
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry , 06 December 2019
Sec. Molecular Psychiatry
Volume 10 - 2019 | https://doi.org/10.3389/fpsyt.2019.00892
This article is part of the Research Topic The Search for Biomarkers in Psychiatry View all 17 articles
Schizophrenia, a multisystem disorder with an unknown etiology, is associated with several immune dysfunctions, including abnormal levels of circulating cytokines. In this review, we investigated the changes of cytokines in schizophrenic patients, their connection with behavioral symptoms severity and their potential clinical implications. We also assessed the possible causative role of abnormal cytokine levels in schizophrenia pathogenesis. Based on meta-analyses, we categorized cytokines according to their changes in schizophrenic patients into four groups: (1) increased cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1β, IL-12, and transforming growth factor (TGF)-β, (2) non-altered cytokines, including IL-2, IL-4, and IL-17, (3) increased or non-altered cytokines, including IL-8 and interferon (IFN)-γ, and (4) IL-10 with increased, decreased, and non-altered levels. Notably, alterations in cytokines may be variable in four different categories of SP, including first-episode and drug-naïve, first-episode and non-drug-naïve, stable chronic, and chronic in acute relapse. Furthermore, disease duration, symptoms severity, incidence of aggression, and cognitive abilities are correlated with levels of certain cytokines. Clinical implications of investigating the levels of cytokine in schizophrenic patients include early diagnosis, novel therapeutic targets development, patient stratification for choosing the best therapeutic protocol, and predicting the prognosis and treatment response. The levels of IL-6, IL-8, IFN-γ, IL-2 are related to the treatment response. The available evidence shows a potential causative role for cytokines in schizophrenia development. There is a substantial need for studies investigating the levels of cytokines before disease development and delineating the therapeutic implications of the disrupted cytokine levels in schizophrenia.
Schizophrenia, a multisystem disorder with a global prevalence of 0.33–0.75%, is one of the top 15 causes of disability (1–4). The underlying etiology of this disease is controversial and not fully understood. Increased dopamine-based activities, together with decreased glutamatergic signaling, are the main suggested etiological hypotheses (5). Abnormalities in the immune system, which are associated with schizophrenia, are one of the other etiological hypotheses.
The immune system is composed of innate and adaptive immune responses. The innate immunity is a rapid-acting antigen-independent response, while the adaptive immunity is an antigen-dependent defense mechanism, with the ability to memorize the antigens. The immune response is mainly mediated by cytokines, which are mostly produced by a critical component of the adaptive immunity, T-lymphocytes. These mediators can be divided into 5 groups; (1) pro-inflammatory cytokines; interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1 family, and IL-8, which are involved in initiation and aggravating inflammatory responses (6); (2) T-helper 1 cytokines; IL-2, interferon (IFN)-γ, and IL-12, which create a pro-inflammatory response and function in autoimmune diseases and defense against intracellular parasites; (3) T-helper 2 cytokines; IL- 4, IL-5, and IL-13, which counterbalance the effects of T-helper1 cytokines; (4) T-helper 17 cytokines; Il-17, and IL-23, which are chiefly involve in pro-inflammatory processes and defense against extracellular pathogens; (5) T-regulatory cytokines; IL-10 and transforming growth factor (TGF)-β, which primarily suppress immune responses (7, 8).
The associated immune disorders in schizophrenia have been investigated for more than a century (9–11). These abnormalities include increased activity and density of microglia cells, abnormal profiles of peripheral leukocytes, serum cytokines, and cerebrospinal fluid (CSF) cytokines (12, 13). Based on genome-wide association studies, schizophrenia is also associated with specific major histocompatibility complex (MHC) region genes (14). Furthermore, this disease is significantly linked with enhancers having a strong role in the immune functions, even after excluding the MHC region genes (15). These findings support the clinical and genetic aspects of the connection between schizophrenia and the immune system.
One group of components of the immune system affecting the brain by several mechanisms are cytokines which are either produced outside of the central nervous system (CNS) or within the CNS. Peripheral cytokines, presented in the circulation, can access the CNS and affect it via four major ways: (1) binding to specific transporters, (2) stimulating afferent vagal fibers, (3) accessing areas such as circumventricular organs, and (4) passing the damaged blood-brain barrier (BBB) which has an increased permeability (16, 17). Notably, elevated levels of the peripheral markers of BBB damage, such as S100B, indicate BBB damage in schizophrenic patients (18). In addition to the peripheral cytokines, microglia, astrocytes, endothelial cells, and even neurons can produce different cytokines within the CNS (17, 19). Moreover, despite the prevailing view that the brain is an immune-privileged area, several studies have shown that immune responses can be established within CNS by several mechanisms, one of which is transferring the immune cells located within the meninges, which are sources of different immune mediators, into the brain’s parenchyma in a pathologic state. These cells physiologically transfer through the blood-meningeal barrier in order to pass the meninges as it is more permeable than BBB (20, 21).
These cytokines can have various roles in neurodevelopment, neuroendocrine activities, and neurotransmission. Their role in neurodevelopment is mainly through affecting microglia which are the chief cells responsible for this task (11, 16, 17, 19). Cell migration, water balance, body temperature regulation and synthesis and release of neurotransmitters can be influenced by these mediators (16).
A role for cytokines in schizophrenia was proposed almost three decades ago (22, 23). Ever since, an increasing number of studies investigated alterations of cytokine levels in schizophrenic patients, their changes following antipsychotic treatment, and their relationship with clinical manifestations. However, not only are the results of these studies controversial, but they also do not clearly answer whether the changes in the levels of these cytokines can have a causative role in the development of psychotic symptoms. Moreover, diagnostic and therapeutic implications of these alterations are not defined.
This review aims to answer four questions: A) What are the changes in the serum levels of cytokines in schizophrenic patients? B) What is the relationship between the levels of cytokines and the severity of clinical symptoms? C) How antipsychotics affect the baseline levels of cytokines? and D) What is the potential role of cytokines as predictors of treatment response?
Using the answers to these questions, we investigate whether abnormal cytokine levels are the culprit in the pathogenesis of schizophrenia and also provide clinical implications in terms of diagnosis and treatment.
A considerable number of studies, including meta-analyses, found increased levels of IL-6 in different groups of patients including, first-episode and drug-naive (FEDN) psychosis patients and cases with first-episode psychosis (FEP), majority of whom were using antipsychotics (24–28). Similar findings were observed in chronic patients, including those without any significant inflammation (29), patients in an acute relapse or recovering from it, and stable outpatients (27, 30–32). Recently, Hartwig et al. found increased levels of soluble IL-6 receptors in a clinical two-sample Mendelian randomization study, which can be explained as a compensatory response to the increased levels of IL-6 in schizophrenia (33).
Conversely, some studies found no significant changes in the levels of IL-6 in schizophrenic patients (34, 35).
Multiple studies, including meta-analyses, reported elevated levels of TNF-α, one of the other pro-inflammatory cytokines, in FEDN patients (24, 25), (both adult and pediatric) FEP patients, majority of whom were using antipsychotics (26, 27, 36) and chronically ill patients using antipsychotics, regardless of their status in terms of acute relapse (24, 37). Higher levels of TNF-α have also been reported in chronic patients taking atypical antipsychotics having no major inflammation (29).
On the contrary, Potvin et al. conducted a meta-analysis and found no significant alteration in the levels of TNF-α in in vivo and in vitro studies (30). Their finding may be explained by the scarcity of studies until 2005. Decreased levels of TNF-α are seen in FEDN patients with a disease duration of under two years (37) and chronic patients with a disease duration of more than five years taking typical and atypical antipsychotics (38, 39).
Regarding the next pro-inflammatory cytokine, IL-8, despite meta-analyses supporting elevated levels of it in FEP patients (27) and chronic patients who are stable or are experiencing an acute relapse or are recovering from one (24, 32), a recent meta-analysis showed no significant alterations in the levels of IL-8 in FEP patients versus HC’s (32). This is concordant with the findings of another study in FEP patients, most of whom were medicated (36). Concordantly, in a study in which less than a quarter of patients were taking benzodiazepine and others were drug-naive, except for obese cases who had increased levels of IL-8, other patients showed no significant alterations in the IL-8 level (40).
IL-1 family is one of the major pro-inflammatory cytokines, one of the members of which is IL-1β. A large number of studies, including several meta-analyses, found elevated levels of IL-1ß in FEDN patients (25) and adult and pediatric FEP patients, majority of whom were taking atypical antipsychotics (26, 28, 36), and chronically ill patients who were stable or were experiencing an acute relapse or were recovering from one (24, 27, 32, 37). Interestingly, it seems that the levels of IL-1ß mRNA do not change in FEP patients (36).
In contrast to a substantial number of studies suggesting an increase in the levels of IL-1ß, the meta-analysis conducted by Potvin et al. in 2008 found no significant alterations in the levels of IL-1ß in in vivo and in vitro studies (30). No significant elevation has been reported in chronic patients with a disease period of more than six years, either (41). Interestingly, a recent study has found decreased levels of IL-1ß in FEDN patients with a disease period of shorter than 2 years (37).
Other members of the IL-1 family may be elevated in schizophrenic patients as well. The levels of IL-1α and its leukocyte mRNA were found to be higher in FEP patients who were mostly medicated (36). However, other investigators did not find the same pattern in long-term chronically ill patients (41). A recent study has reported increased levels of IL-33, one of the other members of IL-1 family, and its soluble receptor (sST2) in FEDN patients and patients in an acute relapse compared to patients in remission or HC’s (42). Several studies, including a number of meta-analyses, reported increased levels of IL-1 receptor antagonist (IL-1RA) in patients with first-episode psychosis, whether most of the patients used antipsychotics (43) or whether they were drug-naive (40), in chronic patients who were experiencing an acute relapse or had multiple episodes of schizophrenia (24, 27, 32), and in in vitro studies (30).
According to the majority of studies (including meta-analyses), the levels of IL-2, one of the cytokines produced by T-helper 1, do not alter in schizophrenic patients. These studies were performed on FEDN patients, FEP patients (with a history of using antipsychotics in many of them), and chronic patients who were experiencing an acute relapse, were recovering from it or were stable (24, 25, 27, 35, 36).
However, several studies found contradictory results. Some of these studies reported increased levels of IL-2 in FEDN patients with a normal BMI (44), FEP patients, majority of whom were using atypical antipsychotics (28), and chronic patients with stable antipsychotic medication regimens combined with a disease period of more than six years (41). Furthermore, some in vitro studies found a substantial decrease in the levels of IL-2 (30). Studies also found a decrease in the mRNA levels of this cytokine in the peripheral blood of chronically ill patients who were using antipsychotics for at least a year (45).
Moreover, several studies (including meta-analyses) found a significant increase in the levels of soluble IL-2 receptor (sIL-2R), levels of which might affect the level of IL-2 by binding with it, in FEDN patients and chronic patients who were stable or were experiencing an acute relapse or were recovering from one (24, 25, 27).
Alterations in IFN-γ, the next T-helper 1 cytokine, levels are very controversial. The correlation between IFN-γ and BMI, previously found in patients with first-episode schizophrenia, may explain this inconsistency (32). Due to this great controversy, the reported changes in the levels of this cytokine in each group of patients are discussed separately.
In FEDN patients, several studies, including a meta-analysis, found no significant alterations in the levels of IFN-γ (40, 46–49) while some other studies, including another meta-analysis, showed increased levels of IFN-γ (24, 50). Conversely, Reale et al. found decreased levels of IFN-γ in FEDN patients (51).
Studies showed elevated levels of this cytokine in adult or pediatric FEP patients, most of whom had a history of using antipsychotic medications (26–28). However, Di Nicola et al. found non-altered levels in adult FEP patients, most of whom were on atypical antipsychotics (36).
Several studies, including some meta-analyses, found elevated levels of IFN-γ in chronic schizophrenic patients who were stable or were experiencing an acute relapse (24, 27, 32). However, no significant disruption was found in patients recovering from an acute relapse (27, 46). Decreased levels of IFN-γ were also reported in patients with acute psychotic symptoms who were drug-naive for at least 6 months (52) and in chronic patients. The details of the medications that were used were not reported in these studies (53, 54).
Investigation of the alterations in the levels of IL-12, one of the other T-helper 2 cytokines, showed that several studies (including meta-analyses) found elevated levels in FEP patients, whether drug-naive or not, and chronic patients who were stable or were experiencing an acute relapse, or were recovering from one (24, 27, 55). In the study by Bedrossian et al., the patients were treated with clozapine for at least one year (55).
In contrast to the studies reporting elevated levels of IL-12, some other studies reported non-altered levels of this cytokine in FEP (28, 43), and in chronic patients who had a history of using antipsychotic medications (32, 45).
Compared to other T-helper 2 cytokines, there are a larger number of studies performed on the alterations of IL-4. Several studies, including two meta-analyses, found no major differences in the levels of IL-4 between HC’s and FEDN or FEP patients, majority of whom were taking atypical antipsychotics, or chronic patients who had schizophrenia for a long time (including treatment-resistant patients) (25, 27, 30, 36, 48, 56). However, like other cytokines, a small number of studies reported inconsistent results. Decreased levels of IL-4 were reported in chronic patients experiencing an acute relapse, during their treatment after the relapse (supported by a meta-analysis) (27), and in stable chronic patients (41, 57). In addition, elevated levels were reported in FEP pediatric patients taking antipsychotics (26) and in adult chronic patients taking clozapine (58).
Few studies have investigated other T-helper 2 cytokines, including IL-5 and IL-13. Increased levels of IL-5 were found in chronic adult patients with multiple episodes of unsuccessful treatment and FEP pediatric patients who were mostly taking antipsychotics (26, 59). Similarly, the levels of IL-13 have been reported to be elevated in adults with multiple episodes of schizophrenia (32, 59).
The literature is inconsistent about IL-17 alterations in schizophrenic patients. A recent meta-analysis found no significant changes in the levels of IL-17 in FEDN patients (60). Non-disturbed levels are also reported in chronic patients experiencing an acute relapse (46).
On the contrary, some studies reported increased levels of this cytokine in FEDN (50) and chronic hospitalized patients who were medication free for at least four weeks (61).
However, decreased levels have also been reported in FEDN patients (46) and in chronic patients using different antipsychotics (62).
One of the other main T-helper 17 cytokines is IL-23, which has been reported to be elevated in FEDN and chronic patients in an acute relapse (8, 61, 63).
Goldsmith et al. found reduced levels of IL-10 in FEP patients and chronic patients who were in an acute relapse in their meta-analysis (27). Decreased levels are also reported in FEDN patients (64).
However, this meta-analysis showed that the levels of this cytokine do not change in stable chronic patients (27, 45). Other studies have reported no significant alterations in the levels of IL-10 in comparison with HC in FEDN (44), FEP (36), and chronic patients experiencing an acute relapse (65).
Conversely, many investigators have reported elevated levels of this cytokine in FEDN (49, 66), FEP (26), and chronic patients (41, 57, 67).
Several studies, including meta-analyses, found elevated levels of TGF-β in FEDN, FEP patients (27, 46), and in chronic patients experiencing an acute relapse (47).
However, some studies found other findings. Non-disturbed levels of TGF-β have been detected in FEDN and chronic patients who were medication free for four months before the study (18, 44). Interestingly, some studies found decreased levels of TGF-β in chronic treatment-resistant patients (56).
A summary of alterations in the levels of cytokines in schizophrenia based on the meta-analyses is shown in Table 1.
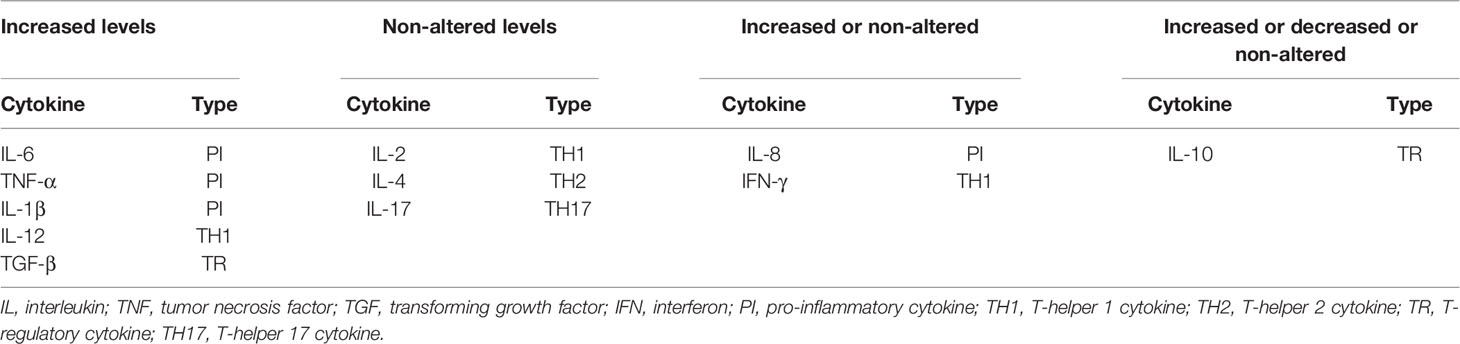
Table 1 Summary of alterations in serum levels of cytokines in schizophrenia, based on meta-analyses [18, 20, 21).
The levels of cytokines seem to be correlated with both the disease duration and symptom severity. Patients with elevated levels of IL-6, IL-8, and IlL-4 tend to have a longer disease duration and longer hospitalizations (31, 65, 68).
Furthermore, higher levels of IL-6, IL-1ß, IL-33, and IL-17 are associated with more severe positive symptoms (28, 42, 50, 69, 70). In chronic patients using a stable dose of antipsychotics, decreased levels of TNF-α are similarly associated with more severe positive symptoms (38, 71), while no correlation has been found in FEDN patients (37).
Exacerbated negative symptoms are seen in patients with elevated levels of IL-6, TNF-α, IL-1ß, IL-8, IFN-γ, IL-4, and TGF-β as well as patients with decreased levels of IL-2 and IL-17 (50, 61, 64, 65, 72–75). Interestingly, the correlation between TNF-α and IL-1ß and negative symptoms is only seen in chronic patients and is not reported in FEDN patients (37). Additionally, in FEDN patients, the levels of IL-10 are negatively correlated with negative symptoms, while in chronic patients, they are positively correlated with these symptoms (54, 76).
Increased levels of IL-6, IL-33, sIL-2R, IL-17, and TGF-β are positively correlated with PANSS (positive and negative syndrome scale) general psychopathology sub-score (42, 50, 61, 65). PANSS is a widely used tool to determine the severity of the psychotic symptoms. It is a clinical interview assessing the severity of positive symptoms, negative symptoms, and general psychopathology in schizophrenic patients via 30 items. Higher scores indicate more severe conditions (77). The total PANSS score is positively correlated with the levels of IL-6, sIL-2R, IL-1β, IFN-γ, IL-13, TGF-β1 and IL-17 (61, 65, 78, 79). Interestingly, the levels of IL-6 and IL-17 correlate with the total score in both chronic and FEDN patients, while the levels of IFN-γ correlate with the total score only in FEDN patients (50). Moreover, only in chronic patients, decreased levels of TNF-α are associated with higher general and total sub-scores, and no association has been reported in FEDN patients (37, 38, 71).
Regarding the correlation between IL-8 and severity of symptoms, Dahan and his colleagues did not find any association between the levels of IL-8 and PANSS sub-scores. Instead, they reported that patients with higher levels of IL-8 had higher scores of the Clinical Global Impression (CGI) severity scale (a subjective assessment tool to determine the severity of the mental illness by clinicians) (65, 80). However, the levels of IL-8 are reported to correlate with the PANSS mean score positively (79).
The levels of cytokines may be relevant to behavior disorders as well. Surprisingly, worse cognitive abilities are associated with higher levels of IL-6, IL-1RA, IL-33, and IL-12 or lower levels of TNF-α in chronic patients, and with lower levels of IL-10 in FEDN patients (31, 38, 64, 81–86). Moreover, patients with higher levels of IL-10 had a higher total score of the RBANS (Repeatable Battery for the Assessment of Neuropsychological Status) and a worse performance in the attention domain (83, 87). Furthermore, better performance on the memory and intelligence tests has been reported to be associated with higher levels of IL-2 (72). Aggressive behavior is more common among patients with higher levels of IL-17 and IL-10 (54, 61, 76). In addition, in FEDN schizophrenic patients, depressive behaviors are more prevalent among those who have higher levels of IL-4 and TNF-α (79).
Table 2 summarizes the relationship between levels of various cytokines and severity of clinical symptoms.
Shortly (up to 2 months) after treatment with typical or atypical antipsychotics (such as risperidone), the levels of IL-6 and IL-1β seem to decrease (16, 88, 89). However, in the long term, their levels either rise or do not change compared to the baseline levels (70, 90–92).
The increasing trend of IL-6 and Il-1β can be explained by antipsychotics (especially atypical antipsychotics) side effects such as metabolic syndrome in the long period (93), as increased levels of IL-6, IL-1β, TNF-α, IL-2, IFN-γ, and IL-4 have been reported in patients with metabolic syndrome (94, 95).
After using typical or atypical antipsychotics for up to two months, the levels of TNF-α and IL-8 do not change (24, 70, 89, 91, 96). The IL-8 levels seem to remain unchanged even after three months of therapy with risperidone and haloperidol (90). However, one study found that taking typical or atypical antipsychotics or a combination of them in FEDN patients for seven months causes a significant decrease in the level of IL-8 while patients’ BMI also increased (97). Furthermore, it has been reported that the levels of TNF-α significantly increase following taking risperidone for more than three months (possibly because of its side effects resulting in induction of metabolic syndrome) (92), or after taking adjunct mood stabilizers with typical or atypical antipsychotics for an average of six weeks of treatment (98). Interestingly, Amisulpride seems to decrease the levels of TNF-α after six weeks of treatment (52).
The level of the anti-inflammatory cytokine, IL-1RA, is reported to decrease following 6 weeks of treatment with olanzapine or risperidone (48) or 8 weeks of antipsychotic therapy adjusted with patients’ clinical status (99). Nonetheless, these studies are in contrast with meta-analyses that found no significant alterations in the levels of this cytokine after an average of eight weeks of treatment (70, 91).
The levels of IL-2 seem to decrease in the first month after antipsychotic therapy (atypical or typical antipsychotics or mixed) (89). Particularly, olanzapine and haloperidol decrease the level of IL-2 significantly (70). However, studies with longer average treatment periods found no significant changes in the level of IL-2 (24, 70, 91).
Moreover, the levels of IL-12 seem to significantly increase following the use of risperidone for 7–8 weeks (24, 91). Similarly, an elevation in the levels of IL-12 has been reported after six weeks of treatment with olanzapine and haloperidol (100). Surprisingly, aripiprazole (a third-generation antipsychotic) seems to decrease IL-12 levels in chronic patients after four weeks of treatment (101). This is in contrast with the results of a meta-analysis of studies with a treatment period of 4–10 weeks using typical or atypical antipsychotics that found no significant changes in the level of IL-12 after medication (70).
IFN-γ levels do not significantly change in the first month following antipsychotic treatment (89). However, findings after antipsychotic therapy for an average of approximately 2 months are inconsistent. Two meta-analyses found decreased levels following treatment with typical or atypical or mixed antipsychotics while a meta-analysis by Miller et al., in which more than half of the included studies had non-standardized antipsychotic treatment, suggested the levels of IFN-γ remained constant in this period (16, 70, 91). Olanzapine seems to be the main medication that decreases IFN-γ levels (70). Interestingly, assessing the effect of atypical antipsychotics for 3 months revealed increased levels of IFN-γ, which may be because of their side effects such as metabolic disorder (73).
Several meta-analyses have confirmed no disturbances in the level of IL-4 after an average of two months of antipsychotic treatment (70, 91). In contrast, some studies found that treatment with typical or atypical antipsychotics or a combination of them for 1 month or treatment with risperidone for 10 weeks led to decreased levels of IL-4 (102, 46, 68). Thus, IL-4 might be a trait marker that decreases at first but then increases after a certain time due to the metabolic side effects of antipsychotics, which can result in normal levels in the long term. However, to the best we know, no meta-analysis has evaluated the short-term effects. Furthermore, it has been reported that treatment with atypical antipsychotics for eight weeks causes a significant reduction in the levels of IL-13 (78).
IL-17 and IL-23 seem to be a trait marker whose level does not change after one month of treatment with typical or atypical antipsychotics or a combination of them compared to the baseline level (47, 63, 89). Assessing the effect of risperidone for 10 weeks has shown the same result (102). Interestingly, one study found that a 4-week treatment course with risperidone decreased the number of T-h17 cells while it had no significant effects on the IL-17 levels (50).
The levels of IL-10 do not change following an average of 8 weeks of treatment compared to the baseline levels (70, 91). However, it has been reported that in chronic patients, treatment with aripiprazole, risperidone, or clozapine for 4–6 weeks increased the IL-10 levels (101, 103). The results of treatment with atypical antipsychotics, particularly risperidone, in FEDN patients are different. In these patients, the levels of IL-10 are lower after treatment compared to baseline. Similarly, an average of eight weeks of treatment does not cause a significant alteration in the level of TGF-β (70, 91). However, a meta-analysis by Miller et al., in which more than half of the studies had non-standardized antipsychotic treatment, found decreased levels of TGF-β after 8 weeks of therapy (24). Four weeks of treatment with aripiprazole in chronic patients and 6 weeks of therapy, mostly with atypical antipsychotics in FEDN patients are associated with decreased levels of TGF-β as well (101, 104). However, elevated levels of TGF-β have been reported after four weeks of typical or atypical or mixed antipsychotic treatment in FEDN patients (46).
There is a growing body of evidence on the clinical implications of cytokines in schizophrenia. Considering the lack of predictor biomarkers of the treatment response in psychosis, one of the suggested applications is using cytokines to predict response to treatment (73). Furthermore, the relationship between cytokine levels and response to treatment can support the hypothesis that cytokines may play a role in schizophrenia pathogenesis.
Increased levels of IL-6 and IFN-γ are associated with treatment resistance (105, 73). Treatment resistance is defined by not achieving the remission criteria proposed by the Schizophrenia Working Group Consensus (106) or Kane’s criteria (107). Patients with higher levels of IL-8 and IL-2 experience less improvement in PANSS compared to other patients after twelve weeks of therapy with risperidone or haloperidol (90). Moreover, even though the level of TGF-β is not related to treatment response, TGF-ß1 polymorphism is reported to be associated with PANSS score improvement after antipsychotic treatment (108).
The results showed that schizophrenic patients had an inflammatory cytokine profile and imbalanced T-helper 1, T-helper 2, and regulatory cytokines. The severity of symptoms and abnormal behaviors in addition to antipsychotic therapies may affect abnormal cytokine levels in these patients. However, there were significant inconsistencies regarding the cytokine profile in the reviewed studies in schizophrenic patients. Six reasons may explain these controversies: 1) Diverse Patients’ characteristics; 2) Different sampling methods; 3) Heterogeneous patient populations; 4) The difference between cytokine profile of plasma, serum or whole blood; 5) Different specifications of assay kits; 6) Small sample size.
1. Diverse Patients’ characteristics: Several studies did not evaluate factors such as the BMI, diet, diurnal rhythm, smoking habits, psychological stress, and lifestyle in their patients, which may affect the cytokines profile (109–112). However, elevated levels of IL-6 are reported in a study excluding patients with a body mass index (BMI) > 25, which gave rise to the conclusion that the changes of IL-6 did not seem to be related to obesity (44).
2. Different sampling methods: The method and duration of storage and the used anticoagulant may affect the measurement of levels of cytokines. Moreover, as the levels of cytokines are influenced by the circadian pattern, the time of sampling is of great importance. Mornings are suggested to be the best time to take samples (113).
3. Heterogeneous patient populations: In some studies, FEDN patients were not separated from patients with a history of antipsychotic treatment. Not only do antipsychotics affect cytokine levels, but they can also cause weight gain, which is considered a low-grade inflammation (93). Thus, studies in a FEDN population are more reliable.
4. The difference between cytokine profile of plasma, serum or whole blood: The cytokine profile of each of these can be different from the other ones as the coagulation process may trigger release of some inflammatory cytokines (113).
5. Different specifications of assay kits: The quality of antibody used in ELISA kits, kit manufacture, and the operator’s skills may affect the measurement (113). For example, Hope et al. indicated that different assay kits could affect the measurement of serum levels of different cytokines. In their study, the IL-6 level was higher than the detection limit of the kit in more than half of the samples (82).
6. Small sample size: A considerable number of the studies had small sample sizes (less than 30 participants in each of their groups) that limited their statistical power (54, 114).
The question is whether abnormalities in cytokine levels have a causative role in schizophrenia or are merely associated with the disease? We used the Bradford Hill criteria (115, 116) to answer this question. These criteria assess nine factors, including the strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, analogy, and experimental effect to differentiate causality from association. As for the strength of association, the levels of several cytokines (specifically pro-inflammatory cytokines) are found to be significantly disturbed in schizophrenic patients. The second criterion, consistency, is not met in most of the cytokine level alterations. These changes are not specific either and can cause a wide variety of diseases. However, in the modern context, specificity is a less important factor in determining or refusing causality (116). Temporality, as the next criterion, was assessed by only a few studies. In two studies that measured the levels of cytokines before schizophrenia presentation, individuals who developed the disease in the following years had higher baseline levels of IL-6 compared to others (117, 118). This association is strengthened by the study of Khandaker et al. in 2018, finding a strong association between a genetic variant of IL-6 receptor relating to the levels of IL-6 and CRP and development of schizophrenia (119). Moreover, individuals with higher levels of inflammatory markers, including ESR (erythrocyte sedimentation rate) and CRP (C-reactive protein), were more likely to develop schizophrenia (120, 121). The severity of the psychotic symptoms correlated with abnormalities in the levels of cytokines, particularly pro-inflammatory cytokines. So, the biological gradient criterion was met. Regarding plausibility and coherence, there are three suggested major ways by which pro-inflammatory cytokines can contribute to schizophrenia development. First, they can increase kynurenic acid (a metabolite of tryptophan) formation. This metabolite function as an N-Methyl-D-aspartate (NMDA) receptor antagonist, which based on the glutamate hypothesis of schizophrenia (122), might play a causative role in schizophrenia together with decreased glutamatergic signaling. Second, these cytokines increase oxidative stress leading to increased neurodegeneration, which can be seen in schizophrenia. Third, pro-inflammatory cytokines may disturb neurodevelopment (particularly when there is a prenatal inflammation), increasing the risk of psychosis (12, 123). Furthermore, the interplay of cytokines and neurotransmitters may be one of the mechanisms by which cytokines can play a causative role in schizophrenia. Inflammatory cytokines can affect synthesis of monoamine neurotransmitters, increase reuptake of dopamine, serotonin, and norepinephrine, and influence on the release of neurotransmitters (16). Increased levels of soluble IL-6 receptors can be explained as a compensatory response to increased levels of IL-6 (33), and elevated levels of TNF-α mRNA suggest systemic blood immune cells as the main source of higher levels of TNF-α (36). These two findings provide more experimental evidence for this causation. In terms of analogy, disrupted cytokine levels can be seen in depression and bipolar disorder (114). There is considerable evidence supporting the causal role of cytokines in depression (124). Finally, decreasing inflammation using adjuvant anti-inflammatory agents is reported to improve the symptoms in schizophrenic patients. Consequently, the experimental effect criterion was met as well (125, 126).
The association between cytokine alterations and schizophrenia may have potential clinical implications. Currently, the diagnosis of schizophrenia is based on clinical symptoms, and there is no standard diagnostic biomarker that allows early recognition of this disease, while cytokines such as IL-6, TNF-α, IL-1ß, and IL-RA can be used as potential biomarkers for early detection of at least a subgroup of schizophrenic patients (118, 127, 128). Furthermore, IL-6, sIL-2r, TNF-α, IL-1RA, and IL-4t can also be useful in detecting the acute-relapse phase of disease (27, 46).
Targeting inflammatory pathways may lead to new treatment options in schizophrenia. A growing body of evidence supports the effect of immune modulators on ameliorating the symptoms of schizophrenia. (12, 129). A recent meta-analysis showed that a variety of medications can reduce the severity of symptoms of schizophrenia. These medications are aspirin (by reducing inflammation by modifying cyclooxygenase-2 enzyme), estrogens (through immunomodulatory effects), minocycline (by inhibiting microglia), and N-acetylcysteine (as an anti-inflammatory agent) (130).
Prediction of response to treatment is another application of cytokine levels. Patients with increased levels of IL-6, IL-8, IFN-γ, and soluble TNF-α receptor 1 and decreased levels of IL-2 exhibit less improvement after standard antipsychotic therapy (73, 90, 131). Moreover, patients with higher levels of CRP, an inflammatory marker, tend to have lower quality of life (132).
It is possible that patients who have higher levels of pro-inflammatory cytokines benefit more from adding specific drugs to the standard therapeutic regimen. Stratifying patients on this basis can potentially help the physicians choose the best treatment option. A randomized clinical trial study in treatment-resistant depressive patients supports this hypothesis. Depression is also associated with a disrupted cytokine profile. This study showed that in a subgroup of patients who had higher baseline inflammation, the use of infliximab—a TNF-α antagonist—led to better results after the treatment. However, infliximab had no significant effects on another subgroup of patients (133).
Although current studies provide a wealth of information on the cytokines profile in schizophrenic patients, several major shortcomings cannot be overlooked. There is a substantial need for longitudinal studies investigating the levels of cytokines before the development of clinical manifestations of schizophrenia in individuals with a strong positive family history of schizophrenia. Moreover, the potential confounding factors such as age, sex, smoking, obesity, and individuals’ diet should be rigorously controlled in the future studies (134).
More studies also need to be performed on the diagnostic and the prognostic applications of measurement of levels of cytokines. The relationship between severity of symptoms and levels of cytokines in FEDN patients can be one of the examples of the diagnostic applications. Identifying treatment-resistant patients based on their cytokines profile can be noted as the prognostic applications, on which more studies are needed. Last but not the least, future studies should be based on defined categories of schizophrenic patients.
Lastly, there is a significant shortcoming in the studies that investigate the therapeutic applications. As an example, selection of patients for adjuvant therapy with medications targeting immune modulatory pathways can be guided by inflammatory cytokine levels (135). Moreover, the role of adjuvant monoclonal antibody immunotherapy, which in contrast to NSAIDs only targets immune pathways, needs to be more investigated (134).
This was a narrative review. Therefore, all the limitations of narrative reviews may apply to this study (136).
Schizophrenia is associated with several abnormalities in the levels of cytokines, which are the mediators of the immune system. A deeper understanding of this association can be useful in clinical practice in terms of early diagnosis and treatment.
SM developed the concept and design, collected the data, drafted the article, critically revised the manuscript for important intellectual content, and approved the final version. AZ-S critically revised the manuscript for important intellectual content and approved the final version. NR supervised the project, critically revised the manuscript for important intellectual content, and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Saha S, Chant D, Welham J, Mcgrath JJPM. A systematic review of the prevalence of schizophrenia. PloS Med (2005) 2:e141. doi: 10.1371/journal.pmed.0020141
2. Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, Mcgrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry (2014) 71:573–81. doi: 10.1001/jamapsychiatry.2014.16
3. Moreno-Kustner B, Martin C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PloS One (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687
4. Pillinger T, D'ambrosio E, Mccutcheon R, Howes OD. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry 24(6):776–794. doi: 10.1038/s41380-018-0275-2
5. Tamminga C. Schizophrenia and other psychotic disorders: Introduction and overview. In: Sadock VA, Sadock BJ, Ruiz P. Md, editors. Kaplan and Sadock"s Comprehensive Textbook of Psychiatry, tenth ed. Wolters Kluwer Health. Philadelphia: Lippincott Williams & Wilkins (2017). 3613–7 p.
7. Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol (2011) 7(Suppl 1):S1. doi: 10.1186/1710-1492-7-S1-S1
8. Debnath M, Berk M. Functional implications of the IL-23/IL-17 immune axis in Schizophrenia. Mol Neurobiol (2017) 54:8170–8. doi: 10.1007/s12035-016-0309-1
9. Heath RG. Proceedings of the annual meeting of the American Psychopathological Association. In: Schizophrenia: evidence of a pathologic immune mechanism. United States: Baltimore Md: Johns Hopkins University Press (1969). 234–52 p.
10. Delisi L. Is immune dysfunction associated with schizophrenia? A review of the data. Psychopharmacol Bull (1984) 20:509–13.
11. Khandaker GM, Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacol (Berl) (2016) 233:1559–73. doi: 10.1007/s00213-015-3975-1
12. Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry (2015) 2:258–70. doi: 10.1016/S2215-0366(14)00122-9
13. Muller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci (2015) 9:372. doi: 10.3389/fnins.2015.00372
14. Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature (2009) 460:744–7. doi: 10.1038/nature08186
15. Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature (2014) 511:421–7. doi: 10.1038/nature13595
16. Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety (2013) 30:297–306. doi: 10.1002/da.22084
17. Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci (2014) 68:21–36. doi: 10.1111/pcn.12089
18. Hong W, Zhao M, Li H, Peng F, Wang F, Li N, et al. Higher plasma S100B concentrations in schizophrenia patients, and dependently associated with inflammatory markers. Sci Rep (2016) 6:27584. doi: 10.1038/srep27584
19. Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol (2012) 33:116–25. doi: 10.1016/j.yfrne.2011.12.002
20. Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol (2013) 13:206–18. doi: 10.1038/nri3391
21. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest (2017) 127:3210–9. doi: 10.1172/JCI90603
22. Libikowa H, Stancek D, Wiedermann V, Hasto J, Breier S. Psychopharmaca and electroconvulsive therapy in relation to viral antibodies and interferon. Experimental and clinical study. Arch Immunol Ther Exp (Warsz) (1977) 25:641–9.
23. Smith RJMH. A comprehensive macrophage-T-lymphocyte theory of schizophrenia. Med Hypotheses (1992) 39:248–57. doi: 10.1016/0306-9877(92)90117-U
24. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick BJBP. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
25. Upthegrove R, Manzanares-Teson N, Barnes NMJSR. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res (2014) 155:101–8. doi: 10.1016/j.schres.2014.03.005
26. Falcone T, Carlton E, Lee C, Janigro M, Fazio V, Forcen FE, et al. Does systemic inflammation play a role in pediatric psychosis? Clin Schizophr Relat Psychoses (2015) 9:65–78B. doi: 10.3371/CSRP.FACA.030813
27. Goldsmith D, Rapaport M, Miller BJMP. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry (2016) 21:1696. doi: 10.1038/mp.2016.3
28. Lesh TA, Careaga M, Rose DR, Mcallister AK, Van De Water J, Carter CS, et al. Cytokine alterations in first-episode schizophrenia and bipolar disorder: relationships to brain structure and symptoms. J Neuroinflammation (2018) 15:165. doi: 10.1186/s12974-018-1197-2
29. Al-Hakeim HK, Al-Rammahi DA, Al-Dujaili AH. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J Affect Disord (2015) 182:106–14. doi: 10.1016/j.jad.2015.04.044
30. Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi EJBP. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry (2008) 63:801–8. doi: 10.1016/j.biopsych.2007.09.024
31. Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci (2015) 265:449–59. doi: 10.1007/s00406-014-0533-5
32. Frydecka D, Krzystek-Korpacka M, Lubeiro A, Stramecki F, Stanczykiewicz B, Beszlej JA, et al. Profiling inflammatory signatures of schizophrenia: a cross-sectional and meta-analysis study. Brain Behav Immun (2018) 71:28–36. doi: 10.1016/j.bbi.2018.05.002
33. Hartwig FP, Borges MC, Horta BL, Bowden J, Smith GDJJP. Inflammatory biomarkers and risk of schizophrenia: a 2-sample mendelian randomization study. JAMA Psychiatry (2017) 74:1226–33. doi: 10.1001/jamapsychiatry.2017.3191
34. Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord (2009) 11:726–34. doi: 10.1111/j.1399-5618.2009.00757.x
35. Wei L, Du Y, Wu W, Fu X, Xia QJJOaD. Elevation of plasma neutrophil gelatinase-associated lipocalin (NGAL) levels in schizophrenia patients. J Affect Disord (2018) 226:307–12. doi: 10.1016/j.jad.2017.10.002
36. Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, et al. Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun (2013) 31:90–5. doi: 10.1016/j.bbi.2012.06.010
37. Zhu F, Zhang L, Liu F, Wu R, Guo W, Ou J, et al. Altered serum tumor necrosis factor and interleukin-1ß in first-episode drug-naive and chronic schizophrenia. Front Neurosci (2018) 12:296. doi: 10.3389/fnins.2018.00296
38. Lv MH, Tan YL, Yan SX, Tian L, Tan SP, Wang ZR, et al. Decreased serum TNF-alpha levels in chronic schizophrenia patients on long-term antipsychotics: correlation with psychopathology and cognition. Psychopharmacol (Berl) (2015) 232:165–72. doi: 10.1007/s00213-014-3650-y
39. Turhan L, Batmaz S, Kocbiyik S, Soygur AHJNJOP. The role of tumour necrosis factor alpha and soluble tumour necrosis factor alpha receptors in the symptomatology of schizophrenia. Nord J Psychiatry (2016) 70:342–50. doi: 10.3109/08039488.2015.1122079
40. Lin Y, Peng Y, He S, Xu J, Shi Y, Su Y, et al. Serum IL-1ra, a novel biomarker predicting olanzapine-induced hypercholesterolemia and hyperleptinemia in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2018) 84:71–8. doi: 10.1016/j.pnpbp.2018.01.020
41. Balõtšev R, Koido K, Vasar V, Janno S, Kriisa K, Mahlapuu R, et al. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur Psychiatry (2017) 39:1–10. doi: 10.1016/j.eurpsy.2016.05.010
42. Borovcanin MM, Janicijevic SM, Jovanovic IP, Gajovic N, Arsenijevic NN, Lukic ML. IL-33/ST2 Pathway and Galectin-3 as a new analytes in pathogenesis and cardiometabolic risk evaluation in psychosis. Front Psychiatry (2018) 9:271. doi: 10.3389/fpsyt.2018.00271
43. Zhou Y, Peng W, Wang J, Zhou W, Zhou Y, Ying BJP, et al. Plasma levels of IL-1Ra is associated with schizophrenia. Psychiatry Clin. Neurosci. (2018) 73: 109–15. doi: 10.1111/pcn.12794
44. Petrikis P, Voulgari PV, Tzallas AT, Archimandriti DT, Skapinakis P, Mavreas VJJOPR. Cytokine profile in drug-naive, first episode patients with psychosis. J Psychosom Res (2015) 79:324–7. doi: 10.1016/j.jpsychores.2015.06.011
45. Boerrigter D, Weickert TW, Lenroot R, O’donnell M, Galletly C, Liu D, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation (2017) 14:188. doi: 10.1186/s12974-017-0962-y
46. Borovcanin M, Jovanovic I, Radosavljevic G, Dejanovic SD, Bankovic D, Arsenijevic N, et al. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res (2012) 46:1421–6. doi: 10.1016/j.jpsychires.2012.08.016
47. Borovcanin M, Jovanovic I, Radosavljevic G, Dejanovic SD, Stefanovic V, Arsenijevic N, et al. Antipsychotics can modulate the cytokine profile in schizophrenia: attenuation of the type-2 inflammatory response. Schizophr Res (2013) 147:103–9. doi: 10.1016/j.schres.2013.03.027
48. De Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res (2014) 154:23–9. doi: 10.1016/j.schres.2014.02.005
49. Noto C, Ota VK, Santoro ML, Ortiz BB, Rizzo LB, Higuchi CH, et al. Effects of depression on the cytokine profile in drug naive first-episode psychosis. Schizophr Res (2015) 164:53–8. doi: 10.1016/j.schres.2015.01.026
50. Ding M, Song X, Zhao J, Gao J, Li X, Yang G, et al. Activation of Th17 cells in drug naïve, first episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2014) 51:78–82. doi: 10.1016/j.pnpbp.2014.01.001
51. Reale M, Patruno A, De Lutiis MA, Pesce M, Felaco M, Di Giannantonio M, et al. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci (2011) 12:13. doi: 10.1186/1471-2202-12-13
52. Na K-S, Kim Y-KJN. Monocytic, Th1 and th2 cytokine alterations in the pathophysiology of schizophrenia. Neuropsychobiology (2007) 56:55–63. doi: 10.1159/000111535
53. Al-Asmari A, Khan MWJH, Toxicology E. Inflammation and schizophrenia: alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol (2014) 33:115–22. doi: 10.1177/0960327113493305
54. Das S, Deuri SK, Sarmah A, Pathak K, Baruah A, Sengupta S, et al. Aggression as an independent entity even in psychosis-the role of inflammatory cytokines. J Neuroimmunol (2016) 292:45–51. doi: 10.1016/j.jneuroim.2016.01.012
55. Bedrossian N, Haidar M, Fares J, Kobeissy FH, Fares YJFIMN. Inflammation and elevation of interleukin-12p40 in patients with schizophrenia. Front Mol Neurosci (2016) 9:16. doi: 10.3389/fnmol.2016.00016
56. Kartalci S, Erbay LGJTPD. IL-4, TGF-?, NF-? B and MPO levels in patients with treatment resistant schizophrenia. Turk Psikiyatri Derg (2016) 27:170. doi: 10.5080/u13642
57. Noto C, Maes M, Ota VK, Teixeira AL, Bressan RA, Gadelha A, et al. High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. World J Biol Psychiatry (2015) 16:422–9. doi: 10.3109/15622975.2015.1062552
58. Eftekharian MM, Omrani MD, Arsang-Jang S, Taheri M, Ghafouri-Fard SJHA. Serum cytokine profile in schizophrenic patients. Hum Antibodies (2018) 27(1):1–7. doi: 10.3233/HAB-180344
59. Maxeiner H-G, Schneider EM, Kurfiss S-T, Brettschneider J, Tumani H, Bechter KJC. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine (2014) 69:62–7. doi: 10.1016/j.cyto.2014.05.008
60. Fang X, Zhang Y, Fan W, Tang W, Zhang CJMN. Interleukin-17 alteration in first-episode psychosis: a meta-analysis. Mol Neuropsychiatry (2018) 3:135–40. doi: 10.1159/000481661
61. Li H, Zhang Q, Li N, Wang F, Xiang H, Zhang Z, et al. Plasma levels of Th17-related cytokines and complement C3 correlated with aggressive behavior in patients with schizophrenia. Psychiatry Res (2016) 246:700–6. doi: 10.1016/j.psychres.2016.10.061
62. Dimitrov DH, Lee S, Yantis J, Valdez C, Paredes RM, Braida N, et al. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: potential role for IL-17 pathway. Schizophr Res (2013) 151:29–35. doi: 10.1016/j.schres.2013.10.019
63. Borovcanin M, Jovanovic I, Dejanovic SD, Radosavljevic G, Arsenijevic N, Lukic ML. Increase systemic levels of IL-23 as a possible constitutive marker in schizophrenia. Psychoneuroendocrinology (2015) 56:143–7. doi: 10.1016/j.psyneuen.2015.03.003
64. Xiu MH, Yang GG, Tan YL, Tan SP, Wang ZR, De Yang F, et al. Decreased interleukin-10 serum levels in first-episode drug-naïve schizophrenia: relationship to psychopathology. Schizophr Res (2014) 156:9–14. doi: 10.1016/j.schres.2014.03.024
65. Dahan S, Bragazzi NL, Yogev A, Bar-Gad M, Barak V, Amital H, et al. The relationship between serum cytokine levels and degree of psychosis in patients with schizophrenia. Psychiatry Res (2018) 268:467–72. doi: 10.1016/j.psychres.2018.07.041
66. Noto MN, Maes M, Nunes SOV, Ota VK, Rossaneis AC, Verri WA Jr., et al. Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol (2019) 29:416–31. doi: 10.1016/j.euroneuro.2018.12.008
67. Fu G, Zhang W, Dai J, Liu J, Li F, Wu D, et al. Increased peripheral interleukin 10 Relate to white matter integrity in Schizophrenia. Front Neurosci (2019) 13:52. doi: 10.3389/fnins.2019.00052
68. Szymona K, Zdzisinska B, Karakula-Juchnowicz H, Kocki T, Kandefer-Szerszen M, Flis M, et al. Correlations of Kynurenic Acid, 3-Hydroxykynurenine, sIL-2R, IFN-alpha, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res (2017) 32:17–26. doi: 10.1007/s12640-017-9714-0
69. Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YCJSR. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res (2002) 57:247–58. doi: 10.1016/S0920-9964(01)00296-1
70. Romeo B, Brunet-Lecomte M, Martelli C, Benyamina AJIJON. Kinetics of cytokine levels during antipsychotic treatment in schizophrenia: a meta-analysis. Int J Neuropsychopharmacol (2018) 21:828–36. doi: 10.1093/ijnp/pyy062
71. Tian L, Tan Y, Chen D, Lv M, Tan S, Soares JC, et al. Reduced serum TNF alpha level in chronic schizophrenia patients with or without tardive dyskinesia. Prog Neuropsychopharmacol Biol Psychiatry (2014) 54:259–64. doi: 10.1016/j.pnpbp.2014.06.012
72. Asevedo E, Rizzo LB, Gadelha A, Mansur RB, Ota VK, Berberian AA, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav (2014) 129:194–8. doi: 10.1016/j.physbeh.2014.02.032
73. Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, et al. Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. Schizophr Bull (2015) 41:1162–70. doi: 10.1093/schbul/sbv028
74. Simsek S, Yildirim V, Çim A, Kaya SJJOC, Psychopharmacology A. Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with first episode, early onset schizophrenia. J Child Adolesc Psychopharmacol (2016) 26:721–6. doi: 10.1089/cap.2015.0220
75. Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ. TNF-alpha and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res (2018) 199:281–4. doi: 10.1016/j.schres.2018.02.048
76. Zhang Q, Hong W, Li H, Peng F, Wang F, Li N, et al. Increased ratio of high sensitivity C-reactive protein to interleukin-10 as a potential peripheral biomarker of schizophrenia and aggression. Int J Psychophysiol (2017) 114:9–15. doi: 10.1016/j.ijpsycho.2017.02.001
77. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
78. Pae C-U, Yoon C-H, Kim T-S, Kim J-J, Park S-H, Lee C-U, et al. Antipsychotic treatment may alter T-helper (TH) 2 arm cytokines. Int Immunopharmacol (2006) 6:666–71. doi: 10.1016/j.intimp.2005.10.004
79. Noto CS, Gadelha A, Belangero SI, Smith MaC, De Aguiar BW, Panizzuti B, et al. Association of biomarkers and depressive symptoms in schizophrenia. Neurosci Lett (2011) 505:282–5. doi: 10.1016/j.neulet.2011.10.042
80. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) (2007) 4:28–37.
81. Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO, et al. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res (2013) 145:36–42. doi: 10.1016/j.schres.2012.12.023
82. Hope S, Hoseth E, Dieset I, Mørch RH, Aas M, Aukrust P, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res (2015) 165:188–94. doi: 10.1016/j.schres.2015.04.004
83. Xiu MH, Tian L, Chen S, Tan YL, Chen J, Chen N, et al. Contribution of IL-10 and its-592 A/C polymorphism to cognitive functions in first-episode drug-naive schizophrenia. Brain Behav Immun (2016) 57:116–24. doi: 10.1016/j.bbi.2016.03.005
84. De Campos-Carli SM, Miranda AS, Dias IC, De Oliveira A, Cruz BF, Vieira EL, et al. Serum levels of interleukin-33 and its soluble form receptor (sST2) are associated with cognitive performance in patients with schizophrenia. Compr Psychiatry (2017) 74:96–101. doi: 10.1016/j.comppsych.2017.01.008
85. Kogan S, Ospina LH, Kimhy DJB., Behavior, and Immunity. Inflammation in individuals with schizophrenia–implications for neurocognition and daily function. Brain Behav Immun (2018) 74:296–9. doi: 10.1016/j.bbi.2018.09.016
86. Misiak B, Stanczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: a systematic review. Schizophr Res (2018) 192:16–29. doi: 10.1016/j.schres.2017.04.015
87. Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol (1998) 20:310–9. doi: 10.1076/jcen.20.3.310.823
88. Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry (2009) 65:481–8. doi: 10.1016/j.biopsych.2008.10.018
89. Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà GJN, Reviews B. Acute variations of cytokine levels after antipsychotic treatment in drug-naive subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev (2017) 77:122–8. doi: 10.1016/j.neubiorev.2017.03.003
90. Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YCJTJOCP. Changes in serum interleukin-2,-6, and-8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry (2004) 65:940–7. doi: 10.4088/JCP.v65n0710
91. Tourjman V, Kouassi É, Koué M-È, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res (2013) 151:43–7. doi: 10.1016/j.schres.2013.10.011
92. Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, et al. Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naive, first-episode schizophrenia. Psychopharmacol (Berl) (2014) 231:319–25. doi: 10.1007/s00213-013-3382-4
93. Dikec G, Arabaci LB, Uzunoglu GB, Mizrak SD. Metabolic side effects in patients using atypical antipsychotic medications during hospitalization. J Psychosoc Nurs Ment Health Serv (2018) 56:28–37. doi: 10.3928/02793695-20180108-05
94. Mirhafez SR, Pasdar A, Avan A, Esmaily H, Moezzi A, Mohebati M, et al. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br J Nutr (2015) 113:1911–9. doi: 10.1017/S0007114515001038
95. Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west virginian population. Int J Med Sci (2016) 13:25–38. doi: 10.7150/ijms.13800
96. Kubistova A, Horacek J, Novak TJA. Increased interleukin-6 and tumor necrosis factor alpha in first episode schizophrenia patients versus healthy controls. Psychiatr Danub (2012) 1:P2.
97. Haring L, Koido K, Vasar V, Leping V, Zilmer K, Zilmer M, et al. Antipsychotic treatment reduces psychotic symptoms and markers of low-grade inflammation in first episode psychosis patients, but increases their body mass index. Schizophr Res (2015) 169:22–9. doi: 10.1016/j.schres.2015.08.027
98. Dunjic-Kostic B, Jasovic-Gasic M, Ivkovic M, Radonjic NV, Pantovic M, Damjanovic A, et al. Serum levels of interleukin-6 and tumor necrosis factor-alpha in exacerbation and remission phase of schizophrenia. Psychiatr Danub (2013) 25:0–61.
99. Chu C-S, Li D-J, Chu C-L, Wu C-C, Lu TJPI. Decreased IL-1ra and NCAM-1/CD56 serum levels in unmedicated patients with schizophrenia before and after antipsychotic treatment. Psychiatry Invest (2018) 15(7):727–732. doi: 10.30773/pi.2017.11.10
100. Crespo-Facorro B, Carrasco-Marin E, Perez-Iglesias R, Pelayo-Teran JM, Fernandez-Prieto L, Leyva-Cobian F, et al. Interleukin-12 plasma levels in drug-naive patients with a first episode of psychosis: effects of antipsychotic drugs. Psychiatry Res (2008) 158:206–16. doi: 10.1016/j.psychres.2006.08.005
101. Sobis J, Rykaczewska-Czerwinska M, Swietochowska E, Gorczyca PJPR. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep (2015) 67:353–9. doi: 10.1016/j.pharep.2014.09.007
102. Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola L, Honda PH, et al. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. Int J Neuropsychopharmacol (2014) 18(4):pyu042. doi: 10.1093/ijnp/pyu042
103. Ajami A, Abedian F, Hosseini SH, Akbarian E, Alizadeh-Navaei R, Taghipour MJIJOI. Serum TNF-α, IL-10 and IL-2 in schizophrenic patients before and after treatment with risperidone and clozapine. Iran J Immunol (2014) 11:200–9. doi: IJIv11i3A6
104. Petrikis P, Voulgari PV, Tzallas AT, Boumba VA, Archimandriti DT, Zambetas D, et al. Changes in the cytokine profile in first-episode, drug-naïve patients with psychosis after short-term antipsychotic treatment. Psychiatry Res (2017) 256:378–83. doi: 10.1016/j.psychres.2017.07.002
105. Lin A, Kenis G, Bignotti S, Tura GJB, De Jong R, Bosmans E, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res (1998) 32:9–15. doi: 10.1016/S0920-9964(98)00034-6
106. Andreasen NC, Carpenter WT Jr., Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry (2005) 162:441–9. doi: 10.1176/appi.ajp.162.3.441
107. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry (1988) 45:789–96. doi: 10.1001/archpsyc.1988.01800330013001
108. Lee H-Y, Kim Y-KJaN. Effect of TGF-ß1 polymorphism on the susceptibility to schizophrenia and treatment response to atypical antipsychotic agent. Acta Neuropsychiatr (2010) 22:174–9. doi: 10.1111/j.1601-5215.2009.00435.x
109. Rodrigues FMM, Ramos D, Xavier RF, Ito JT, De Souza AP, Fernandes RA, et al. Nasal and systemic inflammatory profile after short term smoking cessation. Respir Med (2014) 108:999–1006. doi: 10.1016/j.rmed.2014.04.020
110. Aziz N. Measurement of circulating cytokines and immune-activation markers by multiplex technology in the clinical setting: what are we really measuring? For Immunopathol Dis Therap (2015) 6:19–22. doi: 10.1615/ForumImmunDisTher.2015014162
111. Cox AJ, West NP, Cripps AWJTLD, . Endocrinology. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
112. Marsland AL, Walsh C, Lockwood K, John-Henderson NaJB, . Behavior and Immunity. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun (2017) 64:208–19. doi: 10.1016/j.bbi.2017.01.011
113. Zhou X, Fragala MS, Mcelhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care (2010) 13:541–7. doi: 10.1097/MCO.0b013e32833cf3bc
114. Manu P, Correll CU, Wampers M, Mitchell AJ, Probst M, Vancampfort D, et al. Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry (2014) 13:189–92. doi: 10.1002/wps.20117
115. Hill AB. The environment and disease: association or causation? Proc R Soc Med (1965) 58:295–300. doi: 10.1177/003591576505800503
116. Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol (2015) 12:14. doi: 10.1186/s12982-015-0037-4
117. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry (2014) 71:1121–8. doi: 10.1001/jamapsychiatry.2014.1332
118. Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology (2014) 41:23–32. doi: 10.1016/j.psyneuen.2013.12.005
119. Khandaker GM, Zammit S, Burgess S, Lewis G, Jones PB. Association between a functional interleukin 6 receptor genetic variant and risk of depression and psychosis in a population-based birth cohort. Brain Behav Immun (2018) 69:264–72. doi: 10.1016/j.bbi.2017.11.020
120. Metcalf SA, Jones PB, Nordstrom T, Timonen M, Maki P, Miettunen J, et al. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav Immun (2017) 59:253–9. doi: 10.1016/j.bbi.2016.09.008
121. Kappelmann N, Khandaker GM, Dal H, Stochl J, Kosidou K, Jones PB, et al. Systemic inflammation and intelligence in early adulthood and subsequent risk of schizophrenia and other non-affective psychoses: a longitudinal cohort and co-relative study. Psychol Med (2019) 49:295–302. doi: 10.1017/S0033291718000831
122. Hu W, Macdonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci (2015) 1338:38–57. doi: 10.1111/nyas.12547
123. Ratnayake U, Quinn T, Walker DW, Dickinson H. Cytokines and the neurodevelopmental basis of mental illness. Front Neurosci (2013) 7:180. doi: 10.3389/fnins.2013.00180
124. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience (2013) 246:199–229. doi: 10.1016/j.neuroscience.2013.04.060
125. Sommer IE, Van Westrhenen R, Begemann MJ, De Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull (2014) 40:181–91. doi: 10.1093/schbul/sbt139
126. Cho M, Lee TY, Kwak YB, Yoon YB, Kim M, Kwon JS. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry (2019) 53(8):742–759. doi: 10.1177/0004867419835028
127. Dubois T, Reynaert C, Jacques D, Lepiece B, Patigny P, Zdanowicz N. Immunity and psychiatric disorders: variabilities of immunity biomarkers are they specific? Psychiatr Danub (2018) 30:447–51.
128. Herron JW, Nerurkar L, Cavanagh J. Neuroimmune biomarkers in mental illness. In: Pratt J, Hall J (eds), Springer, Cham: Biomarkers in Psychiatry Current Topics in Behavioral Neurosciences (2018). vol 40. doi: 10.1007/7854_2018_45
129. Muller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr Bull (2018) 44:973–82. doi: 10.1093/schbul/sby024
130. Cakici N, Van Beveren NJM, Judge-Hundal G, Koola MM, Sommer IEC. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med (2019) 49:2307–19. doi: 10.1017/S0033291719001995
131. Nishimon S, Ohnuma T, Takebayashi Y, Katsuta N, Takeda M, Nakamura T, et al. High serum soluble tumor necrosis factor receptor 1 predicts poor treatment response in acute-stage schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2017) 76:145–54. doi: 10.1016/j.pnpbp.2017.03.006
132. Faugere M, Micoulaud-Franchi JA, Alessandrini M, Richieri R, Faget-Agius C, Auquier P, et al. Quality of life is associated with chronic inflammation in schizophrenia: a cross-sectional study. Sci Rep (2015) 5:10793. doi: 10.1038/srep10793
133. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
134. Miller BJ, Goldsmith DR. Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neuropsychopharmacology (2017) 42:299–317. doi: 10.1038/npp.2016.211
135. Upthegrove R, Khandaker GM. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. In: Current topics in behavioral neurosciences. Berlin, Heidelberg: Springer (2019). doi: 10.1007/7854_2018_88
Keywords: schizophrenia, cytokines, inflammation, behavioral symptoms, treatment outcome, antipsychotic agents
Citation: Momtazmanesh S, Zare-Shahabadi A and Rezaei N (2019) Cytokine Alterations in Schizophrenia: An Updated Review. Front. Psychiatry 10:892. doi: 10.3389/fpsyt.2019.00892
Received: 08 September 2019; Accepted: 13 November 2019;
Published: 06 December 2019.
Edited by:
Maria S. Garcia-Gutierrez, Universidad Miguel Hernández de Elche, SpainReviewed by:
Stefania Schiavone, University of Foggia, ItalyCopyright © 2019 Momtazmanesh, Zare-Shahabadi and Rezaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nima Rezaei, cmV6YWVpX25pbWFAdHVtcy5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.