- 1Department of Neurology, Charité University Hospital, Berlin, Germany
- 2Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
The capacity to efficiently control motor output, by either refraining from prepotent actions or disengaging from ongoing motor behaviors, is necessary for our ability to thrive in a stimulus-rich and socially complex environment. Failure to engage in successful inhibitory motor control could lead to aberrant behaviors typified by an excess of motor performance. In tic disorders and Tourette syndrome (TS) — the most common tic disorder encountered in clinics — surplus motor output is rarely the only relevant clinical sign. A range of abnormal behaviors is often encountered which are historically viewed as “disinhibition phenomena”. Here, we present the different clinical features of TS from distinct categorical domains (motor, sensory, complex behavioral) that evoke the concept of disinhibition and discuss their associations. We also present evidence for their consideration as phenomena of inhibitory dysfunction and provide an overview of studies on TS pathophysiology which support this view. We then critically dissect the concept of disinhibition in TS and illuminate other salient aspects, which should be considered in a unitary pathophysiological approach. We briefly touch upon the dangers of oversimplification and emphasize the necessity of conceptual diversity in the scientific exploration of TS, from disinhibition and beyond.
Inhibitory Control in Health and Disorder
The capacity to efficiently control motor output in order to fulfill a desired outcome is a fundamental characteristic of our behavior. Beyond the selection and initiation of context-specific behaviors (e.g., waving the hand to signal familiarity), the ability to either refrain from executing prepotent actions (e.g., not slamming a door) or to timely disengage from an ongoing motor behavior (e.g., stop speaking at the beginning of an opera) is necessary for our capacity to thrive in a stimulus-rich and socially complex environment. The importance of the inhibitory qualities of our behavior is further emphasized by religious, moral, social, and legal regulatory codes, which typically penalize lack of self-control, often manifesting as non-conforming or inappropriate behaviors. Importantly, effective self-regulatory control constitutes a potential neurodevelopmental marker of well-being, as people who were able to better control their actions early in life were found to have improved psychosocial functioning, including resilience and coping with stressors, sense of self-worth and higher degree of education as adults (1).
A particular, and perhaps the most basic aspect in the behavioral science of self-regulatory control (or simply “self-control”) (2), is the study of motor responses. However, the scientific exploration of motor inhibition is notoriously difficult, as successful control over movement implies a behavior that never occurs (3). Therefore, psychology often examines the effects of insufficient behavioral inhibition in certain neuropsychiatric and movement disorders typified by an excess of behavioral motor output (4). On the one hand, these disorders exemplify the importance of efficient inhibitory control over motor output. On the other hand, they also provide the unique opportunity to scientifically examine the neural circuitry of motor inhibition. Indeed, in hyperkinetic movement disorders, loss of inhibition at different neuronal levels and networks has been associated with the manifestation of different abnormal motor behaviors (4). For example, loss of inhibition in spatial and temporal sensorimotor processing is a common pathophysiological finding in some forms of isolated dystonia (5–7) and loss of cerebellar inhibitory control over the motor cortex is suggested to be an underlying factor driving the manifestation of cortical myoclonus (8, 9)
Perhaps the most popular example of inhibitory dysfunction in medical literature and movement disorders are tic disorders, and particularly Tourette syndrome (TS), the most common tic disorder encountered in clinics. TS is defined by the presence of at least two motor tic behaviors and one vocal tic behavior for a minimum period of a year, manifesting before the age of 18 (10). Although there are several clinical features of TS that evoke the concept of disinhibition, scientific studies assessing different inhibitory functional domains have provided mixed results (11–14). Thus, the unitary concept of TS as a disorder of inhibitory control remains controversial (15–18). In order to provide some clarity to this critical issue, we here first present a list of clinical features which evoke the concept of disinhibition classified in distinct categorical domains. We then provide evidence and an overview of studies on TS pathophysiology which support the view of inhibitory dysfunction. We subsequently critically dissect the concept of disinhibition in TS and highlight additional aspects that should be considered in TS pathophysiology. Finally, we discuss the dangers of oversimplification and emphasize the necessity of conceptual diversity in the scientific exploration of TS. This should incorporate additional evidence-driven approaches, including pathophysiological frameworks such as enhanced reinforcement learning, impaired predictive coding for action control, and abnormal body-focused metacognitions.
The Motor Manifestations of TS That Evoke the Concept of Disinhibition
Simple Tics – Uncontrollable Surplus Fragments of Behavior
Tics are the prototypical and defining manifestation in TS and indeed the main clinical feature evoking the notion of disinhibition. Historically, there has been some confusion as to the exact classification of tic behaviors and their distinction from other pathological conditions of excessive motor output, as spasms or jerks (19). Moreover, there has been a long dispute, — which in some regard is still ongoing (20)— as to whether tics can be truly distinguished from voluntary actions. Following Meige and Feindel's scholarly treatise on “Les tics et leur traitement”, a first definition of tics was provided, and surprisingly little has changed since then. Tics are movements or sounds that resemble physiological actions, but appear repetitive and are inopportune to contextual cues from the environment (21, 22). Accordingly, tics represent excessive motor behaviors that are superimposed to ongoing voluntary motor output, but are inflexible and often appear exaggerated in intensity and frequency (23). There is marked intra- and interpersonal variability of tic behaviors; any possible movement or sound can constitute a tic. However, there are specific features, particularly at the onset of tic behaviors, which are characteristic for TS. First, individual tic behaviors are typically brief, occur suddenly and involve only a limited number of muscles (also termed simple tics), often resembling fragments of normal voluntary motor behavior. Second, many tics involve the face. Third, tics are amenable to a specific cognitive process of effortful voluntary inhibitory control and can thereby be typically suppressed on demand (17). These specific characteristics of simple tic behaviors, particularly their surplus and exaggerated nature superimposed to ongoing voluntary actions, as well as their brief, sudden and socially inopportune character (e.g., facial tics appear as conspicuous behaviors in the absence of conveyed social meaning), and their susceptibility to voluntary control evoke the concept of motor disinhibition.
Complex Tics – Seemingly Purposeless But Socially Disruptive Actions
Beyond simple tics, patients with TS may also often exhibit complex tic behaviors. These resemble goal-directed actions (e.g., shrugging shoulders, waving hello, jumping on the spot, uttering words or short sentences etc.) but are repetitive and without apparent purpose. Although the prevalence and clinical characteristics of the entire range of complex tics compared to simple tics remains underdetermined, certain behaviors, to include echo-, pali-, and coprophenomena have been better studied. Among these and perhaps owing to their vexing and socially disruptive nature, coprophenomena received more clinical attention, and are typically viewed as the pivotal example of behavioral disinhibition in TS.
Coprophenomena are defined as the unintended expression of socially obscene actions (copropraxia) and/or utterances (coprolalia) (24). Despite their notoriety, they only occur in less than 30% of TS patients (24). Coprophenomena typically first appear around the age of 11 years (24), indeed with a lag of several years after overall tic onset. Coprolalia is more prevalent than copropraxia, and most patients who exhibit copropraxia also have coprolalia (24). No sex differences were found in the expression of coprophenomena in TS, but there were strong associations with tic severity, as well as the overall number of neuropsychiatric comorbidities such as OCD, self-injurious behavior (SIB, also see below), and anger control issues (24, 25). Moreover, a link was established between coprophenomena and non-obscene socially inappropriate type of behaviors, as well as sexually inappropriate behaviors (also see below). The prevalence of these types of behaviors in patients who exhibit coprophenomena and are severely affected by tics lend support to the notion of a broader inhibitory deficit, which afflicts both patterns of movement, and also overall expressions of behavior.
Echophenomena denote the imitation of actions (echopraxia) or sounds (echolalia), without the prerequisite of explicit awareness over their occurrence (26). Although in TS echophenomena fall within the rubric of complex tic behaviors, they are encountered in many other neuropsychiatric disorders, also in the overt absence of tics (Figure 1). Thereby, it remains unclear whether the pathophysiological underpinnings of echophenomena are similar to that of other tic behaviors. Indeed, echophenomena are characterized by their immediacy with the external environment, as they are directly generated by it as externally triggered behaviors. Despite methodological differences between studies (e.g., direct observation using standardized protocols vs clinical examination vs self-report), echophenomena appear to be present in about a third of patients with TS and similar to coprophenomena, echolalia is noted more commonly than echopraxia (25). One study provided experimental evidence on the exact characteristics of echopraxia and showed that patients most commonly echoed behaviors, which belonged to their tic repertoire (27). Compared to patients who do not report echophenomena, the presence of echo- behaviors was positively associated with tic severity and the overall burden of neuropsychiatric comorbidities (25). Given the transient neurodevelopmental nature of echophenomena in healthy development, and their persistence or reemergence later in life in TS, as well as the overall burden of behavioral difficulties in these patients, a putative inhibitory deficit over the control of pre-wired patterns of socially-triggered motor behaviors has been suggested (26).
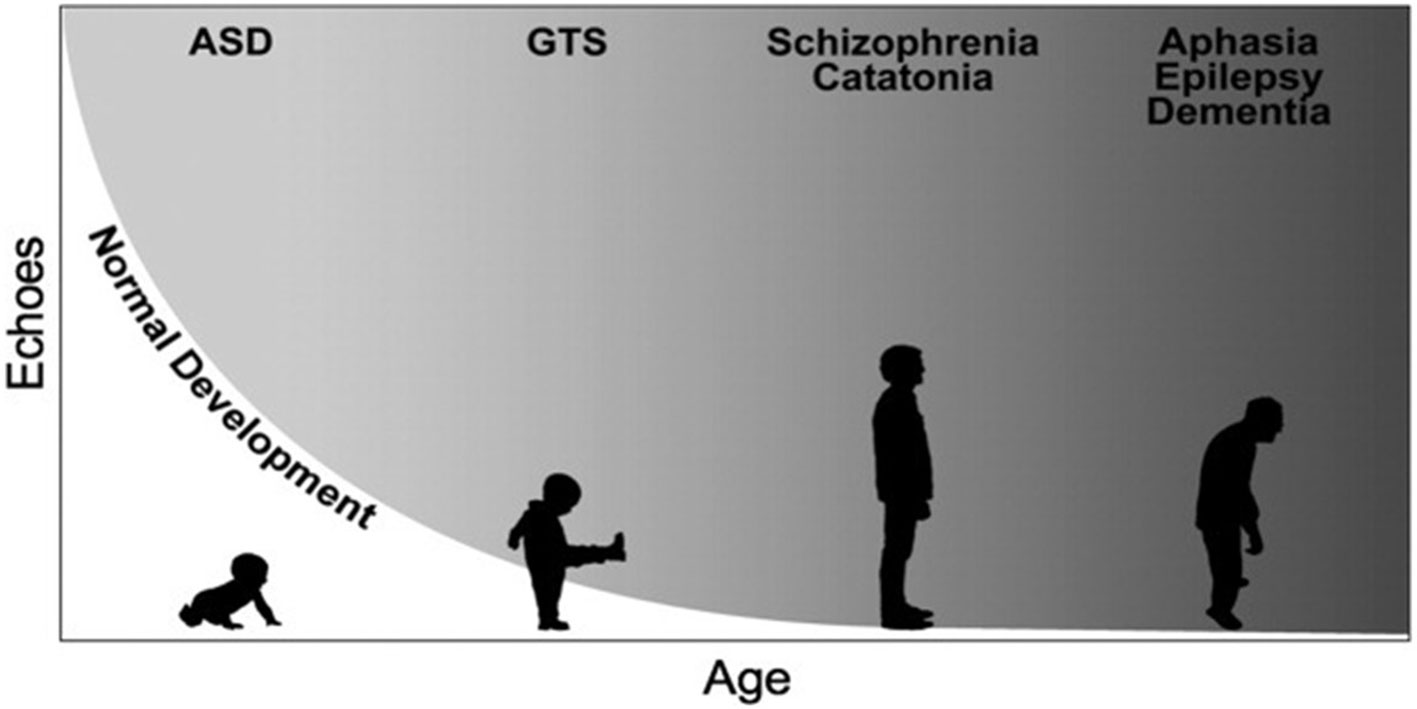
Figure 1 Simplified representation of the developmental trajectories of echophenomena in health and disease. Echophenomena are present in normal childhood development, with a gradual reduction throughout the first three years of life (depicted trajectory in white). Gray shades demonstrate the persistence or reemergence of echophenomena as a sign of underlying neuropsychiatric disorders, e.g., in autism spectrum disorders (ASD), Gilles de la Tourette syndrome (GTS) or others [figure republished with permission from Wiley publishing, (26)].
Paliphenomena refer to the repetition of self-generated movements (palipraxia) or sounds/words (paliphonia/palilalia). Similar to echophenomena, paliphenomena may present in a whole range of neuropsychiatric conditions, particularly in disorders, such as those affecting the frontal lobes, where loss of behavioral control and social disinhibition are characteristic (28, 29). However, not all cases with brief repetitive utterances (e.g., the repetition of phonemes or syllables) are palilalic behaviors, and indeed a distinction from stuttering should be considered. In TS paliphenomena are often induced through an echo-behavior. For example, saying “hello” to a patient may result in the repetitive utterance of several “hellos” in return. Although it is unclear why patients need to repeat certain phonemes or actions, the sheer phenomenology of paliphenomena resembles the observable inflexible behavioral output of patients with OCD, where compulsions such as repetitive checking, washing, or touching are characteristic. Therefore, studies have sought to explore similarities and differences between the two phenomena, as indeed paliphenomena may also occur in TS in the absence of overt obsessive-compulsive behaviors (30, 31). Compared to patients with OCD, TS-only patients tend to display more egosyntonic automatic repetitive behaviors like mental play (e.g., repetitive, often intended as pastime and mostly not unpleasant impulses or cognitions, including images or sounds) (32) touching and just-right phenomena (e.g., the need to perform a tic or a compulsion until it is experienced “just right”) (33). This is in contrast to the distressing, egodystonic and occasionally aggressive repetitive thoughts, contamination worries, and washing behaviors of OCD (31). There is a distinction within the spectrum of repetitive behavior in TS (30). On one side are some of the repetitive behaviors, which are goal-directed and volitional to reduce anxiety, such as the OCD-like checking, washing hands, and on the other side are tic-like repetitive behaviors, which are more egosyntonic, automatic, sometimes even referred as “impulsions”, which belong to paliphenomena (30–32). As in the previous two types of complex tic behaviors (copro- and echophenomena), patients with more severe tics and neuropsychiatric comorbidities will often exhibit paliphenomena, particularly palilalia (25). The repetitive, excessive, inflexible and purposeless nature of paliphenomena has, therefore, also contributed to the view that in TS inhibitory deficits are related with an inability to properly measure and dosage behavioral output.
The Sensory Manifestations of TS That Evoke the Concept of Disinhibition
Premonitory Urges – Pathological Interoceptive Experiences in Excess
Beyond the motor manifestations of simple and complex tic behaviors evoking the concept of disinhibition described above, patients with TS also report sensory abnormalities, which may equally serve as markers of inhibitory dysfunction. Firstly, the majority of adolescents and adults with tics describe sensations preceding their tic behaviors, which are commonly known as premonitory urges. Although descriptions of these excessive bodily experiences often lack precision, patients report a range of perceived phenomena, spanning from a somatic urge to move, to increased tension, an urge to apply pressure or stretch particular body parts, an ache, itch, tingle, and others (34). Most importantly, premonitory urges are often viewed as the defining pathophysiological element, which drives and/or perpetuates tic behaviors (35). Indeed, most patients, particularly in adolescence and beyond, report premonitory urges preceding their tics (25, 36) and often view their tic behaviors as a voluntary response to the unpleasant sensory experience. Despite the apparent straightforward relation between premonitory urges and tics (i.e., tics are the result of premonitory urges), the exact pathophysiological relevance of these phenomena remains unclear (37, 38), and several models have been proposed to explain the presence of excessive bodily sensations (35, 36, 39–41). According to one promising line of research, the capacity to perceive premonitory urges is related to the overall capacity to perceive interoceptive signals (41, 42), possibly providing an explanatory framework to understand the associations of premonitory urges with other clinical symptoms, such as anxiety or obsessive-compulsive behaviors (25, 43–46). Interestingly, patients with TS are less well able to perceive their own physiological interoceptive signals (41, 42, 47).
Sensory Hypersensitivity – Perceived Exteroceptive Surplus
Heightened sensitivity to multimodal exteroceptive stimuli is another salient clinical feature of TS that evokes the concept of disinhibition. For example, increased perception of tactile, auditory and visual stimuli was noted in adolescents and adults with TS (48–50). Crucially, one of the studies identified four perceptual domains which discriminated TS subjects from healthy controls. These domains included perceived stimulus intensity, distractibility, stimulus discrimination and the capacity to attend to sensory stimuli during phases of stress/fatigue (49). Given neurophysiological evidence of deficient gating of sensory input in studies of prepulse inhibition in TS (reduced inhibition of response to a single stimulus, e.g. startle-response following a prepulse stimulus) (49, 51–54), a framework of sensorimotor disinhibition at the basis of tic disorders was further supported. However, the only three studies that clinically examined sensory thresholds in adolescents and adults with TS did not identify significant differences from healthy controls, despite patient reports of increased somatic experiences (55–57).
Other Behavioral Manifestations of TS That Evoke the Concept of Disinhibition
Impulsivity and Attention-Deficit Hyperactivity Disorder
Already in 1902 Meige and Feindel commented on the role of impulsivity as a distinctive feature of people with tics and TS. Their observation has since been confirmed in numerous clinical studies (58, 59). Together with inattention and hyperactivity – the major clinical subtypes of attention deficit hyperactivity disorders (ADHD) – the lifetime prevalence of these behaviors exceeds 50% (59). Beyond the typical neurocognitive difficulties due to ADHD affecting executive functions (e.g. response inhibition), overall academic performance and psychosocial functioning (60), patients with tics and ADHD more often exhibit disruptive behaviors like those related to oppositional defiant disorder (ODD) and conduct disorder (61) and episodic rage outbursts, also known as rage attacks, compared to patients with tics without ADHD. This latter set of behaviors is characterized by discrete episodes of failure to resist aggressive impulses leading to overt behaviors, where the degree of aggression is grossly disproportionate to any precipitating stressors (62). A body of evidence supports the association between these different behavioral manifestations and ADHD comorbidity in patients with tic disorders. For example the presence of explosive outbursts in patients with tics has been repeatedly documented in observational clinical studies that reported how the vast majority of patients with rage attacks had more than one comorbid disorder, specifically ADHD or OCD, alongside TS (63–65). Apart from being more prevalent in TS children and adolescents with co-existing ADHD, ODD, and conduct disorder display a greater stability of symptom severity in this patient subgroup compared to TS patients without ADHD, indicating that the development of ODD and conduct disorder in TS is influenced by ADHD comorbidity (66). Another study reported a differential impact on ODD symptom domains exerted by ADHD (associated with headstrong or defiant domain of ODD) and obsessive-compulsive behaviors (associated with the irritability domain of ODD) (67).
Non-Obscene and Other Socially Inappropriate Behaviors
Another putative aspect of behavioral disinhibition in TS are non-obscene inappropriate behaviors (68). These sets of behaviors are characterized by the impulse to make insulting or inappropriate comments or actions related to an individual or a situation. They differ from coprophenomena in two main aspects. First, coprophenomena are typically purposeless, unintended behaviors, whereas non-obscene socially inappropriate behaviors are externally triggered and stimulus-bound. Second, the content of these behaviors does not involve swearing or obscene derogatory actions. However, non-obscene socially inappropriate behaviors and coprophenomena may often manifest together (68, 69). A carefully conducted study by Eddy and Cavanna (69) in 60 adults with TS found that that presence of non-obscene socially inappropriate behaviors was associated with obsessive-compulsive behaviors, ADHD, mental coprolalia, and poor quality of life.
A brief note should also be made to inappropriate sexual behaviors in TS, which were indeed commonly described in older literature (70–72). Different from non-obscene socially inappropriate behaviors, reported inappropriate sexual behaviors range from inappropriate talks and jokes about sex, over to exhibitionism and paraphilic behaviors. Although this area of research remains underexplored, these behaviors were shown to be more prevalent in samples of TS patients compared to controls, particularly in the presence of comorbid ADHD and coprophenomena (70, 73).
Self-Injurious Behaviors (SIB)
The umbrella term of SIBs encompasses different behaviors leading to bodily self-harm that are neither characteristic for patients with TS nor uniform in their clinical presentation or underlying pathophysiology. However, patients with tics and TS often exhibit SIBs, with an overall prevalence estimated between 15% (73) and 39% (25). SIBs in TS include repetitive skin picking or scratching, head-banging, head or body punching/slapping, body-to-hard-object banging, and poking sharp objects into body parts (74). In a carefully conducted study by Mathews and colleagues, 29% of 297 individuals with TS showed some form of SIB. Importantly, the severity of SIB allowed discriminating between clinical determinants of self-harm, further corroborating pathophysiological diversity. In milder forms of SIBs there was a correlation with obsessive-compulsive symptoms, including violent and aggressive obsessions/compulsion. Impaired impulse control (rage and risk-taking behaviors) and tic severity were associated with severe SIB (75), in line again with the notion of an extensive inhibitory deficit governing both inappropriate and self-harming behavioral output, as well as the overall expression of tics.
Disinhibition as a Pathophysiological Framework in TS: Sides of a Controversy
A Congruent View of Disinhibition as the Core Abnormality of Tics and TS
A pathophysiological framework of disinhibition could explain the illustrated motor, sensory, and other behavioral characteristics of TS (Figure 2). Interestingly, this view has a strong historical background. Tics and their associations were considered to afflict those with “weak control over their actions” (21), with the extent of clinical abnormalities suggested to correlate to the severity of the underlying inhibitory dysfunction. Indeed, after a century of clinical research, experimental data provided strong support for the disinhibition theory (76, 77).
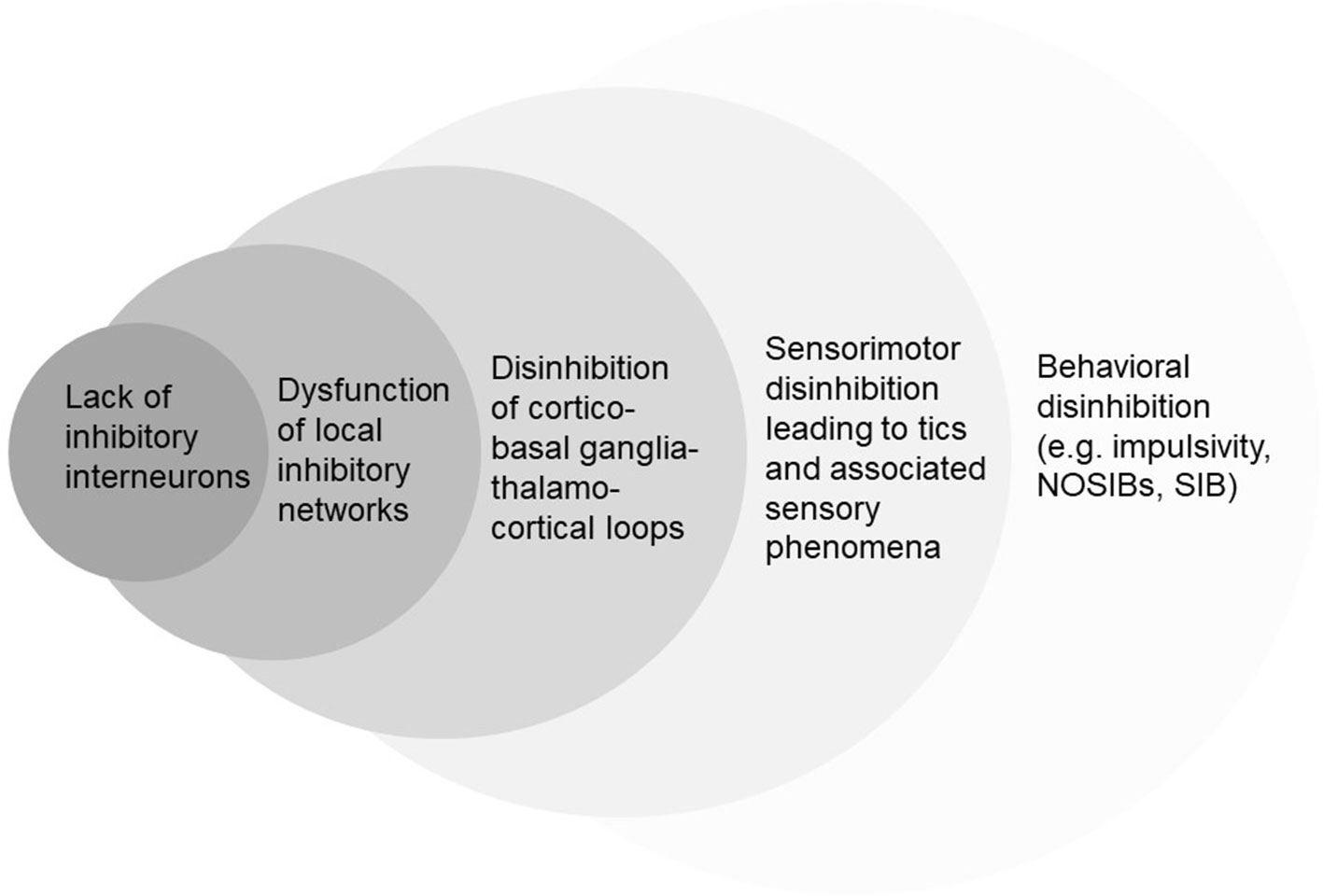
Figure 2 Simplified hierarchic representation of different conceptual levels of disinhibition in TS. NOSIBs; Non-Obscene Socially Inappropriate Behaviors. SIB; Self-Injurious Behaviors.
From a neuroanatomical perspective, two neuropathological studies demonstrated a reduction of inhibitory gamma-aminobutyric acid-ergic (GABA) and cholinergic interneurons in the striatum of patients with TS (78, 79). The abnormalities of the cholinergic interneuronal population were specifically found in the associative and sensorimotor parts of the striatum, in areas that were previously predicted to be involved in the pathophysiology of abnormal tic behaviors (80). The functional relevance of these findings was further supported by animal models of tic disorders. Indeed, pharmacological striatal GABAergic disinhibition has been associated with motor and behavioral abnormalities that may well fall within the tic disorder spectrum (81–83).
Cortical neurophysiology has also provided evidence of inhibitory dysfunction in TS. Transcranial magnetic stimulation measures of both short-interval intracortical inhibition (SICI) and short-afferent inhibition (SAI) were found reduced in TS (84–89). Also, using magnetoencephalographic recordings during the execution of brief voluntary actions, an imbalance between local inhibitory-excitatory neuronal populations in motor and sensory cortical circuits has been previously demonstrated (90, 91). In addition, and even though not specific to TS, prepulse inhibition of the startle response was found deficient in people with tic disorders (49, 51–53). Finally, both behavioral and heritability studies also supported a notion of a pervasive disinhibition trait. A recent meta-analysis on the behavioral performance of patients with TS compared to controls in different inhibitory tasks revealed a mild but significant excess of inhibitory deficits in patients (15) compared to control subjects. The difference was more pronounced for tasks exploring verbal rather than pure motor inhibitory measures, and showed a positive correlation between the degree of these deficits and tic severity. An examination of a large data-set of 1,191 people with TS and 2,303 of their first-degree relatives concluded that behaviors such as copropraxia, palilalia, and others (also noted above; termed in study as “socially disinhibited behaviors”) represent a heritable TS subphenotype, associated with more neuropsychiatric comorbidities (e.g., ADHD, OCD), earlier tic onset and overall higher tic severity (92).
Challenging the View of Disinhibition as the Core Abnormality of Tics and TS
Despite the wealth of presented data, not all efforts to establish disinhibition as an overarching pathophysiological theme in TS have been successful. Importantly, a critical dissection of some of the aforementioned studies provides more complex insights into the underlying circumstances that lead to tics and their associations. First, the aforementioned neuropathological evidence stems from brain samples of a small cohort of five patients. These patients were on average 18 years younger compared to healthy controls (79) and had been previously chronically exposed to psychotropic medications, including antipsychotics. Second, although the pharmacological striatal GABAergic disinhibition model for tics has provided invaluable information to the putative pathophysiological mechanisms of the syndrome (93), it is important to note that it remains unclear whether the observed animal behaviors following disinhibition are indeed tics. In fact earlier studies of similar pharmacologic models referred to the generated abnormal motor output as myoclonus or dyskinesias (94–96), which highlights the difficulty of using a consistent phenomenological labeling for these toxin-induced motor behaviors. Most importantly, not only GABAergic but also other neurotransmitter animal models (97, 98) have been proposed to explain clinical findings, and thereby contribute to the pathophysiology of the syndrome. Overall, it may be argued that the inability to examine whether some of the abnormal motor output is preceded by premonitory urges, or whether it is actively suppressible question the validity of all animal models of tic behaviors. Third, the neurophysiological study of tic disorders has not only provided evidence suggestive of disinhibition, but has also revealed normal or supra-normal inhibitory functions. Some general measures of motor cortical excitability, e.g. resting and active motor thresholds, are not different in TS patients from those in healthy controls (84), whereas other measures such as the recruitment of motor-evoked potentials (input/output curves) suggest reduced baseline excitability (84, 88, 99). Similarly, some magnetoencephalographic cortical event-related studies also revealed patterns of enhanced inhibitory function (100, 101). Although these findings are often suggested to reflect active symptom compensation (102), they do highlight that the functionality of relevant inhibitory networks in TS is intact and that these can be recruited when necessary. This view is also supported by a wealth of neuroimaging studies, the majority of which examined cross-sectional samples, where it is often difficult to distinguish whether findings (e.g., prefrontal cortical volumes or task-related activations) point towards deficient inhibition, enhanced compensation, or both (16, 76). A crucial factor in this regard is also the age of the studied population. The inhibitory capacity of children and adolescents with tic disorders and TS could well differ from adults and indeed drawing firm conclusions from comparing data between cross-sectional samples of patients is strongly hampered by this limitation. Finally, although an overall mild effect was found with regard to behavioral evidence of disinhibition of action, the significant heterogeneity of the examined studies does not allow drawing firm conclusions (15). The conceptual fallacy of a framework where tics represent fragments of disinhibited actions was previously discussed (17).
Why Is Investigating Disinhibition in TS Important? Conclusive Remarks
The ongoing discussion on whether motor and behavioral disinhibition is a pivotal mechanism in tic disorders should not remain a mere academic exercise but should be channeled towards a more granular description of the clinical spectrum of TS and other chronic tic disorders. There is little doubt that the debate is fueled by evidence partly supporting and partly refuting a unitary conceptualization of tics and related repetitive behaviors as the result of insufficient capacity to ‘hold in' unwanted actions. Moreover, recent work within the field of cognitive neuropsychology proposed alternative conceptualizations of tic genesis, among which are the formation of pathologically reinforced stimulus-response associations or sensorimotor programs (103–105), the generation of physiological motor commands to match abnormal sensorimotor priors (106), or the release of motor output that results from excessive body-focused metacognitions (107). None of these alternative conceptualizations is either based on defective inhibition, or categorically excludes the contribution of defective inhibition to the genesis of tics. For example, modulation of striatal GABAergic interneurons was demonstrated to mediate reinforcement-related cholinergic striatal interneuronal function (108). This could explain the presence of both quantitative abnormalities in GABAergic interneuronal populations in neuropathological studies in people with TS (78, 79) and the existence of enhanced reinforcement learning (103, 105). A system with these critical alterations in its basic sensorimotor circuitry may also be fraught in the relative weighing of information processing and monitoring (109). In turn this could lead to the presence of abnormal metacognitions for sensorimotor events (107, 110), including changes in the experience of interoceptive signals (41) and voluntary actions (111, 112).
The limited evidence of numerically defective or dysfunctional inhibitory interneurons in the brain of patients with TS, as well as the legitimate doubt that the striatal disinhibition animal model of tics yields sufficient face validity vis-à-vis human disease, indicate that preclinical or post mortem studies did not provide a solution to the debate in object. In the wake of neuropathological research yielding more generalizable results and animal models demonstrating greater validity for tic disorders in humans, clinical research should invest more in the exploration of biological markers or endophenotypes related to disinhibition, and evaluate how these correlate with specific phenotypic subtypes of the TS spectrum. Importantly, clinical research has already shown that the comorbidity profile should be incorporated in the definition of these phenotypic subtypes, with particular attention to ADHD. Neurophysiological markers of defective inhibition, e.g. those based on TMS-EMG and pre-pulse inhibition paradigms, should be assessed in association with specific clinical characteristics of the syndrome, such as presence/absence of echo-, pali-, coprophenomena, socially inappropriate behaviors, or self-injurious behaviors. Conversely, a similar clinic-physiological correlation should be performed for those markers (e.g., event-related potentials) that yielded evidence of preserved or even enhanced inhibition. Moreover, it would be interesting to assess if this enhanced inhibition truly correlates with a performance gain in specific executive tasks for which compensation by increased cognitive control has been hypothesized in this condition.
With this approach in mind, the puzzling dilemma of disinhibition in TS would be re-converted into a multidisciplinary experimental factory that could lead to the identification of specific clinical and pathophysiological subtypes differing on the basis of decreased or increased motor and behavioral inhibitory capacity. Such a novel course of phenotype subgrouping within the TS spectrum would bear important implications for the personalization and the selection of appropriate behavioral interventions for tics, and for the identification of biomarkers that would help monitoring treatment efficacy and forecasting the evolution of tic behaviors when patients transition from neurodevelopmental stages to maturity.
Author Contributions
LK drafted the manuscript. DM revised the manuscript. CG made substantial contributions to the conception of the work and revised the manuscript.
Funding
This research project was supported by a grant from the VolkswagenStiftung (Freigeist) held by CG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, et al. ‘Willpower' over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci (2011) 6(2):252–56. doi: 10.1093/scan/nsq081
3. Filevich E, Kühn S, Haggard P. Intentional inhibition in human action: the power of ‘No.'. Neurosci Biobehav Rev (2012) 36(4):1107–18. doi: 10.1016/j.neubiorev.2012.01.006
4. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A Fronto–Striato–Subthalamic–Pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci (2015) 16(12):719–32. doi: 10.1038/nrn4038
5. Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis (2011) 42(2):177–84. doi: 10.1016/j.nbd.2010.08.025
6. Ganos C, Ferrè ER, Marotta A, Kassavetis P, Rothwell J, Bhatia KP, et al. Cortical inhibitory function in cervical dystonia. Clin Neurophysiol (2018) 129(2):466–72. doi: 10.1016/j.clinph.2017.11.020
7. Conte A, Rocchi L, Latorre A, Belvisi D, Rothwell JC, Berardelli A. Ten-year reflections on the neurophysiological abnormalities of focal dystonias in humans. Mov Disord (2019) October:mds.27859. doi: 10.1002/mds.27859
8. Tijssen MAJ, Thom M, Ellison DW, Wilkins P, Barnes D, Thompson PD, et al. Cortical myoclonus and cerebellar pathology. Neurology (2000) 54(6):1350–56. doi: 10.1212/WNL.54.6.1350
9. Ganos C, Kassavetis P, Erro R, Edwards MJ, Rothwell J, Bhatia KP. The role of the cerebellum in the pathogenesis of cortical myoclonus: THE CEREBELLUM AND CORTICAL MYOCLONUS. Mov Disord (2014) 29(4):437–43. doi: 10.1002/mds.25867
10. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth Edition. (Arlington, VA: American Psychiatric Publishing) (2013). doi: 10.1176/appi.books.9780890425596
11. Ganos C, Kühn S, Kahl U, Schunke O, Feldheim J, Gerloff C, et al. Action inhibition in Tourette syndrome: action Inhibition in Tourette syndrome. Mov Disord (2014) 29(12):1532–38. doi: 10.1002/mds.25944
12. Thomalla G, Jonas M, Bäumer T, Siebner HR, Biermann-Ruben K, Ganos C, et al. Costs of control: decreased motor cortex engagement during a Go/NoGo task in Tourette's syndrome. Brain (2014) 137(1):122–36. doi: 10.1093/brain/awt288
13. Jung J, Jackson SR, Nam K, Hollis C, Jackson GM. Enhanced saccadic control in young people with tourette syndrome despite slowed pro-saccades. J Neuropsychol (2015) 9(2):172–83. doi: 10.1111/jnp.12044
14. Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced cognitive control in young people with Tourette's syndrome. Curr Biol (2006) 16(6):570–73. doi: 10.1016/j.cub.2006.01.064
15. Morand-Beaulieu S, Grot S, Lavoie J, Leclerc JB, Luck D, Lavoie ME. The puzzling question of inhibitory control in Tourette syndrome: a meta-analysis. Neurosci Biobehav Rev (2017) 80:240–62. doi: 10.1016/j.neubiorev.2017.05.006
16. Jackson GM, Draper A, Dyke K, Pépés SE, Jackson SR. Inhibition, disinhibition, and the control of action in Tourette syndrome. Trends Cogn Sci (2015) 19(11):655–65. doi: 10.1016/j.tics.2015.08.006
17. Ganos C, Rothwell J, Haggard P. Voluntary inhibitory motor control over involuntary tic movements: VOLUNTARY INHIBITION OF TICS. Mov Disord March (2018) 33(6):937–46. doi: 10.1002/mds.27346
18. Maigaard K, Nejad AB, Andersen KW, Herz DM, Hagstrøm J, Pagsberg AK, et al. A superior ability to suppress fast inappropriate responses in children with tourette syndrome is further improved by prospect of reward. Neuropsychologia (2019) 131:342–52. doi: 10.1016/j.neuropsychologia.2019.05.012
19. Kushner HI. A cursing Brain?: The Histories of Tourette Syndrome. Cambridge, Mass: Harvard University Press (2000).
20. Paszek J, Pollok B, Biermann-Ruben K, Müller-Vahl K, Roessner V, Thomalla G, et al. Is it a tic?-twenty seconds to make a diagnosis. Mov Disord (2010) 25(8):1106–8. doi: 10.1002/mds.23053
21. Meige H, Feindel E. Les Tics et Leur Traitement. ÉDITEURS LIBRAIRES DE L'ACADEMIE DE MEDECINE 120, BOULEVARD SAINT-GERMAIN: PRÉFACE DE M. LE Pl BRISSAUD PARIS MASSON ET Cie (1902).
22. Ganos C, Martino D. Tics and Tourette syndrome. Neurol Clinics (2015) 33(1):115–36. doi: 10.1016/j.ncl.2014.09.008
23. Beste C, Münchau A. Tics and Tourette syndrome - surplus of actions rather than disorder?: Tourette syndrome - surplus of action. Mov Disord (2018) 33(2):238–42. doi: 10.1002/mds.27244
24. Freeman RD, Zinner SH, Müller-Vahl KR, Fast DK, Burd LJ, Kano Y, et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol (2009) 51(3):218–27. doi: 10.1111/j.1469-8749.2008.03135.x
25. Sambrani T, Jakubovski E, Müller-Vahl KR. New insights into clinical characteristics of Gilles de La Tourette syndrome: findings in 1032 patients from a single German center. Front Neurosci (2016) 10:415. doi: 10.3389/fnins.2016.00415
26. Ganos C, Ogrzal T, Schnitzler A, Münchau A. The pathophysiology of echopraxia/echolalia: relevance to Gilles De La Tourette syndrome. Mov Disord (2012) 27(10):1222–29. doi: 10.1002/mds.25103
27. Finis J, Moczydlowski A, Pollok B, Biermann-Ruben K, Thomalla G, Heil M, et al. Echoes from childhood-imitation in Gilles de La Tourette syndrome: echoes from childhood-imitation in GTS. Mov Disord (2012) 27(4):562–65. doi: 10.1002/mds.24913
28. Kluin KJ, Foster NL, Berent S, Gilman S. Perceptual analysis of speech disorders in progressive supranuclear palsy. Neurology (1993) 43(3, Part 1):563–3. doi: 10.1212/WNL.43.3_Part_1.563
29. Mendez MF, Perryman KM. Neuropsychiatric features of frontotemporal dementia: evaluation of consensus Criteria and review. J Neuropsychiatry Clin Neurosci (2002) 14(4):424–29. doi: 10.1176/jnp.14.4.424
30. Worbe Y, Mallet L, Golmard J-L, Béhar C, Durif F, Jalenques I, et al. “Repetitive behaviours in patients with Gilles de La Tourette syndrome: tics, compulsions, or both?” Edited by Angela Sirigu. PLoS One (2010) 5(9):e12959. doi: 10.1371/journal.pone.0012959
31. Cath DC, Spinhoven P, Hoogduin CA, Landman AD, van Woerkom TC, van de Wetering BJ, et al. Repetitive behaviors in Tourette's syndrome and OCD with and without tics: what are the differences? Psychiatry Res (2001) 101(2):171–85. doi: 10.1016/S0165-1781(01)00219-0
32. Cath DC, van de Wetering BJM, van Woerkom TCAM, Hoogduin CAL, Roos RAC, Rooijmans HGM. Mental play in Gilles de La Tourette's syndrome and obsessive-compulsive disorder. Br J Psychiatry (1992) 161(4):542–45. doi: 10.1192/bjp.161.4.542
33. Leckman JF, Walker DE, Goodman WK, Pauls DL, Cohen DJ. ‘Just right' perceptions associated with compulsive behavior in Tourette's syndrome. Am J Psychiatry (1994) 151(5):675–80. doi: 10.1176/ajp.151.5.675
34. Kwak C, Vuong KD, Jankovic J. Premonitory sensory phenomenon in Tourette's syndrome. Mov Disord (2003) 18(12):1530–33. doi: 10.1002/mds.10618
35. Capriotti MR, Brandt BC, Turkel JE, Lee H-J, Woods DW. Negative reinforcement and premonitory urges in youth with Tourette syndrome: an experimental evaluation. Behav Modification (2014) 38(2):276–96. doi: 10.1177/0145445514531015
36. Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. Am J Psychiatry (1993) 150(1):98–102. doi: 10.1176/ajp.150.1.98
37. Cox JH, Seri S, Cavanna AE. Sensory aspects of Tourette syndrome. Neurosci Biobehav Rev (2018) 88:170–76. doi: 10.1016/j.neubiorev.2018.03.016
38. Ganos C. Tics and Tourette's: update on pathophysiology and tic control. Curr Opin Neurol (2016) 29(4):513–18. doi: 10.1097/WCO.0000000000000356
39. Bliss J. Sensory experiences of Gilles de La Tourette syndrome. Arch Gen Psychiatry (1980) 37(12):1343–47. doi: 10.1001/archpsyc.1980.01780250029002
40. Jackson SR, Parkinson A, Kim SY, Schüermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cogn Neurosci (2011) 2(3–4):227–43. doi: 10.1080/17588928.2011.604717
41. Ganos C, Garrido A, Navalpotro-Gómez I, Ricciardi L, Martino D, Edwards MJ, et al. Premonitory urge to tic in Tourette's is associated with interoceptive awareness. Mov Disord (2015) 30(9):1198–202. doi: 10.1002/mds.26228
42. Rae CL, Larsson DEO, Garfinkel SN, Critchley HD. Dimensions of interoception predict premonitory urges and tic severity in Tourette syndrome. Psychiatry Res (2019) 271:469–75. doi: 10.1016/j.psychres.2018.12.036
43. Conelea CA, Woods DW. Examining the impact of distraction on tic suppression in children and adolescents with Tourette syndrome. Behav Res Ther (2008) 46(11):1193–200. doi: 10.1016/j.brat.2008.07.005
44. Rozenman M, Johnson OE, Chang SW, Woods DW, Walkup JT, Wilhelm S, et al. Relationships between premonitory urge and anxiety in youth with chronic tic disorders. Children's Health Care (2015) 44(3):235–48. doi: 10.1080/02739615.2014.986328
45. Eddy CM, Cavanna AE. Premonitory urges in adults with complicated and uncomplicated Tourette syndrome. Behav Modification (2014) 38(2):264–75. doi: 10.1177/0145445513504432
46. Kano Y, Matsuda N, Nonaka M, Fujio M, Kuwabara H, Kono T. Sensory phenomena related to tics, obsessive-compulsive symptoms, and global functioning in Tourette syndrome. Compr Psychiatry (2015) 62, 141–46. doi: 10.1016/j.comppsych.2015.07.006
47. Pile V, Lau JYF, Topor M, Hedderly T, Robinson S. Interoceptive accuracy in youth with tic disorders: exploring links with premonitory urge, anxiety and quality of life. J Autism Dev Disord (2018) 48(10):3474–82. doi: 10.1007/s10803-018-3608-8
48. Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de La Tourette's syndrome. J Clin Psychiatry (1992) 53(9):319–23.
49. Sutherland Owens AN, Miguel EC, Swerdlow NR. Sensory gating scales and premonitory urges in Tourette Syndrome. Sci World J (2011) 11:736–41. doi: 10.1100/tsw.2011.57
50. Cavanna AE, Seri S. Misophonia: current perspectives. Neuropsychiatr Dis Treat (2015). 2117. doi: 10.2147/NDT.S81438
51. Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry (1996) 39(1):33–41. doi: 10.1016/0006-3223(95)00101-8
52. Buse J, Beste C, Herrmann E, Roessner V. Neural correlates of altered sensorimotor gating in boys with Tourette syndrome: a combined EMG/FMRI Study. World J Biol Psychiatry (2016) 17(3):187–97. doi: 10.3109/15622975.2015.1112033
53. Zebardast N, Crowley MJ, Bloch MH, Mayes LC, Wyk BV, Leckman JF, et al. Brain mechanisms for prepulse inhibition in adults with tourette syndrome: initial findings. Psychiatry Res: Neuroimaging (2013) 214(1):33–41. doi: 10.1016/j.pscychresns.2013.05.009
54. Swerdlow NR. Update: studies of prepulse inhibition of startle, with particular relevance to the pathophysiology or treatment of Tourette syndrome. Neurosci Biobehav Rev (2013) 37(6):1150–56. doi: 10.1016/j.neubiorev.2012.09.002
55. Belluscio BA, Jin L, Watters V, Lee TH, Hallett M. Sensory sensitivity to external stimuli in tourette syndrome patients. Mov Disord (2011) 26(14):2538–43. doi: 10.1002/mds.23977
56. Schunke O, Grashorn W, Kahl U, Schöttle D, Haggard P, Münchau A, et al. Quantitative sensory testing in adults with Tourette syndrome. Parkinsonism Related Disord (2016) 24:132–36. doi: 10.1016/j.parkreldis.2016.01.006
57. Weisman H, Parush S, Apter A, Fennig S, Benaroya-Milshtein N, Steinberg T. A study of sensory dysregulation in children with tic disorders. J Neural Transm (2018) 125(7):1077–85. doi: 10.1007/s00702-018-1858-4
58. Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain (2000) 123(3):425–62. doi: 10.1093/brain/123.3.425
59. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry (2015) 72(4):325. doi: 10.1001/jamapsychiatry.2014.2650
60. Rizzo R, Gulisano M, Pellico A, Calì PV, Curatolo P. Tourette syndrome and comorbid conditions: a spectrum of different severities and complexities. J Child Neurol (2014) 29(10):1383–89. doi: 10.1177/0883073814534317
61. Eapen V, Robertson M. Are Tthere distinct subtypes in tourette syndrome? Pure-tourette syndrome versus tourette syndrome-plus, and simple versus complex tics. Neuropsychiatr Dis Treat (2015) June:1431. doi: 10.2147/NDT.S72284
62. Budman CL, Rosen M, Shad S. Fits, tantrums, and rages in TS and related disorders. Curr Dev Disord Rep (2015) 2(4):273–84. doi: 10.1007/s40474-015-0059-1
63. Mol Debes NMM, Hjalgrim H, Skov L. Validation of the presence of comorbidities in a danish clinical cohort of children with Tourette syndrome. J Child Neurol (2008) 23(9):1017–27. doi: 10.1177/0883073808316370
64. Budman CL, Bruun RD, Park KS, Lesser M, Olson M. Explosive outbursts in children with Tourette's disorder. J Am Acad Child Adolesc Psychiatry (2000) 39(10):1270–76. doi: 10.1097/00004583-200010000-00014
65. Rizzo R, Curatolo P, Gulisano M, Virzì M, Arpino C, Robertson MM. Disentangling the effects of Tourette syndrome and attention deficit hyperactivity disorder on cognitive and behavioral phenotypes. Brain Dev (2007) 29(7):413–20. doi: 10.1016/j.braindev.2006.12.003
66. Gadow KD, Nolan EE, Sprafkin J, Schwartz J. Tics and psychiatric comorbidity in children and adolescents. Dev Med Child Neurol (2002) 44(5):330–38. doi: 10.1111/j.1469-8749.2002.tb00820.x
67. Thériault M-CG, Lespérance P, Achim A, Tellier G, Diab S, Rouleau GA, et al. ODD irritability is associated with obsessive–compulsive behavior and not ADHD in chronic tic disorders. Psychiatry Res (2014) 220(1–2):447–52. doi: 10.1016/j.psychres.2014.07.039
68. Kurlan R, Daragjati C, Como PG, McDermott MP, Trinidad KS, Roddy S, et al. Non-obscene complex socially inappropriate behavior in Tourette's syndrome. J Neuropsychiatry Clin Neurosci (1996) 8(3):311–17. doi: 10.1176/jnp.8.3.311
69. Eddy CM, Cavanna AE. ‘It's a Curse!': coprolalia in Tourette Syndrome. Eur J Neurol (2013) June:n/a–a. doi: 10.1111/ene.12207
70. Comings DE. Role of genetic factors in human sexual behavior based on studies of Tourette syndrome and ADHD probands and their relatives. Am J Med Genet (1994) 54(3):227–41. doi: 10.1002/ajmg.1320540309
71. Moldofsky H, Tullis C, Lamon R. Multiple tic syndrome (Giles de La Tourette's syndrome). J Nervous Ment Dis (1974) 159(4):282–92. doi: 10.1097/00005053-197410000-00007
72. Nee LE, Caine ED, Polinsky RJ, Eldridge R, Ebert MH. Gilles de La Tourette syndrome: clinical and family study of 50 cases. Ann Neurol (1980) 7(1):41–9. doi: 10.1002/ana.410070109
73. Freeman RD, Tourette Syndrome International Database Consortium. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry (2007) 16(S1):15–23. doi: 10.1007/s00787-007-1003-7
74. Robertson MM, Trimble MR, Lees AJ. Self-injurious behaviour and the Gilles de La Tourette syndrome: a clinical study and review of the literature. Psychol Med (1989) 19(3):611–25. doi: 10.1017/S0033291700024211
75. Mathews CA. Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry (2004) 75(8):1149–55. doi: 10.1136/jnnp.2003.020693
76. Ganos C, Roessner V, Münchau A. The functional anatomy of Gilles de La Tourette syndrome. Neurosci Biobehav Rev (2013) 37(6):1050–62. doi: 10.1016/j.neubiorev.2012.11.004
77. Hashemiyoon R, Kuhn J, Visser-Vandewalle V. Putting the pieces together in gilles de la tourette syndrome: exploring the link between clinical observations and the biological basis of dysfunction. Brain Topogr (2017) 30(1):3–29. doi: 10.1007/s10548-016-0525-z
78. Kalanithi PSA, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci (2005) 102(37):13307–12. doi: 10.1073/pnas.0502624102
79. Kataoka Y, Kalanithi PSA, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol (2010) 518(3):277–91. doi: 10.1002/cne.22206
80. Leckman JF, Riddle MA. Tourette's syndrome. Neuron (2000) 28(2):349–54. doi: 10.1016/S0896-6273(00)00114-8
81. Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex (2009) 19(8):1844–56. doi: 10.1093/cercor/bhn214
82. McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain (2009) 132(8):2125–38. doi: 10.1093/brain/awp142
83. Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Front Syst Neurosci (2013) 7:50. doi: 10.3389/fnsys.2013.00050
84. Heise K-F, Steven B, Liuzzi G, Thomalla G, Jonas M, Muller-Vahl K, et al. Altered modulation of intracortical excitability during movement preparation in Gilles de La Tourette syndrome. Brain (2010) 133(2):580–90. doi: 10.1093/brain/awp299
85. Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de La Tourette syndrome. J Neurol Neurosurg Psychiatry (2009) 80(1):29–34. doi: 10.1136/jnnp.2008.149484
86. Orth M, Amann B, Robertson MM, Rothwell JC. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain (2005) 128(6):1292–300. doi: 10.1093/brain/awh473
87. Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, et al. Association of cortical disinhibition with Tic, ADHD, and OCD severity in Tourette syndrome. Mov Disord (2004) 19(4):416–25. doi: 10.1002/mds.20044
88. Orth M, Münchau A, Rothwell JC. Corticospinal system excitability at rest is associated with tic severity in Tourette syndrome. Biol Psychiatry (2008) 64(3):248–51. doi: 10.1016/j.biopsych.2007.12.009
89. Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry (1997) 154(9):1277–84. doi: 10.1176/ajp.154.9.1277
90. Franzkowiak S, Pollok B, Biermann-Ruben K, Südmeyer M, Paszek J, Jonas M, et al. Altered pattern of motor cortical activation-inhibition during voluntary movements in tourette syndrome. Mov Disord (2010) 25(12):1960–66. doi: 10.1002/mds.23186
91. Biermann-Ruben K, Miller A, Franzkowiak S, Finis J, Pollok B, Wach C, et al. Increased Sensory sensory feedback in Tourette syndrome. NeuroImage (2012) 63(1):119–25. doi: 10.1016/j.neuroimage.2012.06.059
92. Hirschtritt ME, Darrow SM, Illmann C, Osiecki L, Grados M, Sandor P, et al. Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology (2016) 87(5):497–504. doi: 10.1212/WNL.0000000000002910
93. Bronfeld M, Bar-Gad I. Tic disorders: what happens in the bsal ganglia? Neurosci (2013) 19(1):101–8. doi: 10.1177/1073858412444466
94. Marsden CD, Meldrum BS, Pycock C, Tarsy D. Focal myoclonus produced by injection of picrotoxin into the caudate nucleus of the rat. J Physiol (1975) 246(2):96P.
95. Tarsy D, Pycock CJ, Meldrum BS, Marsden CD. Focal contralateral myoclonus produced by inhibition of gaba action in the caudate nucleus of rats. Brain (1978) 101(1):143–62. doi: 10.1093/brain/101.1.143
96. Muramatsu S, Yoshida M, Nakamura S. Electrophysiological study of dyskinesia produced by microinjection of picrotoxin into the striatum of the rat. Neurosci Res (1990) 7(4):369–80. doi: 10.1016/0168-0102(90)90011-3
97. McCairn KW, Isoda M. Pharmacological animal models of tic disorders. In: . International Review of Neurobiology, vol. 112. (San Diego: Elsevier Science Publishing Co Inc.). (2013) p. 179–209. doi: 10.1016/B978-0-12-411546-0.00007-X
98. Godar SC, Mosher LJ, Giovanni GD, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods (2014) 238:54–69. doi: 10.1016/j.jneumeth.2014.09.008
99. Pépés SE, Draper A, Jackson GM, Jackson SR. Effects of age on motor excitability measures from children and adolescents with Tourette syndrome. Dev Cogn Neurosci (2016) 19:78–86. doi: 10.1016/j.dcn.2016.02.005
100. Niccolai V, van Dijk H, Franzkowiak S, Finis J, Südmeyer M, Jonas M, et al. Increased beta rhythm as an indicator of inhibitory mechanisms in Tourette syndrome: OSCILLATORY CHARACTERISTICS OF TOURETTE SYNDROME. Mov Disord (2016) 31(3):384–92. doi: 10.1002/mds.26454
101. Franzkowiak S, Pollok B, Biermann-Ruben K, Südmeyer M, Paszek J, Thomalla G, et al. “Motor-cortical interaction in Gilles de La Tourette syndrome.” Edited by Paul L. Gribble. PLoS One (2012) 7(1):e27850. doi: 10.1371/journal.pone.0027850
102. Ganos C, Rocchi L, Latorre A, Hockey L, Palmer C, Joyce EM, et al. Motor cortical excitability during voluntary inhibition of involuntary tic movements: the motor neurophysiology of tic inhibition. Mov Disord (2018) 33(11):1804–9. doi: 10.1002/mds.27479
103. Palminteri S, Pessiglione M. Reinforcement learning and Tourette syndrome. In: International Review of Neurobiology, vol. 112. (San Diego: Elsevier Science Publishing Co Inc.). (2013). p. 131–53. doi: 10.1016/B978-0-12-411546-0.00005-6
104. Worbe Y. Reinforcement learning and Gilles de La tourette syndrome: dissociation of clinical phenotypes and pharmacological treatments. Arch Gen Psychiatry (2011) 68(12):1257. doi: 10.1001/archgenpsychiatry.2011.137
105. Delorme C, Salvador A, Valabrègue R, Roze E, Palminteri S, Vidailhet M, et al. Enhanced habit formation in Gilles de La Tourette syndrome. Brain (2016) 139(2):605–15. doi: 10.1093/brain/awv307
106. Rae CL, Critchley HD, Seth AK. A Bayesian account of the sensory-motor interactions underlying symptoms of tourette syndrome. Front Psychiatry (2019) 10:29. doi: 10.3389/fpsyt.2019.00029
107. O'Connor K, St-Pierre-Delorme M-È, Leclerc J, Lavoie M, Blais MT. Meta-cognitions in Tourette syndrome, tic disorders, and body-focused repetitive disorder. Can J Psychiatry (2014) 59(8):417–25. doi: 10.1177/070674371405900804
108. English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsáki G, Deisseroth K, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci (2012) 15(1):123–30. doi: 10.1038/nn.2984
109. Diederen KMJ, Spencer T, Vestergaard MD, Fletcher PC, Schultz W. Adaptive prediction error coding in the human midbrain and striatum facilitates behavioral adaptation and learning efficiency. Neuron (2016) 90(5):1127–38. doi: 10.1016/j.neuron.2016.04.019
110. Sherman MT, Seth AK, Barrett AB, Kanai R. Prior expectations facilitate metacognition for perceptual decision. Conscious Cogn (2015) 35:53–65. doi: 10.1016/j.concog.2015.04.015
111. Delorme C, Salvador A, Voon V, Roze E, Vidailhet M, Hartmann A, et al. Illusion of agency in patients with Gilles de La Tourette Syndrome. Cortex (2016) 77:132–40. doi: 10.1016/j.cortex.2016.02.003
Keywords: Tourette syndrome, tics, inhibitory control, disinhibition, Gamma aminobutyric acid, basal ganglia
Citation: Kurvits L, Martino D and Ganos C (2020) Clinical Features That Evoke the Concept of Disinhibition in Tourette Syndrome. Front. Psychiatry 11:21. doi: 10.3389/fpsyt.2020.00021
Received: 05 July 2019; Accepted: 09 January 2020;
Published: 25 February 2020.
Edited by:
Clare Margaret Eddy, Birmingham and Solihull Mental Health NHS Foundation Trust, United KingdomReviewed by:
Macià Buades-Rotger, Universität zu Lübeck, GermanyKerstin Jessica von Plessen, Centre Hospitalier Universitaire Vaudois, Switzerland
Copyright © 2020 Kurvits, Martino and Ganos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christos Ganos, Y2hyaXN0b3MuZ2Fub3NAY2hhcml0ZS5kZQ==
 Lille Kurvits
Lille Kurvits Davide Martino
Davide Martino Christos Ganos
Christos Ganos