Corrigendum: Correlation Analysis Between Attentional Bias and Somatic Symptoms in Depressive Disorders
- 1Department of Psychiatry, Guangzhou Panyu Central Hospital, Guangzhou, China
- 2Mental Health Centre, People’s Hospital of Wuhan University, Wuhan, China
- 3Department of Pathology, Shenzhen Baoan District People’s Hospital, Shenzhen, China
- 4Department of Clinical Psychology, Second People’s Hospital of Wuhu, Wuhu, China
Objective: To investigate the relationship between attentional bias and the severity of depression as assessed by the TORAWARE state and physical symptoms.
Methods: We enrolled 55 patients with depression and 60 healthy people. The Hamilton Depression Scale (HAMD-24), Somatic Self-Rating Scale (SSS), and the Chinese version of the Self-Rating Scale for the TORAWARE State of Neurosis (SSTN) were selected to assess the severity of psychological symptoms. Dot-probe tasks were used to detect attentional bias. We then analyzed the correlation of attentional bias with the total scores on the symptom scales.
Results: The negative attentional bias and negative disengaging index scores were both greater than 0 (t = 3.15 and 2.78, respectively; all P < 0.01). The negative attention bias score was positively correlated with the SSTN and negative disengaging index scores (r = 0.29 and 0.53, respectively; all P < 0.05). SSTN score was positively correlated with the total HAMD and SSS scores (r = 0.34 and 0.38, respectively; all P < 0.05).
Conclusion: There is no direct correlation between negative attentional bias and depression. It may be through the intermediate mechanism of TORAWARE state to influence symptoms.
Introduction
Approximately one third of patients with depression experience chronic or recurrent symptoms (1). In a previous study by Geden, 69% of patients with depression reported experiencing somatic symptoms only, while 11% denied experiencing depressive symptoms even when directly questioned regarding such symptoms (2). Additional studies have indicated that up to 50% of patients with depressive tendencies may be misdiagnosed due to the manifestation of physical/somatic symptoms rather than typical depressive symptoms (3, 4). Typical symptoms of depression include low mood, pessimism, feelings of meaninglessness, decreased energy, fatigue, low self-evaluation, guilt, and physical or biological symptoms such as loss of appetite and sleep disturbance. Previous meta-analyses have suggested that painful physical symptoms can be an important part of depression (5). Indeed, physical symptoms such as fatigue, weakness, chronic pain, and gastrointestinal disturbances (6, 7) often mask depressive symptoms, complicating the diagnosis and treatment of depression. Recent studies have demonstrated that rates of improvement in depressive symptoms are lower in patients with depression who exhibit somatic symptoms or comorbid physical illness than in those without comorbidities (8, 9). In addition, patients with poor physical health experience higher rates of relapse following recovery from the initial depressive episode. Evidence suggests that the degree of relief from somatic symptoms such as chronic pain is directly associated with higher rates of remission among patients with depressive symptoms (10). Such findings highlight the importance of treating somatic and physical symptoms associated with depression.
According to the cognitive model of depression, patients with depression exhibit a negative attentional bias that is manifested by their prioritization of negative cognitive stimuli. This attention bias of information processing plays a crucial role in the pathogenesis, maintenance, and development of depression (11, 12). One meta-analysis has suggested that patients with depression exhibit an attentional bias toward negative information (13). Negative attentional bias has been proven to predict the occurrence and recurrence of depressive symptoms (14, 15). Furthermore, patients with depression exhibit decreases in attentional bias toward positive information (16). Researchers have hypothesized that deficits in the processing of positive emotional information lead to decreases in the sensitivity to incentive stimuli, in turn leading to impairments in attentional orientation to such stimuli (17). In contrast, mentally healthy individuals exhibit attentional bias toward positive information, along with the tendency to avoid negative information (16). Prolonged absence of the attentional bias toward positive information leads to cognitive distortions (e.g., the attribution of negative events to oneself), exacerbating and maintaining depressive symptoms (18). However, the relationships among negative attentional bias, depressive symptoms, and physical discomfort in patients with depression remain unclear.
Dr. Morita Masaki, professor of Japanese psychiatry, first described the psychopathological characteristics of TORAWARE, a Japanese term referring to a state in which one’s attention and ideas are bound to a symptom or concept (i.e., neurotic preoccupation). TORAWARE is regarded as both a spiritual interaction and ideologically contradictory state (19, 20). Although patients may first experience conscious hyperprosexia, this state leads to decreases in the awareness of one’s surroundings, which in turn inhibits the ability to shift one’s attention, resulting in TORAWARE (21). Based on this theory, Morita therapy is a psychological therapy method invented by Japan, which is widely used in Asian countries and has significant clinical effects in treating neurosis, somatic symptoms of depression, generalized anxiety disorder, and other diseases (22).There are some studies on the application of Morita therapy, a research recruited 68 major depressive disorder participants (34 control and 34 intervention) provided 4-month follow-up data. We randomized participants on a 1:1 basis stratified by symptom severity to receive treat as usual (TAU) (control) or 8–12 sessions of Morita therapy plus TAU (intervention). Results compared with the control group, the symptom scale score of the control group was significantly improved. Morita therapy shows promise in treating depression and may provide patients with a distinct alternative to current treatments (23). He did a similar study before in 2016 (24). Morita therapists help patients to move away from symptom preoccupation and resistance, which are thought to interfere with the natural recovery process and lead to further preoccupation with and worsening of symptoms (25). Previous studies have demonstrated that pharmacological treatment combined with Morita therapy is more effective than treatment with medication alone, and that patients undergoing combined treatment exhibit more significant improvements in depressive symptoms and quality of life, along with fewer adverse effects (26, 27). While Morita therapy aims to break the cycle of attentional fixation on one’s symptoms, helping to accelerate improvements in both depressive and physical symptoms, some evidence suggests that depressive symptoms may also be present in the TORAWARE state (28).
In the nearly four years from 2015 to 2019 alone, a total of 180 papers on the application of Morita therapy have been retrieved from Chinese core journals. There must be a lot of studies in Japan, but there are few articles on Morita therapy in international scientific literature. After analyzing the reasons, we believe that: 1) apart from cultural factors, such as the difficult translation of its theory into English, it is not really easily understood by the international medical community; 2) the theory of Morita therapy is mainly derived from the summary of individual cases, and there are few large samples and experimental studies, so its theory does not exclude subjectivity and one-sidedness, and may lack of representativeness. So it didn’t get a lot of international attention. In order to avoid these disadvantages in the present study, we adopted the research method of medical psychology to discuss the attention characteristics of patients with depression and the correlation with somatic symptoms, and explore the occurrence and development mechanism of somatic symptoms of depression, and verify the scientific nature of Morita therapy theory. It is hoped that the international academic field has a new understanding of Morita therapy theory. According to the above theory, we hypothesized (a) that patients with depression would exhibit greater attentional bias toward negative emotional stimuli, as well as avoidance of positive stimuli; (b) that attentional bias toward negative emotional stimuli is positively correlated with the severity of depressive/somatic symptoms in patients with depression; (c) and that increases in negative attentional bias are associated with increases in the severity of the TORAWARE state and depressive/somatic symptoms.
Patients and Methods
Study Participants
The present cross-sectional study included 55 patients with depression and 60 healthy controls. Depression patients were recruited from the Clinical Psychology Department at Wuhu City Second People’s Hospital (Wuhu, China). Control participants with no current or prior psychiatric disorders were recruited from the Physical Examination Center. All prospective participants with depression were diagnosed by their treating psychiatrists using the Mini-International Neuropsychiatric Interview (MINI) in accordance with criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (29). The MINI is used to evaluate the mental state of depressed patients, including the degrees of cognitive impairment, depression and its duration, self-worth, and deterioration of physical and physiological states.
Inclusion criteria were as follows: (i) ≥15 years of age; (ii) all depression met the diagnostic criteria of DSM-IV for depressive disorder, Hamilton Depression Scale (HAMD-24) score >20; (iii) no history of organic brain disease, serious physical disease, or mental illnesses other than depression; (iv) no drug or alcohol use in the previous 2 weeks; (v) normal or corrected-to-normal vision. Exclusion criteria were as follows: (i) color blindness or presence of concurrent eye disease; (ii) history of alcohol/drug dependence; (iii) comorbid neuropsychiatric disorders; (iv) severe depression inhibiting completion of the study. All study participants provided written informed consent. The study was approved by the ethics committee at Wuhu Second People’s Hospital, Wannan Medical College (Wuhu, China).
Self-Report Measures
The Hamilton Depression Scale (HAMD) was developed by Hamilton in 1960 and was the most commonly used scale in clinical evaluation of depression. This scale was conducted by two trained evaluators to conduct HAMD joint examination on patients. Generally, the method of conversation and observation was adopted. After the examination, the two evaluators scored independently. The severity and therapeutic effect of the disease can be evaluated by comparing the scores before and after treatment. The Somatic Self-Rating Scale (SSS) is a highly valid and reliable self-report questionnaire that is primarily used to evaluate emotional responses and somatic symptoms in patients treated at general hospitals (30). The SSS consists of 20 items divided into four domains, although we primarily utilized the somatization (S) factor to assess the severity of somatic symptoms in the present study. The Japanese version of the “Self-Rating Scale for the TORAWARE State of Neurosis” (SSTN) (31) was translated into Chinese in 2016 (32), exhibiting good internal consistency (α = .81). The SSTN is used to evaluate the severity of the TORAWARE state and consists of 20 items across the following six dimensions: mental interaction, mental conflict, attentional fixation, low social and body functioning, poor symptom tolerance, and perfectionism. Participants were asked to provide responses regarding symptoms experienced during the previous week using a four-point scale. Higher total scores were considered indicative of more severe symptoms.
Dot-Probe Task
Materials
Forty positive, negative, and neutral pictures each were selected from the Chinese Affective Picture System (CAPS) (33). Positive and negative images were matched according to the degree of pleasure and polarization: The average pleasure rating of positive pictures was 7.68 ± 0.45, with an average degree of arousal of 5.72 ± 0.56. The average pleasure rating of negative pictures was 3.20 ± 0.35, with an average degree of arousal of 4.32 ± 0.66. Average pleasure and arousal ratings for neutral pictures were 5.34 ± 0.21 and 4.53 ± 0.34, respectively. Each picture was converted to a Bitmap (BMP) image with a resolution of 480 × 250 pixels using image editing software. Images were matched into negative–neutral, positive–neutral, neutral–neutral three types of combinations while taking the brightness and tonal proximity of the picture into consideration. The visual probe task was programmed using the E-prime software package, which was also used to present the experimental stimuli. Response times (RTs) and error rates were automatically recorded by the software, and the task was run on a computer with a 14-inch color monitor (resolution: 1366 × 768 pixels).
Procedures
All participants were tested in individual sessions lasting approximately 30 min each. Participants first completed the questionnaires, following which the experiment was conducted.
The dot-probe task consisted of 108 test trials (20 neutral–neutral, 40 negative–neutral trials, and 40 positive–neutral trials) and eight practice trials. Neutral and emotional picture pairs and probes appeared equally often to the left or right. Participants were seated at a viewing distance of approximately 60 cm from the computer screen, which displayed the experimental instructions. At the beginning of each trial, a fixation cross (“+”; 10 mm × 10 mm) was presented in the middle of the screen for 500 ms, following which two pictures (each 80 mm × 100 mm) were presented symmetrically on either side of the middle line of the screen for 500 ms. The distance between the inner edges of the pictures was 44 mm. Directly after the offset of the two pictures, a small probe point (“*”; 10 mm × 10 mm) appeared in the center of the location of either the left or right picture. Participants were asked to respond as quickly as possible by identifying the location of the probe and pressing one of two keys: the “A” key for left and the “L” key for right. The dot remained in view until participants provided a response or did not respond within 2,000 ms. A blank gray screen was presented for 1,000 ms prior to the onset of the next trial. Pictures were presented in random order.
Analysis Indicators
Total HAMD-24, SSS-S, and SSTN scores, as well as attentional bias scores, were compared between the two groups. Bias scores were calculated by subtracting the mean RT for congruent trials from the mean RT for incongruent trials: bias score = RTinconsistent – RTconsistent. RTinconsistent represents the average response time when the detection point was not in the same location as the emotional picture, while RTconsistent represents the average response time when the detection point was in the same location as the emotional picture. Positive scores are considered indicative of attentional bias towards emotional stimuli. We also calculated the orienting index (OI) and disengaging index (DI) (34) for each group, as follows: OI = RTneutral pair – RTconsistent; DI = RTinconsistent – RTneutral pair. Positive OI values indicate that detection of emotional pictures occurred more quickly, while positive DI values indicate difficulty in disengaging attention from emotional pictures. Negative bias scores, OI values, and DI values were considered indicative of attentional avoidance of emotional pictures.
Data Analysis
All statistical analyses were performed using SPSS version 20.0. After visual inspection of the data, RTs <200 ms and >1000 ms were considered outliers indicative of anticipatory and delayed responding, respectively. Trials with errors were discarded from the analyses, as we observed no significant differences in the number of erroneous responses between the two groups (F = 1.85, p > .1). Five participants with depression were excluded due to unusually slow RTs or the absence of 40% of RT data due to errors and outliers. Average individual RTs were computed for each emotion in each of the five conditions. Chi-square tests and t-tests were used to investigate between-group differences in demographic characteristics and symptom scores. One-sample t-tests were used to compare bias scores, OI values, and DI values to 0 (indicating a lack of attentional bias). Pearson correlation analyses were used to explore the relationships between attentional bias indices and symptoms.
Results
Participant Characteristics
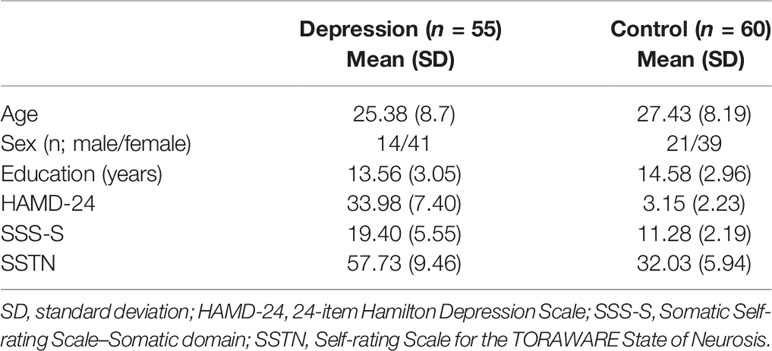
As shown in Table 1, participants in the depression group reported significantly greater symptoms of depression and somatic discomfort than those in the control group (t [62.97] = 29.69, t [69.17] = 10.14; all P < 0.001). In addition, symptoms of the TORAWARE state were significantly greater in the depression group than in the control group (t [89.38] = 17.26; P < 0.001). No significant differences in age (t [113] = –1.3; P = 0.20), education (t [109.11] = –1.3; P = 0.07), or gender ratio (χ2 [1, n = 50] = 1.24; P = 0.27) were observed between the groups.
Attentional Indices for Positive and Negative Pictures
We compared the bias scores, OI values, and DI values for positive and negative stimuli to a no-bias criterion (zero) in each group. Statistically significant difference in negative attention bias score and negative DI in the control group (t = −4.18 and −2.19, respectively; P = 0.000 and 0.033, respectively), indicating that the control group exhibited avoidance of negative stimuli; while these two indicators were both significant >0 in the depression group (t = 3.15 and 2.78, respectively; P = 0.003 and 0.008, respectively), indicating that patients with depression exhibited attentional bias towards negative images as well as difficulty disengaging their attention from such stimuli. The positive bias score of the depression group was statistically significant (t = −2.29, P = 0.026), indicating that the participants in the depression group avoided positive stimuli (Table 2).
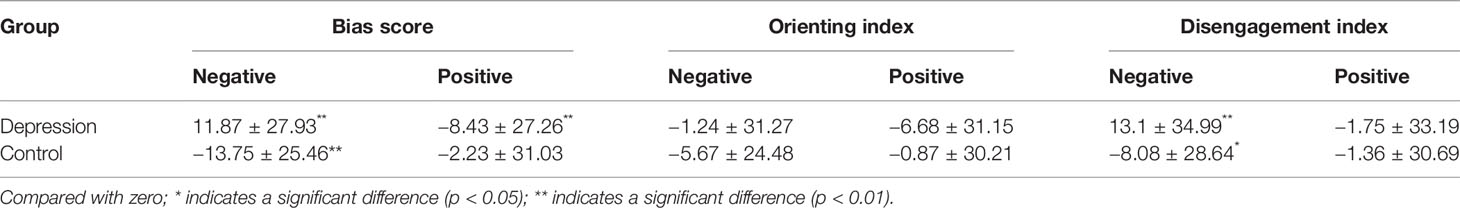
Table 2 Bias score, orienting index, and disengagement index (s) for positive and negative stimuli [(x ± s)/ms)].
Relationships Between Attentional Indices and Symptoms in the Depression Group
We conducted a series of bivariate correlation analyses to explore whether attentional bias scores were associated with the severity of depressive or somatic symptoms. We further investigated the interaction among attentional bias, the TORAWARE state, and symptoms among patients with depression. Table 3 shows the zero-order correlation coefficients. Our analyses revealed that the positive bias scores have no correlation with other indicators, negative bias scores were positively associated with negative DI values (r = 0.53, P < 0.001) and SSTN scores (r = 0.29, P = 0.035). SSTN scores were positively associated with HAMD-24 and SSS-S scores (r = 0.34 and 0.38, respectively; P = 0.011 and 0.005, respectively). No other significant associations were observed (Table 3).
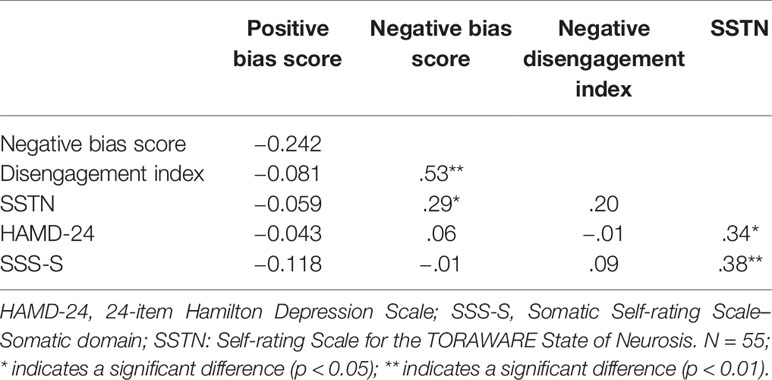
Table 3 Bivariate correlations between attentional bias indices and symptoms in the depression group.
Discussion
Although scientists are trying to study “medical unexplained symptoms” (MUS) disease etiology, mechanisms, but so far MUS is still a difficult problem in the international medical. Most depression patients with MUS, like often accompanied by physically weakening symptoms, such as loss of appetite, fatigue, and weight loss. This results in a reluctance to partake in social activities, which further affects the patient’s relationships. Many patients’ somatoform discomforts disappeared after the depression cured, but why depression are prone to body symptom? We found that Morita therapy can achieve good results in the treatment of somatic symptoms of depression, and the psychological mechanism is worth studying. Clinical depression is generally found to have negative thinking tendency (looking at negative aspects of things and ignoring positive aspects). Is this thinking tendency related to debilitating symptoms? In the present study, we investigated the associations among attentional bias, the TORAWARE state, and somatic/depressive symptoms in patients with clinical depression. Our findings indicated that patients with depression exhibit obvious attentional bias towards negative image information, which is mainly manifested as difficulty in disengaging attention, consistent with the results of previous studies (35, 36). In addition, control participants exhibited attentional avoidance with regard to negative stimuli, consistent with previous findings as well as our first hypothesis. Previous research has suggested that such avoidance exerts a protective effect in healthy individuals confronted with negative stimuli (37); however, excessive processing occurs in patients with depression, making it difficult to refocus attention and increasing vulnerability to negative information (25).
Saskia et al. (38) explored the relationship between attentional bias and the severity/specificity of symptoms in patients with depression using the Beck Depression Inventory-II (BDI-II), which evaluates the following three dimensions of depression: negative cognition, depressive symptoms, and somatic symptoms. The authors demonstrated that only negative cognition is a main predictor of negative attentional bias among patients with depression. Disner et al. (39) reported that negative attentional bias is related to the course of depressive symptoms, although they observed no significant relationship between such bias and baseline levels of depressive symptoms. Such results suggest that there is no direct correlation between negative attentional bias and depressive symptoms in patients with depression, in accordance with our findings. Although we observed no direct correlation between negative attentional bias and somatic discomfort or depressive symptoms, such bias was positively correlated with the severity of the TORAWARE state, and the TORAWARE state was positively correlated with the severity of symptoms. These findings suggest that the TORAWARE state of patients with depression may play a mediating role in the effect of attention bias of negative stimuli on depressive and somatic symptoms.
The current hypothesis regarding the pathology of the TORAWARE state proposes that patients with neuroses exhibit cognitive biases, which Morita referred to as “ideological contradictions”. Such biases are thought to lead to involuntarily or consciously excessive attention (attentional fixation) on symptoms such as physical discomfort or annoyance, thereby exacerbating these feelings. This in turn leads to involuntary fixation on or hypersensitivity to such symptoms, decreasing social functioning and maintaining the TORAWARE state (20, 28). The somatic discomfort experienced by patients with depression is often similar to neurosis in that patients may excessively focus on physical discomfort and depressive symptoms. In the present study, we observed that SSTN scores were significantly higher among patients with depression than in the control group, suggesting that the psychopathology of depression can also involve the TORAWARE state. We also observed a significant positive correlation between negative attentional bias and the severity of the TORAWARE state in the depression group. Furthermore, the severity of the TORAWARE state was significantly positively correlated with depression and somatosensory symptom scores, in accordance with our hypotheses. These results suggest that attentional bias toward negative information in patients with depression can reinforce depressive symptoms and somatic discomfort, and that this process is mediated by the TORAWARE state. Moreover, the negative DI value in the depression group was significantly larger than 0, consistent with the results of Zhu et al. (40). These results suggest that patients with depression exhibit difficulty disengaging from negative stimuli, which may underlie attentional fixation. Such attentional fixation may aggravate depressive/somatic symptoms, thereby leading to the development and maintenance of the depressive state.
Our findings support the notion that patients with depression exhibit negative attentional bias. Our results further suggest that the presence of depressive and somatic symptoms may lead to the development of attentional bias and the TORAWARE state. The results of our study are exciting. It is the first time to demonstrate the mechanism of the TORAWARE state in role of depression with somatic symptoms by experimental method, and it also helps us find a new target for the treatment of somatic symptoms of depression, which is beneficial to the research and treatment of somatic symptoms of depression. Further studies are required to determine whether resolution of the TORAWARE state can improve depressive/somatic symptoms in patients with depression. Our research subjects are limited to Chinese patients with depression. Due to cultural and ethnic factors, our conclusions may be limited or lack of representation in different ethnic and cultural backgrounds worldwide. However, one fourth of the world’s population in China has such research conclusion on depression, which may suggest that this study has important reference value for the worldwide exploration of somatic symptoms of depression or the pathogenesis of MUS.
Ethics Statement
This study was carried out in accordance with the recommendations of Ethical guidelines for biomedical research involving people, Wuhu Second People's Hospital Ethics Committee. The protocol was approved by the Wuhu Second People's Hospital Ethics Committee. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
The completion of the experiment and the writing of the article were mainly by YW. YH helped with data processing and analysis, and proofread the manuscript. GW helped strictly check the quality of the article and his project fund supported the successful conduct and completion of the research. LJ helped with corrections and revisions of this paper and also have given me a lot of advice on the shortcomings of the article. HZ provided partial fund support and academic guidance.
Funding
This project received funding from the Panyu District Science and Technology Plan Project (Grant No.: 2017-Z04-02) and Mental Health Okamoto Memorial Consortium Research Support Project, Pingcheng 29 years graduate 8. NSFC: Study on the role and mechanism of NLRP3 inflammatory cortisone/il-18/NF-B neuroinflammatory pathway in mediating the occurrence and outcome of depression, and 81871072 to GW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Klein DN. Chronic Depression: Diagnosis and Classification. Curr Dir Psychol Sci (2010) 19:96–100. doi: 10.1177/0963721410366007
2. Greden JF. Physical symptoms of depression: unmet needs. J Clin Psychiatry (2003) 64:5–11. doi: 10.1016/S0887-6185(02)00239-6
3. Pincus HA, Pettit AR. The societal costs of chronic major depression. J Clin Psychiatry (2001) 6:5–9. doi: 10.1076/jcen.23.6.829.1022
4. Sartorius N. Physical symptoms of depression as a public health concern. J Clin Psychiatry (2003) 7:3–4. doi: 10.1111/1469-7610.00108_2
5. Garciacebrian A, Gandhi P, Demyttenaere K, et al. The association of depression and painful physical symptoms–a review of the European literature. Eur Psychiatry (2006) 6:379–88. doi: 10.1016/j.eurpsy.2005.12.003
7. Stahl SM. The psychopharmacology of painful physical symptoms in depression. J Clin Psychiatry (2002) 5:382–3. doi: 10.4088/JCP.v63n0501
8. Greco T, Eckert G, Kroenke K. The Outcome of Physical Symptoms with Treatment of Depression. J Gen Intern Med (2004) 32(8):813–21 doi: 10.1111/j.1525-1497.2004.30531.x
9. Iosifescu DV, Nierenberg AA, Alpert JE, et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am J Psychiatry (2003) 12:2122–7. doi: 10.1176/appi.ajp.160.12.2122
10. Fava M, Mallinckrodt CH, Detke MJ, Watkin JG, Wohlreich MM. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry (2005) 4:521–30. doi: 10.4088/JCP.v65n0411
11. Beck AT. Depression: Clinical, experimental, and theoretical aspects. Harper and Row: New York (1967) p. 232–3. doi: 10.1037/0010175
12. Jaffe B. Cognitive Therapy and the Emotional Disorders. Psychosomatics (1977) 18(1):57–60. doi: 10.1016/S0033-3182(77)71107-7
13. Peckham AD, Mchugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety (2010) 27(12):1135–42. doi: 10.1002/da.20755
14. Beevers CG, Lee HJ, Wells TT, et al. Association of predeployment gaze bias for emotion stimuli with later symptoms of PTSD and depression in soldiers deployed in Iraq. Am J Psychiatry (2011) 168(7):735–41. doi: 10.1176/appi.ajp.2011.10091309
15. Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol (2015) 116(4):80–3. doi: 10.1037/0021-843X.116.1.80
16. Ellis AJ, Beevers CG, Wells TT. Attention allocation and incidental recognition of emotional information in dysphoria. Cognit Ther Res (2011) 5:425–33. doi: 10.1007/s10608-010-9305-3
17. Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol (1991) 3:316–36. doi: 10.1037//0021-843X.100.3.316
18. Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry (2008) 8:969–77. doi: 10.1176/appi.ajp.2008.08050721
20. Li J, Huang J, Kubota M, Zhong CJ, Niu DX. The Research on Psychopathology of Neurosis TORAWARE. Jpn J Morita Ther (2001) 12:137–42.
21. Hiroshi K. An Addendum to psychopathology of the Morita therapy. Genet Soc Gen Psychol Monogr (1952) 74:1214–30.
22. Mei L, Xiang LI, Qing-Gang C, et al. Efficacy analysis of the outpatient type Morita therapy for neurosis. J Sichuan Ment Health (2015) 28:34–6. doi: 10.1159/000283934
23. Sugg HVR, Richards DA, Frost J. Morita Therapy for depression (Morita Trial): a pilot randomised controlled trial. J BMJ Open (2018) 8:1–13. doi: 10.1136/bmjopen-2018-021605
24. Sugg HVR, Richards DA, Frost J. Morita therapy for depression and anxiety (Morita Trial): study protocol for a pilot randomised controlled trial. J Trials (2016) 17:161–74. doi: 10.1186/s13063-016-1279-3
25. Nakamura K, Kitanishi K, Maruyama S, et al. Guidelines for practicing outpatient Morita therapy Vol. 32. Japanese Society for Morita Therapy: Tokyo (2010) p. 43–8.
26. Sun Y, Song S. Effect of Modified Morita Therapy on Patients with Depression. China J Health Psychol (2013) 21(5):255–58.
27. Wang SC. Analysis of efficacy and safety of modified Morita therapy combined with drug in treatment of depression. Contemp Med (2017) 23(3):273–82. doi: 10.3969/j.issn.1009-4393.2017.03.080
28. Zhong CJ, Shi W, Li J, Jiang B. Morita therapy for depression Vol. 2. Xian, China: The Fourth Military Medical University press, (2015) p. 35–6.
29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV Internet. 4th ed. American Psychiatric Association: Washington (DC). (1994).
30. Zhuang Q, Mao JL, Li CB, He B. Developing of somatic self-rating scale and its reliability and validity. China J Behav Med Brain Sci (2010) 9:847–9. doi: 10.3760/cma.j.issn.16746554.2010.09.026
31. Li J, Huang J, Kubota M, et al. Usefulness of the self-evaluation scale of Neurosis TORAWARE State. Jpn J Morita Ther (2003) 14:167–77.
32. Li J, Liu P, Rong W, et al. The Reliability and Validity of the Chinese Version of Self-rating Scale for the TORAWARE State of Neurosis. China J Health Psychol (2016) 6:897–900.
33. Bai L, Ma H, Huang YX, et al. The development of native Chinese affective picture system-a pretest in 46 college students. Chin Ment Health J (2005) 11:719–22. doi: 10.1016/j.molcatb.2005.02.001
34. Salemink E, van den Hout MA, Kindt M. Selective attention and threat: quick orienting versus slow disengagement and two versions of the dot probe task. Behav Res Ther (2007) 3:607–15. doi: 10.1016/j.brat.2006.04.004
35. Le H, Ma S, Cheng X, Gao C, Gan J, Liang X, Zhu X. Attentional bias for negative emotional facial expressions in major depressive disorder. Chin Ment Health J (2009) 11:795–99.
36. Jiang Q, Fei, Gao C, Gan J, Liang X, Zhu X. Reaction time and attention bias characteristics of patients with first-episode depressive disorder. China J Behav Med Brain Sci (2017) 8:699–703. doi: 10.3760/cma.j.issn.1674-6554.2017.08.00
37. Mccabe SB, Gotlib IH, Martin RA. Cognitive vulnerability for depression: deployment of attention as a function of history of depression and current mood state. Cognit Ther Res (2000) 4:427–44. doi: 10.1080/02699930903043461
38. Baert S, De Raedt R, Koster EH. Depression-related attentional bias: the influence of symptom severity and symptom specificity. Cogn Emotion (2010) 6:1044–52. doi: 10.1080/02699931.2016.1146123
39. Disner SG, Shumake JD, Beevers CG. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cognit Emot (2016) 2:1–13.
Keywords: depressive disorder, attentional bias, TORAWARE state, depression, somatic discomfort
Citation: Wang Y, He Y, Wang G, Li J and Zhu H (2019) Correlation Analysis Between Attentional Bias and Somatic Symptoms in Depressive Disorders. Front. Psychiatry 10:903. doi: 10.3389/fpsyt.2019.00903
Received: 15 June 2018; Accepted: 15 November 2019;
Published: 13 December 2019.
Edited by:
Gianluca Serafini, San Martino Hospital (IRCCS), ItalyReviewed by:
Jin Pyo Hong, Sungkyunkwan University, South KoreaPeter Kyriakoulis, Swinburne University of Technology, Australia
Copyright © 2019 Wang, He, Wang, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaohua Wang, d2doNjQwMkAxNjMuY29t; Jiangbo Li, MTAxNTk1MDk3M0BxcS5jb20=; Haibing Zhu, emhiMkAxNjMuY29t
†These authors have contributed equally to this work
 Yun Wang
Yun Wang Yajun He3
Yajun He3 Gaohua Wang
Gaohua Wang