- 1St. Edmund Hall College, University of Oxford, Oxford, United Kingdom
- 2Department of Psychiatry, University of Oxford, Oxford, United Kingdom
Schizophrenia is a debilitating psychiatric disorder, leading to both physical and social morbidity. Despite its importance, the etiology of schizophrenia remains poorly understood. Furthermore, its mainstream treatments fail to address all aspects of the disorder and are associated with significant side-effects. Recently, there has been growing interest in the relationship between the gut microbiome and mental health, including in schizophrenia. In this article, we review the existing evidence implicating dysbiosis in schizophrenia and discuss how the presumed dysbiosis could fit within known hypotheses of its pathogenesis, focusing on inflammation, tryptophan metabolites, and BDNF levels. We also evaluate the clinical potential of manipulating the gut microbiome with probiotics and prebiotics as adjunctive treatments in schizophrenia, based on existing clinical and pre-clinical studies. Overall, the current data showing microbiome alterations in schizophrenia are highly discrepant and insufficient to conclude whether microbiome changes are associated with increased risk of the disorder, or are simply the result of external factors or treatment. Despite some encouraging results of pro/prebiotic supplementation, there is also inconclusive evidence for their efficacy in schizophrenia. Thus, further research and more clinical trials are needed to test the validity of manipulating the gut microbiome to improve the treatment of this disorder.
Introduction
Schizophrenia is a debilitating psychiatric disorder, characterized by positive symptoms (delusions, hallucinations, aberrant flow of thoughts), and negative symptoms (apathy, withdrawal, slowness) (1). It affects ~21 million people worldwide, and causes significant physical and social morbidity (2, 3). Its heritability is estimated at ~80% based on concordance rates in monozygotic twins (4), while risk profile scores constructed from alleles associated with schizophrenia explain ~7% of variation in the liability to schizophrenia (5). Thus, genetics cannot fully explain its etiology, and so other factors, particularly the environment, need to be explored (6, 7). Furthermore, the current treatments for schizophrenia do not address all aspects of this disorder (8) and are associated with considerable side-effects (9), necessitating development of improved treatment strategies.
The gut microbiome has been implicated in psychiatric disorders, and is well-described for depression, with dysbiosis suggested in clinical studies (10) and fecal transplants from depressed patients to germ-free rats shown to induce depressive-like behaviors (11). The gut microbiome is a rich community of microbes which are diverse and personalized, though dominated by phyla Bacteroidetes and Firmicutes (12). It is also dynamic, influenced by lifestyle factors such as diet (13), sleep (14), or stress (15). The personal and dynamic nature of the gut microbiome is why dysbiosis remains a poorly defined concept, despite the common use of this term in the literature. Accurate definition of a healthy baseline microbiome is near impossible to establish, so it is unclear what qualitative and quantitative changes in the microbiome composition constitute functionally significant deviations from the “norm,” what thresholds should be set when assessing the importance of relative deviations from controls, and whether these should be different for various populations. Bearing these limitations in mind, we take the term dysbiosis to mean microbiome profiles which are significantly different from controls and which could have functional significance in pathological processes if conclusively proven. Research into the role of the microbiome in schizophrenia is still in its infancy. In this article, the evidence implicating dysbiosis in schizophrenia, and how it could fit within existing hypotheses of schizophrenia pathogenesis will be appraised. Furthermore, the therapeutic potential of pre/probiotics in schizophrenia will be discussed.
Evidence for Dysbiosis in Schizophrenia
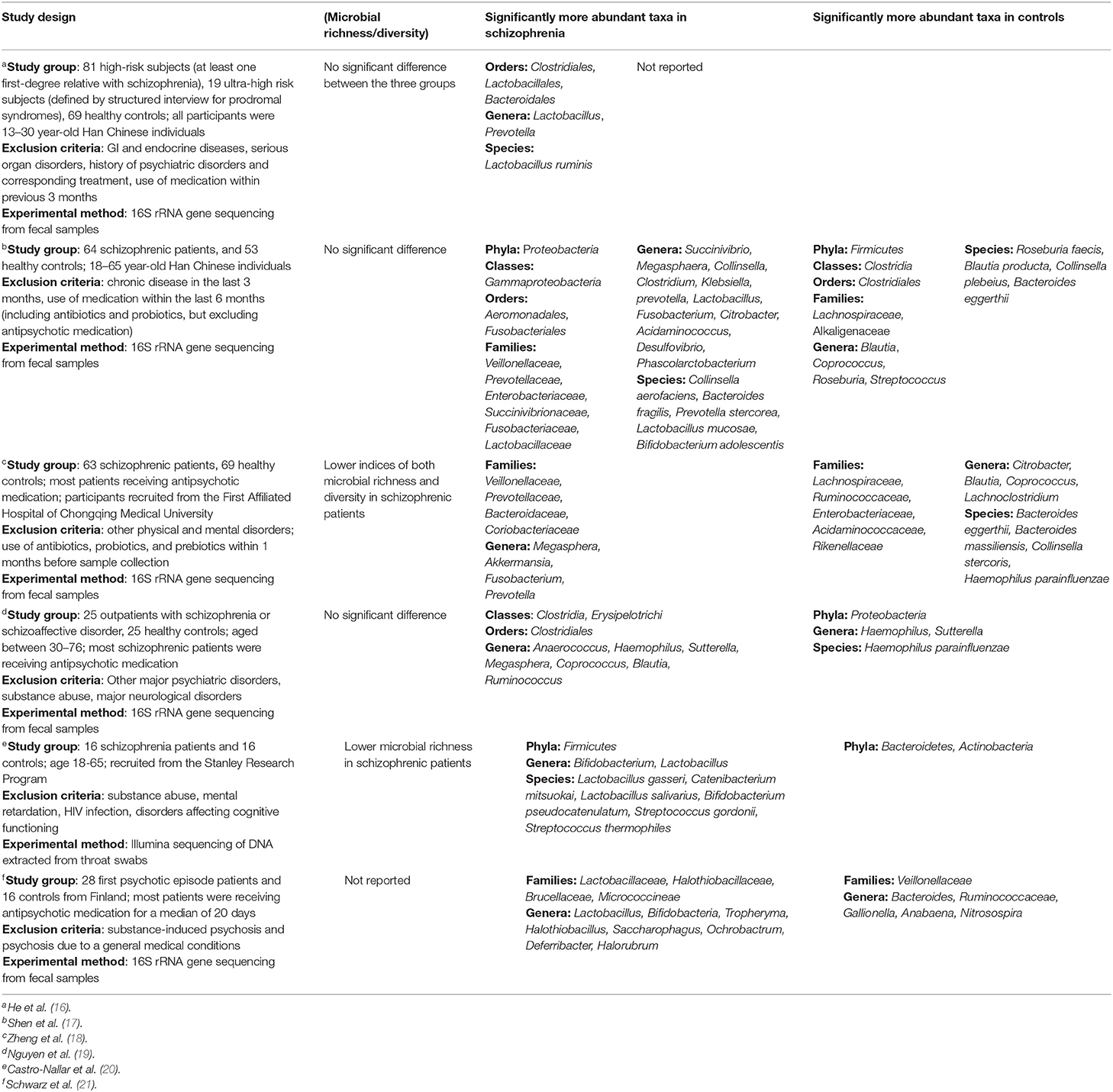
To date, six studies have been published investigating microbiome differences between healthy controls and individuals with schizophrenia (Table 1). Unaltered microbial richness/diversity was predominantly reported, but with significant differences in the abundance of specific taxa between groups. Interestingly, one study reported that among patients who clustered with controls at baseline, 70% experienced remission within 12 months, compared to only 28% with “abnormal” microbiota—an association which was not weakened by including baseline GAF score as a covariate (21). There is, however, considerable discord between these results. At phylum level, Proteobacteria and Firmicutes were found both significantly elevated and reduced in schizophrenia. This is also the case for taxa within the class Clostridia, despite one study identifying this class as a whole to be enriched in schizophrenia (19). Importantly, one of these studies investigated the oropharyngeal microbiome which is structurally distinct from the gut microbiome (20). Perhaps the only truly consistent finding is a significant elevation of Lactobacilli in schizophrenia and people at increased risk of schizophrenia, which even correlated with symptom severity (21). This is perplexing, considering that Lactobacilli are common components of probiotics, thought to confer benefits for mental health.
Several factors could explain these inconsistencies. Firstly, all studies used small sample sizes (<100 patients), which could explain why certain differences did not reach statistical significance, and why differences are reported at selected taxonomic levels, thus hindering comparisons between studies. Reporting differences at selected taxonomic levels has an additional pitfall—members of the same taxon may differ significantly in terms of their physiological role and impact on pathological processes. This is obviously true for higher taxonomic levels which include increasingly dissimilar groups of bacteria, but is likely also true at genus or even species level. Such variation could explain why Lactobacilli can be implicated in both the pathogenesis of psychiatric disorders and confer benefits in them, depending on which specific subtype of is present. Secondly, the studies differed in exclusion of potential confounders, such as smoking, or co-morbid metabolic and cardiovascular illnesses, all of which affect the microbiome. Patient groups also differed in age, stage of illness, and treatment status. Indeed, antipsychotics have significant anti-commensal activity (22), and in rodents, olanzapine has been shown to elevate Firmicutes and decrease Bacteroidetes (23, 24), which was paralleled in human antipsychotic trials (25, 26), although not universally (27). These results bear resemblance to some of those reported in Table 1, and suggest that the microbiome was affected by treatment status. However, one implication of this is that if antipsychotic treatment impairs the microbiome, then auxiliary interventions aimed at restoring microbiome function could prove beneficial. Thirdly, the methods used in these papers are not consistent, which might alter the obtained taxonomic profiles and further hinder comparisons. While most studies utilized 16S rRNA gene sequencing as the taxonomic marker, different regions of the gene were used (V3 and V4) along with different primers, which is known to affect the outcomes of 16S rRNA gene-based diversity studies due to variation in the evolutionary rates of different regions of this gene (28). Additionally, one study used shotgun metagenomic sequencing on throat swab samples (20). Aside from the problem of differences between oropharyngeal and intestinal microbiomes, an important caveat of this approach is that the abundance of host DNA in swab samples may impair the accuracy of microbiome profiling (29). Considering the intrinsic variations in microbiome profiles, methodological consistency is necessary to generate data which will allow useful and accurate comparisons across studies.
The existing studies implicating dysbiosis in schizophrenia are all cross-sectional, hence causal relations cannot be inferred. Thus, large-scale prospective studies are needed, to identify whether certain microbiome profiles are associated with increased risk of developing schizophrenia. Fecal microbiome transplants have also been used to demonstrate causal roles of microbiome in disease, but will be challenging to implement in schizophrenia due to the lack of accurate animal behavioral correlates. In one study, fecal transplantation from schizophrenia patients to GF mice led to hyperactivity, reduced immobility in the forced swimming test (behavioral despair), and exaggerated startle response (18). Although these behaviors are seen in schizophrenia, they are not unique to it, and certain tests such as the Y-maze, sociability test, and pre-pulse inhibition test did not reveal differences, suggesting either that the microbiome does not affect all aspects of schizophrenia, or that the findings were not actually indicative of a schizophrenia-like phenotype. Finally, there is very little data on possible sources of dysbiosis, with C-section—a commonly suggested cause of dysbiosis (30)—found not to be associated with schizophrenia (31, 32).
Potential Diagnostic Application
Two key studies have explored whether microbiome differences could serve as biomarkers for schizophrenia. One investigation demonstrated that the disorder is associated with changes in Gammaproteobacteria at class level, Enterobacteriales at order level, and Bacteroides fragilis at species level (17), whereas another determined that a panel consisting of Aerococcaceae, Bifidobacteriaceae, Brucellaceae, Pasteurellaceae, and Rikenellaceae is sufficient to distinguish patients from controls (18). However, much like with data in Table 1, there is discord in the bacterial taxa identified as markers in these studies, which could again reflect small sample sizes and insufficient overlap between studied populations. Furthermore, investigations of the gut microbiome in depression identified similarly discordant patterns of alterations, and showed a degree of overlap to changes seen in schizophrenia (33). This lack of specificity limits the potential diagnostic usefulness of the data and indeed, its robustness in demonstrating dysbiosis that is characteristic to schizophrenia.
How Could Dysbiosis Contribute to Schizophrenia?
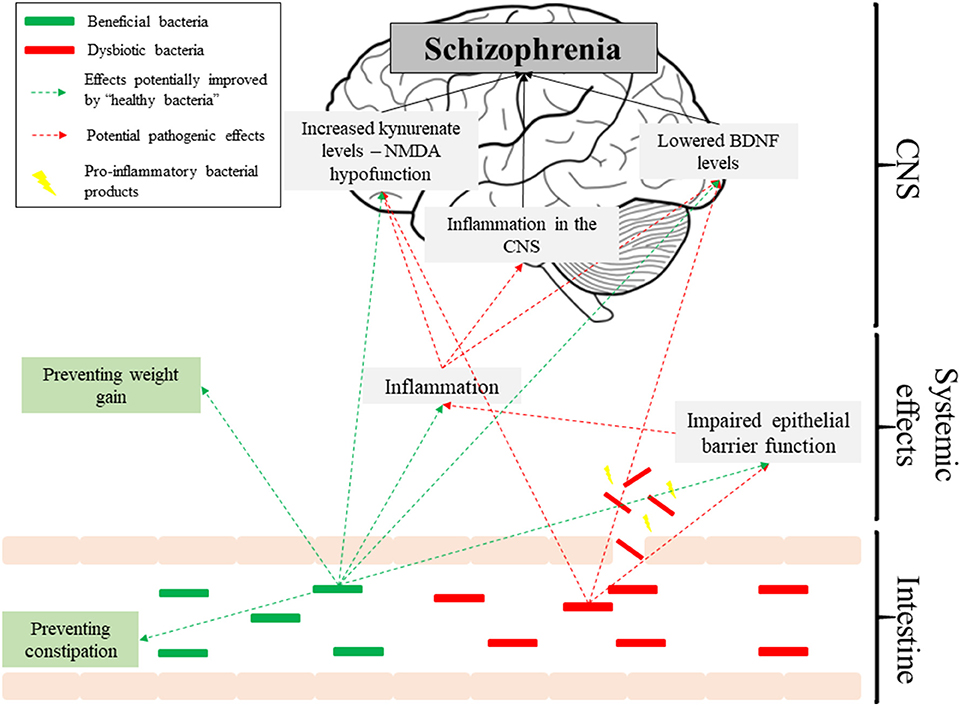
Inflammatory responses have long been implicated in schizophrenia [reviewed in (34)], although their origins are not understood. Schizophrenia is associated with elevated levels of IL-6, IL-8, and TNF-α, and reductions in the anti-inflammatory IL-10 (35). Moreover, elevated antibodies to Saccharomyces cerevisiae (markers of intestinal inflammation) have been identified (36), which is consistent with GI pathologies being among the most common co-morbidities of schizophrenia (37). Dysbiosis could exacerbate inflammation in the disorder through increased intestinal permeability. Indeed, elevated levels of the bacterial translocation marker sCD14 have been observed in schizophrenia (38), and in another study Roseburia, Coprococcus, and Blautia were reduced (17). These species mediate butyrate production (39), which has been shown to enhance the intestinal barrier function by facilitating tight junction assembly in monolayers intestinal cell-lines (40). In addition, genes involved in immunity are among the most commonly appearing in GWAS analyses of schizophrenia (5), suggesting that perhaps a genetic component driving inflammation is more significant. It is also possible that dysbiosis facilitates bacterial infections and contributes to inflammation—a hypothesis consistent with a study indicating bacterial infections as risk factors for schizophrenia (41). Additionally, the gut microbiome has been reported to regulate blood-brain barrier permeability (42), and so dysbiosis could potentially facilitate CNS infection and inflammation, which are also associated with schizophrenia (43).
There also exists a link between the immune system and the conversion of tryptophan to kynurenate (44). Kynurenate is a broad-spectrum glutamate receptor antagonist, and N-Methyl-D-Aspartate Receptor (NMDAR) hypofunction is implicated in schizophrenia [reviewed in (45)]. Elevated kynurenate levels have been observed in post-mortem schizophrenia brain tissue (46), while rats treated with kynurenine-3-monooxygenase inhibitors have reduced pre-pulse inhibition (reflecting the inability of schizophrenia patients to filter out extraneous stimuli), along with increased firing rate and burst firing activity of VTA dopaminergic neurons, indicating the dopamine hyperactivity associated with schizophrenia (47). Indoleamine-2,3-dioxygenase (IDO) is responsible for the rate-limiting step in conversion of tryptophan down the kynurenine pathway. Its key function is depleting tryptophan during immune responses to prevent pathogenic growth and dampen immune responses (44). Hence, IDO is upregulated by cytokines such as IFN-γ and TNF-α (44), and in schizophrenia, increased TNF-α has been observed.
The gut microbiome might affect the balance of tryptophan metabolism directly. A study comparing germ-free to control mice identified that GF mice had significantly higher tryptophan availability and decreased kynurenine:tryptophan ratio, which was increased upon colonization (48). In another study, administration of Bifidobacterium infantis to rats elevated plasma kynurenate (49). However, a decrease in the kynurenine:tryptophan ratio was observed, indicating reduced overall tryptophan metabolism down the kynurenine pathway. Importantly, there are no studies directly linking specific microbiome profiles in schizophrenia to kynurenate alterations. It is also important to mention that quinolinic acid is an NMDAR agonist, so it is difficult to extrapolate alterations of the kynurenine pathway directly to NMDAR dysfunction.
The Microbiome and Brain-Derived-Neurotrophic Factor (BDNF)
BDNF is a key neurotrophin involved in neurodevelopment, particularly in learning and memory processes, and neurodevelopmental models of schizophrenia often include BDNF alterations, focusing on its role in the cognitive dysfunction in the illness [reviewed in (50)]. Reduced BDNF levels have been observed both in post-mortem hippocampal samples (51), and in the plasma of drug-naïve patients with schizophrenia (52, 53), while low baseline BDNF levels are associated with worse response to antipsychotic treatment (54).
In rodent studies, broad-spectrum antimicrobials have been found to significantly lower BDNF and TrkB expression in mouse hippocampus (55), though another study with similar design found significantly increased BDNF levels in the hippocampus, paralleled by increased abundance of Lactobacilli and Actinobacteria, and decreased abundance of γ-Proteobacteria and Bacteroidetes (56). These conflicting results are problematic, and it remains unclear whether the BDNF changes were mediated by the microbiome and/or the antibiotics themselves. GF mice were found to have lower BDNF expression in the hippocampus in two separate studies (57, 58), however colonization of GF NIH Swiss mice with BALB/c microbiota was found to reduce BDNF expression at 1 week post-transfer compared to NIH Swiss microbiota, but not at 3 weeks (56). Again, none of these studies is directly related to the microbiome in schizophrenia, and more convincing data could be obtained from studies analyzing the effects of FMT from schizophrenia patients on BDNF expression. Interestingly, inflammatory responses have also been associated with decreased hippocampal BDNF expression, providing an additional potential link between BDNF levels and altered gut microbiome (59).
Prebiotics in Schizophrenia
Prebiotics are substrates utilized by host microorganisms, providing favorable conditions for “beneficial” bacteria (60). They commonly include non-digestible fructan oligosaccharides (FOS) and galactan oligosaccharides (GOS), selectively degraded by Bifidobacteria. Recently, a study has shown the potential of using prebiotics as an adjunctive treatment in schizophrenia, and was based on animal studies that explored two aspects of schizophrenia: cognitive dysfunction and antipsychotic-mediated weight gain.
There is strong evidence that schizophrenia and its treatment are associated with impaired cognitive functions. Schizophrenia subjects perform 1.5–2.0 standard deviations below healthy controls in a number of neurocognitive tasks, with largest impairments observed in working memory, attention, problem solving, processing speed, and social cognition (61). Particularly alarming are studies showing that antipsychotic drugs can induce cognitive impairments—risperidone was found to decrease spatial working memory despite clinical improvement (62), while lowering the dose of risperidone or olanzapine was found to significantly improve RBANS and DIEPSS scores (63). These changes can have significant functional implications such as poor work performance, hence addressing them is important in effective treatment.
Ingestion of the B-GOS® prebiotic formulation was found to significantly improve cognitive flexibility in rats (64). Although reduced cognitive flexibility is not unique to schizophrenia, B-GOS® supplementation in this disorder improved global cognitive performance measured by the Brief Assessment of Cognition in Schizophrenia [BACS, (61)], compared to placebo (65). This clearly requires replication with a larger study which should also include assessments of positive and negative symptoms so that the full therapeutic potential of prebiotics in schizophrenia is understood.
Although the mechanisms underlying the pro-cognitive effect of B-GOS® in schizophrenia are not clear, prebiotic supplementation in rats was also found to increase responses of PFC pyramidal neurones to the application of NMDA, and elevate cortical expression of GluN2B and GluN2A NMDA receptor subunits (64, 66). Furthermore, elevated hippocampal levels of BNDF have been reported (66). These changes are highly pertinent to schizophrenia, as NDMA hypofunction and decreased BDNF levels are thought to be involved in its pathogenesis, and its associated cognitive impairment (67).
The Problem of Obesity in Schizophrenia, and the Potential Benefits of Prebiotics
Antipsychotic treatment is associated with increased body weight, and it is thought to be a key reason for higher rates of obesity among people with schizophrenia, along with lifestyle and social factors (68). This was robustly demonstrated in a meta-analysis of studies investigating this phenomenon, with clozapine and olanzapine increasing weight by >4 kg after 10 weeks of treatment (69). Furthermore, second-generation antipsychotics were found to induce extreme weight gain (≥7% body weight gain) in 7.7–17% of patients within the first year of treatment (70).
The relationship between obesity and schizophrenia is complicated and deserves further attention when discussing the role of the microbiome in this disorder. Obesity is known to be associated with altered gut microbiota, with most convincing evidence showing that transfer of “obese” microbiota to GF mice is associated with weight gain (71). However, the precise nature of those alterations is unclear, much like those in schizophrenia. Some studies identified a decrease in the abundance of Bacteroidetes and increase in Firmicutes [reviewed in (72)], which is interestingly a pattern also reported following olanzapine treatment (as discussed above)—a drug known to induce weight-gain. However, as there is such inconsistency among studies on the microbiomes in both conditions, it is impossible to make useful comparisons between them. Interestingly, there is evidence that the pathways discussed above as potentially affected by the microbiome in schizophrenia might also be affected in obesity. This pertains primarily to the inflammatory hypotheses of schizophrenia, as obesity is known to induce a chronic low-grade inflammatory state, with elevated levels of cytokines including IL-6 and TNF-α [reviewed extensively in (73)]. The parallels extend, however, further. Mutations in the BDNF gene have been found to be associated with hyperphagia and obesity in people (74, 75), while exogenous BDNF administration was shown to induce weight loss in animal models (76), although studies on serum BDNF levels in obese individuals have yielded ambiguous results (77). Likewise, metabolomic studies have shown elevated levels of kynurenate associated with high BMI in both adults (78) and children (79).
Unfortunately, interpreting the complicated relationship between schizophrenia, obesity, and the microbiome is made almost impossible by the lack of data indicating causal relationships. Perhaps surprisingly, two studies found a negative correlation between body weight and symptom severity in schizophrenia (80, 81), but this was likely an artifact of the effects of atypical antipsychotics on both weight and symptoms. On its own, however, obesity was found to be associated with lower cognitive function, although it is unclear which is antecedent, and whether obesity triggers specific mechanisms affecting cognition (82). Thus, it is unknown whether obesity could have a direct impact on symptoms of schizophrenia, or whether it is merely a side-effect of treatment or an associated morbidity with shared pathophysiological aspects, like BDNF alterations and inflammation. Likewise, there is no clear indication whether the changes in BDNF and kynurenate levels are a contributing cause or a consequence of obesity, whether they are of clinical significance, and whether the microbiome has any impact on them. Nonetheless, considering the importance of obesity in schizophrenia, it would be useful to see detailed studies analyzing the impact of obesity on specific pathophysiological correlates of this disorder, as well as methodologically consistent studies comparing microbiome profiles between schizophrenia and obesity, searching for common signatures.
Regardless of the impact of obesity on the pathophysiology of schizophrenia, the problem of obesity in the disorder needs to be addressed due to its medical implications, and prebiotics could prove useful for this purpose. The administration of B-GOS® reduced olanzapine-induced weight gain in rats (65), whereas in a mouse model of metabolic syndrome, an oligofructose prebiotic caused a trend decrease in weight gain, reductions in food and liquid intake and improved glucose tolerance (83). Prebiotic metabolism by gut bacteria produces short-chain fatty acids (SCFAs), which are agonists of G-protein coupled receptors GPR41 and GPR43 (84). These receptors are expressed in adipocytes, inhibit lipolysis and reduce plasma levels of free fatty acids (85). This could provide a potential mechanism for prebiotic-induced weight normalization and protection against metabolic syndrome, and is also consistent with reduced SCFA-producing bacteria reported in one study in subjects with schizophrenia (17). However, the metabolic effects of the B-GOS® prebiotic in rats were not observed in schizophrenia subjects following a 12 week supplementation (65). In this instance it is noteworthy that in rats the B-GOS® was administered 7 days prior to antipsychotic, whereas the schizophrenia patients had been on medications for several months or years prior to B-GOS® intake. Perhaps prebiotic supplementation would have more beneficial effects of started in parallel with antipsychotic prescription.
Probiotics in Schizophrenia
Probiotics contain living beneficial bacteria, typically from genera Lactobacilli and Bifidobacteria (86). A randomized, placebo-controlled trial of a combination of Lactobacillus rhamnosus and Bifidobacterium lactis Bb12 in schizophrenia did not change PANSS scores over the course of the 14 week trial (87), though a trend increase in plasma BDNF was observed (88). This effect was also reported in a separate trial of Bifidobacterium breve in rats (89). The treated patients also reported less severe bowel movement difficulties, an important observation considering that constipation is a common side-effect of antipsychotic treatment (90) and can be fatal (91).
Recently, a probiotic supplement containing Lactobacilli and Bifidobacterium bifidum was given with vitamin D to schizophrenia subjects, which resulted in a significant improvement in the general and total PANSS scores, decreased circulating CRP levels and enhanced total antioxidant capacity of plasma, indicating symptomatic improvement and reduced inflammation (92). However, it is uncertain which component of the intervention was responsible for those changes. In another study of schizophrenia, consumption of Bifidobacterium breve A-1 for 4 weeks improved PANSS and anxiety/depression scores, increased levels of IFN-γ, IL-1R1, IL-10, IL-22, and decreased levels of TNF-α (93). The psychological effects are significant as the prevalence of depression and panic disorder in schizophrenia patients is 50 and 15% respectively (93). The increase in IL-22 is perplexing, as IL-22 is associated with both inflammatory reactions, and maintenance of the intestinal barrier and host responses against intestinal bacterial pathogens (94). The increased expression of IL-10 and decreased expression of TNF-α may further suggest anti-inflammatory effects, but is countered by elevated pro-inflammatory TNF-β and IL-1R1 (95, 96).
Taken together, the results of probiotic trials are highly discrepant, which could reflect differences in the treatments used. There is, however, preliminary evidence that probiotic supplementation could benefit people with schizophrenia both in terms of symptoms and co-morbid conditions, despite the apparent lack of effect on core aspects of the disorder. What is truly needed are larger-scale studies with consistency in treatment choices and measured outcomes.
Conclusion
The existing evidence for dysbiosis as a factor in the pathogenesis of schizophrenia is underwhelming. None of the available studies are prospective, making causal relationships unfeasible, and the data is plagued with methodological inconsistencies. However, if future studies confirm that dysbiosis predicts schizophrenia, then it could link a number of observations in schizophrenia patients, such as raised inflammatory markers or altered BDNF and kynurenate levels, and thus contribute to the increasingly complex picture of its etiology (Figure 1). Despite limited evidence, there is promise in the use of pre/probiotics as auxiliary treatments in schizophrenia, aimed at improving side-effects of antipsychotics and complementing their action, particularly in terms of cognitive impairments. Overall, we should look with anticipation toward new studies published in this field.
Author Contributions
TS and PB made an equal contribution to the conceptualization and drafting of the manuscript. TS drafted the final version of the document and constructed the accompanying figure and table. BL provided a significant contribution to clinical content and proofreading. AL and PB made equal contributions to scientific content proofreading.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
GAF, global assessment of functioning; GF mice, germ-free mice; VTA, ventral tegmental area; LBP, LPS-binding protein; FMT, fecal microbiota transplant; PFC, pre-frontal cortex; BDNF, brain-derived neurotrophic factor; RBANS, repeatable battery for assessment of neuropsychological status; DIEPSS, drug-induced extrapyramidal symptoms scale; PANSS, positive and negative syndrome scale; GWAS, genome wide association study; TrkB, tropomyosin receptor kinase B (the BDNF receptor); hs-CRP, high-sensitivity C-reactive protein.
References
1. Cowen P, Harrison P, Burns T. Shorter Oxford Textbook of Psychiatry. Oxford: Oxford University Press (2017). doi: 10.1093/med/9780198747437.001.0001
2. Hjorth P, Medici CR, Juel A, Madsen NJ, Vandborg K, Munk-Jorgensen P. Improving quality of life and physical health in patients with schizophrenia: a 30-month program carried out in a real-life setting. Int J Soc Psychiatry. (2017) 63:287–96. doi: 10.1177/0020764017702172
3. Marwaha S, Johnson S. Schizophrenia and employment. Soc Psychiatry Psychiatr Epidemiol. (2004) 39:337–49. doi: 10.1007/s00127-004-0762-4
4. Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM, et al. Heritability of schizophrenia and schizophrenia spectrum based on the Nationwide Danish Twin Register. Biol Psychiatry. (2018) 83:492–8. doi: 10.1016/j.biopsych.2017.08.017
5. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. (2014) 511:421–7. doi: 10.1038/nature13595
6. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. (2003) 60:1187–92. doi: 10.1001/archpsyc.60.12.1187
7. Dean K, Murray RM. Environmental risk factors for psychosis. Dialogues Clin Neurosci. (2005) 7:69–80.
8. Hill SK, Bishop JR, Palumbo D, Sweeney JA. Effect of second-generation antipsychotics on cognition: current issues and future challenges. Expert Rev Neurother. (2010) 10:43–57. doi: 10.1586/ern.09.143
9. Uçok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World Psychiatry. (2008) 7:58–62. doi: 10.1002/j.2051-5545.2008.tb00154.x
10. Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making sense of the microbiome in psychiatry. Int J Neuropsychopharmacol. (2019) 22:37–52. doi: 10.1093/ijnp/pyy067
11. Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
12. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. (2011) 473:174–80. doi: 10.1038/nature09944
13. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. doi: 10.1126/science.1208344
14. Benedict C, Vogel H, Jonas W, Wooting A, Blaut M, Schumann A, et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab. (2016) 5:1175–86. doi: 10.1016/j.molmet.2016.10.003
15. Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. (2017) 312:G571. doi: 10.1152/ajpgi.00066.2017
16. He Y, Kosciolek T, Tang J, Zhou Y, Li Z, Ma X, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur Psychiatry. (2018) 53:37–45. doi: 10.1016/j.eurpsy.2018.05.011
17. Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr Res. (2018) 197:470–7. doi: 10.1016/j.schres.2018.01.002
18. Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. (2019) 5:eaau8317. doi: 10.1126/sciadv.aau8317
19. Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. (2018) 204:23–9. doi: 10.1016/j.schres.2018.09.014
20. Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. (2015) 3:e1140. doi: 10.7717/peerj.1140
21. Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. (2018) 192:398–403. doi: 10.1016/j.schres.2017.04.017
22. Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. (2018) 555:623–8. doi: 10.1038/nature25979
23. Davey KJ, O'Mahony SM, Schellekens H, O'Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. (2012). 221:155–69. doi: 10.1007/s00213-011-2555-2
24. Morgan AP, Crowley JJ, Nonneman RJ, Quackenbush CR, Miller CN, Ryan AK, et al. The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS ONE. (2014) 9:e115225. doi: 10.1371/journal.pone.0115225
25. Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM, et al. Effects of atypical antipsychotic treatment and resistant starch supplementation on gut microbiome composition in a cohort of patients with bipolar disorder or schizophrenia. Pharmacotherapy. (2019) 39:161–70. doi: 10.1002/phar.2214
26. Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Burns TL, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. (2015) 5:e652. doi: 10.1038/tp.2015.135
27. Yuan X, Zhang P, Wang Y, Liu Y, Li X, Kumar BU, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. (2018) 201:299–306. doi: 10.1016/j.schres.2018.05.017
28. Ghyselinck J, Pfeiffer S, Heylen K, Sessitsch A, De Vos P. The effect of primer choice and short read sequences on the outcome of 16S rRNA gene based diversity studies. PLoS ONE. (2013) 8:e71360. doi: 10.1371/journal.pone.0071360
29. Pereira-Marques J, Hout A, Ferreira RM, Weber M, Pinto-Ribeiro I, Van Doorm LJ, et al. Impact of host DNA and sequencing depth on the taxonomic resolution of whole metagenome sequencing for microbiome analysis. Front Microbiol. (2019) 10:1277. doi: 10.3389/fmicb.2019.01277
30. Goedert JJ, Hua X, Yu G, Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: analysis of the American Gut Project. EBioMedicine. (2014) 1:167–72. doi: 10.1016/j.ebiom.2014.11.004
31. O'Neill SM, Curran EA, Dalman C, Kenny LC, Kearney PM, Clarke G, et al. Birth by caesarean section and the risk of adult psychosis: a population-based cohort study. Schizophr Bull. (2016) 42:633–41. doi: 10.1093/schbul/sbv152
32. Fond G, Bulzacka E, Boyer L, Llorca PM, Godin O, Brunel L, et al. Birth by cesarean section and schizophrenia: results from the multicenter FACE-SZ data-set. Eur Arch Psychiatry Clin Neurosci. (2017) 267:587–94. doi: 10.1007/s00406-016-0708-3
33. Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. (2019) 10:34. doi: 10.3389/fpsyt.2019.00034
34. Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. (2015) 9:372. doi: 10.3389/fnins.2015.00372
35. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
36. Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. (2012) 138:48–53. doi: 10.1016/j.schres.2012.02.025
37. Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. (2015) 17:27. doi: 10.1007/s11920-015-0574-0
38. Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, et al. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. (2013) 148:130–7. doi: 10.1016/j.schres.2013.05.018
39. Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5:14. doi: 10.1186/s40168-016-0222-x
40. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
41. Benros ME, Mortensen PB, Nielsen PR. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish National Registers. Schizophr Bull. (2013) 40:1526–32. doi: 10.1093/schbul/sbt200
42. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
43. Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. (2012) 139:161–8. doi: 10.1016/j.schres.2012.05.023
44. Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. (2012) 119:197–209. doi: 10.1007/s00702-011-0681-y
45. Balu DT. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Adv Pharmacol. (2016) 76:351–82. doi: 10.1016/bs.apha.2016.01.006
46. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. (2001) 50:521–30. doi: 10.1016/S0006-3223(01)01078-2
47. Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. (2007) 92:203–9. doi: 10.1016/j.physbeh.2007.05.025
48. Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. (2013) 18:666–73. doi: 10.1038/mp.2012.77
49. Desbonnet L, Garrett L, Clarke G, Bienestock J, Dinan TG. The probiotic Bifidobacteria infantis : an assessment of potential antidepressant properties in the rat. J Psychiatr Res. (2008) 43:164–74. doi: 10.1016/j.jpsychires.2008.03.009
50. Nieto R, Kukuljan M, Silva H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry. (2013) 4:45. doi: 10.3389/fpsyt.2013.00045
51. Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. (2001) 52:79–86. doi: 10.1016/S0920-9964(00)00084-0
52. Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. (2007) 91:1–5. doi: 10.1016/j.schres.2006.12.026
53. Rizos EN, Rontos I, Laskos E, Arsenis G, Michalopoulou PG, Vasilopoulos D, et al. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1308–11. doi: 10.1016/j.pnpbp.2008.04.007
54. Lee BH, Kim YK. Increased plasma brain-derived neurotropic factor, not nerve growth factor-Beta, in schizophrenia patients with better response to risperidone treatment. Neuropsychobiology. (2009) 59:51–8. doi: 10.1159/000205518
55. Bistoletti M, Caputi V, Baranzini N, Marchesi N, Filpa V, Marsilio O, et al. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS ONE. (2019) 14:e0212856. doi: 10.1371/journal.pone.0212856
56. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. (2011) 141:599–609.e3. doi: 10.1053/j.gastro.2011.04.052
57. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
58. Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. xNormal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. (2014) 108:3047–52. doi: 10.1073/pnas.1010529108
59. Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci. (2014) 8:430. doi: 10.3389/fncel.2014.00430
60. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
61. Keefe RSE, Harvey PD, Goldberg TE, Gold JM, Walker TM, Courtney K, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. (2008) 102:108–15. doi: 10.1016/j.schres.2008.03.024
62. Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. (2006) 63:1189–97. doi: 10.1001/archpsyc.63.11.1189
63. Takeuchi H, Suzuki T, Remington G, Bies RR, Abe T, Graff-Guerrero A, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. (2013) 39:993–8. doi: 10.1093/schbul/sbt090
64. Gronier B, Savignac HM, Di Miceli M, Idriss SM, Tzortzis G, Anthony D, et al. Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS®) ingestion. Eur Neuropsychopharmacol. (2018) 28:211–24. doi: 10.1016/j.euroneuro.2017.11.001
65. Kao AC-C, Safarikova J, Marquardt T, Mullins B, Lennox BR, Burnet PWJ. Pro-cognitive effect of a prebiotic in psychosis: a double blind placebo controlled cross-over study. Schizophr Res. (2019) 208:460–61. doi: 10.1016/j.schres.2019.03.003
66. Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int. (2013) 63:756–64. doi: 10.1016/j.neuint.2013.10.006
67. Islam F, Mulsant BH, Voineskos AN, Rajji TK. Brain-derived neurotrophic factor expression in individuals with schizophrenia and healthy aging: testing the accelerated aging hypothesis of schizophrenia. Curr Psychiatry Rep. (2017) 19:36. doi: 10.1007/s11920-017-0794-6
68. Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatrica Scandinavica. (2015) 132:97–108. doi: 10.1111/acps.12445
69. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. (1999) 156:1686–96.
70. Arterburn D, Wood GC, Theis MK, Westbrook EO, Anau J, Rusktalis M, et al. Antipsychotic medications and extreme weight gain in two health systems. Obes Res Clin Pract. (2016) 10:408–23. doi: 10.1016/j.orcp.2015.08.012
71. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. doi: 10.1038/nature05414
72. Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. (2018) 2018:4095789. doi: 10.1155/2018/4095789
73. Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr. (2013) 16:143–52. doi: 10.5223/pghn.2013.16.3.143
74. Gray J, Yeo GSH, Cox JJ, Morton J, Adlam AR, Keogh JM, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. (2006) 55:3366–71. doi: 10.2337/db06-0550
75. Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. (2008) 359:918–27. doi: 10.1056/NEJMoa0801119
76. Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. (2005) 146:5612–20. doi: 10.1210/en.2005-0419
77. Sandrini L, Di Minno A, Amadio P, Ieraci A, Tremoli E, Barbieri SS. Association between obesity and circulating brain-derived neurotrophic factor (BDNF) levels: systematic review of literature and meta-analysis. Int J Mol Sci. (2018) 19:2281. doi: 10.3390/ijms19082281
78. Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. (2019) 29:488–500. doi: 10.1016/j.cmet.2018.09.022
79. Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr. (2015) 102:256–67. doi: 10.3945/ajcn.115.111872
80. Li Q, Du X, Zhang Y, Yin G, Zhang G, Walss-Bass C, et al. The prevalence, risk factors and clinical correlates of obesity in Chinese patients with schizophrenia. Psychiatry Res. (2017) 251:131–6. doi: 10.1016/j.psychres.2016.12.041
81. Kemp DE, Correll CU, Tohen M, Delbello MP, Ganocy SJ, Findling RL, et al. Associations among obesity, acute weight gain, and response to treatment with olanzapine in adolescent schizophrenia. J Child Adolesc Psychopharmacol. (2013) 23:522–30. doi: 10.1089/cap.2012.0099
82. Coumot M, Marquie JC, Ansiau D, Martinaud C, Fonds H, Ferrieres J, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. (2006) 67:1208–14. doi: 10.1212/01.wnl.0000238082.13860.50
83. de Cossío LF, Fourrier C, Sauvant J, Everard A, Capuron L, Cani PD, et al. Impact of prebiotics on metabolic and behavioral alterations in a mouse model of metabolic syndrome. Brain Behav Immun. (2017) 64:33–49. doi: 10.1016/j.bbi.2016.12.022
84. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. (2003) 278:11312–9. doi: 10.1074/jbc.M211609200
85. Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. (2008) 149:4519–26. doi: 10.1210/en.2008-0059
86. Lara-Villoslada F, Olivares M, Sierra S, Rodriguez JM, Boza J, Xaus J. Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr. (2007) 98(Suppl. 1):96. doi: 10.1017/S0007114507832910
87. Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. (2014) 16:PCC.13m01579. doi: 10.4088/PCC.13m01579
88. Tomasik J, Yolken R, Bahn S, Dickerson F. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: a randomised placebo-controlled trial. Biomark Insights. (2015) 10:47–54. doi: 10.4137/BMI.S22007
89. O'Sullivan E, Barrett E, Grenham S, Fitzgerald P, Stanton C, Ross RP, et al. BDNF expression in the hippocampus of maternally separated rats: does Bifidobacterium breve 6330 alter BDNF levels? Benef Microbes. (2011) 2:199–207. doi: 10.3920/BM2011.0015
90. De Hert M, Hudyana H, Dockx L, Bernagie C, Sweers K, Tack J, et al. Second-generation antipsychotics and constipation: a review of the literature. Eur Psychiatry. (2010) 26:34–44. doi: 10.1016/j.eurpsy.2010.03.003
91. Hibbard KR, Props A, Fran DE, Wys J. Fatalities associated with clozapine-related constipation and bowel obstruction: a literature review and two case reports. Psychosomatics. (2011) 50:416–9. doi: 10.1176/appi.psy.50.4.416
92. Ghaderi A, Banafshe HR, Mirhosseini N, Moradi M, Karimi MA, Mehrzad F, et al. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry. (2019) 19:77. doi: 10.1186/s12888-019-2059-x
93. Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord. (2019) 245:377–85. doi: 10.1016/j.jad.2018.11.011
94. Parks OB, Pociask DA, Hodzic Z, Kolls JK, Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front Cell Dev Biol. (2016) 3:85. doi: 10.3389/fcell.2015.00085
95. Calmon-Hamaty F, Combe B, Hahne M, Morel J. Lymphotoxin α revisited: general features and implications in rheumatoid arthritis. Arthritis Res Ther. (2011) 13:232. doi: 10.1186/ar3376
Keywords: inflammation, microbial communities, psychosis, antipsychotics, supplementation
Citation: Szeligowski T, Yun AL, Lennox BR and Burnet PWJ (2020) The Gut Microbiome and Schizophrenia: The Current State of the Field and Clinical Applications. Front. Psychiatry 11:156. doi: 10.3389/fpsyt.2020.00156
Received: 09 December 2019; Accepted: 18 February 2020;
Published: 12 March 2020.
Edited by:
Kelly Anne Allott, University of Melbourne, AustraliaReviewed by:
Eduardo Castro-Nallar, Andres Bello University, ChileLuis Vitetta, University of Sydney, Australia
Copyright © 2020 Szeligowski, Yun, Lennox and Burnet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip W. J. Burnet, cGhpbC5idXJuZXQmI3gwMDA0MDtwc3ljaC5veC5hYy51aw==
 Tomasz Szeligowski1
Tomasz Szeligowski1 Belinda R. Lennox
Belinda R. Lennox Philip W. J. Burnet
Philip W. J. Burnet