- 1Laboratory of Research, Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan
- 2Department of Psychiatry, Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan
- 3Graduate Institute of Medical Science, National Defense Medical Center, Taipei, Taiwan
- 4Department of Nursing, Shu Zen Junior College of Management and Medicine, Kaohsiung, Taiwan
- 5Chi-hung Clinic, Kaohsiung, Taiwan
- 6School of Medicine, Griffith University, Gold Coast, Australia
- 7Calo Psychiatric Center, Pingtung County, Taiwan
Objects: The aim of our study was to investigate whether major depressive disorder (MDD) increased the risk of hypertension using propensity score matching (PSM) in patients with MDD in Taiwan.
Methods: In this study, we recruited all samples from a random sample sub-dataset of one million insured individuals from 2005. A total of 743,114 outpatients were included in our study. We used PSM (nearest neighbor matching) stratified by age, hospital level, insurance amount, and Charlson Comorbidity Index score.
Results: The hazard ratio (HR) of hypertension was significantly greater in the male MDD outpatients (HR = 1.116, P = 0.004) than in the female MDD outpatients (HR = 0.93, P = 0.02). Using PSM, we selected 27,988 outpatients with hypertension and 27,988 outpatients without hypertension for a nested case–control study. In this analysis, female outpatients with MDD (relative risk = 0.852) had lower risks of hypertension. Male outpatients without/with MDD (relative risk = 1.987/3.018) showed a synergistic interaction with gender in which male patients had a higher risk of hypertension in a multiplicative model. Furthermore, MDD appeared to have an interaction effect with gender (HR = 1.82, P < 0.001) in the proportional hazards model analysis. Antidepressant use also increased the risk of hypertension (HR = 1.16, P < 0.001).
Conclusions: There was gender disparity in the risk of hypertension in subjects with MDD. MDD outpatients who used antidepressants had a higher risk of suffering from hypertension. A large-scale, population-based study is warranted to generalize these results in the future.
Background
The World Mental Health Survey estimates that depression affects 350 million people worldwide (1) with a lifetime risk of 7% (2). Depression will become the second global disease burden after ischemic heart disease by 2020. Several studies have shown that major depressive disorder (MDD) is one of the most common mental disorders worldwide, with a prevalence ranging between 2.1 and 7.6% (3–6). In Taiwan, the prevalence of MDD increased from 0.167 to 1.724% from 1996 to 2003, which was lower than the prevalence reported in Western countries (7–10).
Hypertension is associated with at least 7.6 million deaths annually worldwide, which accounts for 13.5% of all deaths (11), and people with hypertension have a higher risk of all types of cardiovascular diseases (12–15). A cross-sectional study (16) of 153,996 adults aged from 35 to 70 years from 17 countries showed that 40.8% of the subjects had hypertension. Another study (17) showed that the prevalence of hypertension in six European countries, Canada, and the USA ranged from 29.8 to 60.2% in men and from 23.8 to 50.3% in women. The prevalence of hypertension in Taiwan was 23.2% (26.5% in men and 19.0% in women) from 1993 to 1996 and decreased to 17.6% (20.9% in men and 14.4% in women) from 2005 to 2008. From 2013 to 2014, the prevalence rate increased to 25.6% (29.6% in men and 21.8% in women) (18).
MDD and hypertension are both important issues in Taiwan and globally. Past studies have shown an association between MDD and hypertension (19–27), although other studies have found no such association (28, 29). Two studies determined that the risk of hypertension was increased years after the occurrence of MDD (24–27).
To the best of our knowledge, only one large population-based study has investigated this topic; this study was a random sample of 1 million individuals registered in the Taiwanese National Health Insurance Database (25). The results are in agreement with those of the present study, which observed an association between MDD (on a 1-year basis) and a subsequent diagnosis of hypertension with an odds ratio (OR) of 1.22. Another study (30) showed that the age-adjusted OR for depression in persons with hypertension was 1.293 (95% CI 1.256–1.331) in the general population using administrative healthcare data from Stockholm County, Sweden. Our previous study (31) showed that hypertension was a possible vulnerability marker for depression in patients with end-stage renal disease.
Based on the above studies, we hypothesize that an association exists between depression and hypertension. The aim of our study was to investigate whether MDD increased the risk of hypertension and to analyze the related risk factors of hypertension among patients with MDD in Taiwan’s National Health Insurance Database.
Methods
Data Resources
The National Health Insurance (NHI) system, which is the source of the study data, provides insurance coverage for more than 98% of the 23 million Taiwanese people, and has contracted with more than 93% of medical institutions since 1996. The National Health Research Institute (NHRI) administers all medical claims information recorded from the contracted health care facilities. We retrieved all sampled subjects from the Longitudinal Health Insurance Database (LHID). The LHID includes all original medical claims and registration files for the one million enrollees in the NHI program. The one million random sample enrollees in the LHID were taken from all insured persons registered in the 2015 registry of beneficiaries (N = 23.72 million) (32). This dataset contained the registry of medical facilities, orders for inpatients and outpatients, dental services, and prescriptions linked to anonymous identifiers. Thousands of published SCI articles have demonstrated the high validity of NHI data (33–35). Because all patient identifiers were released to the public for research purposes, the LHID was omitted from full review by the Institutional Review Board in Taiwan. However, we obtained an ethics certificate (protocol number 105-049) from the Institutional Review Board of Kaohsiung Armed Forces General Hospital in Taiwan.
Study Samples
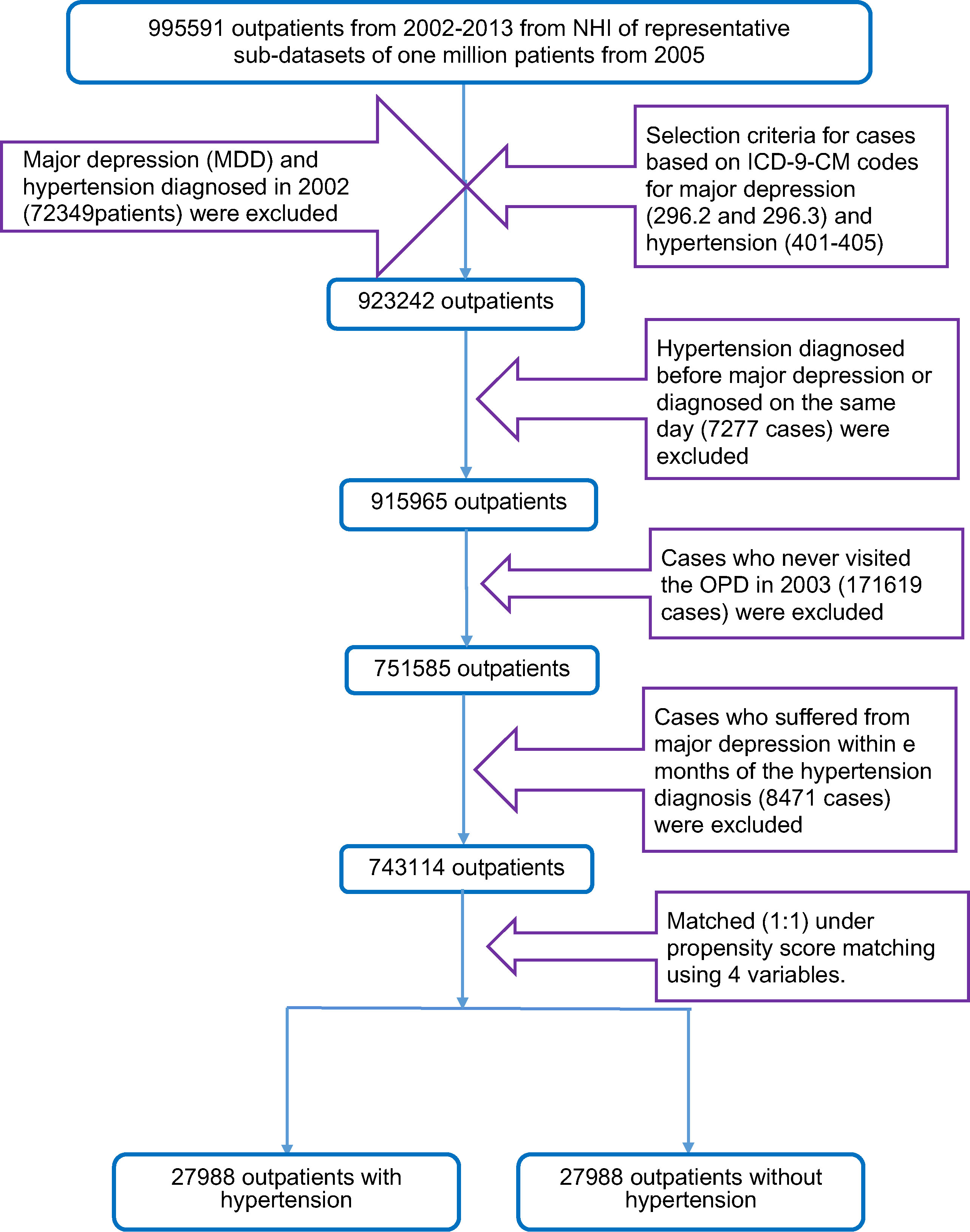
When selecting samples for analysis in this retrospective study, we first recruited 995,591 outpatients between 2002 and 2013 from a representative one million sub-datasets. We selected the samples using the ICD-9-CM (International Classification of Diseases, 9th revision, Clinical Modification) codes for hypertension (ICD-9-CM codes 401 X-405 X) and major depression (ICD-9-CM codes 296.2X and 296.3X). Our goal was to include new cases in our study, but we could not clarify whether these cases (major depression and hypertension diagnosed in 2002) had visited the outpatient department (OPD) before 2002. Therefore, major depression and hypertension cases diagnosed in 2002 (72,349 patients) were excluded. To explore whether MDD increased the risk of hypertension, MDD needed to occur before hypertension. Therefore, cases in which MDD was diagnosed after hypertension or diagnosed on the same day (7,277 cases) were excluded (Figure 1). Because the observations in our study occurred starting in 2003, cases that did not visit the OPD in 2003 (171,619 cases) were excluded. To prevent confusion from cases who suffered from major depression and hypertension over a time span that was too small to determine whether these cases suffered from MDD before hypertension, cases who suffered from hypertension within 3 months of the diagnosis of major depression (8,471 cases) were excluded. Finally, 743,114 outpatients were included in our study. Additionally, we used propensity score matching to select 27,988 outpatients with hypertension and 27,988 outpatients without hypertension.
Measurements
Information on demographic factors was obtained, including age, gender, antipsychotic use (divided into first- and second-generation antipsychotics), antidepressant use, mood stabilizer use, Charlson comorbidity index (CCI) score, hospital level (divided into medical centers, regional hospitals, district hospitals, and local clinics), and insurance amount. The ICD-9-CM version of the CCI was used to measure the comorbid disease status in the study; this version has been a valuable resource for several health services researchers (36–40). The insurance amount (also known as the insured wage) represented the salaried income and was used instead of the socioeconomic status in our study; this measure was divided into the following three categories: <640 US$, 640–1,280 US$, and >1,280 US$.
Study Starting Point, Endpoint, and Follow-Up Duration
We defined our starting point, endpoint, and follow-up duration as follows. The follow-up period for the cases was between January 1, 2003 and December 31, 2013. For the cases without major depression, the starting point was the first ambulatory care visit (including outpatient departments of hospitals or clinics); for the cases with major depression, the starting point was the time of the first diagnosis of depression. For the cases with hypertension, the endpoint was the time of the first diagnosis of hypertension; for the cases without hypertension, the endpoint was the last ambulatory care visit. The follow-up duration was calculated from the starting point to the endpoint.
Statistical Analysis
The data were analyzed using the IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp. Released 2012. Armonk, NY: IBM Corp). Baseline characteristics were compared using an independent t test or the chi-square test. Taking into account the factor of follow-up duration, Cox’s regression analysis with hazard ratios (HRs) was used to estimate the association of the risk of hypertension with major depression after adjusting for related variables.
The propensity score exact matching method presented by Thoemmes (41) was adapted in this study. The propensity score matching method is a statistical technique that is used to select samples by capturing the relevant differences between any two units into a single (propensity) score that encapsulates multiple characteristics. This method provides a natural weighting scheme that yields unbiased estimates of the impact by interested factors (42). Variables in the stratum that were matched and controlled included age, CCI score, hospital level, and insurance amount.
Results
In total, 743,114 outpatients were included in our study, of which 12,570 cases had MDD and 730,544 cases did not have MDD. The t test analysis (Table 1) found significant differences in age (T = 2,341.9, P < 0.001) and the CCI score (T = 1,702.4, P < 0.001) between the MDD and non-MDD groups. The MDD group was older than the non-MDD group. The CCI score was higher in the MDD group than in the non-MDD group. The chi-square test (Table 1) found significant differences in gender (χ2 = 1,191, P < 0.001), the hospital level visited by the cases (χ2 = 17,384, P < 0.001), and the insurance amount (χ2 = 13.44, P = 0.001) between the MDD and non-MDD groups. A higher ratio of female patients was noted in the MDD group. Fewer MDD outpatients visited clinics relative to the other hospital levels.
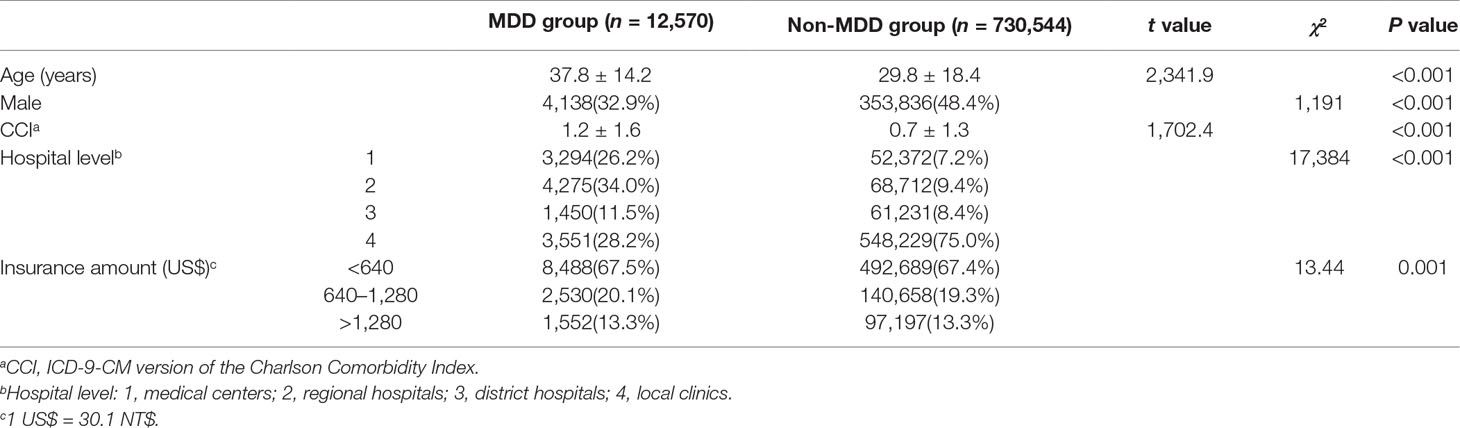
Table 1 Comparison of demographic data between the major depressive disorder (MDD) and non-MDD groups.
We used proportional hazards model analysis to explore the risk of hypertension for the 743,114 outpatients. Table 2 showed that the hazard ratio of hypertension did not significantly increase in the MDD group (ICD-9-CM codes 296.2X and 296.3X) (HR = 0.95, P = 0.134) after adjusting for other confounders. However, the hazard ratio for hypertension was significantly greater for the male MDD outpatients (HR = 1.12, P = 0.004) than for the female MDD outpatients (HR = 0.93, P ≤ 0.001) after adjusting for the other confounders. Therefore, we observed an interaction between the MDD and gender (HR = 1.14, P = 0.009).
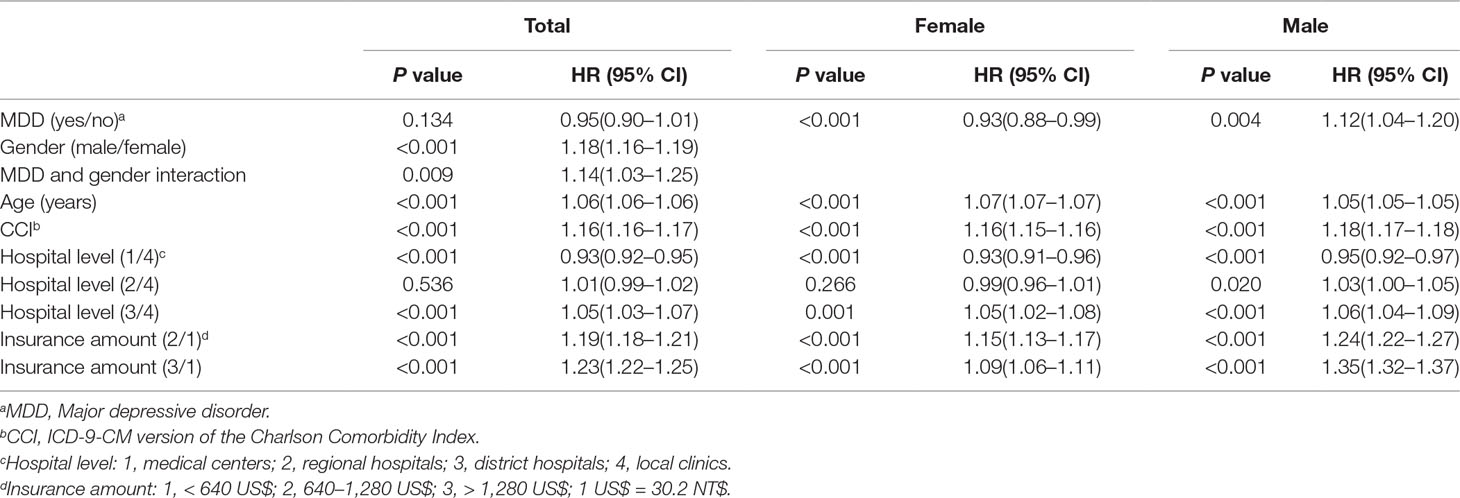
Table 2 Proportional hazards model analysis of the MDD and non-MDD groups adjusted for related factors of hypertension.
Male (HR = 1.18, P < 0.001) and older (HR = 1.06, P < 0.001) outpatients had a higher risk of suffering from hypertension. Additionally, outpatients with a higher CCI score (HR = 1.16, P < 0.001) had a higher risk of suffering from hypertension. Relative to the local clinics, the outpatients who visited medical centers (HR = 0.93, P < 0.001) had a lower risk of suffering from hypertension, whereas the outpatients who visited district hospitals (HR = 1.05, P < 0.001) had a higher risk of suffering from hypertension. Relative to an insurance amount <640 US$, outpatients with insurance amounts in the range from 640 to 1,280 US$ (HR = 1.19, P < 0.001) and >1,280 US$ (HR = 1.23, P < 0.001) both had higher risks of suffering from hypertension.
The mean follow-up time was 9.46 ± 2.64 years (after the first OPD in 2003), the minimum follow-up period was 0.3 years, and the maximum follow-up period was 11.0 years for the 743,114 outpatients.
After propensity score matching, which used nearest neighbor matching by age, CCI score, hospital level, and insurance amount, we selected 27,988 outpatients with hypertension and 27,988 outpatients without hypertension. The demographic data between the two groups are shown in Supplementary Table 1. We found that there was no difference between age, CCI score, hospital level, and insurance amount after propensity score matching. Because our aim was to confirm the gender disparity in the risk of hypertension, we did not control for gender in the propensity score matching analysis. Relative to female outpatients without MDD, female outpatients with MDD had a lower risk of hypertension (relative risk = 0.852), whereas male outpatients without MDD (relative risk = 1.987) or with MDD (relative risk = 3.018) had higher risks of hypertension in a multiplicative model (Table 3).
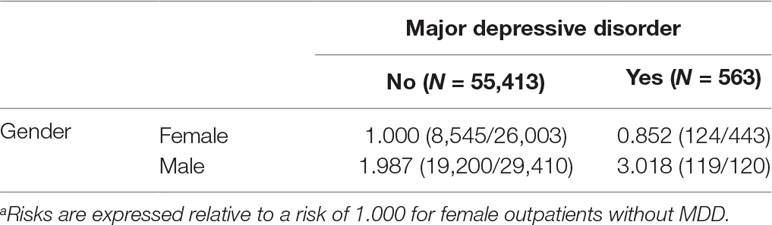
Table 3 Relative risksa of hypertension among three groups (female outpatients with MDD, male outpatients without MDD, and male outpatients with MDD) in comparison with the female outpatients without MDD for 27,988 outpatients with hypertension and 27,988 outpatients without hypertension (nearest neighbor matching by age, CCI, hospital level, and insurance amount) in a multiplicative model.
In order to consider the factor of follow-up duration, we also used proportional hazard model analysis to explore the risk of hypertension in the nested case–control study (Table 4). We observed that the hazard ratio (HR) of hypertension was significantly greater for male MDD outpatients (HR = 2.69, P < 0.001) and female MDD outpatients (HR = 1.53, P < 0.001). Additionally, MDD appeared to have an interaction effect with gender (HR = 1.82, P < 0.001) in the proportional hazards model analysis. On the other hand, the survival curve of suffering hypertension between four groups (female outpatients without MDD, female outpatients with MDD, male outpatients without MDD, and male outpatients with MDD) are shown in Supplementary Figure 1. Male outpatients with MDD had highest risk of hypertension and female without MMD had lowest risk of hypertension. First-generation (P = 0.466) and second-generation antipsychotic use (P = 0.141) had no significant effects on the risk of hypertension in the total cases. However, second-generation antipsychotic use increased the risk of hypertension in the female outpatients (HR = 1.38, P = 0.014). Antidepressant use increased the risk of hypertension in the male outpatients (HR = 1.44, P = 0.001), female outpatients (HR = 1.20, P = 0.003), and total cases (HR = 1.16, P < 0.001) (Table 4). Mood stabilizer use only increased the risk of hypertension in the female outpatients (HR = 1.31, P = 0.016) and total cases (HR = 1.21, P = 0.012) and not in the male outpatients (HR = 1.73, P = 0.151).
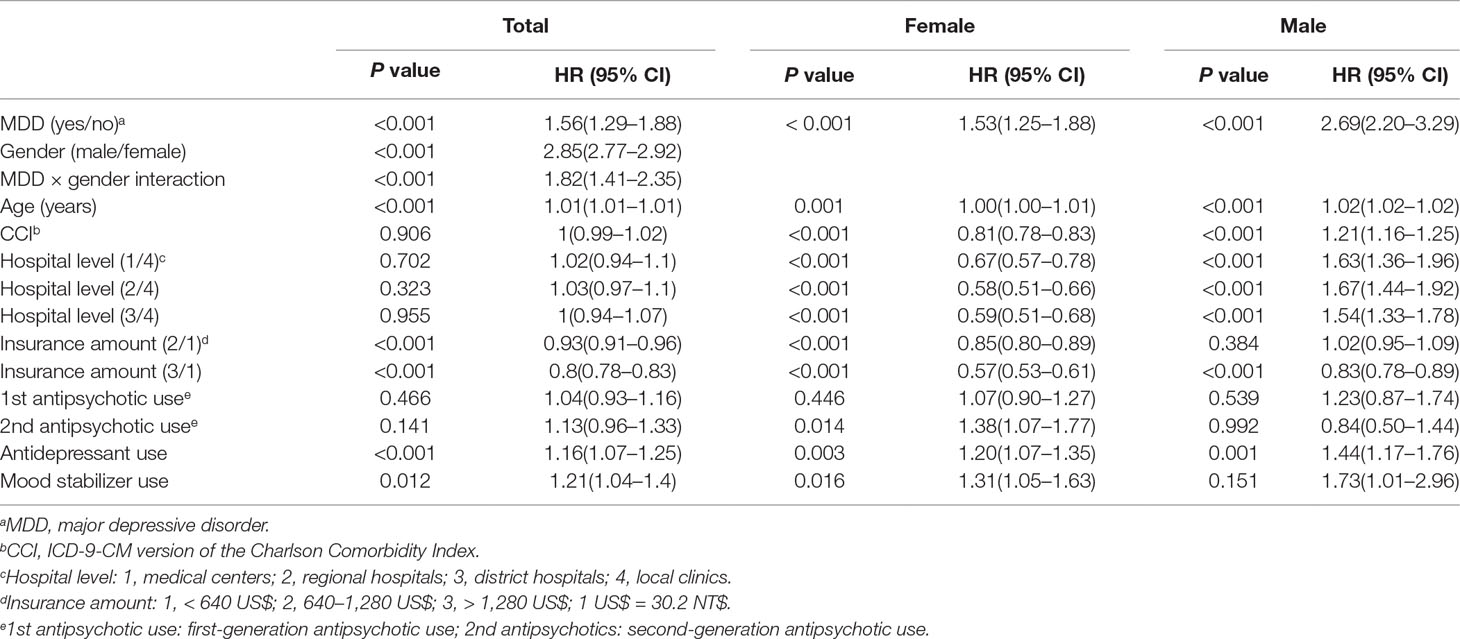
Table 4 Proportional hazards model analysis for 27,988 outpatients with hypertension and 27,988 outpatients without hypertension (nearest neighbor matching by age, CCI, hospital level, and insurance amount).
Discussion
A gender difference in the prevalence of MDD which is more than twice in women than in men has been well-established in multiple studies (43–47). Our study showed a similar result (32.9% in men vs. 67.1% in women) in Table 1. We also found that the prevalence of MDD were 1.2% in 357,974 male outpatients and 2.2% in 385,140 female outpatients between 2002 and 2013.
A previous study (25) showed an increased risk of hypertension in patients with major depressive disorder. However, this cross-sectional study only presented a possible association and not a temporal relationship. Although Wu et al. found that the prevalence and incidence of hypertension were higher among MDD patients than among the general population in their case–control study, they could not clarify whether depression occurred before the onset of hypertension or as a consequence of hypertension. On the other hand, they did not consider the factor of follow-up duration. Cox’s regression analysis with hazard ratios (HRs) was not used in their study.
In our study, outpatients who received a diagnosis of hypertension before major depression or diagnoses for both diseases on the same day (7,277 cases) were excluded. Additionally, outpatients who suffered from hypertension within 3 months of the diagnosis of major depression (8,471 cases) were excluded. After these procedures, we confirmed that MDD occurred before the onset of hypertension in our study.
Our study used proportional hazards model analysis to explore risk of hypertension for 743,114 outpatients; the time factor was added to the analysis, and the observation duration was from 2003 to 2013. This approach was superior to the approach used in the previous study (25), which used risk ratios to analyze the prevalence (only in 2005) and incidence (2006–2008) of hypertension in patients with major depression. In the proportional hazards model analysis of the total cases, the results of our study were similar to previous research (48, 49), with male and older subjects having a higher rate of hypertension.
In the retrospective analysis of the male and female outpatients (Table 2), we found different effects of MDD on the occurrence of hypertension between the two groups. MDD increased the risk of hypertension in the male outpatients. In contrast, MDD did not increase the risk of hypertension in the female outpatients. Table 3 shows that female outpatients with MDD had a lower risk (relative risk = 0.852) of hypertension than female outpatients without MDD and that male outpatients with MDD (relative risk = 3.018) had a higher risk than the male outpatients without MDD (relative risk = 1.987). Moreover, the male and female outpatients with MDD both had higher risks of hypertension in the proportional hazards model analysis of the 27,988 outpatients with hypertension and 27,988 outpatients without hypertension after propensity score matching (Table 4). In Table 4, it indicates that female outpatients with MDD had a higher risk (HR = 1.53, P < 0.001) than female outpatients without MDD. The results in Tables 2 and 3 were the opposite. The possible reasons were discussed below.
However, the use of large samples like in Table 2 can lead to the following potential issues: 1) studies with large samples are likely to be more sensitive in the detection of significant results, and 2) a large sample size may cause false positives or negatives during the statistical analysis of control charts (50). Hence, the validity of large samples needs to be examined to decrease the type I error (false positives). Therefore, we used propensity score matching (nearest neighbor matching) for four variables in our study to select 27,988 outpatients with hypertension and 27,988 outpatients without hypertension in an effort to prevent issues due to the large sample size. The different effects of MDD on the risk of hypertension among female outpatients before and after propensity score matching (Tables 2 and 4) may be a result of this approach.
Even after propensity score matching, different risks of hypertension were observed among female MDD outpatients between the multiplicative model and proportional hazards model analysis (Tables 3 and 4), which might be due to control-related variables associated with hypertension and the follow-up duration.
However, the hazard ratio of hypertension was higher in the male MDD outpatients than in the female outpatients, and an interaction was found between MDD and gender in Table 4. One possible explanation for this gender disparity among MDD patients and increased hypertension is that sex hormones are responsible for the difference because premenopausal women are relatively protected against hypertension compared to men and postmenopausal women (51). Another possible explanation for the results is gender differences stemming from social factors. Men and women tend to have different perceptions of healthy behaviors, and men tend to have unhealthier lifestyles (i.e., smoking and alcohol consumption) than women. Furthermore, men are less likely to perceive themselves as being at risk for health problems (52–54). In clinical, there were differences in the symptom of depression between men and women (55). Depressive men more commonly had serious symptoms than depressive women. This may also explain that the hazard ratio of hypertension was higher in the male MDD outpatients than in the female outpatients.
Regarding the effects of drug use on the risk of hypertension among patients with MDD, antidepressants (29) and antipsychotics (56) were shown to elevate the risk of hypertension among MDD patients in past studies. One study also showed that antidepressant use increased the risk of hypertension (29). In our study, antidepressant use increased the risk of hypertension in male outpatients, female outpatients, and the total cases. However, the data about different kinds of antidepressants use were not included in our study, so we cannot analyze the effects of different kinds of antidepressants to the risk of hypertension.
Limitations
Our study had several limitations. Because our cases were selected from the Longitudinal Health Insurance Database (LHID2005), certain risk factors, such as smoking (57, 58) and alcohol consumption (59), could not be included in our analysis. On the other hand, it also led us to select the samples using the ICD-9-CM codes for hypertension and MDD instead of using standardized instrument to diagnose hypertension and MDD. Therefore, a large-scale, population-based study that includes complete risk factors is necessary. Furthermore, large data are likely to be more sensitive when detecting significant results. We used the propensity score matching method to control variables associated with hypertension, and the results before and after propensity score matching were consistent.
Conclusions
Using proportional hazards model analysis in a retrospective study and a nested case–control study, we observed gender disparity in the risk of hypertension in subjects with MDD. The hazard ratio of hypertension was higher in the male MDD outpatients than in the female outpatients. Furthermore, a large population should be considered in the future to generalize these results.
Author Contributions
W-TK and C-LC, with help of F-WL, planned the present study’s content and analysis, interpreted the data, and wrote the paper. W-TK, C-LC, C-HL, S-LW, S-LL, and F-WL initiated and performed the whole survey, analyzed the data, and helped to interpret the findings and to write the paper. All authors read and approved the final manuscript.
Funding
The study was supported by a research grant (넰KAFGH 105-049) from the Outpatient Foundation of Kaohsiung Armed Forces General Hospital (Kaohsiung, Taiwan).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The authors would like to thank all researchers in Kaohsiung Armed Forced General Hospital who assisted in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00541/full#supplementary-material
References
1. World Health Organization. (2012). Sixty-fifth world health assembly 2012. Available at: http://www.who.int/mediacentre/events/2012/wha65/journal/en/index4.html (Accessed December 11, 2014).
2. Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry (2004) 49:124–38. doi: 10.1177/070674370404900208
3. Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry (2015) 54:37–44. e32. doi: 10.1016/j.jaac.2014.10.010
4. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA (1996) 276:293–9. doi: 10.1001/jama.276.4.293
5. Oakley-Browne MA, Joyce PR, Wells JE, Bushnell JA, Hornblow AR. Christchurch Psychiatric Epidemiology Study, Part II: six month and other period prevalences of specific psychiatric disorders. Aust N Z J Psychiatry (1989) 23:327–40. doi: 10.3109/00048678909068290
6. Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry (1994) 151:979–86. doi: 10.1176/ajp.151.7.979
7. Hwu HG, Chang IH, Yeh EK, Chang CJ, Yeh LL. Major depressive disorder in Taiwan defined by the Chinese diagnostic interview schedule. J Nerv Ment Dis (1996) 184:497–502. doi: 10.1097/00005053-199608000-00007
8. Liao SC, Chen W, Lee MB, Lung FW, Lai TJ, Liu CY, et al. Low prevalence of major depressive disorder in Taiwanese adults: possible explanations and implications. Psychol Med (2012) 42:1227–37. doi: 10.1017/S0033291711002364
9. Lin E, Chen PS, Chang HH, Gean PW, Tsai HC, Yang YK, et al. Interaction of serotonin-related genes affects short-term antidepressant response in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33:1167–72. doi: 10.1016/j.pnpbp.2009.06.015
10. Fan PL, Chen CD, Kao WT, Shu BC, Lung FW. Protective effect of the apo ɛ2 allele in major depressive disorder in Taiwanese. Acta Psychiatr Scand (2006) 113:48–53. doi: 10.1111/j.1600-0447.2005.00686.x
11. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet (2008) 371:1513–8. doi: 10.1016/S0140-6736(08)60655-8
12. Faraco G, Iadecola C. Hypertension. Hypertension (2013) 62:810–7. doi: 10.1161/HYPERTENSIONAHA.113.01063
13. van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. N Engl J Med (2000) 342:1–8. doi: 10.1056/NEJM200001063420101
14. Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet (2012) 380:1649–61. doi: 10.1016/S0140-6736(12)61272-0
15. Schillaci G, Pucci G, Perlini S. From hypertension to hypertrophy to heart failure: the role of cardiotrophin-1. J Hypertens (2013) 31:474–6. doi: 10.1097/HJH.0b013e32835ed4bb
16. Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA (2013) 310:959–68. doi: 10.1001/jama.2013.184182
17. Wolf-Maier K, Cooer RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA (2003) 289:2363–9. doi: 10.1001/jama.289.18.2363
18. Chiang KM. Trends in prevalence, awareness, and control of hypertension and associated dietary factors in Taiwan from 1993 to 2014: results of Nutrition and Health Survey in Taiwan (NAHSIT). In: 143rd APHA Annual Meeting and Exposition; 2015 Oct 31–Nov 4; Chicago: APHA. (2015).
19. Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Arch Intern Med (2000) 160:1495–500. doi: 10.1001/archinte.160.10.1495
20. Jonas BS, Franks P, Ingram DD. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med (1997) 6:43–9. doi: 10.1001/archfami.6.1.43
21. Meyer CM, Armenian HK, Eaton WW, Ford DE. Incident hypertension associated with depression in the Baltimore Epidemiologic Catchment area follow-up study. J Affect Disord (2004) 83:127–33. doi: 10.1016/j.jad.2004.06.004
22. Piane GM, Smith TC. Building an evidence base for the co-occurrence of chronic disease and psychiatric distress and impairment. Prev Chronic Dis (2014) 11:E188. doi: 10.5888/pcd11.140211
23. Carroll D, Phillips AC, Gale CR, Batty GD. Generalized anxiety and major depressive disorders, their comorbidity and hypertension in middle-aged men. Psychosom Med (2010) 72:16–9. doi: 10.1097/PSY.0b013e3181c4fca1
24. Nabi H, Chastang JF, Lefèvre T, Dugravot A, Melchior M, Marmot MG, et al. Trajectories of depressive episodes and hypertension over 24 years: the Whitehall II prospective cohort study. Hypertension (2011) 57:710–6. doi: 10.1161/HYPERTENSIONAHA.110.164061
25. Wu EL, Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of hypertension in patients with major depressive disorder: a population-based study. J Psychosom Res (2012) 73:169–74. doi: 10.1016/j.jpsychores.2012.07.002
26. Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res (2011) 70:145–54. doi: 10.1016/j.jpsychores.2010.07.010
27. Patten SB, Williams JV, Lavorato DH, Campbell NR, Eliasziw M, Campbell TS. Major depression as a risk factor for high blood pressure: epidemiologic evidence from a national longitudinal study. Psychosom Med (2009) 71:273–9. doi: 10.1097/PSY.0b013e3181988e5f
28. Wiehe M, Fuchs S, Moreira LB, Moraes RS, Pereira GM, Gus M, et al. Absence of association between depression and hypertension: results of a prospectively designed population-based study. J Hum Hypertens (2006) 20:434–9. doi: 10.1038/sj.jhh.1002017
29. Licht CM, De Geus EJ, Seldenrijk A, Van Hout HP, Zitman FG, Van Dyck R, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension (2009) 53:631–8. doi: 10.1161/HYPERTENSIONAHA.108.126698
30. Sandstrom YK, Ljunggren G, Wandell P, Wahlstrom L, Carlsson AC. Psychiatric comorbidities in patients with hypertension—a study of registered diagnoses 2009–2013 in the total population in Stockholm County, Sweden. J Hypertens (2016) 34:414–20. doi: 10.1097/HJH.0000000000000824
31. Fan PL, Shu CH, Shiang JC, Kuo TS, Lung FW. Hypertension—a possible vulnerability marker for depression in patients with end-stage renal disease. Nephron Clin Pract (2006) 102:c43–50. doi: 10.1159/000088314
32. Administration NHI (2015). Longitudinal Health Insurance Database (LHID) in 2015. Available at:https://www.gender.ey.gov.tw/gecdb/Stat_Statistics_Query.aspx?sn=Jvwu1Ndiotx2AzKr6MD1kg%3d%3d&statsn=EcfUJy%2fsRRPbnOe4TvO%2fJg%3d%3d&d=m9ww9odNZAz2Rc5Ooj%2fwIQ%3d%3d(Accessed November 11, 2016).
33. Chen YC, Yeh HY, Wu JC, Haschler I, Chen TJ, Wetter T. Taiwan’s National Health Insurance Research Database: administrative health care database as study object in bibliometrics. Scientometrics (2011) 86:365–80. doi: 10.1007/s11192-010-0289-2
34. Yeh ST, Ng YY, Wu SC. Risk of suicide according to the level of psychiatric contact in the older people: analysis of national health insurance databases in Taiwan. Compr Psychiatry (2017) 74:189–95. doi: 10.1016/j.comppsych.2017.01.016
35. Chen YC, Wu JC, Chen TJ, Wetter T. A publicly available database accelerates academic production. BMJ (2011) 342:d637. doi: 10.1136/bmj.d637
36. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol (1992) 45:613–9. doi: 10.1016/0895-4356(92)90133-8
37. Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol (1993) 46:1075–9. doi: 10.1016/0895-4356(93)90103-8
38. D’hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med (1993) 32:382–7. doi: 10.1055/s-0038-1634956
39. D’hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol (1996) 49:1429–33. doi: 10.1016/S0895-4356(96)00271-5
40. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol (2004) 57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012
41. Thoemmes F (2012). Propensity score matching in SPSS; 2012. Available at: http://arxiv.org/abs/1201.6385 (Accessed July 24, 2013).
42. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
43. Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr Scand (2003) 108:163–74. doi: 10.1034/j.1600-0447.2003.00204.x
44. Ferrari A, Somerville A, Baxter A, Norman R, Patten S, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med (2013) 43:471–81. doi: 10.1017/S0033291712001511
45. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: lifetime prevalence, chronicity and recurrence. J Affect Disord (1993) 29:85–96. doi: 10.1016/0165-0327(93)90026-G
46. Schuch JJ, Roest AM, Nolen WA, Penninx BW, de Jonge P. Gender differences in major depressive disorder: results from the Netherlands study of depression and anxiety. J Affect Disord (2014) 156:156–63. doi: 10.1016/j.jad.2013.12.011
47. Silverstein B, Ajdacic-Gross V, Rossler W, Angst J. The gender difference in depressive prevalence is due to high prevalence of somatic depression among women who do not have depressed relatives. J Affect Disord (2017) 210:269–72. doi: 10.1016/j.jad.2017.01.006
48. McEniery C, Yasmin Y, McDonnell B, Cockcroft J, Wilkinson I. Impact of age and gender on the determinants of pulse pressure and isolated systolic hypertension. FASEB J (2017) 31:1012.1012.
49. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol (2011) 589:5285–97. doi: 10.1113/jphysiol.2011.212753
50. Runkel P (2012). Large samples: too much of a good thing? Available at: http://blog.minitab.com/blog/statistics-and-quality-data-analysis/large-sames-too-much-of-a-good-thing (Accessed July 4, 2012).
51. Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens (2011) 20:133–8. doi: 10.1097/MNH.0b013e3283431921
52. Boehm S, Selves EJ, Raleigh E, Ronis D, Butler PM, Jacobs M. College students’ perception of vulnerability/susceptibility and desire for health information. Patient Educ Couns (1993) 21:77–87. doi: 10.1016/0738-3991(93)90062-2
53. Flynn J, Slovic P, Mertz CK. Gender, race, and perception of environmental health risks. Risk Anal (1994) 14:1101–8. doi: 10.1111/j.1539-6924.1994.tb00082.x
55. Poutanen O, Koivisto AM, Mattila A, Joukamaa M, Salokangas RK. Gender differences in the symptoms of major depression and in the level of social functioning in public primary care patients. Eur J Gen Pract (2009) 15:161–7. doi: 10.3109/13814780903186423
56. Liao CH, Chang CS, Wei WC, Chang SN, Liao CC, Lane HY, et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res (2011) 126:110–6. doi: 10.1016/j.schres.2010.12.007
57. Leone A. Smoking and hypertension: carbon monoxide or nicotine? A meta-analysis study. J Am Soc Hypertens (2015) 9:e117–8. doi: 10.1016/j.jash.2015.03.272
58. Gao K, Shi X, Wang W. The life-course impact of smoking on hypertension, myocardial infarction and respiratory diseases. Sci Rep (2017) 7:4330. doi: 10.1038/s41598-017-04552-5
Keywords: major depressive disorder (MDD), propensity score matching (PSM), hypertension, risk factors, National Health Insurance Database
Citation: Kao W-T, Chang C-L, Lin C-H, Wu S-L, Lin S-L and Lung F-W (2019) Gender Disparity in the Risk of Hypertension in Subjects With Major Depressive Disorder. Front. Psychiatry 10:541. doi: 10.3389/fpsyt.2019.00541
Received: 27 December 2018; Accepted: 12 July 2019;
Published: 02 August 2019.
Edited by:
Renerio Fraguas, University of São Paulo, BrazilReviewed by:
Marsal Sanches, University of Texas Health Science Center at Houston, United StatesBrett Silverstein, City College of New York (CUNY), United States
Copyright © 2019 Kao, Chang, Lin, Wu, Lin and Lung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: For-Wey Lung, Zm9yd2V5QHNlZWQubmV0LnR3
 Wei-Tsung Kao
Wei-Tsung Kao Chen-Lin Chang
Chen-Lin Chang Chi-Hung Lin
Chi-Hung Lin Shang-Liang Wu6
Shang-Liang Wu6 For-Wey Lung
For-Wey Lung