- 1Division of Psychiatry, Department of Brain Sciences, Imperial College London, London, United Kingdom
- 2Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom
- 3Medical Sciences Division, Oxford Institute of Clinical Psychology Training, University of Oxford, Oxford, United Kingdom
- 4Copenhagen Affective Disorder Research Centre (CADIC), Psychiatric Centre Copenhagen, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
- 5Division of Psychology, Department for Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
- 6Department of Psychology, Uppsala University, Uppsala, Sweden
- 7Department of Psychology, University of Copenhagen, Copenhagen, Denmark
Background: Mental imagery abnormalities feature across affective disorders including bipolar disorder (BD) and unipolar depression (UD). Maladaptive emotional imagery has been proposed as a maintenance factor for affective symptomatology and a target for mechanism-driven psychological treatment developments. Where imagery abnormalities feature beyond acute affective episodes, further opportunities for innovation arise beyond treatments, such as for tertiary/relapse prevention (e.g., in remitted individuals) or primary prevention (e.g., in non-affected but at-risk individuals). The aim of our study was to investigate for the first time the presence of possible mental imagery abnormalities in affected individuals in remission and at-risk individuals for affective disorders using a familial risk design.
Methods: A population-based cohort of monozygotic twins was recruited through linkage between the Danish national registries (N=204). Participants were grouped as: affected (remitted BD/UD; n = 115); high-risk (co-twin with history of BD/UD; n = 49), or low-risk (no co-twin history of BD/UD; n = 40). Twins completed mental imagery measures spanning key subjective domains (spontaneous imagery use and emotional imagery) and cognitive domains (imagery inspection and imagery manipulation).
Results: Affected twins in remission reported enhanced emotional mental imagery compared to both low- and high-risk twins. This was characterized by greater impact of i) intrusive prospective imagery (Impact of Future Events Scale) and ii) deliberately-generated prospective imagery of negative scenarios (Prospective Imagery Task). There were no significant differences in these key measures between affected BD and UD twins in remission. Additionally, low- and high-risk twins did not significantly differ on these emotional imagery measures. There were also no significant differences between the three groups on non-emotional measures including spontaneous imagery use and cognitive stages of imagery.
Conclusions: Abnormalities in emotional prospective imagery are present in monozygotic twins with affective disorders in remission—despite preserved cognitive stages of imagery—but absent in unaffected high-risk twins, and thus do not appear to index familial risk (i.e., unlikely to qualify as “endophenotypes”). Elevated emotional prospective imagery represents a promising treatment/prevention target in affective disorders.
Introduction
Mental imagery refers to the experience of perception in the absence of external sensory input, for example “seeing in the mind’s eye” (1). Cognitive science suggests that mental imagery is more emotionally-evocative than its verbal-based counterpart (2). Emotional imagery symptoms feature across affective disorders (3–6). Clinical formulation in psychology suggests such mental imagery contributes to the disorder maintenance, and as such imagery presents opportunities for treatment innovation, especially for areas of considerable clinical challenge such as bipolar disorder/BD (7) and anhedonia in unipolar depression/UD (8). In psychopathology, adaptive positive imagery can also be promoted (9–11), and conversely, maladaptive negative imagery can be disrupted (12–15).
Imagery—in models of psychological treatments—has been primarily conceptualized as a maintenance (“proximal”) factor, i.e., keeping the disorder going once it developed. Delineating the role of imagery throughout illness progression and outside of the illness episode can help map other areas of application that stand to gain from a focus on imagery. For example, emotional imagery abnormalities, which are (causal) risk factors proceeding disorder onset, might be addressed to prevent disorder emergence (i.e., primary prevention). Likewise, imagery abnormalities that persist in remission can serve as target for keeping the individual well and preventing relapse (i.e., tertiary prevention). Importantly, identifying potential cognitive maintenance and/or risk factors paves the way for testing mechanistic hypotheses and mechanism-based interventions—both psychological and pharmacological (16).
Affective disorders (UD and BD, defined as disorders in which the fundamental disturbance is a change in affect or mood to depression) (17) hold one of the highest burdens of disease worldwide (18). There is a need for better identification of risk and resilience markers to develop better therapeutic interventions at different stages of disease. Affective disorders present variable degrees of heritability, from high rates in BD (19, 20) to moderate rates in UD (20).
Biases in cognition—in both “cold” (non-emotional) and “hot” (emotional-laden) information processing spanning domains of perception, attention, memory, and learning—have been associated with acute disorder episodes (21, 22) well as after recovery from acute episodes (23, 24) and in familial risk (25). Cognitive abnormalities that persist in remitted states (i.e. are state-independent) may represent illness-related traits, conferring cognitive vulnerability for relapse (26, 27). If trait-abnormalities also meet further criteria such as heritability and higher frequency in individuals at familial risk compared to the general population, these may constitute “endophenotypes” of the disorder—i.e., potentially lying along the causal pathway between genes and disorder (28)—which could guide the discovery of genetic etiological mechanisms and inform clinical efforts including preventative strategies targeting such mechanisms in disorders with high genetic risk.
Emotional imagery has been proposed to be an “emotional amplifier” that drives both depressive/anxiety and manic symptoms in BD (29). Such images may depict aspects of the future, thought to underline associated emotions and behaviors such as wellbeing, prediction, and planning (30, 31). BD and UD have both been associated with heightened involuntary and intrusive prospective imagery (32–34)—more recurrent and impactful images of personally-relevant future real-world scenarios that spring to mind unbidden (e.g., an upcoming job interview). BD and UD have also been associated with more vivid and “real” future negative images that are deliberately-generated, such as under direct instructions in the laboratory to imagine in response to statements such as “someone close will reject you” (32). Some phenomenological aspects may show more disorder-specific profiles. For instance, imagery can be “overactive” in positive states in BD only (32, 35) possibly driving escalation to mania similar to other positive emotion biases (36), and suicidal imagery may be more “compelling” in BD compared to UD (37) in line with higher suicidal rates in BD (38, 39).
There is a paucity of studies of cognitive (non-emotional) aspects of mental imagery in affective disorders, such as studies based on a key computational model that identifies four cognitive stages of mental imagery (1, 40). In this model, mental images are thought to be initially created from either short-term or long-term memory (i.e., generation); once held in mind temporarily avoiding immediate decay (i.e., maintenance), such images’ characteristics can be interpreted/scanned (i.e., inspection) and further transformed (i.e., manipulation). Available evidence from one study in UD points to potential deficits in both imagery generation and manipulation (41). The only study simultaneously assessing emotional and cognitive domains of imagery across affective disorders showed that in BD performance on non-emotional imagery tasks may vary depending on the cognitive stage tested, with deficits present in imagery manipulation alongside superior performance in imagery maintenance (32).
To date, imagery research on BD and UD has primarily involved individuals with mix of acute and recovered depression episodes (32, 33, 35, 37), and abnormalities remain untested for remitted states in affective disorders.
Imagery may also play a role in the etiology of affective disorders, i.e., involved also in the initial emergence of the disorder. This is supported by abnormalities detected in non-clinical samples with subclinical features of BD and UD (42–45) and UD (30, 46, 47). Nevertheless, it is unclear whether or not such imagery-based abnormalities reflect familial risk and represent candidate “endophenotypes” for affective disorders (28).
The present study investigated the presence of imagery-based abnormalities in a population-based cohort of monozygotic twins, grouped as affected (remitted or partially-remitted twins with personal history of BD/UD), high-risk (unaffected twins with co-twin history of BD/UD), and low-risk (unaffected twins with no co-twin history of BD/UD). Participants completed assessments of subjective domains of mental imagery and cognitive (non-emotional) imagery stages, informed by our previous research (32,40).
Our primary aims were to delineate whether imagery abnormalities i) persist in remission (by comparing affected twins in remission versus unaffected low-risk twins); and ii) are present in twins at high familial risk for affective disorders (by comparing high-risk twins versus both remitted and low-risk twins). If both i) and ii) were true, this would be consistent with the proposition that imagery abnormalities are candidate “endophenotypes” of affective disorders. Further, we conducted exploratory analyses separating BD from UD; comparing BD vs. UD affected twins directly; and assessing imagery-symptom links transdiagnostically.
Method
Participants
A nationwide record linkage of the Danish Twin Registry (48) and the Danish Psychiatric Central Research Register (DPCRR) (49) identified eligible monozygotic twins. In addition to monozygosity, eligibility criteria were i) a personal or co-twin history of an affective spectrum diagnosis (i.e. International Classification for Diseases ICD-10 codes F30-34.0 and F38.0) (17) or for low-risk twins neither a personal nor a co-twin history affective spectrum diagnosis from January 1995 to June 2014, and ii) age 18–50 years.
Exclusion criteria for all groups were: birth weight under 1.3 kg, history of brain injury; current severe somatic illness, current substance abuse; current mood episode defined by Hamilton Depression Rating Scale (HDRS-17) (50) or Young Mania Rating Scale (51) (YMRS; scores >14), current pregnancy, or being dizygotic. The low-risk twins were also excluded if they reported other first-degree relatives with organic mental disorder, schizophrenia spectrum, or affective disorders.
Participants provided their written and informed consent to the study in accordance with the Helsinki declaration. The study was approved by the ethics committee for the Capital Region of Denmark (H-3-2014-003) and the Danish data protection agency (2014–331–0751).
Recruitment took place from December 2014 until January 2017. From an initial sample of 215 participants, 11 participants were excluded due to missing diagnoses (n = 6), affective disorder (n = 4), or alternative high-risk affective disorder (n=1). The final sample for this paper included 204 participants, with each classified as affected (n = 115; BD = 31 and UD = 84), high-risk (n = 49; BD = 11 and UD = 38), or low-risk (n = 40). There were 25 concordant affected twin-pairs (BD/BD: n = 5; UD/UD: n = 11; BD/UD: n = 9), 45 discordant twin-pairs (high-risk/UD: n = 36; high-risk/BD: n = 9), 19 low-risk twin pairs, and 26 single twins.
Overall Procedure
Participants attended a 1-day assessment at the Danish Research Centre for Magnetic Resonance at Copenhagen University Hospital Hvidovre. Further data from this sample have been reported elsewhere, including neurocognitive (52–55), clinical/psychological (56), and biological outcomes (57). Here we report data on assessments related to mental imagery for the first time.
Assessments
Clinical Characteristics
Diagnoses of psychiatric illness were assessed with the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) (58). Ratings and a SCAN interview were conducted by two PhD students blinded to the DPCRR register diagnoses. The research diagnoses obtained from the SCAN interviews determined the final assignment to groups. Pre-morbid intelligence (IQ) was assessed with the Danish Adult Reading Task (59). Depressive symptoms were assessed with the HDRS-17 (50). State anxiety was assessed with the State-Trait Anxiety Inventory form-Y (STAI-Y) (60). Manic symptoms were assessed with the YMRS (51).
Subjective Domains of Mental Imagery
Spontaneous Imagery Use
The Spontaneous Use of Imagery Scale (SUIS) (61) is a 12-item self-report scale which measures the use of non-emotional mental imagery in daily life. Each item is rated on a 5-point Likert-type scale. Total scores range from 12 to 60, with higher scores indicating greater use of imagery. An example item is “When I think about a series of errands I must do, I visualize the stores I will visit”.
Emotional Mental Imagery
Three assessments were considered to gauge various aspects of emotional imagery. The Impact of Future Events Scale (IFES) (62) is a 24-item self-report scale which is used to assess intrusive (involuntary) future-oriented imagery. Participants were asked to identify three future events which they had thought about or imagined over the previous week, and state whether each was positive or negative. Participants then responded to 24 statements about prospective imagery in relation to these events. Each item is rated on a 5-point Likert-type scale. Total scores range from 0 to 96, with higher scores indicating greater emotional impact of involuntarily-generated prospective imagery.
The Prospective Imagery Task (PIT) (63) is used to assess aspects of deliberately-generated (voluntary) imagery for imagined future events. Participants were presented with 10 positive and 10 negative hypothetical future scenarios and asked to generate a mental image of each. They were then asked to rate each image of a 5-point Likert-type scale, assessing vividness, likelihood of the event occurring to them, and how much they felt as though they were experiencing the event while imagining it. Total scores for each dimension range from 20 to 100, with higher total scores indicating greater subjective experience of deliberately-generated prospective imagery.
The Mental Imagery Interview (MII) (32) is a semi-structured interview which contains 12-items each for low-mood, anxious, and high-mood states. It explored the subjective experience, occurrence, and content of a significant mental image in each mood state, retrospectively. Each item is rated on a 7-point scale (from -3 “not at all” to 3 “extremely”), or 9-point scale (from 1 “not at all” to 9 “extremely”). Higher scores indicate greater emotional impact of the image.
Cognitive (Non-Emotional) Stages of Mental Imagery
Imagery Inspection
This is considered the third stage of cognitive mental imagery (1) and can be assessed with the Letter Corner Classification Task (LCCT) (64). This task involves assessing the object-based spatial characteristics of four block capital letters (F, N, Z, G). Each letter was marked with an asterisk in the bottom left corner, and an arrow travelling clockwise around the letter. The task was split into three stages, each involving the letters being presented, which participants were asked to memorize, followed by the letters being removed from sight. In stage one, participants were then asked to reproduce each letter, starting at the asterisk and moving clockwise. In stage two, participants were asked to categorize the corners of each letter as “top” or “bottom” by moving clockwise around the letter, starting from the asterisk and indicating “yes” if the corner is at the extreme top or bottom of the letter, and indicating “no” otherwise. In stage three, participants were asked to categorize the corners of each letter as “outside” or “inside”, by moving clockwise around the letter, starting from the asterisk and indicating “yes” if the corner is at the extreme left or right of the letter, and indicating “no” otherwise. Error rates and response times were recorded.
Imagery Manipulation
This is considered the fourth/final stage of cognitive mental imagery (1) and can be assessed with the Mental Rotation Task (MRT) (65). In a computerized version of the MRT, participants were shown pairs of three-dimensional line drawings and asked to decide whether the drawings were of the same rotated object or of different objects. Participants completed a practice trial followed by three progressively more difficult levels of transformation, based on the angular disparity between the two shapes (easy: 50; medium: 100; difficult: 150). Error rates and response times per difficulty level were recorded. Two performance parameters were derived based on response times (41, 66): i) the intercept-index, deemed to represent the sensory-motor component of task response, and ii) the slope-index, deemed to represent the spatial-ability (imagery-based) component of task response.
Statistical Analyses
Outliers above or below three standard deviations (SDs) of the mean were excluded. Groups were compared on baseline and clinical characteristics and on key outcomes from each imagery assessment based on previous literature (32). Each continuous dependent variable was examined with mixed-model analysis of variance with groups as fixed factors and modeling random effects for twin pairs to account for dependence within these. Categorical variable (sex only) was examined with logistic regression with groups as predictors and within twin-pair dependence adjusted for using generalized estimating equations estimates of the standard errors.
For our primary analyses, we included our three key groups, i.e., affected in remission (combining BD and UD), high-risk and low-risk, followed by pairwise comparisons when relevant. Comparing the affected (remitted) group with the low-risk group would help determine whether and which imagery abnormalities persist in remission; comparing the high-risk group with the other two groups would help determine whether and which imagery abnormalities are present in individuals who have not developed BD/UD despite genetic liability (consistent with the notion of an endophenotype for affective disorders). Effect sizes as Cohen d (67) were included to aid interpretations (0.30 = small; 0.50 = medium; 0.80 = large).
We conducted three additional sets of secondary (exploratory) analyses. First, we repeated the above analyses with only BD or UD on selected imagery measures for which previous literature indicates discrepancy between disorders (and hence combining both groups may not be always appropriate): i) emotional imagery of positive valence (PIT and MII) may be enhanced in BD only (35, 68, 69) and ii) aspects of mental rotation performance may be affected in BD (32) and UD (41). Second, we directly contrasted the BD and UD affected (remitted) groups, as previous studies have contrasted imagery measures between BD and UD groups predominantly during depression illness at the time of assessment (32). Finally, we conducted a series of multiple linear regressions with baseline clinical characteristics (HDRS and STAI-Y) as predictors, and key imagery outcomes (emerging as significant from our primary analyses) as dependent variables, based on our previous research demonstrating associations between emotional imagery and symptoms transdiagnostically (32). Manic symptoms (YMRS) were not included as these were matched between groups (see Results). Initially both predictors were entered simultaneously, and then non-significant predictors were removed from the model stepwise until only significant predictors remained.
Significance level was set to alpha = .05 for two-sided hypothesis-testing (unless otherwise stated for directional hypothesis-testing). As our primary analyses involved multiple comparisons, we also applied the Benjamini-Hochberg procedure (70) to control for family-wise false discovery rate (q = .10), and we reported these when they changed the pattern of results (for one pairwise comparison only). Data analyses were conducted using SPSS (71).
Results
Baseline and Clinical Characteristics
Means and SDs are presented in Table 1. There were no significant group differences in sex, age, education, or IQ. As expected, the affected twins scored higher (than high-risk and low-risk groups) on baseline symptoms of depression and anxiety, but there were no significant differences between high-risk and low-risk twins. There were no significant group differences in symptoms of mania. A high proportion of affected twins in remission reported current pharmacological medications (Table 1). Restricting our primary analyses to twins without medication did not change the pattern of results, hence we report results including all twins.
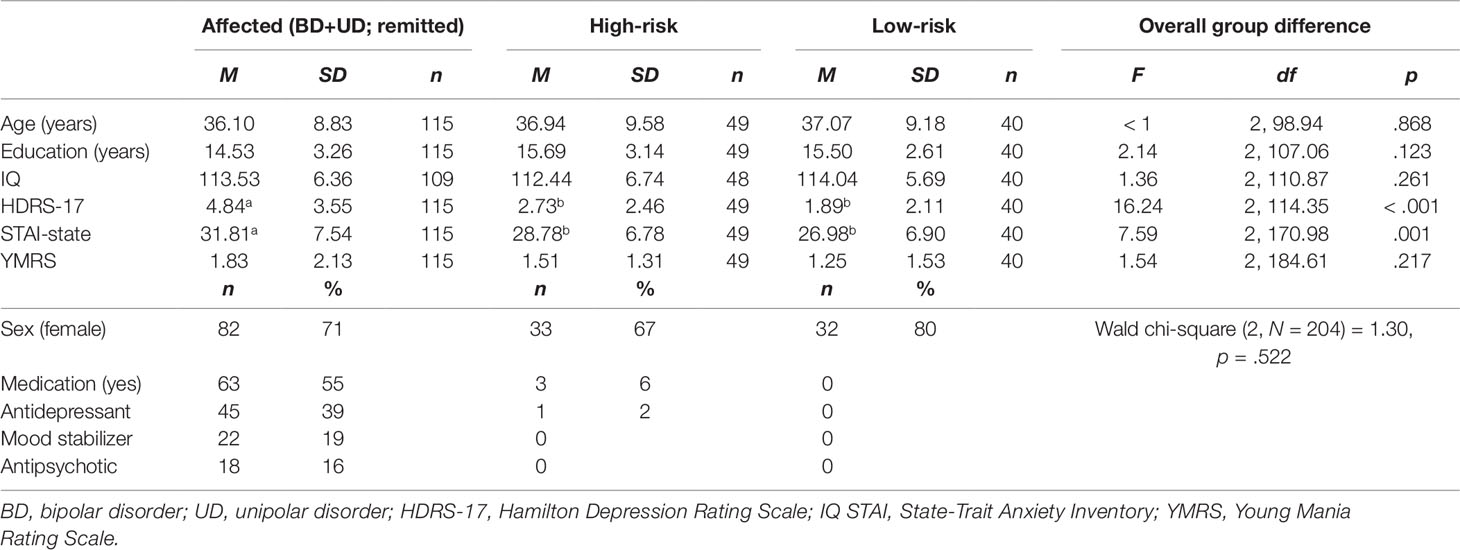
Table 1 Baseline and clinical characteristics of affected, high-risk, and low-risk monozygotic twins for affective disorders.
Subjective Domains of Mental Imagery
Spontaneous Imagery Use
Overall group difference in SUIS scores was not statistically significant (Table 2), indicating an absence of between-group differences in spontaneous use of non-emotional imagery in everyday life.
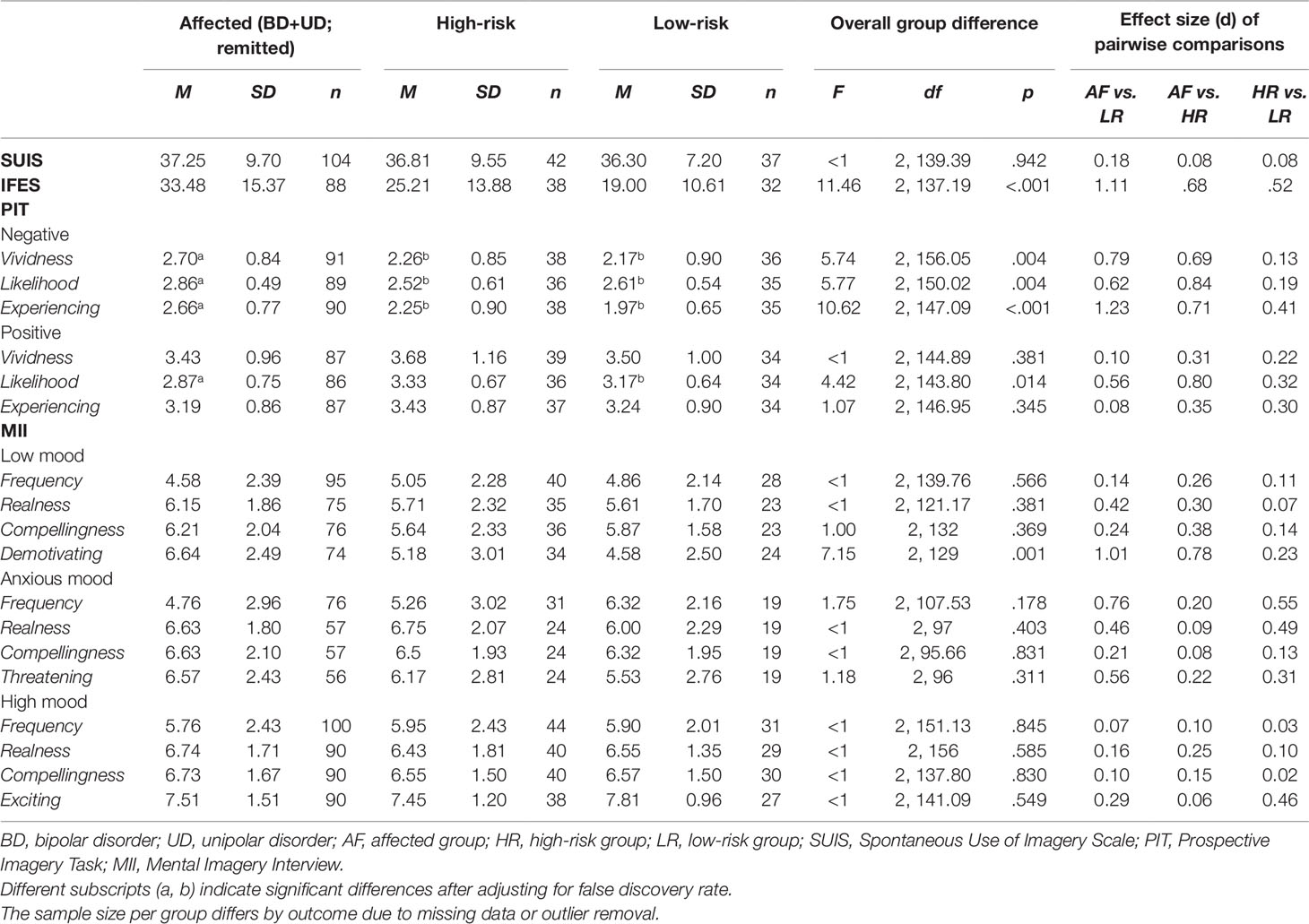
Table 2 Subjective domains of mental imagery in affected, high-risk, and low-risk monozygotic twins for affective disorders.
Emotional Mental Imagery
For the IFES (assessing the impact of intrusive future imagery), the overall group difference in total scores was statistically significant (Table 2). Affected twins in remission had higher IFES scores (indicating higher impact of intrusive prospective imagery) both relative to low-risk twins (F(1, 89.93) = 8.60, p = .004), and to high-risk twins (F(1, 85.70) = 15.49, p < .001). Low- and high-risk twins did not differ significantly in IFES scores (F(1, 52.28) = 2.77, p = .102).
For the PIT (assessing the impact of deliberately-generated future imagery), overall statistically significant group differences were consistently found in response to imagined negative future scenarios (Table 2). Affected twins in remission rated negative future scenarios as more vivid than low-risk twins (F(1, 72,58) = 7.64, p = .007), and also than high-risk twins (F(1, 98.21) = 6.54, p= .012). Affected twins in remission rated these scenarios also as more likely to occur to them than low-risk twins (F(1, 70.70) = 4.70, p= .034), and also than high-risk twins (F(1, 91.31) = 10.05, p = .002). Finally, affected twins in remission reported “experiencing” these scenarios while imagining them, more so than low-risk twins (F(1, 55.08) = 19.16, p < .001), and also than high-risk twins, (F(1, 126) = 6.91, p = .010). The low- and the high-risk twins did not statistically significantly differ in ratings of vividness, likelihood (F’s <1), or “experiencing” (F(1, 41.10) = 1.93, p = .173).
In contrast, overall statistically significant group differences in response to deliberately-generated imagery of positive future scenarios were found for ratings of likelihood only, but not for ratings of vividness or “experiencing” (Table 2). Pairwise comparisons showed affected twins in remission rated positive future scenarios as less likely to occur to them than low-risk twins (F(1, 63.1) = 3.74, p = .058), and also than high-risk twins (F(1,94.66)= 6.90, p = .010), but there were no statistically significant differences in the latter two groups (F < 1). When controlling for false discovery rate, the latter pairwise comparison between affected twins and low-risk twins was no longer significant.
In the MII for low-mood states, there were no statistically significant overall group differences in time spent thinking in images (frequency), nor in ratings of images as “real” and “compelling” (Table 2). However, there were statistically significant group differences in how “demotivating” the image was. Affected twins in remission rated finding their images during low-mood more demotivating low-risk twins (F(1,69.05) = 11.65, p= .001), and also than high-risk twins (F(1,106) = 6.99, p = .009), but there were no significant differences in the latter two groups (F< 1). For images during anxious-mood and high-mood states, there were no statistically significant overall group differences in time spent thinking in images (frequency), nor ratings of other phenomenological properties of the images (Table 2).
To assess whether significant group differences in emotional imagery were driven by affected twins in remission reporting higher levels of residual depressive symptoms even during remission (Table 1), we repeated the above analyses with statistically significant findings without participants reporting scores > 8 in HDRS (threshold for full remission) (50), but the same pattern of findings remained (data not shown).
Cognitive (Non-Emotional) Stages of Mental Imagery
Letter Corner Classification Task
The overall group difference in LCCT performance was not statistically significant for both error rates and reaction times (Table 3).
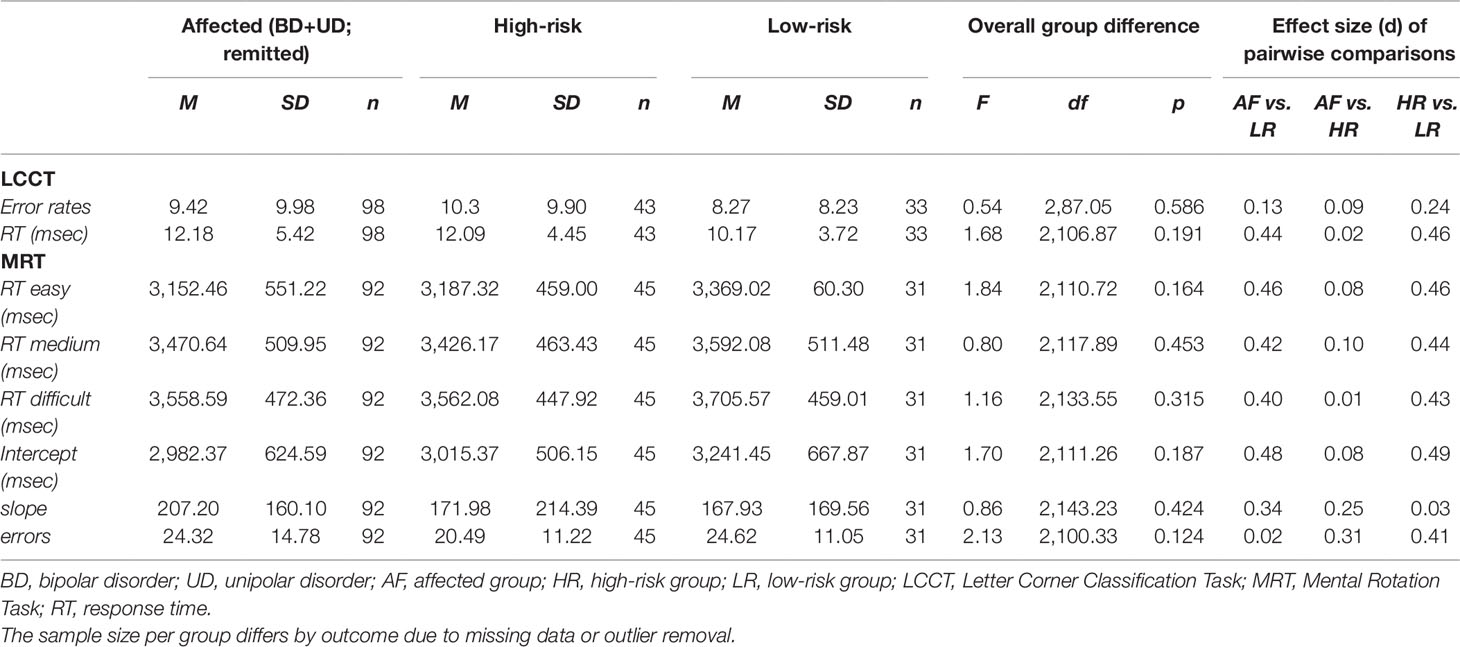
Table 3 Cognitive (non-emotional) domains of mental imagery in affected, high-risk, and low-risk monozygotic twins for affective disorders.
Mental Rotation Task
The overall group difference in MRT performance was not statistically significant, for the intercept (index of sensory-motor processing), slope (index of spatial/imagery processing), nor error rates (Table 3).
Secondary Analyses
Separate Bipolar Disorder/Unipolar Depression Analyses
For the analyses on the BD only (affected-BD, high-risk-BD, and low-risk), there were no overall statistically significant group differences in positive imagery (in PIT and MII) (F’s < 2.54, p’s > .090) or MRT performance (F’s < 1.45, p’s > .246). Similarly, for the analyses on the UD only (affected-UD, high-risk-UD, and low-risk), there were again no statistically significant overall difference in positive imagery (F’s < 2.65, p’s > .075) or MRT performance (F’s < 2.07, p’s > .132).
Bipolar Disorder Vs. Unipolar Depression Within Affected Twins
We compared BD vs. UD twins in remission on all our key subjective and cognitive imagery outcomes. There was only statistically significant group difference in one outcome of the MII. Specifically, affected-BD twins in remission rated their significant image during low-mood states as more “compelling” (M = 7.06, SD = 1.73) than affected-UD twins in remission (M = 5.95, SD = 2.06; F(1,74) = 4.24, p = .043).
Clinical Characteristics Across the Full Sample
Across all groups, higher levels of depressive symptoms were significantly associated with i) higher IFES scores; ii) rating negative imagined scenarios in the PIT as more vivid, more likely to occur and accompanied with more feelings of “experiencing”; iii) rating positive imagined scenarios in the PIT as less likely to occur to them; and iv) reporting (in the MII) that images during low-mood states are more “demotivating”. Additionally, higher levels of anxiety symptoms were significantly associated with i) higher IFES scores; and ii) rating positive imagined scenarios in the PIT as less likely to occur to them.
Discussion
Main Findings
This large study of 204 monozygotic twins investigated—for the first time—whether mental imagery abnormalities are present in i) affected twins with affective disorders in remission and ii) twins with high familial risk for affective disorders. Regarding the first aim, we found that remitted twins with a history of affective disorders reported greater emotional impact of intrusive prospective imagery, and greater vividness, likelihood, and subjective experience of (deliberately-generated) negative prospective imagery compared with unaffected twins at either low-risk or high-risk of affective disorders. That is, affected twins are more prone to imagining the future (whether a desired holiday or a feared examination) and when they did so it felt more real and emotional. The affected twins in remission also reported less likelihood of (deliberately-generated) positive prospective imagery compared to both high-risk and low-risk twins (however, the latter result did not survive correction for multiple comparisons). In contrast, the twin groups did not differ on measures of cognitive stages (non-emotional) of mental imagery.
In relation to the second aim, we found no evidence of mental imagery abnormalities in twins with high familial risk for affective disorders but who were nevertheless not affected. Finally, mood and anxiety symptoms were significantly associated with greater emotional impact of prospective imagery across our entire sample, replicating findings that support a dimensional and transdiagnostic role of imagery abnormalities in psychopathology (32).
Theoretical Implications
Our findings extend previous work identifying abnormalities in prospective emotional mental imagery across affective disorders in the presence of significant depressive symptoms (7, 32, 33, 37). Interestingly, our pattern of results were the same for analyses conducted using a sub-sample restricting the affected group in remission to only those individuals with no residual subclinical symptomatology (HDRS scores <8; n = 89). This suggests that imagery abnormalities are not just related to residual affective symptomatology—these critically may represent a trait-related phenomenon in individuals with affective disorders. The distinction between “state” or “trait” needs to be further delineated, for example using direct comparisons of participants during remission versus during illness episodes (while acutely depressed and/or in manic/mixed episodes). While imagery-based abnormalities that are equivalent during both illness and remission are consistent with the notion of “trait” markers, those abnormalities that more pronounced during illness episodes may additionally represent “state” markers.
The association between biases in prospection and psychopathology remains understudied, although recent research suggests its potential relevance for depression (72) and anxiety (73). Our study indicates, for the first time, that individuals with a history of affective disorders continue to experience vivid intense images of negative future events even during long-term remission, similar to other cognitive-emotional biases including imagery of the past (27). Instead, abnormalities in positive prospective imagery appeared limited to likelihood ratings across affective disorders (although these results did not survive more stringent corrections for multiple comparisons), and did not differentiate BD versus UD (both in remitted states). Future replications need to confirm if the inability to experience vivid positive images of future events as previously reported is only prominent during illness/depressive states (63, 74).
Our findings also highlight that unlike emotional imagery, cognitive (non-emotional) imagery processes appear intact across affective disorders in remission. While we previously found some evidence of both better and worse performance on cognitive stages of imagery in a sample of mixed euthymic and depressed BD (32), the present study confirms that cognitive stages of imagery remain largely preserved in affective disorders (32). These cognitive processes rely on general executive function as well as on more specific visuospatial abilities (1). How these processes—as measured by cognitive imagery tasks—compare to other components and measures of cognition remains unclear. This is relevant as executive function in particular is often compromised in recovered individuals with affective disorders (75, 76). However, affective disorders are largely heterogeneous with regards to cognition (77, 78); for instance, affected twins in our sample did not show any cognitive deficits compared to low-risk twins (54). Answering the above question may be relevant with a view to personalizing therapeutic interventions. As examples, common cognitive therapy techniques such as reappraisal may rely on well-functioning cognition and hence be less efficacious if executive function is compromised, and likewise, imagery-based techniques could be advantageous in those with intact cognitive stages of imagery.
Abnormalities in prospective imagery were not present in high-risk twins compared to low-risk twins, indicating that these do not fulfill the criteria for an illness “endophenotype” (28). Our data are more consistent with the notion that imagery-based abnormalities reflect (neuro)cognitive markers of the disorder itself—abnormalities that are likely to play a role in increasing future relapse given their persistence in remitted states. We note that previous research has shown altered emotional mental imagery in individuals with phenotypic (rather than genetic/familial) risk for BD, mainly based on the presence of hypomanic-like experiences (32, 43, 69, 79). However, our study was the first to focus instead on genetic-based risk using a twin design enabled by the unique Danish registers (49). As a potential explanation for this discrepancy, maladaptive prospective emotional imagery may be associated with phenotypic characteristics of affective disorders that lie on the same dimension of clinical symptoms, such as the actual presence of dysphoric mood (47, 62) or hypomanic-like experiences (32, 43, 69, 79), but we did not measure such features in our high-risk twins. Another potential explanation is that our high-risk twins had an average higher age compared to at-risk groups from previous research. Thus it is also possible that our sample included individuals who had a familial risk but were actually “resilient” to developing affective disorders, at least in terms of mental imagery characteristics. We have previously argued that higher extroversion and lower neuroticism may mediate sensitivity to adverse events and better functioning in this high-risk sample (56), although only future longitudinal studies will be able to clarify factors determining vulnerability or resilience.
Notably, we found no evidence of differences between affective disorders categories of BD vs. UD. The only exception was that remitted BD twins retrospectively described their imagery at times of depressed mood as more compelling compared to remitted UD twins. This is consistent with previous findings on suicidal imagery (37), suggesting that when asking about past mental images during depressive episodes it may be important to distinguish for specific suicidal content to clarify potential phenomenological differences. We also found that greater emotional impact of prospective imagery was associated with anxiety and low mood symptoms across our whole sample. If replicated in samples with a larger number of individuals with BD, overall these results will indicate that individuals with affective disorders may share a trait-like common profile of mental imagery characteristics, in line with a most recent dimensional and transdiagnostic model of the role of emotional imagery in psychopathology (32).
Clinical Implications
If prospective emotional imagery remains abnormally enhanced after recovery from affective disorders episodes, this could represent a vulnerability factor for future relapses through their impact on emotion and mood instability. We have previously proposed that vivid intense mental images may act as an emotional amplifier and maintain mood instability (7, 29). These novel findings highlight the importance of future longitudinal studies investigating whether imagery abnormalities during remission indeed predict subsequent illness recurrence rates.
Prospection has a key functional role in individuals’ daily life: we imagine future scenarios as a way to plan action, anticipate potential events and direct decision-making, manage uncertainty, and strengthen motivation toward goals (30, 31). Future imagery can also support emotion regulation, e.g., by anticipating future rewards to overcome present difficulties (80, 81). If prospection is biased in those with past affective episodes, remitted individuals will experience negative future scenarios more vividly, more likely to happen, and such intrusive images of the future will carry maladaptive emotional impact.
Finally, our findings indicate that imagery-based treatment innovation could be guided toward tertiary rather than primary prevention—at least in relation to familial risk. Building on previous therapy protocols (82–84) and recent experimental studies (85), targeting prospective imagery abnormalities using imagery-based cognitive therapy techniques has potential as a brief relapse prevention intervention, in particular in patients with greater mood instability (86). Emerging work in cognitive science also suggests that simple competing tasks can dampen recurrent “flashforward” imagery by taxing working memory while holding the flashforward image in mind (15, 87). Future studies should measure change in prospective imagery characteristics after imagery-based interventions to develop novel interventions based on mechanistic hypotheses (16).
Limitations and Strengths
Several limitations should be noted in our study. First, we did not include a dizygotic twins group; hence, we could not assess the interaction between environmental and genetic influence on variance, which is preferable when investigating potential endophenotypes. Second, we did not directly compared twins at high-risk for UD versus at high-risk for BD. Such comparison could establish whether familial risk in imagery abnormalities is more evident in high-risk twins given a co-twin history of BD rather than UD, because of the higher heritability of BD (19) relative to UD (20). Unfortunately we had low power to run those analyses because the high-risk for BD group was very small (n = 11). Third, we did not include a full comprehensive assessment of mental imagery (40), so it is possible that biases in other imagery domains—such as trauma memory imagery (5, 14, 88) and/or reward/motivational imagery (89, 90)—are also present in remitted affective disorders and/or high-risk twins. This might be relevant to the prevalence of trauma in affective disorders (91) and of persistent anhedonic symptomatology (92), which are both associated with relapse and worse prognosis. Finally, results from our analyses comparing patients affected with UD versus BD (n = 84 versus n = 31, respectively) and from our secondary analysis should be interpreted with caution, given the small sample size of the separate BD group (n = 31 affected and n = 11 high risk, respectively).
Major strengths of our study are the large and population-based twin sample (enabling a unique design to test hypotheses regarding familial risk), twin groups well-matched on demographic variables, and the use of validated scales and tasks that allow building on previous research and future replication. Further, participants had been in long-term remission providing greater confidence that findings were not state-related (although it remains possible that imagery is also linked to states). Importantly, our findings are unlikely to be attributed to other cognitive confounders, as our groups did not differ in general cognitive function (54). Finally, to our knowledge, this was also the second study ever to combine emotional in tandem with cognitive (non-emotional) testing of imagery abnormalities in a clinical sample.
Conclusions
Our study was the first investigation of mental imagery characteristics in affective disorders using a large population-based monozygotic twin sample. For the first time, we show that abnormalities in emotional prospective imagery also persist after recovery from acute episodes, but are not present in unaffected individuals at familial risk, suggesting that imagery characteristics are unlikely to fulfill the “endophenotype” criterion. Thus, mechanistically, imagery-abnormalities in affective disorders (BD and UD) may be best conceptualized as cognitive markers of the disorders, contributing to both psychopathology maintenance and possibly future relapse. Our findings also highlight that emotional imagery phenomenology can be useable in clinical practice (93), as a hallmark of psychopathology and ongoing vulnerability. Abnormalities in prospective emotional imagery represent a promising target for mechanism-testing in treatment innovation in affective disturbances.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The study was approved by the local ethics committee (H-3-2014-003) and the Danish data protection agency (2014–331–0751). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MS contributed to study design, data analysis, data interpretation, and manuscript writing. AL-Z conducted data analysis and contributed to data interpretation and manuscript writing. PT contributed to data processing, data analysis, and manuscript writing. IM conducted data collection and contributed to study design and data processing. LK and MV contributed to study design. EH contributed to study design, data interpretation, and manuscript writing. MV designed, obtained the ethical permissions, led the register linkage, and supervised together with LK the recruitment of participants and data collection. KM designed and managed the study, including data collection and processing, and contributed to manuscript writing. All authors reviewed and approved the finalmanuscript.
Funding
MD was funded by the United Kingdom Medical Research Council intramural programme to EH (MRC-A060-5PR50). IM was supported by The Lundbeck Foundation (grant number R108-A10015) and the Hørslev Foundation. EH was supported by The Wellcome Trust (WT088217), the Lupina Foundation, and the Swedish Research Council (2017–00957). KM is supported by The Lundbeck Foundation and Weimann Foundation.
Conflict of Interest
MS and EH are co-authors of a book on imagery-based cognitive therapy (Guildford Press, 2019). LK and MV have within recent 3 years been consultants for Lundbeck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the The Danish Twin Registry, and also for Mette Marie Støttrup and Hanne Lie Kjærstad for help with data collection.
References
1. Kosslyn SM, Thompson WL, Ganis G. The case for mental imagery. New York, NY: Oxford University Press (2006). doi: 10.1093/acprof:oso/9780195179088.001.0001
2. Holmes EA, Mathews A. Mental imagery and emotion: a special relationship? Emotion (2005) 5:489–97. doi: 10.1037/1528-3542.5.4.489
3. Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev (2010) 117:210–32. doi: 10.1037/a0018113
4. Holmes EA, Mathews A. Mental imagery in emotion and emotional disorders. Clin Psychol Rev (2010) 30:349–62. doi: 10.1016/j.cpr.2010.01.001
5. Iyadurai L, Visser RM, Lau-Zhu A, Porcheret K, Horsch A, Holmes EA, et al. Intrusive memories of trauma: a target for research bridging cognitive science and its clinical application. Clin Psychol Rev (2019) 69:67–82. doi: 10.1016/j.cpr.2018.08.005
6. Ji JL, Kavanagh DJ, Holmes EA, MacLeod C, Di Simplicio M. Mental imagery in psychiatry: conceptual & clinical implications. CNS Spectr (2019) 24:114–26. doi: 10.1017/S1092852918001487
7. Holmes EA, Bonsall MB, Hales SA, Mitchell H, Renner F, Blackwell SE, et al. Applications of time-series analysis to mood fluctuations in bipolar disorder to promote treatment innovation: a case series. Transl Psychiatry (2016b) 6:e720. doi: 10.1038/tp.2015.207
8. Holmes EA, Blackwell SE, Burnett Heyes S, Renner F, Raes F. Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu Rev Clin Psychol (2016a) 12:249–80. doi: 10.1146/annurev-clinpsy-021815-092925
9. Blackwell SE, Browning M, Mathews A, Pictet A, Welch J, Davies J, et al. Positive imagery-based cognitive bias modification as a web-based treatment tool for depressed adults: a randomized controlled trial. Clin Psychol Sci (2015) 3:91–111. doi: 10.1177/2167702614560746
10. Torkan H, Blackwell SE, Holmes EA, Kalantari M, Neshat-Doost HT, Maroufi M, et al. Positive imagery cognitive bias modification in treatment-seeking patients with major depression in Iran: a pilot study. Cognit Ther Res (2014) 38:132–45. doi: 10.1007/s10608-014-9598-8
11. Williams AD, Blackwell SE, Mackenzie A, Holmes E, Andrews G. Combining imagination and reason in the treatment of depression: a randomized controlled trial of internet-based cognitive-bias modification and internet-CBT for depression. J Consult Clin Psychol (2013) 81:793–9. doi: 10.1037/a0033247
12. Iyadurai L, Blackwell SE, Meiser-Stedman R, Watson PC, Bonsall MB, Geddes JR, et al. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol Psychiatry (2018) 23:674–82. doi: 10.1038/mp.2017.23
13. Kessler H, Holmes EA, Blackwell SE, Schmidt AC, Schweer JM, Bücker A, et al. Reducing intrusive memories of trauma using a visuospatial interference intervention with inpatients with post-traumatic stress disorder (PTSD). J Consult Clin Psychol (2018) 86:1076–90. doi: 10.1037/ccp0000340
14. Lau-Zhu A, Henson RN, Holmes EA. Intrusive memories and voluntary memory of a trauma film: effects of a cognitive interference task after encoding. J Exp Psychol Gen (2019). In Press.
15. Lau-Zhu A, Holmes EA, Butterfield S, Holmes J. Selective association between Tetris game play and visuospatial working memory: a preliminary investigation. Appl Cogn Psychol (2017) 31:438–45. doi: 10.1002/acp.3339
16. Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, et al. The lancet psychiatry commission on psychological treatments research in tomorrow’s science. Lancet Psychiatry (2018) 5:237–86. doi: 10.1016/S2215-0366(17)30513-8
17. World Health Organization. International statistical classification of diseases and related health problems - 10th revision. Geneva: World Health Organization (2004).
18. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (2013) 382:1575–86.
19. McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry (2003) 60:497. doi: 10.1001/archpsyc.60.5.497
20. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry (2000) 157:1552–62. doi: 10.1176/appi.ajp.157.10.1552
21. Miskowiak KW, Carvalho AF. “Hot” cognition in major depressive disorder: a systematic review. CNS Neurol Disord Drug Targets (2014) 13:1787–803.
22. Robinson ESJ, Roiser JP. Affective Biases in Humans and Animals. Curr Top Behav Neurosci (2015) 28: 263–86. doi: 10.1007/7854_2015_5011.
23. LeMoult J, Joormann J, Sherdell L, Wright Y, Gotlib IH. Identification of emotional facial expressions following recovery from depression. J Abnorm Psychol (2009) 118:828–33. doi: 10.1037/a0016944
24. Levens SM, Gotlib IH. Updating emotional content in recovered depressed individuals: Evaluating deficits in emotion processing following a depressive episode. J Behav Ther Exp Psychiatry (2015) 48:156–63. doi: 10.1016/j.jbtep.2015.03.009
25. Miskowiak KW, Kjærstad HL, Meluken I, Petersen JZ, Maciel BR, Köhler CA, et al. The search for neuroimaging and cognitive endophenotypes: a critical systematic review of studies involving unaffected first-degree relatives of individuals with bipolar disorder. Neurosci Biobehav Rev (2017) 73:1–22. doi: 10.1016/j.neubiorev.2016.12.011
26. Teasdale JD. Cognitive vulnerability to persistent depression. Cogn Emot (1988) 2:247–74. doi: 10.1080/02699938808410927
27. Werner-Seidler A, Moulds ML. Autobiographical memory characteristics in depression vulnerability: formerly depressed individuals recall less vivid positive memories. Cogn Emot (2011) 25:1087–103. doi: 10.1080/02699931.2010.531007
28. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry (2003) 160:636–45. doi: 10.1176/appi.ajp.160.4.636
29. Holmes EA, Geddes JR, Colom F, Goodwin GM. Mental imagery as an emotional amplifier: application to bipolar disorder. Behav Res Ther (2008a) 46:1251–8. doi: 10.1016/j.brat.2008.09.005
30. MacLeod AK. Prospection, well-being and memory. Mem Stud (2016) 9:266–74. doi: 10.1177/1750698016645233
31. Schacter DL, Benoit RG, Szpunar KK. Episodic future thinking: mechanisms and functions. Curr Opin Behav Sci (2017) 17:41–50. doi: 10.1016/j.cobeha.2017.06.002
32. Di Simplicio M, Renner F, Blackwell SE, Mitchell H, Stratford HJ, Watson P, et al. An investigation of mental imagery in bipolar disorder: exploring “the mind’s eye.”. Bipolar Disord (2016) 18:669–83. doi: 10.1111/bdi.12453
33. Holmes EA, Deeprose C, Fairburn CG, Wallace-Hadrill SM, Bonsall MB, Geddes JR, et al. Mood stability versus mood instability in bipolar disorder: a possible role for emotional mental imagery. Behav Res Ther (2011) 49:707–13. doi: 10.1016/j.brat.2011.06.008
34. Morina N, Deeprose C, Pusowski C, Schmid M, Holmes EA. Prospective mental imagery in patients with major depressive disorder or anxiety disorders. J Anxiety Disord (2011) 25:1032–7. doi: 10.1016/j.janxdis.2011.06.012
35. Ivins A, Di Simplicio M, Close H, Goodwin GM, Holmes EA. Mental imagery in bipolar affective disorder versus unipolar depression: investigating cognitions at times of ‘positive’ mood. J Affect Disord (2014) 166:234–42. doi: 10.1016/j.jad.2014.05.007
36. Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clin Psychol Psychother (2011) 18:356–65. doi: 10.1002/cpp.776
37. Hales SA, Deeprose C, Goodwin GM, Holmes EA. Cognitions in bipolar affective disorder and unipolar depression: imagining suicide. Bipolar Disord (2011) 13:651–61. doi: 10.1111/j.1399-5618.2011.00954.x
38. De Crescenzo F, Serra G, Maisto F, Uchida M, Woodworth H, Casini MP, et al. Suicide attempts in juvenile bipolar versus major depressive disorders: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry (2017) 56:825–831.e3. doi: 10.1016/j.jaac.2017.07.783
39. Tondo L, Pompili M, Forte A, Baldessarini RJ. Suicide attempts in bipolar disorders: comprehensive review of 101 reports. Acta Psychiatr Scand (2016) 133:174–86. doi: 10.1111/acps.12517
40. Pearson DG, Deeprose C, Wallace-Hadrill SMA, Burnett Heyes S, Holmes EA. Assessing mental imagery in clinical psychology: a review of imagery measures and a guiding framework. Clin Psychol Rev (2013) 33:1–23. doi: 10.1016/j.cpr.2012.09.001
41. Zarrinpar A, Deldin P, Kosslyn SM. Effects of depression on sensory/motor vs. central processing in visual mental imagery. Cogn Emot (2006) 20:737–58. doi: 10.1080/02699930500405600
42. Deeprose C, Malik A, Holmes EA. Measuring intrusive prospective imagery using the impact of future events scale (IFES): psychometric properties and relation to risk for bipolar disorder. Int Cogn J Ther (2012) 4:187–96. doi: 10.1521/ijct.2011.4.2.187
43. Malik A, Goodwin GM, Hoppitt L, Holmes EA. Hypomanic experience in young adults confers vulnerability to intrusive imagery after experimental trauma: Relevance for bipolar disorder. Clin Psychol Sci (2014) 2:675–84. doi: 10.1177/2167702614527433
44. McCarthy-Jones S, Knowles R, Rowse G. More than words? Hypomanic personality traits, visual imagery and verbal thought in young adults. Conscious Cogn (2012) 21:1375–81. doi: 10.1016/j.concog.2012.07.004
45. McGill B, Moulds ML. Characteristics of autobiographical memories and prospective imagery across a spectrum of hypomanic personality traits. Memory (2014) 22:1139–48. doi: 10.1080/09658211.2013.873808
46. Holmes EA, Lang TJ, Moulds ML, Steele AM. Prospective and positive mental imagery deficits in dysphoria. Behav Res Ther (2008b) 46:976–81. doi: 10.1016/j.brat.2008.04.009
47. Ji JL, Holmes EA, MacLeod C, Murphy FC. Spontaneous cognition in dysphoria: reduced positive bias in imagining the future. Psychol Res (2018) 83:817–31. doi: 10.1007/s00426-018-1071-y
48. Skytthe A, Christiansen L, Kyvik KO, Bødker FL, Hvidberg L, Petersen I, et al. The Danish Twin Registry: linking surveys, national registers, and biological information. Twin Res Hum Genet (2013) 16:104–11. doi: 10.1017/thg.2012.77
49. Mors O, Perto GP, Mortensen PB. The danish psychiatric central research register. Scand J Public Health (2011) 39:54–7. doi: 10.1177/1403494810395825
50. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol (1967) 6:278–96.
51. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry J Ment Sci (1978) 133:429–35.
52. Kærsgaard S, Meluken I, Kessing L, Vinberg M, Miskowiak K. Increased sensitivity to positive social stimuli in monozygotic twins at risk of bipolar vs. unipolar disorder. J Affect Disord (2018) 232:212–8. doi: 10.1016/j.jad.2018.02.055
53. Meluken I, Ottesen NM, Phan KL, Goldin PR, Di Simplicio M, Macoveanu J, et al. Neural response during emotion regulation in monozygotic twins at high familial risk of affective disorders. NeuroImage Clin (2019c) 21:101598. doi: 10.1016/j.nicl.2018.11.008
54. Meluken I, Ottesen NM, Harmer CJ, Scheike T, Kessing LV, Vinberg M, et al. Is aberrant affective cognition an endophenotype for affective disorders? – a monozygotic twin study. Psychol Med (2019b) 49:987–96. doi: 10.1017/S0033291718001642
55. Meluken I, Ottesen N, Harmer C, Macoveanu J, Siebner H, Kessing L, et al. Neural response to emotional faces in monozygotic twins: association with familial risk of affective disorders. J Psychiatry Neurosci (2019a) 44:1–10. doi: 10.1503/jpn.170246
56. Ottesen NM, Meluken I, Scheike T, Kessing LV, Miskowiak KW, Vinberg M. Clinical characteristics, life adversities and personality traits in monozygotic twins with, at risk of and without affective disorders. Front Psychiatry (2018) 9:401. doi: 10.3389/fpsyt.2018.00401
57. Vinberg M, Ottesen NM, Meluken I, Sørensen N, Pedersen O, Kessing LV, et al. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta Psychiatr Scand (2019) 139:174–84. doi: 10.1111/acps.12976
58. Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry (1990) 47:589–93.
59. Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex (1978) 14:234–44.
60. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press (1983).
61. Reisberg D, Pearson DG, Kosslyn SM. Intuitions and introspections about imagery: the role of imagery experience in shaping an investigator’s theoretical views. Appl Cogn Psychol (2003) 17:147–60. doi: 10.1002/acp.858
62. Deeprose C, Holmes EA. An exploration of prospective imagery: the impact of future events scale. Behav Cogn Psychother (2010) 38:201–9. doi: 10.1017/S1352465809990671
63. Stöber J. Prospective cognitions in anxiety and depression: replication and methodological extension. Cogn Emot (2000) 14:725–9. doi: 10.1080/02699930050117693
64. Farah MJ, Hammond KM, Levine DN, Calvanio R. Visual and spatial mental imagery: dissociable systems of representation. Cogn Psychol (1988) 20:439–62.
65. Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science (1971) (80-.), 701–3.
66. Borst G, Kievit RA, Thompson WL, Kosslyn SM. Mental rotation is not easily cognitively penetrable. J Cogn Psychol (2011) 23:60–75. doi: 10.1080/20445911.2011.454498
67. Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Routledge Academic (1988).
68. Gregory JD, Brewin CR, Mansell W, Donaldson C. Intrusive memories and images in bipolar disorder. Behav Res Ther (2010) 48:698–703. doi: 10.1016/j.brat.2010.04.005
69. O’Donnell C, Di Simplicio M, Brown R, Holmes EA, Burnett Heyes S. The role of mental imagery in mood amplification: an investigation across subclinical features of bipolar disorders. Cortex (2018) 105:104–17. doi: 10.1016/j.cortex.2017.08.010
70. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (1995) 57:289–300. doi: 10.2307/2346101
72. Roepke AM, Seligman MEP. Depression and prospection. Br J Clin Psychol (2016) 55:23–48. doi: 10.1111/bjc.12087
73. Miloyan B, Bulley A, Suddendorf T. Episodic foresight and anxiety: proximate and ultimate perspectives. Br J Clin Psychol (2016) 55:4–22. doi: 10.1111/bjc.12080
74. Murphy FC, Peers PV, Blackwell SE, Holmes EA, Manly T. Anticipated andimagined futures: prospective cognition and depressed mood followingbrain injury. Br J Clin Psychol (2019) 58:91–109. doi: 10.1111/bjc.12202
75. Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med (2013) 43:2017–26. doi: 10.1017/S0033291712002085
76. Bortolato B, Carvalho AF, McIntyre RS. Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNS Neurol Disord Drug Targets (2014) 13:1804–18.
77. Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry (2008) 69:1122–30. doi: 10.4088/jcp.v69n0712
78. Jensen JH, Knorr U, Vinberg M, Kessing LV, Miskowiak KW. Discrete neurocognitive subgroups in fully or partially remitted bipolar disorder: Associations with functional abilities. J Affect Disord (2016) 205:378–86. doi: 10.1016/j.jad.2016.08.018
79. Ng RM, Heyes SB, McManus F, Kennerley H, Holmes EA. Bipolar risk and mental imagery susceptibility in a representative sample of Chinese adults residing in the community. Int J Soc Psychiatry (2016) 62:94–102. doi: 10.1177/0020764015597951
80. Jing HG, Madore KP, Schacter DL. Worrying about the future: An episodic specificity induction impacts problem solving, reappraisal, and well-being. JExp Psychol Gen (2016) 145:402–18. doi: 10.1037/xge0000142
81. Szpunar KK, Spreng RN, Schacter DL. A taxonomy of prospection: introducing an organizational framework for future-oriented cognition. Proc Natl Acad Sci USA (2014) 111:18414–21. doi: 10.1073/pnas.1417144111
82. Brewin CR, Wheatley J, Patel T, Fearon P, Hackmann A, Wells A, et al. Imagery rescripting as a brief stand-alone treatment for depressed patients with intrusive memories. Behav Res Ther (2009) 47:569–76. doi: 10.1016/j.brat.2009.03.008
83. Hales SA, Di Simplicio M, Iyadurai L, Blackwell SE, Young K, Fairburn CG, et al. imagery-focused cognitive therapy (ImCT) for mood instability and anxiety in a small sample of patients with bipolar disorder: a pilot clinical audit. Behav Cogn Psychother (2018) 46:706–25. doi: 10.1017/S1352465818000334
84. Holmes EA, Hales S, Young K, Di Simplicio M. Imagery-based cognitive therapy for bipolar disorder and mood instability. New York, USA: The Guildford Press (2019).
85. Boland J, Riggs KJ, Anderson RJ. A brighter future: the effect of positive episodic simulation on future predictions in non-depressed, moderately dysphoric & highly dysphoric individuals. Behav Res Ther (2018) 100:7–16. doi: 10.1016/j.brat.2017.10.010
86. Broome MR, Saunders KEA, Harrison PJ, Marwaha S. Mood instability: significance, definition and measurement. Br J Psychiatry (2015) 207:283. doi: 10.1192/BJP.BP.114.158543
87. Engelhard IM, van den Hout MA, Janssen WC, van der Beek J. Eye movements reduce vividness and emotionality of “flashforwards.”. Behav Res Ther (2010) 48:442–7. doi: 10.1016/j.brat.2010.01.003
88. James EL, Lau-Zhu A, Clark IA, Visser RM, Hagenaars MA, Holmes EA. The trauma film paradigm as an experimental psychopathology model of psychological trauma: intrusive memories and beyond. Clin Psychol Rev (2016) 47:106–42. doi: 10.1016/j.cpr.2016.04.010
89. Kavanagh DJ, Andrade J, May J. Imaginary Relish and Exquisite Torture: The Elaborated Intrusion Theory of Desire. Psychol Rev (2005) 112:446–67. doi: 10.1037/0033-295X.112.2.446
90. Renner F, Murphy FC, Ji JL, Manly T, Holmes EA. Mental imagery as a “motivational amplifier” to promote activities. Behav Res Ther (2019) 114:51–9. doi: 10.1016/J.BRAT.2019.02.002
91. Etain B, Henry C, Bellivier F, Mathieu F, Leboyer M. Beyond genetics: childhood affective trauma in bipolar disorder. Bipolar Disord (2008) 10:867–76. doi: 10.1111/j.1399-5618.2008.00635.x
92. Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med (2012) 42:967–80. doi: 10.1017/S0033291711001905
Keywords: mental imagery, future simulation, bipolar disorder, depression, twins, endophenotype
Citation: Di Simplicio M, Lau-Zhu A, Meluken I, Taylor P, Kessing LV, Vinberg M, Holmes EA and Miskowiak KW (2019) Emotional Mental Imagery Abnormalities in Monozygotic Twins With, at High-Risk of, and Without Affective Disorders: Present in Affected Twins in Remission but Absent in High-Risk Twins. Front. Psychiatry 10:801. doi: 10.3389/fpsyt.2019.00801
Received: 04 June 2019; Accepted: 07 October 2019;
Published: 08 November 2019.
Edited by:
Liam Mason, University College London, United KingdomReviewed by:
Sarah Kittel-Schneider, Universitätsklinikum Würzburg, GermanyGeorgie Paulik, University of Western Australia, Australia
Copyright © 2019 Di Simplicio, Lau-Zhu, Meluken, Taylor, Kessing, Vinberg, Holmes and Miskowiak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Di Simplicio, m.di-simplicio@imperial.ac.uk
 Martina Di Simplicio
Martina Di Simplicio Alex Lau-Zhu
Alex Lau-Zhu Iselin Meluken4
Iselin Meluken4 Lars Vedel Kessing
Lars Vedel Kessing Maj Vinberg
Maj Vinberg Kamilla Woznica Miskowiak
Kamilla Woznica Miskowiak