- 1Epidemiology Department, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA, United States
- 2Stanley S. Scott Cancer Center, Louisiana State University Health Sciences Center School of Medicine, New Orleans, LA, United States
- 3Windward Islands Research and Education Foundation, St. George's University, St. George's, Grenada
- 4Behavioral and Community Health Sciences Department, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA, United States
- 5Department of Pediatrics, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA, United States
Background: Low- and middle-income countries are affected disproportionately by the ongoing global obesity pandemic. Representing a middle income country, the high prevalence of obesity among Grenadian adults as compared to US adults is expected as part of global obesity trends. The objective of this study was to determine if Grenadian adolescents have a higher prevalence of overweight compared to their US counterparts, and if a disparity exists between urban and rural adolescents.
Methods: Using a subcohort of participants in the Grenadian Nutrition Student Survey, diet quality and anthropometric measures were collected from 55% of the classrooms of first year secondary students in Grenada (n = 639). Rural or urban designations were given to each school. Body Mass Index (BMI) was calculated and categorized as overweight or obese for each student following CDC classification cutoffs. A standardized BMI (BMIz) was calculated for each school. Sex-specific BMI and overall BMIz were compared to a 1980s US cohort. Multilevel models, overall and stratified by sex, of students nested within schools were conducted to determine if BMIz differed by rural or urban locality, gender, and diet quality.
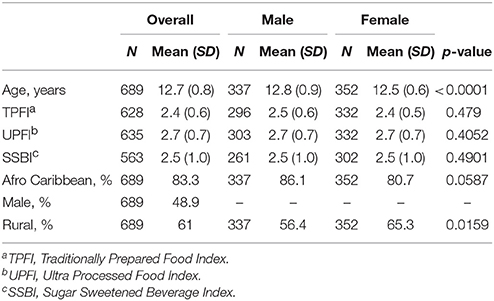
Results: The mean age of this cohort was 12.7 (SD = 0.8) years with 83.8% of the cohort identifying as Afro-Caribbean. Females had nearly twice the prevalence of overweight when compared to males (22.7 vs. 12.2%) but a similar prevalence of obesity (8.2 vs. 6.8%). Grenadian adolescents had lower prevalence of overweight (females: 22.7 vs. 44.7%; males: 12.2 vs. 38.8%, respectively) as compared to US counterparts. Eating a traditional diet was negatively associated with BMIz score among females (β^ = −0.395; SE = 0.123) in a stratified, multilevel analysis. BMIz scores did not differ significantly by rural or urban school designation.
Conclusions: Among Grenadian adolescents, this study identified a lower overweight prevalence compared to US counterparts and no difference in overweight prevalence by urban or rural location. We hypothesize that the late introduction of processed foods to Grenada protected this cohort from obesogenic promoters due to a lack of fetal overnutrition. However, further research in subsequent birth cohorts is needed to determine if adolescent obesity will increase due to a generational effect.
Introduction
The epidemiologic transition from communicable disease and nutritional deficiency to non-communicable disease is occurring in low- and middle-income countries (LMIC) (1). It is widely recognized that this transition is primarily the result of the global obesity pandemic leading to increased rates of cardiovascular disease, diabetes and cancer (1, 2). Developed countries underwent the epidemiologic transition decades ago, and indications of the forthcoming obesity pandemic were seen as early as the 1980s (3). Since then, obesity prevalence has doubled among adults and tripled among children in the United States (US) (4). These increases of obesity highlight the dietary and social changes the US and other developed countries underwent in the last half of the 20th century. Furthermore the obesity pandemic is widely attributed to a global infrastructure that makes highly processed foods widely available in terms of cost and access (5). The proliferation of industrialized food processing, as well as advances in technology that allow for decreased physical activity, have largely been held as the main reasons behind the pandemic (6–9). These obesogenic lifestyle changes have been proliferating globally since normalizing in developed countries, and the health effects are just now being realized in LMIC (3, 5).
In an effort to understand societal exposures driving the global pandemic, Monteiro and Cannon (10) suggest the widespread availability of ultra-processed foods was temporally associated with the obesity epidemic in Brazil. They demonstrated the dramatic rise in obesity in Brazil during the 1990s was not associated with any particular nutrient (e.g., sugar, fat) but with replacement of minimally processed, traditionally prepared foods with the ultra-processed foods produced by transnational food companies (11, 12). The rapid globalization of the corporate food infrastructure over the past decades has made the introduction of ultra-processed foods occur more rapidly in LMIC compared to what was seen with their introduction in high income countries in earlier decades (12). From 1998 to 2012, LMIC more than doubled their spending on processed foods and quadrupled spending on soft drinks. Relative growth of sales of these processed foods had an inverse relationship with gross national income, indicating LMIC increase spending on these products annually (13). Growth in processed food sales have positive implications for the prevalence of underweight, which is declining, but conversely negative implications for prevalence of overweight, which continues to increase worldwide (13). This may account for the rapid transitions now occurring in many LMIC countries.
Studies reviewing childhood obesity trends among LMIC in the Caribbean are inconsistent. The prevalence of overweight/obese (OW/OB) increased from 8.52% in 1981 to 32.5% in 2010 among 9–11 year olds in Barbados, and a slightly older age group (14–19 year olds) in Jamaica had a similar prevalence of OW/OB of 35.5% in 2007 (14, 15). Conversely, in a cohort of 7–18 year olds in Trinidad, only 17% were OW/OB in 2010 (16). These discrepancies in childhood obesity prevalence in the Caribbean may be explained by differences in country income. Out of these three countries, Trinidad has experienced the greatest growth in income over the past few decades, almost double that of Jamaica and five times that of Barbados (17). After reviewing the findings of Monteiro and colleagues mentioned previously, Trinidad may have concurrently experienced decreases or stagnation in spending on processed foods, explaining why childhood obesity prevalence is lowest in this country among the three (13).
The built environment is also an important factor to assess when measuring obesity. In high-income countries, the prevalence of obesity is higher in rural areas as compared to urban (18). With the development of LMIC resulting in the introduction of ultra-processed foods, investigators are seeing the burden of obesity shifting to or equalizing with urban areas (19). A recent study determined that Nicaraguan urban overweight prevalence experienced greater increases than rural, with an annualized change in the prevalence of overweight in the 1990s−2000s of 2.33 vs. 1.00, respectively (20). Yet, in other countries, such as Haiti, increases in obesity prevalence have stabilized between urban and rural environments (0.73 vs. 0.73) (20). This inconclusive information regarding the obesity prevalence disparity between urban and rural environments within LMIC warrants further investigation.
Since 1990, Latin America and the Caribbean have experienced an average increase of 0.31% in OW/OB prevalence annually (20). Grenada represents a LMIC currently undergoing the epidemiologic transition, of which increases in OW/OB prevalence are a direct side effect (21). However, there have been inconsistent findings regarding obesity prevalence among the Grenada population. A recent study indicates the prevalence of overweight and obesity (OW/OB) among adult Grenadians is 57%, which is comparable to US black adults (22). In contrast, a report conducted by the Caribbean Food and Nutrition Institute demonstrated low rates of OW/OB among a sample of Grenadian pre-school children compared to US counterparts (23). Research to determine if Grenadian adolescent obesity has a higher prevalence of OW/OB as compared to a similar US cohort is lacking.
Given these contradictory reports, the objective of the present study was to determine if Grenadian adolescents have a higher prevalence of OW/OB compared to their US counterparts, and if a disparity exists between urban and rural adolescents.
Materials and Methods
Study Participants
In an effort to develop policies to prevent obesity among youth, the Windward Islands Research and Education Foundation (WINDREF) at St. George's University (SGU) conducted a nutrition survey of Grenadian adolescents called the Grenada School Nutrition Study (GSNS). The current study cohort is a subset of adolescents who participated in the GSNS. Institutional review boards at both SGU and Louisiana State University Health Sciences Center approved the protocols. At least one classroom of first year secondary students (i.e., Form 1) was sampled from each of the 23 Grenadian secondary schools. Although the target age range was 11–14 years old, all students within a given class were eligible to participate regardless of age. During the 2012–2013 academic year, 1,004 students were sampled from 35 classrooms, constituting approximately 55% of all Form 1 students in Grenada. Parental refusals (n = 276) and students who were absent or did not complete all the assessments or surveys (n = 39) resulted in a final study cohort of 689 students.
Data Collection
Students from two schools were recruited each week to participate in a demographic survey, an anthropometric assessment, and a food frequency questionnaire specific to the Grenadian diet, adapted from the Eating Habits Questionnaire (EHQ) (24). Assessments and surveys were administered on two successive days in a classroom, activity room, or gym that was allocated by each school for the project. The surveys were interviewer assisted. A team of interventionists implemented the procedures simultaneously. Each interventionist was certified by GSNS co-investigators to ensure performance standards were met. All procedures, testing methods and measurement tools were pilot tested and revised as necessary prior to final data collection. On day 1, anthropometric data (e.g., height, weight, waist circumference) were collected, and nutrition information, such as portion size, was provided to the student, which was necessary to ensure accurate completion of the food frequency questionnaire. On day 2, the demographic survey (including medical/family history) and food frequency questionnaire were administered. Scheduling of data collection days avoided dates subject to potential seasonal biases related to holidays, exams, and school events. Rural and urban designations were defined at the school level, with students attending school in the two largest towns in Grenada (St. Georges and Grenville) defined as urban students.
Anthropometric Measures
Height, weight, and waist circumference were directly measured for each student in a private setting (e.g., trifold blind booth). The students wore school uniforms and were asked to remove all outer clothing, heavy pocket items, and shoes. Height was measured twice using a portable stadiometer (Shorr Productions, Olney, Maryland) and weight was measured three times using an electronic scale (Seca 880, Seca Corporation, Hanover, Maryland), with both devices standardized before each session. The average of both measurements was used for analysis. The reliability of these measurements were established previously (25). Body Mass Index (BMI), calculated by dividing participants' weight in kilograms by height in meters squared, was used to determine excess body weight. The CDC age- and sex-specific cutoffs were used to characterize overweight (OW, BMI ≥ 85th percentile) and obese (OB, BMI ≥ 95th percentile) adolescents in terms of their standardized percentile (26). Furthermore, a standardized BMI (BMIz) for each school was calculated using the above mentioned classification system. CDC classification cutoffs are based on growth charts derived from National Health and Nutrition Examination Survey (NHANES) II (1976–1980) and NHANES III (1988–1994) (27). By using these CDC classification cutoffs, we are considering this cohort (NHANES 1976–1980, NHANES III 1988–1994) as our comparison US adolescent cohort.
Diet Quality Measures
Diet quality indexes were derived from the food frequency questionnaire administered to all students. Several indexes of diet quality were developed including: the Traditional Prepared Food Index (TPFI), the Ultra Processed Food Index (UPFI), and the Sugar Sweetened Beverage Index (SSBI). The food frequency questionnaire was adapted from a previously validated food frequency questionnaire (EHQ) for use in a West Indian population to make it culturally appropriate in terms of inclusion of local dietary items (e.g., callaloo soup), use of proper dietary terms for local foods, and food models depicting typical foods and serving sizes (24). The TPFI was an index of the average number of times per week (i.e., 1 = never eaten; 2 = not eaten last week; 3 = 1–2 times per week; 4 = 3–6 times per week and 5 = almost every day) students stated they had eaten the following seven common traditional prepared foods including: porridge, oil down, pelau, fish broth, cou cou, callaloo, and provisions. The UPFI was an index of the average number of times per week students stated they had eaten the following six common ultra-processed foods including: lunch meat, hot dogs, ice cream, candy bars, presweetened cereal, and potato chips. The UPFI was adapted from processed food classifications defined by Monteiro et al. (13). The SSBI was an index of the average number of times per week students stated they drank regular soda and/or fruit flavored packaged drinks.
Statistical Analysis
All analyses were conducted using SAS statistical software package version 9.4. Summary statistics were produced using either the MEANS or FREQ procedures. Demographic characteristics, diet quality measures, and anthropometrics measures were conducted for the overall study population and also stratified by gender. Differences between genders were established using Wald tests from the MIXED procedure for continuous variables and Chi-Square Tests for Independence for categorical variables. Multilevel models of students nested within schools were developed to characterize the overall and between school BMIz across Grenada. Data were analyzed implementing random intercept models using the MIXED procedure. The outcome modeled was BMIz as a continuous variable. An empty model and various combinations of individual and multilevel measures were analyzed. Individual level variables consisted of age, gender, race, and various indices of diet quality (i.e., TPFI, UPFI, and SSBI). The only multilevel variable considered was urban/rural designation. Another multilevel analysis adjusting for variables found to be significant in the saturated multilevel model was stratified by gender. When a significant interaction between a categorical and quantitative variable was observed, a secondary analysis was conducted to investigate the estimated effect of the quantitative variable within each level of the categorical variable. Significance was defined at p < 0.05.
Results
Study Population Demographics
The average age of the students was 12.7 years (standard deviation (SD) = 0.8) (Table 1). Male students, on average, were significantly older than female (12.8 vs. 12.5 years old, p < 0.0001). In terms of ethnicity, 83.3% of the students identified as Afro Caribbean. Female students were more likely to attend rural schools. There were no statistically significant differences between males and females in terms of the diet quality items.
Overweight/Obese Prevalence
The overall prevalence of OW/OB among Form 1 Grenadian adolescents was 17.6 and 7.6%, respectively (Table 2). Females were at a statistically significant greater risk of being OW than males (i.e., 22.7 vs. 12.2%), indicating a gender difference in the prevalence of OW/OB among Grenadian students. OB prevalence and waist circumference were not significantly different between male and female students. Grenadian female and male adolescents are less OW/OB than their US counterparts, with American girls experiencing an OW/OB prevalence of 44.7% vs. Grenadian girls at 22.7% and American boys experiencing an OW/OB prevalence of 38.8% vs. Grenadian boys at 12.2% (27). In addition, Grenadian adolescents had a significantly lower mean BMIz (−0.097; standard error (SE) = 0.045) compared with the US median BMI established using NHANES data from the 1980s (27).
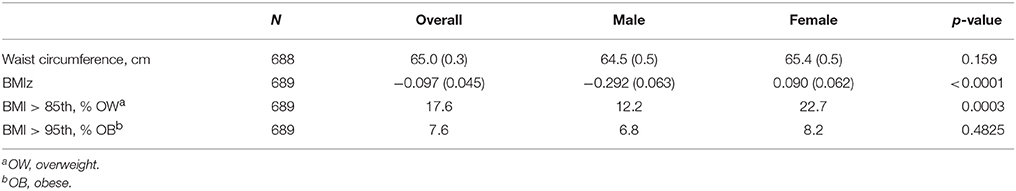
Table 2. Mean (± SE) and prevalence of anthropometric measures for Grenadian adolescents overall and by gender.
Between School BMIz
An empty multilevel model (Table 3) with obesity risk as the outcome yielded an intraclass correlation coefficient of 0.051 indicating that 5.1% of the variance in BMIz was between schools. Adding age ( = −0.123; SE = 0.059) and male gender ( = −0.323; SE = 0.095) into the model explained most of the school level effect. Male gender was negatively associated with BMIz score. Adding urban or rural location of each school did not significantly predict BMIz and only explained a small amount of the school level effect (results not shown).
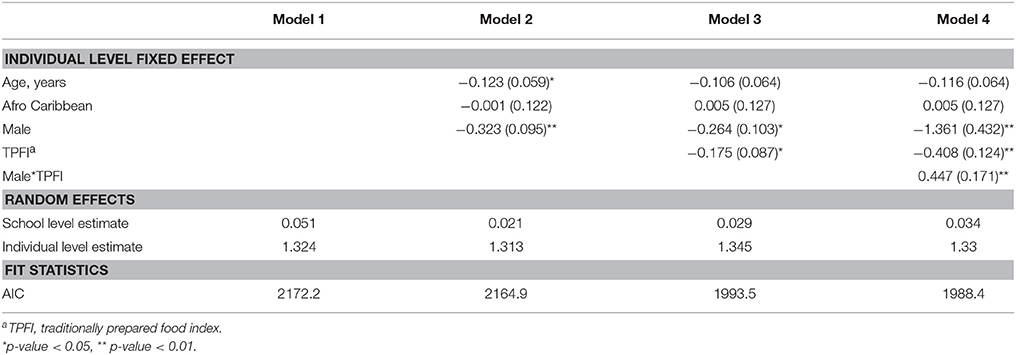
Table 3. Parameter estimates (±SE) from multilevel models predicting BMIz among Grenada School Nutrition Study students nested within 23 schools, (N = 689).
Diet Quality and BMIz
The various diet quality indices (TPFI, UPFI, and SSBI) were included in the models with age, gender, and Afro-Caribbean race. The TPFI was the only diet quality measure significantly associated with BMIz score; an inverse relationship between TPFI and BMIz score was identified ( = −0.175; SE = 0.087) (Table 3). Furthermore, a strong positive interaction was noted between gender and TPFI ( = 0.447; SE = 0.171) (Table 3). Stratified analyses were conducted to characterize this interaction. In the stratified analyses, the negative effect on BMIz score associated with eating a traditional diet was only evident in the female students ( = −0.395; SE = 0.123) (Table 4).
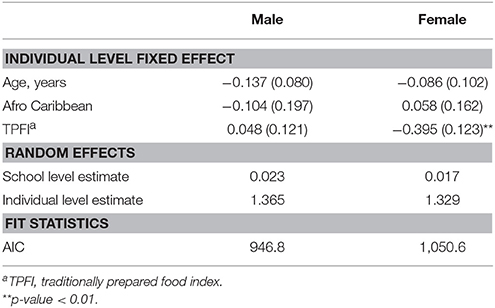
Table 4. Parameter estimates (± SE) from multilevel model 4 predicting BMIz among Grenada School Nutrition Study students stratified by gender nested within 23 schools, (N = 689).
Discussion
The results of this study document a low prevalence of OW/OB (17.6%) among Grenadian adolescents relative to US counterparts (27). Grenadian females demonstrated a prevalence of OW/OB half that of American girls, while Grenadian males showed a prevalence of OW/OB more than 3 times less than that of American boys. Furthermore, the mean BMIz for the study sample (mean = −0.097; SE = 0.045) was significantly lower than zero, indicating that on average, Grenadian adolescents had lower BMI than US adolescents in the 1980s, which is the reference population for calculating BMIz scores (27).
An additional finding in the current study suggests that diet quality is a possible etiologic factor in the epidemiologic transition from communicable disease and nutritional deficiency as the predominant causes of morbidity and mortality to a predominance of noncommunicable diseases (13, 28). Results showed an inverse association between the Grenadian TPFI and BMIz score among females in the study cohort but not in males. The TPFI assessed the number of times per week the adolescents consumed any of the common traditional foods typically prepared at home. The more times they indicated consumption of traditional foods in the past week, the lower the BMIz score. This relationship, which was not seen among males, may be attributed to the low prevalence of OW/OB among the males.
The lack of a significant effect of school location (urban/rural designation) on BMIz may be due to a few reasons. One possibility is the small sample size of urban schools, with only two schools out of the 23 schools classified as urban. In contrast, about one-third of the Grenadian population resides in an urban area (29). Another option is the collective influence of many single-sex schools within the survey cohort and the greater risk of OW/OB among females, thereby attenuating the school level effect after adding sex to the model.
These findings among adolescents are unexpected given the prevalence of OW/OB among Grenadian adults. Bansilal et al. (22) found that adult Grenadian women had a higher prevalence of OW/OB compared with US women in the NHANES (68.1 vs. 58.4%) after standardizing to the Grenadian adult population. Findings indicate that Grenadian adults have transitioned to a more OW/OB population in the absence of a high prevalence of OW/OB among Grenadian adolescents, which is multiple times lower as compared to their US counterparts (27). There is evidence that the infrastructure that permitted the introduction of ultra-processed, sugar-sweetened beverages to Grenada did not arise until the early 2000s (30), therefore the obesogenic effects may not have yet reached adolescents. Popkin et al. (31) found this is a global phenomenon, in that rates of increases in overweight were higher among adults than children in seven countries comprising high-, middle-, and low-income strata. This lag in overweight trends between children and adults may explain why Grenadian adolescents did not have a high OW/OB similar to adults. The absence of a period effect in this cohort is a possible explanation for this observation, suggesting a generational effect may be operating (32).
In populations most influenced by the global obesity pandemic, period and age effects are well-documented. However, it has also been proposed that cohort or generational effects may be operating to amplify the risk of non-communicable diseases (NCDs) for populations in affected countries ill prepared to address the pandemic (3). Hypotheses associated with a potential generational effect involve the developmental origins perspective (e.g., fetal overnutrition) (33, 34). The developmental origins perspective proposes that exposures during critical periods of development (e.g., prenatal period), when there is a high degree of genomic plasticity, can result in permanent physiologic changes predisposing the affected individual to obesity during early life (35, 36). Consequently, a period effect resulting in high rates of obesity among women of childbearing age could theoretically result in a generational effect evidenced as adolescent obesity among their offspring (32). Specifically, women who are either obese or have gestational diabetes during pregnancy provide an in utero environment associated with developmental overnutrition, a condition known to negatively affect a number of physiologic mechanisms (33, 37–40). Phenotypically, affected individuals have increased odds of being obese and may experience an accelerated growth trajectory during childhood due to an altered physiology (32, 40, 41). It is important to note that unlike period effects, which impact all age groups similarly and gradually occur over many years, a generational effect of this type emerges only in the cohort born to the generation of women of childbearing age who have gradually become obese (42). Consequently, the effect should be temporally lagged compared to ongoing period effects. Unfortunately, few studies have followed successive birth cohorts to document the possibility of a generational effect, much less a lag in the initiation of such an occurrence, and the methods previously employed have been contested (32, 43, 44). A better understanding of how this intergenerational amplification of risk is operating is critical to understanding the physiologic processes and societal exposures that drive the global obesity pandemic (3, 33, 37). Such information is necessary for the design of prevention strategies worldwide since it would shift the target to women of child-bearing age rather than the population as a whole.
This cohort of Grenadian adolescents assessed in 2013–2014 may have been unexposed to fetal overnutrition, the postulated origin of the generational effect, which is shown to promote childhood onset of OW/OB (32, 45). Due to the late introduction of processed food and beverages to Grenada, the mothers of this birth cohort may have escaped exposure to this type of food, along with obesity, before or during pregnancy. Thus, offspring may be protected from obesogenic promoters due to a lack of fetal overnutrition. This may explain why, unlike OW/OB in adults, a higher prevalence of OW/OB among Grenadian adolescents as compared to a US cohort was not observed. It is expected this generational effect will reverse in future generations, switching from protective to obesogenic promotion as obesity trends increase.
There are several limitations to the study. The first being its cross-sectional design, which does not allow for a determination of the temporality of OW/OB trends among the study population. Also, there is a lack of a similar comparison cohort for these Grenadian adolescents. Diet quality and adolescent OW/OB in LMIC has not been thoroughly researched, therefore we are unable to determine if our results are typical of this population in similar settings. Lastly, census data regarding Grenada is not readily available, consequently standardizing current NHANES 2013–2014 data to the Grenadian population would not be possible.
The study identified lower prevalence of OW/OB in Grenadian adolescents compared with both their US counterparts and Grenadian adults. In addition, despite low prevalence of OW/OB, adolescent Grenadian females had twice the overweight risk compared with males. Attending a rural or urban school did not have an effect on student OW/OB. Finally, adherence to a diet of traditional prepared foods was associated with lower BMIz scores among the females. However, further research in subsequent birth cohorts is needed determine if adolescent obesity will increase due to a generational effect.
Author's Note
In memory of Romero Benjamin.
Author Contributions
RS made substantial contributions to the conception, design, and analysis of the data, as well as drafting the article and approved the final version. He agrees to be accountable for all aspects of the work with regard to accuracy and integrity. RR and TN made substantial contributions to the conception, design, and data acquisition, as well as revising the article for intellectual content. He approved the final version. He agrees to be accountable for all aspects of the work with regard to accuracy and integrity. AG and TF made substantial contributions to revising the article for intellectual content. She approved the final version. She agrees to be accountable for all aspects of the work with regard to accuracy and integrity. CL made substantial contributions to the data analysis, as well as revising the article for intellectual content. She approved the final version. She agrees to be accountable for all aspects of the work with regard to accuracy and integrity. RA, NA, CR, RF, JB, JJ, and RB made substantial contributions to data acquisition, as well as revising the article for intellectual content. They each approved the final version. They agree to be accountable for all aspects of the work with regard to accuracy and integrity. RW made substantial contributions to the conception, design, and data analysis, as well as revising the article for intellectual content. He approved the final version. He agrees to be accountable for all aspects of the work with regard to accuracy and integrity. MS was the senior researcher on the project. She made substantial contributions to the conception, design, and data acquisition, as well as revising the article for intellectual content. She approved the final version. She agrees to be accountable for all aspects of the work with regard to accuracy and integrity.
Funding
The research was made possible by a grant from the International Research Development Centre (IRDC) Grant #106844.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor and reviewer JGK declared their involvement as co-editors in the Research Topic, and confirm the absence of any other collaboration.
Abbreviations
BMI, body mass index; BMIz, standardized BMI z-score; CDC, Centers for Disease Control; GSNS, Grenada School Nutrition Study; LMIC, low- and middle-income country; NCDs, non-communicable diseases; NHANES, National Health and Nutrition Examination Survey; OW/OB, overweight and obesity; SSBI, sugar sweetened beverage index; TPFI, traditional prepared food index; UPFI, ultra processed food index.
References
1. Ford ND, Patel SA, Narayan KMV. Obesity in low- and middle-income countries: burden, drivers, and emerging challenges. Annu Rev Public Health (2017) 38:145–64. doi: 10.1146/annurev-publhealth-031816-044604
2. GBD 2013 DALYs and HALE Collaborators, Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. (2015) 386:2145–91. doi: 10.1016/S0140-6736(15)61340-X
3. Popkin BM, Adair LS, Ng SW. NOW AND THEN: the global nutrition transition: the pandemic of obesity in developing countries. Nutr Rev. (2012) 70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x
4. The surgeon general's vision for a healthy and fit nation. In: Background on Obesity. Rockville, MD: Office of the Surgeon General (2010).
5. Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. (2009) 90:1453–6. doi: 10.3945/ajcn.2009.28595
6. Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. (2004) 27:205–10. doi: 10.1016/j.amepre.2004.05.005
7. Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. JAMA (2003) 289:450–3. doi: 10.1001/jama.289.4.450
8. Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. (2002) 10:370–8. doi: 10.1038/oby.2002.51
9. Sindelar R. Recess: Is it Needed in the 21st Century?. Early Childhood and Parenting (ECAP) Collaborative. Urbana-Champaign, IL: University of Illinois (2004). p. 6. Available online at: https://www.isbe.net/Documents/recess_is_it_needed.pdf
10. Monteiro CA, Cannon G. The impact of transnational “big food” companies on the south: a view from Brazil. PLoS Med. (2012) 9:e1001252. doi: 10.1371/journal.pmed.1001252
11. Monteiro CA, Levy RB, Claro RM, de Castro IRR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. (2011) 14:5–13. doi: 10.1017/S1368980010003241
12. Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. (2004) 82:940–6.
13. Monteiro CA, Moubarac J-C, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. (2013) 14:21–8. doi: 10.1111/obr.12107
14. Fernandez MA, Kubow S, Gray-Donald K, Knight J, Gaskin PS. Drastic increases in overweight and obesity from 1981 to 2010 and related risk factors: results from the Barbados Children's Health and Nutrition Study. Public Health Nutr. (2015) 18:3070–7. doi: 10.1017/S1368980015002190
15. Barrett SC, Huffman FG, Johnson P, Campa A, Magnus M, Ragoobirsingh D. A cross-sectional study of Jamaican adolescents' risk for type 2 diabetes and cardiovascular diseases. BMJ Open. (2013) 3:e002817. doi: 10.1136/bmjopen-2013-002817
16. Batson YA, Teelucksingh S, Maharaj RG, Cockburn BN. A cross-sectional study to determine the prevalence of obesity and other risk factors for type 2 diabetes among school children in Trinidad, West Indies. Paediatr Int Child Health (2014) 34:178–83. doi: 10.1179/2046905514Y.0000000116
17. GNI Atlas Method (Current US$). Data (2018). Available online at: https://data.worldbank.org/indicator/NY.GNP.ATLS.CD?end=2016andlocations%3DBB-TT-JM-GDandstart%3D1962andview%3Dchart%27%27&locations=BB-GD-JM-TT
18. Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005–2008). J Rural Health Off J Am Rural Health Assoc Natl Rural Health Care Assoc. (2012) 28:392–7. doi: 10.1111/j.1748-0361.2012.00411.x
19. Neuman M, Kawachi I, Gortmaker S, Subramanian SV. Urban-rural differences in BMI in low- and middle-income countries: the role of socioeconomic status123. Am J Clin Nutr. (2013) 97:428–36. doi: 10.3945/ajcn.112.045997
20. Popkin BM, Slining MM. New dynamics in global obesity facing low- and middle-income countries. Obes Rev Off J Int Assoc Study Obes. (2013) 14:11–20. doi: 10.1111/obr.12102
21. Block RC, Dozier AM, Hazel-Fernandez L, Guido JJ, Pearson TA. An epidemiologic transition of cardiovascular disease risk in Carriacou and Petite Martinique, Grenada: the Grenada Heart Project, 2005–2007. Prev Chronic Dis. (2012) 9:E90. doi: 10.5888/pcd9.110167
22. Bansilal S, Vedanthan R, Woodward M, Iyengar R, Hunn M, Lewis M, et al. Cardiovascular risk surveillance to develop a nationwide health promotion strategy. Glob Heart (2012) 7:87–94. doi: 10.1016/j.gheart.2012.06.002
23. The Food and Nutrition Policy and Plan of Action for Grenada. St. George's: The Grenada Food and Nutrition Council (2007).
24. Speck BJ, Bradley CB, Harrell JS, Belyea MJ. A food frequency questionnaire for youth: psychometric analysis and summary of eating habits in adolescents. J Adolesc Health (2001) 28:16–25. doi: 10.1016/S1054-139X(00)00171-3
25. Chumlea C, Roche R. Measurement descriptions and techniques. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics (1988). p. 1–55.
26. Nichols SD, Cadogan F. BMI-based obesity cutoffs and excess adiposity in a Caribbean adolescent population of African origin. Eur J Clin Nutr. (2009) 63:253–8. doi: 10.1038/sj.ejcn.1602913
27. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: Methods and Development. Hyattsville, MD: Public Health Service, Centers for Disease Control and Prevention, National Center for Health Statistics (2002). Vital and Health Statistics; Vol. 26.
28. Popkin BM. Global changes in diet and activity patterns as drivers of the nutrition transition. Nestle Nutr Workshop Ser Paediatr Program. (2009) 63:1–10; discussion 10–14, 259–68. doi: 10.1159/000209967
29. The World Factbook — Central Intelligence Agency (2018). Available online at: https://www.cia.gov/library/publications/the-world-factbook/geos/gj.html
30. Jaleel SM. History SM Jaleel Co. Ltd. (2018). Available online at: http://smjaleel.net/history-smj/
31. Popkin BM, Conde W, Hou N, Monteiro C. Is there a lag globally in overweight trends for children compared with adults? Obesity. (2012) 14:1846–53. doi: 10.1038/oby.2006.213
32. Keyes KM, Utz RL, Robinson W, Li G. What is a cohort effect? Comparison of three statistical methods for modeling cohort effects in obesity prevalence in the United States, 1971–2006. Soc Sci Med. (2010) 70:1100–8. doi: 10.1016/j.socscimed.2009.12.018
33. Fall CH. Evidence for the intra-uterine programming of adiposity in later life. Ann Hum Biol. (2011) 38:410–28. doi: 10.3109/03014460.2011.592513
34. Lawlor DA, Smith GD, O'Callaghan M, Alati R, Mamun AA, Williams GM, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the Mater-University study of pregnancy and its outcomes. Am J Epidemiol. (2006) 165:418–24. doi: 10.1093/aje/kwk030
35. Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Developmental plasticity and human health. Nature (2004) 430:419–21. doi: 10.1038/nature02725
36. Fall CH. Fetal programming and the risk of non-communicable disease. Indian J Pediatr. (2013) 80:S13–20. doi: 10.1007/s12098-012-0834-5
37. Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition—an old hypothesis with new importance? Int J Epidemiol. (2013) 42:7–29. doi: 10.1093/ije/dys209
38. Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort123. Am J Clin Nutr. (2011) 93:780–8. doi: 10.3945/ajcn.110.005801
39. Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, et al. Epigenetic gene promoter methylation at birth is associated with child's later adiposity. Diabetes (2011) 60:1528–34. doi: 10.2337/db10-0979
40. Mathias PCF, Elmhiri G, Oliveira JC, de Delayre-Orthez C, Barella LF, Tófolo LP, et al. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Eur J Nutr. (2014) 53:711–22. doi: 10.1007/s00394-014-0654-7
41. Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. (2011) 158:941–6. doi: 10.1016/j.jpeds.2010.12.007
42. Olsen LW, Baker JL, Holst C, Sørensen TI. Birth cohort effect on the obesity epidemic in Denmark. Epidemiology (2006) 17:292–5. doi: 10.1097/01.ede.0000208349.16893.e0
43. Bell A, Jones K. Don't birth cohorts matter? A commentary and simulation exercise on Reither, Hauser, and Yang's (2009) age-period-cohort study of obesity. Soc Sci Med. (2014) 101:176–80. doi: 10.1016/j.socscimed.2013.09.004
44. Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord J Int Assoc Study Obes. (2004) 28:S2–9. doi: 10.1038/sj.ijo.0802804
Keywords: obesity, body mass index, adolescent, generation, nutrition, cohort effect, diet quality
Citation: Scribner RA, Radix RL, Gilliland AE, Leonardi C, Ferguson TF, Noel TP, Andall RG, Andall NR, Radix C, Frank R, Benjamin J, James J, Benjamin R, Waechter RL and Sothern MS (2018) Absence of Adolescent Obesity in Grenada: Is This a Generational Effect? Front. Public Health 6:204. doi: 10.3389/fpubh.2018.00204
Received: 10 April 2018; Accepted: 03 July 2018;
Published: 03 August 2018.
Edited by:
Giridhara R. Babu, Public Health Foundation of India, IndiaReviewed by:
Jodie Geiger Katon, U.S. Department of Veterans Affairs, United StatesEugenia M. Bastos, Bastos Consulting, United States
Adam Edward Locke, Washington University in St. Louis, United States
Copyright © 2018 Scribner, Radix, Gilliland, Leonardi, Ferguson, Noel, Andall, Andall, Radix, Frank, Benjamin, James, Benjamin, Waechter and Sothern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard A. Scribner, cnNjcmliQGxzdWhzYy5lZHU=
† Deceased
 Richard A. Scribner
Richard A. Scribner Roger L. Radix3
Roger L. Radix3 Aubrey E. Gilliland
Aubrey E. Gilliland Tekeda F. Ferguson
Tekeda F. Ferguson Trevor P. Noel
Trevor P. Noel Christal Radix
Christal Radix