- 1Shanghai Mental Health Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
- 2State Key Laboratory of Microbial Metabolism, Joint International Research Laboratory of Metabolic & Developmental Sciences, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University, Shanghai, China
Introduction: Psychosocial stressors may worsen psychotic symptoms in schizophrenia, while social support could protect against the effects of stress in schizophrenia. Hypothalamus-pituitary-adrenal axis dysfunction has been associated with schizophrenia. Hair cortisol concentrations (HCC) allow assessment of cumulative cortisol secretion over the preceding 3 months. The relationship between HCC, psychosocial stressors, social support, and the clinical characteristics of schizophrenia needs to be explored.
Methods: One hundred nine schizophrenia patients and 86 healthy controls between the ages of 18 and 60 were enrolled in the study. Three-centimeter samples of hair were collected from the scalp and HCC were measured using ELISA kits. Linear regression and factor analysis were employed to examine the relationship between HCC, childhood trauma, the number of stressful life events (SLE), the amount of social support in the 3 months prior to the hair cortisol assessment and clinical characteristics of schizophrenia.
Results: Schizophrenia patients experience more SLE in their lifetime, receive less social support, and have lower HCC in the recent 3 months compared to healthy controls. In the schizophrenia patients, HCC are positively associated with the amount of social support and negatively associated with the severity of delusions. The interaction between social support and SLE predicts decreased HCC. Factor analysis shows that a subgroup of schizophrenia patients who experience childhood trauma and SLE are characterized by decreased HCC.
Conclusions: Findings indicate social support could be a moderator for the relationship between SLE and HCC which may attenuate the effects of SLE in schizophrenia.
Introduction
Psychosocial stressors, such as childhood trauma or stressful life events (SLE), appear to play a significant role for the onset and course of schizophrenia. They have been found to prospectively predict psychotic symptoms exacerbation and be associated with increased risk of relapse (1–3). These data support the neural diathesis-stress model of schizophrenia which implicate a role for stress in the etiology of schizophrenia (4, 5). Social support is usually defined as the existence or availability of people on whom we can rely, people who let us know that they care about, value, and love us (6). Social support can help individuals to cope with everyday life, particularly in response to critical situations. Increased subjective social support shows correlation with a lower degree of psychotic symptoms (7, 8). Social support can provide a buffer against psychosocial stressors and protect against the negative effects of SLE in schizophrenia (7, 9). Furthermore, the beneficial effects of social support on health can be influenced by culture and race (10, 11). The relationship between psychosocial stressors and social support in schizophrenia has seldom been investigated in the Han Chinese population.
Cortisol are essential for an adequate response to stress. Cortisol concentrations have been recognized as the inner indicator of stress response regulated by the hypothalamic-pituitary-adrenal (HPA) axis (12). Abnormal HPA axis functioning in the form of altered cortisol concentrations has been associated with more severe symptoms in schizophrenia (13). Studies have either shown no difference, elevated or attenuated cortisol concentrations in schizophrenia compared to healthy controls (13–16). Meanwhile, both hypo- and hyper-function of cortisol response to stress have been reported in schizophrenia (16–18). An impaired function of the glucocorticoid receptor-mediated negative feedback may account for the dysregulated HPA axis [8]. The inconsistent findings about the cortisol concentrations in schizophrenia could partly be caused by methodological differences between studies, but could also be caused by differential exposure to the number of SLE and the amount of received social support of the participants enrolled in studies. Studies report psychosocial stressors and social support are inversely associated with cortisol concentrations. For example, elevated cortisol concentrations are not only associated with psychosocial stressors (19), but also with lower positive social support (20). Few studies examine the relationship between social support, psychosocial stressors, and cortisol concentrations in schizophrenia.
Cortisol measurements in saliva and plasma are validated measures of acute stress, but they are subject to the normal diurnal variation in cortisol secretion, and easily affected by temporary or transient disturbances in psychosocial stress on the day of measurement (21, 22). In contrast, hair cortisol concentrations (HCC) can reflect long-term cumulative cortisol secretion and chronic stress response for periods of several months. Typically, the average hair grows 1 cm per month, so a 3 cm segment of scalp hair is assessed to determine cortisol secretion during the preceding 3 months (23). As a measure of cortisol changes over time, HCC are suitable for the evaluation of cumulative effects of stress. Thus, HCC may be robust biomarkers in trauma and significant stress-related mental disorders, such as schizophrenia (24). Yet, studies investigating HCC in schizophrenia are scarce and the results are inconclusive (25, 26).
The purpose of this study is to investigate the associations between childhood trauma, the number of SLE, the amount of social support in the 3 months prior to the hair cortisol assessment, clinical characteristics and HCC in schizophrenia patients compared to healthy controls. We also explored the factorial constructs of HCC, psychosocial stressors, social support and symptoms in schizophrenia patients. We hypothesize that schizophrenia patients experiencing more psychosocial stressors and receiving less social support may exhibit more severe symptoms and HPA axis dysfunction indexed by abnormal HCC.
Materials and Methods
Sample
The patients were recruited consecutively from Shanghai Mental Health Center inpatient department. A total of 138 schizophrenia patients were assessed for eligibility and 21 of them did not meet the inclusion criteria and were excluded. Among the 117 eligible schizophrenia patients, 8 of them refused to participate. A total of 109 patients and 86 healthy control subjects were enrolled in the study. Psychiatrists and psychiatrists-in-training performed clinical assessments. All clinical personnel completed training in diagnostics and symptoms rating. Inclusion criteria for patients included age range between 18 and 60 years, had a diagnosis of schizophrenia according to Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (27). They were required to have a total Positive and Negative Syndrome Scale (PANSS) score between 60 and 120.
Controls with similar ethnic background were recruited by means of advertisements in and around the hospital in the same district in Shanghai. Inclusion criteria for the healthy controls were the following: being between 18 and 60 years and having no lifetime diagnosis of psychiatric disorder. Exclusion criteria for all groups included the following: neuroendocrine disorders, neurological disorders, any use of medication that may influence HPA axis function, organic psychosis, unstable or uncontrolled medical conditions interfering with brain function. Moderate to severe brain damage or IQ under 70 were also exclusion criteria for all participants.
All participants were interviewed using a pencil-and-paper version of the interview on average one hour for the patients and 30 minutes for the controls. All participants were informed of the purpose of the study and a written informed consent was established before the interview. The participants were enrolled between January 2018 and December 2019. The ethics committee in Shanghai Mental Health Center approved this study (registration number 2018-13).
Measures
Diagnoses were based on the Structured Clinical Interview for DSM-IV-TR axis I disorders (SCID-I) (28). Psychotic symptoms were rated using PANSS. Items are divided into three symptoms domains which include positive symptoms, negative symptoms and general psychopathology (29). Formal thought disorder was assessed using the Thought, Language, and Communication scale (TLC) (30). Briefly, the TLC contains 18 items and an overall rating (global TLC). Severity ratings of the items 1–9 range from 0 (absent) to 4 (extreme), while severity ratings of the items 10–18 range from 0 (absent) to 3 (severe). Symptom severity and function during the previous three months were rated using the Global Assessment of Functioning Scale (GAF) (31). The GAF evaluates both symptom severity and functioning, ranking a patient from 1 (lowest score) to 100 (highest score) on both scales.
The instrument for assessment of SLE was translated from the interview employed in the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPUD) study, which assessed thirteen negative SLE (32). The thirteen stressful personal events include loss of confidant (death of a spouse, child or sibling), marital difficulties (divorce or marital separation), job loss (laid off from a job or being fired), major financial crisis, legal problems (problems with police or other legal trouble), serious illness, life-threatening accident, natural disaster (fire, flood, etc.), witness of someone being injured or killed, assault (physical assault, rape), and threat (captive, kidnap). The timing of SLE is recorded if the coding is positive.
We assessed childhood emotional neglect (CEN), childhood physical abuse (CPA), and childhood sexual abuse (CSA) through the questions adapted from the Childhood Trauma Questionnaire (CTQ) (33). “Before the age of 16 years old, were you ever sexually abused as a child?”, “Before the age of 16 years old, were you ever physically abused as a child?”, “Before the age of 16 years old, were you ever seriously neglected as a child?”. Sexual abuse refers to any unwanted incidents such as (1) inviting or requesting the child to do something sexual, (2) touching or fondling private parts, (3) making them touch the person in a sexual way, or (4) attempting or having sexual intercourse. Physical abuse refers to bodily assaults on a child by an older person that pose a risk of, or result in, injury. Emotional neglect refers to a lack of emotional support and inadequate attention to a child’s emotional needs, including the need for affection.
Social support was measured using the 6-item short form of the Social Support Questionnaire (SSQ) (34), a psychometrically sound and conveniently administered instrument. The items have two parts. The first part of each item assesses the number of available others the participant has, whom he/she can rely on in times of need. In the second part of the items, the participant is asked to indicate on a 6-point Likert scale how satisfied he/she is with the overall support from the number of people indicated in the first part ranging from 1 (Very dissatisfied) to 6 (Very satisfied).
The age at onset of schizophrenia was assessed retrospectively by reviewing the medical record and was defined as the age at which the first manifestation of psychotic symptoms fulfilling the criteria of schizophrenia occurred. Symptoms reported during the recent episode were regarded as positive and classified according to DSM-IV-TR diagnostic criteria. The duration of illness was defined as the time between the age at onset and age at interview of schizophrenia. The risk of relapse was defined as the ratio between numbers of episodes in lifetime and duration of illness. Medication compliance was defined as the proportion of total duration of medicine-taking over the total duration of illness.
Hair Sample and Hair Cortisol Analysis
Hair sample preparation has been described in detail elsewhere (35). A 3 cm hair segment, approximately 100–150 hairs were cut from the posterior vertex as close to the scalp as possible from the participants. The hairs were cut and stored in envelopes until preparation. As hair grows at an average speed of 1 cm per month, this 3 cm segment can be assumed to reflect cortisol secretion during the preceding 3 months (36). The 3 cm hair segments were minced with small surgical scissors and 50 mg of powered hair were weighed and separated into a glass vial. One ml of methanol was added to extract cortisol from the hair samples and incubated for 16 h at 52°C while gently shaking. Afterwards, the methanol was transferred to a clean glass vial and was evaporated under a constant nitrogen stream until completely dry. The samples were then dissolved in 250 μl phosphate buffered saline (pH 8.0) and vortexed until thoroughly mixed. Cortisol concentrations in the hair extracts were measured using a commercial ELISA kit for salivary cortisol (DRG Instruments GmbH, Marburg, Germany) according to the manufacturer’s instructions. HCC values are presented in pg/mg hair.
Statistics
The statistical analyses were completed using R (version 3.3.1) (37). The descriptive statistics are presented as percentages for discrete variables and as means (standard deviation, S.D.) for continuous variables. T- test or Chi - square test were used to compare HCC and social demographic features between schizophrenia and healthy controls. We applied linear regression to examine the association of HCC, treated as a continuous independent variable, with clinical characteristics of schizophrenia and different phenotypes. Coefficient values were used to quantify the strength of associations. The statistical significance for all tests was set at P < 0.05 as the analyses were exploratory in nature.
To examine the relationship and factorial constructs of HCC, psychosocial stressors, social support and symptoms in schizophrenia patients, exploratory factor analysis was performed by using both varimax and promax rotations. Interpretations of the scree plot and eigenvalue were used to guide decisions on the number of factors to be extracted. Factor loadings ≧ 0.30 were considered to be substantial.
Results
The average age of schizophrenia at interview was 40.8 (S.D. = 12.2) years (range 18–60). The mean age at onset of schizophrenia was 27.7 (S.D. = 11.4) years (range 18–55). The average age of healthy controls at interview was 42.2 (S.D. = 10.9) years (range 18–60). Schizophrenia patients have significantly lower HCC (14.2 pg/mg, S.D. = 11.7) than healthy controls (18.5 pg/mg, S.D. = 10.5) (P < 0.05).
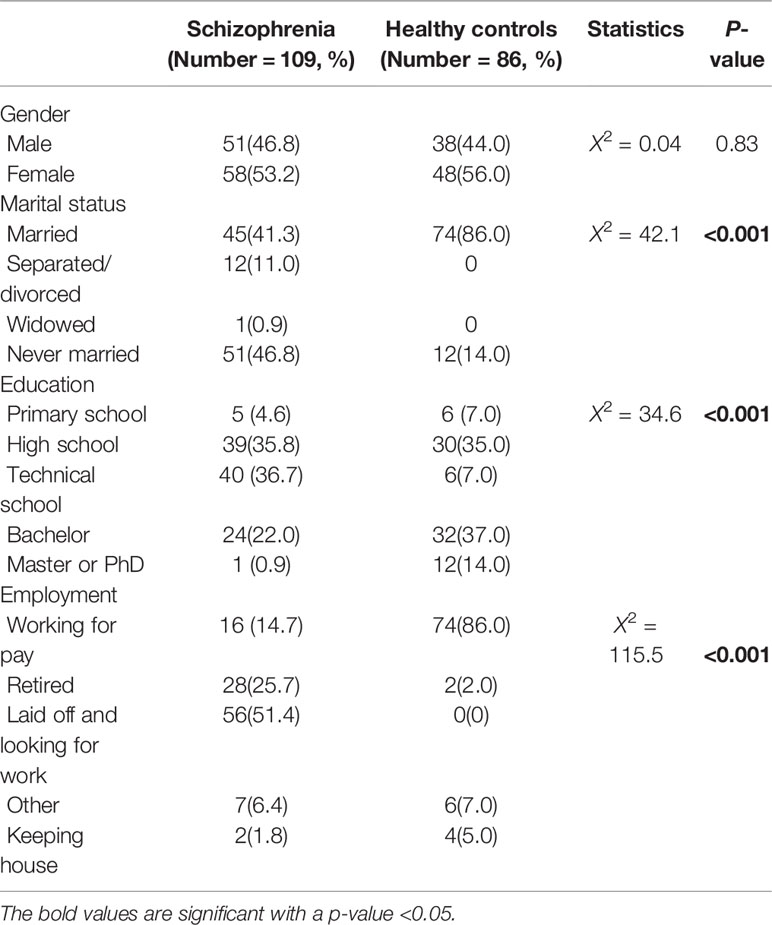
Table 1 shows the results for socio-demographic features of schizophrenia patients and healthy controls. Schizophrenia patients were less likely to be employed, to be married and to receive education (all P < 0.001).
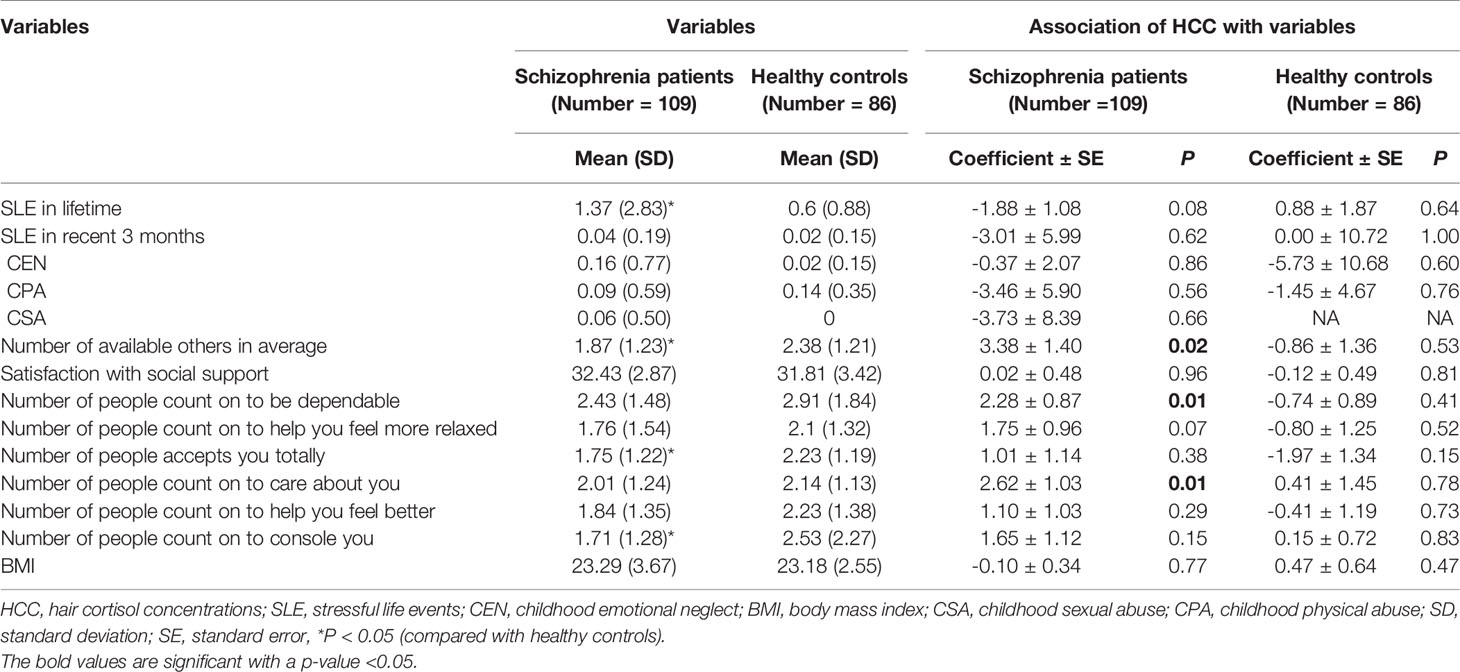
The differences of SLE, childhood trauma, social support and body mass index between schizophrenia patients and healthy controls are presented in Table 2. Compared with healthy controls, schizophrenia patients had more SLE in lifetime, less people who totally accepted them, less people whom they could count on to be consoled and less average number of available others (all P < 0.05). There was a trend toward significance that schizophrenia patients experienced more CEN compared to healthy controls (P = 0.08).
We examined the association between psychosocial stressors, social support, body mass index, and HCC both in schizophrenia patients and healthy controls (Table 2). In the schizophrenia patients, HCC were positively associated with the average number of available others (P = 0.02), including the number of people they can count on to be dependable (P = 0.01) and count on to be cared about (P = 0.01). There was a trend that decreased HCC were associated with more SLE (P = 0.08). HCC were not associated with SLE that happened within the past 3 months (P = 0.62). While in the control group, HCC were not associated with psychosocial stressors, social support and BMI (all P > 0.05).
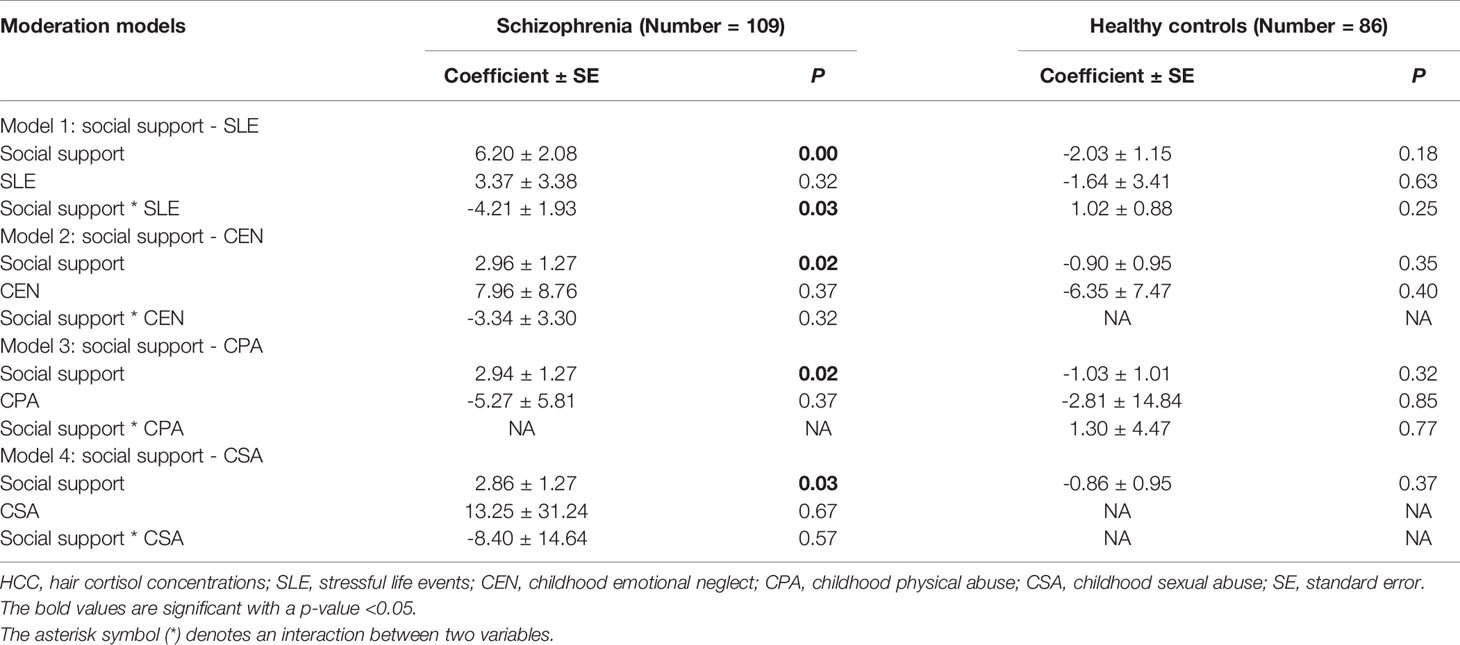
The amount of social support (average number of available others) was calculated as a moderator for the relationship between psychosocial stressors and HCC (Table 3). In the schizophrenia patients, social support was positively associated with HCC in all of the moderation models. The amount of social support predicted HCC (all P < 0.05). SLE and childhood trauma did not predict HCC (all P > 0.05). The interaction term between social support and SLE was significant and predicted decreased HCC [F (3,89) = 3.64, P = 0.02]. The interaction terms between social support and CEN [F (3,89) = 2.00, P = 0.12], between social support and CPA [F (3,89) = 2.87, P = 0.06], between social support and CSA [F (3,89) = 1.80, P = 0.15] were not significant. In the control group, the interaction terms between social support and SLE, between social support and childhood trauma were not significant (all P > 0.05).
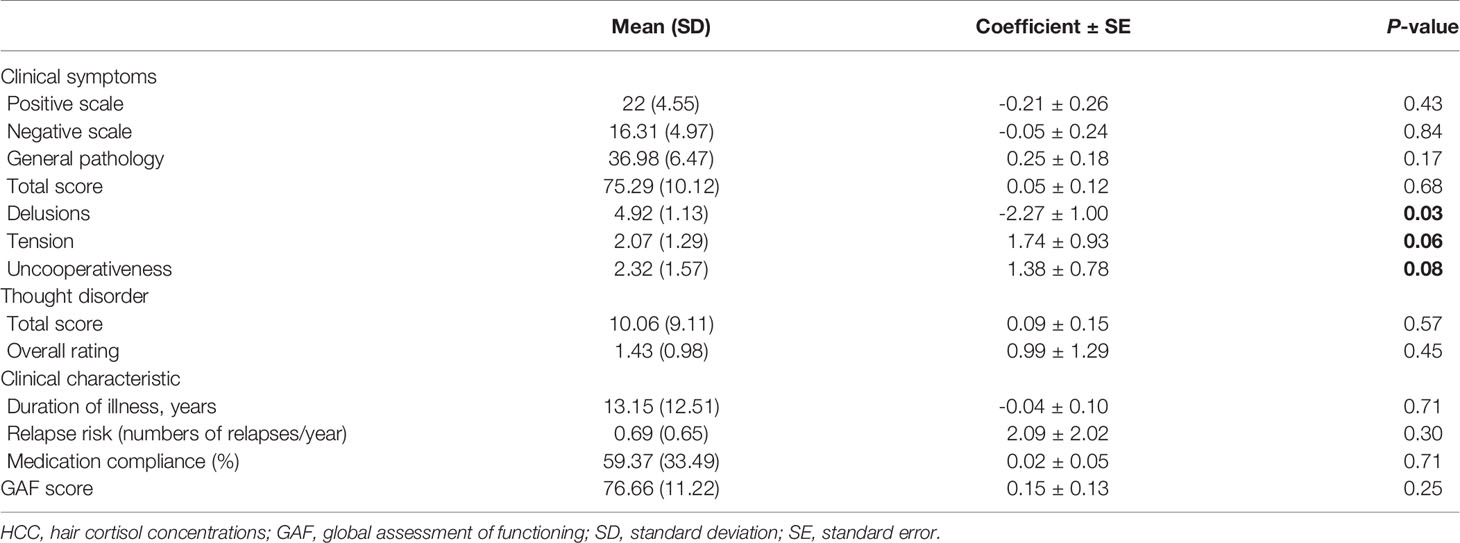
We applied linear regression to explore the association between HCC and a series of clinical characteristics in schizophrenia, including symptoms, thought disorder, duration of illness, risk of relapse and social functioning (Table 4). HCC were negatively associated with the severity of delusions (P = 0.03). We found a trend towards significance for the severity of tension (P = 0.06) and uncooperativeness (P = 0.08). No other significant associations were found between HCC and all the above mentioned clinical characteristics of schizophrenia.
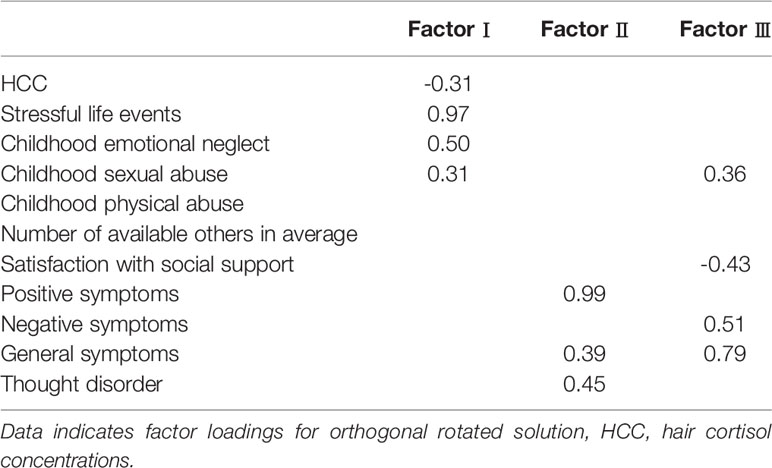
Examination of the scree plot and eigenvalue indicated that a three or four factor solution best fitted the data. We examined both an orthogonal and oblique factor rotation which produced similar results. The orthogonal rotations are more likely to approximate clinical reality (Table 5). Factor analysis of the 11 items yielded three factors accounting for 40% of the variance. SLE, CEN, CSA, and HCC loaded prominently on factor I. Positive symptoms, general symptoms and thought disorder loaded substantially on factor II. General symptoms, negative symptoms, satisfaction with social support and CSA loaded the highest on factor III. Items within a factor are supposed to be inter-related. The factor analysis points to a subgroup of schizophrenia patients who experience childhood trauma and SLE are characterized by decreased HCC.
Discussion
Our study aims to examine the relationship between childhood trauma, the number of SLE, the amount of social support, clinical characteristics, and HCC in schizophrenia. We find that schizophrenia patients have lower HCC, experience more SLE, and receive less social support compared to healthy controls. In schizophrenia patients, decreased HCC are significantly associated with less social support and more severe delusions. Social support is observed to be a moderator for the relationship between SLE and HCC in schizophrenia patients. The interaction between social support and SLE predicts decreased HCC. Factor analysis shows that a subgroup of schizophrenia patients who experience psychosocial stressors are characterized by decreased HCC.
Consistent with previous studies, patients with schizophrenia are more likely to have experienced intense psychosocial adversities than healthy controls (26, 38). It has also been shown that schizophrenia patients receive less social support compared to healthy controls (39, 40). Studies report increased subjective social support shows correlation with a lower degree of psychotic symptoms (7), and emotional and socialization supports are helpful for schizophrenia patients (9). On the contrary, exposure to social stress is strongly associated with onset of psychosis, schizophrenia in particular (41). We think it is important to offer social support to schizophrenia patients in rehabilitation programs to improve their quality of life and decrease chances for relapse in their lifetime (9, 42).
The previous studies report mixed results on the cortisol concentrations in schizophrenia patients, including elevated or normal serum or salivary cortisol concentrations compared to healthy controls (13, 15, 16, 43). HCC reflect long-term cumulative cortisol secretion over weeks to months and studies investigating HCC in schizophrenia are scarce. We find schizophrenia patients experience more SLE in their lifetime, yet they have lower HCC than healthy controls. SLE have a negative trend association with HCC in schizophrenia patients. However, one study reports schizophrenia patients with a history of childhood maltreatment have higher HCC relative to healthy controls (25). Another shows no difference in HCC between schizophrenia patients and healthy controls (26). Since social support has been proved to be associated with increased cortisol concentrations (20), the differences in the amount of social support received by the participants may contribute to the inconsistent results in different studies, including ours.
We find more social support is significantly associated with increased HCC in schizophrenia. Furthermore, the interaction between social support and SLE significantly predicts decreased HCC. Thus, social support is observed to be a moderator for the relationship between SLE and HCC in schizophrenia patients. Our results are consistent with previous findings which indicate SLE and social support may influence cortisol concentrations inversely in schizophrenia patients (20). Social support may have protective effects against psychosocial stressors as indexed by HCC, which corroborate the neural diathesis-stress model of schizophrenia (5, 17). The protective effects of social support against psychosocial stressors and its moderating role for the relationship between SLE and HCC could be further investigated by cohort studies.
Besides the cortisol concentrations, cortisol response is another important index reflecting the HPA axis function. Most of the studies report that cortisol stress reactivity is blunted in schizophrenia, showing reduced HPA axis reactivity to stressors, indicating an impaired activation of HPA axis in facing stressors among schizophrenia patients (44, 45). HCC measure long-term cumulative cortisol level and can be interpreted as promising biomarkers of long-term HPA activation (46). Our results show schizophrenia patients experience more psychosocial stressors in their lifetime history, yet they display lower HCC compared to healthy controls. The impaired activation of HPA axis may indicate schizophrenia does not display physiological readiness following psychosocial stressors (47). On the contrary, schizophrenia patients who do not exhibit attenuated cortisol responses to stress may have generally better social functioning (43).
Abnormal HPA axis function and cortisol concentrations have been shown to be associated with the severity of clinical symptoms or the general severity of schizophrenia (16). For example, cortisol concentrations are positively or negatively associated with the severity of negative symptoms, positive symptoms or the severity of a wide array of symptoms in schizophrenia (13, 48, 49). Our results are consistent with the previous findings and show HCC are negatively associated with the severity of delusions and there is a trend association between HCC and tension and uncooperativeness. The severity and exacerbation of symptoms in schizophrenia are associated with cortisol concentrations, yet the underlying mechanism is still unclear. Glucocorticoids receptors are present throughout the central nervous system and thus can mediate the effects of cortisol on several neural systems (50). The synergistic relation between HPA activity and DA neurotransmission can help us to understand how stress exposure leading to increased cortisol secretion might trigger a neuropathological dopamine-driven process (51).
Factor analysis in our study reveals three latent factors within the schizophrenia patients. One subgroup of patients who experience childhood trauma and SLE are characterized by decreased HCC. The second subgroup of patients who experience CSA, exhibit more severe symptoms and have less satisfaction with social support. The third subgroup of patients have clinical symptoms that are not correlated with HCC or psychosocial stressors. Hence our data suggest mechanistic or biological heterogeneity within the patient population. Psychosocial stressors may contribute to the severity and complexity of a certain subgroup of schizophrenia patients who seem to be vulnerable to stress. This kind of vulnerability indicated by HPA axis dysfunction may be one of the biomarkers of schizophrenia patients since the clinical high risk population also exhibits this tendency (52).
Our study conveys importance messages to help practitioners understanding the role of psychosocial stressors in the etiology and treatment of schizophrenia. Psychiatrist should try to empathize the schizophrenia patients on how much the childhood traumatic events or SLE could have influenced their symptoms, e.g., the severity of delusions (53, 54). Our findings could also be relevant in the treatment of schizophrenia when choosing therapeutic interventions that manipulate cortisol levels (55). Considering the moderating effects of social support for the relationship between SLE and HCC, mental health practitioners should provide more social and psychological support to schizophrenia patients throughout the course of this disease, especially in the stable phase (20, 43, 56). Furthermore, aerobic exercises have been proved to decrease the cortisol levels (57). Taichi is a form of physical exercise and moving meditation originated in China. The role of Taichi and aerobic exercises in the adjunctive treatment of schizophrenia should be investigated further (58).
This study has several strengths. All of the clinical data were collected through face-to-face interviews by trained interviewers with clinical backgrounds. To our knowledge, it is the first study to examine the relationship between psychosocial stressors, social support and HCC in Han Chinese schizophrenia patients.
This study has a number of limitations which should be carefully considered. First, this is a cross sectional study: data were collected retrospectively and recall bias will have affected results. Second, no causal conclusions can be drawn because all the variables were measured only once. Third, we only assessed the significant negative SLE such as the death of significant others and job loss in our study. The influence of everyday living from daily hassles and uplifts which can be measured using the Hassles and Uplifts Scale were not assessed (59). We could not rule out the confounding effects of the above unassessed psychological factors on HPA axis (60). Fourth, we did not examine detailed information on medication for this sample, thus we were not able to adjust for potential effect of medication on HPA axis. Antipsychotic medication may increase (61), decrease (62) or have no influence on cortisol levels in schizophrenia patients (63). Further controlled follow up studies investigating cortisol levels in antipsychotic-naïve patients with schizophrenia treated with certain antipsychotic medication will help to make this clearer.
In summary, our results show HCC are associated with the amount of social support and the severity of delusions in schizophrenia patients. Social support may act as a moderator for the relationship between SLE and HCC which could attenuate the effects of SLE in schizophrenia. We conclude psychosocial stressors and abnormal HCC may contribute to the severity of delusions in a subgroup of stress-vulnerable schizophrenia patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceived and designed the experiments: FY, HX. Performed the experiments: FY, XS, XC, HW, JQ. Analyzed the data: FY. Wrote the paper: FY. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Shanghai Jiaotong University Medical-Engineering Cross Fund (YG2015MS48).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the participants who took part in the study and Zhiguang Lin for his for his help with HCC assays. We appreciate Margaret Cheng Tuttle for the editing of the English-language text.
References
1. Gallagher BJ,3, Jones BJ, Pardes M. Stressful Life Events, Social Class and Symptoms of Schizophrenia. Clin Schizophr Rel Psychoses (2016) 10(2):101–8. doi: 10.3371/1935-1232-10.2.101
2. Fallon P. The role of intrusive and other recent life events on symptomatology in relapses of schizophrenia: a community nursing investigation. J Psychiatr Ment Health Nurs (2009) 16(8):685–93. doi: 10.1111/j.1365-2850.2009.01451.x
3. Muenzenmaier KH, Seixas AA, Schneeberger AR, Castille DM, Battaglia J, Link BG. Cumulative Effects of Stressful Childhood Experiences on Delusions and Hallucinations. J Trauma Dissociation Off J Int Soc Study Dissociation (2015) 16(4):442–62. doi: 10.1080/15299732.2015.1018475
4. Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull (1984) 10(2):300–12. doi: 10.1093/schbul/10.2.300
5. Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev (1997) 104(4):667–85. doi: 10.1037/0033-295X.104.4.667
6. Leistner C, Menke A. How to measure glucocorticoid receptor’s sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology (2018) 91:235–60. doi: 10.1016/j.psyneuen.2018.01.023
7. Peng MM, Zhang TM, Liu KZ, Gong K, Huang CH, Dai GZ, et al. Perception of social support and psychotic symptoms among persons with schizophrenia: A strategy to lessen caregiver burden. Int J Soc Psychiatry (2019) 65(7-8):548–57. doi: 10.1177/0020764019866230
8. Lutgens D, Gariepy G, Malla A. Psychological and psychosocial interventions for negative symptoms in psychosis: systematic review and meta-analysis. Br J Psychiatry J Ment Sci (2017) 210(5):324–32. doi: 10.1192/bjp.bp.116.197103
9. Karanci NA, Gok AC, Yildirim B, Borhan N. Social support perceptions of Turkish people with schizophrenia: What helps and what doesn’t help. Int J Soc Psychiatry (2017) 63(7):657–65. doi: 10.1177/0020764017726931
10. Tan S-h. AuthoritativemasterKong(Confucius)inanauthoritarianage. Dao (2010) 9:137–49. doi: 10.1007/s11712-010-9157-2
11. Sheffler J, Sachs-Ericsson N. Racial Differences in the Effect of Stress on Health and the Moderating Role of Perceived Social Support. J Aging Health (2016) 28(8):1362–81. doi: 10.1177/0898264315618923
12. Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol (2007) 58:145–73. doi: 10.1146/annurev.psych.58.110405.085605
13. Hempel RJ, Tulen JH, van Beveren NJ, Roder CH, de Jong FH, Hengeveld MW. Diurnal cortisol patterns of young male patients with schizophrenia. Psychiatry Clin Neurosci (2010) 64(5):548–54. doi: 10.1111/j.1440-1819.2010.02121.x
14. Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnormal Psychol (2010) 119(2):401–8. doi: 10.1037/a0018399
15. Brenner K, St-Hilaire A, Liu A, Laplante DP, King S. Cortisol response and coping style predict quality of life in schizophrenia. Schizophr Res (2011) 128(1-3):23–9. doi: 10.1016/j.schres.2011.01.016
16. Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol (2010) 24(4 Suppl):91–118. doi: 10.1177/1359786810385491
17. Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev (2017) 73:191–218. doi: 10.1016/j.neubiorev.2016.12.013
18. Lange C, Huber CG, Frohlich D, Borgwardt S, Lang UE, Walter M. Modulation of HPA axis response to social stress in schizophrenia by childhood trauma. Psychoneuroendocrinology (2017) 82:126–32. doi: 10.1016/j.psyneuen.2017.03.027
19. Mayer SE, Lopez-Duran NL, Sen S, Abelson JL. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology (2018) 92:57–65. doi: 10.1016/j.psyneuen.2018.03.020
20. Iob E, Kirschbaum C, Steptoe A. Positive and negative social support and HPA-axis hyperactivity: Evidence from glucocorticoids in human hair. Psychoneuroendocrinology (2018) 96:100–8. doi: 10.1016/j.psyneuen.2018.06.008
21. Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci Biobehav Rev (2010) 35(1):91–6. doi: 10.1016/j.neubiorev.2010.01.010
22. Stalder T, Evans P, Hucklebridge F, Clow A. State associations with the cortisol awakening response in healthy females. Psychoneuroendocrinology (2010) 35(8):1245–52. doi: 10.1016/j.psyneuen.2010.02.014
23. Wester VL, van Rossum EF. Clinical applications of cortisol measurements in hair. Eur J Endocrinol (2015) 173(4):M1–10. doi: 10.1530/EJE-15-0313
24. Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med Med Clin Exp (2007) 30(5):E183–191. doi: 10.25011/cim.v30i5.2894
25. Aas M, Pizzagalli DA, Laskemoen JF, Reponen EJ, Ueland T, Melle I, et al. Elevated hair cortisol is associated with childhood maltreatment and cognitive impairment in schizophrenia and in bipolar disorders. Schizophr Res (2019) 213:65–71. doi: 10.1016/j.schres.2019.01.011
26. Streit F, Memic A, Hasandedic L, Rietschel L, Frank J, Lang M, et al. Perceived stress and hair cortisol: Differences in bipolar disorder and schizophrenia. Psychoneuroendocrinology (2016) 69:26–34. doi: 10.1016/j.psyneuen.2016.03.010
27. Association. AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: Washington, DC (2000).
28. First MBSR, Gibbon MW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP). New York: New York Biometrics Research, New York State Psychiatric Institute (2002).
29. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13(2):261–76. doi: 10.1093/schbul/13.2.261
30. Andreasen NC. Scale for the assessment of thought, language, and communication (TLC). Schizophr Bull (1986) 12(3):473–82. doi: 10.1093/schbul/12.3.473
31. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry J Ment Sci (1995) 166(5):654–9. doi: 10.1192/bjp.166.5.654
32. Kendler K, Prescott C. Genes, Environment, and Psychopathology. Guildford Press: New York (2006).
33. Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl (2003) 27(2):169–90. doi: 10.1016/S0145-2134(02)00541-0
34. Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat (1987) 4(4):497–510. doi: 10.1177/0265407587044007
35. Staufenbiel SM, Koenders MA, Giltay EJ, Elzinga BM, Manenschijn L, Hoencamp E, et al. Recent negative life events increase hair cortisol concentrations in patients with bipolar disorder. Stress (2014) 17(6):451–9. doi: 10.3109/10253890.2014.968549
36. Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int (2010) 196(1-3):32–7. doi: 10.1016/j.forsciint.2009.12.040
37. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria (2013). Available at: http://wwwR-projectorg/.
38. Mauritz MW, Goossens PJ, Draijer N, van Achterberg T. Prevalence of interpersonal trauma exposure and trauma-related disorders in severe mental illness. Eur J Psychotraumatol (2013) 4:1–15. doi: 10.3402/ejpt.v4i0.19985
39. Munikanan T, Midin M, Daud TIM, Rahim RA, Bakar AKA, Jaafar NRN, et al. Association of social support and quality of life among people with schizophrenia receiving community psychiatric service: A cross-sectional study. Compr Psychiatry (2017) 75:94–102. doi: 10.1016/j.comppsych.2017.02.009
40. Xie P, Wu K, Zheng Y, Guo Y, Yang Y, He J, et al. Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. J Affect Disord (2018) 228:41–8. doi: 10.1016/j.jad.2017.11.011
41. Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience (2013) 249:172–91. doi: 10.1016/j.neuroscience.2012.12.017
42. Gorna K, Jaracz K, Rybakowski J. [The role of social support on the quality of life of patients with schizophrenia]. Psychiatria Polska (2004) 38(3):443–52.
43. Tas C, Brown EC, Eskikurt G, Irmak S, Aydin O, Esen-Danaci A, et al. Cortisol response to stress in schizophrenia: Associations with oxytocin, social support and social functioning. Psychiatry Res (2018) 270:1047–52. doi: 10.1016/j.psychres.2018.05.011
44. Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (2000) 149(3):319–25. doi: 10.1007/s002130000381
45. Ciufolini S, Dazzan P, Kempton MJ, Pariante C, Mondelli V. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neurosci Biobehav Rev (2014) 47:359–68. doi: 10.1016/j.neubiorev.2014.09.004
46. Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology (2017) 77:261–74. doi: 10.1016/j.psyneuen.2016.12.017
47. Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, et al. Social cognition in schizophrenia, Part 1: performance across phase of illness. Schizophr Bull (2012) 38(4):854–64. doi: 10.1093/schbul/sbq171
48. Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol (2005) 30(8):1532–8. doi: 10.1038/sj.npp.1300756
49. Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res (2010) 175(1-2):47–53. doi: 10.1016/j.psychres.2009.01.013
50. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev (2007) 87(3):873–904. doi: 10.1152/physrev.00041.2006
51. Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry (2012) 71(6):561–7. doi: 10.1016/j.biopsych.2011.10.009
52. Schifani C, Pruessner J, Tseng HH, Rao N, Tagore A, Wilson AA, et al. Stress-induced cortical dopamine response is altered in subjects at clinical high risk for psychosis using cannabis. Addict Biol (2019) 25(4):e12812. doi: 10.1111/adb.12812
53. Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry (2017) 81(1):9–20. doi: 10.1016/j.biopsych.2016.07.014
54. Walter EE, Fernandez F, Snelling M, Barkus E. Stress induced cortisol release and schizotypy. Psychoneuroendocrinology (2018) 89:209–15. doi: 10.1016/j.psyneuen.2018.01.012
55. Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull (2003) 29(4):671–92. doi: 10.1093/oxfordjournals.schbul.a007038
56. Malinauskas R, Malinauskiene V. The Mediation Effect of Perceived Social Support and Perceived Stress on the Relationship Between Emotional Intelligence and Psychological Wellbeing in Male Athletes. J Hum Kinetics (2018) 65:291–303. doi: 10.2478/hukin-2018-0017
57. Beserra AHN, Kameda P, Deslandes AC, Schuch FB, Laks J, Moraes HS. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother (2018) 40(4):360–8. doi: 10.1590/2237-6089-2017-0155
58. Ho RT, Fong TC, Wan AH, Au-Yeung FS, Wong CP, Ng WY, et al. A randomized controlled trial on the psychophysiological effects of physical exercise and Tai-chi in patients with chronic schizophrenia. Schizophr Res (2016) 171(1-3):42–9. doi: 10.1016/j.schres.2016.01.038
59. DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol (1988) 54(3):486–95. doi: 10.1037//0022-3514.54.3.486
60. Ravindran AV, Griffiths J, Merali Z, Anisman H. Primary dysthymia: a study of several psychosocial, endocrine and immune correlates. J Affect Disord (1996) 40(1-2):73–84. doi: 10.1016/0165-0327(96)00045-6
61. Jakovljevic M, Pivac N, Mihaljevic-Peles A, Mustapic M, Relja M, Ljubicic D, et al. The effects of olanzapine and fluphenazine on plasma cortisol, prolactin and muscle rigidity in schizophrenic patients: a double blind study. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(2):399–402. doi: 10.1016/j.pnpbp.2006.10.007
62. Tanaka K, Morinobu S, Ichimura M, Asakawa A, Inui A, Hosoda H, et al. Decreased levels of ghrelin, cortisol, and fasting blood sugar, but not n-octanoylated ghrelin, in Japanese schizophrenic inpatients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(6):1527–32. doi: 10.1016/j.pnpbp.2008.05.013
Keywords: schizophrenia, hair cortisol concentrations, childhood trauma, stressful life events, social support
Citation: Yang F, Cao X, Sun X, Wen H, Qiu J and Xiao H (2020) Hair Cortisol Is Associated With Social Support and Symptoms in Schizophrenia. Front. Psychiatry 11:572656. doi: 10.3389/fpsyt.2020.572656
Received: 15 June 2020; Accepted: 07 September 2020;
Published: 24 September 2020.
Edited by:
Bernat Kocsis, Harvard Medical School, United StatesReviewed by:
Tertia Purves-Tyson, Neuroscience Research Australia, AustraliaBartlomiej Stanczykiewicz, Wroclaw Medical University, Poland
Copyright © 2020 Yang, Cao, Sun, Wen, Qiu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Xiao, aHVheGlhb0BzanR1LmVkdS5jbg==; Jianyin Qiu, amlhbnlpbl9xaXVAMTYzLmNvbQ==
 Fuzhong Yang
Fuzhong Yang Xinyi Cao
Xinyi Cao Xiujia Sun
Xiujia Sun Hui Wen
Hui Wen Jianyin Qiu
Jianyin Qiu Hua Xiao
Hua Xiao