- 1Clinical Emotion and Cognition Research Laboratory, Inje University, Goyang, South Korea
- 2Department of Psychology, Chung-Ang University, Seoul, South Korea
- 3Department of Psychiatry, Inje University, Ilsan-Paik Hospital, Goyang, South Korea
- 4Department of Psychiatry, Chonbuk National University Medical School, Jeonju, South Korea
Memory impairment, excessive rumination, and increased interpersonal sensitivity are major characteristics of high psychosis risk or first episode psychosis (FEP). Herein, we investigated the relationship between brain volume and self-awareness of psychopathology in patients with FEP. All participants (FEP: 34 and HCs: 34) completed clinical assessments and the following self-reported psychopathology evaluations: prospective and retrospective memory questionnaire (PRMQ), ruminative response scale (RRS), and interpersonal sensitivity measure (IPSM). Structural magnetic resonance imaging was then conducted. The PRMQ, RRS, and IPSM scores were significantly higher in the FEP group than in the healthy controls (HCs). The volumes of the amygdala, hippocampus, and superior temporal gyrus (STG) were significantly lower in the FEP group than in the HCs. There was a significant group-dependent moderation effect between self-awareness of psychopathology (PRMQ, RRS, and IPSM scores) and right STG (rSTG) volume. In the FEP group, self-awareness of psychopathology was positively associated with rSTG volume, while in the HCs, this correlation was negative. Our results indicate that self-awareness of psychopathology impacts rSTG volume in the opposite direction between patients with FEP and HCs. In patients with FEP, awareness of impairment may induce increases in rSTG brain volume. However, HCs showed decreased rSTG volume when they were aware of impairment.
Introduction
First episode psychosis (FEP) is defined as the first time a person outwardly shows symptoms of psychosis. When patients with FEP become aware of their problems, they show distress and confusion, ruminate their symptoms, and have interpersonal problems caused by enhanced sensitivity (1). Conversely, after experiencing chronic overt psychotic symptoms, patients with chronic schizophrenia generally show poor insight into their own symptoms (2).
Self-awareness of psychopathology is one dimension of clinical insight (3). In psychiatric patients, lack of self-awareness of illness is associated with poor psychosocial function (4), poor clinical outcomes (5), and poorer treatment adherence (6), while in healthy controls, greater self-awareness of illness may lead them to adopt the identity of a “psychiatric patient” to themselves (7), which may be associated with poorer social functioning (8) and lower self-esteem (9). Previous studies have found that patients with full-blown schizophrenia lack self-awareness of illness (4, 10). About 46% of FEP patients showed poor insight (11) and insight impairment is associated with multiple cognitive deficits (12).
The subjective evaluation of self-awareness of illness can also be important in the early detection of schizophrenia, because complaints precede prodromal symptoms and are therefore useful in predicting onset and relapse of schizophrenia (13, 14) as well as long-term symptomatic deterioration (15). Subjective measures of cognitive function, such as self-report questionnaires or related scales, shed light on self-perceived cognitive difficulties that occur during daily activity and cannot be observed using behavioral tests (16). Although some self-reported scales measure clinical insight, such as the schedule of assessment of insight-expanded version (SAI-E) (17 1997) and the Beck cognitive insight scale (BCIS) (18), they are too broad to examine self-awareness of illness among patients with FEP. In particular, the SAI-E measures overall relabeling of symptoms, awareness of illness, and need for treatment, while the BCIS measures self-certainty and self-reflectiveness. Therefore, self-awareness of illness should be measured using other self-reporting scales that measure specific cognitive functions related to the pathological problems seen in patients with FEP.
In addition to cognitive deficits, patients with FEP seem to display memory problems, rumination, and interpersonal sensitivity (19–22). Bigdeli et al. (21) revealed that patients with FEP performed more poorly than a healthy group in a self-reporting memory task. In contrast, patients with schizophrenia did not report subjective complaints significantly more often than controls (16).
Rumination is described as a cognitive process that includes repetitive, prolonged, and recurrent thoughts about oneself, one’s concerns, and one’s experiences (23). Rumination is related to hallucination-proneness and to a range of mild abnormal experiences, including unrealistic feelings, perceptual alterations, and temporal disintegration (24, 25), as well as to negative symptoms such as stereotyped thinking and emotional withdrawal (26). One experiment revealed that antecedent rumination and worry predicted persecutory delusions and auditory hallucinations; it also predicted the degree of anguish associated with these psychotic experiences in young adults with psychosis (22). In fact, rumination may be the reason some young people who experience psychotic symptoms become distressed and seek help, while others do not (27, 28).
To understand psychosis, one important aspect of interpersonal interactions is interpersonal sensitivity, which is a personality trait described as excessive awareness of the behavior and feelings of others (29). Interpersonal sensitivity is associated with the risk of paranoid thinking in the general population (30). Early studies indicated that high interpersonal sensitivity occurs among other subjective symptoms and observable behavioral changes during the prodromal phase of schizophrenia (19, 30).
With regards to the brain abnormalities related to psychosis, one meta-analysis found that gray matter decreases were common in the thalamus, the left uncus/amygdala region, the insula bilaterally, and the anterior cingulate in patients with both first episode psychosis (FEP) and chronic schizophrenia (31). Furthermore, the decreases in gray matter volume in the bilateral caudate head and right bilateral superior parietal lobule were more severe in first-episode or early stage schizophrenia than in chronic schizophrenia (32, 31). Another study found that the volumes of the frontal and temporal areas were decreased in first-episode schizophrenia, but less than in chronic schizophrenia (33). The superior frontal gyrus has been associated with anomalies in self-awareness, social cognition, and emotion (34, 35), while the superior temporal gyrus (STG) and subcortical regions such as the thalamus are associated with positive symptoms, including auditory hallucinations, thought disorders (36, 37), deficits in working memory and attention (38, 39), and social processing (40–42).
In the present study, we explored the relationship between brain volume and self-awareness of psychopathology in FEP. We hypothesized that patients with FEP show greater self-reported memory impairment, rumination, and interpersonal sensitivity than healthy participants, and that these abnormalities are associated with brain morphological anomalies that are vulnerable targets of FEP.
Materials and Methods
Participants
A total of 34 patients [male: 16, female: 18, age: 28.353 ± 7.261 (range: 19–47)] who met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) criteria for first-episode schizophrenia, schizophreniform disorder, schizoaffective disorder, or psychotic disorder not otherwise specified (NOS), as assessed using the Structured Clinical Interview for DSM-V Axis I disorders (43) (SCID-I), were eligible to participate in the study. Patients were included if they had symptoms requiring antipsychotic treatment [a score of ≥4 (moderate) on at least one of the following: the PANSS (44) positive items, which were P1, P2, P3, P5, and P6, or Clinical Global Impression (CGI) score ≥4] with illness duration of more than 1 month and less than 60 months (45), and no lifetime history of previous antipsychotic exposure lasting for 2 or more consecutive weeks (46).
A total of 34 healthy control (HC) participants [male: 21, female: 13, age: 31.647 ± 6.764 (range: 21–47)] were recruited from the local community via newspapers and flyers. An initial screening interview was used to exclude subjects with any identifiable neurological disorder, head injury, or any personal or family history of psychiatric illness. After the initial screening, potential HCs were interviewed using the Structured Clinical Interview for DSM V for Axis I Psychiatric Disorders (43) and were excluded if they had any such disorders. All participants were right-handed according to the Edinburgh Handedness Inventory (47). All procedures followed were in accordance with the ethical standards of the Ethics Committee of Chonbuk National University Hospital, Republic of Korea (approval number: CUH2014-11-002) and with the Declaration of Helsinki. All participants provided written informed consent themselves or from their legal guardians if they were the minors.
Psychological Measures
Self-Awareness of Psychopathology: Subjective Symptoms
Self-awareness of psychopathology was assessed using self-report measures, namely, the prospective and retrospective memory questionnaire (PRMQ), the ruminative response scale (RRS), and the interpersonal sensitivity measure (IPSM).
The PRMQ (48, 49) was used to measure subjective complaints of memory impairment. It is a 16-item questionnaire. Each participant was asked to rate how often each type of memory failure occurred in their daily life on a 5-point scale (very often, quite often, sometimes, rarely, and never). The PRMQ comprises six subscales: prospective, retrospective, short-term, long-term, self-cued, and environmentally-cued. Of the 16 PRMQ items, eight inquire about prospective memory and eight inquire about retrospective memory. The questionnaire also contains an equal number of items concerned with self-cued memory and environmentally-cued memory, and with short-term and long-term memory. Thus, each PRMQ item can be categorized along three dimensions. For example, “If you tried to contact a friend or relative who was out, would you forget to try again later?” is categorized as measuring prospective, long-term, self-cued memory. Thus, the PRMQ may have an advantage over other self-report scales in that it balances prospective with retrospective items and measures these constructs systematically over a range of contexts.
The RRS (50, 51) comprises three subscales: depression-related, reflection, and brooding. It consists of 22 items, each of which is rated using a 4-point Likert scale, ranging from 1 (“almost never”) to 4 (“almost always”). Among the 22 RRS items, 12 are concerned with depressive symptoms (e.g., “Think about how alone you feel”), five inquire about brooding (e.g., “Think about a recent situation, wishing it had gone better”), and five address reflective pondering or reflection (e.g., “Analyze recent events to try to understand why you are depressed”) (51).
The IPSM has been used to assess excessive sensitivity to the interpersonal behavior of others, to social feedback, and to negative evaluation by others (29). The original IPSM scale comprises 36 items. However, we used the validated Korean version of the IPSM, which consists of 24 items (52) rated using 4-point Likert scale ranging from 1 (“very unlike me”) to 4 (“very like me”). The Korean version of the IPSM comprises five subscales, namely, interpersonal awareness (four items, e.g., “I worry about the effect I have on other people”), need for approval (four items, e.g., “I will go out of my way to please someone I am close to”), separation anxiety (six items, e.g., “I feel insecure when I say goodbye to people”), timidity (five items, e.g., “I will do something I don’t want to do rather than offend or upset someone”), and fragile inner self (five items, e.g., “My value as a person depends enormously on what others think of me”).
Clinical Assessment of Psychopathology: Objective Symptoms
Psychopathology was assessed clinically using the Positive and Negative Syndrome Scale (PANSS), the Clinical Global Impression Schizophrenia (CGI-SCH), the Calgary Depression Scale for Schizophrenia (CDSS), and the Social and Occupational Functioning Assessment Scale (SOFAS). The PANSS (44, 53) uses interview and reports of family members to assess the severity of the two common symptom types in schizophrenia—positive and negative—as well as the general psychopathology of the patient. The CGI-SCH (54) consists of only two categories: severity of illness and degree of change. Each category contains five different ratings (positive, negative, depressive, cognitive, and global). The CDSS (55–57) was used to measure depressive symptoms of schizophrenia. The SOFAS (58) is a single-item scale used to indicate the individual’s level of social and occupational functioning across a continuum, ranging from a state of optimum functioning to a state of important functional impairment. It measures the level of social and occupational functioning, without taking symptoms into account.
Magnetic Resonance Imaging and Voxel-Based Morphometry Analysis
Magnetic resonance imaging (MRI) was performed using a 3-T scanner (MAGNETOM Verio; Siemens, Erlangen, Germany). To minimize image distortion due to head motion, restraining foam pads were used. High-resolution, T1-weighted structural brain MR images were acquired using the following acquisition parameters: 256 × 246 acquisition matrix, 270 × 270 or 250 × 250 field-of-view, 0.527 × 0.527 × 1 or 0.488 × 0.488 × 1 voxel size, a total of 262,144 voxels, an echo time (TE) of 2.45 ms, a repetition time (TR) of 1900 ms, a 1-mm slice thickness, and a flip angle of 9°. Specifically, among the patients with FEP, 22 were imaged using a voxel size of 0.488 × 0.488 × 1, while 12 had a voxel size of 0.527 × 0.527 × 1). Among the HCs, 19 were imaged using a voxel size of 0.488 × 0.488 × 1), while 15 had a voxel size of 0.527 × 0.527 × 1. Therefore, we controlled for voxel size as covariate factor in the statistical analysis.
Voxel-based volumetry (VBM) was conducted using the Computational Anatomy Toolbox (CAT12; developed by Christian Gaser, University of Jena, http://dbm.neuro.uni-jena.de/cat), which is provided in the SPM12 software package (Wellcome Department of Cognitive Neurology, London, UK) (59, 60) and can be run using MATLAB R2016b (Mathworks Inc.). The structural T1 images were affine registered to an ICBM East Asian template and normalized using the DARTEL algorithm (61). The images were then segmented into gray matter, white matter, and cerebrospinal fluid (62). Jacobian-transformed tissue probability maps were used to modulate the images and estimate volume differences in gray matter. The brain volume was estimated in 148 regions of the Neuromorphometrics atlas, which is available in SPM12 (Neuromorphometrics Inc., http://neuromorphometrics.com). The frontal lobe, amygdala, hippocampus, insula, and STG were analyzed as regions of interest (ROIs) of brain volume, since these areas were associated with major pathology of first episode psychosis (63–69). Among 148 regions of the Neuromorphometrics atlas, 30 regions matched with the five ROIs were selected for analysis (Frontal lobe: L/R frontal operculum, L/R frontal pole, L/R medial frontal cortex, L/R middle frontal gyrus, L/R superior frontal gyrus medial segment, L/R opercular part of the inferior frontal gyrus, L/R orbital part of the inferior frontal gyrus, L/R superior frontal gyrus, L/R triangular part of the inferior frontal gyrus, amygdala: L/R Amygdala, hippocampus: L/R Hippocampus, L/R parahippocampal gyrus, insula: L/R anterior insula, L/R posterior insula, and STG: L/R superior temporal gyrus).
Statistical Analysis
Independent t-tests were used to compare the demographic data. A multivariate analysis of covariance (MANCOVA) was used to compare psychological measures and brain volume between the patients with the FEP and the HC. A partial correlation analysis was performed to examine the relationship between psychological measures and brain volume which had differed significantly by two groups through the preceding MANCOVA analysis. Additional regression analysis using the SPSS Macro PROCESS for SPSS 2.16.3 (70) was performed to examine the moderation effect of the groups. Age, sex, years of education, antipsychotic dosage, status of medication use (taking drugs, drug naïve, drug free, HC), and duration of illness (DI) were considered as covariates. Antipsychotic dosage, status of medication use (taking drugs, drug naïve, drug free, and HC), and duration of illness (DI) were not considered as covariates during partial correlation for the HC group. When volume was included in the analysis, the total intracranial volume (TIV) and voxel size were considered as additional covariates to correct for different brain sizes (71). The significance level was set at p < 0.05 (two-tailed). For multiple correction, 5,000 times resampled bootstrapping method was applied for the MANCOVA, partial correlation, and moderated regression analyses (72). Statistical analyses were performed using SPSS 21 (SPSS, Inc., Chicago, IL, USA).
Results
Demographic and Psychological Characteristics
The patients with FEP did not differ in age and sex from the HCs, although the number of years of education differed significantly between the two groups (p < 0.001), so we controlled for years of education as a covariate in the statistical analysis. The demographic and psychological characteristics of the participants are shown in Table 1. Among the 34 FEP patients, eight were drug naive, six were drug free, and 20 were taking antipsychotic medication (risperidone: n = 3, olanzapine: n = 3, paliperidone: n = 4, aripiprazole: n = 6, blonanserin: n = 2, amisulpride: n = 1, paliperidone palmitate: n = 1) during the study.
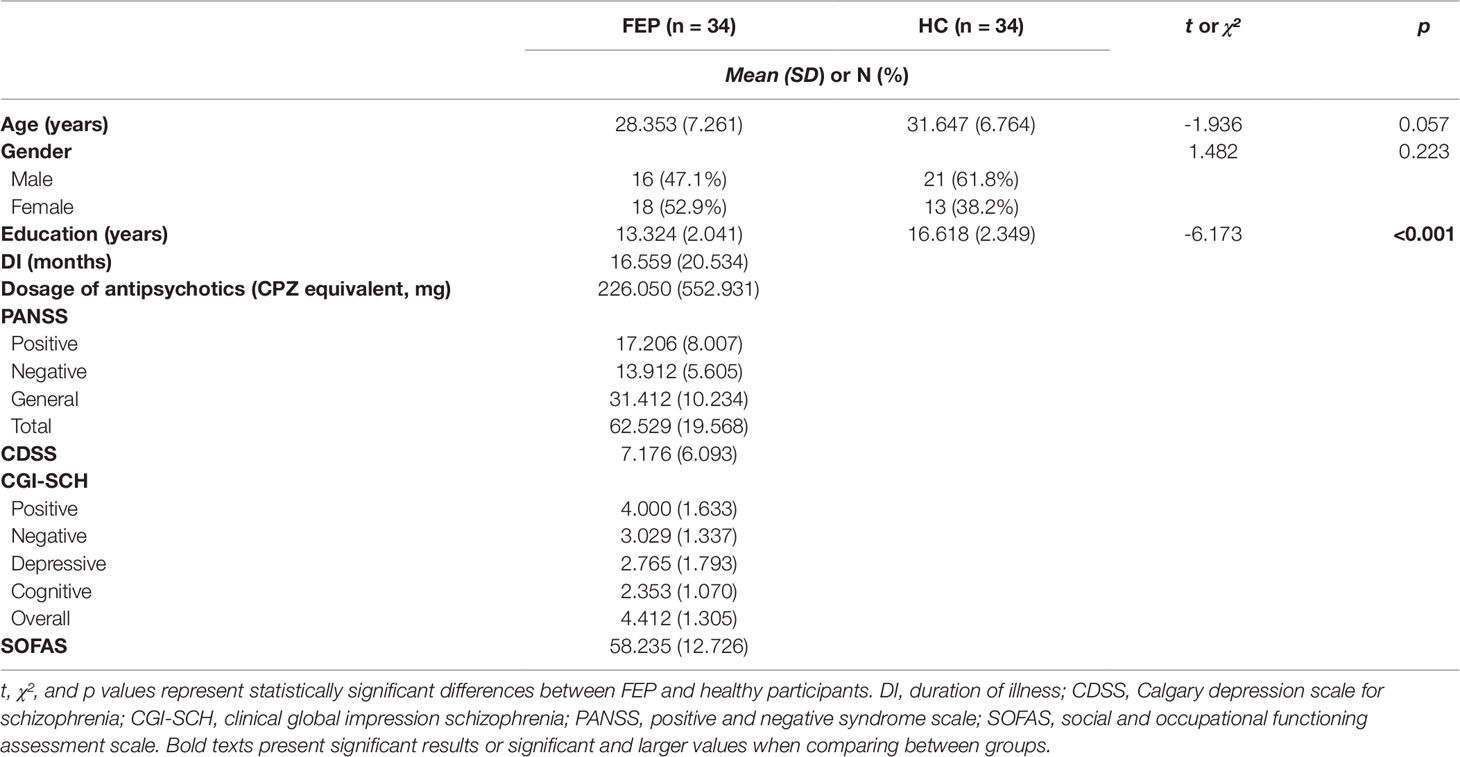
Table 1 Comparison of demographic and clinical characteristics between first episode psychosis (FEP) patients and healthy control (HC) participants.
Self-Awareness of Psychopathology: Subjective Symptoms
MANCOVA analysis was applied to all self-awareness of psychopathology measures, including the six subscales of the PRMQ, the three subscales of the RRS, and the five subscales of the IPSM, to examine the differences between the two groups by generating 5,000 bootstrapped samples for multiple comparison (72). Age, sex, years of education, dosage of antipsychotics, status of medication use, and DI were controlled as covariates. There were significant differences between the two groups in the PRMQ, RRS, and IPSM. In the PRMQ, all six subscale scores were significantly higher in the FEP group than in the HC group: prospective (17.206 ± 6.777 vs. 13.941 ± 5.662, F(1, 59) = 10.108, p = 0.002), retrospective (16.324 ± 5.250 vs. 12.676 ± 4.511, F(1, 59) = 19.001, p < 0.001), short-term (16.882 ± 5.989 vs. 13.235 ± 4.912, F(1, 59) = 12.465, p = 0.001), long-term (16.647 ± 5.969 vs. 13.382 ± 5.176, F(1, 59) = 15.139, p < 0.001), self-cued (17.882 ± 6.158 vs. 13.588 ± 5.286, F(1, 59) = 13.705, p < 0.001), environmentally-cued (15.647 ± 6.075 vs. 13.029 ± 4.796, F(1, 59) = 12.982, p = 0.001), and total memory impairment self-assessment score (33.529 ± 11.471 vs. 26.618 ± 9.912, F(1, 59) = 14.807, p < 0.001). In the RRS, the depression-related rumination scale (26.353 ± 8.790 vs. 21.000 ± 6.325, F(1, 59) = 9.508, p = 0.003) and total ruminative response scale (47.294 ± 15.712 vs. 39.353 ± 11.484, F(1, 59) = 6.153, p = 0.016) were significantly higher in the FEP group than in the HC group. In the IPSM, interpersonal awareness (9.618 ± 3.420 vs. 8.294 ± 2.529, F(1, 59) = 4.181, p = 0.045), separation anxiety (11.647 ± 4.206 vs. 9.294 ± 3.010, F(1, 59) = 5.185, p = 0.026), and fragile inner self (9.353 ± 3.757 vs. 7.294 ± 2.493, F(1, 59) = 7.998, p = 0.006) were significantly higher in the FEP group than in the HC group. These results are presented in Table 2.
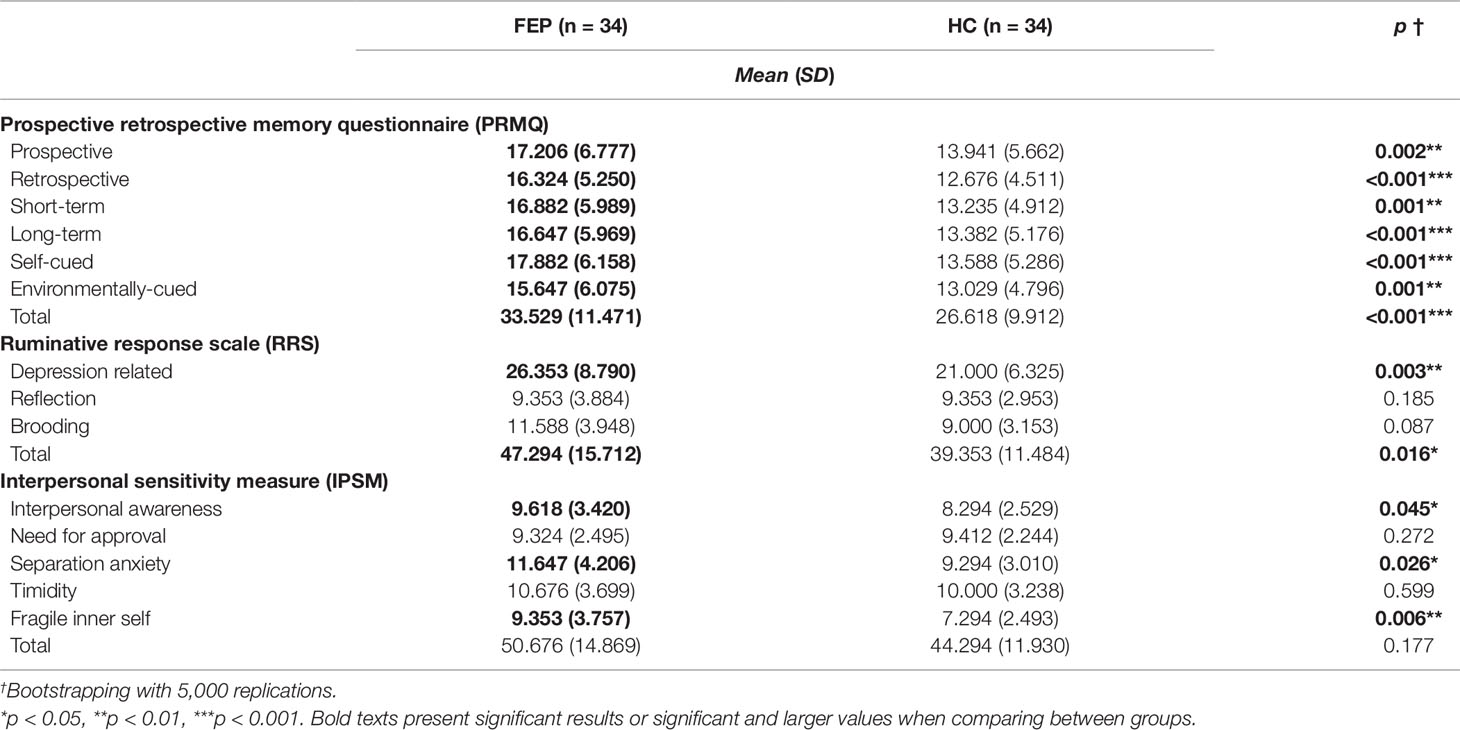
Table 2 Comparison of scores on subjective cognitive assessment scales between first episode psychosis (FEP) patients and healthy control (HC) participants.
Brain Volume
MANCOVA analysis was applied to brain volume in 30 subregions, including the five ROIs that are associated with major pathology in FEP (frontal lobe, amygdala, hippocampus, insula, and STG) (63–69), to examine the differences between the two groups by generating 5,000 bootstrapped samples for multiple comparison (72). Age, sex, years of education, antipsychotic dosage, status of medication use, DI, TIV, and voxel size were controlled as covariates. There were significant volume differences between the two groups in the amygdala, hippocampus, and STG. The volumes of the left amygdala (0.845 ± 0.111 vs. 0.931 ± 0.085, F(1, 58) = 7.001, p = 0.010), right amygdala (0.825 ± 0.106 vs. 0.914 ± 0.085, F(1, 58) = 5.520, p = 0.022), left hippocampus (3.145 ± 0.352 vs. 3.404 ± 0.341, F(1, 58) = 8.559, p = 0.005), right hippocampus (3.494 ± 0.350 vs. 3.763 ± 0.310, F(1, 58) = 5.515, p = 0.022), left parahippocampal gyrus (2.920 ± 0.282 vs. 3.182 ± 0.304, F(1, 58) = 5.106, p = 0.028), left STG (6.241 ± 0.783 vs. 6.821 ± 0.890, F(1, 58) = 6.161, p = 0.016), and right STG (rSTG; 6.618 ± 0.777 vs. 7.255 ± 0.899, F(1, 58) = 4.146, p = 0.046) were significantly lower in the FEP group than in the HC group. These results are presented in Table 3.
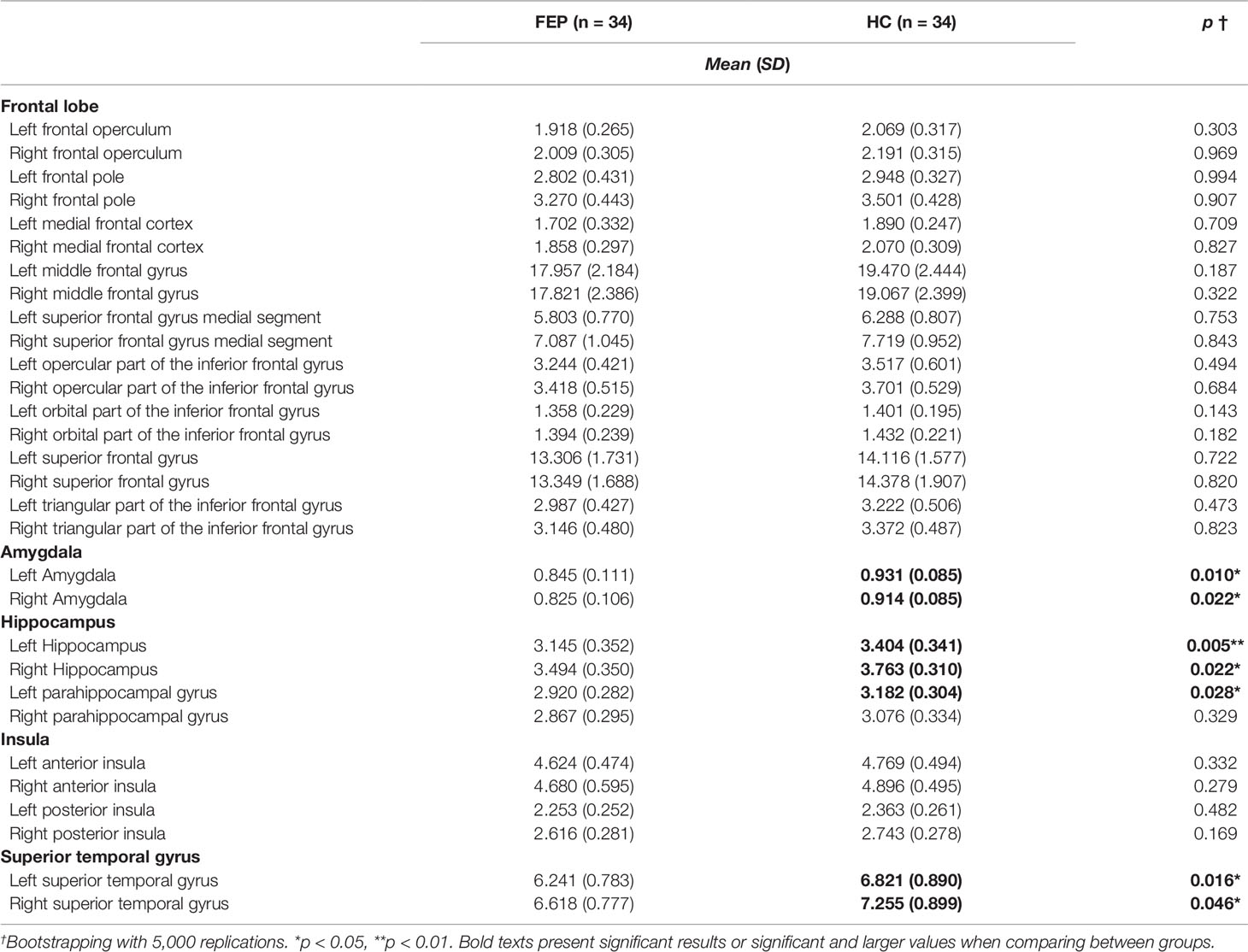
Table 3 Comparison of brain volume between first episode psychosis (FEP) patients and healthy control (HC) participants.
Correlation Analyses of Psychological Measures and Brain Volume
Partial correlation analysis was applied to the psychological measures and to the volume of the left/right amygdala, left/right hippocampus, left hippocampal gyrus, and left/right STG, all of which had differed significantly between the two groups in the preceding MANCOVA analysis, to examine their correlation with psychological symptoms by generating 5,000 bootstrapped samples for multiple comparison (72). Age, sex, years of education, TIV, and voxel size were controlled as covariates in all participants. Antipsychotic dosage, status of medication use, and DI were controlled in FEP patients.
Among all participants, need for approval (IPSM) was significantly associated with the left amygdala (r = 0.284, p = 0.028) and right hippocampus (r = 0.289, p = 0.025). The left STG was correlated with the PRMQ (prospective: r = -0.255, p = 0.050; retrospective: r = -0.310, p = 0.016; short-term: r = -0.259, p = 0.046; long-term: r = -0.303, p = 0.019; self-cued: r = -0.316, p = 0.014; total PRMQ: r = -0.290, p = 0.025) and with the RRS (depression related: r = -0.355, p = 0.005; reflection: r = -0.344, p = 0.007; brooding: r = -0.340, p = 0.008; total RRS: r = -0.370, p = 0.004).
In the FEP group, the volume of the left hippocampus was significantly correlated with the CDSS (r = 0.480, p = 0.013). The right hippocampus was related to the need for approval (IPSM subscale) (r = 0.388, p = 0.050), CDSS (r = 0.543, p = 0.004), and depressive CGI-SCH (r = 0.460, p = 0.018). All correlation results between clinical assessment and brain volume are presented in Table 4. The right STG volume was associated with the PRMQ (prospective: r = 0.393, p = 0.047; short-term: r = 0.419, p = 0.033; environmentally-cued: r = 0.494, p = 0.010; total PRMQ: r = 0.414, p = 0.036).
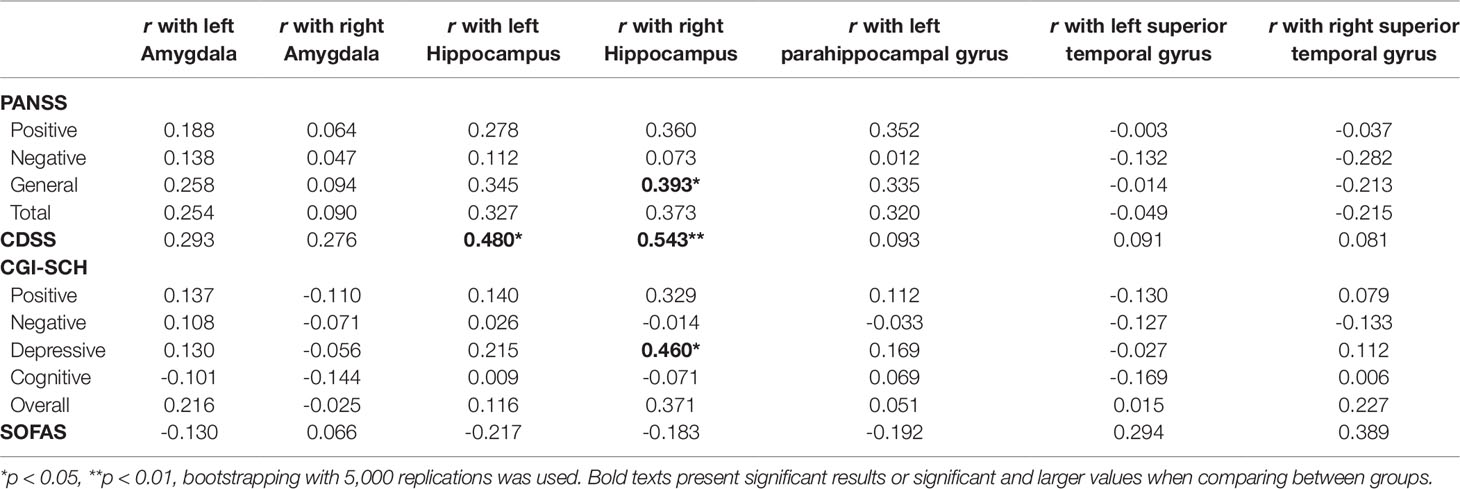
Table 4 Correlation between brain volume and clinical assessment of psychopathology in first episode psychosis (FEP) patients.
In the HC group, the left STG volume was negatively correlated with the PRMQ (retrospective: r = -0.389, p = 0.037) and the RRS (depression related: r = -0.466, p = 0.011; reflection: r = -0.432, p = 0.019; brooding: r = -0.526, p = 0.003; total RRS: r = -0.507, p = 0.005). The rSTG volume was negatively related to the PRMQ (prospective: r = -0.423, p = 0.022; retrospective: r = -0.446, p = 0.015; short-term: r = -0.486, p = 0.007; long-term: r = -0.388, p = 0.038; self-cued: r = -0.401, p = 0.031; environmentally-cued: r = -0.475, p = 0.009; total PRMQ: r = -0.443, p = 0.016), the RRS (depression related: r = -0.431, p = 0.020; total RRS: r = -0.420, p = 0.023), and the IPSM (timidity: r = -0.406, p = 0.029).
No other pairs showed any significant correlation between brain volume of ROIs and psychological measures.
Moderated Regression Analysis of Self-Awareness of Psychopathology and rSTG Volume
From the previous correlation analysis comparing five ROIs (frontal lobe, amygdala, hippocampus, insula, and STG) with self-awareness of psychopathology, only rSTG volume showed a noticeable difference between the FEP and HC groups. To examine the interaction between group and self-awareness of psychopathology (the PRMQ, RRS, and IPSM) on rSTG volume, moderation analyses were used. Each self-awareness scale was set as an independent variable, while rSTG volume was set as the dependent variable and the group (FEP vs. HC) was set as a moderator. The following variables were controlled for as covariates: age, sex, years of education, antipsychotic dosage, status of medication use, DI, TIV, and voxel size.
When the PRMQ score was set as the independent variable, the moderation model was significant (R² = 0.656, p < 0.001), as was the moderation effect, since the R² of the interaction was higher (△R² = 0.074, △F = 12.074, p = 0.001). The coefficient of the PRMQ total (B = -0.028, p = 0.009), the coefficient of group (B = -2.703, p < 0.001), and the interaction between PRMQ total and group (B = 0.049, p = 0.001) were significant. The HC group showed a significant negative effect (Effect = -0.028, p = 0.009), while the FEP group showed a significant positive effect (Effect = 0.021, p = 0.048).
When RRS score was set as the independent variable, the moderation model was significant (R² = 0.624, p < 0.001), as was the moderation effect was significant, since the R² of the interaction was higher (△R² = 0.034, △F = 5.101, p = 0.028). The coefficient of the RRS total (B = -0.024, p = 0.016), the coefficient of group (B = -2.206, p = 0.007), and the interaction between RRS total and group (B = 0.027, p = 0.028) were significant. The HC group showed a significant negative effect (Effect = -0.024, p = 0.016), while the FEP group showed an insignificant positive effect (Effect = 0.004, p = 0.597).
When the IPSM score was set as the independent variable, the moderation model was significant (R² = 0.615, p < 0.001), as was the moderation effect was significant, since the R² of the interaction was higher (△R² = 0.032, △F = 4.608, p = 0.036). Although the coefficient of the IPSM total (B = -0.013, p = 0.181) was not significant, the coefficient of group (B = -2.523, p = 0.004) and the interaction between IPSM total and group (B = 0.027, p = 0.036) were significant. The HC group showed an insignificant negative effect (Effect = -0.013, p = 0.181), while the FEP group showed an insignificant marginally significant positive effect (Effect = 0.014, p = 0.067).
Figure 1 presents the moderation effects of the PRMQ, RRS, and IPSM on the rSTG volume in each moderator group. Figure 2 shows the opposite direction of the effect in each group. In FEP group, the PRMQ, RRS, and IPSM scores positively correlated with the rSTG volume. In HC group, the scores negatively correlated with the rSTG volume.
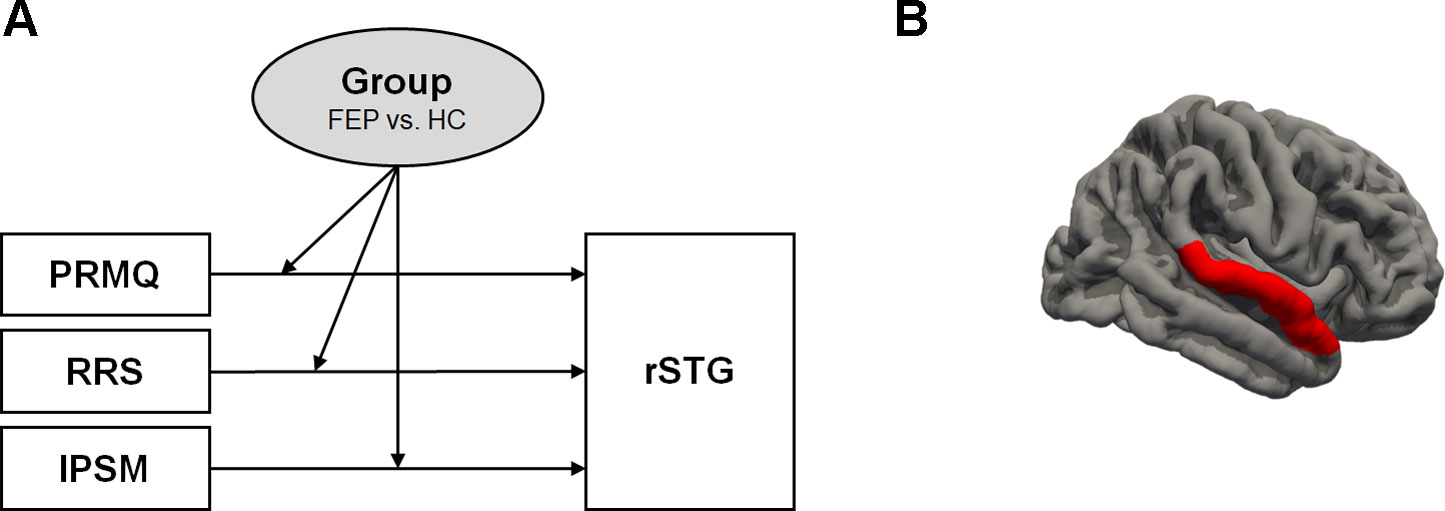
Figure 1 (A) Moderation of the effect of self-reported psychopathology evaluations (prospective and retrospective memory questionnaire (PRMQ), ruminative response scale (RRS), and interpersonal sensitivity measure (IPSM)) on right superior temporal gyrus (rSTG) volume at values of the moderator group. (B) rSTG region (red colored).
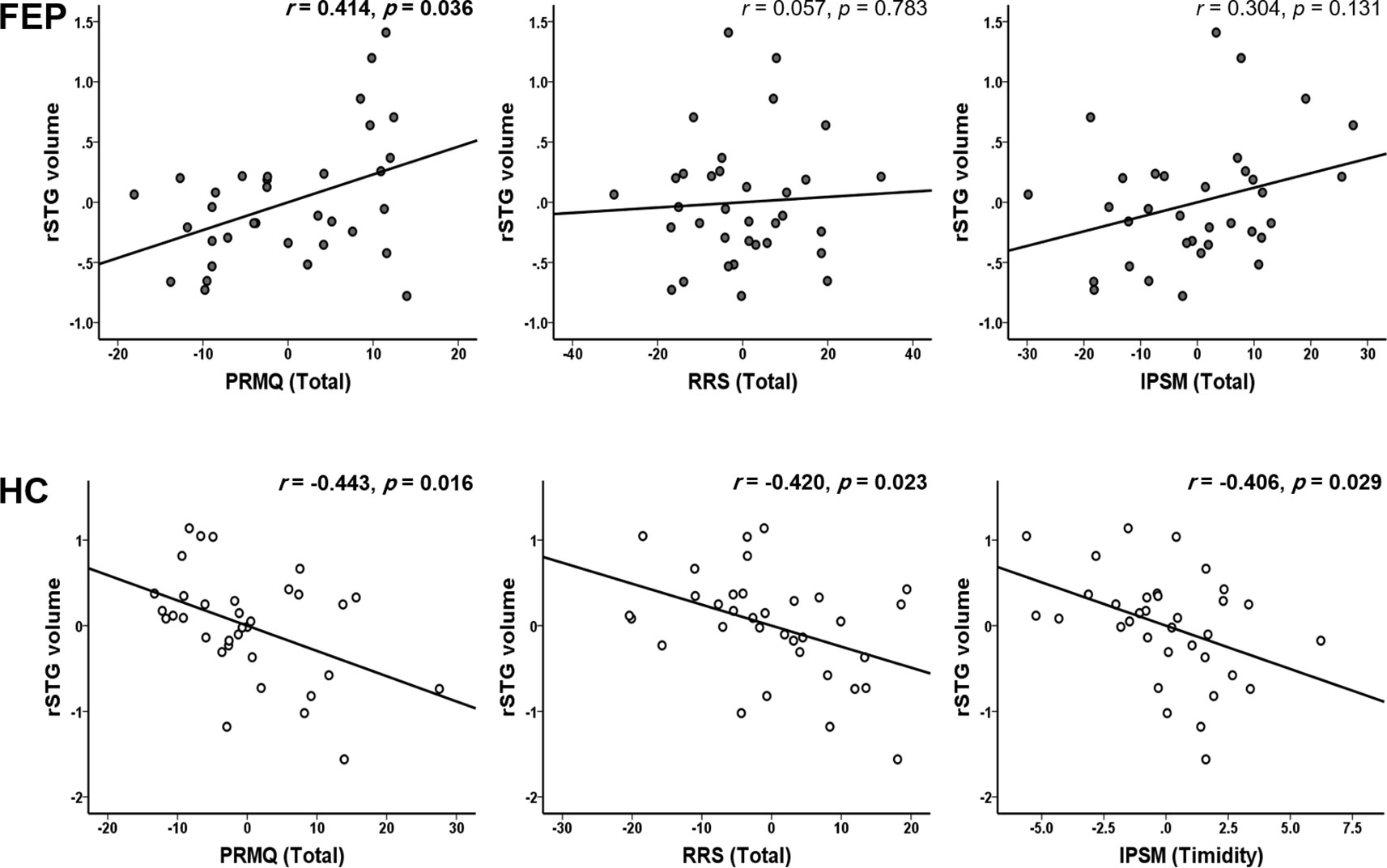
Figure 2 Significant group dependent moderation effect between self-reported psychopathology evaluations (prospective and retrospective memory questionnaire (PRMQ), ruminative response scale (RRS), and interpersonal sensitivity measure (IPSM)) and right superior temporal gyrus (rSTG) volume. In first episode psychosis (FEP) group, the PRMQ score significantly positively correlated with the rSTG volume, also RRS and IPSM, positively correlated with the rSTG volume. In healthy control (HC) group, the PRMQ, RRS, and IPSM scores significantly negatively correlated with the rSTG volume.
Discussion
The present study used the PRMQ, RRS, and IPSM to explore brain volume and self-awareness of psychopathology in patients with FEP. In the FEP group, the volume of the amygdala, hippocampus, and STG were significantly lower than in the HC group, while the PRMQ, RRS, and IPSM scores were significantly higher. Significant group-dependent moderation effects were found between self-awareness of psychopathology (PRMQ, RRS, and IPSM scores) and rSTG volume. The PRMQ score was positively associated with rSTG volume in the FEP group. However, the relationship was negative in the HC group.
In patients with FEP, the amygdala, hippocampus, and STG showed significantly lower volumes than in HCs. Reduced amygdala volume has repeatedly been demonstrated among patients with chronic schizophrenia (73–77), although another study reported no such volume reduction (78). Several studies have found that amygdala volume in patients with early-stage schizophrenia is smaller than in HCs (79, 68), while others have found no significant difference (80, 81). Rich et al. (82) demonstrated smaller amygdala volume during early illness than during chronic-stage schizophrenia. Meta-analyses have confirmed reduced hippocampal volume in patients with schizophrenia (83, 84), and some studies have identified decreased hippocampal volume in patients with chronic schizophrenia (85, 86), as well as in those with early schizophrenia (85, 84). The hippocampus plays a role in cognitive function, particularly memory (87). In schizophrenia, the hippocampus and parahippocampus have been correlated with accuracy and performance speed, memory, and executive function and abstraction (88, 89).
Smaller STG volume occurs in patients with schizophrenia when compared with HCs (90–93, 36, 94, 95). This volumetric reduction in the STG is progressive over time in individuals at ultra-high risk for psychosis, as well as in those with childhood onset and in those with schizophrenia and FEP (96, 97, 94, 95). In addition, a right-side dominant STG volumetric reduction has often been reported in both first-episode (98, 99) and chronic schizophrenia (76, 100). The STG contains the “social brain” network (101, 102, 103) and is responsible for auditory processing, language functions, and auditory memory (104, 105).
In the present study, subjective self-report scales were used to measure psychopathology. Although self-reporting tools have consistently demonstrated high reliability, meta-memory (i.e., individuals’ beliefs about their own memory ability) is not always highly correlated with actual performance in objective memory tests or clinical observation (106, 107). The present results showed that higher self-awareness of psychopathology scores positively associated with rSTG volume in the FEP group (PRMQ was significant, RRS and IPSM were insignificant). However, these correlations were negative in the HC group. This correlation was especially robust in the PRMQ. These results suggest that rSTG volume has significant implications for self-reporting memory. Since the PRMQ assesses a patient’s own amnesia experience, high scores imply high awareness of memory impairment (108). In addition, PRMQ is used to measure cognitive functions, as well as the broader neurobehavioral changes that are likely to occur alongside compromised functioning, such as loss of insight (109–111).
Significant group-dependent moderation effects were found between self-awareness of psychopathology (PRMQ, RRS, and IPSM scores) and rSTG volume, positive effect in the FEP group, and negative effect in the HC group. In patients with schizophrenia, poor insight (lower self-awareness of psychopathology) is associated with reduced total brain volume (112, 113), ventricular enlargement (114), frontal lobe atrophy (115), reduced frontal lobe volume (116–118), and gray matter deficits in the cingulate gyrus (119, 118), temporal lobe (119, 120), parietal lobe (120), and precuneus (120). In addition to these findings, the results of our study could provide further evidence on the characteristics of an individual’s poor insight into his or her psychopathology.
Meanwhile, healthy older adults with subjective memory impairments, defined as the feeling of worsening memory with normal memory performance, show smaller brain structures (121–125), especially in the medial temporal lobe region (122–126), hippocampus (127, 128, 121, 129), and amygdala (122, 130, 131, 124, 132). Additionally, smaller gray matter volume is associated with excessive rumination in healthy adults (133–136). No previous studies have addressed the correlation between interpersonal sensitivity and brain volume. These previous findings suggest that self-awareness of psychopathology would reduce brain volume in healthy individuals.
This study had some limitations. Firstly, we did not measure objective cognitive impairment. Secondly, we could not measure brain MRI in a unified way. Instead, images were acquired in two voxel sizes, even though voxel size has been identified as a covariate to correct for different brain sizes (71).
In conclusion, we found different correlation patterns between brain volume and self-awareness of psychopathology in the FEP and HC groups. In the FEP group, self-awareness of psychopathology was associated with increased rSTG volume. However, the HC group showed decreased rSTG volume when they were aware their discomfort. Our results indicate that self-awareness of psychopathy impacts rSTG volume differently in patients with FEP and HCs.
Data Availability Statement
The datasets for this manuscript are not publicly available because participants and guardians have not given consent for data sharing. Requests to access the datasets should be directed to SL (bHNocHNzQGhhbm1haWwubmV0).
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Chonbuk National University Hospital, Republic of Korea. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
J-YK contributed to analyzing data and wrote the paper. HJ collected the data and analyzed data. AK wrote sections of the manuscript. MJ wrote sections of the manuscript. S-HL and Y-CC supervised the study process and manuscript writing. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This study was supported by a grant from the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2018R1A2A2A05018505), a grant from the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HM14C2608), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and the Ministry of Health & Welfare, Republic of Korea (grant number HI18C2383).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Reed SI. First-episode psychosis: A literature review. Int J Ment Health Nurs (2008) 17(2):85–91. doi: 10.1111/j.1447-0349.2008.00515.x
2. Lincoln TM, Lüllmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull (2007) 33(6):1324–42. doi: 10.1093/schbul/sbm002
3. David AS. Insight and psychosis. Br J Psychiatry (1990) 156(6):798–808. doi: 10.1192/bjp.156.6.798
4. Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, et al. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry (1994) 51(10):826–36. doi: 10.1001/archpsyc.1994.03950100074007
5. Schwartz RC. The relationship between insight, illness and treatment outcome in schizophrenia. Psychiatr Q (1998) 69(1):1–22. doi: 10.1023/A:1022141322657
6. Cuffel BJ, Alford J, Fischer EP, Owen RR. Awareness of illness in schizophrenia and outpatient treatment adherence. J Nervous Ment Dis (1996) 184(11):653–659. doi: 10.1097/00005053-199611000-00001
7. Lally SJ. Does being in here mean there is something wrong with me? Schizophr Bull (1989) 15(2):253–65. doi: 10.1093/schbul/15.2.253
8. McCay EA, Seeman MV. A scale to measure the impact of a schizophrenic illness on an individual’s self-concept. Arch Psychiatr Nurs (1998) 12(1):41–9. doi: 10.1016/S0883-9417(98)80007-1
9. Warner R, Taylor D, Powers M, Hyman J. Acceptance of the mental illness label by psychotic patients: Effects on functioning. Am J Orthopsychiatry (1989) 59(3):398–409. doi: 10.1111/j.1939-0025.1989.tb01675.x
10. van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol M-J, Nolen WA, et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull (2013) 39(6)1288. doi: 10.1093/schbul/sbs122
11. Ayesa-Arriola R, Moríñigo JDL, David AS, Pérez-Iglesias R, Rodríguez-Sánchez JM, Crespo-Facorro B. Lack of insight 3 years after first-episode psychosis: an unchangeable illness trait determined from first presentation? Schizophr Res (2014) 157(1-3):271–7. doi: 10.1016/j.schres.2014.05.011
12. Keshavan MS, Rabinowitz J, DeSmedt G, Harvey PD, Schooler N. Correlates of insight in first episode psychosis. Schizophr Res (2004) 70(2-3):187–94. doi: 10.1016/j.schres.2003.11.007
13. Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull (1984) 10(2):300–12. doi: 10.1093/schbul/10.2.300
14. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry (2001) 58(2):158–64. doi: 10.1001/archpsyc.58.2.158
15. Moritz S, Krausz M, Gottwalz E, Lambert M, Perro C, Ganzer S, et al. Cognitive dysfunction at baseline predicts symptomatic 1-year outcome in first-episode schizophrenics. Psychopathology (2000) 33(1):48–51. doi: 10.1159/000029119
16. Chan RC, Wang Y, Ma Z, Hong X, Yuan Y, Yu X, et al. Objective measures of prospective memory do not correlate with subjective complaints in schizophrenia. Schizophr Res (2008) 103(1-3):229–39. doi: 10.1016/j.schres.2008.02.019
17. Kemp R, David A. Insight and compliance In Treatment Compliance and the Therapeutic Alliance in Serious Mental Illness. Switzerland: Harwood Acad publishers (1997). p. 61–84.
18. Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. (2004). A new instrument for measuring insight: the Beck Cognitive insight scale. Schizophr Res 68(2-3), 319-329. doi: 10.1016/S0920-9964(03)00189-0
19. Subotnik KL, Nuechterlein KH. Prodromal signs and symptoms of schizophrenic relapse. J Abnormal Psychol (1988) 97(4):405–12. doi: 10.1037/0021-843X.97.4.405
20. Hambrecht M, Häfner H, Löffler W. Beginning schizophrenia observed by significant others. Soc Psychiatry Psychiatr Epidemiol (1994) 29(2):53–60. doi: 10.1007/BF00805621
21. Bigdeli I, Farzin A, Talepasand S. Prospective memory impairments in schizophrenic patients. Iranian J Psychiatry Behav Sci (2014) 8(4):57–63.
22. Hartley S, Haddock G, Sa DV, Emsley R, Barrowclough C. An experience sampling study of worry and rumination in psychosis. Psychol Med (2014) 44(8):1605–14. doi: 10.1017/s0033291713002080
23. Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull (2008) 134(2):163–206. doi: 10.1037/0033-2909.134.2.163
24. Jones SR, Fernyhough C. Rumination, reflection, intrusive thoughts, and hallucination-proneness: towards a new model. Behav Res Ther (2009) 47(1):54–9. doi: 10.1016/j.brat.2008.09.008
25. Freeman D, Startup H, Dunn G, Černis E, Wingham G, Pugh K, et al. The interaction of affective with psychotic processes: a test of the effects of worrying on working memory, jumping to conclusions, and anomalies of experience in patients with persecutory delusions. J Psychiatr Res (2013) 47(12):1837–42. doi: 10.1016/j.jpsychires.2013.06.016
26. Halari R, Premkumar P, Farquharson L, Fannon D, Kuipers E, Kumari V. Rumination and negative symptoms in schizophrenia. J nervous Ment Dis (2009) 197(9):703–6. doi: 10.1097/nmd.0b013e3181b3af20
27. van Nierop M, van Os J, Gunther N, Myin-Germeys I, de Graaf R, Have M, et al. Phenotypically continuous with clinical psychosis, discontinuous in need for care: evidence for an extended psychosis phenotype. Schizophr Bull (2012) 38(2):231–8. doi: 10.1093/schbul/sbr129
28. Rapado-Castro M, McGorry PD, Yung A, Calvo A, Nelson B. Sources of clinical distress in young people at ultra high risk of psychosis. Schizophr Res (2015) 165(1):15–21. doi: 10.1016/j.schres.2015.03.022
29. Boyce P, Parker G. Development of a scale to measure interpersonal sensitivity. Aust New Z J Psychiatry (1989) 23(3):341–51. doi: 10.3109/00048678909068294
30. Meisel SF, Garety PA, Stahl D, Valmaggia LR. Interpersonal processes in paranoia: a systematic review. Psychol Med (2018) 48(14):2299–312. doi: 10.1017/s0033291718000491
31. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry (2008) 165(8):1015–23. doi: 10.1176/appi.ajp.2008.07101562
32. Burke L, Androutsos C, Jogia J, Byrne P, Frangou S. The Maudsley Early Onset Schizophrenia Study: the effect of age of onset and illness duration on fronto-parietal gray matter. Eur Psychiatry (2008) 23(4):233–6. doi: 10.1016/j.eurpsy.2008.03.007
33. Wood SJ, Reniers RL, Heinze K. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry (2013) 58(1):13–8. doi: 10.1177/070674371305800104
34. Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron (2006) 50(2):329–39. doi: 10.1016/j.neuron.2006.03.015
35. Sass K, Habel U, Sachs O, Huber W, Gauggel S, Kircher T. The influence of emotional associations on the neural correlates of semantic priming. Hum Brain Mapp (2012) 33(3):676–94. doi: 10.1002/hbm.21241
36. Sun J, Maller JJ, Guo L, Fitzgerald PB. Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Res Rev (2009) 61(1):14–32. doi: 10.1016/j.brainresrev.2009.03.004
37. Huang P, Xi Y, Lu Z-L, Chen Y, Li X, Li W, et al. Decreased bilateral thalamic gray matter volume in first-episode schizophrenia with prominent hallucinatory symptoms: A volumetric MRI study. Sci Rep (2015) 5:14505. doi: 10.1038/srep14505
38. Crespo-Facorro B, Roiz-Santiáñez R, Pelayo-Terán JM, Rodríguez-Sánchez JM, Pérez-Iglesias R, González-Blanch C, et al. Reduced thalamic volume in first-episode non-affective psychosis: correlations with clinical variables, symptomatology and cognitive functioning. Neuroimage (2007) 35(4):1613–23. doi: 10.1016/j.neuroimage.2007.01.048
39. Guerrero-Pedraza A, McKenna P, Gomar J, Sarro S, Salvador R, Amann B, et al. First-episode psychosis is characterized by failure of deactivation but not by hypo-or hyperfrontality. Psychol Med (2012) 42(1):73–84. doi: 10.1017/s0033291711001073
40. Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, et al. Other minds in the brain: a functional imaging study of “theory of mind”. story comprehensionCognition (1995) 57(2):109–28. doi: 10.1016/0010-0277(95)00692-R
41. Schultz J, Imamizu H, Kawato M, Frith CD. Activation of the Human Superior Temporal Gyrus during Observation of Goal Attribution by Intentional Objects. J Cogn Neurosci (2004) 16(10):1695–705. doi: 10.1162/0898929042947874
42. Straube B, Green A, Sass K, Kircher T. Superior Temporal Sulcus Disconnectivity During Processing of Metaphoric Gestures in Schizophrenia. Schizophr Bull (2013) 40(4):936–44. doi: 10.1093/schbul/sbt110
43. First MB, Spitzer R, Gibbon M, Williams JB. Structured clinical interview for DSM-IV clinical version (SCID-I/CV). Washington, DC: American Psychiatric Press (1997).
44. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13(2):261–76. doi: 10.1093/schbul/13.2.261
45. Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley J, Tollefson GD. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry (1999) 156(1):79–87. doi: 10.1176/ajp.156.1.79
46. Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet (2008) 371(9618):1085–97. doi: 10.1016/S0140-6736(08)60486-9
47. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia (1971) 9(1):97–113. doi: 10.1016/0028-3932(71)90067-4
48. Smith G, Del Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory (2000) 8(5):311–21. doi: 10.1080/09658210050117735
49. Crawford J, Smith G, Maylor E, Della Sala S, Logie R. The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory (2003) 11(3):261–75. doi: 10.1080/09658210244000027
50. Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol (1991) 61(1):115–21. doi: 10.1037//0022-3514.61.1.115
51. Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cogn Ther Res (2003) 27(3):247–59. doi: 10.1023/A:102391031
52. Cho H-J. A study on personality and cognitive-behavioral factors vulnerable to depression. Unpublished doctoral dissertation. Sung Kyun Kwan University University: Doctoral (2000).
53. Yi JS, Ahn YM, Shin HK, An SK, Joo YH, Kim SH, et al. Reliability and validity of the Korean version of the Positive and Negative Syndrome Scale. J Korean Neuropsychiatr Assoc (2001) 40(6):1090–105.
54. Guy W. ECDEU assessment manual for psychopharmacology. Rockville, Md: US Department of Health, Education and Welfare (1976).
55. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res (1990) 3(4):247–51. doi: 10.1016/0920-9964(90)90005-r
56. Kim YK, Won SD, Lee KM, Choi HS, Jang HS, Lee BH, et al. A study on the reliability and validity of the Korean version of the Calgary Depression Scale for Schizophrenia (K-CDSS). J Korean Neuropsychiatr Assoc (2005) 44(4):446–55.
57. Kim S-W, Kim S-J, Yoon B-H, Kim J-M, Shin I-S, Hwang MY, et al. Diagnostic validity of assessment scales for depression in patients with schizophrenia. Psychiatry Res (2006) 144(1):57–63. doi: 10.1016/j.psychres.2005.10.002
58. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association (1994).
59. Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage (2000) 11(6):805–21. doi: 10.1006/nimg.2000.0582
60. Ashburner J. Computational anatomy with the SPM software. Magnetic resonance Imaging (2009) 27(8):1163–74. doi: 10.1016/j.mri.2009.01.006
61. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage (2007) 38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007
62. Ashburner J, Friston KJ. Unified segmentation. Neuroimage (2005) 26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018
63. Crespo-Facorro B, Kim J-J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res (2000) 46(1):35–43. doi: 10.1016/s0920-9964(00)00028-1
64. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry (2000) 57(11):1033–8. doi: 10.1001/archpsyc.57.11.1033
65. Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, et al. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cereb Cortex (2002) 12(12):1331–41. doi: 10.1093/cercor/12.12.1331
66. Sumich A, Chitnis XA, Fannon DG, O’Ceallaigh S, Doku VC, Faldrowicz A, et al. Unreality symptoms and volumetric measures of Heschl’s gyrus and planum temporal in first-episode psychosis. Biol Psychiatry (2005) 57(8):947–50. doi: 10.1016/j.biopsych.2004.12.041
67. Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Höschl C. Amygdala volumes in mood disorders—meta-analysis of magnetic resonance volumetry studies. J Affect Disord (2009) 115(3):395–410. doi: 10.1016/j.jad.2008.10.007
68. Watson DR, Bai F, Barrett SL, Turkington A, Rushe TM, Mulholland CC, et al. Structural changes in the hippocampus and amygdala at first episode of psychosis. Brain Imaging Behav (2012) 6(1):49–60. doi: 10.1007/s11682-011-9141-4
69. Qiu A, Gan S, Wang Y, Sim K. Amygdala–hippocampal shape and cortical thickness abnormalities in first-episode schizophrenia and mania. Psychol Med (2013) 43(7):1353–63. doi: 10.1017/s0033291712002218
70. Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther (2017) 98:39–57. doi: 10.1016/j.brat.2016.11.001
71. Segall JM, Turner JA, Van Erp TG, White T, Bockholt HJ, Gollub RL, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophr Bull (2008) 35(1):82–95. doi: 10.1093/schbul/sbn150
72. Westfall PH. On using the bootstrap for multiple comparisons. J biopharmaceutical Stat (2011) 21(6):1187–205. doi: 10.1080/10543406.2011.607751
73. Niu L, Matsui M, Zhou S-Y, Hagino H, Takahashi T, Yoneyama E, et al. Volume reduction of the amygdala in patients with schizophrenia: a magnetic resonance imaging study. Psychiatry Res: Neuroimaging (2004) 132(1):41–51. doi: 10.1016/j.pscychresns.2004.06.002
74. Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? An anatomical likelihood meta-analysis. Front Hum Neurosci (2010) 4:189. doi: 10.3389/fnhum.2010.00189
75. Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res (2011) 127(1-3):46–57. doi: 10.1016/j.schres.2010.12.020
76. Chan RC, Di X, McAlonan GM, Gong Q.-y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull (2011) 37(1):177–88. doi: 10.1093/schbul/sbp073
77. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehavioral Rev (2012) 36(4):1342–56. doi: 10.1016/j.neubiorev.2011.12.015
78. Tanskanen P, Veijola JM, Piippo UK, Haapea M, Miettunen JA, Pyhtinen J, et al. Hippocampus and amygdala volumes in schizophrenia and other psychoses in the Northern Finland 1966 birth cohort. Schizophr Res (2005) 75(2-3):283–94. doi: 10.1016/j.schres.2004.09.022
79. Joyal CC, Laakso MP, Tiihonen J, Syvälahti E, Vilkman H, Laakso A, et al. The amygdala and schizophrenia: a volumetric magnetic resonance imaging study in first-episode, neuroleptic-naive patients. Biol Psychiatry (2003) 54(11):1302–4. doi: 10.1016/s0006-3223(03)00597-3
80. Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res (2006) 82(1):75–88. doi: 10.1016/j.schres.2005.11.004
81. Bodnar M, Malla AK, Czechowska Y, Benoit A, Fathalli F, Joober R, et al. Neural markers of remission in first-episode schizophrenia: a volumetric neuroimaging study of the hippocampus and amygdala. Schizophr Res (2010) 122(1-3):72–80. doi: 10.1016/j.schres.2010.06.013
82. Rich AM, Cho YT, Tang Y, Savic A, Krystal JH, Wang F, et al. Amygdala volume is reduced in early course schizophrenia. Psychiatry Res: Neuroimaging (2016) 250:50–60. doi: 10.1016/j.pscychresns.2016.02.006
83. Steen RG, Mull C, Mcclure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry (2006) 188(6):510–8. doi: 10.1192/bjp.188.6.510
84. Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. Neuroscientist (2012) 18(2):180–200. doi: 10.1177/1073858410395147
85. Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra–high-risk individuals. Arch Gen Psychiatry (2006) 63(2):139–49. doi: 10.1001/archpsyc.63.2.139
86. Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ Jr., Pung CJ, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry (2010) 68(1):41–50. doi: 10.1016/j.biopsych.2010.03.036
87. Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn (2007) 65(3):209–37. doi: 10.1016/j.bandc.2007.02.007
88. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, et al. Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res (1995) 17(1):47–58. doi: 10.1016/0920-9964(95)00028-k
89. Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res (2004) 70(2-3):117–45. doi: 10.1016/j.schres.2003.12.002
90. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry (2000) 57(7):692–9. doi: 10.1001/archpsyc.57.7.692
91. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res (2001) 49(1-2):1–52. doi: 10.1016/s0920-9964(01)00163-3
92. Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee C-U, Ciszewski AA, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry (2003) 160(1):156–64. doi: 10.1176/appi.ajp.160.1.156
93. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry (2005) 162(12):2233–45. doi: 10.1176/appi.ajp.162.12.2233
94. Takahashi T, Suzuki M, Zhou S-Y, Tanino R, Nakamura K, Kawasaki Y, et al. A follow-up MRI study of the superior temporal subregions in schizotypal disorder and first-episode schizophrenia. Schizophr Res (2010) 119(1-3):65–74. doi: 10.1016/j.schres.2009.12.006
95. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Trans Psychiatry (2012) 2(11):e190. doi: 10.1038/tp.2012.116
96. Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci (2001) 98(20):11650–5. doi: 10.1073/pnas.201243998
97. Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry (2009) 66(4):366–76. doi: 10.1001/archgenpsychiatry.2009.12
98. Kong L, Bachmann S, Thomann PA, Essig M, Schröder J. Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res (2012) 134(1):27–32. doi: 10.1016/j.schres.2011.09.015
99. Guo X, Li J, Wang J, Fan X, Hu M, Shen Y, et al. Hippocampal and orbital inferior frontal gray matter volume abnormalities and cognitive deficit in treatment-naive, first-episode patients with schizophrenia. Schizophr Res (2014) 152(2-3):339–43. doi: 10.1016/j.schres.2013.12.015
100. Kong L, Herold CJ, Zöllner F, Salat DH, Lässer MM, Schmid LA, et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: a matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res: Neuroimaging (2015) 231(2):176–83. doi: 10.1016/j.pscychresns.2014.12.004
101. Brothers L, Ring B. A neuroethological framework for the representation of minds. J Cogn Neurosci (1992) 4(2):107–18. doi: 10.1162/jocn.1992.4.2.107
102. Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci (1999) 11(6):1891–8. doi: 10.1046/j.1460-9568.1999.00621.x
103. Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol (2007) 31(2):217–38. doi: 10.1080/87565640701190841
104. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature (2000) 403:309–12. doi: 10.1038/35002078
105. Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G. Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex (2003) 13(10):1034–43. doi: 10.1093/cercor/13.10.1034
106. Morris PE. The validity of subjective reports on memory. In: Harris JE, Morris PE, editors. London: Everyday memory, actions and absent-mindedness. Academic Press (1984). p. 153–72.
107. Craik FIM, Anderson ND, Kerr SA, Li KZH. Memory changes in normal ageing. In: Baddeley AD, Wilson BA, Watts FN, editors. Handbook of memory disorders. New York: John Wiley & Sons (1995). p. 211–41.
108. Eisenacher S, Zink M. The Importance of Metamemory Functioning to the Pathogenesis of Psychosis. Front Psychol (2017) 8:304. doi: 10.3389/fpsyg.2017.00304
109. Bogod NM, Mateer CA, Macdonald SW. Self-awareness after traumatic brain injury: A comparison of measures and their relationship to executive functions. J Int Neuropsychol Soc (2003) 9(3):450–8. doi: 10.1017/s1355617703930104
110. Crawford JR, Henry JD, Ward AL, Blake J. The Prospective and Retrospective Memory Questionnaire (PRMQ): Latent structure, normative data and discrepancy analysis for proxy-ratings. Br J Clin Psychol (2006) 45(1):83–104. doi: 10.1348/014466505x28748
111. Chan RC, Bode RK. Analysis of patient and proxy ratings on the Dysexecutive Questionnaire: an application of Rasch analysis. J Neurol Neurosurgery Psychiatry (2008) 79(1):86–8. doi: 10.1136/jnnp.2007.117184
112. Flashman LA, McAllister TW, Andreasen NC, Saykin AJ. Smaller brain size associated with unawareness of illness in patients with schizophrenia. Am J Psychiatry (2000) 157(7):1167–9. doi: 10.1176/appi.ajp.157.7.1167
113. McEVOY JP, Johnson J, Perkins D, Lieberman JA, Hamer RM, Keefe RS, et al. Insight in first-episode psychosis. Psychol Med (2006) 36(10):1385–93. doi: 10.1017/S0033291706007793
114. Takai A, Uematsu M, Ueki H, Sone K. Insight and its related factors in chronic schizophrenic patients: A preliminary study. Eur J Psychiatry (1992) 6(3):159–70.
115. Larøi F, Fannemel M, Rønneberg U, Flekkøy K, Opjordsmoen S, Dullerud R, et al. Unawareness of illness in chronic schizophrenia and its relationship to structural brain measures and neuropsychological tests. Psychiatry Res: Neuroimaging (2000) 100(1):49–58. doi: 10.1016/s0925-4927(00)00063-9
116. Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J neuropsychiatry Clin Neurosci (2001) 13(2):255–7. doi: 10.1176/jnp.13.2.255
117. Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res (2006) 86(1-3):54–70. doi: 10.1016/j.schres.2006.06.006
118. Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res (2007) 89(1-3):22–34. doi: 10.1016/j.schres.2006.09.016
119. Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res: Neuroimaging (2004) 132(3):251–60. doi: 10.1016/j.psychresns.2004.05.001
120. Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res (2008) 103(1-3):40–51. doi: 10.1016/j.schres.2008.04.022
121. van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, Mutsaers ER, Bollen EL, Admiraal-Behloul F, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol (2004) 251(6):671–5. doi: 10.1007/s00415-004-0390-7
122. Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging (2006) 27(12):1751–6. doi: 10.1016/j.neurobiolaging.2005.10.010
123. Saykin A, Wishart H, Rabin L, Santulli R, Flashman L, West J, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology (2006) 67(5):834–42. doi: 10.1212/01.wnl.0000234032.77541.a2
124. Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dementia geriatric Cogn Disord (2010) 29(1):75–81. doi: 10.1159/000264630
125. Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry (2011) 198(3):199–205. doi: 10.1192/bjp.bp.110.078683
126. Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology (2012) 79(13):1332–9. doi: 10.1212/wnl.0b013e31826c1a8d
127. de Groot JC, De Leeuw F-E, Oudkerk M, Hofman A, Jolles J, Breteler M. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology (2001) 56(11):1539–45. doi: 10.1212/wnl.56.11.1539
128. Grön G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW. Subjective memory complaints: objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Ann Neurol: Off J Am Neurological Assoc Child Neurol Soc (2002) 51(4):491–8. doi: 10.1002/ana.10157
129. Van Norden A, Fick W, De Laat K, van Uden I, van Oudheusden L, Tendolkar I, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology (2008) 71(15):1152–9. doi: 10.1212/01.wnl.0000327564.44819.49
130. Stewart R, Dufouil C, Godin O, Ritchie K, Maillard P, Delcroix N, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology (2008) 70(18):1601–7. doi: 10.1212/01.wnl.0000310982.99438.54
131. Tepest R, Wang L, Csernansky JG, Neubert P, Heun R, Scheef L, et al. Hippocampal surface analysis in subjective memory impairment, mild cognitive impairment and Alzheimer’s dementia. Dementia geriatric Cogn Disord (2008) 26(4):323–9. doi: 10.1159/000161057
132. Kim M-J, Seo SW, Kim GH, Kim ST, Lee J-M, Qiu A, et al. Less depressive symptoms are associated with smaller hippocampus in subjective memory impairment. Arch gerontology geriatrics (2013) 57(1):110–5. doi: 10.1016/j.archger.2013.01.005
133. Kühn S, Vanderhasselt M-A, De Raedt R, Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. J Affect Disord (2012) 141(2-3):352–60. doi: 10.1016/j.jad.2012.03.024
134. Wang K, Wei D, Yang J, Xie P, Hao X, Qiu J. Individual differences in rumination in healthy and depressive samples: association with brain structure, functional connectivity and depression. Psychol Med (2015) 45(14):2999–3008. doi: 10.1017/s0033291715000938
135. Ismaylova E, Di Sante J, Gouin J-P, Pomares FB, Vitaro F, Tremblay RE, et al. Associations between Daily Mood States with Brain Gray Matter Volume, Resting-state Functional Connectivity and Task-based Activity in Healthy Adults. Front Hum Neurosci (2018) 12:168. doi: 10.3389/fnhum.2018.00168
Keywords: schizophrenia, prospective and retrospective memory questionnaire, ruminative response scale, interpersonal sensitivity measure, right superior temporal gyrus
Citation: Kim J-Y, Jeon H, Kwon A, Jin MJ, Lee S-H and Chung Y-C (2019) Self-Awareness of Psychopathology and Brain Volume in Patients With First Episode Psychosis. Front. Psychiatry 10:839. doi: 10.3389/fpsyt.2019.00839
Received: 25 June 2019; Accepted: 22 October 2019;
Published: 15 November 2019.
Edited by:
Neeltje E. M. Van Haren, Erasmus University Rotterdam, NetherlandsReviewed by:
Daniel Jonas Hauke, University of Basel, SwitzerlandAndré Schmidt, University of Basel, Switzerland
Copyright © 2019 Kim, Jeon, Kwon, Jin, Lee and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung-Hwan Lee, bHNocHNzQHBhaWsuYWMua3I=; bHNocHNzQGhhbm1haWwubmV0; Young-Chul Chung, Y2h1bmd5Y0BqYm51LmFjLmty
 Jeong-Youn Kim
Jeong-Youn Kim Hyeonjin Jeon1
Hyeonjin Jeon1 Min Jin Jin
Min Jin Jin Seung-Hwan Lee
Seung-Hwan Lee Young-Chul Chung
Young-Chul Chung