- 1Department of Cardiology, The Second Hospital of Jilin University, Changchun, China
- 2Department of Pediatrics, The Second Hospital of Jilin University, Changchun, China
- 3Department of Developmental Pediatrics, The Second Hospital of Jilin University, Changchun, China
- 4Department of Developmental and Behavioral Pediatrics, The First Hospital of Jilin University, Changchun, China
- 5Department of Physiology, College of Basic Medical Sciences, Jilin University, Changchun, China
The implication of different dietary n-3/n-6 polyunsaturated fatty acids (PUFAs) ratios has been investigated in some neurodevelopmental disorders (including autism and depression). However, the mechanisms underlying the effects of different PUFAs ratios on the autism are still poorly understood. In the present study, a valproic acid (VPA) rat model of autism was used to study the effects of diet with different n-3/n-6 PUFA ratios on the autism, and the underlying mechanisms explored. Our results showed that rats with prenatal administration of VPA took less response time to sniff three odorants in the olfactory habituation/dishabituation tests, had lower frequency of pinning and following patterns, and had decreased hippocampal 5-hydroxytryptamine (5-HT), increased serum 5-HT and downregulated expression of tight junction protein (occludin and claudin-1) in the colon. However, supplementation of n-3/n-6 PUFAs (1:5) in the VPA treated rats ameliorated the autistic behaviors, increased hippocampal 5-HT and tight junction expression in the colon, and decreased serum 5-HT. In conclusion, dietary supplementation of n-3/n-6 PUFAs (1:5) significantly improves VPA-induced autism-like behaviors in rats, which may be, at least partially, related to the increased hippocampal 5-HT. Furthermore, this diet can increase the expression of tight junction proteins to improve intestinal barrier impairment.
Introduction
The autism spectrum disorder (ASD), a type of developmental neurobiological disorders, is characterized by the pervasive impairments of social interaction and communication capabilities, and the restricted, repetitive, and stereotyped behaviors, activities, and interests (1). Generally, the prevalence of autism is about 1% in young and adult population (2). It is has been revealed that both environmental and genetic factors are related to the pathogenesis of ASDs (3).
Clinically, valproic acid (VPA) is used as an anticonvulsant and mood-stabilizing drug, and there is evidence showing that prenatal VPA exposure may cause maladaptive behaviors and birth defects and increase the risk of autism (4). It has been reported that epileptic mothers with VPA treatment during the pregnancy have autistic offspring (5). In our study, results showed rats treated with VPA at embryo day 12.5 (E12.5) developed ASD syndromes. Prenatal administration of VPA (Pre-VPA) in rats is an acceptable practice in the establishment of animal model of autism, and these autistic rodents have a wide range of characteristics similar to those in humans (6).
It has been reported that gastrointestinal symptoms (such as abdominal pain, diarrhea, vomiting, chronic constipation, and gastroesophageal reflux) are common in ASD children, but the prevalence of these symptoms varies in ASD children of different studies. As compared to healthy children, ASD children are more susceptible to gastrointestinal symptoms (7). The intestinal tract is also referred as “second brain” due to its abundance of enteric nerves, because it has vital roles and functions in the immune system as well as neurological system. Increasing evidence shows that signals of the intestinal immune disturbances can be transduced to the brain through multiply pathways, influencing the emotions and behaviors (8). Normal intestinal barrier function plays a vital role in maintaining immunity and overall wellbeing (9). At least, over 50 proteins have been identified to play significant roles in the regulation of tight junction in the mucosal endothelial layer and the intestinal permeability (10). The tight junction complexes include four families of transmembrane proteins. They are tricellulin, occludin, junctional adhesion molecules, and claudins. Normally, the tight junction proteins maintain the polarization of intestinal barrier to regulate the paracellular passage to transport small molecules only, such as leukocytes, ions, solutes and water.
Some studies, including ours, have shown that prenatal administration of VPA in rats may cause some cerebral and intestinal abnormalities similar to those in ASD patients (11–13).
Dietary supplementation of n-3 PUFAs can improve the intestinal barrier function in rats. It has been reported that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) can prevent deoxynivalenol-induced intestinal cell injury and reinforce barrier function, which is associated with the inhibition of necroptosis signaling pathway (14). Furthermore, Zhu et al. found that marine oil supplementation was beneficial for the improvement of intestinal barrier function and inhibition of CRH/CRHR1 signaling pathway and mast cell density (15). Thus, we speculate that dietary supplementation of n-3/n-6 PUFAs may be conducive to improve the impaired intestinal barrier in autistic rats.
Dietary supplementation of n-3 PUFAs has been studied in the treatment of neurologic disorders such as ASD. n-3/n-6 PUFAs are abundant in the central nervous system (CNS), particularly DHA (n-3) and to a lesser extent arachidonic acid (AA, n-6) (16). PUFAs cannot be produced in humans and should be obtained via diet (17). However, the intake of n-3 PUFAs in the diet has declined. In Western diet, n-6/n-3 PUFAs ratio is up to 16:1, which is significantly higher than 1:1 in the traditional diet. This is of special concern because competition between dietary PUFAs from plant sources and long-chain PUFAs for the same enzymes functioning for elongation and desaturation. Therefore, excess n-6 PUFAs may lead to the translocation of n-3 PUFAs in cellular membranes. n-6 and n-3 PUFAs are derived from eicosaoids generated by EPA and AA, respectively, and may exert almost opposite effects. Thus, the balance between n-3 and n-6 PUFAs is important for the homeostasis. It has been found that the imbalance between n-3 and n-6 PUFAs may cause vasoconstriction (and subsequent restricted or decreased bloodstream), thrombosis, and inflammation (18). In children with attention-deficit hyperactivity disorder (ADHD) or ASD, the serum levels of AA, EPA, and DHA are low, while the ratio of n-6/n-3 PUFAs is higher, which is significantly associated with ASD symptoms (19). Therefore, supplementation of dietary n-3/n-6 PUFAs may affect the autistic behaviors.
In this study, rats received prenatal administration of VPA to induce ASD-relevant symptoms, following behavioral assessment, colonic pathology, and biochemistry. In addition, the effects of daily supplementation of n-3/n-6 PUFAs (1:5) on the autism were further investigated by behavioral tests and detections of serum and hippocampal 5-hydroxytryptamine (5-HT) and the expression of tight junction proteins in the colon. The present study was under-taken to investigate the potential of daily supplementation of n-3/n-6 PUFAs in prenatal VPA induced autism in rats.
Materials and Methods
Animal Model
The animal study was reviewed and approved by the Ethics Committee of the Second Hospital and College of Basic Medical Sciences of Jilin University, and all procedures were conducted according to the Guideline for Animal Care and Use of Jilin University. Five adult male Wistar rats (220–240 g) were selected to mate with 10 female ones (2 weeks old and 350–500 g). After mating, gestational day 1 (G1) was defined as the day of presence of sperms on a vaginal smear. The pregnant rats at E12.5 were grouped as follow: Control group (n = 3) (rats were intraperitoneally administered with 100 μl of 0.9% saline) and VPA group (n = 7) (rats were intraperitoneally administered with VPA at 600 mg/kg once).
On the 12th day after birth, 8 male young offspring of mothers in the control group served as controls. In addition, 24 male young offspring of mothers with VPA treatment were divided into VPA group, A group and B group (n = 8 per group).
Diet
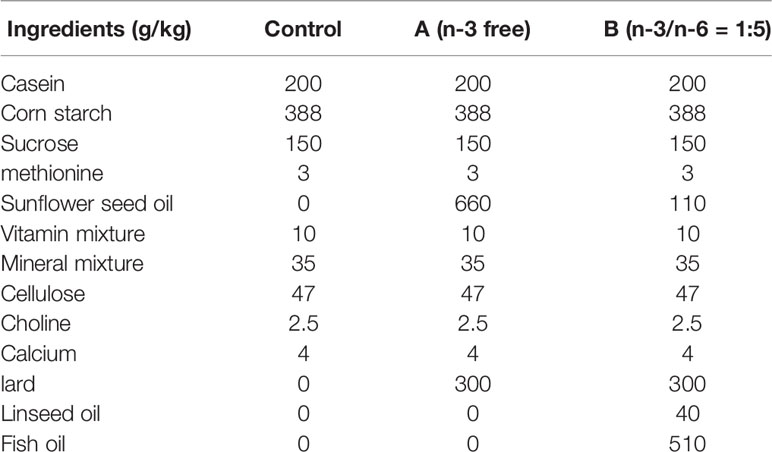
All neonatal rats received breast-feeding for 21 days. Then, these rats were given ad libitum access to standard chow containing 11.6% fat, 18.8% protein, and 66.7% carbohydrate in the control group and VPA group. In the “A” group, rats were given ad libitum access to n-3 free chow; in the “B” group, rats were given ad libitum access to chow supplemented with n-3/n-6 PUFAs at 1:5. Nutrients of each type of chow are as shown in Table 1.
Behavioral Tests
Olfactory Habituation/Dishabituation
The behavior test begins at the 11th week after birth. Olfaction is a major communicative way between rats (20). Rats were separately housed in clean plastic cages (24 cm × 14 cm × 13 cm). A detachable plexiglas covered with a 1.25-cm hole was used for behavioral habituation testing on the 30th day. After 2-min acclimation, a saline-saturated swab was placed through the hole to a 2.5-cm deep position and remained for 5 min with 3 cycles. Then, a swab saturated with another rat’s urine was used for habitation testing for 5 min with 3 cycles. Subsequently, a beer-soaked cotton swab was used for 5 min with 3 cycles. Before and after each test, 75% ethanol was used to sterile the plexiglas cover. A digital stopwatch was employed to record the response time to active sniffing. The mean response time was calculated.
Social Play Behaviors
The experiments were done in a sound attenuated but dimly-lit space. A Plexiglas cage (40 cm × 40 cm × 60 cm) was used as the testing arena. The cage floor covered with 2-cm-deep wood shavings. A zoom video equipped with DVD and LCD monitor was employed to monitor and record the behaviors of tested rats.
The rats aged 3 months were placed into the experimental apparatus individually and allowed to accommodate to the environment for 10 min, which repeated two days. On the day of testing, in order to promote their social interaction motivation and further boost the expression of social play behaviors during the testing, the individual rat stayed in a separated environment for 3 h till the testing (21). In each test, two rats from the same group were placed in the experimental apparatus and stayed for 15 min. The difference of body weight between two rats was no more than 10 g and these rats had no common social experience previously.
The following parameters were scored: (1) Following: moving forward to or tailing after the other experimental subject, who was running away; (2) Frequency of pinning: one rat lying on its back on the floor while the other rat standing over it. In the social play of rats, such posture is most characteristic one when one tested subject is under a playing plea solicited by its counterpart who rotates to tis dorsal surface.
Detection of Hippocampal and Serum Serotonin Levels
After behavioral test, rats were anesthetized with chloroform and killed by decapitation, the brain and blood were collected, and hippocampus was carefully separated. The blood was centrifuged (1500 × g, 10 min), and the supernatant was collected for the detection of 5-HT level. In addition, 500 μl of ice-cold Tissue Protein Extraction solution (Beijing CoWin Biotech) was used to homogenize rat hippocampus (50 mg). The homogenates were centrifuged (10,000 × g, 20 min at 4°C). BCA Kit (Beijing CoWin Biotech) was employed to determine the total protein concentration. According to the manufacturer instructions, an ELISA kit (Tianjin Anoric Biotech, China) was employed to detect hippocampal and serum 5-HT level.
Hematoxylin and Eosin (H&E) Staining
After behavioral assessment, animals were sacrificed, and the colons were harvested for further H&E staining. The colonic tissues were fixed in 10% buffered neutral formalin and then embedded in paraffin. Colon sections (5 μm) were obtained for routine HE staining with standard protocol. These sections were evaluated independently in a blinded way.
Western Blotting
The colonic tissues were homogenized in lysis buffer for 30 min, followed by centrifugation. The supernatant was collected, and the protein concentration was determined with BCA method. Then, the supernatant containing 50 μg of protein was boiled for 5 min, and then subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis for protein separation. Then, the proteins were transferred onto nitrocellulose membranes which were then treated with 5% non-fat milk in TBST containing 10 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, and 0.05% Tween-20 at room temperature to block nonspecific reactivity for 1 h. Following primary antibodies were used: occludin (1:1,000, ABclonal, no: A12621, Wuhan, China), claudin-1(1:1000, ABclonal, no: A2196, Wuhan, China) and anti-GAPDH (1:1,000, Boster Biotechnology, BM1985, Wuhan, China).
Statistical Analysis
SPSS version 22.0 (Statistical Product and Service Solutions Inc., Chicago, IL) was used for statistical analysis. Data are expressed as mean ± standard error (SEM). One way analysis of variance (ANOVA), followed by least significant difference (LSD) test was employed for comparisons among groups. A value of P < 0.05 was considered statistically significant.
Results
Effects of Dietary n3/n6 PUFAs Supplementation on the Behaviors
Rats in the B group with n3/n6 = 1:5 supplementation were more sensitive to three odorants in the olfactory capability test (Figure 1A). Compared with control rats, the urine sniffing time reduced significantly in the VPA group (P < 0.01). In social play behavior testing, the following frequency in the B group was markedly higher than in the VPA group; as compared to the control group, the following frequency reduced markedly in the VPA group (Figure 1B). In the pinning frequency test, male offspring with prenatal VPA exposure had no difference with control ones (Figure 1C).
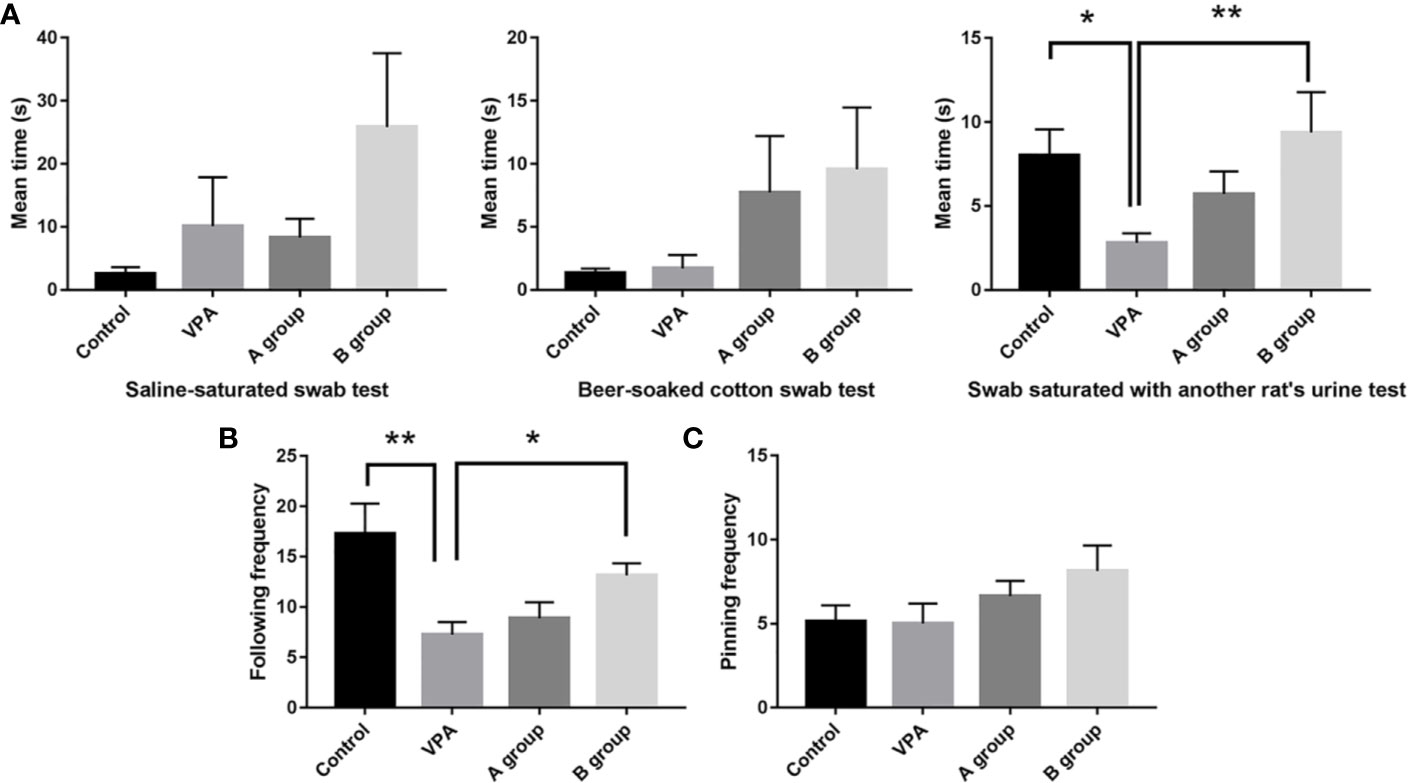
Figure 1 Effect of dietary n3/n6 PUFAs supplementation on the olfactory habituation/dishabituation and social play behavior. (A) Olfactory habituation/dishabituation; (B) Following frequency; (C) Pinning frequency. Data are expressed as mean ± SEM, n = 8. *P < 0.05 vs. VPA group. **P < 0.01 vs. VPA group.
Effects of Dietary n3/n6 PUFAs (1:5) Supplementation on the Hippocampal and Serum 5-HT Levels
As shown in Figure 2, as compare to the control group, the hippocampal 5-HT level decreased significantly in the VPA group (P < 0.01, ANOVA). The hippocampal 5-HT level in the B group was markedly higher than in the ASD group (P < 0.01, ANOVA). Additionally, the serum 5-HT level in the VPA group was significantly higher than in the control group (P < 0.01, ANOVA) and the serum 5-HT level in the B group was markedly lower than in the VPA group (P < 0.01, ANOVA).
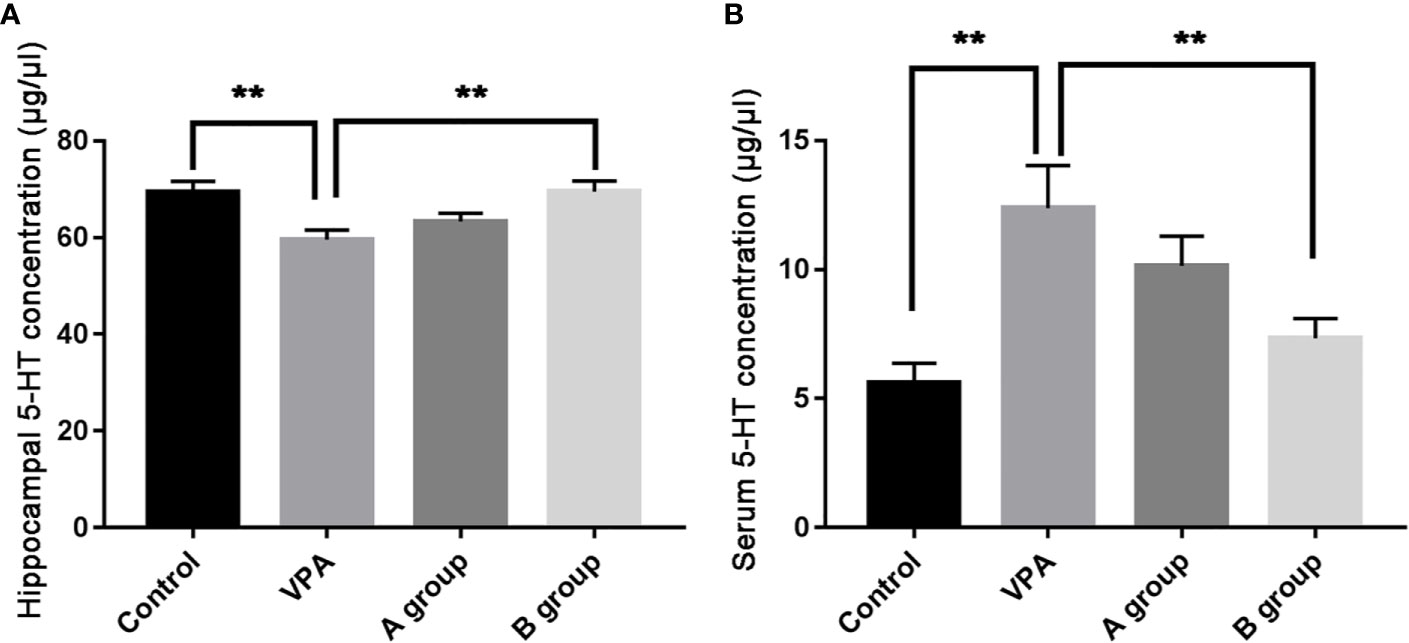
Figure 2 Hippocampal and serum 5-HT levels (μg/μl) in different groups. (A) Hippocampal 5-HT level; (B) serum 5-HT level. Data are expressed as means ± SEM. **P < 0.01: vs. VPA group. Control group, n = 7; VPA group, n = 6; A group, n = 5; B group, n = 5.
Effects of Dietary n3/n6 (1:5) Supplementation on the Colonic Histology and Tight Junction Protein Expression
H&E staining of colon sections was employed to evaluate the histological changes in 12 weeks-old rats. As shown in H&E staining, more inflammatory cells were observed in the colon of rats with VPA treatment. However, the inflammatory was ameliorated after dietary n3/n6 PUFAs (1:5) supplementation (Figure 3).
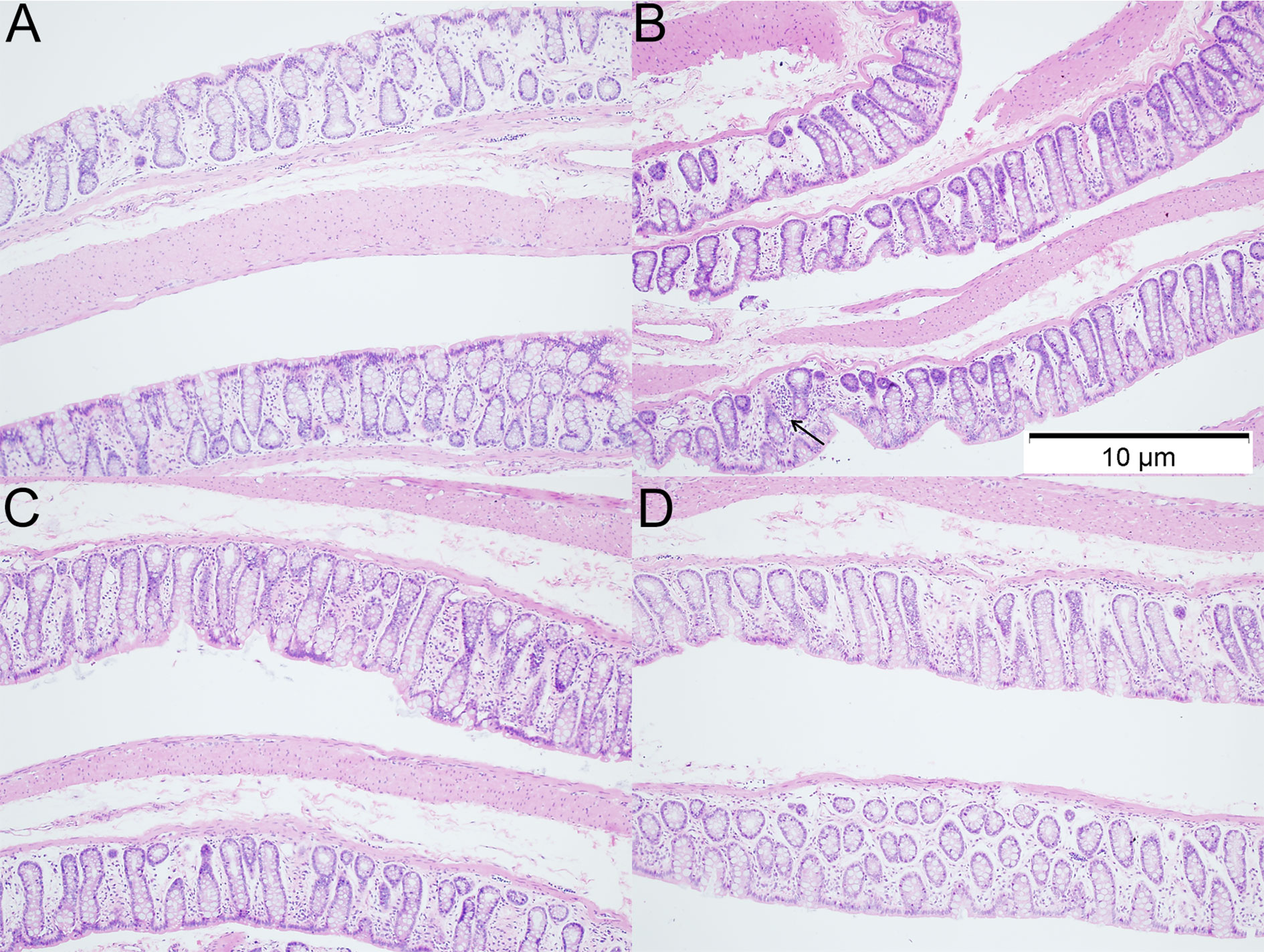
Figure 3 Histology of colonic tissues in different groups (HE staining; ×100). (A) control group; (B) VPA group; (C) A group; (D) B group. Arrows indicate more inflammatory cells were observed in the colon of rats with VPA treatment.
In the family of epithelial tight junction proteins, occludin and claudin-1 are important. As shown in Western blotting, the expression of occludin was significantly downregulated in the VPA group as compared to the control group. However, dietary n3/n6 PUFAs (1:5) supplementation significantly up-regulated the expression of occludin and claudin-1 in the VPA-treated rats (Figure 4).
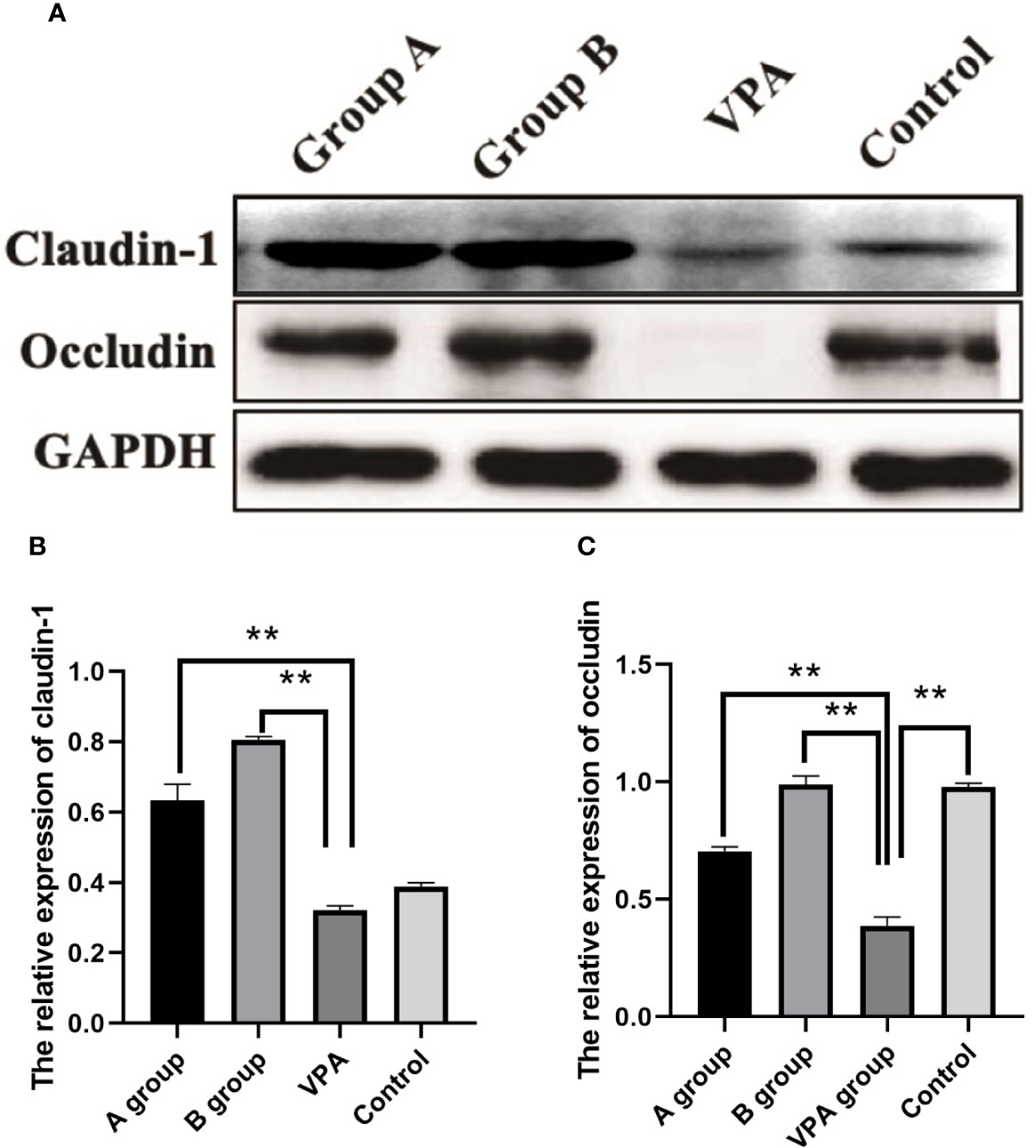
Figure 4 Expression of claudin-1 and occludin in the colonic tissues of different groups. Downregulated expression of occludin after VPA treatment. Upregulated expression of claudin-1 and occludin after dietary n3/n6 (1:5) supplementation. (A) Representative Western blot images of the protein bands. (B) Fold change of Claudin-1. (C) Fold change of occludin. Results are presented as mean ± SEM. Differences were analyzed using one-way ANOVA followed by LSD test. n = 3 in each group. **P < 0.01, compared with VPA group.
Discussion
Serotonin (or 5‐HT) is mainly found in the gastrointestinal tract (60%–90%), platelets and CNS of animals and humans, and it plays important roles in the regulation of intestinal movement, intestinal sensing and signaling, and secretion (22). In vitro studies have indicated that 5‐HT affects tight junction expression in Caco‐2 intestinal epithelial cells (23). 5-HT is involved in the modulation of hypotonicity-induced increase of duodenal mucosal permeability (24). Dong et al. found that the intestinal and plasma 5-HT levels were higher in the stressed animals, and the elevated 5-HT level could lead to diarrhea. 5-HT and diarrhea delayed the renewal of intestinal epithelial cells and impaired the mucosal barrier and absorption (25). In autism population and their relatives, the blood 5-HT level increases, implicating the involvement of 5-HT in the pathophysiology of autism (26). Therefore, the elevated serum 5-HT level in ASD patients may ameliorate the gastrointestinal dysfunction. Our results also showed the serum 5-HT level increased in the VPA-treated rats. However, after dietary supplementation of PUFAs, the serum 5-HT level in the VPA group decreased significantly, which may be associated with the improvement of intestinal barrier function.
H&E staining indicated the increased inflammatory cells and the loss of crypt structure in the colonic tissues of VPA group. However, after dietary supplementation of PUFAs in the VPA-treated rats, inflammatory cells in colonic tissues reduced, the structural impairment was lessened, the expression of junction proteins (occludin and claudin-1) in the colon was also upregulated significantly, which may be related to the decreased serum 5-HT level. In the in vitro model of enterocytes, 5-HT (0–2.5 mM) was able to regulate the expression of occludin in the basolateral cells in a dose dependent manner: exogenous 5-HT was able to decrease the occludin expression (23). This was consistent with our findings: the serum 5-HT level reduced after supplementation of PUFAs in the VPA group, which was accompanied by the elevated expression of occludin in the colon.
Compared with non-autistic population, the brain 5-HT concentration reduces significantly in the ASD population (27). Lower 5-HT level in the early brain development of rats may result in the neuroanatomical defects, such as abnormally tiny somatosensory barrels and dendritic arbors, fewer dendritic spines, and decreased synaptic density. In male young adults with ASD, a negative correlation has also been observed between low serotonergic neurotransmission and high blood 5-HT concentration, which is also referred as serotonin anomaly (28). According to the causal relationship between low serotonin and autism, rats lacking TPH2 gene (a protein coding gene and known to cause low 5-HT concentration in the brain) have abnormal serotonin synthesis in the brain and present autism-related symptoms, including the impairment of social communication and interaction capabilities, and disposition for restricted, repetitive behaviors and interests (29, 30). The behavioral complexity of ASD, characterized by deficits in learning, memory, emotion, and social functioning, suggests underlying alterations in multiple brain area (31). Hippocampus is an important brain region related to the etiology of ASD. It has been reported that decreased hippocampal neuronal size with increased cell packing density and the presence of less complex dendritic arborization are related to the disrupted neuronal maturation in ASD (32). Intriguingly, Narita et al. reported that prenatal VPA exposure increased hippocampal 5-HT level and pre-frontal DA level in rats (33). Our study also showed that hippocampal 5-HT level decreased and autism-related behaviors (olfactory habituation/dishabituation and social play behavior) were present in the VPA-treated rats, which may be associated with the decreased hippocampal 5-HT level. After dietary n-3/n-6 PUFAs (1:5) supplementation, the autistic behaviors in the VPA group were ameliorated and the hippocampal 5-HT level increased, suggesting that the behavioral improvement of autistic rats is associated with elevated hippocampal 5-HT level. n-3 PUFAs are crucial for the development and function of CNS. Increasing evidence from randomized placebo-controlled trials, animal experiments, and epidemiological studies has shown that n-3 PUFAs deficiency in the diet may lead to the development of affective mental disorders, and dietary supplementation with n-3 PUFAs may be helpful for the treatment of these disorders. It has been reported that n-3 PUFAs are better to increase the cell membrane fluidity as compared to n-6 PUFAs (34). It seems that patients with depressive disorder have decreased membrane fluidity due to the composition changes of PUFAs, which affects neurotransmitter binding, membrane enzymes and receptor activities (35, 36). It is reported a reversal diet with adequate n-6 and n-3 PUFA given during the lactating period to rats originating from alpha-linolenic acid-deficient dams was able to restore both the fatty acid composition of brain membranes and several parameters of the dopaminergic and serotonergic neurotransmission (37). Hereby, the membrane composition of n-3/n-6 PUFAs may exert significant influence on the monoaminergic systems, and the normalization of membrane structure by regulating n-3/n-6 PUFAs ratio may be employed to ameliorate ASD symptoms.
In the future, more studies are required to confirm the importance of n-3/n-6 PUFAs in the treatment of ASD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of Second Hospital and College of Basic Medical Sciences of Jilin University.
Author Contributions
JW designed the work, collected and analyzed data, and drafted the manuscript. JW, DZ, BHZ, JZ, HL, JL, ZZ, and BLZ collected and analyzed data and revised the manuscript; and PL contributed to the concept and design of this study, reviewed, and revised the manuscript.
Funding
This study was supported by the Natural Science Foundation of China (No. 81602847), Youth Foundation of Health Commission of Jilin Province (No. 2015Q007), and Youth Foundation of Norman Bethune Medical College of Jilin University (No. 2015026021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hollocks MJ, Jones CR, Pickles A, Baird G, Happe F, Charman T, et al. The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Res (2014) 7(2):216–28. doi: 10.1002/aur.1361
2. Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet (2006) 368(9531):210–5. doi: 10.1016/S0140-6736(06)69041-7
3. Currenti SA. Understanding and determining the etiology of autism. Cell Mol Neurobiol (2010) 30(2):161–71. doi: 10.1007/s10571-009-9453-8
4. Vinten J, Bromley RL, Taylor J, Adab N, Kini U, Baker GA, et al. The behavioral consequences of exposure to antiepileptic drugs in utero. Epilepsy Behav (2009) 14(1):197–201. doi: 10.1016/j.yebeh.2008.10.011
5. Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, Garcia-Finana M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry (2013) 84(6):637–43. doi: 10.1136/jnnp-2012-304270
6. Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology (2005) 30(1):80–9. doi: 10.1038/sj.npp.1300518
7. Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord (2014) 44(5):1117–27. doi: 10.1007/s10803-013-1973-x
8. Kennedy PJ, Clarke G, Quigley EM, Groeger JA, Dinan TG, Cryan JF. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev (2012) 36(1):310–40. doi: 10.1016/j.neubiorev.2011.07.001
9. de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med (2014) 44 Suppl 1:S79–85. doi: 10.1007/s40279-014-0153-2
10. Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med (2008) 8(4):274–81. doi: 10.2174/156652408784533760
11. Kim JW, Choi CS, Kim KC, Park JH, Seung H, Joo SH, et al. Gastrointestinal tract abnormalities induced by prenatal valproic Acid exposure in rat offspring. Toxicol Res (2013) 29(3):173–9. doi: 10.5487/TR.2013.29.3.173
12. Kumar H, Sharma BM, Sharma B. Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem Int (2015) 91:34–45. doi: 10.1016/j.neuint.2015.10.007
13. Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res (2016) 1630:83–97. doi: 10.1016/j.brainres.2015.10.052
14. Xiao K, Liu C, Qin Q, Zhang Y, Wang X, Zhang J, et al. EPA and DHA attenuate deoxynivalenol-induced intestinal porcine epithelial cell injury and protect barrier function integrity by inhibiting necroptosis signaling pathway. FASEB J (2020) 34(2):2483–96. doi: 10.1096/fj.201902298R
15. Zhu H, Liu Y, Chen S, Wang X, Pi D, Leng W, et al. Fish oil enhances intestinal barrier function and inhibits corticotropin-releasing hormone/corticotropin-releasing hormone receptor 1 signalling pathway in weaned pigs after lipopolysaccharide challenge. Br J Nutr (2016) 115(11):1947–57. doi: 10.1017/S0007114516001100
16. Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem (2013) 24(5):725–43. doi: 10.1016/j.jnutbio.2013.01.002
17. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) (2008) 233(6):674–88. doi: 10.3181/0711-MR-311
18. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. BioMed Pharmacother (2002) 56(8):365–79. doi: 10.1016/s0753-3322(02)00253-6
19. Parletta N, Niyonsenga T, Duff J. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Levels and Correlations with Symptoms in Children with Attention Deficit Hyperactivity Disorder, Autistic Spectrum Disorder and Typically Developing Controls. PloS One (2016) 11(5):e0156432. doi: 10.1371/journal.pone.0156432
20. Stack CM, Lim MA, Cuasay K, Stone MM, Seibert KM, Spivak-Pohis I, et al. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol (2008) 211(1):67–84. doi: 10.1016/j.expneurol.2008.01.003
21. Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev (1984) 8(4):465–92. doi: 10.1016/0149-7634(84)90005-8
22. Keszthelyi D, Troost FJ, Masclee AA. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil (2009) 21(12):1239–49. doi: 10.1111/j.1365-2982.2009.01370.x
23. Haub S, Kanuri G, Volynets V, Brune T, Bischoff SC, Bergheim I. Serotonin reuptake transporter (SERT) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. Am J Physiol Gastrointest Liver Physiol (2010) 298(3):G335–344. doi: 10.1152/ajpgi.00088.2009
24. Nylander O, Pihl L. Luminal hypotonicity increases duodenal mucosal permeability by a mechanism involving 5-hydroxytryptamine. Acta Physiol (Oxf) (2006) 186(1):45–58. doi: 10.1111/j.1748-1716.2005.01507.x
25. Dong Y, Yang C, Wang Z, Qin Z, Cao J, Chen Y. The injury of serotonin on intestinal epithelium cell renewal of weaned diarrhoea mice. Eur J Histochem (2016) 60(4):2689. doi: 10.4081/ejh.2016.2689
26. Muller CL, Anacker AMJ, Weele J VV. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience (2016) 321:24–41. doi: 10.1016/j.neuroscience.2015.11.010
27. Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci (2005) Apr-23(2-3):171–82. doi: 10.1016/j.ijdevneu.2004.08.002
28. McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, et al. Serotonergic responsivity in male young adults with autistic disorder. Results Pilot Study Arch Gen Psychiatry (1989) 46(3):213–21. doi: 10.1001/archpsyc.1989.01810030019003
29. Kane MJ, Angoa-Perez M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, et al. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PloS One (2012) 7(11):e48975. doi: 10.1371/journal.pone.0048975
30. Yang SY, Yoo HJ, Cho IH, Park M, Kim SA. Association with tryptophan hydroxylase 2 gene polymorphisms and autism spectrum disorders in Korean families. Neurosci Res (2012) 73(4):333–6. doi: 10.1016/j.neures.2012.05.012
31. Müller R-A. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev (2007) 13:85–95. doi: 10.1002/mrdd.20141
32. Kemper TL, Bauman ML. The Contribution of Neuropathologic Studies to the Understanding of Autism. Neurol Clin (1993) 11(1):175–87. doi: 10.1016/S0733-8619(18)30176-2
33. Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res (2002) 52(4):576–9. doi: 10.1203/00006450-200210000-00018
34. Haag M. Essential fatty acids and the brain. Can J Psychiatry (2003) 48(3):195–203. doi: 10.1177/070674370304800308
35. Fernando WM, Martins IJ, Goozee KG, Brennan CS, Jayasena V, Martins RN. The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: potential mechanisms of action. Br J Nutr (2015) 114(1):1–14. doi: 10.1017/S0007114515001452
36. Müller CP, Reichel M, Mühle C, Rhein C, Gulbins E, Kornhuber J. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta (2015) 1851(8):1052–65. doi: 10.1016/j.bbalip.2014.12.014
Keywords: polyunsaturated fatty acids, autism spectrum disorder, valproic acid, intestinal barrier, serotonin
Citation: Wang J, Zheng B, Zhou D, Xing J, Li H, Li J, Zhang Z, Zhang B and Li P (2020) Supplementation of Diet With Different n-3/n-6 PUFA Ratios Ameliorates Autistic Behavior, Reduces Serotonin, and Improves Intestinal Barrier Impairments in a Valproic Acid Rat Model of Autism. Front. Psychiatry 11:552345. doi: 10.3389/fpsyt.2020.552345
Received: 30 April 2020; Accepted: 24 August 2020;
Published: 09 September 2020.
Edited by:
Trevor Ronald Norman, The University of Melbourne, AustraliaReviewed by:
Caroline Menard, Laval University, CanadaYanling Zhou, Guangzhou Medical University, China
Copyright © 2020 Wang, Zheng, Zhou, Xing, Li, Li, Zhang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bF9waW5nQGpsdS5lZHUuY24=
 Jinpeng Wang1
Jinpeng Wang1 Honghua Li
Honghua Li Beilin Zhang
Beilin Zhang Ping Li
Ping Li