
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 05 June 2020
Sec. Mood Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.00494
 Claudia von Zimmermann1*†
Claudia von Zimmermann1*† Merle Winkelmann1
Merle Winkelmann1 Tanja Richter-Schmidinger1†
Tanja Richter-Schmidinger1† Christiane Mühle1†
Christiane Mühle1† Johannes Kornhuber1†
Johannes Kornhuber1† Bernd Lenz1,2†
Bernd Lenz1,2†Background: Physical activity and a healthy body composition are said to reduce the risk of major depressive disorder. Nonetheless, deeper insight is needed into which specific forms of physical activity (and their relation to body composition) are effective in improving and preventing depressive symptoms.
Methods: We compared different self-reported physical activities of the Global Physical Activity Questionnaire and body composition measures between patients with a current major depressive episode (MDE; N = 130) and healthy control subjects (N = 61). These parameters were also tested for correlations with depression severity and serum lipid levels in patients and controls.
Results: Patients with a current MDE reported significantly fewer hours spent on total physical activity, walking or bicycling for travel, and vigorous-intensity activities at leisure than healthy control subjects. More time spent on vigorous-intensity activities at work, less time spent on walking or bicycling for travel, higher body fat mass, and lower body muscle mass correlated significantly with stronger depression severity. Physical activity and body measures correlated significantly with serum lipid levels.
Limitations: Self-reports of physical activity, only short-term follow-up of 20 days, cross-sectional study design without examination of causal role of exercise.
Conclusions: More time spent on traveling by foot or by bike is especially associated with a lower risk of and milder depression. These results highlight the differential role of physical activity in depression.
Major depressive disorder (MDD) is one of the five leading causes for years lived with disability (1), and 30% of the depressed patients are considered therapy refractory. Suffering from depression entails immense individual burden and creates high economic costs for society. Hence, there is urgent need for effective prevention strategies.
The World Health Organization (WHO) recommends at least 150 min of moderate-intensity physical activity throughout the week to reduce the risk of noncommunicable diseases (NCDs) like depression (2). Some studies show a reduction of depressive symptoms following resistance exercise training (3). However, it seems that sport administered in a therapeutical setting has only a minor positive impact on depressive symptoms overall (4). Instead, physical activity appears to be more effective in prevention of depression. In contrast to physical activity at work, physical activity during leisure-time might protect against the development of a depressive disorder depending on the amount but regardless of the intensity (5). Using the genetic instrument of Mendelian randomization, Choi et al. (6) clarified that reduced physical activity is a risk factor for depressive symptoms, not only a consequence. Overall, we have only limited knowledge about which subforms of physical activity are likely to help preventing depression.
Physical activity is also important for weight control and prevention of obesity. On the one hand, especially the atypical subtype of MDD is a strong predictor of obesity and weight gain in the future (7). On the other hand, obesity increases the risk of future depression (8). Higher body mass index (BMI) was associated with increased risk of depression in a large prospective population survey (9). In addition, abdominal fat distribution may be a key mediator in the relationship between obesity and depression (10). Based on these data, we would expect that patients with a current major depressive episode (MDE) show body measures related to obesity, i.e., primarily a higher BMI.
Moreover, physical activity reduces the likelihood of NCDs and depressive comorbidities, particularly cardiovascular disease (11). There is evidence that physical training improves cholesterol metabolism, e.g., lowers LDL lipoprotein levels (12, 13). Cholesterol metabolisms and serum lipids seem to play an important role in depression. It has been shown that high triglycerides, high total cholesterol, and high LDL cholesterol are related to depression per se (14). By contrast Ancelin et al. (15) found out that lower LDL cholesterol is a risk factor for depressive symptoms in elderly males. Using lipid lowering statins as an additional medication to antidepressants, Salagre et al. (16) found a better Hamilton Depression Rating Scale (HAMD) outcome in patients compared to those with antidepressants and placebo in a large meta-analysis of epidemiological studies. Interestingly, cerebral lipids, especially ceramides, have been shown to be a potential target of antidepressants and influence depression like behavior in animal experiments (17). Considering the possible LDL lowering effect of physical activity, lipid pathways might thus be involved in the relationship between physical activities and depression.
In this study, we aimed to investigate which physical activities in everyday life (total physical activity, vigorous- and moderate-intensity activities at work and at leisure, and time spent walking or bicycling for travel; sitting as a control condition) are associated with the risk and severity of depressive disorders. We further analyzed which related body composition measures (BMI, body weight and height, waist circumference, body fat and muscle mass, and visceral adipose tissue) are associated with the risk and severity of depressive disorders. We also analyzed the association of physical activity with serum lipids. We have previously found in the same cohort that higher serum lipids, especially higher LDL cholesterol, are positively associated with depression severity (18).
Sex differences in depression, like the two-fold increased lifetime prevalence in women compared with men, are well known (19). Obesity seems to be more prevalent in women. Worldwide, 11% of all men and 15% of all women are classified as obese (20). Li et al. (21) found a positive correlation between depression and BMI in women, but not in men.
Hagströmer et al. (22) showed men spending more time than women in moderate and vigorous physical activity, but no sex-specific differences for inactivity in a large population based study.
Based on these sex differences, we also explored whether the effects were present in analyses specific to women and men.
Our primary hypothesis was that more time spent on physical activity is associated with a lower risk of a current MDE and lower depression severity. Second, we hypothesized that lower serum lipids, especially LDL cholesterol, are associated with protective physical activities. Third, we hypothesized that higher body composition measures, especially higher BMI and visceral adipose tissue, are positively associated with higher risk of depression.
We used data from participants of the CeraBiDe (“Ceramide-associated Biomarkers in Depression”) study (18, 23). The recruitment took place between 01/2014 and 01/2017. Patients with a current MDE were recruited from in- and outpatients of the Department of Psychiatry and Psychotherapy at the University Hospital Erlangen and further interested persons fulfilling the inclusion criteria. Healthy control subjects were local citizens informed about the study via letters, local newspapers, flyers, and via internet advertisement; we matched both groups by age and gender. Participants underwent a multi-step screening procedure to exclude severe physical (e.g., autoimmune disorder, cancer) and psychiatric morbidity (with the exclusion of nicotine dependence and comorbid anxiety disorder), and the use of corticosteroids or anti-inflammatory drugs in the last 7 days, pregnancy, and breastfeeding (see Supplementary Material). The study sample characteristics are provided in Table 1. In total, we included 130 patients with a current MDE (64 without any antidepressants for at least 2 weeks and 66 persons taking antidepressants in a stable regime for at least 2 weeks) and 61 healthy control subjects. Inclusion criteria were BMI 18.5–35 kg/m², age 18–75 years, and written informed consent. We screened all participants using a shortened German version of the structured clinical interview for DSM-IV (SCID-I) and quantified depression severity using the self-report Beck Depression Inventory (BDI)-II and the clinician-administered 17-item HAMD. The patients had to fulfill the criteria for a MDD in the SCID-I.
One hundred twenty of the 130 patients with a current MDE participated in a direct follow-up (study visit 2) [14–63 days post inclusion, median 20 days, interquartile range (IQR) 16–27]. All patients received treatment as usual during the follow-up period. At the first time point (study visit 1), blood, clinical, behavioral, and body composition parameters [including SCID-I, BDI-II, HAMD, Global Physical Activity Questionnaire (GPAQ), body composition] were collected. During the follow-up visit (study visit 2), a blood taking and a second clinical interview (including BDI-II and HAMD) took place.
Physical activity was measured by the GPAQ (24, 25). Participants were asked about the time spent on different types of physical activity in a typical week in the last six months. Total physical activity per week was calculated using metabolic equivalents to activity levels according to the recommendations of the WHO (26). Moderate physical activity at leisure includes activities with moderate physical effort, which cause small increases in breathing or heart rate such as brisk walking, cycling, swimming, or volleyball; vigorous-intensity activities at leisure require hard physical effort and cause large increases in breathing or heart rate, like running or football. Vigorous-intensity activities at work include carrying or lifting heavy loads, digging, and construction work. Moderate-intensity activity at work causes small increases in breathing or heart rate such as brisk walking or carrying light loads.
For the judgment of the GPAQ, it is important to equalize vigorous-intensity to moderate-intensity physical activity. As recommended by the WHO, we doubled the time spent with vigorous-intensity activity to equalize it with moderate level activity. Time spent traveling is considered a moderate activity. Total physical activity means the sum of all single activities per week (without sitting).
For body composition analysis (BMI, body weight and height, waist circumference, body fat and muscle mass, visceral adipose tissue), a classical measuring tape and body analyzer (Omron BF511, Krell Precision, Jiangsu, China) applying bio-impedance measurement were used.
Blood samples were taken (after fasting overnight) in the morning. Serum lipid levels were quantified at the Central Laboratory of the University Hospital Erlangen, Germany (DIN EN ISO 15189 accredited) by enzymatic photometric assays.
The data were analyzed using SPSS for Windows 24.0 (SPSS Inc., Chicago, IL) and visualized using Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA). The descriptive statistics report median, IQR, and frequencies. The χ2 test was used to evaluate differences in the frequency of nominal variables. Because the Kolmogorov-Smirnov test showed significant deviations from normal distributions in most variables of interest in patients with a current MDE and/or healthy control subjects (all depression scores, all times spent on physical activity, all body measures except for body fat mass), we decided to use non-parametric methods. Group differences were tested using the Mann-Whitney U tests for independent samples. Spearman's method was applied to evaluate bivariate correlations. P < 0.05 for two-tailed tests was considered significant. All P-values are uncorrected for multiple hypothesis testing. We used Receiver Operating Characteristic (ROC) and Youden's J statistic (J = sensitivity + specificity − 1) to evaluate the thresholds of hours of physical activity per week to best discriminate patients with a current MDE from healthy control subjects (27). The results of sex-specific analyses are shown in the Supplemental Tables.
This study was approved by the Ethics Committee of the Medical Faculty of the Friedrich-Alexander University Erlangen-Nürnberg (ID 148_13 B).
We did not find any significant between-group differences in age, sex, education, and time spent working (i.e., paid hours at work per week and paid months during the previous year). Patients with a current MDE were significantly more frequently divorced (OR 5.0) and significantly more often single (OR 2.0) than controls. They also scored significantly higher on the BDI-II and HAMD scores (Table 1).
Patients with current MDE spent significantly less time on total physical activity, on traveling to and from places by bike or by foot, and on activities with vigorous physical effort in leisure time than healthy control subjects (Table 1). Neither time invested in activities at work (vigorous-intensity and moderate-intensity), nor moderate intensity activities at leisure, varied significantly between healthy control subjects and patients with a current MDE. There was also no significant difference in our control parameter, the daily time used for sitting. Moreover, the groups did not significantly vary in body composition measurements (Table 1). Most of the effects seen in the total cohort were also present in the sex-specific sub-groups. In addition, we found longer sitting in female and longer vigorous-intensity activities at work in male patients with a current MDE than in sex-specific healthy control subjects. Moreover, male patients with a current MDE showed significantly higher body fat mass and visceral adipose tissue and lower body muscle mass (Supplemental Tables 1 and 2).
Because of significant differences in the between-group comparisons for total physical activity, time spent walking or bicycling for travel, and vigorous-intensity activities at leisure, we further conducted ROC analyses to evaluate the thresholds of minutes per week to discriminate patients with MDE from healthy control subjects. These showed Youden cut-off points of 465 min per week of total physical activity (N = 188, AUC 0.669, P < 0.001, sensitivity 0.543, specificity 0.852), of 123 min per week spent walking or bicycling for travel (N = 191, AUC 0.724, P < 0.001, sensitivity 0.662, specificity 0.738), and of 113 min per week with vigorous-intensity activities at leisure (N = 191, AUC 0.725, P < 0.001, sensitivity 0.854, specificity 0.590) (Figure 1). In sex-specific analyses, we found Youden cut-off points for total physical activity of 245 min per week in females (N = 97, AUC 0.683, P = 0.004, sensitivity 0.379, specificity 0.968) and 450 min per week in males (N = 91, AUC 0.646, P = 0.024, sensitivity 0.492, specificity 0.967). We found Youden cut-off points for walking or bicycling for travel of 130 min per week in females (N = 100, AUC 0.752, P < 0.001, sensitivity 0.710, specificity 0.742) and 165 min per week in males (N = 91, AUC 0.693, P = 0.003, sensitivity 0.689, specificity 0.667), and for vigorous-intensity activities at leisure of 113 min per week in females (N = 100, AUC 0.680, P = 0.004, sensitivity 0.884, specificity 0.452) and of 95 min per week in males (N = 91, AUC 0.765, P < 0.001, sensitivity 0.820, specificity 0.733). In our study population, the cut-off point next to the 150 min/week of total physical activity recommended by WHO was 162.5 min/week. For this value, we found a sensitivity of 0.236 and a specificity of 0.967.
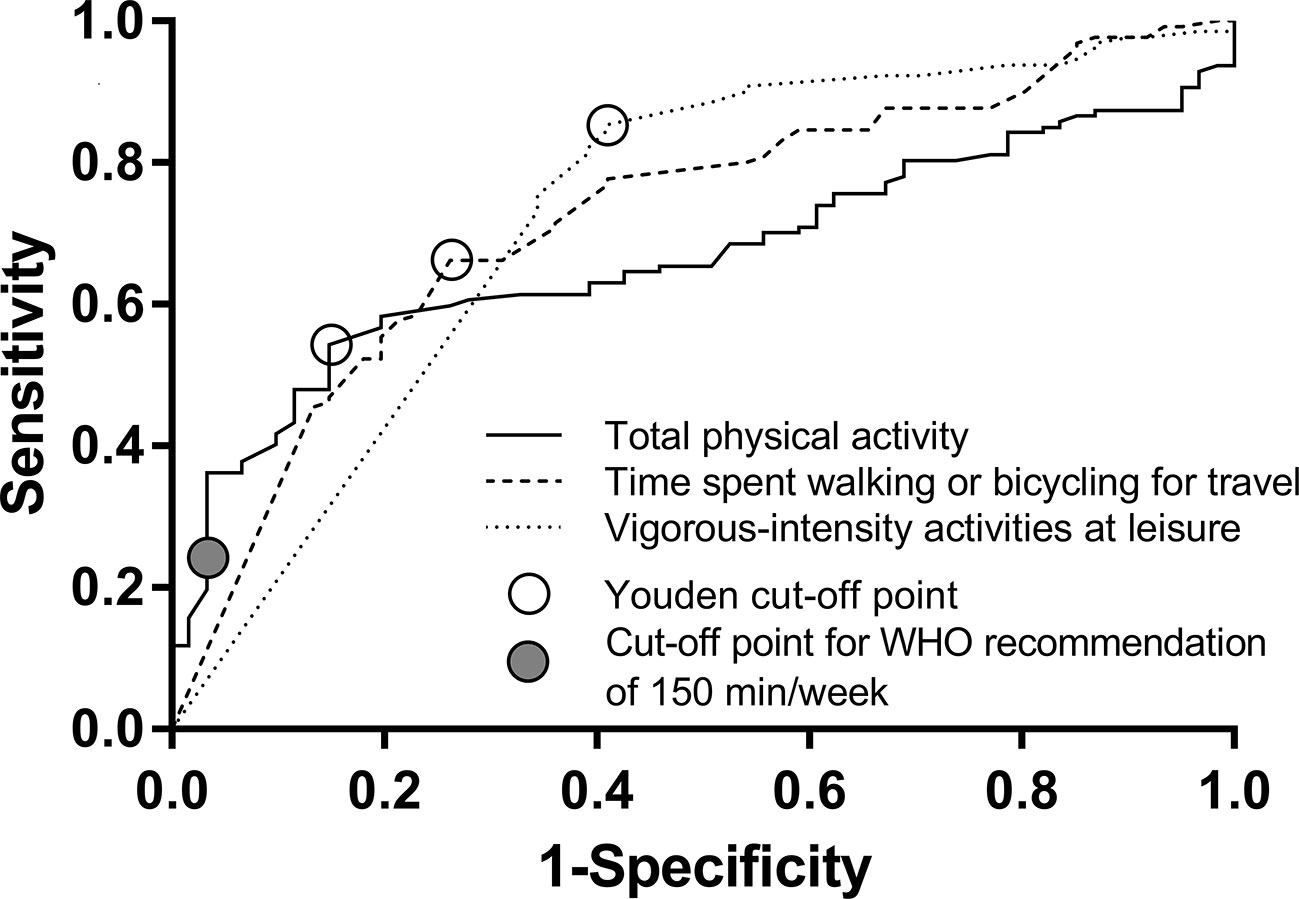
Figure 1 The results of the Receiver Operating Characteristic analysis with open circles indicating Youden's cut-off points.
Time with vigorous-intensity activities at work correlated significantly positively with HAMD scores at baseline. On the contrary, the time spent on walking or bicycling for travel correlated significantly negatively with BDI-II and HAMD scores. Consistent with that, we also found significant positive relationships between body fat mass and depression scores and significant negative relationships between body muscle mass and depression scores (Table 2). Physical activity and body measures were not significantly associated with a change in depression severity from study visit 1 to study visit 2. However, in sex-specific analyses, such effects were found in the group of female patients with an MDE. In this group, lower BMI, body weight, waist circumference, body fat mass, and visceral adipose tissue were associated with a stronger reduction on the HAMD score (Supplemental Tables 3 and 4). In line with the findings in the patients group, we also detected in the control group that HAMD scores correlated significantly positively with activities at work and significantly negatively with vigorous-intensity activities at leisure (Supplemental Table 5).
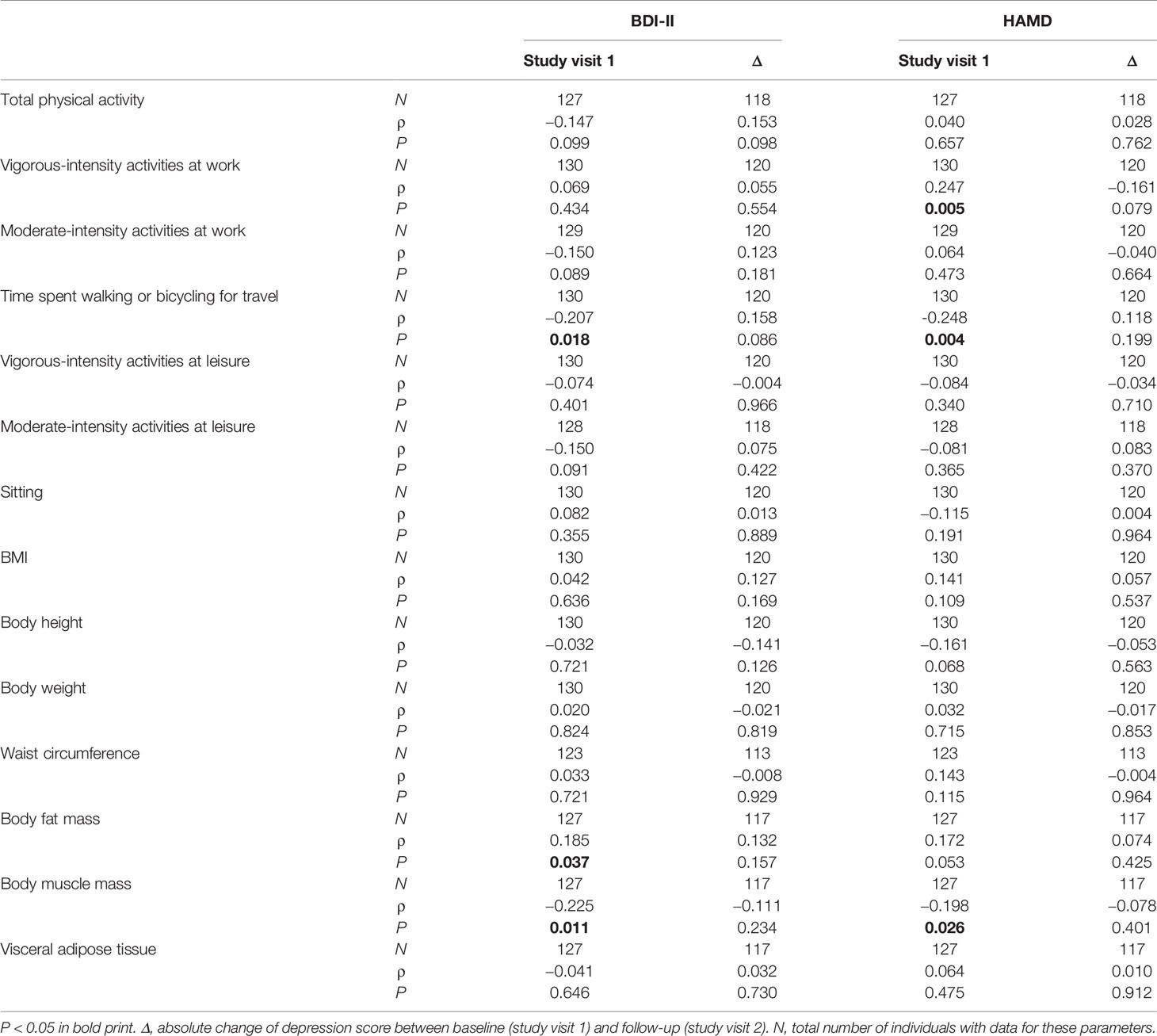
Table 2 Spearman correlations between physical activity, body measures, and depression severity in patients with a current MDE.
Details on lipid serum levels and the use of lipid lowering agents in the sample analyzed here have already been published in Wagner et al. (18). In patients with a current MDE, vigorous-intensity activities at work correlated significantly positively with triglyceride levels and LDL/HDL ratios, and time spent walking or bicycling for travel correlated significantly negatively with triglycerides, total and LDL cholesterol, and LDL/HDL ratios (Table 3). The sex-specific analyses confirmed the significant negative correlations between time spent walking or bicycling for travel and triglycerides, total and LDL cholesterol, and LDL/HDL ratio in male patients with MDE (Supplemental Table 6). As expected, there were several significant associations between body measures and serum lipid profile (Table 3 and Supplemental Table 6). In the group of healthy control subjects, moderate-intensity activities at leisure correlated significantly positively with triglycerides and total and LDL cholesterol in the total cohort and the female and male sub-cohorts. In line with the patients with a current MDE, body measures were significantly related to the serum lipid profile (Supplemental Tables 7 and 8).
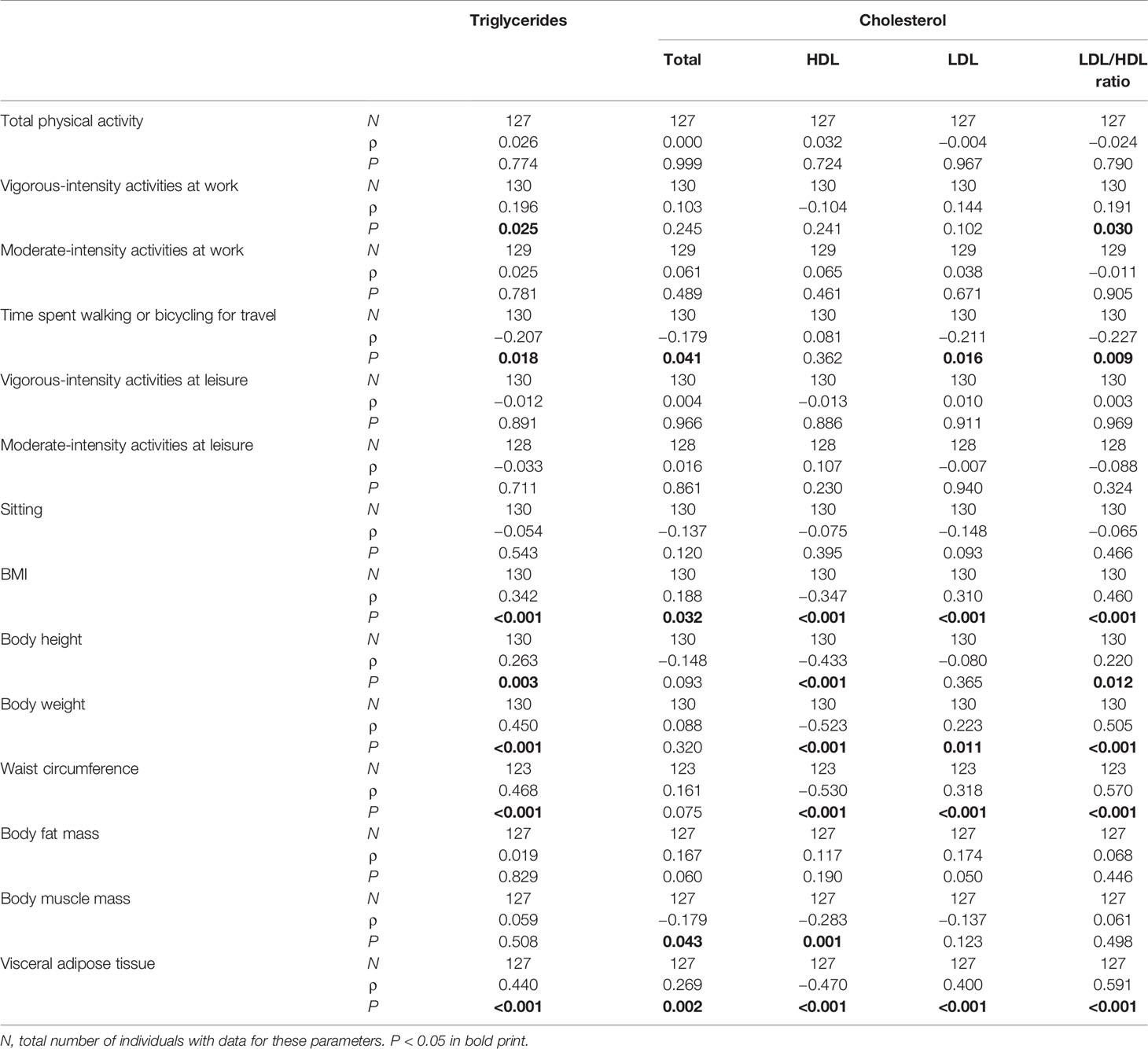
Table 3 Spearman correlations between physical activity, body measures, and serum lipid profile at baseline in patients with current MDE.
We found various significant correlations between physical activities and body measures (Table 4). In sex-specific analyses, these were found only in males but not in females (Supplemental Tables 9 and 10).
Physical activity is widely recommended to promote health and to protect against a broad range of diseases. In support of this, here we found a significantly higher amount of physical activity in healthy control subjects than in patients with a current MDE. Vigorous-intensity activities in leisure and time spent to travel to and from places by bike or on foot were especially more extensive in healthy control subjects. Interestingly, neither moderate-intensity activities at work nor at leisure varied significantly and consistently between patients with a current MDE and the controls in all three groups (total, female, and male groups). As the groups did not significantly differ with regard to time spent working (i.e., paid hours at work per week and paid months during the previous year), disability, unemployment, and sick leave are unlikely to account for this observation. In the total cohort and the male sub-cohort, there was no significant difference in our control parameter (daily time used for sitting) which might in part be due to recall bias in patients with a current MDE. The time spent sitting (480 min/day for patients with an MDE vs. 420 min/day for healthy control subjects) in our cohort agrees with data from Hagströmer et al. (22) who found 459 min of inactivity per day for adults in a huge population based study. Helgadottir et al. (28) found 546 min of sedentary behavior per day in persons with mild to moderate symptoms of depressive and/or anxiety disorders. They showed that depression severity is positively associated with time spent sedentary (28); we were not able to replicate this association.
We calculated ROC analysis and demonstrated that less than 465 min per week of total physical activity (sensitivity 0.543, specificity 0.852), less than 123 min per week spent walking or bicycling for travel (sensitivity 0.662, specificity 0.738), and less than 113 min per week with vigorous-intensity activities at leisure (sensitivity 0.854, specificity 0.590) optimally discriminates between patients with MDE and healthy control subjects. The WHO recommends at least 150 min of moderate physical activity per week to prevent NCDs (2). Our data suggest that longer times spent on physical activity might be more beneficial. The results of our cross-sectional study are not applicable to make any inferences about causality. It is also very important to mention the bidirectional relationship of physical activity and mental health. On the one hand, physical activity reduces the risk of future depression (29, 30). On the other hand, depression may also lead to inactivity and increased sedentary behavior (31). Using Mendelian randomization, Choi et al. (6) found evidence that physical activity demonstrated a potential causal relationship with depression. While the physical activities' impact on depressive symptoms seems not to be crucial (4), it appears to be more important in the prevention of mental illness. Although mainly leisure time associated physical activity has been shown to be helpful to prevent depression, office-based workplace physical activity interventions seem to be effective in improving well-being (32). Since office-based interventions seem to be applicable, they should be broader analyzed in future studies.
In patients with a current MDE, more time spent on traveling was associated with milder depression severity, while vigorous-intensity activities at work were associated with a higher HAMD score. Also, in the group of healthy control subjects, activities at work related to higher HAMD scores and vigorous activities at leisure were linked to lower HAMD scores. These results partly agree with those of Harvey at al. (5), who found on the one hand a protective impact of leisure-time physical activity in general but on the other hand a harming effect of physical activities at work. Here, the causality remains unclear as monotonous work or socioeconomic status might act as confounder.
In our study cohort, we found significant differences in the between-group comparisons. Healthy control subjects spent more time for total physical activity, walking or bicycling for travel, and vigorous-intensity activities at leisure. This agrees with Chekroud et al. (33) who found a significant reduction in mental health burden especially for popular sports, cycling, and aerobic and gym exercises compared with no exercise, preferably 30 to 60 min, three to five times per week, in a large cross-sectional study. In accordance with Cooney et al. (4), we also show that, once a patient has developed an MDE, physical activity seems not to be associated with the prospective course of depression per se, but does seem to be associated with milder depression severity.
As shown in Figure 2, we also explored the role of body measures and serum lipids in the relationship between physical activity and depression severity. The investigated body measurements did not significantly differ between the two groups, although some showed statistical trends. As expected, depressive patients tended to have higher body fat mass, higher visceral adipose tissue mass, and lower body muscle mass than the controls. These differences reached significance in the male sub-cohort. Altogether, this supports our results showing less physical activity in patients with a current MDE. The rather small number of overall study subjects might account for missing a significant effect. Moreover, the inconsistencies between the associations with self-reported physical activity and objective body measure might be due to recall bias in patients with a current MDE. Surprisingly, in the group of female patients with an MDE, we found that lower BMI, body weight, waist circumference, body fat mass, and visceral adipose tissue were associated with a stronger reduction in HAMD score in sex-specific analyses. Further studies for replication of this result are necessary.
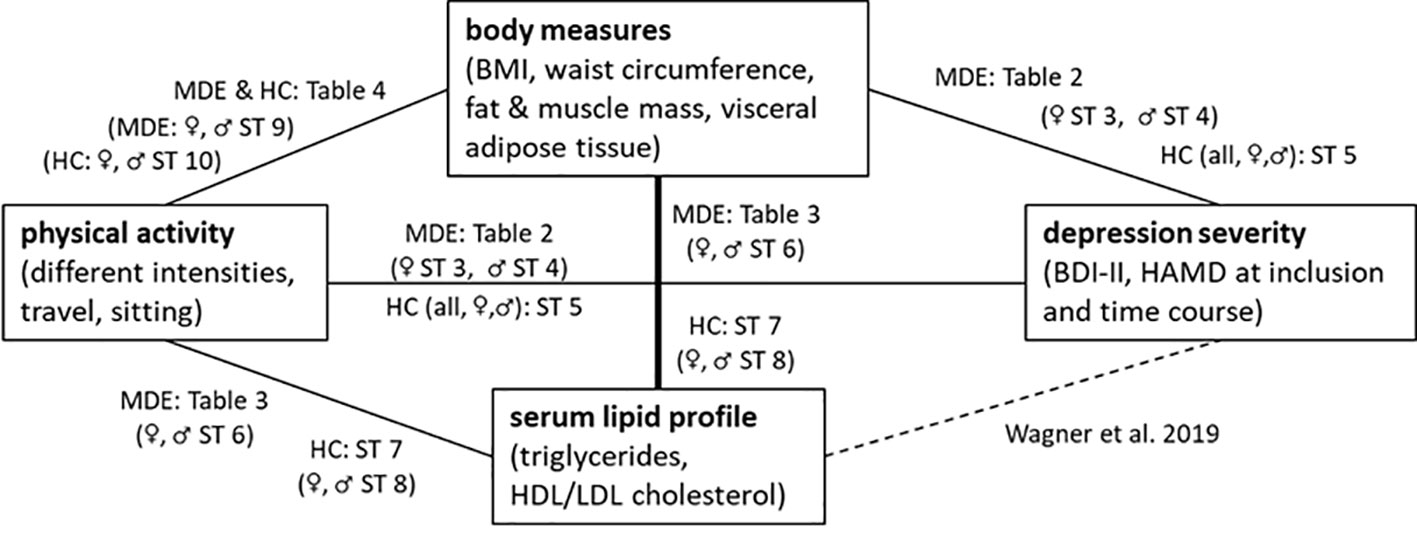
Figure 2 An overview of the correlations shown in the tables and supplemental tables (ST) for patients with a current MDE and healthy controls (HC).
Both physical activity and body measurements were strongly related to serum lipid levels. As described earlier, serum lipids, especially LDL cholesterol, are positively associated with depression and depression severity in our cohort (18). In this former study, we observed that treatment with antidepressants did not have a strong effect on the association of lipid levels with depression severity (18). In support of this Kuel et al. (34) did not find different cardiovascular risk profiles among depressed patients with and without antidepressant medication.
For patients with a current MDE, vigorous-intensity activities at work correlated positively with triglyceride levels and LDL/HDL ratios. Furthermore, time spent walking or bicycling for travel correlated negatively with triglyceride, total and LDL cholesterol, and LDL/HDL ratio. These associations suggest that serum lipids are involved in the relationship between physical activity and MDE. This also supports the assumption that subforms of physical activity differ in their impact on mental health with both helpful and harming effects. MDD, dyslipidemia, and insulin resistance are said to share immunoinflammatory alterations (35). Exercise training is potent to interrupt the obesity-induced inflammatory mechanisms (36) and can be utilized to improve cholesterol levels (37). As a result, physical activity might prevent depressive symptoms.
Body fat mass correlated positively and body muscle mass correlated negatively with depression severity, supporting the described associations between physical activity and depression. This agrees with Alshehri et al. (38), who detected a positive association between total body fat and depressive mood both in men and women. As expected, we found strong correlations between physical activity and body composition measurements. These support the validity of the self-reported physical activities. In line with our results, Choi et al. (6) found a protective effect against depression of objectively assessed but not self-reported physical activity.
Our study is limited by the associational study design, which does not allow causal conclusions. We analyzed a rather small study population and did not differentiate clinical characteristics of the episode like duration of the episodes or former episodes. We used self-reports of physical activity for our analyses without any direct objective measurements. Future studies might use pedometers. We also had only a small control population, which might account for distortion of the results. Moreover, we did not correct for multiple hypothesis testing, which might have resulted in some false positive findings. The strengths of our study include the systematic analyses of subforms of physical activity, the measurements of several body composition parameters, and the analyses of serum lipids.
In summary, our results support the recommendation that traveling by foot or by bike and vigorous-intensive physical activity at leisure should be widely promoted to prevent MDE.
The datasets are available on request. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical Faculty of the Friedrich-Alexander University Erlangen-Nürnberg (ID 148_13 B). The patients/participants provided their written informed consent to participate in this study.
Conceived and designed the study: CZ, MW, TR-S, CM, JK, and BL. Performed the experiments: CZ, MW, CM, and BL. Analyzed the data and wrote the paper: CZ, MW, CM, and BL. Commented on the manuscript and provided intellectual input: TR-S and JK.
This work was supported by grants of the German Federal Ministry of Education and Research (01EE1401C), the German Research Foundation (KO 947/13-3), and intramural grants from the University Hospital of the Friedrich-Alexander University Erlangen-Nürnberg (FAU). CM is an associated fellow of the research training group 2162 funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 270949263/GRK2162. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the patients and control persons for their participation in this research project. We gratefully appreciate the support of Lea Böhm, Katharina Färber, Dr. Anna-Isabell Fischer, Cornelia Musenbichler, Felicitas von Nippold in recruiting patients and healthy control subjects. We are thankful to Alexander Gagel, Bruno Gegenhuber, and Hedya Riesop for excellent technical support with the blood samples. The present work was performed in partial fulfillment of the requirements for obtaining the degree “Dr. med.” for MW.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00494/full#supplementary-material
1. GBD 2016 disease and injury incidence and prevalence collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. WHO. Global strategy on diet, physical activity and health. Physical activity and adults. (2019).
3. Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms: Meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry (2018) 75:566–76. doi: 10.1001/jamapsychiatry.2018.0572
4. Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Systemat Rev (2013) (9):Cd004366. doi: 10.1002/14651858.CD004366.pub6
5. Harvey SB, Hotopf M, Øverland S, Mykletun A. Physical activity and common mental disorders. Br J Psychiatry (2010) 197:357–64. doi: 10.1192/bjp.bp.109.075176
6. Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: A 2-sample Mendelian randomization study. JAMA Psychiatry (2019) 76:399–408. doi: 10.1001/jamapsychiatry.2018.4175
7. Lasserre AM, Glaus J, Vandeleur CL, Marques-Vidal P, Vaucher J, Bastardot F, et al. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: A prospective, population-based study. JAMA Psychiatry (2014) 71:880–8. doi: 10.1001/jamapsychiatry.2014.411
8. Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry (2010) 67:220–9. doi: 10.1001/archgenpsychiatry.2010.2
9. Bjerkeset O, Romundstad P, Evans J, Gunnell D. Association of adult body mass index and height with anxiety, depression, and suicide in the general population: The HUNT study. Am J Epidemiol (2007) 167:193–202. doi: 10.1093/aje/kwm280
10. Rivenes AC, Harvey SB, Mykletun A. The relationship between abdominal fat, obesity, and common mental disorders: results from the HUNT study. J Psychosom Res (2009) 66:269–75. doi: 10.1016/j.jpsychores.2008.07.012
11. Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med (2019) 6:69. doi: 10.3389/fcvm.2019.00069
12. Pedersen LR, Olsen RH, Anholm C, Walzem RL, Fenger M, Eugen-Olsen J, et al. Weight loss is superior to exercise in improving the atherogenic lipid profile in a sedentary, overweight population with stable coronary artery disease: A randomized trial. Atherosclerosis (2016) 246:221–8. doi: 10.1016/j.atherosclerosis.2016.01.001
13. Murphy MH, Lahart I, Carlin A, Murtagh E. The effects of continuous compared to accumulated exercise on health: A meta-analytic review. Sports Med (Auckland NZ) (2019) 49:1585–607. doi: 10.1007/s40279-019-01145-2
14. Wysokiński A, Strzelecki D, Kłoszewska I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab Syndrome: Clin Res Rev (2015) 9:168–76. doi: 10.1016/j.dsx.2015.04.004
15. Ancelin M-L, Carrière I, Boulenger J-P, Malafosse A, Stewart R, Cristol J-P, et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in community-dwelling elderly (the ESPRIT study). Biol Psychiatry (2010) 68:125–32. doi: 10.1016/j.biopsych.2010.04.011
16. Salagre E, Fernandes BS, Dodd S, Brownstein DJ, Berk M. Statins for the treatment of depression: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord (2016) 200:235–42. doi: 10.1016/j.jad.2016.04.047
17. Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. Nat Med (2013) 19:934–8. doi: 10.1038/nm.3214
18. Wagner CJ, Musenbichler C, Böhm L, Färber K, Fischer AI, von Nippold F, et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog Neuropsychopharmacol Biol Psychiatry (2019) 92:405–11. doi: 10.1016/j.pnpbp.2019.01.010
19. Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology (2019) 44:111–28. doi: 10.1038/s41386-018-0148-z
21. Li L, Gower BA, Shelton RC, Wu X. Gender-specific relationship between obesity and major depression. Front Endocrinol (Lausanne) (2017) 8:292. doi: 10.3389/fendo.2017.00292
22. Hagstromer M, Oja P, Sjöström M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc (2007) 39:1502–8. doi: 10.1249/mss.0b013e3180a76de5
23. Mühle C, Wagner CJ, Färber K, Richter-Schmidinger T, Gulbins E, Lenz B, et al. Secretory acid sphingomyelinase in the serum of medicated patients predicts the prospective course of depression. J Clin Med (2019) 8(6):846. doi: 10.3390/jcm8060846
24. Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Activity Health (2009) 6:790–804. doi: 10.1123/jpah.6.6.790
25. Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the global physical activity questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health (2014) 14:1255. doi: 10.1186/1471-2458-14-1255
27. Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr (Oslo Norway: 1992) (2007) 96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x
28. Helgadóttir B, Forsell Y, Ekblom Ö. Physical activity patterns of people affected by depressive and anxiety disorders as measured by accelerometers: a cross-sectional study. PLoS One (2015) 10:e0115894. doi: 10.1371/journal.pone.0115894
29. Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: A meta-analysis of prospective cohort studies. Am J Psychiatry (2018) 175:631–48. doi: 10.1176/appi.ajp.2018.17111194
30. Fernandez-Montero A, Moreno-Galarraga L, Sánchez-Villegas A, Lahortiga-Ramos F, Ruiz-Canela M, Martínez-González M.Á., et al. Dimensions of leisure-time physical activity and risk of depression in the “Seguimiento Universidad de Navarra” (SUN) prospective cohort. BMC Psychiatry (2020) 20:98–8. doi: 10.1186/s12888-020-02549-5
31. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry (2009) 31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002
32. Abdin S, Welch RK, Byron-Daniel J, Meyrick J. The effectiveness of physical activity interventions in improving well-being across office-based workplace settings: a systematic review. Public Health (2018) 160:70–6. doi: 10.1016/j.puhe.2018.03.029
33. Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus M, Krumholz HM, Krystal JH, et al. Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry (2018) 5:739–46. doi: 10.1016/S2215-0366(18)30227-X
34. Kuehl LK, Muhtz C, Hinkelmann K, Dettenborn L, Wingenfeld K, Spitzer C, et al. Association between major depression and cardiovascular risk: the role of antidepressant medication. Psychopharmacology (2016) 233:3289–95. doi: 10.1007/s00213-016-4361-3
35. de Melo LGP, Nunes SOV, Anderson G, Vargas HO, Barbosa DS, Galecki P, et al. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry (2017) 78:34–50. doi: 10.1016/j.pnpbp.2017.04.027
36. Soltani N, Marandi SM, Kazemi M, Esmaeil N. The exercise training modulatory effects on the obesity-induced immunometabolic dysfunctions. Diabetes Metab Syndr Obes (2020) 13:785–810. doi: 10.2147/DMSO.S234992
37. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med (Auckland NZ) (2014) 44:211–21. doi: 10.1007/s40279-013-0110-5
Keywords: depression, physical activities and sports, body composition, serum lipid concentration, mental health
Citation: von Zimmermann C, Winkelmann M, Richter-Schmidinger T, Mühle C, Kornhuber J and Lenz B (2020) Physical Activity and Body Composition Are Associated With Severity and Risk of Depression, and Serum Lipids. Front. Psychiatry 11:494. doi: 10.3389/fpsyt.2020.00494
Received: 10 January 2020; Accepted: 15 May 2020;
Published: 05 June 2020.
Edited by:
Bruno Etain, Université Paris Diderot, FranceReviewed by:
Nefize Yalin, King's College London, United KingdomCopyright © 2020 von Zimmermann, Winkelmann, Richter-Schmidinger, Mühle, Kornhuber and Lenz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia von Zimmermann, Q2xhdWRpYS52b24uWmltbWVybWFubkB1ay1lcmxhbmdlbi5kZQ==
†ORCID: Claudia von Zimmermann, orcid.org/0000-0003-3522-754X; Tanja Richter-Schmidinger, orcid.org/0000-0001-5426-8342; Christiane Mühle, orcid.org/0000-0001-7517-9154; Johannes Kornhuber, orcid.org/0000-0002-8096-3987; Bernd Lenz, orcid.org/0000-0001-6086-0924
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.