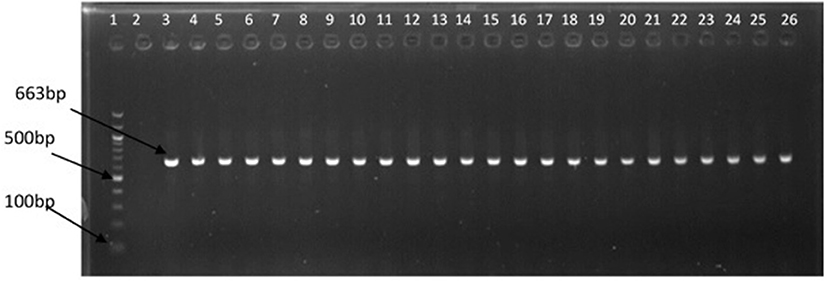
Figure 3. PCR products of the amplification of Vibrio specific region of 16S rRNA gene. Lane 1, molecular weight marker (100 bp); lane 2, negative control; lane 3, positive control (DSM 19130); lanes 4–26, positive isolates.
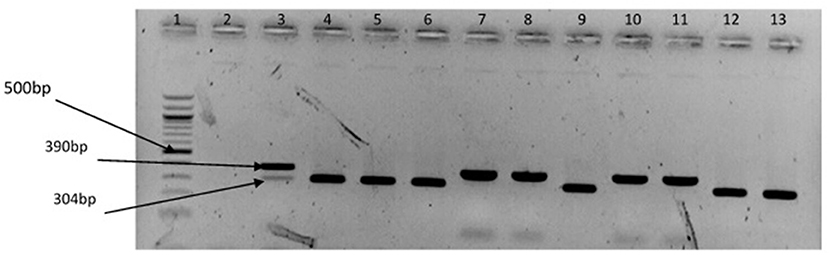
Figure 4. Duplex PCR products of the amplification of OmpW and vhm genes regions specific for V. cholerae and V. mimicus, respectively. Lane 1, molecular weight marker (100 bp); lane 2, negative control; lane 3, positive control (locally isolated V. cholerae and DSM 19130); lanes 4–6, 9, 12 &13, V. cholerae positive isolates; lanes 7 & 8, 10 &11, V. mimicus positive isolates.
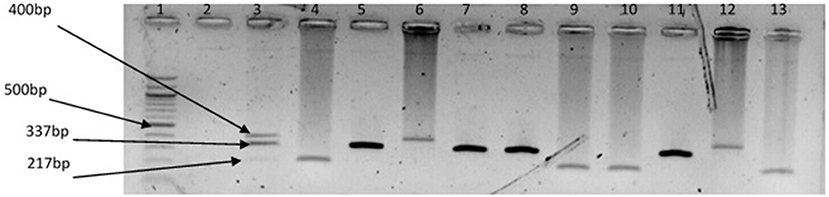
Figure 5. Triplex PCR products of the amplification of GroEl, ToxR, and GyrB genes regions specific for V. vulnificus, V. fluvialis, and V. alginolyticus, respectively. Lane 1, molecular weight marker (100 bp); lane 2, negative control; lane 3, positive control (DSM 19130, DSM 10143, DSM 19283); lanes 4, 9 &10, 13, V. fluvialis positive isolates; lanes 5, 7 & 8, 11, V. alginolyticus positive isolates; lanes 6 &12, V. vulnificus positive isolates.
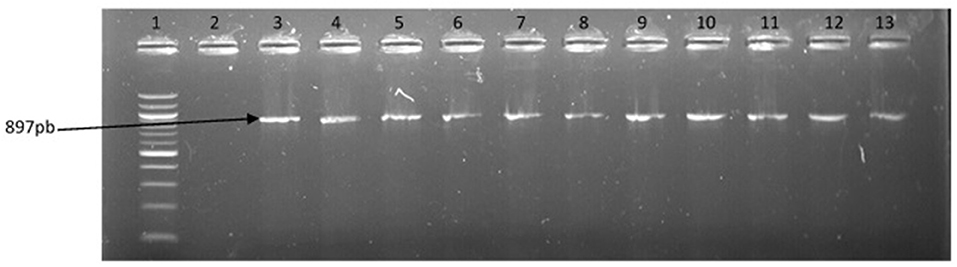
Figure 6. PCR products of the amplification of a region of fla E gene specific for V. parahaemolyticus. Lane 1, molecular weight marker (100 bp); lane 2, negative control; lane 3, positive control; lanes 4–13, positive isolates.
Discussion and Conclusion
Halophilic bacteria are a group of organism that has the ability to survive in the hypersaline environment (38). The occurrence of this group of bacteria and their involvement in human infections and the industrial application have been studied over the years (39–41). As an example, (42) reported a more cost-effective application of halophile in the bio-treatment of saline wastewater while gastroenteritis outbreak linked to halophile has been reported as far back as 1958 by Takikawa, in Yokohama, Japan. Although the occurrence of halophile in saline water has been well documented in the literature, the uniqueness of the finding of this study lies in the fact that halophile's density in limpet samples was significantly higher than that observed in the source water samples at the three sampling points (Table 3). A similar observation was made for Vibrio spp. except for the density of the bacteria in limpet samples and source water samples from site EL5 which were not significantly different (Table 3). This observation showed that limpet has the potential to concentrate halophile especially Vibrio species some of which have been implicated in various human infections such as cholera, cholera-like infections, wound infections, and gastroenteritis. The isolation of five (V. cholerae, V. fluvialis, V. alginolyticus, V. vulnificus, V. parahaemolyticus) out of the six key Vibrio pathogens investigated in this study from the limpet samples further confirmed the aforementioned. Literally, Figure 2 showed that the five Vibrio pathogens were more prevalent in limpet samples than they are in source water samples but this was only significant for V. parahaemolyticus and V. alginolyticus. This observation suggests that while limpets found in the sampling sites could serve as maintenance reservoir for V. parahaemolyticus and V. alginolyticus, they could serve as transient reservoir for V. cholerae, V. fluvialis, and V. vulnificus. These findings explain to an extent while V. parahaemolyticus and V. alginolyticus are the most reported etiological agents of foodborne infections associated with seafood (43). Interestingly, V. vulnificus was only isolated from limpet samples while V. mimicus was isolated from only the source water samples. This observation suggests that V. vulnificus in the aquatic environment studied better adapt and proliferate within limpet than in the limpet source water where they might be existing in the viable but nonculturable state (VBNC). Ability of V. vulnificus which is responsible for 95% of all seafood-associated fatality in the United States (44), to undergo viable but nonculturable state at slightest of unfavorable conditions in an attempt to withstands a whole lot of stress that could occur in aquatic milieu, has been severally reported (45, 46). The fact that limpet source water studied serves as receiving watershed for industrial final effluents, run-offs and different types of wastewater which when poorly treated becomes toxic to aquatic ecology (47–49) supports our assertion. On the other hand, the isolation of V. mimicus which was only in source water samples suggests that the limpet recovered from the sampling points are not concentrating the organism. Although the presence of virulence determinants in the six Vibrio species focused on in this study was not consider here, the fact that vibrio easily undergo inter and intragenic exchange of genetic materials via horizontal and gene transfer (50–52) makes them potentially dangerous pathogens which if manage to enter human population, could cause serious vibrio infection outbreaks. Furthermore, the distribution of the Vibrio spp. targeted in this study per season (Table 4) suggests that vibrio related infections will be common during warm season of the year in our sampling area. Outbreaks of vibrio infections commonly occur in the warm months of the year (53) therefore public health officers should be on a lookout for vibrio infections outbreaks during this period. The general populace need to be careful in the course of their recreational activities during this period as well.
It is worthy of note that the mollusk herein studied is at the lower trophic level of the aquatic food chain and thus will definitely contribute significantly to the occurrence, distribution and prevalence of any organism it is concentrating in aquatic animals such as crab, fish, water birds etc. that consume it at the higher trophic level of the food chain due to the process called biomagnification. Moreover, the finding of this study is of importance to public health and ecology of the aquatic environment since limpet serves as seafood recipe for human and an important member of the lower trophic level of the aquatic food chain (13, 54). Foodborne Vibrio infection outbreaks caused by species of Vibrio isolated from limpet samples processed in this study have been reported (55–60). It is important to note that cooking limpet at high temperature for 30 sec or at low temperature as recommended by Fitzgerald (11) will increase chances of contracting vibrio infection from vibrio contaminated limpet. This is because the cooking temperature is either sub-leather to the pathogens or the cooking duration is not long enough to achieve total destruction of all Vibrio pathogen cells that may be present in the limpet.
Therefore, in the best interest of the public health and aquatic ecology, we recommend more studies on the role that limpet, an abundant mollusc in South Africa aquatic environment, plays in the maintenance of pathogens in the aquatic milieu. It is anticipated that the information from such studies will shed more light on other possible diseases outbreaks aside cholera and vibriosis, which could emanate from the consumption of limpet recipe.
Finally, this is the first study that attempts differentiating between transient and maintenance reservoir of pathogens by employing the combination of MPN-PCR method and statistical analysis. Furthermore, although isolation of key Vibrio pathogens from various types of mollusc has been severally reported (5, 19, 31, 61–63), to the best of our knowledge no report has implicated limpet. Since a comparative high abundance of Vibrio spp. which was statistically significant was observed in limpet than in source water in this study, and that the five of the six Vibrio pathogens targeted in this study were more prevalent in limpet than in source water samples, we concluded that limpets found in the estuaries are not just a transient reservoirs but a maintenance reservoirs for Vibrio species.
Ethics Statement
The animal samples used in this study were invertebrates from the wild and the samples were purchased from fishermen. Also, the quantity sampled per time (10–15 pieces) could not be considered to affect the population of organism in the wild and limpet (Scutellastra cochlear) is not an endangered species.
Author Contributions
AO initiated the research topic, provided materials for the study, and proof-read the manuscript. OA structured the methods used in the study, carried out the experiments, and write the manuscript.
Funding
South Africa Medical Research Council (SAMRC) and Water Research Commission (WRC K5-2432).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Alam MT, Weppelmann TA, Weber CD, Johnson JA, Rashid MH, Birch CS, et al. Monitoring water sources for environmental reservoirs of toxigenic Vibrio cholerae O1, Haiti. Emerg Infect Dis. (2014) 20:356–63. doi: 10.3201/eid2003.131293
2. Stanislas R, Renaud P. Monitoring water sources for environmental reservoirs of toxigenic Vibrio Cholerae O1. (2015) 21:169–70. doi: 10.3201/eid2101.140627
3. Morris JG Jr. Cholera–modern pandemic disease of ancient lineage. Emerg Infect Dis. (2011) 17:2099–104. doi: 10.3201/eid1711.111109
4. Garrido-Maestu A, Lozano-León A, Rodríguez-Souto RR, Vieites-Maneiro R, Chapela MJ, Cabado AG. Presence of pathogenic Vibrio species in fresh mussels harvested in the southern Rias of Galicia (NW Spain). Food Control (2016) 59:759–65. doi: 10.1016/j.foodcont.2015.06.054
5. Lopez-Joven C, de Blas I, Furones MD, Roque A. Prevalences of pathogenic and non-pathogenic Vibrio parahaemolyticus in mollusks from the Spanish Mediterranean Coast. Front Microbiol. (2015) 6:736. doi: 10.3389/fmicb.2015.00736
6. Lopatek M, Wieczorek K, Osek J. Prevalence and antimicrobial resistance of Vibrio parahaemolyticus isolated from raw shellfish in Poland. J Food Protect. (2015) 78:1029–33. doi: 10.4315/0362-028X.JFP-14-437
7. Mannas H, Mimouni R, Chaouqy N, Hamadi F, Martinez-Urtaza J. Occurrence of Vibrio and Salmonella species in mussels (Mytilus galloprovincialis) collected along the Moroccan Atlantic coast. SpringerPlus (2014) 3:265. doi: 10.1186/2193-1801-3-265
8. Bakr WM, Hazzah WA, Abaza AF. Detection of Salmonella and Vibrio species in some seafood in Alexandria. J Am. Sci. (2011) 7:663–8.
9. MolluscaBase. Scutellastra cochlear (Born, 1778). Accessed through: World Register of Marine Species. (2018). Available online at: http://marinespecies.org/aphia.php?p=taxdetails&id=456669 on 2018-03-20
10. Maneveldt GW, Keats DW. Effects of herbivore grazing on the physiognomy of the coralline alga Spongites yendoi and on associated competitive interactions. African J Mar Sci. (2008) 30:581–93. doi: 10.2989/AJMS.2008.30.3.11.645
11. Fitzgerald A. Slipper Limpet Utilisation and Management, Final Report. Port of Truro Oyster Management Group (Cornwall) (2007).
12. Beachcomber Guide (undated). The Amazing Limpets of South Africa. Available online at: http://beachcomberguide.co.za/index.php/general-rss/29-amazing-limpets-south-africa
13. Limpet. New World Encyclopedia, 7 Aug 2014, 17:24 UTC. 1 Feb 2018, 05:26 (2014) Available online at: http://www.newworldencyclopedia.org/p/index.php?title=Limpet&oldid=983527
14. Home (undated). Limpet at A-Frame. https://www.ispotnature.org/communities/southern-africa/view/observation/607769/limpet-at-a-frame [Accessed March 20, 2018].
15. Patella (Scutellastra) cochlear. http://www.gastropods.com/1/Shell_1261.shtml [Accessed March 20, 2018].
16. Encyclopedia of Life (undated). Pear Limpet - Scutellastra cochlear – Overview. http://eol.org/pages/4792779/overview [Accessed March 20, 2018].
17. Noorlis A, Ghazali FM, Cheah YK, Tuan Zainazor TC, Ponniah J, Tunung R, et al. Prevalence and quantification of Vibrio species and Vibrio parahaemolyticus in freshwater fish at hypermarket level. Int Food Res J. (2011) 18:689–95. Available online at: http://www.ifrj.upm.edu.my/18%20(02)%202011/(31)%20IFRJ-2010-266.pdf
18. Copin S, Robert-Pillot A, Malle P, Quilici ML, Gay M. Evaluation of most-probable-number–PCR method with internal amplification control for the counting of total and pathogenic Vibrio parahaemolyticus in frozen shrimps. J Food Protect. (2012) 75:150–3. doi: 10.4315/0362-028X.JFP-11-165
19. Ramos RJ, Miotto LA, Miotto M, Silveira Junior N, Cirolini A, Silva HSD, et al. Occurrence of potentially pathogenic Vibrio in oysters (Crassostrea gigas) and waters from bivalve mollusk cultivations in the South Bay of Santa Catarina. Rev Soc Brasil Med Trop. (2014) 47:327–33. doi: 10.1590/0037-8682-0069-2014
20. Tunung R, Margaret SP, Jeyaletchumi P, Chai LC, Tuan Zainazor TC, Ghazali FM, et al. Prevalence and quantification of Vibrio parahaemolyticus in raw salad vegetables at retail level. J Microbiol Biotechnol. (2010) 20:391–6.
21. Ntema VM. Detection of Vibrio cholerae and Vibrio Parahaemolyticus by Molecular and Culture Methods From Source Water to Household Container-Stored Water at the Point-of-use in Rural Vhembe Communities in South Africa. Doctoral dissertation, University of Johannesburg (2009).
22. Maugeri TL, Carbone M, Fera MT, Gugliandolo C. Detection and differentiation of Vibrio vulnificus in seawater and plankton of a coastal zone of the Mediterranean Sea. Res Microbiol. (2006) 157:194–200. doi: 10.1016/j.resmic.2005.06.007
23. Kwok AY, Wilson JT, Coulthart M, Ng LK, Mutharia L, Chow AW. Phylogenetic study and identification of human pathogenic Vibrio species based on partial hsp60gene sequences. Can J Micriobiol. (2002) 48:903–10. doi: 10.1139/w02-089
24. Okoh AI, Sibanda T, Nongogo V, Adefisoye M, Olayemi OO, Nontongana N. Prevalence and characterisation of non-cholerae Vibrio spp. in final effluents of wastewater treatment facilities in two districts of the Eastern Cape Province of South Africa: implications for public health. Environ Sci Poll Res. (2015) 22:2008–17. doi: 10.1007/s11356-014-3461-z
25. Goel AK, Jain M, kumar P, Kamboj DV, Singh L. Virulence profile and clonal relationship among the Vibrio cholera isolates from ground and surface water in a cholera endemic area during rainy season. Folia Microbiol. (2010) 55:69–74. doi: 10.1007/s12223-010-0011-z
26. Menezes FGRD, Neves SDS, Sousa OVD, Vila-Nova CMVM, Maggioni R, Theophilo GND, et al. Detection of virulence genes in environmental strains of Vibrio cholerae from estuaries in northeastern Brazil. Rev Inst Med Trop Sao Paulo. (2014). 56:427–32. doi: 10.1590/S0036-46652014000500010
27. Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp CA, Strockbine NA. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol. (2007) 45:134–40. doi: 10.1128/JCM.01544-06
28. Wong RS, Chow AW. Identification of enteric pathogens by heat shock protein 60 kDa (HSP60) gene sequesnces. FEMS Microbiol Lett. (2002) 206:107–13. doi: 10.1111/j.1574-6968.2002.tb10994.x
29. Osorio CR, Klose KE. A region of the transmembrane regulatory protein ToxR that tethers the transcriptional activation domain to the cytoplasmic membrane displays wide divergence amongVibrio species. J Bacteriol. (2000) 182:526–8. doi: 10.1128/JB.182.2.526-528.2000
30. Chakraborty R, Sinha S, Mukhopadhyay AK, Asakura M, Yamasaki S, Bhattacharya SK, et al. Species-specific identification of Vibrio fluvialis by PCR targeted to the conserved transcriptional activation and variable membrane tether regions of the toxR gene. J Med Microbiol. (2006) 55:805–8. doi: 10.1099/jmm.0.46395-0
31. Guardiola-Avila I, Noriega-Orozco L, Acedo-Félix E, Lara AE, Tapia-Olea MM. Presence of the hemolysin gene of Vibrio mimicus in fish and seafood products in Sonora, México. J Food Res. (2014) 4:66. doi: 10.5539/jfr.v4n1p66
32. Shinoda S, Nakagawa T, Shi L, Bi K, Kanoh Y, Tomochika K, et al. Distribution of virulence-associated genes in Vibrio mimicus isolates from clinical and environmental origins. Microbiol Immunol. (2004) 48:547–51. doi: 10.1111/j.1348-0421.2004.tb03551
33. Sultan Z, Mizuno T, Sakurai A, Takata N, Okamoto K, Miyoshi S. Growth phase dependant activation of the precursor of Vibrio mimicus hemolysin (Pro-VMH). J Health Sci. (2007) 53:430–4. doi: 10.1248/jhs.53.430
34. Shi L, Miyoshi S, Bi K, Nakamura M, Hiura M, Tomochika K, Shinoda S. Presence of hemolysin genes (vmh, tdh and hlx) in isolates of Vibrio mimicus determined by polymerase chain reaction. J Health (2000) 46:63–5. doi: 10.1248/jhs.46.63
35. Shuang W, Hui Z, Yuyin X, Hussain MK, Xiyang W. Multiplex PCR assays for the dectection of Vibrio alginolyticus, Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio chokerae with an internal amplification control. Diag Infect Dis. (2014) 79:115–8. doi: 10.1016/j.diagmicrobio.2014.03.012
36. Zhou S, Hou Z, Li N, Qin Q. Development of a SYBR Green I real-time PCR for quantitative detection of Vibrio alginolyticus in seawater and seafood. J Appl Microbiol. (2007) 103:1897–906. doi: 10.1111/j.1365-2672.2007.03420.x
37. Sint D, Raso L, Traugott M. Advances in multiplex PCR: balancing primer efficiencies and improving detection success. Methods Ecol Evol. (2012) 3:898–905. doi: 10.1111/j.2041-210X.2012.00215.x
38. Nguyen A. Isolation, Characterization, and Antibiotic Susceptibility Testing of Halophilic Bacteria from the Grand Saline Salt Marsh. Doctoral dissertation, Baylor University, WACO (2015).
39. Sarwar MK, Azam I, Iqbal T. Biology and applications of halophilic bacteria and archaea: a review. Electron J Biol. (2015) 11:98–103.
40. Miyamoto Y, Nakamura K, Takizawa K. Seasonal distribution of Oceanomonas spp., halophilic bacteria, in the coastal sea. Its significance in epidemiology and marine industry. Jap J Microbiol. (1962) 6:141–58. doi: 10.1111/j.1348-0421.1962.tb00231.x
42. Kargi F. Enhanced biological treatment of saline wastewater by using halophilic bacteria. Biotechnol Lett. (2002) 24:1569–72. doi: 10.1023/A:1020379421917
43. CDC-COVIS. Summary of Human Vibrio Cases Reported to CDC, 2012. National Enteric Disease Surveillance: COVIS Annual Summary (2009–2014).
44. Nowakowska J, Oliver JD. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol Ecol. (2013) 84:213–22. doi: 10.1111/1574-6941.12052
45. Oliver JD. The Viable but Nonculturable State and Cellular Resuscitation. Microbial Biosystems: New Frontiers. Halifax: Atlantic Canada Society for Microbial Ecology (2000), p. 723–30.
46. Zhao X, Zhong J, Wei C, Lin CW, Ding T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front Microbiol. (2017) 8:580. doi: 10.3389/fmicb.2017.00580
47. Akpor OB. “Wastewater effluent discharge: effects and treatment processes,” In: International Conference on Chemical, Biological and Environmental Engineering. Singapore (2011). p. 85–91.
48. Obire O, Ogan A, Okigbo RN. Impact of fertilizer plant effluent on water quality. Int. J. Environ. Sci. Technol. (2008) 5:107–18. doi: 10.1007/BF03326003
49. Emmanuel E, Perrodin Y, Keck G, Blanchard JM, Vermande P. “Effects of hospital wastewater on aquatic ecosystem,” In: Proceedings of the XXVIII Congreso Interamericano de Ingenieria Sanitaria y Ambiental. Cancun (2002) p. 27–31. Available online at: https://pdfs.semanticscholar.org/60ed/95e04abb895351c6781ff0bee70869e5fff9.pdf
50. Zahid HM, Mahal Z, Chowdhury MR. Intergenic and intragenic conjugal transfer of multiple antibiotic resistance determinants among bacteria in the aquatic environment of Bangladesh. Afr J Biotechnol. (2009) 8:155–60.
51. Li M, Kotetishvili M, Chen Y, Sozhamannan S. Comparative genomic analyses of the vibrio pathogenicity island and cholera toxin prophage regions in nonepidemic serogroup strains of Vibrio cholerae. Appl Environ Microbiol. (2003) 69:1728–38. doi: 10.1128/AEM.69.3.1728-1738.2003
52. Chakraborty S, Mukhopadhyay AK, Bhadra RK, Ghosh AN, Mitra R, Shimada T, et al. Virulence genes in environmental strains ofVibrio cholerae. Appl Environ Microbiol. (2000) 66:4022–8. doi: 10.1128/AEM.66.9.4022-4028.2000
53. Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis. (2008) 46:970–6. doi: 10.1086/529148
54. House Leisure A Recipe for Limpets Cooked in Vermouth. Available online at: https://www.houseandleisure.co.za/food/vermouth-limpets/ (Accessed March (2016) 20, 2018).
55. CDC. Increase in Vibrio Parahaemolyticus Illnesses Associated With Consumption of Shellfish From Several Atlantic Coast Harvest Areas, United States, 2013. (2017). Available online at: https://www.cdc.gov/vibrio/investigations/vibriop-09-13/index.html
56. Gonzalez-Escalona N, Gavilan RG, Toro M, Zamudio ML, Martinez-Urtaza J. Outbreak of Vibrio parahaemolyticus sequence type 120, Peru, 2009. Emerg Infect Dis. (2016) 22:1235. doi: 10.3201/eid2207.151896
57. Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. (2015) 7:5901. doi: 10.4081/idr.2015.5901
58. McLaughlin JB, DePaola A, Bopp CA, Martinek KA, Napolilli NP, Allison CG, et al. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med. (2005) 353:1463–70. doi: 10.1056/NEJMoa051594
59. Rabbani GH, Greenough III WB. Food as a vehicle of transmission of cholera. J Diarrhoeal Dis Res. (1999) 17:1–9.
60. Albert MJ, Neira M, Motarjemi Y. The role of food in the epidemiology of cholera. world health statistics quarterly. Rapport Trimestriel Statistiques Sanitaires Mondiales (1997) 50:111–8.
61. Romalde JL, Diéguez AL, Lasa A, Balboa S. New Vibrio species associated to molluscan microbiota: a review. Front Microbiol. (2014) 4:413. doi: 10.3389/fmicb.2013.00413
62. Beaz-Hidalgo R, Balboa S, Romalde JL, Figueras MJ. Diversity and pathogenecity of Vibrio species in cultured bivalve molluscs. Environ Microbiol Rep. (2010) 2:34–43. doi: 10.1111/j.1758-2229.2010.00135.x
Keywords: public-health, MPN-PCR, reservoir types, Vibrio species, limpet, prevalence, transient reservior, maintanance reservoir
Citation: Abioye OE and Okoh AI (2018) Limpet (Scutellastra cochlear) Recovered From Some Estuaries in the Eastern Cape Province, South Africa Act as Reservoirs of Pathogenic Vibrio Species. Front. Public Health 6:237. doi: 10.3389/fpubh.2018.00237
Received: 03 April 2018; Accepted: 08 August 2018;
Published: 31 August 2018.
Edited by:
Nur A. Hasan, University of Maryland, United StatesReviewed by:
Durg Vijai Singh, Institute of Life Sciences (ILS), IndiaYan Boucher, University of Alberta, Canada
Copyright © 2018 Abioye and Okoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oluwatayo E. Abioye, YWJpb3lldGhheW9yQGdtYWlsLmNvbQ==
 Oluwatayo E. Abioye
Oluwatayo E. Abioye Anthony I. Okoh
Anthony I. Okoh