- 1Division of Psychotherapy and Psychosomatic Medicine, Department of Psychiatry, Wroclaw Medical University, Wroclaw, Poland
- 2Department of Endocrinology, Diabetes and Isotope Therapy, Wroclaw Medical University, Wroclaw, Poland
- 3Department of Psychiatry, Wroclaw Medical University, Wroclaw, Poland
Background: Approximately half of all patients with posttraumatic stress disorder (PTSD) also suffer from major depressive disorder (MDD). This co-occurrence might lead to an impairment of cognitive functions, worse response to antidepressant medications, and an increased risk of suicide in comparison to patients with PTSD alone. Prognosis for people with PTSD and MDD co-occurrence is poorer than for either one alone; therefore, researchers look for novel, effective treatments.
Case Presentation: A patient with MDD with the co-occurrence of PTSD was admitted to the Department of Endocrinology with suspicion of adrenal insufficiency. In order to assess the adrenocorticotropin/cortisol axis, a standard insulin tolerance test was performed. After inducing a hypoglycemic episode with intravenous short-acting insulin, PTSD symptoms were reduced. To the best of our knowledge, this is the first report on the reduction of PTSD symptoms after performing an insulin tolerance test.
Conclusion: Reduction of PTSD symptoms in PTSD and MDD comorbidity has been noticed after a hypoglycemic episode. This demonstrates the mutual dependencies between the endocrine and nervous systems, covered extensively by psychoneuroendocrinology.
Background
Meta-analysis data indicate that in as many as 52% patients with a posttraumatic stress disorder (PTSD), major depressive disorder (MDD) co-occurs, which might lead to an impairment of cognitive functions, worse response to antidepressant medications, and an increased risk of suicide in comparison to patients with PTSD alone (1). Neuroimaging studies reveal differences between people with PTSD and MDD and those with PTSD alone (2–4). In the PTSD/MDD group, medial prefrontal cortex and amygdala activation by threatening stimuli are lower than in the PTSD group. People with PTSD/MDD also exhibit a lower functional connectivity between the insula and hippocampus when compared to those with PTSD alone. Thus, it can be hypothesized that PTSD and MDD comorbidity can even represent a subtype of PTSD (5).
Food and Drug Administration (FDA)–approved pharmacological treatment for PTSD produces a response rate of approximately 60% (6). Prognosis for people with PTSD and MDD co-occurrence is poorer than for either one alone. Dropout rates in the PTSD/MDD group are more common; therefore, researchers look for novel, effective treatments. For example, the results of the Yehuda et al. study reveal that hydrocortisone augmentation during prolonged exposure therapy may result in a greater retention in the treatment group, thereby improving PTSD symptom response (7).
Here we describe a case of a female patient with MDD with the co-occurrence of PTSD diagnosed in the Department of Endocrinology, where she was admitted with suspicion of adrenal insufficiency. The insulin tolerance test (ITT) was performed to diagnose adrenal insufficiency. After inducing a hypoglycemic episode with intravenous short-acting insulin, PTSD symptoms were reduced.
Case Presentation
The patient was a 36-year-old woman, married, with no children; having completed a higher education, she is a lawyer by profession. In October 2013, the patient sought psychiatric help in an outpatient clinic due to depressed mood, decreased interest, insomnia, feelings of worthlessness, and significant weight change (−10%). MDD, 296.22 [Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)] was diagnosed, and the patient received escitalopram 20 mg/d, with no improvement after 5 weeks’ treatment. Thus, the treatment changed to paroxetine 60 mg/d and trazodone 150 mg/d, which resulted in a marked improvement within 6 weeks (difficulties in starting activities remained).
In January 2014, the patient was diagnosed with Hashimoto autoimmune thyroiditis. Introduction of levothyroxine 75 mg/d was temporally connected to remission of depressive symptoms. The diagnosis was updated to mood disorder due to hypothyroidism with major depressive-like episode, 293.83 (DSM-IV-TR). In March 2015, psychiatric treatment was concluded.
In May 2015, the patient became pregnant. Ultrasound examination revealed multiple birth defects of the fetus, which led to its death in week 16 of the pregnancy. The patient was hospitalized in the Department of Gynaecology and Obstetrics, where she gave birth to a dead fetus. During the event, she experienced intense fear and helplessness. Following the incident, the patient sought psychiatric help, again due to symptoms she had experienced earlier and which had returned, that is, depressed mood, decreased interest, insomnia, and feelings of worthlessness. Additional symptoms of PTSD, unreported previously, ensued: recurrent distressing dreams of the event, the sensation that the traumatic event was recurring, and the inability to recall an important aspect of the trauma (the patient did not remember a part of her stay at the gynecological ward). Attempts at broaching the subject of losing her child were met with defiance and emotional withdrawal. Her general state was characterized by an increased arousal typical of PTSD cases (irritability, outbursts of anger, difficulty concentrating, hypervigilance). The patient no longer managed to function normally in social contexts (workplace, home). MDD, 296.22, was diagnosed with the co-occurrence of acute PTSD, 309.81. Given that selective serotonin reuptake inhibitor (SSRI) medications such as sertraline and paroxetine are approved by the FDA for the treatment of PTSD and with her previous treatment response, we decided to start with paroxetine 20 mg/d and trazodone 75 mg/d. However, no marked improvement was noted following 5 weeks of treatment. Paroxetine was increased up to 60 mg/d and trazodone up to 150 mg/d, the same dose prescribed previously, which has resulted in marked improvement of her MDD.
Due to a massive sleep dysfunction consisting in troubles with falling asleep, frequent waking periods during the night, and a very high level of irritability, as well as multiple outbursts of anger and anxiety, it was necessary to increase the trazodone dose to 200 mg/d and to introduce diazepam in the dose of 5 mg/tds. Moreover, the patient’s somatic health condition was deteriorating rapidly. The patient lost weight [−10% within a month; body mass index (BMI) = 19 kg/m2]; she complained of general weakness, dizziness and fainting, as well as of episodes of hyperventilation and paresthesia. Physical examination revealed dehydration and low blood pressure (100/60 mmHg), which plummeted even further when standing (orthostatic hypotension). Assessments of both morning adrenocorticotropin (ACTH) and morning cortisol levels in the blood performed on an outpatient basis revealed slightly decreased results (ACTH = 6.00 pg/ml; cortisol = 4.90 ng/ml). Therefore, in April 2016, the patient was admitted to the Department of Endocrinology with suspicion of adrenal insufficiency.
During the patient’s stay at the ward, examination revealed correct circadian rhythm in serum cortisol (6:00, 5.60 μg/dl; 8:00, 11.30 μg/dl; 20:00, 4.40 μg/dl; 24:00, 4.20 μg/dl), as well as correct levels of the hormone in a 24-h sample of urine: 28.2 μg/24. Also, the ACTH concentration in the daily profile of the serum was correct (6:00, 6.12 pg/ml; 8:00, 10.20 pg/ml; 20:00, <5.40 pg/ml; 24:00, 7.07 pg/ml). Thyrotropin concentration (TSH) with both free thyroxine (fT4) and free triiodothyronine (fT3) were within normal range, with elevated anti-thyroid peroxidase (TPO) antibodies, 253 IU/ml (reference range < 35 IU/ml).
In order to assess the ACTH/cortisol axis for detecting secondary adrenal insufficiency, a standard ITT was performed. Symptomatic hypoglycemia, with blood glucose values below 40 mg/dl, was required to evoke a reliable central stress response with the activation of the hypothalamic–pituitary–adrenal (HPA) axis. Intravenous insulin was administered (0.1 units/kg; 6j aspart insulin). Symptomatic hypoglycemia, with blood glucose values of 29 mg/dl within 30 min of the test, was achieved. The patient experienced mild palpitations, massive hot flushes, and sweating, which disappeared within several minutes of the procedure. The results of the test excluded any abnormalities of the ACTH/cortisol and growth hormone secretion (Table 1).
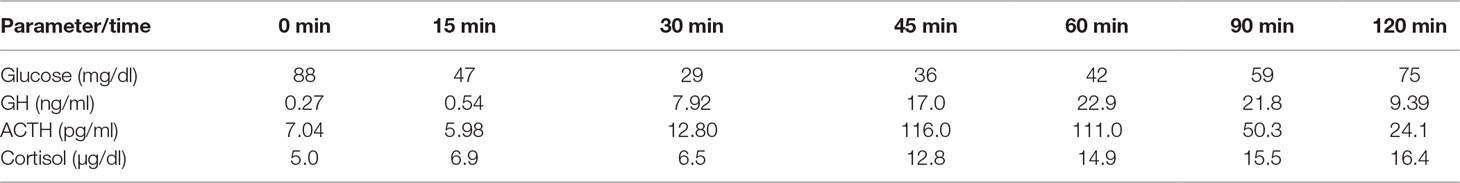
Table 1 Insulin tolerance test: documented hypoglycemia; subsequent rise of adrenocorticotropin (ACTH), cortisol, and growth hormone (GH) concentrations.
Four days after hospitalization, the patient had a consultation with her psychiatrist (the first author of this manuscript). During the examination, a marked improvement of the patient’s mental state was noted. Symptoms of PTSD were reduced: increased arousal diminished, sleep was normalized, and bouts of anxiety became much less frequent and less severe. The patient was able to recall an important aspect of the trauma, and recurrent distressing dreams of the event disappeared. MDD symptoms persisted in the clinical picture, including depressed mood, decreased interest, and feelings of worthlessness. Such results enabled the psychiatrist to take the patient off diazepam (in the course of 10 days).
Over the following month, MDD symptoms gradually became less intense; consequently, trazodone was reduced from 200 to 100 mg/d, and paroxetine from 60 to 40 mg/d. In July 2017, 14 months after the ITT, pharmacological treatment was concluded, and presently, the patient remains in remission and is planning a pregnancy.
Discussion
To the best of our knowledge, this is the first report on the reduction of PTSD symptoms after hypoglycemia induced during a standard ITT. The effects of insulin on the central nervous system were first reported by the Austrian-American psychiatrist Manfred Sakel, who applied insulin so as to decrease anxiety and agitation in patients on a withdrawal course from opioids. This was further expanded into insulin shock therapy, originally used to treat schizophrenia and later also depressive disorders (8). The clinical mechanism of what seemingly rendered the procedure successful has not been scientifically proven.
In the present case report, the improvement of the patient’s mental condition might stem both from systemic functions of insulin, which cause hypoglycemia, and from the effect of insulin on the central nervous system. Florez et al. describe the impact of hypoglycemia on the intrinsic CA (cornu ammonis region) 3 rhythm of the hippocampus (9). A drop in the glucose perfusate concentration produced seizure-like events (SLEs) originating in the CA3 region and spreading into the CA1 region. Prior to the onset of the SLE in the CA3 area, decreased gamma-aminobutyric acid (GABA)–correlated baseline sharp wave activity was observed, simultaneously with increased large-amplitude sharp wave activity from synchronous pyramidal cell firing. Hypoglycemia induces the release of aspartate and glutamate, and transiently GABA, which moves the balance of the circuitry towards excitation. Significantly lower GABA levels have been detected in PTSD (10), which seems to explain why benzodiazepines remain popular forms of treatment in PTSD—they enhance the effect of GABA.
Patients with PTSD/MDD resemble those with PTSD alone in their HPA axis response (11). In patients with PTSD/MDD, peripheral cortisol levels are generally lower, with enhanced glucocorticoid receptor (GR) sensitivity as inferred from results of the dexamethasone suppression test (DST). Patients with PTSD have an exaggerated cortisol suppression response to dexamethasone (DEX), which indicates that the negative-feedback system of the HPA axis is overly sensitive. In healthy subjects, the administration of intranasal insulin prior to a psychosocial stressor was found to diminish saliva and plasma cortisol without affecting heart rate or blood pressure stress reactivity, suggesting that insulin blunts the responsiveness of the stress-induced HPA axis (12).
Conclusions
In this case report, it has been observed that induction of a hypoglycemic episode with intravenous short-acting insulin was temporally connected to improvement of PTSD symptoms in PTSD and MDD comorbidity. The present case study demonstrates the mutual dependencies between the endocrine and nervous systems, covered extensively by psychoneuroendocrinology.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this case report.
Author Contributions
Specific author contributions: study design (TP, AC), data collection (TP, JD), analysis (TP, JD, JR, AC), manuscript writing (TP, JD, JR, CA).
Funding
Funding has been provided through grant ST.C230.18.014 from Wroclaw Medical University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress (2013) 26(3):299–309. doi: 10.1002/jts.21814
2. Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, et al. Influence of comorbid depression on fear in posttraumatic stress disorder: an fMRI study. Psychiatry Res (2007) 155(3):265–9. doi: 10.1016/j.pscychresns.2007.01.010
3. Lanius RA, Frewen PA, Girotti M, Neufeld RW, Stevens TK, Densmore M. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res (2007) 155(1):45–56. doi: 10.1016/j.pscychresns.2006.11.006
4. Kennis M, Rademaker AR, van Rooij SJ, Kahn RS, Geuze E. Altered functional connectivity in posttraumatic stress disorder with versus without comorbid major depressive disorder: a resting state fMRI study. F1000Res (2013) 2:289. doi: 10.12688/f1000research.2-289.v1
5. Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci (2015) 17(2):141–50.
6. Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33(2):169–80. doi: 10.1016/j.pnpbp.2008.12.004
7. Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology (2015) 51:589–97. doi: 10.1016/j.psyneuen.2014.08.004
8. Zyss T, Hese RT, Zieba A. Shock therapy in psychiatry—historical feature. Psychiatr Pol (2008) 42:797–818.
9. Florez CM, Lukankin V, Sugumar S, McGinn R, Zhang ZJ, Zhang L, et al. Hypoglycemia-induced alterations in hippocampal intrinsic rhythms: decreased inhibition, increased excitation, seizures and spreading depression. Neurobiol Dis (2015) 82:213–25. doi: 10.1016/j.nbd.2015.06.005
10. Meyerhoff DJ, Mon A, Metzler T, Neylan TC. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep (2014) 37(5):893–900. doi: 10.5665/sleep.3654
11. Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry (2015) 77(4):356–64. doi: 10.1016/j.biopsych.2014.02.006
Keywords: psychoneuroendocrinology, posttraumatic stress disorder, major depressive disorder, acute hypoglycemia, insulin tolerance test
Citation: Pawlowski T, Daroszewski J, Czerwinska A and Rymaszewska J (2019) Reduction of Posttraumatic Stress Disorder (PTSD) Symptoms in PTSD and Major Depressive Disorder Comorbidity After Acute Hypoglycemia—A Case Report. Front. Psychiatry 10:530. doi: 10.3389/fpsyt.2019.00530
Received: 01 August 2018; Accepted: 08 July 2019;
Published: 26 July 2019.
Edited by:
Beata Godlewska, University of Oxford, United KingdomReviewed by:
Ann Sharpley, University of Oxford, United KingdomJolanta Sylwia Masiak, Medical University of Lublin, Poland
Copyright © 2019 Pawlowski, Daroszewski, Czerwinska and Rymaszewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pawlowski Tomasz, dG9tYXN6LnBhd2xvd3NraUB1bWVkLndyb2MucGw=
 Tomasz Pawlowski
Tomasz Pawlowski Jacek Daroszewski
Jacek Daroszewski Agnieszka Czerwinska
Agnieszka Czerwinska Joanna Rymaszewska
Joanna Rymaszewska