- 1Department of Anesthesiology, Chinese Academy of Medical Sciences and Peking Union Medical College, Fuwai Hospital, Beijing, China
- 2Department of Anesthesiology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, China
Background: The aim of this systematic review and meta-analysis of clinical trials was to investigate the effects of perioperative sleep disturbances on postoperative delirium (POD).
Methods: Authors searched for studies (until May 12, 2020) reporting POD in patients with sleep disturbances following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results: We identified 29 relevant trials including 55,907 patients. We divided these trials into three groups according to study design: Seven retrospective observational trials, 12 prospective observational trials, and 10 randomized controlled trials. The results demonstrated that perioperative sleep disturbances were significantly associated with POD occurrence in observational groups [retrospective: OR = 0.56, 95% CI: [0.33, 0.93], I2 = 91%, p for effect = 0.03; prospective: OR = 0.27, 95% CI: [0.20, 0.36], I2 = 25%, p for effect < 0.001], but not in the randomized controlled trial group [OR = 0.58, 95% CI: [0.34, 1.01], I2 = 68%, p for effect = 0.05]. Publication bias was assessed using Egger's test. We used a one-by-one literature exclusion method to address high heterogeneity.
Conclusions: Perioperative sleep disturbances were potential risk factors for POD in observational trials, but not in randomized controlled trials.
Introduction
Postoperative delirium (POD) is a state of brain dysfunction following surgery, and it features acute onset and fluctuating occurrence (1). The typical clinical manifestations of POD include alterations of consciousness, attention, and cognition. According to reports, POD affects 11–51% of patients after major surgery, and it is independently associated with prolonged intensive care, long-term postoperative cognitive dysfunction, and increased mortality (2–5). However, the pathogenesis of POD remains unclear, so it is particularly important to identify risk factors to prevent its occurrence.
Perioperative sleep disturbances are common among surgery patients. The disturbances include obstructive sleep apnea (OSA), reduced total sleep time, sleep fragmentation, circadian rhythm disruption, and so on (6–8). Over 40% of patients complained about poor sleep quality during the first night following surgery, and the sleep problems continued several days post-operation (9). Some observational studies reported that patients with poor sleep quality were predisposed to mental disorders including delirium and cognitive dysfunction (10–12). In addition, several randomized controlled trials (RCTs) found that improving sleep quality, be it through medication or other interventions, strikingly decreased the incidence of delirium (13–15). Although multiple studies have supported the viewpoint that sleep problems are significantly associated with delirium, some studies obtained negative results (16–18). Thus, we designed this systematic review and meta-analysis to clarify the effect of sleep disturbance on the incidence of delirium in adult surgery patients.
Methods
This systematic review and meta-analysis was performed according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Table 1) (19).
Search Strategy
Hongbai Wang and Liang Zhang were responsible for document retrieval. We searched the databases of Pubmed, Embase, Cochrane Library, and Web of Science using the PICOS (Population, Intervention, Comparison, Outcome, Study design) method. Our last search was completed on May 12, 2020. The search terms included “sleep” OR “insomnia” OR “sleep disturbance” OR “night” OR “circadian” AND “surgery” OR “operation” OR “postoperative” OR “anesthesia” OR “anesthesia” AND “delirium” OR “confusion” OR “agitation” OR “acute confusional state” OR “acute confusional syndrome,” and the search scope was “title and abstract.” Because we sought to examine all studies about the effect of sleep disturbances on POD incidence in adult patients undergoing surgery, we did not constrain the search terms for study designs.
Study Selection
Zhe Zhang and Yinan Li performed the screening process for titles and abstracts, while Hongbai Wang and Su Yuan performed the screening process for full texts. The inclusion criteria were (1) participants aged 18 years or older; (2) patients undergoing surgery; and (3) articles reporting the effect of sleep on delirium. The exclusion criteria were: (1) duplicate articles; (2) participants younger than 18 years old; (3) review or meta-analysis; (4) articles published as an abstract, letter, case report, basic research, editorial, note, method, or protocol; (5) articles presented in a non-English language; (6) studies without statistical differences in sleep quality between intervention and control groups; (7) studies without a specific number of patients with sleep problems (observational studies) and/or delirium; and (8) studies including some patients not undergoing surgery.
Quality Assessment of Included Studies
Qipeng Luo and Su Yuan independently assessed the quality of included studies. For retrospective and prospective observational trials, risk of bias was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS), which comprises the following three domains: selection, comparability, and outcome for cohort studies (20). There were four stars in the selection domain, two stars in the comparability domain, and three stars in the exposure domain. Trials with seven or more cumulative stars were considered to be of high quality, those with six stars of moderate quality, and those with <6 stars of low quality (20). For RCTs, risk of bias was assessed using the Cochrane Collaboration Risk of Bias Assessment tool, which included the following seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and others (bias due to vested financial interest and academic bias). If a trial was found to have one or more of the items associated with high or unclear risk of bias, this trial was classified as high risk (21). If the two authors disagreed on their assessment, they consulted the third or fourth author. Eventually, we reached a consensus.
Data Extraction
Yinan Li and Qipeng Luo were responsible for extracting the following information: (1) authors; (2) publication year; (3) total number of participants in each study; (4) age range of all the participants; (5) country of publication; (6) percentage of males; (7) procedures that the participants underwent; (8) methods of sleep disturbance assessment; (9) methods of POD assessment; (10) number of patients with and without POD; (11) number of patients with good and poor sleep quality; and (12) the follow-up time. Hongbai Wang and Liang Zhang were responsible for adjusting data discrepancies.
Outcome Measures
The sole aim of this meta-analysis was to determine whether perioperative sleep disturbances were associated with increased POD in adult surgery patients.
Data Synthesis
We divided all the included trials into three groups according to their study design to facilitate data synthesis. These were retrospective observational trials (ROTs), prospective observational trials (POTs), and RCTs.
Data Analysis
RevMan Review Manager version 5.3 (Cochrane collaboration, Oxford, UK) and Stata version 12.0 (Stata Corp, College Station, TX, USA) were used to perform statistical analyses. We assessed the heterogeneity of included studies using the values of I2 and the Mantel-Haenszel chi-square test (p-value for heterogeneity). The values of I2 <40%, I2 = 40–60%, and I2 > 60% indicated low, moderate, and high heterogeneity, respectively (22). If we identified I2 > 50% or a p-value for heterogeneity <0.1, we used a random-effect model to analyze the data. Conversely, if we identified I2 <50% or a p-value for heterogeneity ≥ 0.1, we used a fixed-effect model to analyze the data (23). The dichotomous outcomes were reported as odds ratios (OR) with 95% confidence intervals (CI). Publication bias was assessed using Begg's test, and studies with a p < 0.05 were adjusted using trim-and-fill analysis (24). The statistical tests were two-sided, and overall effects with a p < 0.05 were considered to exhibit significant differences.
We conducted sensitivity analysis to address high heterogeneity (I2 > 40%) through the methods of subgroup analysis or one-by-one article removal. We used meta-regression to identify the sources of high heterogeneity according to possible risk factors (25). Meta-regression analyses that produced a risk factor of p < 0.05 were followed by subgroup analysis, while those that produced a risk factor of p ≥ 0.05 were followed by one-by-one article removal (26).
Results
Study Selection
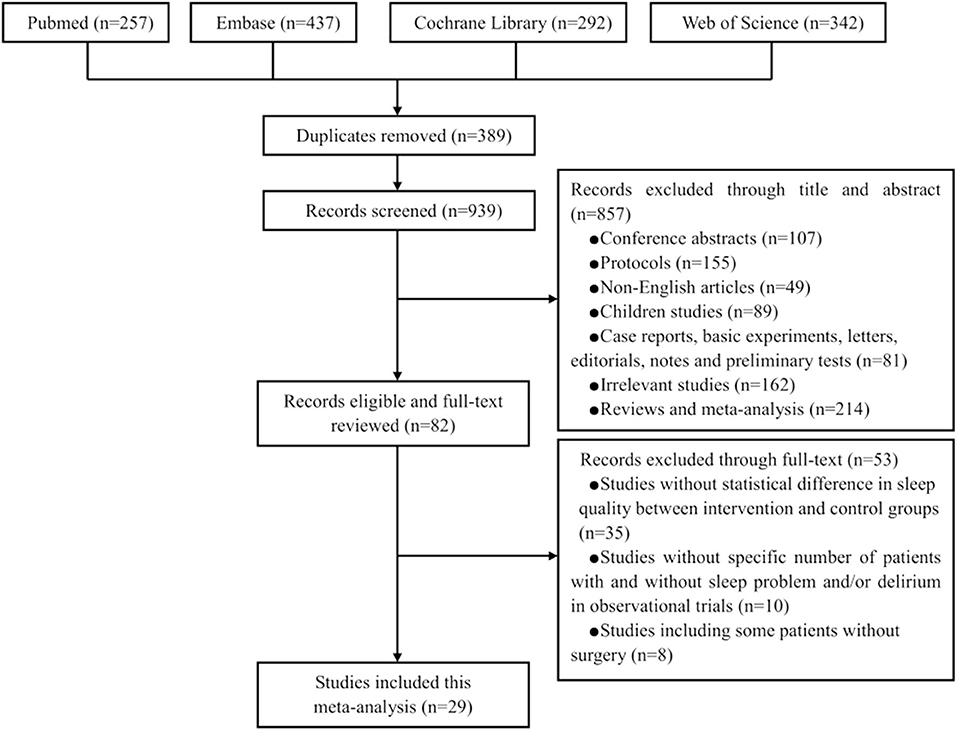
Figure 1 presents the PRISMA flow chart for our screening process. We obtained 257 trials from Pubmed, 437 from Embase, 292 from Cochrane Library, and 342 from Web of Science. We removed 389 duplicate trials and excluded 857 trials at the title-and-abstract review stage based on our exclusion criteria. We excluded 53 trials at the full-text review stage, including 35 without statistical differences in sleep quality between intervention and control groups, 10 without a specific number of patients with sleep problems and/or delirium, and eight that enrolled some patients without surgery. Eventually, our search strategy yielded 29 relevant trials with a total of 55,907 patients (Figure 1) (16–18, 27–52).
Study Characteristics
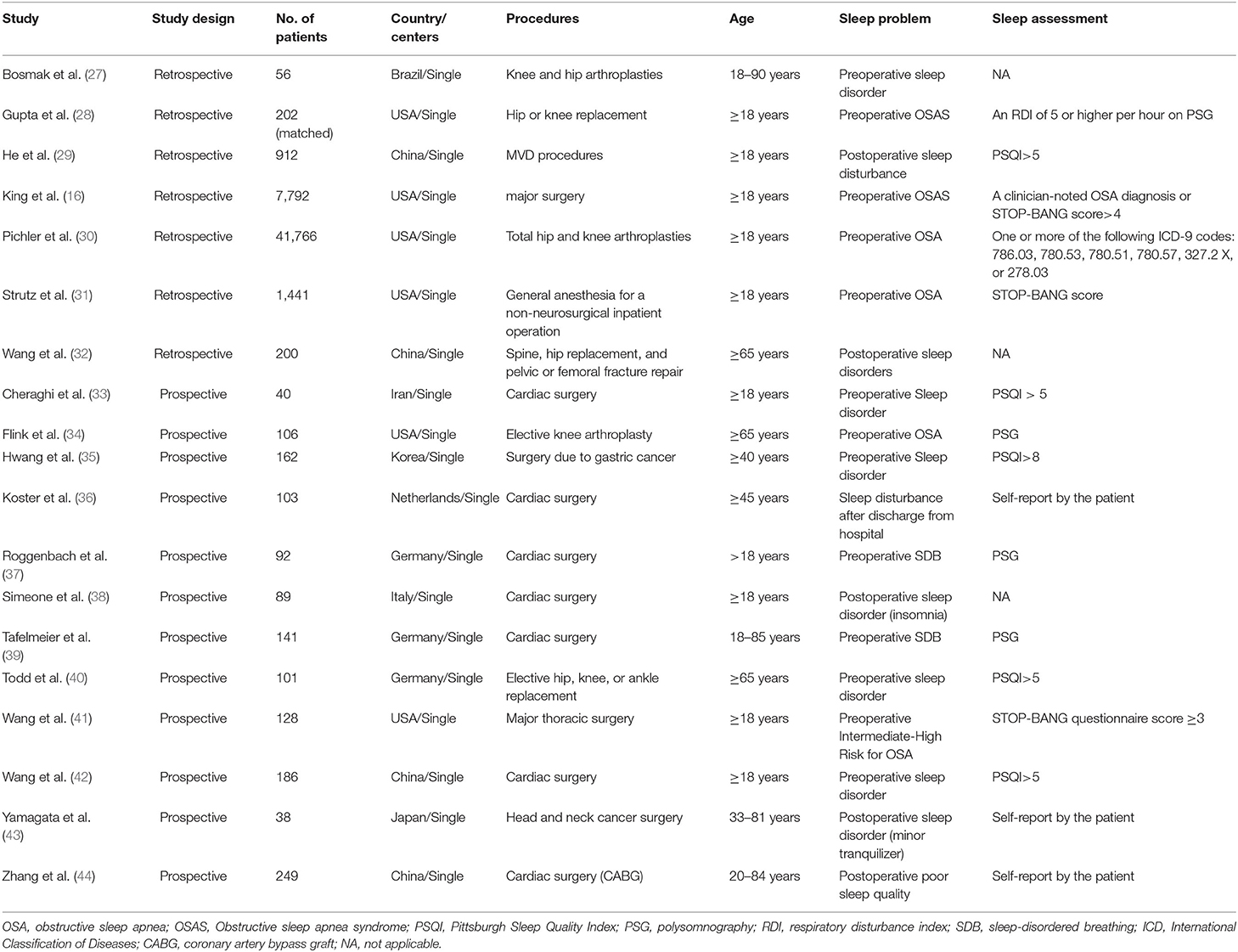
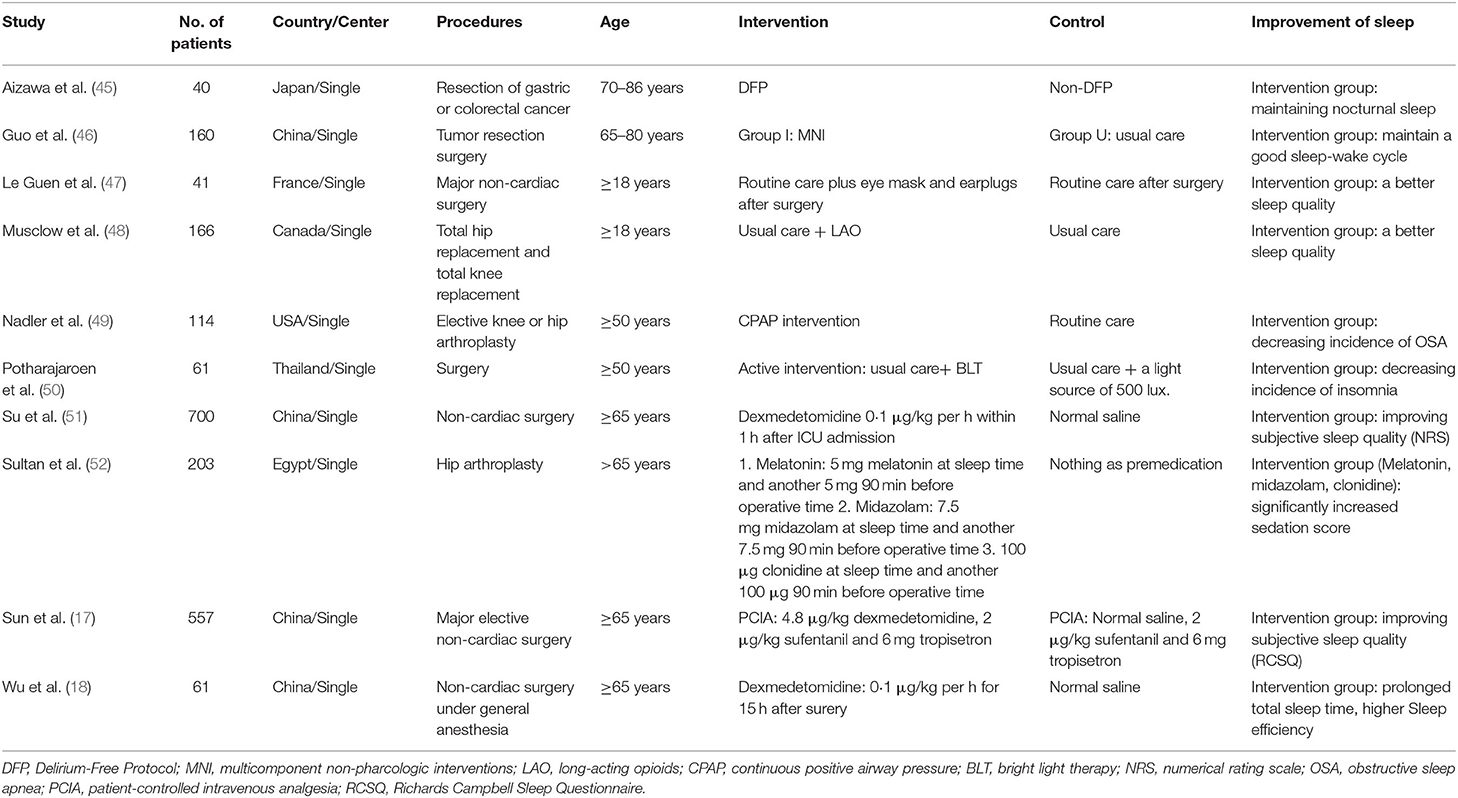
There were seven trials and 52,369 patients in the ROT group (16, 27–32), 12 trials and 1,435 patients in the POT group (33–44), and 10 trials and 2,103 patients in the RCT group (17, 18, 45–52). Tables 1, 2 presented the basic characteristics of the observational studies (retrospective and prospective) and RCTs, respectively. In one retrospective trial, the control group was matched for age, sex, operated side, type of operation, mode of component fixation, year of operation, surgeon, and type of anesthesia (28). In seven trials, patients only underwent cardiac surgery (33, 36–39, 42, 44), in 20 trials, only non-cardiac surgeries (17, 18, 27–30, 32, 34, 35, 40, 41, 43, 45–52), and in two trials, both cardiac and non-cardiac surgeries (16, 31). All the patients in the included RCTs underwent non-cardiac surgeries. The most prevalent type of non-cardiac surgery was orthopedic surgery. We obtained the mean age of patients in each study by adding the mean age of patients in each group and then dividing by two. In 19 trials, the mean age was 65 years or older (17, 18, 28, 30–32, 34, 35, 37–40, 45, 46, 48–52), though one trial did not provide participants' ages (36). Males accounted for 50% or more of all patients in 16 trials (16–18, 28, 31, 33, 35, 37–39, 41–45, 47), though two trials did not provide sex-related information (36, 51). Three trials did not disclose their method for assessing sleep disturbance (27, 32, 38). Nine trials focused on patients with sleep-disorder breathing or OSA (16, 28, 30, 31, 34, 37, 39, 41, 49), and the other trials studied patients with several sleep problems. Thirteen trials described the effect of preoperative sleep problems on the incidence of POD (16, 27, 28, 30, 31, 33–35, 37, 39–42), and the other trials described postoperative sleep quality.
We divided the patients in each study into two groups according to their sleep quality: one good sleep quality group and one poor sleep quality group (Table 3). The numbers of patients with good and poor sleep quality are shown in Table 3, alongside the numbers of patients with and without POD in each group. The onset time of POD was more than 3 days after surgery in 21 trials (16–18, 27–30, 32, 33, 35–38, 40–45, 48, 51).
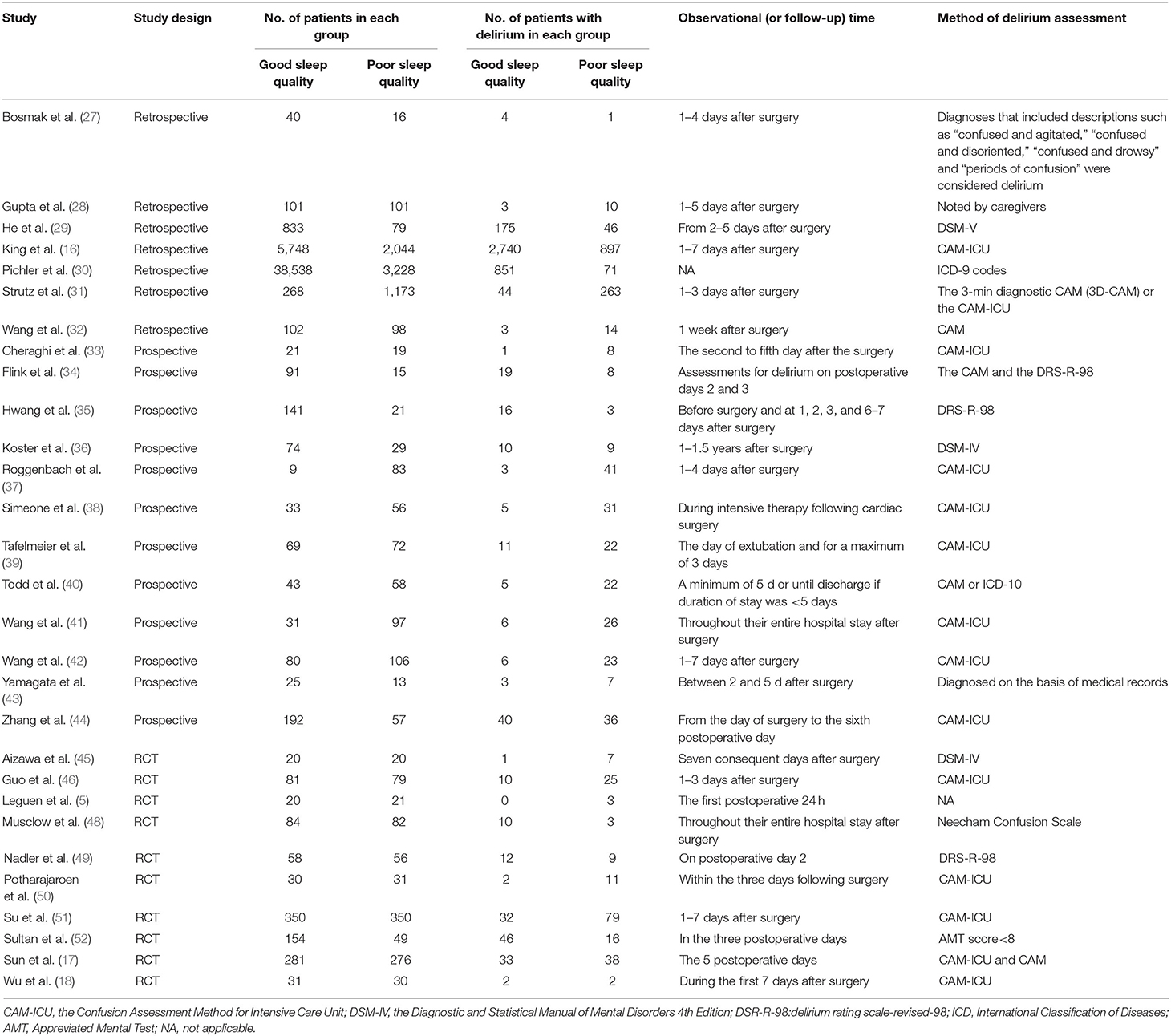
Table 3. The number of patients with POD under different sleep quality and assessment methods of POD.
Study Quality
We used NOS to assess the risk of bias in observational studies (retrospective and prospective), and 15 trials obtained seven stars or more, indicating high quality (Supplementary Table 2) (16, 28–32, 34–37, 39–42, 44). We used the Cochrane Collaboration Risk of Bias Assessment tool to assess the risk of bias in RCTs. Many of the included studies demonstrated low risk of bias, as they clearly assessed random sequence generation (seven studies-70%), allocation concealment (eight studies-80%), blinding of participants (seven studies-70%), blinding of outcome assessment (10 studies-100%), incomplete outcome data (nine studies-90%), and selective outcome reporting (nine studies-90%). Six RCTs were found to be high quality (Supplementary Figures 1, 2) (17, 18, 46–48, 51).
Publication Bias
We assessed publication bias using Egger's test by Stata 12.0 software. We did not find publication bias in the ROT (p = 0.085), POT (p = 0.764), or RCT (p = 0.933) groups (Figure 2 and Supplementary Table 3).
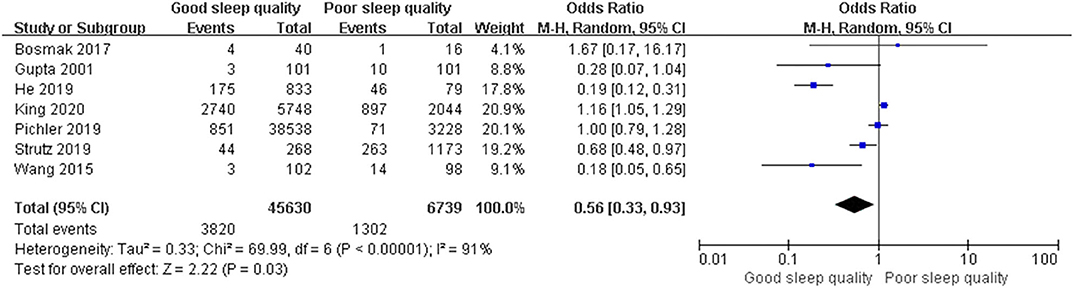
Figure 2. Publication bias of included trials by Egger's test. (A) ROTs group; (B) POTs group; (C) RCTs group.
Post-operative POD
We used a random-effect model with OR in the ROT (I2 = 91%) and RCT (I2 = 68%) groups due to high heterogeneity, and a fixed-effect model with OR in the POT group (I2 = 25%) due to low heterogeneity. The pooled results of the ROT group (OR = 0.56, 95% CI: [0.33, 0.93], I2 = 91%, p for effect = 0.03) and POT group (OR = 0.27, 95% CI: [0.20, 0.36], I2 = 25%, p for effect <0.001) demonstrated significant differences between patients with good and poor sleep quality in incidence of POD after surgery (Figures 3, 4). However, the pooled results of the RCT group (OR = 0.58, 95% CI: [0.34, 1.01], I2 = 68%, p for effect = 0.05) showed no significant differences between patients with good and poor sleep quality (Figure 5).
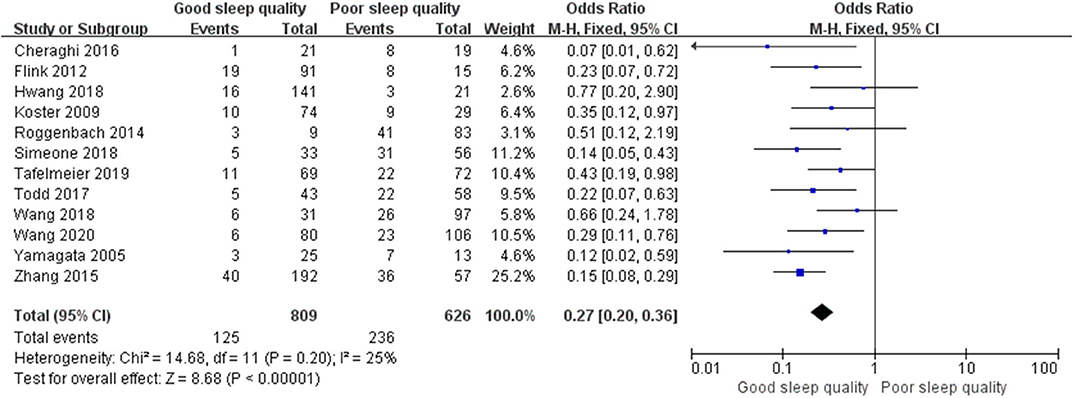
Figure 3. The pooled results of POD incidence after surgery between the patients with good and poor sleep quality in ROTs group.
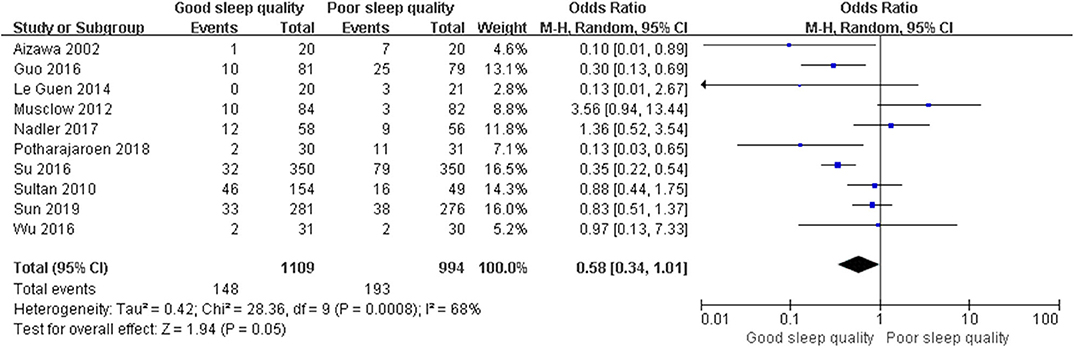
Figure 4. The pooled results of POD incidence after surgery between the patients with good and poor sleep quality in POTs group.
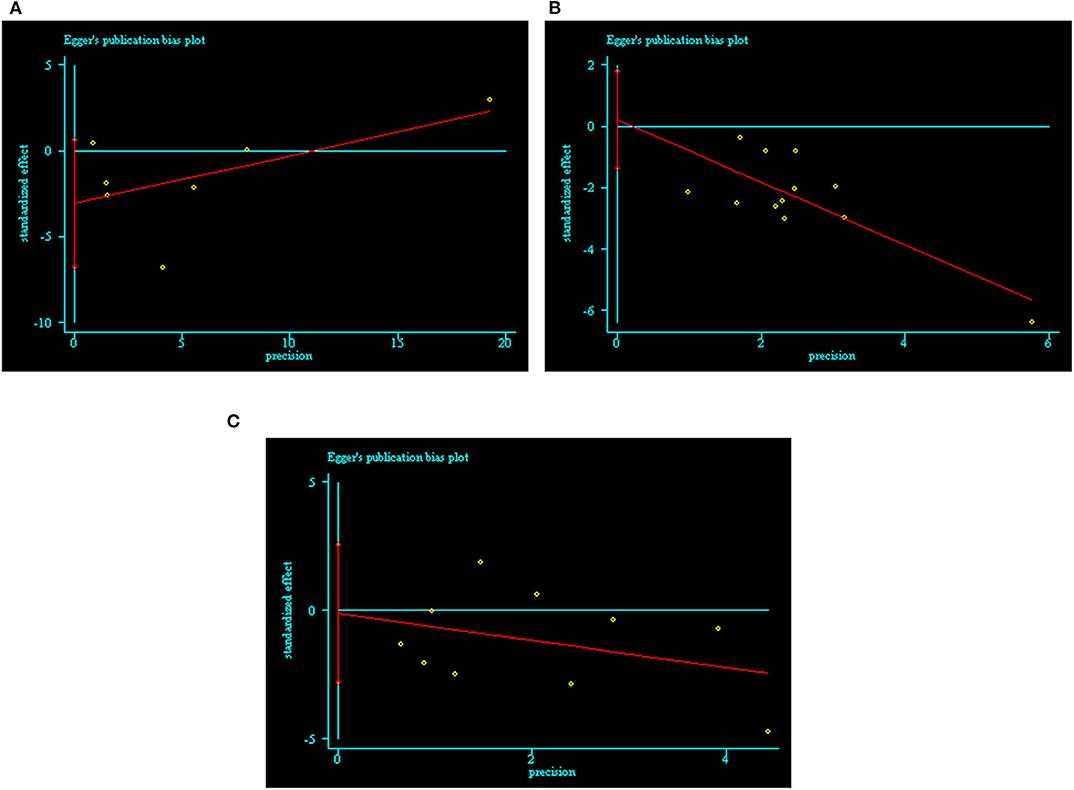
Figure 5. The pooled results of POD incidence after surgery between the patients with good and poor sleep quality in RCTs group.
Sensitivity Analysis
We performed meta-regression to identify the sources of heterogeneity in the ROT and RCT groups, assessing possible risk factors including publication year, average age (≥65 years and <65 years), male proportion (≥50% and <50%), surgery types (non-cardiac surgery, cardiac surgery, and cardiac and non-cardiac surgeries), onset time for POD (>3 days and ≤ 3 days), and study quality (low quality and high quality). Unexpectedly, all p-values for these risk factors were over 0.05 (Supplementary Tables 4, 5). Afterwards, we used the method of one-by-one literature removal and found that 4 trials were the main sources of heterogeneity in the ROT group (I2 dropped from 91 to 12%) and four trials were in the RCT group (I2 dropped from 68% to 19%).
We conducted post hoc meta-analysis for the remaining literature in these groups using a fixed-effects model with OR, and the pooled results were consistent with those prior to sensitivity analysis (ROT group: OR = 0.65, 95% CI: [0.47, 0.91], I2 = 12%, p for effect = 0.01; RCT group: OR = 0.82, 95% CI: [0.52, 1.29], I2 = 19%, p for effect = 0.39) (Figures 6, 7).
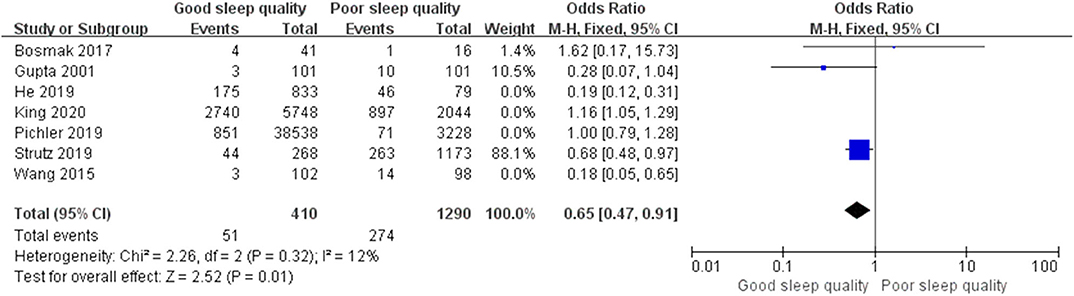
Figure 6. The pooled results of POD incidence after surgery between the patients with good and poor sleep quality in ROTs group after sensitivity analysis.
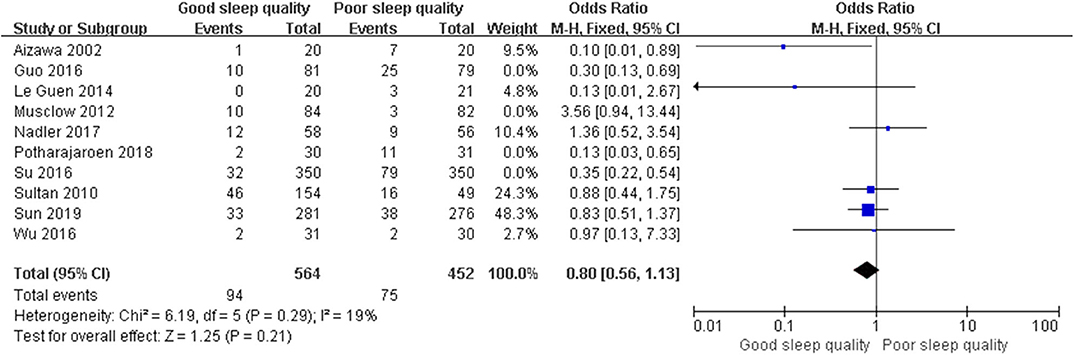
Figure 7. The pooled results of POD incidence after surgery between the patients with good and poor sleep quality in RCTs group after sensitivity analysis.
Discussion
This meta-analysis investigated the effect of perioperative sleep disturbance on the incidence of POD. The results from observational trials (retrospective and prospective) demonstrated that perioperative sleep disturbances were significantly associated with elevated POD incidence, while those from RCTs did not confirm this positive association.
POD, as a kind of mental disorder, is a knotty problem that patients may face following major surgery. It arises as the combined effect of multiple factors, which include advanced age, low education level, preoperative impaired cognition, alcohol abuse, smoking, cardiac or macrovascular surgery, major non-cardiac surgeries, perioperative administration of sedative and analgesic drugs, and postoperative imperfect analgesia, among others (53–55). Sleep problems are a hot topic in current clinical research due to their prevalence and potential negative impact on cognitive functions, including learning, memory, spatial orientation, behavioral capacity, and so on (9, 56–58). Furthermore, long-term sleep disturbances are intimately associated with major depression and dementia in adult populations, especially the aged (59, 60). The mechanism underlying sleep-disturbance-related cognitive dysfunction is still unclear. Some studies reported that poor sleep quality (OSA, disordered circadian rhythms, and psychologically-based sleep deprivation) could lead to neuronal apoptosis in those areas of the brain related to cognition, by means of neuroinflammation, changes in neurotransmitter activity (e.g., adenosine), and cerebral hypoxic and hypoperfusion injury (61–64). Other studies confirmed that sleep disturbance was rather common in the perioperative period, and that it seriously affected postoperative cognitive function (65, 66). A great number of studies have reported the effects of perioperative sleep disturbance on POD, and most of them concluded that poor perioperative sleep quality was an important risk factor of POD (67–69). We performed this meta-analysis to clarify and substantiate these findings, collecting as many articles as possible, listing their trial characteristics, and synthesizing their results.
All enrolled observational trials reported the POD incidence in patients exposed to sleep disturbances and non-sleep disturbances. Because the aim of this meta-analysis was to investigate the effect of perioperative sleep quality on POD, we selected studies with significant differences in sleep quality between intervention and control groups in RCTs. Different from the observational trials, in RCT group, some patients in the intervention group suffered sleep disturbances and some in the control group exhibited good sleep quality; thus, the negative pooled result of RCTs may be unreliable. We detected high heterogeneity in the ROT and RCT groups, which could affect the reliability of our meta-analysis results. We used sensitivity analysis—namely subgroup analysis and one-by-one literature exclusion—to address high heterogeneity (70, 71). In addition, though meta-analysis using a random-effect model did not solve heterogeneity, it did decrease the impact of significant heterogeneity on the pooled results (72). As a result, we conjectured that high heterogeneity may be the result of a combination of factors, and we used the method of one-by-one literature exclusion to solve this problem. Consequently, eight trials were excluded from the ROT and RCT groups (that is, 4 trials each). We then used a fixed-effects model with OR to conduct meta-analyses for the remaining literature in these groups, and the pooled results were consistent with those prior to sensitivity analysis.
Publication bias was another problem affecting the reliability of meta-analysis results (73). Detecting and adjusting publication bias is indispensable for meta-analysis. Currently, the primary methods for detecting publication bias include the rank correlation test (Begg's test, Schwarzer's test, and arcsine Begg's test), the regression test (Egger's test, Macaskill's regression, Harbord's test, Peters' test, and arcsine regression methods), and funnel plots. Funnel plots are not suitable for meta-analyses with few traits and high heterogeneity due to their tendency to produce asymmetric graphs (74, 75). In this meta-analysis, there were only seven trials in the ROT group, so we did not draw a funnel plot. Of the other methods, Egger's test has the highest power and the most accurate p-value, and it is easy to understand. As such, it is the most popular method for detecting publication bias (24). If the p-value for a given group was <0.05 following Egger's test, we determined that this group exhibited significant publication bias. We used trim-and-fill analysis to adjust publication bias (24). Unexpectedly, there was no evidence of significant publication bias in any of the three groups in this meta-analysis.
The advantages of this meta-analysis were as follows. First, this meta-analysis included as many trials as possible, and it did not exclude trials based on study methods. Second, we selected observational trials with exposure and non-exposure factors (sleep disturbances) and RCTs with significant differences in risk factors (sleep quality) between intervention and control groups; thus, the results were more convincing. Third, grouping the studies according to their different study methods helped us to synthesize data. Fourth, different sleep disturbances (OSA, disordered circadian rhythms, and psychologically-based sleep deprivation) were included in this meta-analysis, allowing us to analyze the effects of perioperative sleep quality on POD more comprehensively.
Several limitations should also be taken into consideration in our meta-analysis. First of all, although we observed a positive correlation between sleep disturbance and POD in the observational study groups (retrospective and prospective), this finding may be less reliable than it could be because of the inevitable selection bias (76). Meanwhile, the most enrolled observational studies in this meta-analysis presented small sample size, which may attenuate the reliability of synthesized results as well (77). Furthermore, the study from Gupta et al. (28) only provided the number of matched patients in the control group, therefore we were not sure whether real-world research would affect the pooled results. In addition, although there was a striking difference in sleep quality between the RCTs' intervention and control groups, some patients in the intervention group suffered sleep disturbances and some in the control group exhibited good sleep quality; thus, the negative pooled result of RCTs may be unreliable. Therefore, a real-world prospective observational study with large sample size can significantly elevate the validity and reliability of results in spite of selection bias (78, 79). Different onset time and POD assessment methods may also have affected the reliability of our pooled results. Lastly, the low-quality literature in each of the three groups likely compromised the reliability of our pooled results as well.
Conclusion
This systematic review and meta-analysis demonstrated that perioperative sleep disturbances were significantly associated with elevated POD incidence in observational trials (retrospective and prospective), but not in RCTs. Despite this inconsistency, we suggest that perioperative sleep disturbance could be a potential risk factor for POD, and that clinicians should pay careful attention to this phenomenon. In the future, the high-quality real-world prospective observational trial with large sample size will be required to further prove the effect of perioperative sleep disturbances on POD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HW and LZ were responsible for document retrieval and were responsible for adjusting data discrepancies. ZZ and YL performed the screening process for titles and abstracts. HW and SY performed the screening process for full texts. YL and QL were responsible for extracting the data. QL and SY independently assessed the quality of included studies. QL conducted the statistical analysis and made the figures and tables. HW prepared the manuscript. FY supervised the whole process and ensured the effectiveness of the meta-analysis. All authors read and approved the submission of the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Dr. Yang Wang (Department of Biostatistics, the Chinese Academy of Medical Sciences, Fuwai Hospital, China) for his help with statistical data management.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.570362/full#supplementary-material
Supplementary Figure 1. Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included RCT study.
Supplementary Figure 2. Risk of bias summary: review authors' judgements about each risk of bias item for each included RCT study.
Supplementary Table 1. The guidelines of the 2009 PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses).
Supplementary Table 2. Bias risk of observational studies (retrospective and prospective) by NOS.
Supplementary Table 3. Egger's test for publication bias of included trials.
Supplementary Table 4. Retrospective observational studies-meta regression based on risk factors of high heterogenicity.
Supplementary Table 5. RCT-meta regression based on risk factors of high heterogenicity.
References
1. Lipowski ZJ. Delirium (acute confusional states). JAMA. (1987) 258:1789–92. doi: 10.1001/jama.258.13.1789
2. Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. (2017) 390:267–75. doi: 10.1016/S0140-6736(17)31467-8
3. Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium. F1000Res. (2019) 8:F1000 Faculty Rev−607. doi: 10.12688/f1000research.16780.1
4. Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. (2012) 367:1164. doi: 10.1056/NEJMoa1112923
5. Luger MF, Muller S, Kammerlander C, Gosch M, Luger TJ. Predictors of postoperative cognitive decline in very old patients with hip fracture: a retrospective analysis. Geriatr Orthop Surg Rehabil. (2014) 5:165–72. doi: 10.1177/2151458514548577
6. Cok OY, Seet E, Kumar CM, Joshi GP. Perioperative considerations and anesthesia management in patients with obstructive sleep apnea undergoing ophthalmic surgery. J Cataract Refract Surg. (2019) 45:1026–31. doi: 10.1016/j.jcrs.2019.02.044
7. Madsen MT, Rosenberg J, GaGenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. (2013) 9:387–94. doi: 10.5664/jcsm.2598
8. Rhon DI, Snodgrass SJ, Cleland JA, Cook CE. Comorbid insomnia and sleep apnea are associated with greater downstream health care utilization and chronic opioid use after arthroscopic hip surgery. Pain Physician. (2019) 22:E351–60.
9. Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. (2014) 18:273–82. doi: 10.1016/j.smrv.2013.07.002
10. Slatore CG, Goy ER, O'hearn DJ, Boudreau EA, O'Malley JP, Peters D, et al. Sleep quality and its association with delirium among veterans enrolled in hospice. Am J Geriatr Psychiatry. (2012) 20:317–26. doi: 10.1097/JGP.0b013e3182487680
11. Waters F, Chiu V, Atkinson A, Blom JD. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry. (2018) 9:303. doi: 10.3389/fpsyt.2018.00303
12. Lam EWK, Chung F, Wong J. Sleep-disordered breathing, postoperative delirium, and cognitive impairment. Anesth Analg. (2017) 124:1626–35. doi: 10.1213/ANE.0000000000001914
13. Hu RF, Jiang XY, Chen J, Zeng Z, Chen XY, Li Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. (2015) 2015:CD008808. doi: 10.1002/14651858.CD008808.pub2
14. Tabet N, Howard R. Pharmacological treatment for the prevention of delirium: review of current evidence. Int J Geriatr Psychiatry. (2009) 24:1037–44. doi: 10.1002/gps.2220
15. Smithburger PL, Patel MK. Pharmacologic considerations surrounding sedation, delirium, and sleep in critically Ill adults: a narrative review. J Pharm Pract. (2019) 32:271–91. doi: 10.1177/0897190019840120
16. King CR, Fritz BA, Escallier K, Ju YES, Lin N, McKinnon S, et al. Association between preoperative obstructive sleep apnea and preoperative positive airway pressure with postoperative intensive care unit delirium. JAMA Netw Open. (2020) 3:e203125. doi: 10.1001/jamanetworkopen.2020.3125
17. Sun Y, Jiang M, Ji Y, Sun Y, Liu Y, Shen W. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther. (2019) 13:2911–22. doi: 10.2147/DDDT.S208703
18. Wu XH, Cui F, Zhang C, Meng ZT, Wang DX, Ma J, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit. Anesthesiology. (2016) 125:979–91. doi: 10.1097/ALN.0000000000001325
19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21. Koster G, Wetterslev J, Gluud C, Zijlstra JG, Scheeren TW, van der Horst IC, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. (2015) 41:203–21. doi: 10.1007/s00134-014-3604-1
22. Aziz O, Athanasiou T, Darzi A. Minimally invasive conduit harvesting: a systematic review. Eur J Cardiothorac Surg. (2006) 29:324–33. doi: 10.1016/j.ejcts.2005.11.032
23. Chen P, Wu X, Wang Z, Li Z, Tian X, Wang J, et al. Effects of levosimendan on mortality in patients undergoing cardiac surgery: a systematic review and meta-analysis. J Card Surg. (2018) 33:322–9. doi: 10.1111/jocs.13716
24. Jin ZC, Zhou XH, He J. Statistical methods for dealing with publication bias in meta-analysis. Stat Med. (2015) 34:343–60. doi: 10.1002/sim.6342
25. Ma PL, Peng XX, Du B, Hu XL, Gong YC, Wang Y, et al. Sources of heterogeneity in trials reporting hydroxyethyl starch 130/0.4 or 0.42 associated excess mortality in septic patients: a systematic review and meta-regression. Chin Med J (Engl). (2015) 128:2374–82. doi: 10.4103/0366-6999.163387
26. Yan G, Wang J, Zhang J, Gao K, Zhao Q, Xu X. Long-term outcomes of macrovascular diseases and metabolic indicators of bariatric surgery for severe obesity type 2 diabetes patients with a meta-analysis. PLoS ONE. (2019) 14:e0224828. doi: 10.1371/journal.pone.0224828
27. Bosmak FS, Gibim PT, Guimarães S, Ammirati AL. Incidence of delirium in postoperative patients treated with total knee and hip arthroplasty. Rev Assoc Med Bras 1992. (2017) 63:248–51. doi: 10.1590/1806-9282.63.03.248
28. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. (2001) 76:897–905. doi: 10.1016/S0025-6196(11)62108-3
29. He Z, Cheng H, Wu H, Sun G, Yuan J. Risk factors for postoperative delirium in patients undergoing microvascular decompression. PLoS ONE. (2019) 14:e0215374. doi: 10.1371/journal.pone.0215374
30. Pichler L, Weinstein SM, Cozowicz C, Poeran J, Liu J, Poultsides LA, et al. Perioperative impact of sleep apnea in a high-volume specialty practice with a strong focus on regional anesthesia: a database analysis. Reg Anesth Pain Med. (2019) doi: 10.1136/rapm-2018-000038. [Epub ahead of print].
31. Strutz PK, Kronzer V, Tzeng W, Arrington B, McKinnon SL, Ben Abdallah A, et al. The relationship between obstructive sleep apnoea and postoperative delirium and pain: an observational study of a surgical cohort. Anaesthesia. (2019) 74:1542–50. doi: 10.1111/anae.14855
32. Wang J, Li Z, Yu Y, Li B, Shao G, Wang Q. Risk factors contributing to postoperative delirium in geriatric patients postorthopedic surgery. Asia Pac Psychiatry. (2015) 7:375–82. doi: 10.1111/appy.12193
33. Cheraghi MA, Hazaryan M, Bahramnezhad F, Mirzaeipour F, Haghani H. Study of the relationship between sleep quality and prevalence of delirium in patients undergoing cardiac surgery. World J. Med. Sci. (2016) 13:60–64.
34. Flink BJ, Rivelli SK, Cox EA, White WD, Falcone G, Vail TP, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. (2012) 116:788–96. doi: 10.1097/ALN.0b013e31824b94fc
35. Hwang H, Lee KM, Son KL, Jung D, Kim WH, Lee JY, et al. Incidence and risk factors of subsyndromal delirium after curative resection of gastric cancer. BMC Cancer. (2018) 18:765. doi: 10.1186/s12885-018-4681-2
36. Koster S, Hensens AG, Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. (2009) 87:1469–74. doi: 10.1016/j.athoracsur.2009.02.080
37. Roggenbach J, Klamann M, von Haken R, Bruckner T, Karck M, Hofer S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit Care. (2014) 18:477. doi: 10.1186/s13054-014-0477-1
38. Simeone S, Pucciarelli G, Perrone M, Teresa R, Gargiulo G, Guillari A, et al. Delirium in ICU patients following cardiac surgery: an observational study. J Clin Nurs. (2018) 27:1994–2002. doi: 10.1111/jocn.14324
39. Tafelmeier M, Knapp M, Lebek S, Floerchinger B, Camboni D, Creutzenberg M, et al. Predictors of delirium after cardiac surgery in patients with sleep disordered breathing. Eur Respir J. (2019) 54:1900354. doi: 10.1183/13993003.00354-2019
40. Todd OM, Gelrich L, MacLullich AM, Driessen M, Thomas C, Kreisel SH. Sleep disruption at home as an independent risk factor for postoperative delirium. J Am Geriatr Soc. (2017) 65:949–57. doi: 10.1111/jgs.14685
41. Wang S, Sigua NL, Manchanda S, Gradney S, Khan SH, Perkins A, et al. Preoperative STOP-BANG scores and postoperative delirium and coma in thoracic surgery patients. Ann Thorac Surg. (2018) 106:966–72. doi: 10.1016/j.athoracsur.2018.05.089
42. Wang H, Zhang L, Luo Q, Li Y, Yan F. Effect of sleep disorder on delirium in post-cardiac surgery patients. Can J Neurol Sci. (2020) 47:627–33. doi: 10.1017/cjn.2020.62
43. Yamagata K, Onizawa K, Yusa H, Wakatsuki T, Yanagawa T, Yoshida H. Risk factors for postoperative delirium in patients undergoing head and neck cancer surgery. Int J Oral Maxillofac Surg. (2005) 34:33–6. doi: 10.1016/j.ijom.2004.03.005
44. Zhang W, Wu W, Gu J, Sun Y, Ye X, Qiu W, et al. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: a prospective cohort study. J Crit Care. (2015) 30:606–12. doi: 10.1016/j.jcrc.2015.02.003
45. Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, et al. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg Today. (2002) 32:310–14. doi: 10.1007/s005950200044
46. Guo Y, Sun L, Li L, Jia P, Zhang J, Jiang H, et al. Impact of multicomponent, nonpharmacologic interventions on perioperative cortisol and melatonin levels and postoperative delirium in elderly oral cancer patients. Arch Gerontol Geriatr. (2016) 62:112–7. doi: 10.1016/j.archger.2015.10.009
47. Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. (2014) 112:89–95. doi: 10.1093/bja/aet304
48. Musclow SL, Bowers T, Vo H, Glube M, Nguyen T. Long-acting morphine following hip or knee replacement: a randomized, double-blind, placebo-controlled trial. Pain Res Manage. (2012) 17:83–88. doi: 10.1155/2012/704932
49. Nadler JW, Evans JL, Fang E, Preud'Homme XA, Daughtry RL, Chapman JB, et al. A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia. (2017) 72:729–36. doi: 10.1111/anae.13833
50. Potharajaroen S, Tangwongchai S, Tayjasanant T, Thawitsri T, Anderson G, Maes M. Bright light and oxygen therapies decrease delirium risk in critically ill surgical patients by targeting sleep and acid-base disturbances. Psychiatry Res. (2018) 261:21–27. doi: 10.1016/j.psychres.2017.12.046
51. Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. (2016) 388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3
52. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. (2010) 4:169–73. doi: 10.4103/1658-354X.71132
53. Kang SY, Seo SW, Kim JY. Comprehensive risk factor evaluation of postoperative delirium following major surgery: clinical data warehouse analysis. Neurol Sci. (2019) 40:793–800. doi: 10.1007/s10072-019-3730-1
54. Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS ONE. (2015) 10:e0136071. doi: 10.1371/journal.pone.0136071
55. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. (2007) 120:807–13. doi: 10.1016/j.amjmed.2007.02.026
56. Cordeira J, Kolluru SS, Rosenblatt H, Kry J, Strecker RE, McCarley RW. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res. (2018) 339:124–29. doi: 10.1016/j.bbr.2017.11.033
57. Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of experimental sleep deprivation on anxiety-like behavior in animal research: systematic review and meta-analysis. Neurosci Biobehav Rev. (2016) 68:575–89. doi: 10.1016/j.neubiorev.2016.06.028
58. Valera S, Guadagni V, Slone E, Burles F, Ferrara M, Campbell T, et al. Poor sleep quality affects spatial orientation in virtual environments. Sleep Sci. (2016) 9:225–31. doi: 10.1016/j.slsci.2016.10.005
60. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. (2018) 40:4–16. doi: 10.1016/j.smrv.2017.06.010
61. Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. (2007) 64:934–46. doi: 10.1007/s00018-007-6457-8
62. Trošt Bobić T, Šečić A, Zavoreo I, Matijević V, Filipović B, Kolak Ž, et al. The impact of sleep deprivation on the brain. Acta Clin Croat. (2016) 55:469–73. doi: 10.20471/acc.2016.55.03.17
63. Yin M, Chen Y, Zheng H, Pu T, Marshall C, Wu T, et al. Assessment of mouse cognitive and anxiety-like behaviors and hippocampal inflammation following a repeated and intermittent paradoxical sleep. Behav Brain Res. (2017) 321:69–78. doi: 10.1016/j.bbr.2016.12.034
64. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. J Neurosci Res. (2017) 95:943–72. doi: 10.1002/jnr.23777
65. Sonobe S, Inoue S, Kawaguchi M. The effects of intensive care environment on postoperative nightmare. J Anesth. (2016) 30:970–6. doi: 10.1007/s00540-016-2237-7
66. Mansano-Schlosser TC, Ceolim MF, Valerio TD. Poor sleep quality, depression and hope before breast cancer surgery. Appl Nurs Res. (2017) 34:7–11. doi: 10.1016/j.apnr.2016.11.010
67. Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. (2018) 46:e1204–12. doi: 10.1097/CCM.0000000000003400
68. Leung JM, Sands LP, Newman S, Meckler G, Xie Y, Gay C, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med. (2015) 11:907–13. doi: 10.5664/jcsm.4944
69. Lu Y, Li YW, Wang L, Lydic R, Baghdoyan HA, Shi XY, et al. Promoting sleep and circadian health may prevent postoperative delirium: a systematic review and meta-analysis of randomized clinical trials. Sleep Med Rev. (2019) 48:101207. doi: 10.1016/j.smrv.2019.08.001
70. Brunetti ND, Santoro F, Correale M, De Gennaro L, Conte G, Di Biase M. Incidence of atrial fibrillation is associated with age and gender in subjects practicing physical exercise: a meta-analysis and meta-regression analysis. Int J Cardiol. (2016) 221:1056–60. doi: 10.1016/j.ijcard.2016.07.133
71. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. (2008) 37:1148–57. doi: 10.1093/ije/dyn065
72. Moreno E, Vázquez-Polo FJ, Negrín MA. Bayesian meta-analysis: the role of the between-sample heterogeneity. Stat Methods Med Res. (2018) 27:3643–57. doi: 10.1177/0962280217709837
73. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
74. Biljana M, Jelena M, Branislav J, Milorad R. Bias in meta-analysis and funnel plot asymmetry. Stud Health Technol Inform. (1999) 68:323–8.
75. Barone P, Corradi G, Gomila A. Infants' performance in spontaneous-response false belief tasks: a review and meta-analysis. Infant Behav Dev. (2019) 57:101350. doi: 10.1016/j.infbeh.2019.101350
76. Dimick JB, Livingston EH. Comparing treatments using observational study designs: what can we do about selection bias? Arch Surg. (2010) 145:927. doi: 10.1001/archsurg.2010.223
77. Harding G, Coyne K, Barrett RJ, Pixton GC. Modified visual analog scale symptom-intensity and overall-bother measures for the assessment of symptoms in studies of pharmacologic stress agents. Clin Ther. (2009) 31:889–901. doi: 10.1016/j.clinthera.2009.04.009
78. Du X, Khamitova A, Kyhlstedt M, Sun S, Sengoelge M. Utilisation of real-world data from heart failure registries in OECD countries - A systematic review. Int J Cardiol Heart Vasc. (2018) 19:90–97. doi: 10.1016/j.ijcha.2018.02.006
Keywords: sleep disturbances, surgery, postoperative delirium, adult, meta-analysis
Citation: Wang H, Zhang L, Zhang Z, Li Y, Luo Q, Yuan S and Yan F (2020) Perioperative Sleep Disturbances and Postoperative Delirium in Adult Patients: A Systematic Review and Meta-Analysis of Clinical Trials. Front. Psychiatry 11:570362. doi: 10.3389/fpsyt.2020.570362
Received: 07 June 2020; Accepted: 14 September 2020;
Published: 14 October 2020.
Edited by:
Jihui Zhang, The Chinese University of Hong Kong, ChinaReviewed by:
Brian K. Gehlbach, The University of Iowa, United StatesAxel Steiger, Ludwig Maximilian University of Munich, Germany
Copyright © 2020 Wang, Zhang, Zhang, Li, Luo, Yuan and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Yuan, ZnV3YWl5cyYjeDAwMDQwOzEyNi5jb20=
 Hongbai Wang1
Hongbai Wang1 Liang Zhang
Liang Zhang Su Yuan
Su Yuan Fuxia Yan
Fuxia Yan