- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Neuromodulation, Beijing, China
- 3Center of Epilepsy, Beijing Institute for Brain Disorders, Laboratory of Brain Disorders, Capital Medical University, Ministry of Science and Technology, Beijing, China
Background: Repetitive transcranial magnetic stimulation (rTMS) is a safe and efficacious technique to stimulate specific areas of cortical dysfunction in several neuropsychiatric diseases; however, it is not known whether high-frequency rTMS (HF-rTMS) over the left inferior parietal lobule, in low functioning children with autism spectrum disorder (ASD), improves core symptoms.
Method: Eleven low-functioning children with ASD completed two separate HF-rTMS treatment courses, 6 weeks apart. Each treatment course involved five 5-s trains at 20 Hz, with 10-min inter-train intervals, on left inferior parietal lobule each consecutive weekday for a 3-week period (15 treatments per course). Subjects were assessed at five time points: immediately before and after the first HF-rTMS course, immediately before and after the second HF-rTMS course, and 6 weeks after the second rTMS treatment course. Treatment effectiveness was evaluated using the Verbal Behavior Assessment Scale (VerBAS) and Autism Treatment Evaluation Checklist (ATEC). The latter test consists of four subtest scales: Language, Sociability, Sensory, and Behavior. In addition, daily treatment logbooks completed by parents were considered as one of the outcome measures.
Results: Participants showed a significant reduction in language- and social-related symptoms measured by ATEC from pretreatment to the 6-week follow-up after the second treatment course. Moreover, some possible improvements in imitation and cognition were reported by caregivers.
Conclusions: Our findings suggest that HF-rTMS over the left parietal cortex might improve core deficits in low-functioning children with ASD.
Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social communication and stereotyped behaviors (1). Despite the spectrum’s extreme heterogeneity, deficits in social cognition, including reduced social responsiveness, difficulty interacting with others, and recognizing others’ intentions and emotions, are core features of ASD (1).
Dysfunction of the mirror neuron system (MNS) has been postulated in the pathophysiology of ASD (2). Mirror neurons are visuomotor cells that discharge not only when an individual performs a particular action but also when a similar action is observed (2, 3). The mirror neuron system (MNS) enables individuals to interpret motor acts of others and promotes the development of social cognition, such as emotion and empathy (3). Besides, MNS facilitates motor coordination and participates in memory, speech, and action planning (3–5).
MNS predominantly comprises the inferior frontal gyrus, inferior parietal lobule (IPL), and posterior superior temporal sulcus (6). Recent studies suggest that a dysfunction of the MNS might generate social and cognitive impairments related to ASD (7). It has been found that motor neurons of the IPL can code motor goals (8) and process the congruence between the executed and the observed motor act (8, 9). It has also been demonstrated that any damage to the parietal cortex affects the imitation or understanding of an observed action (10). Therefore, IPL is a likely neurobiological target for the treatment of ASD.
Repetitive transcranial magnetic stimulation (rTMS) offers a noninvasive approach for modifying cortical excitability. It potentially evokes a short-term functional reorganization in the brain (11). Effects of rTMS are not limited to the primarily stimulated cortex, because of anatomical and functional connections of cortical regions within a distributed network (11–13). Studies have suggested that low-frequency rTMS (<1 Hz) decreases cortical excitability, whereas HF-rTMS (>5 Hz) increases it (14, 15). Neuroenhancement of MNS in typically developing individuals has been reported using high-frequency (20 Hz) rTMS (HF-rTMS) (16).
A limited number of research studies have evaluated the therapeutic effects of rTMS in ASD. For example, it has been reported that applying low-frequency rTMS to the dorsolateral prefrontal cortex causes a reduction in stereotypical behaviors (17). Stimulation of IPL, however, has not been undertaken in ASD. Moreover, few studies investigated the effects of rTMS in children with ASD and intellectual disability. In the present study, we examined the effects of HF-rTMS on IPL in autism associated with severe intellectual disability. We hypothesized that HF-rTMS application to IPL would result in improvements in social functioning.
Methods
Participants
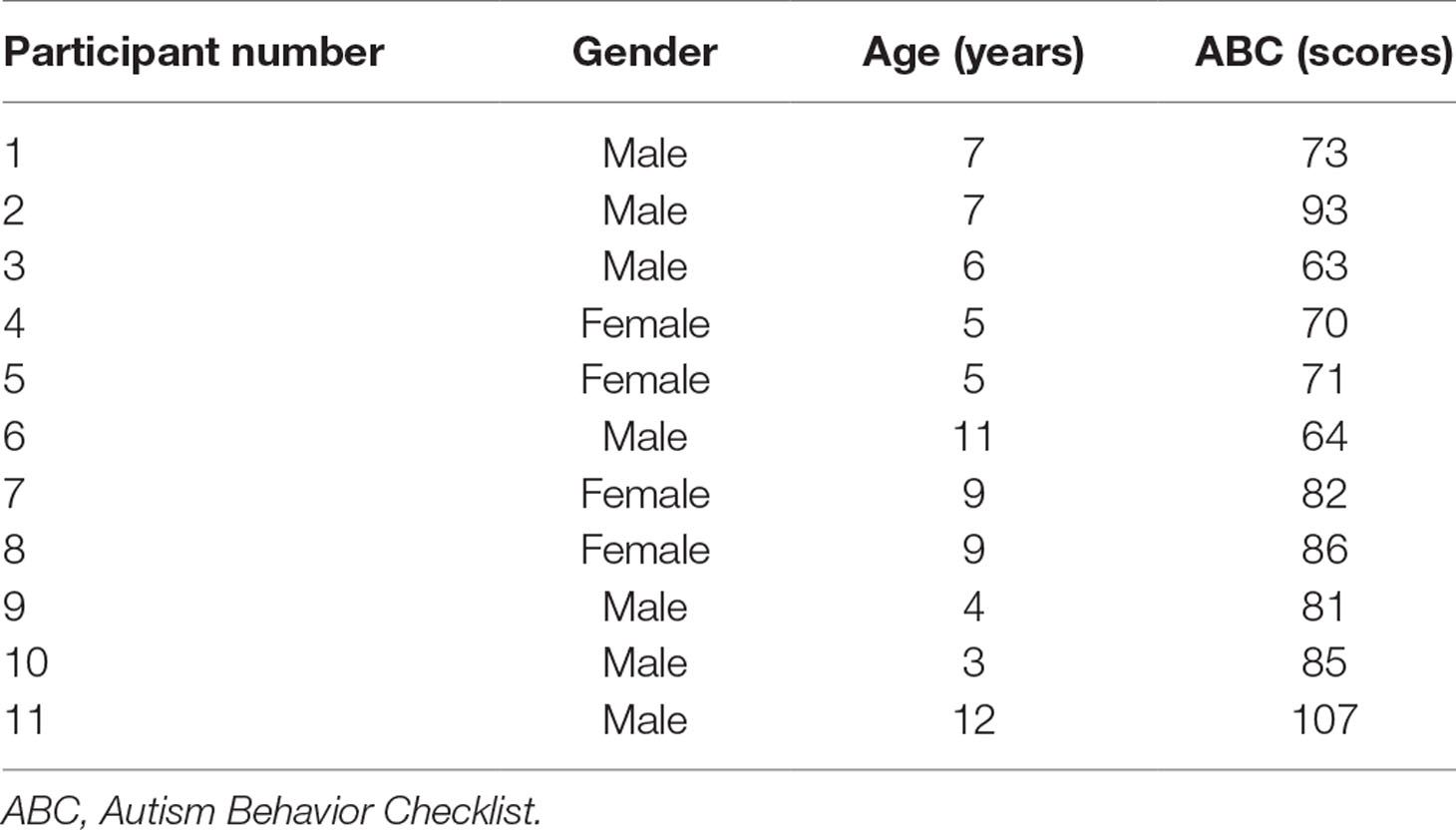
Thirteen participants with ASD (age range 3–12 years) were recruited from Xuanwu Hospital, Capital Medical University, Beijing, China. Diagnosis was made by an experienced physician according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) (1), and further confirmed with the Autism Diagnostic Interview–Revised (ADI-R) (18) and Autism Behavior Checklist (ABC) (19), administered by physicians trained to clinical reliability. Cases with a personal and family history of seizure, the presence of metal implants, were excluded. No subjects were on psychotropic medications. All 13 participants had IQ <70 measured by the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (20). Two patients withdrew from the study during the first course of the treatment due to family reasons. The data of these two individuals were excluded in the final sample. Participant information is summarized in Table 1.
This study had the approval of the ethics committee of Xuanwu Hospital, and all participants’ parents provided written informed consent before the study.
Procedures
A Magstim Super Rapid stimulator (The Magstim Company Ltd., Whitland, UK) connected to a 70-mm figure-of-eight coil was used to perform rTMS. The stimulation was applied on the left IPL [electrode P3 on the electroencephalography (EEG) cap] (21). Participants completed two separate courses that were 6 weeks apart. Each treatment course consisted of five 5-s trains at 20 Hz, with 10-min intertrain intervals, each consecutive weekday for a 3-week period (15 treatments per course). Because most participants could not participate in motor threshold assessments, we referred to the resting motor thresholds (RMT) measured in children (7–13 years old) with Tourette syndrome and children (8–13 years old) with attention deficit hyperactivity disorder in our laboratory. RMT of these children mostly ranged from 40% to 50%. Thus, the stimulation intensity was set uniformly at 50% of stimulator output.
Subjects were evaluated at five time points: immediately before the first HF-rTMS course (“pre-1”), immediately after the first HF-rTMS course (“post-1”), immediately before the second HF-rTMS course (“pre-2”), immediately after the second HF-rTMS course (“post-2”), and 6 weeks after the second rTMS treatment course (6 weeks later, “6wl”). Treatment effectiveness was assessed using the Verbal Behavior Assessment Scale (VerBAS) (22) and Autism Treatment Evaluation Checklist (ATEC) (23). ATEC consists of four subtest scales: Scale I (Speech/Language/Communication), Scale II (Sociability), Scale III (Sensory/Cognitive awareness), and Scale IV (Health/Physical/Behavior) (23). In addition, daily logbooks completed by caregivers were considered as one of the outcome measures.
Moreover, we monitored any side effects during the stimulation courses and instructed caregivers to report any side effects they noted during and after treatment. The protocol flow diagram is shown in Figure 1.
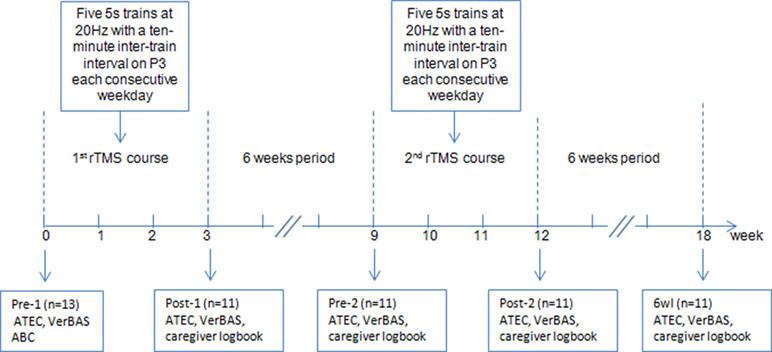
Figure 1 Flow diagram of the experiment. Note: ATEC, Autism Treatment Evaluation Checklist; VerBAS, Verbal Behavior Assessment Scale; ABC, Autism Behavior Checklist; P3, left parietal electrode.
Data Analysis
Statistical analysis was completed using Statistical Product and Service Solutions (SPSS) software (version 19.0, SPSS Inc., IL, USA). A P value <0.05 was considered significant for all analyses. One-way ANOVA with repeated measures was used to examine differences in the effects of HF-rTMS on the four ATEC scale scores, as well as VerBAS scores among different time points (pre-1 vs. post-1 vs. pre-2 vs. post-2 vs. 6wl). Bonferroni correction was used to adjust P values in post hoc analyses.
Results
Eleven individuals (7 boys, 4 girls) with a mean age of 7.09 ± 2.88 years completed the two treatment courses and follow-up assessments. There were no reports of serious adverse events. Transient irritability during or after HF-rTMS was reported in three cases by caregivers (Table 2). One participant became irritable during the first 3 days in each treatment course. Another was more emotional after the second treatment course and recovered in 5 days. A third was hyperactive and irritable during the first 5 days of the first course.

Table 2 Side effects reported by caregivers during and after high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) treatment courses.
The repeated-measures ANOVA revealed significant changes over time in the ATEC language scale [F(4,50) = 2.685, P = 0.042] and ATEC social scale [F(4,50) = 2.636, P = 0.045]. The least significant difference (LSD) method was used in post hoc analysis. The ATEC language scale significantly decreased from “pre-1” to “post-2” (P = 0.048) and from “pre-1” to “6wl” (P = 0.003). There was also a significant reduction in ATEC social scale from “pre-1” to “post-2” (P = 0.021) and from “pre-1” to “6wl” (P = 0.005).
However, after P value correction by Bonferroni method, the difference between “pre-1” and “post-2” did not achieve statistical significance in the ATEC language and social scales. The ATEC language scale significantly decreased from “pre-1” to “6wl” (P = 0.025) (lower ATEC scores reflect reduced impairments). The ATEC social scale significantly decreased from “pre-1” to “6wl” (P = 0.048).
No statistically significant changes over time were found in ATEC sensory and cognitive awareness scale [F(4,50) = 0.234, P = 0.918], ATEC health and behavioral scale [F(4,50) = 0.398, P = 0.809], or VerBAS [F(4,50) = 1.086, P = 0.374]. Summary data for clinical measures were presented in Table 3.
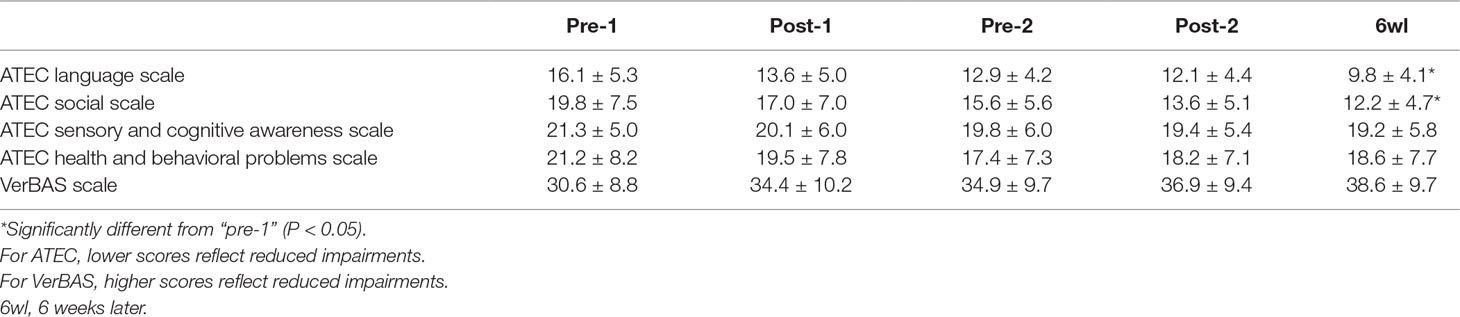
Table 3 Autism Treatment Evaluation Checklist (ATEC) scale scores and Verbal Behavior Assessment Scale (VerBAS) scores at each assessment time point (mean ± SD).
According to the clinical observations and caregiver reports, HF-rTMS might be more effective in male children than in female children. Most caregivers reported their children displayed possible improvements in imitation and cognition (e.g., language imitation and behavior imitation) after the HF-rTMS treatments. We summarized some improvements from caregiver logbooks in 11 participants in Table 4.
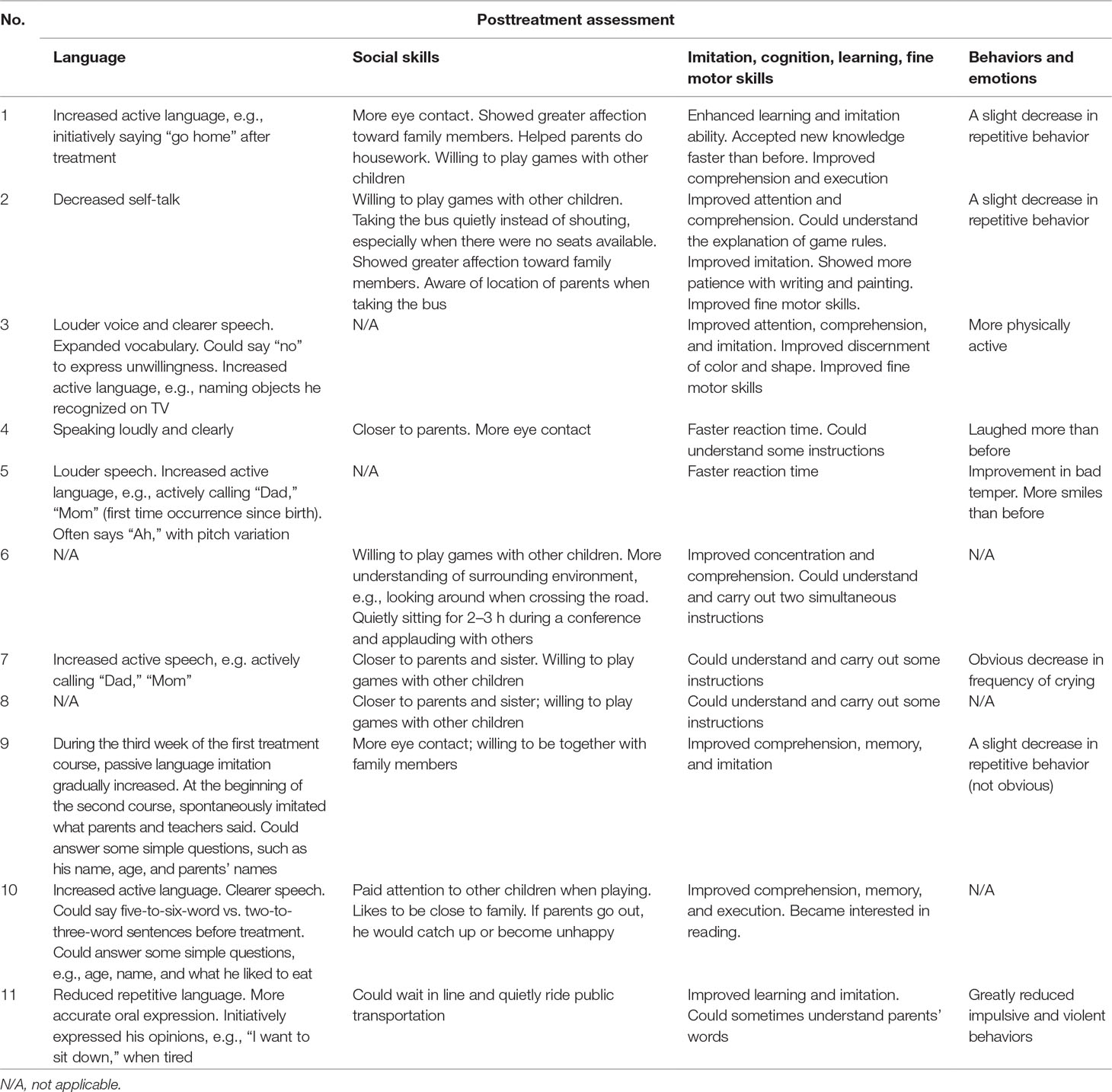
Table 4 Improvements in the quality of life of 11 participants, following HF-rTMS treatments. Records from caregiver’s logbooks.
Discussion
This study provides preliminary evidence for the effectiveness and safety of HF-rTMS over the left IPL as a treatment option to improve core symptoms in low-functioning autism. Specially, HF-rTMS significantly reduced social and speech deficits as measured by ATEC and parents’ report. At the same time, children’s imitation and cognition might be improved following treatment.
The specific mechanisms underlying these effects may reflect specific neuroplastic effects associated with high-frequency stimulation and will require further investigation. Physiological experiments in humans indicate that HF-rTMS evokes long-term potentiation (LTP) of synaptic transmission (24, 25). These changes are not only restricted to the site of stimulation but also observed in a widespread cortical and subcortical network (26, 27). The use of HF-rTMS (20 Hz) to adaptively modulate properties of the MNS in humans has been reported in typically developing individuals (16).
From a neurophysiological perspective, we hypothesize that these clinical effects resulted from stimulation of IPL and associated MNS, which have been linked to ASD (7). Stimulation of the IPL may induce long-lasting changes in the excitability of regions within the MNS network (28). Such alterations may improve one’s understanding of social environment and may reinforce the capacity for imitation. Thus, an enhanced interpretation of social context may lead to improvements in language and social skills, as shown in the current trial (28).
Growing evidence suggests that dopaminergic dysfunction is implicated in the pathogenesis of ASD (29, 30). Human studies show that HF-rTMS of the frontal cortex induces the release of dopamine in the cortical, limbic, and striatal brain regions (31). In this study, HF-rTMS on parietal cortex might alter dopamine activity in specific brain regions, which is related to social cognition in ASD (30).
To the best of our knowledge, this study is the first attempt at HF-rTMS over the IPL in intellectually disabled individuals with ASD. According to clinical observations and caregiver reports, HF-rTMS in this trial might be more effective in boys than in girls. The underlying reasons for the significant gender disparities in treatment outcome are not clear. However, female individuals with ASD seem to exhibit lower IQ (32), more severe phenotypes (33), overall autistic symptoms (34), and psychopathological problems (35). Moreover, it is important to note that the four girls in our study were two sets of twins; thus, genetic factors may play a critical role in their pathogenesis.
It may be confusing that the clinical effects measured by scales did not turn up at the time point of “immediately after the treatment course.” We thought there may be two reasons to account for this. Firstly, the number of participants in our study was relatively small, which may have a great impact on statistical analysis. Despite the limited validity of parental reports as outcome measures, the improvements in social cognition and speech were indeed observed during and after the treatment course. However, the improvements were not reflected by statistical analysis, as we expected. Secondly, repeated sessions of HF-rTMS could produce remodeling with an increase in active synapses (36), which may be responsible for cumulative rTMS effects. This may explain why the difference achieved statistical significance only at the time point of “six weeks after the second treatment course.”
Our study had several limitations that should be mentioned, including the fact that the study does not contain a control group (e.g., sham HF-rTMS), the conclusions are limited by the small sample size, limited validity of parental reports as outcome measures, and lack of neuroimaging and/or neurophysiological assessments. In future follow-up studies, large case–control clinical trials are necessary to explore the use of HF-rTMS as a unique treatment for improving core symptoms in ASD.
Conclusion
Our original findings suggest that HF-rTMS on IPL has the potential to become a distinct therapeutic method aimed at treating core symptoms of ASD.
Ethics Statement
This study was carried out in accordance with the ethics committee of Xuanwu Hospital with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the ethics committee of Xuanwu Hospital, Capital Medical University (LYS2017063).
Author Contributions
YY participated in the design of the study, conducted the analyses, and wrote the manuscript; HW was involved in the study design, supervised the data analysis, and revised the manuscript; QX and ZH helped collected participants and coordinated the study; YW conceived and coordinated the design of the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (81801124) and the Beijing Municipal Hospital Research and Development Plan (PX2017069).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Some parts of the research were presented at the “International Neuromodulation Society’s 13th World Congress Neuromodulation: Technology Changing Lives Edinburgh, Scotland, United Kingdom May 27-June 1, 2017.”
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-V. Arlington, TX: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596
2. Root NB, Case LK, Burrus CJ, Ramachandran VS. External self-representations improve self-awareness in a child with autism. Neurocase (2015) 21(2):206–10. doi: 10.1080/13554794.2014.888455
3. Saffin JM, Tohid H. Walk like me, talk like me. Neurosciences (2016) 21(2):108–19. doi: 10.17712/nsj.2016.2.20150472
4. Rizzolatti G, Fabbri-Destro M, Cattaneo L. Mirror neurons and their clinical relevance. Nat Clin Pract Neurol (2009) 5(1):24–34. doi: 10.1038/ncpneuro0990
5. Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev (2001) 25(4):287–95. doi: 10.1016/S0149-7634(01)00014-8
6. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol (2010) 20(8):750–6. doi: 10.1016/j.cub.2010.02.045
7. Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp Brain Res (2010) 200(3-4):223–37. doi: 10.1007/s00221-009-2002-3
8. Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci (2008) 28(8):1569–88. doi: 10.1111/j.1460-9568.2008.06395.x
9. Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science (2005) 308(5722):662–7. doi: 10.1126/science.1106138
10. Fontana AP, Kilner JM, Rodrigues EC, Joffily M, Nighoghossian N, Vargas CD, et al. Role of the parietal cortex in predicting incoming actions. Neuroimage (2012) 59(1):556–64. doi: 10.1016/j.neuroimage.2011.07.046
11. Sokhadze E, Baruth J, Tasman A, Mansoor M, Ramaswamy R, Sears L, et al. Low-frequency repetitive transcranial magnetic stimulation (rTMS) affects event-related potential measures of novelty processing in autism. Appl Psychophysiol Biofeedback (2010) 35(2):147–61. doi: 10.1007/s10484-009-9121-2
12. Rossi S, Rossini PM. TMS in cognitive plasticity and the potential for rehabilitation. Trends Cogn Sci (2004) 8(6):273–9. doi: 10.1016/j.tics.2004.04.012
13. Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci (2004) 15(4):253–66. doi: 10.1515/REVNEURO.2004.15.4.253
14. Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol (2002) 19(4):322–43. doi: 10.1097/00004691-200208000-00006
15. Filipovic SR, Rothwell JC, Bhatia K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson’s disease. Clin Neurophysiol (2010) 121(7):1129–37. doi: 10.1016/j.clinph.2010.01.031
16. Mehta UM, Waghmare AV, Thirthalli J, Venkatasubramanian G, Gangadhar BN. Is the human mirror neuron system plastic? Evidence from a transcranial magnetic stimulation study. Asian J Psychiatr (2015) 17:71–7. doi: 10.1016/j.ajp.2015.06.014
17. Sokhadze EM, El-Baz AS, Sears LL, Opris I, Casanova MF. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front Syst Neurosci (2014) 8:134. doi: 10.3389/fnsys.2014.00134
18. Couteur AL, Lord C, Rutter M. ADI-R Autism Diagnostic Interview-Revised. Los Angeles: Western Psychological Services (2003).
19. Krug DA, Arick J, Almond P. Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. J Child Psychol Psychiatry (1980) 21(3):221–9. doi: 10.1111/j.1469-7610.1980.tb01797.x
20. Jacobson LA, Mahone EM. Wechsler intelligence scale for children. New York: Springer (2011). doi: 10.1007/978-0-387-79948-3_1605
21. Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr (2003) 16(2):95–9. doi: 10.1023/B:BRAT.0000006333.93597.9d
22. Duker PC. The Verbal Behavior Assessment Scale (VerBAS): construct validity, reliability, and internal consistency. Res Dev Disabil (1999) 20(5):347–53. doi: 10.1016/S0891-4222(99)00016-5
23. Geier DA, Kern JK, Geier MR. A comparison of the Autism Treatment Evaluation Checklist (ATEC) and the Childhood Autism Rating Scale (CARS) for the quantitative evaluation of autism. J Ment Health Res Intellect Disabil (2013) 6(4):255–67. doi: 10.1080/19315864.2012.681340
24. Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull (2006) 69(1):86–94. doi: 10.1016/j.brainresbull.2005.11.003
25. Rajji TK, Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Neuroplasticity-based brain stimulation interventions in the study and treatment of schizophrenia: a review. Can J Psychiatry (2013) 58(2):93–8. doi: 10.1177/070674371305800206
26. Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage (2012) 62(4):2232–43. doi: 10.1016/j.neuroimage.2012.03.035
27. Shafi MM, Westover MB, Fox MD, Pascual-Leone A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur J Neurosci (2012) 35(6):805–25. doi: 10.1111/j.1460-9568.2012.08035.x
28. Enticott PG, Fitzgibbon BM, Kennedy HA, Arnold SL, Elliot D, Peachey A, et al. A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimul (2014) 7(2):206–11. doi: 10.1016/j.brs.2013.10.004
29. Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci (2012) 7(2):160–72. doi: 10.1093/scan/nsq095
30. Paval D. A dopamine hypothesis of autism spectrum disorder. Dev Neurosci (2017) 39(5):355–60. doi: 10.1159/000478725
31. Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev (2010) 34(4):559–74. doi: 10.1016/j.neubiorev.2009.11.006
32. Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism (2015) 6:36. doi: 10.1186/s13229-015-0019-y
33. Banach R, Thompson A, Szatmari P, Goldberg J, Tuff L, Zwaigenbaum L, et al. Brief Report: relationship between non-verbal IQ and gender in autism. J Autism Dev Disord (2009) 39(1):188–93. doi: 10.1007/s10803-008-0612-4
34. Tsai LY, Beisler JM. The development of sex differences in infantile autism. Br J Psychiatry (1983) 142:373–8. doi: 10.1192/bjp.142.4.373
35. Holtmann M, Bolte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol (2007) 49(5):361–6. doi: 10.1111/j.1469-8749.2007.00361.x
Keywords: repetitive transcranial magnetic stimulation, autism spectrum disorder, inferior parietal lobule, social relating, language
Citation: Yang Y, Wang H, Xue Q, Huang Z and Wang Y (2019) High-Frequency Repetitive Transcranial Magnetic Stimulation Applied to the Parietal Cortex for Low-Functioning Children With Autism Spectrum Disorder: A Case Series. Front. Psychiatry 10:293. doi: 10.3389/fpsyt.2019.00293
Received: 06 December 2018; Accepted: 15 April 2019;
Published: 09 May 2019.
Edited by:
Dirk Dhossche, University of Mississippi Medical Center, United StatesReviewed by:
Paul Croarkin, Mayo Clinic, United StatesEstate M. Sokhadze, University of South Carolina, United States
Eva Ceskova, Masaryk University, Czechia
Copyright © 2019 Yang, Wang, Xue, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuping Wang d2FuZ3l5cGluZ0B5ZWFoLm5ldA==
 Yingxue Yang
Yingxue Yang Hongxing Wang1,2,3
Hongxing Wang1,2,3