
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 19 August 2020
Sec. Addictive Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.00827
This article is part of the Research TopicNeurobiological Biomarkers for Developing Novel Treatments of Substance and Non-Substance AddictionView all 45 articles
Background: Opioid use disorders (OUDs) are an epidemic causing catastrophic consequences to individuals, families, and society despite treatments including psychotherapy, substitution therapy or receptor blockers, and psychoeducation. We have developed a novel treatment that combines unilateral transcranial photobiomodulation (t-PBM) to the hemisphere with a more positive valence by Dual Brain Psychology (DBP).
Methods: We used a randomized, double blind, placebo-controlled protocol in which 22 patients with significant opioid cravings and a history of recent or current OUD attended three 1-h weekly sessions. After baseline measures of opioid craving and other psychometrics, subjects received two unilateral t-PBM applications (810 nm CW LED, 250 mW/cm2, 60 J/cm2, 4 min) or a sham (foil-covered LED) at F3 or F4. Prior to any treatment we used two tests to determine which hemisphere was more associated with a negative outlook and cravings and treated that side before the more positive hemisphere. Primary outcome measure was an opioid craving scale (OCS). Secondary outcomes were weekly Hamilton Depression (HDRS) and Anxiety (HARS) Rating Scales prior to treatments and at follow-up.
Results: Immediately after treatment the OCS improved significantly for both the sham and active treatments, but one week later the active treatment showed a 51.0% (SD 33.7) decrease in OCS while a week after the sham treatments there was a decrease of only 15.8% (SD 35.0) (by Wilcoxon Sign Rank Test, p = 0.004) and by a mixed model it was p = 0.0071. The effect size for the differences between active and sham was 0.73. For the active treatment from before and after treatment the effect size was 1.51 and for the sham, 0.45. The HDRS improved from a baseline of 15.1 to 8.8 (SD 10.3) a week after the active treatment and to 13.3 (SD 12.9) after the sham (p = 0.0071). HARS improved from 14.7 to 8.0 (SD 13.2) after the active treatments and to 14.3 (SD 16.0) after the sham, p = 0.08. Active treatment of the positive hemisphere after the negative hemisphere significantly improved the OCS, but there was no significant difference after the sham treatment. One patient complained of 2 h of abdominal bloating and dropped out; no other adverse effects were observed.
Discussion: Unilateral t-PBM to the hemisphere with a more positive hemispheric emotional valence was an effective and safe treatment for opioid cravings as well as for depression and anxiety. Our results also lend support to the underlying premises of DBP.
According to the National Institute on Drug Abuse, “The combined healthcare, crime-related, and productivity costs of tobacco, alcohol, and illicit drugs exceed $700 billion a year, but dollars only poorly approximate the devastating human cost of substance use disorders” (1). In the US in 2015, there were over 52,000 drug overdose deaths, an increase of 11% over 2014 (2–4). Drug abusing patients demonstrate mental distress, health complications, loss of productivity, and increased criminality, which are injurious not only to patients and their families, but also to society as a whole. We have obtained preliminary data (5) that a simple, painless, inexpensive, and safe treatment called unilateral transcranial photobiomodulation (tPBM) using near-infrared light (NIR) may effectively benefit patients with a history of opiate dependence by improving their psychological well-being.
The physiological benefits to the brain from tPBM have been the subject of a rich literature of >400 papers on PubMed and several reviews (6–10). The reviews describe in detail how NIR light is absorbed by cytochrome-C oxidase, which stimulates ATP formation in the mitochondria (11). tPBM also increases neurotrophic factors in the brain, increases blood flow, and decreases inflammation (8, 12).
Hemispheric emotional valence has generally been considered in three hypotheses. The first is the right brain hypothesis, which posits that the right hemisphere is specialized for all emotional responses (13, 14). The second is the motivational hypothesis (15, 16), which suggests that approach emotions (including anger) are associated with the left hemisphere and that withdraw emotions are associated with the right. The third hypothesis, the valence hypothesis (17, 18), proposes that the left hemisphere is associated with positive emotions and the right with negative emotions.
Schiffer originally proposed Dual-brain Psychology (DBP) in 1997 (19–22), which posits that one brain hemisphere tends to be relatively mature and healthy, while the other hemisphere may be more affected by past traumas and supports a personality that is more prone to immature and/or destructive beliefs and/or behaviors. Schiffer has been concerned not simply with positive or negative emotions, but with entire personalities that differ in that one associated with one hemisphere tends to be associated with a disposition that is more mature and grounded in present reality and that the other, associated with the opposite hemisphere, seems to be more affected by past traumas, especially childhood traumas, and has a more childlike disposition that is more strongly influenced by past adversities. His hypothesis came out of his clinical observations, his rereading of the split-brain studies (23), studies by Wittling (24), and a number of published experiments by him and his associates on split-brain patients (25), fMRI imaging during lateral visual field stimulation (26), probe auditory evoked potentials during emotional memories (27), the use of HEV to successfully predict rTMS outcome in two studies on depression (28, 29), and a study using tPBM to treat anxiety and depression (5). The present study combined the physiological benefits of tPBM with the use of Schiffer’s HEV to guide the treatment to the positive hemisphere.
Contrary to the established models that have generally associated negative emotions with the right hemisphere, several findings from WADA studies (30, 31), metanalyses of functional imaging studies (32, 33), and studies of post-stroke patients (34–36) indicated that negative emotions can often be left hemisphere dominant. Schiffer has reported that the side that is less symptomatic, which thereby has a positive HEV varies between individuals, but strongly tends to be a trait for any individual. Schiffer’s HEV is determined simply by asking the patient to look out of either the left or the right lateral visual field by blocking his vision with safety glasses taped to allow vision out of one visual field and reporting his feelings and thoughts. He might be asked to look at a provocative photograph of an angry person while looking out that visual field, based in part on the pioneering work by Wittling and associates (24). The patient might be asked to rate this level of depression from 0 to 10. Then he is asked to look out the other lateral visual field and repeat the requests. In about 70% of patients there is at least a 2-point difference between the sides. Further, the patient is apt to see the therapist as critical (perhaps, as his mother was), to dislike himself, and to develop drug or gambling cravings while looking out of the negative side, all of which are reversed out of the other visual field. The visual field that is less symptomatic relates to the contralateral hemisphere which is then considered positive. See Figure 1. Recently, we have developed a computer test for HEV that correlates with lateralized volumes of the n. accumbens and amygdala. In two studies of rTMS, those patients who by this simple visual test had a positive left hemisphere had a far superior outcome compared to those with a negative left hemisphere from a course of two weeks rTMS, which only by tradition is only given to the left side of the head (28, 29).
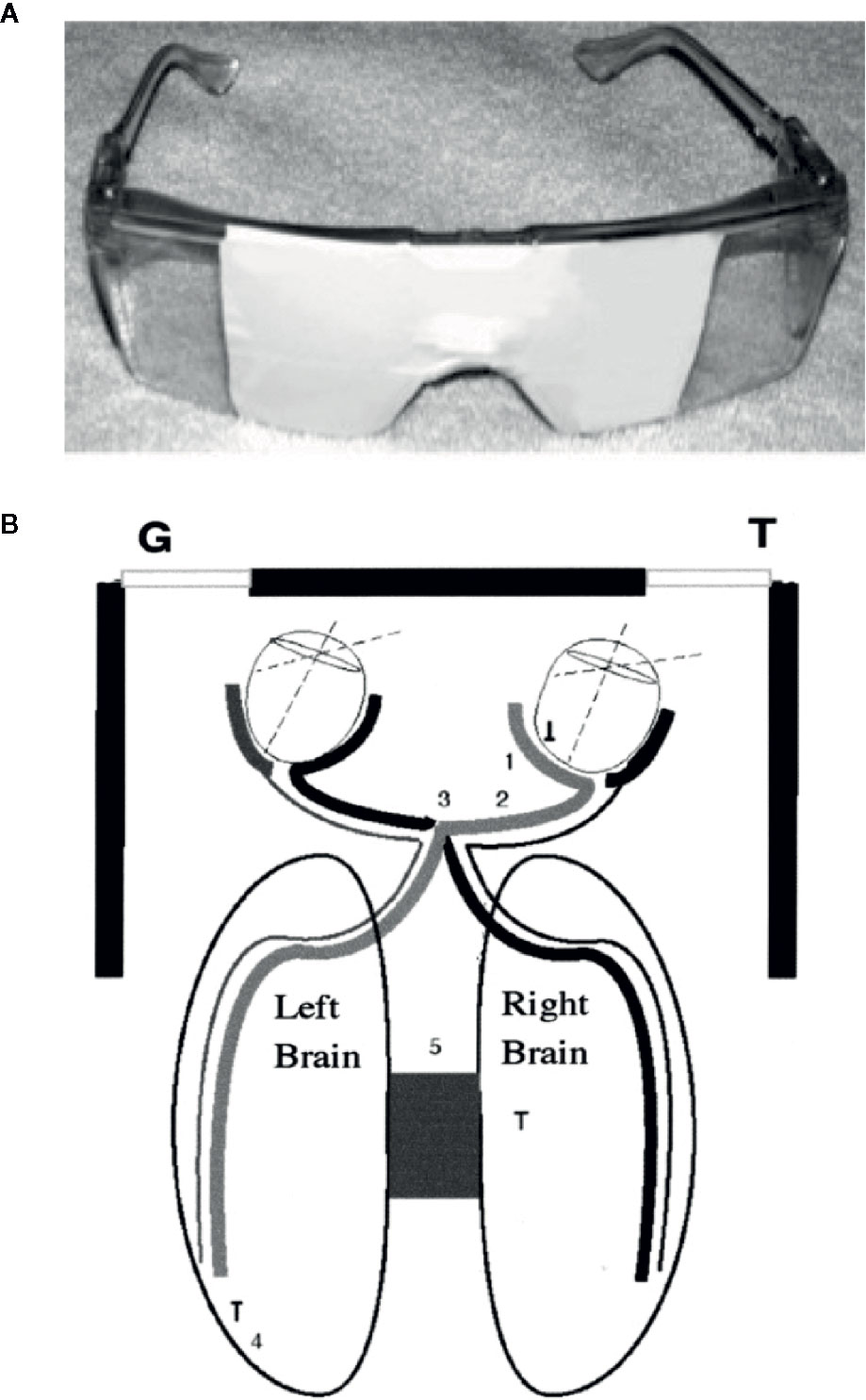
Figure 1 (A) Taped safety glasses used in the experiment. (B) A diagram of how looking to the left allowed vision primarily out of the LVF and looking to the right allowed vision primarily out of the RVF. (1) Nasal retina; (2) optic nerve; (3) optic chiasm; and (4) occipital cortex. The diagram shows how the medial retina receives light from the lateral visual field and transfers information to the contralateral hemisphere. These neurological facts were used extensively in the split-brain studies, and we assume that the personality changes described here and in our other publications use a similar mechanism (26).
Schiffer found that in his practice using unilateral tPBM to the positive hemisphere at either F3 or F4 gave very superior results compared with bilateral tPBM at F3 and F4 (5). See Table 1.
Firstly, to test whether unilateral tPBM to stimulate the positive hemisphere in patients with a current or recent history of opioid use disorders (OUD) could significantly reduce opioid cravings in a randomized, double blind, placebo-controlled design.
Secondly, to evaluate the effects of unilateral tPBM on affect as measured by the Hamilton Depression Rating Scale (HDRS) and the Hamilton Anxiety Rating Scale (HARS).
Thirdly, to test the hypothesis that treating the positive hemisphere is superior to treating the hemisphere with a more negative HEV using active treatment. Applying the sham treatment to the positive hemisphere should not be superior to applying it to the negative hemisphere. We wanted this aim to further test Schiffer’s DBP hypothesis. Schiffer had found clinically that applying tPBM to the negative hemisphere before the positive resulted in the patient’s still having the positive benefit of the treatment of the positive hemisphere.
Our fourth aim was to confirm that there were no significant side effects of tPBM as found in other reports (5, 6, 8–10, 37, 38).
This single site, double blind, placebo-controlled study was approved by the New England IRB, Needham, MA. Twenty-two patients who reported active opioid cravings and had a current or recent opioid use disorder were recruited from online advertising between July and September 2019. Each went through the consent procedure and gave written consent for the study, following which they were given a clinical medical and psychiatric history.
Inclusion criteria were that the patients complained of opioid cravings and were between the ages of 18 and 65. Each met the criteria for a history of opioid dependence by DSM V. Patients who were receiving treatment for opioid dependence or other psychiatric disorders, either psychological or pharmacological treatments, could continue their treatment during the time of the study but were asked to try not to alter their treatment from the onset of the experiment until its conclusion. No patient requested to alter his regular treatment. Subjects were recruited without regard to gender or ethnicity and were enrolled on a first come basis. Exclusion criteria were past history of psychotic disorder (including schizophrenia or schizoaffective disorder), a history of violent behavior, a history of a past suicide attempt, a history of current suicidal ideation, a history of a neurological condition (e.g. epilepsy, traumatic brain injury, stroke), pregnancy, any current acute or chronic medical condition that might confound the study. Any patient judged by an investigator to have an impaired decision-making capacity would have been excluded. In actuality, no patient seeking recruitment was excluded after a screening phone call or clinical interview.
The patients attended the laboratory for three consecutive weekly visits. Patients were not allowed to reschedule a missed session so that the timings of the treatments would be consistent. At the first visit the experimenters gave the patients two tests for HEV to determine the positive hemisphere. The two tests for HEV are the lateral visual field test (LVFT) and a novel computer test for HEV (CTHEV), which has been submitted for a US Patent (patent application number: 16703937). The LVFT consists of asking each patient to put on taped safety goggles that allowed vision out of only one visual field, when the patient looked as far as possible to the left or the right (5, 26, 27). See Figure 1. While looking out of each visual field the patient was asked to rate from 0 to 10 his level of distress and his level of opioid cravings while he looked at a photograph of a very angry man, designed so that each half of the face was identical. The CTHEV, showed a video of alternating, symmetrical photographs of angry men to one visual field (by having the patient fixate on a central dot for 1 min) after which he was asked to rate from 0 to 10 his level of distress and his level of opioid cravings. As with the LVFT, the CTHEV was then repeated to the other visual field and the side with the lower scores was considered the positive visual field, which suggested that the contralateral hemisphere was the positive hemisphere. The HEV with either test was the numeric difference between the recorded scores from each visual field.
At each of the three weekly visits before treatment each patient was also given a urine drug screen, and if female, a pregnancy test, as well as each of the baseline outcome measures, which were the Hamilton Depression Rating Scale-17 (HDRS) (39), the Hamilton Anxiety Rating Scale (HARS) (40), and the Opioid Craving Scale (OCS) (41). The OCS was our primary endpoint and was given before and after each treatment and the follow-up session. It consisted of a mean score from three questions each rated from 0 to 9. (1) Please rate how strong your desire for an opiate is right now. (2) Please rate how strong your urges would be for an opiate if something in the environment had reminded you (examples; seeing a spoon), a needle, a mirror, or an alcohol advertisement), and (3) Please imagine yourself in the environment in which you previously used drugs and/or alcohol (a bar, your dealer’s house, a shooting gallery, or whatever situation reminds you most strongly of active drug use). If you were in this environment right now, what is the likelihood that you would use an opiate?
At the beginning of each of the three weekly sessions, we obtained a detailed verbal report from the patient of his daily use of opioids over the previous week. We used this data (which we felt was honestly and accurately reported and was consistent with the urine drug screen) to calculate a timeline follow-back score using a typical unit of opioid use for the patient multiplied by the number of units and days of use.
After the baseline measures each patient then was given either a sham treatment or an active treatment (unilateral tPBM) in subsequent weeks on a randomized basis. That is, on the first visit the patient was treated with either an active device or a sham device. On his second visit, he was treated with the opposite device. On the third weekly visit, he received only a follow-up evaluation. See Figure 2. Before and after each treatment (first to the negative hemisphere and then to the positive hemisphere), the patient was administered the OCS. At the follow-up session we gave the patients the OCS, UDS, HDRS, HARS, and a report of opioid use during the prior week. Using preliminary data from his private practice FS found that treating the positive hemisphere after the negative relieved any negative effects that the first treatment may have caused and left the patient in a more positive psychological state, while allowing a comparison of the effects of treatments to the negative and the positive hemispheres under active and sham conditions. See Figure 2.
All treatments were randomized by a research assistant (EF) who also administered all the patient treatments, which were blinded to the two data recorders (HM) and (FS). The randomization procedure used computer generated random numbers blocked so that 60% would be given the sham condition before the active to try to avoid crossover effects.
We used the same light source that was used in our 2009 study (6). The active treatment consisted of applying tPBM from a light emitting diode (LED) array (Marubeni America Corp, Santa Clara, CA) with a peak wavelength of 810 nm (+40 nm), delivering 250 mW/cm2 when applied to the skin. tPBM for 4 min (total delivered fluence per site of 60 J/cm2) at each of two sites on the forehead that correspond to the 10–20 EEG sites, F3, and F4. Based on a penetration of 3.7% of the light to the dura, we calculated that 2.1 J/cm2 was delivered to each of the treated areas of the brain. The level of light exposure at the skin was well below the irradiance allowed by the ANSI standard of 320 mW/cm2. The New England IRB currently and the Partners IRB in 2009 both determined that the device posed no significant risk. In other published tPBM studies no significant side effects have been reported to date (5, 8, 9, 12, 37, 42–47).
The LED was attached to a heatsink and a fan for cooling. If the patient wanted further cooling, he could tilt the device so that the LED was a few millimeters from the skin and allowed more air flow from the fan. The power supply was constructed by a product engineer so that the LED would deliver 250 mW/cm2 at the skin. The sham device was the same device, except that the LED was covered with aluminum foil so that the patient felt the same warmth but received no light as verified by a photon detector. In this and other trials, subjects could not detect whether they were receiving active or sham treatment other than by their psychological response.
A biostatistician (WR) selected and conducted all of our statistical tests using R (48). Our main statistical tests were mixed model analyses and Wilcoxon Sign Rank Tests, depending on the statistical need. Because our hypothesis was that the active condition would obtain better clinical results, we used one-sided tests. A copy of all study data and of the statistical tests performed and their results are contained in the Supplementary Material (SM). In the Results section, we present the results extracted from the SM that we feel most accurately present our findings without repetition or data overload. In the SM, we noted which tables were included in the main paper as well as their table numbers which were different from the numbers in the SM.
We initially enrolled 22 patients whose overall baseline characteristics are described in Table 2 and a more complete description is in the SM. We categorized those patients who received the active treatment the first week and the sham the second week as Active–Sham and the other group as Sham–Active. We attempted to enroll female subjects but were only able to enroll two, one of whom dropped out after the two treatments but before the follow-up week because she got a job with a conflicting schedule. For males, one patient dropped out before the follow-up session. Three others, who were all in the Active–Sham group, dropped out after the first treatment. Of the five patients who dropped out, four gave reasons that we felt, and the patient reported, were unrelated to the study. For example, one patient reported that he dropped out because he “had to get out of town.” Only one patient dropped out because of a possible adverse reaction. He had what was described in a phone conversation, initiated by (FS), as “bloating in his abdomen,” which resolved quickly with self-treatment with Pepto-Bismol the evening of the study.
Immediately after the active and the sham treatments we found large and significant decreases in the OCS in both groups. Active had a mean (SD) percent decrease from immediately before treatment of 44.1% (39.6), and the sham had a decrease of 45.5% (35.6), both of which had a p value < 0.0001, by a one-sided Wilcoxon Test. The difference between conditions was not significant, p = 0.445. However, we found that when we compared the percent decrease in OCS one week after the treatments, there were significant differences between the active and the sham treatments.
When we compared the active and sham conditions for all remaining 17 patients we found, as shown in Table 3 and Figure 3, a percent decrease in OCS of 51.0% (33.7) for the week following the active treatment and only 15.8% (35.0) for the week following the sham, p = 0.0040, one-sided Wilcoxon test. For this analysis we chose using the week 1 baseline because the values were common for all patients and were measured before receiving any study intervention. More patients (71%) received the active treatment in week 2, because we wanted to avoid follow through effects with a blocked randomization. For the active over the sham the effect size, Cohen’s ∂, was.73. For the active treatments from their baseline the Cohen’s ∂ was 1.51, while for the sham treatments it was 0.45.
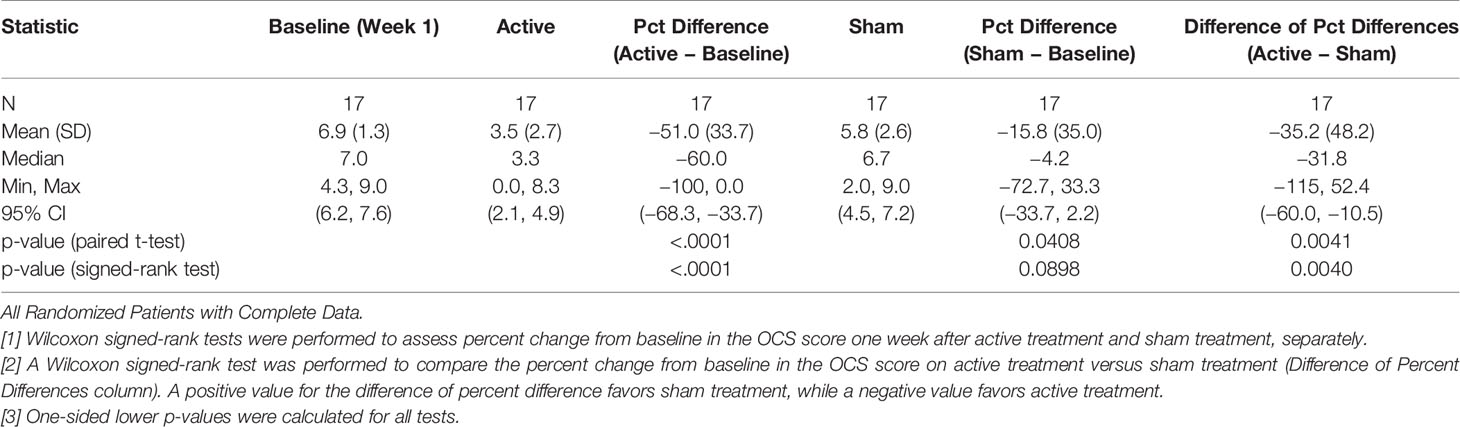
Table 3 Comparison of mean OCS scores (percent change) between active and sham treatments one week after treatment.
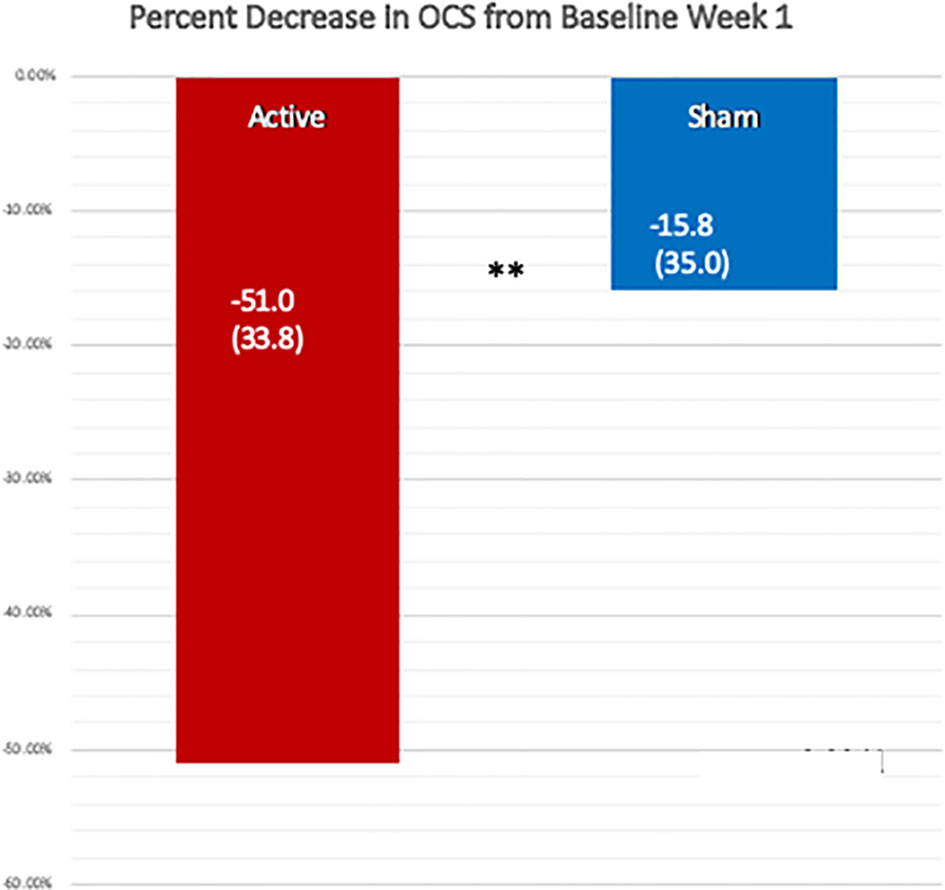
Figure 3 Comparison of the percent reduction in the Opioid Craving Scale one week after the active and the sham treatments, using the OCS from week 1 before treatment as the baseline. The change after the active treatment, −51.0% (SD 33.7) was significantly greater than that following the sham treatment, −15.8% (SD 35.0), by a one-sided, Wilcoxon Test, **p = 0.004. Cohen’s ∂ was 0.73 for the active treatment over sham.
Nine of 17 (53%) in the active group had at least a 60% improvement in the OCS, using the week 1 baseline. Two additional patients who did not have a sham treatment also had greater than a 60% improvement, 11/19 (58%). In the sham group, including one patient who did not have an active treatment, only three of 18 (17%) achieved at least a 60% reduction in the OCS. As shown in Table 4, by McNemar’s test, a paired, one-sided test of N = 17, we compared the paired percent differences and found that it was significant, p = 0.029.
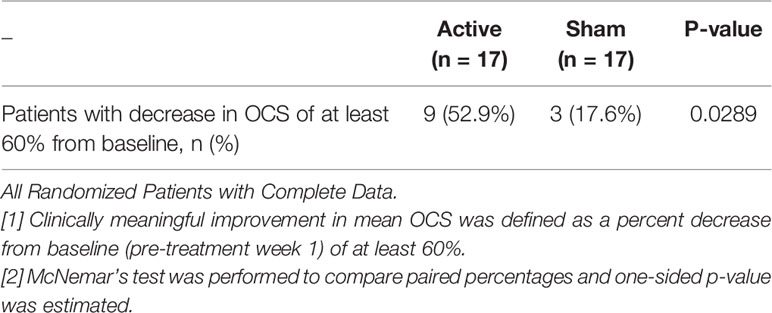
Table 4 Comparison of Clinically Meaningful Improvement in Mean OCS Between Active and Sham Treatments One Week After Treatment.
Table 5A shows the results of a mixed model analysis of the percent change in mean OCS prior to treatment each week that included treatment, treatment sequence and week as fixed effects and subject level as a random effect. The active treatment versus the sham treatment had a one-sided p value of 0.0078. Table 5B shows that the active treatment had an adjusted mean of −51.93% (95% CI −69.21, 34.64), and the sham treatment had an adjusted mean of −22.17% (95% CI −41.22, −3.12).
Table 6 shows the data from a mixed model for the percent change in mean HDRS, and the active treatment had an adjusted mean of −7.97 (95% CI −57.50, 41.56), and the sham had 52.53 (95% CI −0.55, 105.60) with a one-sided p value = 0.0078. Figure 4 shows a comparison of the raw HDRS scores at baseline and after the active and sham treatments and the p values from the one-sided Wilcoxon tests, including an active versus sham difference with a p value of 0.0053.
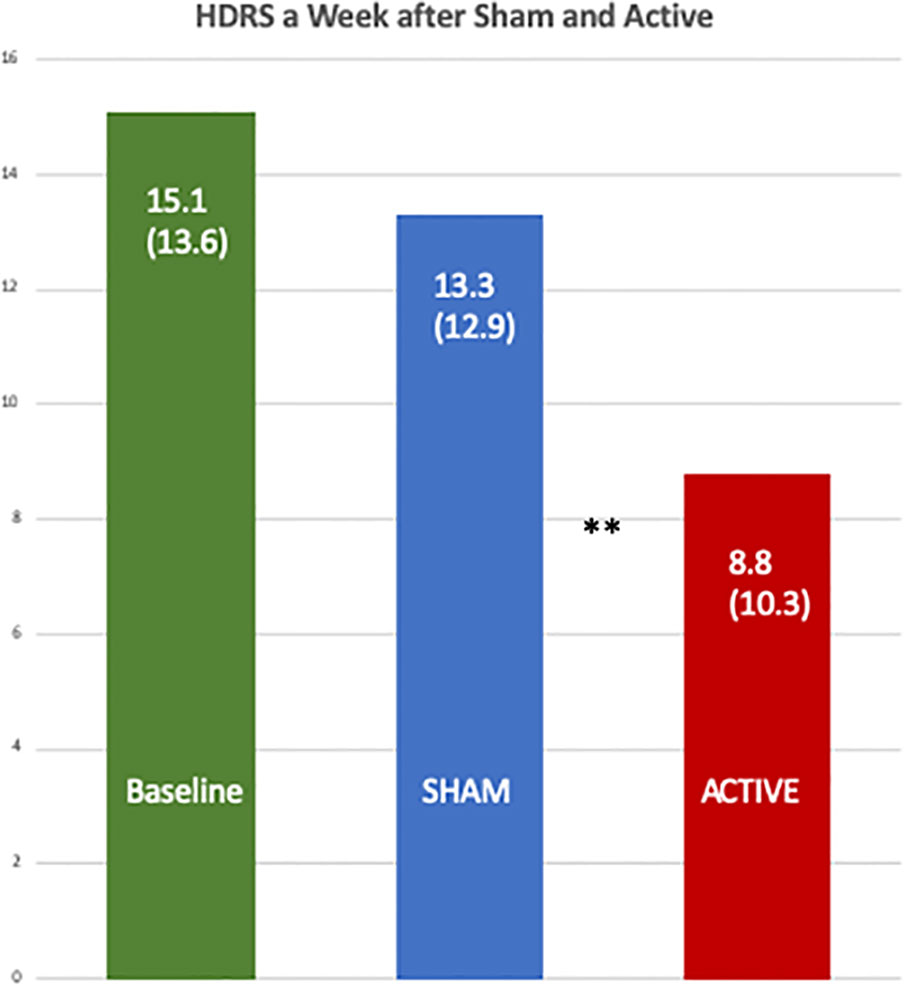
Figure 4 Comparison of the baseline Hamilton Depression Rating Scales with those one week after the active and the sham treatments. The differences between the scores after the active [8.8 (SD 10.3)] and sham treatments [13.3 (SD 12.9)] were significant by a one-sided, Wilcoxon Test, **p = 0.0053.
Table 7 shows the data from a mixed model for the percent change in mean HARS and the active treatment had an adjusted mean of 2.96 (95% CI −79.13, 85.06), and the sham had 53.96 (95% CI −33.54, 141.46) with a one-sided p value = 0.039. Figure 5 shows a comparison of the raw HDRS scores at baseline and after the active and sham treatments and the p values from one-sided Wilcoxon tests, including an active versus sham difference with a p value of 0.0124.
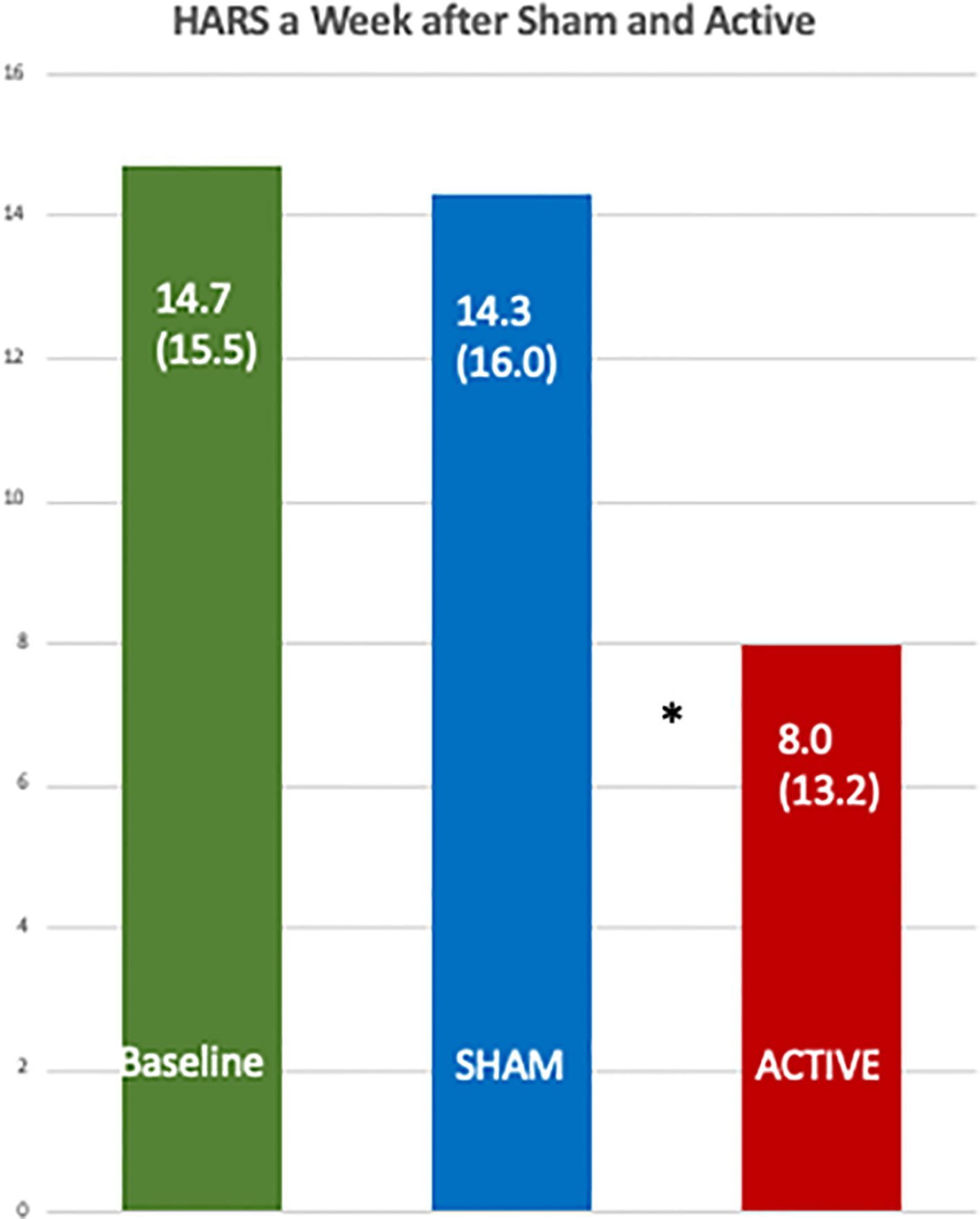
Figure 5 Comparison of the baseline Hamilton Anxiety Rating Scales with those one week after the active and the sham treatments. The differences between the scores after the active [8.0 (SD 13.2)] and sham treatments [14.3 (SD 16.0)] were significant by a one-sided, Wilcoxon Test, *p = 0.012.
Table 8 shows that when the more positive hemisphere (HEV by the LVFT and/or CTHEV tests) was treated during the active treatments, the OCS scores were lower (better) than when the hemisphere with the more negative HEV was treated. When the positive hemisphere was treated there was an improvement of −2.3 (SD 2.6) on the 9-point OCS compared with an improvement of −1.6 (SD 2.2) points when the negative hemisphere was treated, which by a one-sided Wilcoxon test had a p = 0.0087. During the sham treatments there were some placebo improvements on both the correct and the incorrect hemispheres, but the difference between them was not significant by a one-sided Wilcoxon test, p = 0.446. The hemispheric differences between the active and the sham conditions were significant, p = 0.0437, (Figure 6).
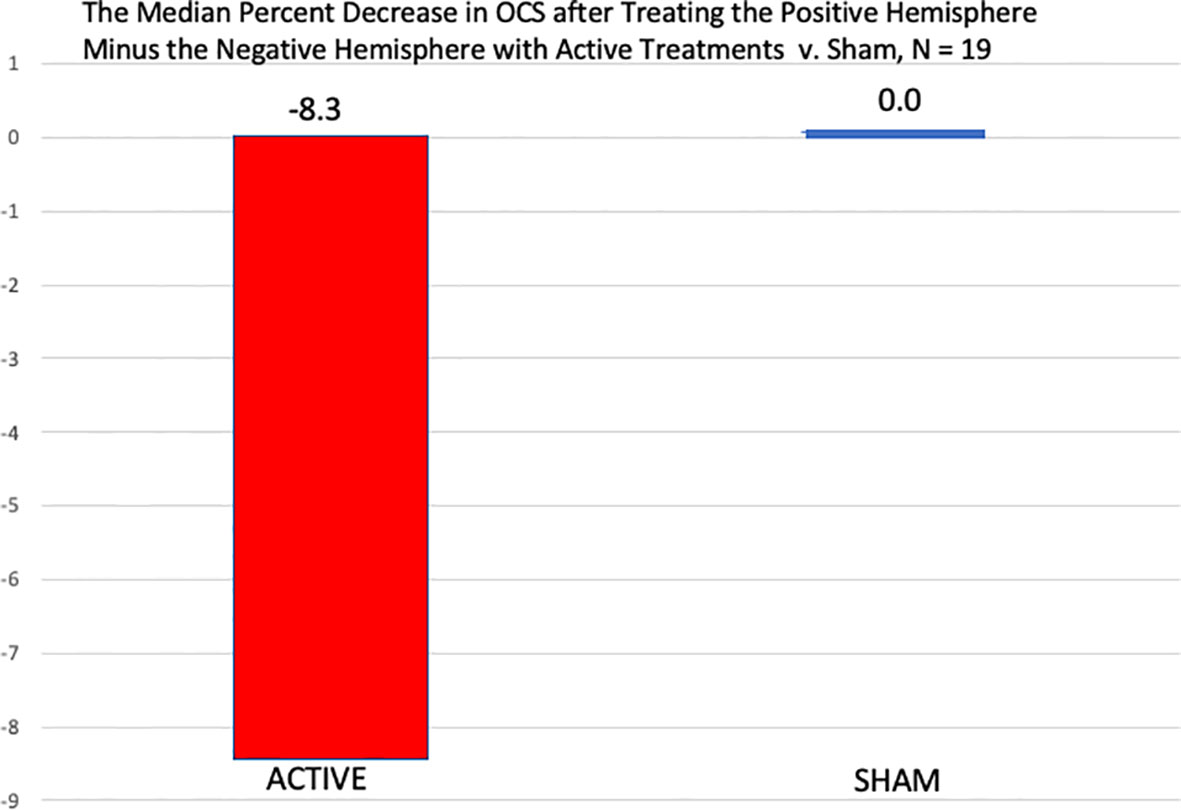
Figure 6 Comparison of the median percent reduction in the OCS measures immediately after the treatment of the negative hemisphere followed by the treatment of the positive hemisphere in both the active conditions and the sham conditions. In the active conditions there was a significantly greater percent decrease in OCS when the positive hemisphere was treated than the negative, by a one-sided, Wilcoxon Test, p = 0.021, but after the sham treatments, there was no difference between the two hemispheric sham treatments.
We counted the number of urine drug screens that were positive for morphine or opioids and found five were positive for either at baseline, and of these four were in the Active–Sham group and none of the five were positive at either week 2 or 3. Two patients who were negative at baseline were positive at week 2 or at week 3. One was after an active treatment and one after a sham.
Using the timeline follow-back scores we found:
● 14/22 patients had zero opioid use at baseline over the preceding 7 days
● two of the 14 patients had on-study usage—both at week 2 after receiving sham treatment at week 1. They returned to zero at the week 3 assessment after receiving active treatment at week 2
● 12 of the 14 had no on-study usage
● Among the eight users at baseline (numbers in parentheses are quantitative data calculated from the followback timeline, not patient numbers)
● one patient was a “heavy” user at baseline (126). He received the active treatment at week 1 and saw his usage drop to (1.5) after the active treatment and then (zero) the following week
● four patients were “moderate” users at baseline (7, 14, 14, 8). All received active treatment at week 1, and all saw a decrease in usage at the week 2 assessment. Two of the four saw another decrease at the week 3 assessment. Another patient did not have a week 3 assessment while the fourth patient increased (8 at week 1, 6 at week 2, 10 at week 3, after sham at week 2).
● three patients were “low” users at baseline (1 or 2) and remained low users at each of the subsequent assessments
We purposely did not use a formal side-effect questionnaire to avoid negatively influencing the patients. All but one patient had a very positive relationship with the treatment team. The one exception was the patient mentioned above who complained of abdominal bloating the night of the active treatment. We know of one other report of a patient treated with t-PBM experiencing abdominal bloating possibly in response to t-PBM (37), who recovered rapidly.
The opioid crisis is well known and widely appreciated (1–4), but while current treatments can be effective when skillfully applied by both the patient and caregivers, they still leave a profound need for further improvements. We have developed a novel treatment that attempts to address the underlying depression and anxiety symptoms likely arising from unappreciated (or appreciated) past traumas and which can be both promoters of the addiction and consequences of it. The treatment tested in this study is based on DBP which suggests that in a large majority of patients, one hemisphere (left or right) is a trait associated with a mental state that manifests a relationship to past traumas (5, 20, 22, 25) causing depression, anxiety, and an immature cognition that leads to adverse behavior, including substance abuse. DBP also suggests that the opposite positive hemisphere is less affected by past trauma and is less prone to negative affects and behaviors (20). Based on Teicher’s (49, 50) work suggesting that the neuropsychiatric consequences of maltreatment relate to its type and timing (sensitive periods) in development, we suspect but have not demonstrated that the development of the negative, more traumatized hemisphere may also relate to these factors: type and timing. We have suggested that unilateral treatments to the positive hemisphere (5, 28, 29) might stimulate it and further promote its dominance thereby inducing a state of greater wellbeing. DBP therapy focuses on using the healthier hemisphere to assist the troubled side, like a parent, with love, insight, and limits. Our innovation is to combine DBP with unilateral tPBM to stimulate the healthy hemisphere with NIR light. Our results, documented in this paper, indicate that this new treatment, compared to sham, had an effect size of −.73, and the benefits were usually obvious to the patients and to our staff. A recent publication in the American Journal of Psychiatry described using CBD to reduce opioid cravings. They found a 3 to 5% decrease in craving in CBD group which was superior to placebo (51). We now have a follow-up blind, placebo-controlled trial offering patients twice a week treatments for 4 weeks underway, which was funded by a NIDA/SBIR grant (#1R43DA050358-01).
The mechanisms of tPBM have been well reviewed in many papers (5, 6, 8, 12) and include the absorption of photons by cytochrome-c to stimulate ATP formation (11) in the mitochondria, increased cerebral blood flow (5), decreased inflammatory factors (52), and increased brain neurotropic factors (53), but none of these mechanisms explains our observations of the often profound decreases in cravings, anxiety, and depression within minutes after a treatment has begun. Caldieraro and Cassano have speculated that tPBM might act through induced electromagnetic fields (54), and Schiffer has speculated that these changes might involve quantum effects due to alterations in biophoton emissions from tubulin molecules encoding brain information related to experiences (55), but these speculations have not yet been tested. What is clear, however, is that several studies have reported psychological benefits from bilateral tPBM (9, 37, 42, 44), including one case report of a 31-month treatment (56).
Because of his success in accurately predicting patients’ responses to left-sided rTMS using his DBP hypotheses, Schiffer was motivated to treat the right hemisphere with 10 Hz in patients in whom the visual test suggested that their right hemisphere was more positive, but he was unable to find an interested collaborator. His first study of tPBM was a bilateral pilot study of 10 patients with a history of trauma, anxiety and depression, seven of whom have a past history of substance abuse. All had been in remission for at least 4 years, and none had active cravings (5). Immediately after treatment and in follow-up these patients did not notice the improvement that was observed on the Hamilton Scales. Schiffer then used unilateral t-PBM in his private practice as an off-label treatment and found that the unilateral treatment to the positive hemisphere seemed to be far superior to the bilateral application as shown in Table 8. This paper reports the first double-blind, placebo-controlled study of unilateral t-PBM to a hemisphere with a more positive HEV. That unilateral tPBM to the positive hemisphere is superior to that to the negative hemisphere, and the fact that unilateral tPBM was effective in reducing cravings, depression, and anxiety offer support for Schiffer’s hypotheses as expressed in DBP and for his clinical observations from his practice and his research indicating that unilateral tPBM is superior to bilateral tPBM. Further study needs to directly compare unilateral and bilateral tPBM.
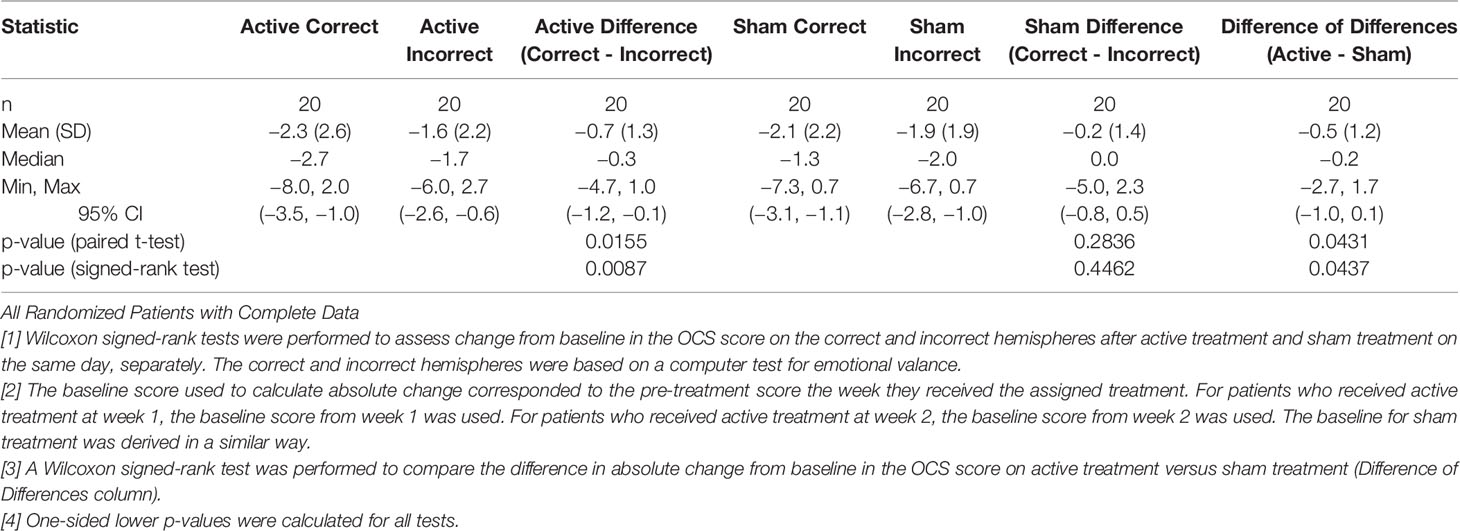
Table 8 Comparison of Absolute Change in Mean OCS Scores Between Active and Sham Treatment by Correct vs. Incorrect Hemisphere.
The main limitation of the study is its small number of patients and its short duration. t-PBM is a relatively new field, and many initial studies have had small numbers (9, 37, 56–58), and larger and longer studies are needed. We have completed 31 of 40 patients in a NIDA sponsored double-blind RTC study (Grant #1R43DA050358-01), which has been temporarily interrupted by the pandemic, but to date shows a highly significant treatment * time effect in regard to opioid craving reduction.
All datasets presented in this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by New England IRB, Needham, MA WIRB-Copernicus IRB Group. The patients/participants provided their written informed consent to participate in this study.
FS made substantial contributions to the conception or design of the work as well as to the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content. WR performed the statistical analyses for the work. EF and HM made substantial contributions to the acquisition of data for the work. MH made substantial contributions to the conception or design of the work; analysis or interpretation of data for the work and revising it critically for important intellectual content. All authors have provided approval for publication of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
WR was a freelance statistician who was referred to MindLight by Horizon Data Science Applications. FS is Founder and President of MindLight, LLC, which has been awarded a NIDA/SBIR grant (1 R43 DA050358-01) for further research and commercialization of the methods and device described in the paper. FS has been issued 2 US patents which cover the method of unilateral tPBM to a positive hemisphere as described in this study: U.S. Patent No. 8303636, Issued 11/06/2012: Methods for treating psychiatric disorders using light energy; and U.S. Patent No. 8574279, Issued 11/05/2013: Methods for treating psychiatric disorders using light energy. FS has filed on December 5, 2019, a US patent application, #16/703,937, Method and Apparatus for Determining Hemispheric Emotional Valence.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00827/full#supplementary-material
1. N.I.o.D. Abuse, Fiscal Year 2018 Budget Information - Congressional Justification for National Institute on Drug Abuse. (2018). Available at: https://www.drugabuse.gov/about-nida/legislative-activities/budget-information/fiscal-year-2018-budget-information-congressional-justification-national-institute-drug-abuse
2. Rudd RA, Paulozzi LJ, Bauer MJ, Burleson RW, Carlson RE, Dao D, et al. and Prevention, Increases in heroin overdose deaths - 28 States, 2010 to 2012. MMWR Morb Mortal Wkly Rep (2014) 63:849–54.
3. Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep (2016) 65:1445–52. doi: 10.15585/mmwr.mm655051e1
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. Am J Transplant (2018) 18:1556–68. doi: 10.1111/ajt.14905
5. Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct (2009) 5:46. doi: 10.1186/1744-9081-5-46
6. Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol (2018) 55:6601–36. doi: 10.1007/s12035-017-0852-4
7. Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol (2013) 86:447–57. doi: 10.1016/j.bcp.2013.06.012
8. Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics (2016) 3:031404. doi: 10.1117/1.NPh.3.3.031404
9. Caldieraro MA, Cassano P. Transcranial and systemic photobiomodulation for major depressive disorder: A systematic review of efficacy, tolerability and biological mechanisms. J Affect Disord (2019) 243:262–73. doi: 10.1016/j.jad.2018.09.048
10. Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience (2013) 230:13–23. doi: 10.1016/j.neuroscience.2012.11.016
11. Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg (2007) 25:180–2. doi: 10.1089/pho.2007.2064
12. Hamblin MR. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin (2016) 6:113–24. doi: 10.1016/j.bbacli.2016.09.002
13. Gainotti G. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neuroscientist (2019) 25:258–70. doi: 10.1177/1073858418785342
14. Packheiser J, Rook N, Dursun Z, Mesenholler J, Wenglorz A, Gunturkun O, et al. Embracing your emotions: affective state impacts lateralisation of human embraces. Psychol Res (2019) 83:26–36. doi: 10.1007/s00426-018-0985-8
15. Harmon-Jones E. Early Career Award. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology (2003) 40:838–48. doi: 10.1111/1469-8986.00121
16. Harmon-Jones E. On motivational influences, moving beyond valence, and integrating dimensional and discrete views of emotion. Cognit Emot (2019) 33:101–8. doi: 10.1080/02699931.2018.1514293
17. Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cognit Behav Neurol (2004) 17:9–17. doi: 10.1097/00146965-200403000-00002
18. Roesmann K, Dellert T, Junghoefer M, Kissler J, Zwitserlood P, Zwanzger P, et al. The causal role of prefrontal hemispheric asymmetry in valence processing of words - Insights from a combined cTBS-MEG study. Neuroimage (2019) 191:367–79. doi: 10.1016/j.neuroimage.2019.01.057
19. Schiffer F. Affect changes observed with right versus left lateral visual field stimulation in psychotherapy patients: possible physiological, psychological, and therapeutic implications. Compr Psychiatry (1997) 38:289–95. doi: 10.1016/S0010-440X(97)90062-6
20. Schiffer F. Of Two Minds: The Revolutionary Science of Dual-Brain Psychology. The Free Press: New York (1998).
21. Schiffer F, Anderson C, Teicher M. EEG, Bilateral Ear Temperature, and Affect Changes Induced by Lateral Visual Field Stimulation. Compr Psychiatry (1999) 40:221–5. doi: 10.1016/S0010-440X(99)90007-X
22. Schiffer F. Can the Different Cerebral Hemispheres Have Distinct Personalities? Evidence and Its Implications for Theory and Treatment of PTSD and Other Disorders. J Trauma Dissociation (2000) 1:83–104. doi: 10.1300/J229v01n02_06
23. Sperry RW, Zaidel E, Zaidel D. Self recognition and social awareness in the deconnected minor hemisphere. Neuropsychologia (1979) 17:153–66. doi: 10.1016/0028-3932(79)90006-X
24. Wittling W, Schweiger E. Neuroendocrine brain asymmetry and physical complaints. Neuropsychologia (1993) 31:591–608. doi: 10.1016/0028-3932(93)90054-4
25. Schiffer F, Zaidel E, Bogen J, Chasan-Taber S. Different psychological status in the two hemispheres of two split brain patients. Neuropsychiatry Neuropsychol Behav Neurol (1998) 11:151–6.
26. Schiffer F, Mottaghy F, Vimal R, Renshaw P, Cowan R, Pascual-Leone A, et al. Lateral Visual Field Stimulation Reveals Extrastriate Cortical Activation In The Contralateral Hemisphere: An fMRI Study. Psychiatry Res: Neuroimaging (2004) 131:1–9. doi: 10.1016/j.pscychresns.2004.01.002
27. Schiffer F, Teicher M, Anderson C, Tomoda A, Polcari A, Navalta C, et al. Determination of hemispheric emotional valence in individual subjects: a new approach with reasearch and therapeutic implications. Behav Brain Funct (2007) 3:13. doi: 10.1186/1744-9081-3-13
28. Schiffer F, Glass I, Lord J, Teicher MH. Prediction of clinical outcomes from rTMS in depressed patients with lateral visual field stimulation: a replication. J Neuropsychiatry Clin Neurosci (2008) 20:194–200. doi: 10.1176/jnp.2008.20.2.194
29. Schiffer F, Stinchfield Z, Pascual-Leone A. Prediction of Clinical Response to Transcranial Magnetic Stimulation for Depression by Baseline Lateral Visual Stimulation. Neuropsychiatry Neuropsychol Behav Neurol (2002) 15:18–27.
30. Branch C, Milner B, Rasmussen T. Intracarotid Sodium Amytal for the Lateralization of Cerebral Speech Dominance; Observations in 123 Patients. J Neurosurg (1964) 21:399–405. doi: 10.3171/jns.1964.21.5.0399
31. Stabell KE, Andresen S, Bakke SJ, Bjornaes H, Borchgrevink HM, Heminghyt E, et al. Emotional responses during unilateral amobarbital anesthesia: differential hemispheric contributions? Acta Neurol Scand (2004) 110:313–21. doi: 10.1111/j.1600-0404.2004.00329.x
32. Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognit Affect Behav Neurosci (2003) 3:207–33. doi: 10.3758/CABN.3.3.207
33. Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage (2002) 16:331–48. doi: 10.1006/nimg.2002.1087
34. Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, et al. Depression after stroke and lesion location: a systematic review. Lancet (2000) 356:122–6. doi: 10.1016/S0140-6736(00)02448-X
35. Ibrahimagic OC, Smajlovic D, Kunic S, Dostovic Z, Custovic A, Sehanovic A, et al. Post-Stroke Depression. Mater Sociomed (2019) 31:31–4. doi: 10.5455/msm.2019.31.31-34
36. Laures-Gore JS, Defife LC. Perceived stress and depression in left and right hemisphere post-stroke patients. Neuropsychol Rehabil (2013) 23:783–97. doi: 10.1080/09602011.2013.811087
37. Cassano P, Petrie SR, Mischoulon D, Cusin C, Katnani H, Yeung A, et al. Transcranial Photobiomodulation for the Treatment of Major Depressive Disorder. The ELATED-2 Pilot Trial. Photomed Laser Surg (2018) 36(12):634–46. doi: 10.1089/pho.2018.4490
38. Lampl Y, Zivin J, Fisher M, Lew R, Welin L, Dahlof B, et al. Infrared Laser Therapy for Ischemic Stroke: A New Treatment Strategy. Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1). Stroke (2007) 38(6):1843–9. doi: 10.1161/STROKEAHA.106.478230
39. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry (1960) 32:56-62. doi: 10.1037/t04100-000
40. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
41. McHugh RK, Fitzmaurice GM, Carroll KM, Griffin ML, Hill KP, Wasan AD, et al. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend (2014) 145:121–6. doi: 10.1016/j.drugalcdep.2014.10.002
42. Cassano P, Dording C, Thomas G, Foster S, Yeung A, Uchida M, et al. Effects of transcranial photobiomodulation with near-infrared light on sexual dysfunction. Lasers Surg Med (2019) 51:127–35. doi: 10.1002/lsm.23011
43. Chao LL. Improvements in Gulf War Illness Symptoms After Near-Infrared Transcranial and Intranasal Photobiomodulation: Two Case Reports. Mil Med (2019) 184(9-10):e568–74. doi: 10.1093/milmed/usz037
44. Eshaghi E, Sadigh-Eteghad S, Mohaddes G, Rasta SH. Transcranial photobiomodulation prevents anxiety and depression via changing serotonin and nitric oxide levels in brain of depression model mice: A study of three different doses of 810 nm laser. Lasers Surg Med (2019) 51(7):634–42. doi: 10.1002/lsm.23082
45. Hamilton C, Hamilton D, Nicklason F, El Massri N, Mitrofanis J. Exploring the use of transcranial photobiomodulation in Parkinson’s disease patients. Neural Regener Res (2018) 13:1738–40. doi: 10.4103/1673-5374.238613
46. Salehpour F, Rasta SH. The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder. Rev Neurosci (2017) 28:441–53. doi: 10.1515/revneuro-2016-0087
47. Zomorrodi R, Loheswaran G, Pushparaj A, Lim L. Pulsed Near Infrared Transcranial and Intranasal Photobiomodulation Significantly Modulates Neural Oscillations: a pilot exploratory study. Sci Rep (2019) 9:6309. doi: 10.1038/s41598-019-42693-x
48. Knoblauch K, Maloney LT. Modeling psychophysical data in R, Use R!. Springer: New York, NY (2012). p. 1. online resource.
49. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci (2016) 17:652–66. doi: 10.1038/nrn.2016.111
50. Schalinski I, Teicher MH, Nischk D, Hinderer E, Muller O, Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry (2016) 16:295. doi: 10.1186/s12888-016-1004-5
51. Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, et al. Cannabidiol for the Reduction of Cue-Induced Craving and Anxiety in Drug-Abstinent Individuals With Heroin Use Disorder: A Double-Blind Randomized Placebo-Controlled Trial. Am J Psychiatry (2019) 176:911–22. doi: 10.1176/appi.ajp.2019.18101191
52. Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg Med (2009) 41:282–90. doi: 10.1002/lsm.20766
53. Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, et al. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma (2007) 24:651–6. doi: 10.1089/neu.2006.0198
54. Caldieraro MA, Cassano P. Transcranial photobiomodulation for major depressive and anxiety disorders and for posttraumatic stress disorder. In: Hamblin MR, Huang Y-Y, editors. Photobiomodulation in the Brain. Cambridge, MA, USA: Academic Press (2019).
55. Schiffer F. The physical nature of subjective experience and its interaction with the brain. Med Hypotheses (2019) 125:57–69. doi: 10.1016/j.mehy.2019.02.011
56. Caldieraro MA, Sani G, Bui E, Cassano P. Long-Term Near-Infrared Photobiomodulation for Anxious Depression Complicated by Takotsubo Cardiomyopathy. J Clin Psychopharmacol (2018) 38:268–70. doi: 10.1097/JCP.0000000000000883
57. Cassano P, Cusin C, Mischoulon D, Hamblin MR, De Taboada L, Pisoni A, et al. Near-Infrared Transcranial Radiation for Major Depressive Disorder: Proof of Concept Study. Psychiatry J (2015) 2015:352979. doi: 10.1155/2015/352979
58. Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, et al. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma (2014) 31:1008–17. doi: 10.1089/neu.2013.3244
Keywords: opioid craving, depression, anxiety, photobiomodulation, brain hemispheres, laterality, dual-brain psychology
Citation: Schiffer F, Reichmann W, Flynn E, Hamblin MR and McCormack H (2020) A Novel Treatment of Opioid Cravings With an Effect Size of .73 for Unilateral Transcranial Photobiomodulation Over Sham. Front. Psychiatry 11:827. doi: 10.3389/fpsyt.2020.00827
Received: 05 April 2020; Accepted: 30 July 2020;
Published: 19 August 2020.
Edited by:
Jianhua Chen, Shanghai Jiao Tong University, ChinaReviewed by:
Leandro Da Costa Lane Valiengo, University of São Paulo, BrazilCopyright © 2020 Schiffer, Reichmann, Flynn, Hamblin and McCormack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fredric Schiffer, ZnJlZC5zY2hpZmZlcm1kQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.