- 1School of Psychology, University of Sussex, Brighton, United Kingdom
- 2Sussex Partnership NHS Foundation Trust, Worthing, United Kingdom
- 3Center of Excellence for Psychosocial and Systemic Research, Massachusetts General Hospital, Boston, MA, United States
- 4Faculty of Health and Medical Sciences, University of Surrey, Guildford, United Kingdom
Introduction: Research has demonstrated that functional outcome in psychosis is predicted by factors such as neurocognition, functional capacity, symptoms and, more recently, metacognition. Metacognitive ability has been demonstrated to mediate between neurocognition and functional outcome in First Episode Psychosis (FEP). Whether metacognition also predicts longer-term recovery in first episode psychosis is unknown. This study assessed whether neurocognition, functional capacity and metacognitive ability in FEP predicted functional outcome three years later.
Methods: Eighty individuals with First Episode Psychosis were re-contacted after an average 3 years (range: 26–45 month follow-up) from baseline. Twenty-six participants (33%) completed completed measures of neurocognition, metacognition, functional capacity, functional outcome (hours spent in structured activity per week) and psychopathology at baseline and at follow-up.
Results: Individual regression analyses demonstrated neurocognition, functional capacity, and metacognitive ability at baseline significantly predicted functional outcome at three years. However, when baseline functional outcome was controlled, only metacognitive ability was a significant predictor of change in functional outcome from baseline to follow-up, p < 0.001. This model explained 72% (adjusted r2 = 0.69) of the variance in functional outcome at follow-up. Negative symptoms did not change the model.
Discussion: This study demonstrated that better metacognitive ability significantly predicted improvement in functioning in FEP across a 3-year period. This highlights the potential value of clinical interventions that focus on improving metacognitive ability at first point of illness to maximize recovery.
Introduction
Clinical recovery can be considered improvement in symptomatology and social/occupational functioning (1, 2). Although clinical recovery after an experience of psychosis was previously considered poor (3), recent research has demonstrated that around 50% of individuals with psychosis had favorable outcomes after long follow-up periods (4–6) and this has also been demonstrated with First Episode Psychosis (FEP) (7, 8). A 10-year follow-up study showed 77% of participants showed at least one period of recovery (defined as sustained symptom remission for at least two years) with 46% symptom-free for at least two years (9). However, for (10) and Robinson et al. (8) only 6.6% and 14% (respectively) met the criteria for full recovery after 1 and 5 year(s) (respectively), suggesting clinical recovery during early stages of illness may be slow. There is clear interest in understanding factors that influence clinical recovery in psychosis, particularly FEP where recovery may be more likely (4, 9). Birchwood et al. (11) named this the critical-period, highlighting the importance of early and targeted interventions to prevent further decline (12–14).
Social and occupational functioning, an aspect of clinical recovery, is a measurable aspect of an individual's specific activities of daily living. Hodgekins et al. (15) suggested assessing functioning using time spent in structured activity per week (4, 16), including employment, education, sports, and leisure. Research has demonstrated that time spent in structured activity is on average 63.5 h in the healthy population, 25.2 h in FEP sample (17), and 19.7 h in a psychosis sample with delayed recovery (15). Importantly, engaging in more hours of activity, e.g., paid work, has been associated with reduced symptoms and improved overall functioning in interventions studies (18, 19).
Research has demonstrated difficulties in functioning across the course of recovery in psychosis and, therefore, the importance of focusing on understanding and improving poor functional outcome. Despite advances in psychological interventions for psychosis, outcome remains poor. There is value in the identification of those with psychosis who are at risk of poor functioning across time, to target interventions to reduce this disability. There are four selected lines of evidence which will be discussed here to suggest factors which predict poor functioning: (i) neurocognition, (ii) functional capacity, (iii) negative symptoms and (iv) metacognition.
Research assessing functional outcome within individuals with psychosis has focused on the influence of neurocognitive difficulties (20–22). The relationship between neurocognition and functional outcome has been demonstrated cross-sectionally (23, 24) and longitudinally in schizophrenia (8), FEP (25, 26) and Ultra-High Risk groups (27). However, studies have demonstrated that predicting those who would have poor outcome with neurocognitive variables is substantially more straight-forward than predicting those who would recover (28, 29). This suggests a more complex relationship with additional factors to be explored.
Functional capacity has also been shown to predict real-world functional outcome within schizophrenia (30, 31) and FEP (32). Neurocognitive ability has been shown to be associated with functional capacity (33–35) and functional capacity has been shown to mediate between neurocognition and functional outcome in schizophrenia (36) and FEP (32). A longitudinal study demonstrated functional capacity predicted real-world functioning in psychosis for those with positive, but not for those with negative symptoms (37). The authors suggested that negative symptoms are distinct and can impact functioning, more so than functional capacity.
Models have highlighted that negative symptoms predict functional outcome in psychosis (38, 39). Specifically, negative community symptoms (e.g., amotivation or social withdrawal), when combined with cognitive deficits, have the largest impact on functioning in schizophrenia (40). Longitudinal studies have demonstrated that lower levels of negative symptoms predicted better outcomes in FEP in a large study of 304 participants (41). When assessing positive and negative symptom trajectories, poorer negative symptom trajectories were associated with poorer social functioning, disorganized symptoms, and schizophrenia diagnosis, compared to positive symptom trajectories which were associated with DUP and substance use (42). However, Alvarez-Jimenez et al. (43) demonstrated that whilst symptom remission predicted functional recovery, negative symptoms had little predictive value for long-term functioning. Studies have noted an overlap in the variance in outcome explained by cognition and negative symptoms (25, 44) and, when taking into consideration the role of cognition, symptoms are shown not to predict functioning cross-sectionally (45) and later longitudinally (46). These studies highlight that functional outcome is the product of a complex array of abilities and symptoms.
Following this research, models in psychosis suggest that neurocognition, functional capacity, and negative symptoms influence functional outcome (33, 47, 48). The path between neurocognition and functioning has been shown to be mediated by functional capacity and cognitive processes (36, 47–49), including defeatist performance beliefs and self-stigma (50) and, most recently, metacognition (32).
Metacognition is considered “thinking about thinking” (51) or the way one thinks about one's experience (52). Metacognition involves forming an integrated representation of oneself, others, and the world and using these representations to implement an effective action strategy to perform or accomplish a task (53). Nelson and Narens (54) outlined a metacognitive model suggesting an object-level which (cognitive processes) and a meta-level (an abstract view of the object-level) which are connected by metacognitive processes. Metacognition may be fractionated and three levels of metacognition have been proposed. Firstly, metacognitive ability: capacity to think about one's own cognitions, emotions and behavior, and to use this reflection to respond to challenges (55, 56). Secondly, metacognitive experience: an online appraisal of one's experience, and thirdly, metacognitive sensitivity: a sub-conscious awareness of performance during a task. The first level, metacognitive ability, measured using Metacognitive Assessment Scale (MAS) (57) or Metacognitive Assessment Interview (MAI) (58), is shown to predict real-life functioning in schizophrenia (59–61). In particular, metacognitive ability is show to be a mediator between neurocognition and functioning in schizophrenia (62) and FEP (32, 63). Metacognitive ability is shown to have a large, key role in functioning in psychosis, although this relationship is also influenced by neurocognition and functional skills.
Whether the relationship between metacognition and functional outcome persists across time is unknown. Intervention studies focusing on improving metacognition have demonstrated an increase in real-world functioning (64–67). Longitudinal studies demonstrated that metacognitive ability, particularly one's ability to use representations of self or other to implement effective strategies, predicted social functioning across a 5-month period in schizophrenia (68) and McLeod et al. (69) demonstrated that metacognitive ability predicted negative symptoms at 12 months in FEP, independent of known factors, e.g., gender, DUP and premorbid academic/social adjustment. However, no study has yet assessed the role of metacognitive ability on functioning over a longer follow-up period; particularly within FEP.
It may be suggested that metacognitive ability enables the use of appropriate skills and abilities to perform a task or challenge. For example, an individual may have poor neurocognitive ability, but if they have appropriate metacognitive ability then they may be able to use their available resources and strategies in order to overcome challenges in the environment. Successful outcome, following the utilization of metacognitive ability, may predict engagement in more activities over that predicted by neurocognition, functional capacity skills and negative symptoms. Lysaker et al. (70) demonstrated that those with schizophrenia and high metacognitive ability display better work performance across 6-months, as those with high metacognitive ability were able to see their conclusions as fallible and were able to learn and adapt to the changing demands of work. Therefore, metacognitive ability may be the key predictor of a change in functional outcome across time.
From this, it is hypothesized that functional outcome in FEP at 3-year follow-up will be predicted by metacognitive ability at baseline, independent of neurocognition, negative symptoms, and functional capacity. It is also hypothesized that a change in functional outcome will be predicted by metacognitive ability at baseline, independent of neurocognition, negative symptoms, and functional capacity.
Materials and Methods
Procedure
Ethical and Health Research Authority approval was obtained through Camberwell St. Giles Research Ethics Committee (reference number: 17/LO/0055). All participants provided written informed consent at first entry to the study. Participants who gave consent to be re-contacted were contacted after the 3-year period and provided written informed consent at follow-up.
Design
This was a longitudinal follow-up study exploring the contribution of metacognitive ability to functional outcome at 3-year follow-up with individuals with First Episode Psychosis. Full details of the study design and ethical approval is provided in the protocol (71). Details of the baseline study are provided in an earlier publication (32).
Participants
Participants with First Episode Psychosis were recruited, via a convenience sample, from outpatient Early Intervention in Psychosis services in Sussex Partnership NHS Foundation Trust Sussex, UK. All participants had been within Early Intervention Services for at least 3 months before entry into the study. All participants received a diagnosis of First Episode Psychosis (F29) by a psychiatrist at entry to the study. Participants with a primary diagnosis of substance misuse disorder or organic neurological impairment were excluded.
Measures
Metacognitive Ability
This was assessed using the Metacognitive Assessment Interview (MAI) (58), which requires the participant to reflect on a recent difficult interpersonal experience and to answer a series of questions reflecting on this experience. The measure assesses the individual's ability for (i) monitoring, identification of feelings and thoughts, (ii) differentiation, distinguishing between dreams, beliefs or assumptions, (iii) integration, reflection on different mental states and rules governing them, and (iv) decentralization, describing the mental state of the other which is independent of their own view. These four subscales are scored between 0 and 5, depending on spontaneity, use of aids/prompts and the sophistication of the answer. The scores for the sub-domains are averaged to provide one multidimensional score. This measure has demonstrated good inter-rater reliability and internal consistency (α = 0.91 for total metacognition), and construct reliability showing correlations amongst the MAI scales (r = 0.62–0.9) (58).
Function
Functional outcome
Time-Use Survey (TUS) (72) is a structured interview [inter-rater reliability 0.99; (15)] during which participants are asked questions regarding the number of hours spent engaged in specific structured activities for the preceding month (D 15), including hours spent in paid work, voluntary work, educational activity, childcare, sports, leisure, and housework activities. A weekly average was calculated for each activity. Two total scores can be produced: (i) constructive economic activity (CEA) including the total hours per week in employment, education, voluntary work, childcare and housework and chores and (ii) structured activity (SA) including the total hours per week in constructive economic activity, leisure activities, and sports activities. Within this study, we used structured activity as the total score. This measure is able to capture differences across clinical groups (15, 73).
Functional capacity
The UCSD Performance-Based Skills Assessment (74) provides a total score for real-life performance skills based on role-play tasks. This measure is divided into five sections: (i) finance, e.g., counting money, (ii) communication, e.g., re-arranging a medical appointment, (iii) comprehension/planning, e.g., planning a visit to a theme park, (iv) transport, e.g., reading a bus timetable, and (v) household, e.g., creating a shopping list from a recipe. During each role-play the individual is given points by the researcher from the manual guidelines. These raw scores are totaled for each domain, converted into 0–20 scale then multiplied by 2 and summed to provide a total out of 100. This measure demonstrates high internal consistency (α = 0.88), good validity with other scales (Direct Assessment of Functional Status scale, r = 0.86) and good test-retest reliability (r = 0.91) (75–77).
Neurocognitive Ability
Participants completed a battery of neurocognitive measures, including Verbal and working memory [Logical Memory and Letter-Number Sequencing subscales from the Wechsler Memory Scale (WMS-III)], executive function (Trail-Making Task and Verbal Fluency), Verbal and Performance IQ (Vocabulary and Matrix reasoning tasks). All scores were converted into Z scores using age-scaled population means and standard deviations (78, 78–80). A neurocognitive composite score at baseline was produced from the measures outlined above. IQ was assessed at follow-up using Vocabulary task and Matrix reasoning task (78).
Symptoms
Positive and Negative Syndrome Scale (81) was used as this is the most widely used standardized instrument for assessing symptom severity in schizophrenia (82). This measure provided three separate scores for positive, negative and general psychopathology symptoms.
Planned Analysis
G power estimation was used for a power calculation based on a power of 0.80, effect size of 0.31 (32) and alpha of 0.05. This suggested a total of 44 participants were required when including 4 predictors.
Hypothesis Testing
Descriptive statistics were produced for neurocognitive ability, metacognitive ability, functional capacity, functional outcome, and symptoms, and scores were compared from baseline to 3-year follow-up using t-tests. At baseline, a large battery of neurocognitive measures was collected. At follow-up, only matrix reasoning and vocab measures (as a two-part IQ score) were collected. Therefore, the difference tests (comparing baseline and follow-up) only assessed differences in these measures.
For the main analyses, predictors of functional outcome at three follow-up were explored (predictors included: neurocognition, negative symptoms, functional capacity, and metacognition). In light of the small sample size, and in order to reduce the number of predictors in the model, a series of single regression analyses were used to assess the predictive value of each variable at baseline on functional outcome at 3-year follow-up. After this, a stepwise regression was conducted using only the significant predictors as covariates and metacognitive ability was added to the model in block 2, to assess the independent contribution of metacognitive ability.
Next, the predictive value of variables on change in functional outcome from baseline to follow-up was assessed. The change was assessed by using baseline functional outcome as a covariate, adjusting the mean for the baseline levels, to explore differences in functional outcome at follow-up. Then, significant predictors were used as covariates and metacognitive ability added to the model in block 3. It is important to note that the Time-Use survey has been demonstrated to be sensitive to change over time in clinical trials [see (83, 84)]. Due to the sample size and to minimize the number of predictors within a single model, the independent role of neurocognition, negative symptoms, and functional capacity were used as covariates within three parallel analyses.
Results
Data and Assumption Checking
Missing data was considered as “Missing Completely At Random” (MAR), as missing data was not associated with data within the study. All predictor and outcome data were checked for skewness, kurtosis, and outliers. MAI total at baseline displayed a multimodal distribution. There were no significant differences on cognition, functional capacity, functional outcome, symptoms, and metacognitive ability for those who participated in follow-up and those who did not.
Sample Characteristics
The first recruitment phase took place during 2013–2015. The follow-up recruitment phase took place within 2017, after 3 years (average 36-month; range 26–45-month follow-up). The baseline sample included 80 participants with FEP (49 men, 31 female) with a mean age of 26.08 years (SD = 5.53). Seventy-seven people provided consent to re-contact. Twenty-six participants from the baseline study took part in the follow-up assessment (see Figure 1 for flowchart of participation). The mean age at follow-up was 28.93 (SD = 5.55, range 22–43) with 23 males and 8 females (see Table 1). See Supplement A for distribution of months between baseline and 3 years for the sample followed-up.
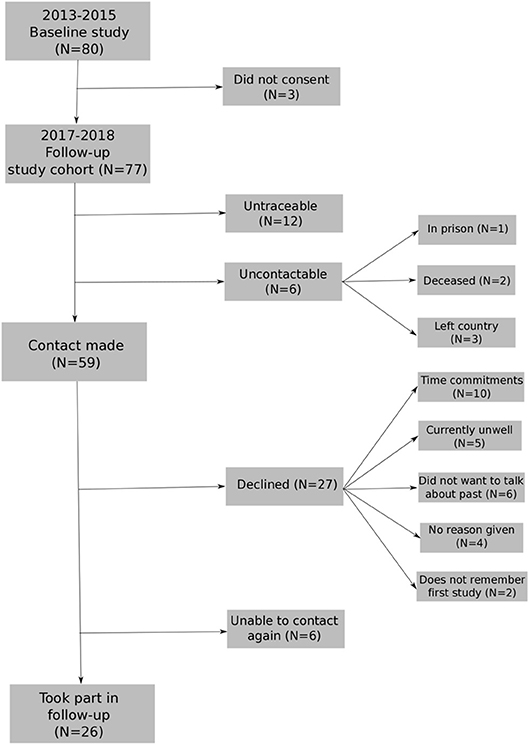
Figure 1. Flowchart for re-recruiting individuals from baseline study into longitudinal study1.
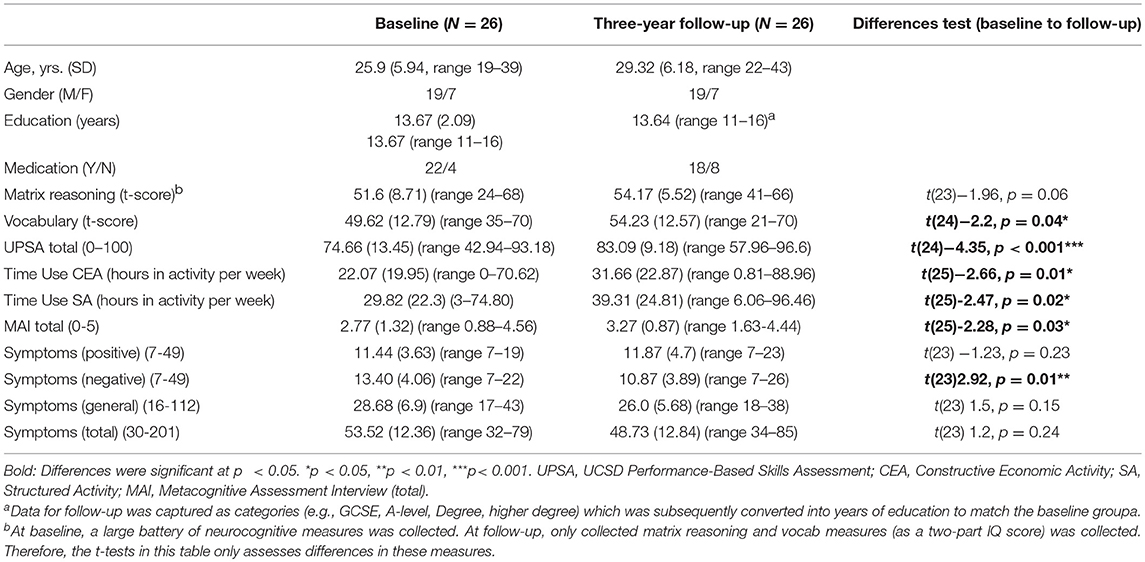
Table 1. Sample characteristics and descriptive and change statistics for neurocognitive measures, functional capacity, functional outcome, metacognitive ability, and symptoms.
Descriptive Statistics
See Table 1 for descriptive statistics. A number of variables increased over the two timepoints: vocabulary score (p = 0.04), functional capacity (UPSA) (p < 0.001), functional outcome (time-use structured activity) (p = 0.01) and metacognitive ability (MAI) (p = 0.03). Negative symptoms decreased (p = 0.01) (see Table 1 for descriptive statistics).
Associations Between Predictor Variables
See Table 2 for correlation matrix for neurocognition, metacognitive ability, symptoms, functional capacity and functional outcome at baseline and follow-up. Age at baseline was significantly associated with functional outcome at follow-up (r = 0.4, p = 0.027) and included as a covariate in subsequent analyses. For neurocognition, a Confirmatory Factor Analysis was conducted on the z scores of the cognitive variables at baseline and converted into a neurocognitive factor score for each participant.
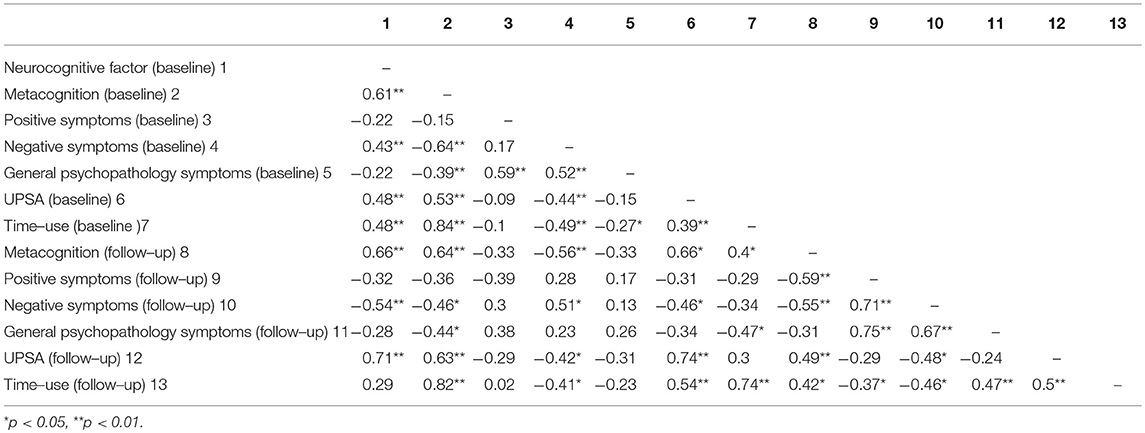
Table 2. Correlation matrix for neurocognition, metacognition, symptoms, functional capacity, and functional outcome at baseline and follow-up.
Hypothesis 1
In order to test predictors (neurocognition, functional capacity, negative symptoms and metacognition) of functional outcome at 3-year follow-up, individual regression analyses were conducted. Neurocognitive ability at baseline did not significantly predict functioning at 3 years, p = 0.24. Functional capacity, F(2, 25) 6.66, p = 0.01, negative symptoms, F(2, 23) 5.69, p = 0.01, and metacognitive ability, F(2, 24) 27.97, p < 0.001, were significant predictors of functioning at 3 years. Including negative symptoms as a covariate in the above analyses did not substantially change the significance levels for neurocognition and metacognitive ability (p = 0.44 and p < 0.001, respectively). However, when controlling for negative symptoms, functional capacity1 at baseline was no longer a significant predictor of functional outcome at follow-up (ΔR2 = 0.07 p = 0.13).
Next, in order to test whether metacognition predicted functional outcome at follow-up independent of other known factors, significant predictors (functional capacity and negative symptoms) were included in the first block of the stepwise regression then the independent contribution of metacognitive ability (MAI) to functioning at three-year follow-up was assessed (see Table 3). This model explained 77.1% (adjusted r2 = 0.72) of the variance in functional outcome at follow-up (R = 0.77, F(4, 23) 16.01, p < 0.001). MAI significantly improved the model (ΔR2 = 0.35, p < 0.001) explaining 34.6% of the 77% (adjusted r2 = 0.72) total variance explained.
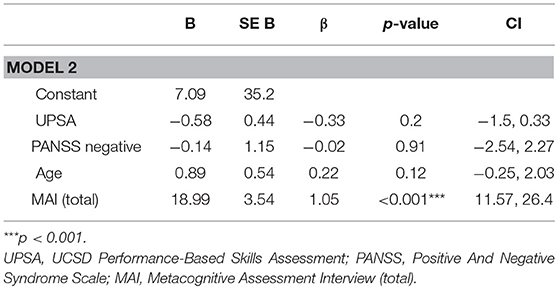
Table 3. Full regression model for predictive value of metacognitive ability on functional outcome at three years, whilst controlling for age, UPSA, and PANSS negative symptoms.
Hypothesis 2
In order to assess predictors (neurocognition, functional capacity, negative symptoms, and metacognition) of a change in functional outcome from baseline to 3-year follow-up, individual regression analyses were conducted, controlling for baseline functional outcome. Neither neurocognition (p = 0.22), functional capacity (p = 0.57) nor negative symptoms (p = 0.35) predicted change in functional outcome. Metacognitive ability (MAI) was a significant predictor of change in functional outcome at follow-up, when including baseline functional outcome as a covariate. This model explained 72% (adjusted r2 = 0.69) of the variance in functional outcome at follow-up (R = 0.72, F(3, 25) 19.22, p < 0.001). MAI significantly improved the model (ΔR = 0.12, p = 0.01), explaining 12% of the 72% total variance explained (see Table 4). VIF values were inspected to check multicollinearity and the score was acceptable (85, 86). Even when separately controlling for negative symptoms, neurocognition, and functional capacity in parallel analyses, metacognitive ability was still a significant predictor of change in functional outcome from baseline to follow-up [controlling for neurocognition (p = 0.005), negative symptoms (p = 0.006), and functional capacity (p = 0.001)].
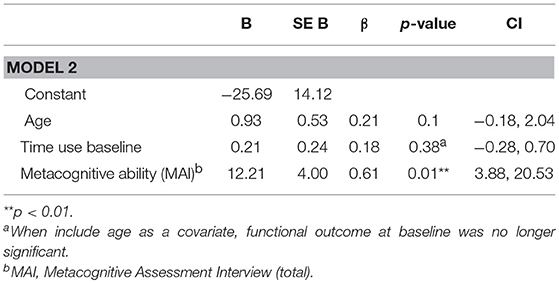
Table 4. Full regression model for predictive value of metacognitive ability on change in functional outcome from baseline to follow-up, whilst controlling for baseline functional outcome, and age.
For those participants who were followed-up, metacognitive ability (MAI) at baseline demonstrated a bivariate distribution (Supplement B). We, therefore, compared those with FEP and either high or low metacognitive ability graphically, with other previous samples. Specifically, we divided participants into two groups: high MAI at baseline (N = 14) or low MAI at baseline (N = 12), using mean split, to assess the changes in time-use scores between the groups. Individuals in the high MAI group demonstrated a difference in hours spent in structured activity between baseline (M = 50.32, SD = 23.98) and follow-up (M = 58.13, SD = 19.29) (p = 0.05), but for the low MAI group there was no significant difference (p = 0.17) in structured activity between baseline (M = 13.17, SD = 7.7) and follow-up (M = 15.53, SD = 7.63) (see Figure 2).
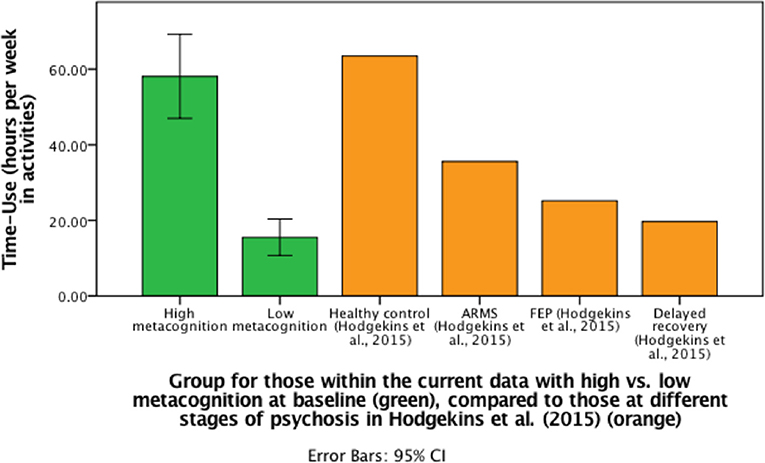
Figure 2. Bar graph to demonstrate differences in mean follow-up time-use scores (including CI for current data) for those with high or low metacognitive ability at baseline compared to previous data from Hodgekins et al. (15).
Discussion
This was the first study to assess the role of metacognitive ability on functional outcome longitudinally across three-years in First Episode Psychosis. This study was able to demonstrate that functional outcome improved over time and, whilst negative symptoms and functional capacity predicted functioning at 3 years, the improvement in functioning was largely predicted by metacognitive ability and baseline functioning.
Within this study, individuals with FEP significantly improved in neuro cognitive ability (vocabulary), real-life functional capacity skills, metacognitive ability and negative symptoms over 3 years. At baseline, individuals within this sample were demonstrating typical mean activity levels for an FEP sample (29.82 h per week) [see (15)], but there was an improvement of 9 hours in structured activity at three-year follow-up, resulting in a time-use score similar to an ARMS group [see (15)]. There was an increase in functional capacity, in that individuals at follow-up were similar to those typically residing independently and employed (76). There was a significant improvement in verbal neuro cognitive ability (vocabulary). Studies in the general population suggest vocabulary is stable over time (87), including studies within schizophrenia (88, 89). The increase in verbal cognition may be the consequence of an initial drop in neuro cognitive ability, particularly verbal IQ (90, 91), which then recovered throughout the follow-up period.
Findings demonstrated that functional capacity and negative symptoms, but not neurocognition, at baseline predicted functional outcome at 3 years after FEP, supporting and furthering research (33, 37, 41, 42). Neurocognition did not directly predict functional outcome at 3-years. However, previous studies have suggested that neurocognitive factors only predict a small amount of variance in real-world functioning (36) and other factors have a more substantial role. Alternatively, neurocognition may have an indirect role, via functional capacity, as the present study demonstrated an association between neurocognition and functional capacity, or neurocognition and negative symptoms as Fervaha et al. (92) demonstrated, using a factor analysis, that negative symptoms may be divided into amotivation and diminished expression and it is the former factor, combined with cognitive deficits, that has the largest impact on functioning in schizophrenia.
Importantly, this study demonstrated that functional outcome at three-years was predicted by metacognitive ability, supporting previous cross-sectional studies (32, 62, 93). Metacognitive ability was also the only significant predictor of improvement in functioning, accounting for a significant change in functioning over time. Therefore, whilst negative symptoms and functional capacity skills predicted functioning at 3-years, this study highlights that those with higher metacognitive ability at baseline may be better able to make use of strategies and resources (e.g., from the early intervention service) to improve their functioning over time, compared to those with lower metacognitive ability who may need more guidance in order to utilize the services available to them.
In further exploring metacognitive ability at baseline, it was evident that individuals who displayed low metacognitive ability at baseline demonstrated limited change in functioning at 3-year follow-up, compared to individuals who displayed adequate metacognitive ability. This may suggest that those with better metacognition were better able to reflect on their thoughts, strengths, as well as perspectives of others, and use appropriate strategies to implement in the real world. Metacognitive ability was a longitudinal predictor of functional outcome, independent of IQ. Whilst the sample size in the group is small, this supports the main hypothesis that early metacognitive factors influence change in functioning.
A large amount of the variance in time-use at follow-up was predicted by baseline time-use and age. Therefore, individuals with better initial functioning are more likely to show an improvement later on, compared to those who had lower functioning who showed no change in already poor functioning. This finding may suggest that those with poor functioning at baseline have poorer metacognitive abilities. The poor metacognitive ability then may predict lower prospective functioning or strategies to improve their poor functioning or are less motivated, due to poor cognitive and metacognitive ability (94, 95), both of which lead to less change or improvement in functioning over time. This study defined functional outcome as a measurable aspect of an individual's specific activities of daily living and social and occupational functioning. The Time-Use Survey is a relatively objective and specific measure of functioning, sensitive to change over time [see (16, 83, 84) and across stages of psychosis see (15)]. Future studies could look at changes in subjective recovery outcomes [see (96)], following the service user movement (97), to identify longitudinal relationships with metacognition. These findings highlight the importance of the Early Interventions services to focus on improving functioning and encouraging early help-seeking to prevent low levels of functioning initially.
These findings can be taken forward in two ways: (i) poor metacognitive ability may be a marker for poor outcome in psychosis later on, and (ii) metacognitive ability may be a key ability for interventions to target in early psychosis to improve subsequent functioning. If metacognitive ability does play a role, metacognitive interventions which have previously demonstrated to be associated with decrease in symptoms (98), may also be useful for improving functional outcome in psychosis (67). Metacognition Reflection and Insight Therapy (MERIT) is specifically aimed at improving metacognitive ability (99). However, De Jong et al. (100) recently demonstrated, in a trial of MERIT for individuals with schizophrenia, evidence of improved metacognitive ability but not functioning. The lack of improvement in functioning may be due to shorter follow-up period or may be accounted for by other factors, e.g., functional capacity or negative symptoms [see (32, 101)]. Therefore, new interventions, such as cognitive remediation, should continue to aim to improve both cognitions and real-life skills and additionally consider training metacognitive ability [e.g., Cella et al. (102)].
Limitations and Future Studies
Firstly, there was a low follow-up rate. This may be due to the long period between the two assessment points and during this time the participant either moved out of area, lost contact with study team, or could not remember the first study due to length of time or being unwell during the first assessment. A consequence of this low follow-up rate was the small sample size, which limited power and it was not possible to fully explore the role of negative symptoms, alongside metacognitive ability. Future studies using a large sample can confirm the results whilst (i) controlling for all symptoms and (i) exploring the interaction between symptoms and metacognitive ability. Follow-up rate could be improved by continuity of the researcher across research visits, regular contact to maintain rapport, and updating of contact information.
In terms of measurement, this study used the PANSS to assess negative symptoms which may not align with current negative symptoms conceptualizations [see (103)], e.g., negative symptoms are a distinct therapeutic area of interest and negative symptoms are independent from depression, medication side effects and neurocognition. Future studies could assess negative symptoms using Clinical Assessment Interview for Negative Symptoms (CAINS) (104) or Brief Negative Symptom Scale (BNSS) (105) and could assess, or control for, other psychotic symptoms which could be deemed as secondary negative symptoms (40).
This sample was on average lower on symptoms of psychosis at baseline and follow-up (106) compared to other FEP samples (69, 107), which may explain the lack of change in positive symptoms. Whilst this study demonstrated that metacognitive ability, measured using Metacognitive Assessment Interview, had a large predictive role on functioning, other measures of metacognition, e.g., metacognitive experience (online appraisal) has been associated with social, real-world and work functioning (108–110). Future studies should aim to replicate this follow-up study with addition of other metacognitive measures. Finally, age was used as a covariate within the main analyses assessing predictors of outcome, as it was associated with functional outcome at follow-up. Age was recently demonstrated as a positive predictor of structured activity in At Risk Mental State (ARMS) group (111) and it may be suggested that age is a proxy for illness severity as those who have an earlier psychosis onset may have more difficulties in functioning later on (112). However, age of onset is difficult to measure and research suggests that premorbid IQ accounts for this difference (113). Analyses without age as a covariate demonstrated no difference in the results.
Conclusion
The present 3-year follow-up study was able to demonstrate that metacognitive ability at baseline significantly predicted improvement in functioning after 3 years, in FEP. This was independent of neurocognition, functional capacity, and negative symptoms. This study highlighted the importance of intervening early to enhance metacognitive ability over neurocognitive ability or functional capacity, in order to improve functioning later on, and to target interventions to improve functioning in those with poor metacognitive ability in the early stages of psychosis. Future studies should aim to replicate this within a larger sample.
Ethics Statement
Ethical and Health Research Authority approval was obtained through Camberwell St. Giles Research Ethics Committee (reference number: 17/LO/0055). All participants provided informed consent at first entry to the study and participants who gave consent to be re-contacted were contacted after the three-year period.
Author Contributions
AW, KG, and DF developed the hypotheses for the study. GD collected the baseline data and AW collected the follow-up data. AW produced the manuscript with reviewing and editing from all authors (KG, DF, and GD).
Funding
This work was supported by Sussex Partnership NHS Foundation Trust and Economic Social Research Council, through a Ph.D studentship awarded to the first author (Reference: ES/J500173/1).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participants for taking part in the study. This study was included within a doctoral thesis by the first author at University of Sussex. This study was presented at IEPA-11 conference 2018.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00182/full#supplementary-material
Footnotes
1. ^11 people were untraceable: 1 participant was not on the records system and 10 people provided contact details which were now out-of-date and were no longer connected to services.
References
1. Andreasen NC, Carpenter WT Jr, Kane JM, Lasser R, Marder SR, Weinberger DR, et al. Remission in schizophrenia: propsed criteria and raionale for consensus. Am J Psychiatry. (2005) 162:441–9. doi: 10.1176/appi.ajp.162.3.441
2. Harvey PD, Bellack AS. Toward a terminology for functional recovery in schizophrenia: is functional remission a viable concept? Schizophr Bull. (2009) 35:300–6. doi: 10.1093/schbul/sbn171
3. May PRA, Tuma H, Wilfrid J, Collaboration I, Coralee W, Thiele DA. Schizophrenia: a follow-up study of the results of five forms of treatment. Arch Gen Psychiatry. (1981) 38:776–84. doi: 10.1001/archpsyc.1981.01780320056006
4. Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, et al. Recovery from psychotic illness: a 15-and 25-year international followup study. Br J Psychiatry. (2001) 178:506–17. doi: 10.1192/bjp.178.6.506
5. Harrow M, Grossman LS, Jobe TH, Herbener ES. Do patients with schizophrenia ever show periods of recovery? A 15-year multi-follow-up study. Schizophr Bull. (2005) 31:723–34. doi: 10.1093/schbul/sbi026
6. Wunderink L, Sytema S, Nienhuis FJ, Wiersma D. Clinical recovery in first-episode psychosis. Schizophr Bull. (2009) 35:362–9. doi: 10.1093/schbul/sbn143
7. Henry LP, Amminger PG, Harris MG, Yuen HP, Harrigan SM, Prosser AL, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. (2010) 71:716–28. doi: 10.4088/JCP.08m04846yel
8. Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. (2004) 161:473–79. doi: 10.1176/appi.ajp.161.3.473
9. Morgan C, Lappin J, Heslin M, Donoghue K, Lomas B, Reininghaus U. Reappraising the long-term course and outcome of psychotic disorders : the AESOP-10 study. Psychol Med. (2014) 44:2713–26. doi: 10.1017/S0033291714000282
10. Edwards J, Maude D, McGorry PD, Harrigan SM, Cocks JT. Prolonged recovery in first-episode psychosis. Br J Psychiatry. (1998) 172:107–16. doi: 10.1034/j.1600-0447.106.s413.1_120.x
11. Birchwood M, Todd P, Jackson C. Early intervention in psychosis: the critical period hypothesis. Br J Psychiatry. (1998) 172 (Suppl. 33): 53–59.
12. Bertolote J, McGorry P. Early intervention and recovery for young people with early psychosis: consensus statement. Br J Psychiatry. (2005) 187(Suppl. 48):9–12. doi: 10.1192/bjp.187.48.s116
13. McGorry P, Killackey E, and Yung A. Early intervention in psychosis : concepts, evidence and future directions. World Psychiatry. (2008) 7:148–56. doi: 10.1002/j.2051-5545.2008.tb00182
14. Marshall M, Rathbone J. Early intervention for psychosis. Schizophr Bull. (2011) 37:1111–4. doi: 10.1093/schbul/sbr110
15. Hodgekins J, French P, Birchwood M, Mugford M, Christopher R, Marshall M, et al. Comparing time use in individuals at different stages of psychosis and a non-clinical comparison group. Schizophr Res. (2015) 161:188–93. doi: 10.1016/j.schres.2014.12.011
16. Fowler DJ, Hodgekins M, Painter T, Reilly C, Crane I, Macmillan M, et al. Cognitive behaviour therapy for improving social recovery in psychosis: a report from the ISREP MRC trial platform study (improving social recovery in early psychosis). Psychol Med. (2009) 39:1627–36. doi: 10.1017/S0033291709005467
17. Hodgekins J, Birchwood M, Christopher R, Marshall M, Coker S, Everard L, et al. Investigating trajectories of social recovery in individuals with first-episode psychosis: a latent class growth analysis. Br J Psychiatry. (2015) 207:536–43. doi: 10.1192/bjp.bp.114.153486
18. Bell MD, Paul H, Robert M. Clinical benefits of paid work activity in schizophrenia. Schizophr Bull. (1996). 22, 51–67.
19. Eklund M, Hansson L, Ahlqvist C. The importance of work as compared to other forms of daily occupations for wellbeing and functioning among persons with long-term mental illness. Commun Ment Health J. (2004) 40:465–77. doi: 10.1023/B:COMH.0000040659.19844.c2
20. Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophrenia Bull. (2011) 37 (Suppl. 2): 33–40. doi: 10.1093/schbul/sbr084
21. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia : implications for MATRICS. Schizophr Res. (2004) 72:41–51. doi: 10.1016/j.schres.2004.09.009
22. Lepage M, Bodnar M, Bowie CR. Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry. (2014) 59:5–12. doi: 10.1177/070674371405900103
23. Allott K, Liu P, Proffitt TM, Killackey E. Cognition at illness onset as a predictor of later functional outcome in early psychosis: systematic review and methodological critique. Schizophr Res. (2011) 125:221–35. doi: 10.1016/j.schres.2010.11.001
24. Carrión RE, McLaughlin D, Goldberg TE, Auther AM, Olsen RH, Olvet DM, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. (2013) 70:1133–42. doi: 10.1001/jamapsychiatry.2013.1909
25. Milev P, Ho B, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outco. Am J Psychiatry. (2005) 162:495–506. doi: 10.1176/appi.ajp.162.3.495
26. Stirling J, White C, Lewis S, Hopkins R, Tantam D, Huddy A, et al. Neurocognitive function and outcome in first-episode schizophrenia: a 10-year follow-up of an epidemiological cohort. Schizophr Res. (2003) 65:75–86. doi: 10.1016/S0920-9964(03)00014-8
27. Lin A, Wood SJ, Nelson B, Brewer WJ, Spiliotacopoulos D, Bruxner A, et al. Neurocognitive predictors of functional outcome two to 13years after identification as ultra-high risk for psychosis. Schizophr Res. (2011) 132:1–7. doi: 10.1016/j.schres.2011.06.014
28. Faber G, Smid HG, Van Gool AR, Wunderink L, Wiersma D, van den Bosch RJ. Neurocognition and recovery in first episode psychosis. Psychiatry Res. (2011) 188, 1–6. doi: 10.1016/j.psychres.2010.11.010
29. Gonzalez-Blanch C, Perez-Iglesias RG, Pardo-Garci'a JM, Rodri'guez-Sa'nchez O, Marti'nez-Garci'a JL, and Va'zquez-Barquero, Crespo-Facorro B. Prognostic value of cognitive functioning for global functional recovery in first-episode Schizophrenia. (2010) 40:935–44. doi: 10.1017/S0033291709991267
30. Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia : the in fluences of cognitive impairment, functional capacity, and symptoms. Schizophr Res. (2009) 115:82–7. doi: 10.1016/j.schres.2009.09.004
31. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ‘right stuff’? Schizophr. Bull. (2000) 26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430
32. Davies G, Fowler D, Greenwood K. Metacognition as a mediating variable between neurocognition and functional outcome in first episode psychosis. Schizophr Bull. (2017) 43:824–32. doi: 10.1093/schbul/sbw128
33. Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, et al. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. (2008) 63:505–11. doi: 10.1016/j.biopsych.2007.05.022
34. Evans JD, Heaton RH, Paulsen JS, Palmer BW, Patterson T, Jeste DV. The relationship of neuropsychological abilities to specific domains of functional capacity in older schizophrenia patients. Biol Psychiatry. (2003) 53:422–30. doi: 10.1016/S0006-3223(02)01476-2
35. Vesterager L, Christensen TT, Olsen BB, Krarup G, Melau M, Forchhammer HB, et al. Cognitive and clinical predictors of functional capacity in patients with first episode schizophrenia. Schizophr Res. (2012) 141:251–6. doi: 10.1016/j.schres.2012.08.023
36. Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia. Am J Psychiatry. (2006) 163:418. doi: 10.1176/appi.ajp.163.3.418
37. Best MW, Gupta M, Bowie CR, Harvey PD. a longitudinal examination of the moderating effects of symptoms on the relationship between functional competence and real world functional performance in schizophrenia. Schizophr Res. (2014) 1:90–5. doi: 10.1016/j.scog.2014.03.002
38. Rector NA, Beck AT, Stolar N. The negative symptoms of schizophrenia: a cognitive perspective. Can J Psychiatry. (2005) 50:247–57. doi: 10.1177/070674370505000503
39. Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. (2009) 113:189–99. doi: 10.1016/j.schres.2009.03.035
40. Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. (2014) 29:449–55. doi: 10.1016/j.eurpsy.2014.01.007
41. Austin SF, Mors O, Secher RG, Hjorthøj CR, Albert N, Bertelsen M, et al. Predictors of recovery in first episode psychosis: the OPUS cohort at 10year follow-up. Schizophr Res. (2013) 150:163–8. doi: 10.1016/j.schres.2013.07.031
42. Austin SF, Mors O, Budtz-Jørgensen E, Secher RG, Hjorthøj CR, Bertelsen M, et al. Long-Term trajectories of positive and negative symptoms in first episode psychosis: a 10year follow-up study in the OPUS cohort. Schizophr Res. (2015) 168:84–91. doi: 10.1016/j.schres.2015.07.021
43. Álvarez-Jiménez M, Gleeson JF, Henry LP, Harrigan SM, Harris MG, Killackey E, et al. Road to full recovery: longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 Years. Psychol Med. (2012) 42:595–606. doi: 10.1017/S0033291711001504
44. Villalta-Gil V, Vilaplana M, Ochoa S, Haro JM, Dolz M, Usall J, et al. Neurocognitive performance and negative symptoms: are they equal in explaining disability in schizophrenia outpatients? Schizophr Res. (2006) 87:246–53. doi: 10.1016/j.schres.2006.06.013
45. Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth LC, et al. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? J Nerv Ment Dis. (2000) 188:518–24. doi: 10.1097/00005053-200008000-00007
46. Peña J, Segarra R, Ojeda N, García J, Eguiluz JI, Gutiérrez M. Do the same factors predict outcome in schizophrenia and non-schizophrenia syndromes after first-episode psychosis? a two-year follow-up study. J Psychiat Res. (2012) 46:774–81. doi: 10.1016/j.jpsychires.2012.03.014
47. Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. (2009) 35:798–806. doi: 10.1093/schbul/sbn008
48. Rector NA, Stolar N, Grant P. Schizophrenia: Cognitive Theory, Research, and Therapy. New York, NY: Guilford Press (2011).
49. Couture SM, Blanchard JJ, Bennett ME. Negative expectancy appraisals and defeatist performance beliefs and negative symptoms of schizophrenia. Psychiatry Res. (2011) 189:43–8. doi: 10.1016/j.psychres.2011.05.032
50. Berry C, Greenwood K. Direct and indirect associations between dysfunctional attitudes, self-stigma, hopefulness and social inclusion in young people experiencing psychosis. Schizophr Res. (2018) 193:197–203. doi: 10.1016/j.schres.2017.06.037
51. Flavell JH. Metacognition and cognitive monitoring: a new area of cognitive-developmental inquiry. Am Psychol. (1979) 34:906–11. doi: 10.1037/0003-066X.34.10.906
52. Dimaggio G, Vanheule S, Lysaker PH, Carcione A, Nicolò G. Impaired self-reflection in psychiatric disorders among adults: a proposal for the existence of a network of semi independent functions. Conscious Cogn. (2009) 18:653–64. doi: 10.1016/j.concog.2009.06.003
53. Semerari A, Carcione A, Dimaggio G, Falcone M, Nicol ò G, Procacci M, et al. How to evaluate metacognitive functioning in psychotherapy? the metacognition assessment scale and its applications. Clin Psychol Psychother. (2003) 10:238–61. doi: 10.1002/cpp.362
54. Nelson T, Narens L. Metamemory: a theoretical framework and new findings. Psychol. Learn Motivat. (1990) 26:125–73. doi: 10.1016/S0079-7421(08)60053-5
55. Lysaker PH, Ringer J, Maxwell C, Mcguire A, Lecomte T. Personal narratives and recovery from schizophrenia. Schizophr Res. (2010) 121:271–6. doi: 10.1016/j.schres.2010.03.003
56. Lysaker PH, Erickson M, Ringer J, Buck KD, Semerari A, Carcione A, et al. Metacognition in schizophrenia: the relationship of mastery to coping, insight, self-esteem, social anxiety, and various facets of neurocognition. Br J Clin Psychol. (2011) 50:412–24. doi: 10.1111/j.2044-8260.2010.02003.x
57. Lysaker PH, Carcione A, Dimaggio G, Johannesen JK, Nicolò G, Procacci M, et al. Metacognition amidst narratives of self and illness in schizophrenia: associations with neurocognition, symptoms, insight and quality of life. Acta Psychiatr Scand. (2005) 112:64–71. doi: 10.1111/j.1600-0447.2005.00514.x
58. Semerari A, Cucchi M, Dimaggio G, Cavadini D, Carcione A, Battelli V, et al. The Development of the metacognition assessment interview: instrument description, factor structure and reliability in a non-clinical sample. Psychiatry Res. (2012) 200:890–5. doi: 10.1016/j.psychres.2012.07.015
59. Lysaker PH, Gumley A, Luedtke M, Buck KD, Ringer JM, Olesek K, et al. Social cognition and metacognition in schizophrenia: evidence of their independence and linkage with outcomes. Acta Psychiatr Scand. (2013) 127:239–47. doi: 10.1111/acps.12012
60. Arnon-Ribenfeld N, Hasson-Ohayon I, Lavidor M, Atzil-Slonim D, Lysaker P H. The association between metacognitive abilities and outcome measures among people with schizophrenia: a meta-analysis. Eur Psychiatry. (2017) 46:33–41. doi: 10.1016/j.eurpsy.2017.08.002
61. Davies G, Greenwood K. A meta-analytic review of the relationship between neurocognition, metacognition and functional outcome in schizophrenia. J Mental Health. (2018). doi: 10.1080/09638237.2018.1521930. [Epub ahead of print].
62. Lysaker PH, Shea AM, Buck KD, Dimaggio G, Nicolò G, Procacci M, et al. Metacognition as a mediator of the effects of impairments in neurocognition on social function in schizophrenia spectrum disorders. Acta Psychiatr Scand. (2010) 122:405–13. doi: 10.1111/j.1600-0447.2010.01554.x
63. Wright AC, Davies G, Fowler D, Greenwood KE. Self-defining memories predict engagement in structured activity in first episode psychosis, independent of neurocognition and metacognition. Schizophr Bull. (2018). doi: 10.1093/schbul/sby155. [Epub ahead of print].
64. Moritz S, Andreou C, Schneider BC, Wittekind CE, Menon M, Balzan RL, et al. Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev. (2014) 34:358–66. doi: 10.1016/j.cpr.2014.04.004
65. Rocha NBF, Queirós C. Metacognitive and Social Cognition Training (MSCT) in schizophrenia: a preliminary efficacy study. Schizophr Res. (2013) 150:64–8. doi: 10.1016/j.schres.2013.07.057
66. Briki M, Monnin J, Haffen E, Sechter D, Favrod J, Netillard C, et al. Metacognitive training for schizophrenia: a multicentre randomised controlled trial. Schizophr Res. (2014) 157:99–106. doi: 10.1016/j.schres.2014.06.005
67. Dubreucq J, Delorme C, Roure R. Metacognitive therapy focused on psychosocial function in psychosis. J Contemp Psychother. (2016) 46:197–206. doi: 10.1007/s10879-016-9334-7
68. Lysaker PH, Erickson MA, Buck B, Buck KD, Olesek K, Grant MLA, et al. Metacognition and social function in schizophrenia: associations over a period of five months. Cogn Neuropsychiatry. (2011) 16:241–55. doi: 10.1080/13546805.2010.530470
69. McLeod HJ, Gumley AI, MacBeth A, Schwannauer M, Lysaker PH. Metacognitive functioning predicts positive and negative symptoms over 12 months in first episode psychosis. J Psychiat Res. (2014) 54:109–15. doi: 10.1016/j.jpsychires.2014.03.018
70. Lysaker PH, Dimaggio G, Carcione A, Procacci M, Buck KD, Davis LW, et al. Metacognition and schizophrenia: the capacity for self-reflectivity as a predictor for prospective assessments of work performance over six months. Schizophr Res. (2010) 122:124–30. doi: 10.1016/j.schres.2009.04.024
71. Wright AC, Fowler D, Greenwood KE. Developing a dynamic model of unusual experiences and function in young people with or without psychosis: a cross-sectional and longitudinal study protocol. BMJ Open. (2018) 8:1–9. doi: 10.1136/bmjopen-2018-022546
72. Short S. Review of the UK 2000 Time Use Survey. London, UK: Office for National Statistics (2006) 53.
73. Cella M, Edwards C, Wykes T. A question of time: a study of time use in people with schizophrenia. Schizophr Res. (2016) 176:480–4. doi: 10.1016/j.schres.2016.06.033
74. Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally Ill adults. Schizophr Bull. (2001) 27:235–45. doi: 10.1093/oxfordjournals.schbul.a006870
75. Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: usefulness in clinical treatment studies. Schizophr Bull. (2007) 33:1138–48. doi: 10.1093/schbul/sbm040
76. Mausbach BT, Depp CA, Bowie CR, Harvey PD, McGrath JA, Thronquist MH, et al. Sensitivity and specificity of the UCSD performance-based skills assessment (UPSA-B) for identifying functional milestones in schizophrenia. Schizophr Res. (2011) 132:165–70. doi: 10.1016/j.schres.2011.07.022
77. Mausbach BT, Moore R, Bowie C, Cardenas V. A review of instruments for measuring functional recovery in those diagnosed with psychosis. Schizophr Bull. (2009) 35:307–18. doi: 10.1093/schbul/sbn152
78. Wechsler D. WMS-R: Wechsler Memory Scale – Revised Manual. New York, NY: Psychological Corporation (1987).
79. Tombaugh TN. Trail making test A, and B: normative data stratified by age and education. Arch Clin Neuropsychol. (2004) 19:203–14. doi: 10.1016/S0887-6177(03)00039-8
80. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. (1999) 14:167–77.
81. Kay SR, Fiszbein A, and Opler LA. The positive and negative syndrome scale for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
82. Hermes ED, Sokoloff D, Stroup S, Rosenheck RA. Minimum clinically important difference in the positive and negative syndrome scale with data from the clinical antipsychotic trials of intervention effectiveness (CATIE). J Clin Psychiatry. (2012) 73:526–32. doi: 10.4088/JCP.11m07162
83. Fowler D, Hodgekins J, French P, Marshall M, Freemantle N, McCrone P, et al. Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): a single-blind, randomised controlled trial. Lancet Psychiatry. (2018) 5:41–50. doi: 10.1016/S2215-0366(17)30476-5
84. Fowler D, Hodgekins J, French P. Social recovery therapy in improving activity and social outcomes in early psychosis: current evidence and longer term outcomes. Schizophr Res. (2019) 203:99–104. doi: 10.1016/j.schres.2017.10.006
85. Hair JF, Hult GTM, Ringle CM, Sarstedt M. A Primer on Partial Least Squares Structural Equation Modeling. Second Edition. Thousand Oaks, CA: Sage (2017).
86. Hair JF Jr, Anderson RE, Tatham RL, Black W, et al. (1995). Multivariate data analysis, 3rd Ed. New York, NY: Macmillan.
87. Scheider W, Niklas F, Schmeideler S. Intellectual development from early childhood to early adulthood: the impact of early IQ differences on stability and change over time. Learn Individ Differ. (2010) 20:14–18. doi: 10.1016/j.lindif.2014.02.001
88. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia 315. Arch Gen Psychiatry. (2001) 58:24–32. doi: 10.1001/archpsyc.58.1.24
89. Ginett LE, Moran LJ. Stability of vocabulary performance by schizophrenics. J Consult Psychol. (1964) 28:178–9. doi: 10.1037/h0045133
90. Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. (2015) 72:377–85. doi: 10.1001/jamapsychiatry.2014.2671
91. Leeson VC, Barnes TRE, Hutton SB, Ron MA, Joyce EM. IQ as a predictor of functional outcome in schizophrenia: a longitudinal, four-year study of first-episode psychosis. Schizophr Res. (2009) 107:55–60. doi: 10.1016/j.schres.2008.08.014
92. Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. (2014) 130:290–9. doi: 10.1111/acps.12289
93. Saeedi H, Addington J, Addington D. The association of insight with psychotic symptoms, depression, and cognition in early psychosis: a 3-year follow-up. Schizophr Res. (2007) 89:123–8. doi: 10.1016/j.schres.2006.09.018
94. Luther L, Firmin RL, Vohs JL, Buck KD, Rand KL, Lysaker PH. Intrinsic motivation as a mediator between metacognition deficits and impaired functioning in psychosis. Br J Clin Psychol. (2016). 55:332–47. doi: 10.1111/bjc.12104
95. Tas C, Brown EC, Esen-Danaci A, Lysaker PH, Brüne M. Intrinsic motivation and metacognition as predictors of learning potential in patients with remitted schizophrenia. J Psychiatr Res. (2012) 46:1086–92. doi: 10.1016/j.jpsychires.2012.04.027
96. Neil ST, Kilbride M, Pitt L, Nothard S, Welford M, Sellwood W, et al. The Questionnaire about the Process of Recovery (QPR): a measurement tool developed in collaboration with service users. (2009) 2012, 37–41. doi: 10.1080/17522430902913450
97. Shepherd G, Boardman J, Slade M. Making recovery a reality. Scott Recov Network. London: Sainsbury Centre (2008) 1–23.
98. Lysaker PH, Buck KD, Ringer J. The recovery of metacognitive capacity in schizophrenia across 32 months of individual psychotherapy: a case study. Psychother. Res. (2007) 17:713–20. doi: 10.1080/10503300701255932
99. Lysaker PH, Buck KD, Carcione A, Procacci M, Salvatore G, Nicolò G, et al. Addressing metacognitive capacity for self reflection in the psychotherapy for schizophrenia: a conceptual model of the key tasks and processes. Psychol Psychother. (2011) 84:58–69. doi: 10.1348/147608310X520436
100. de Jong S, van Donkersgoed RJM, Timmerman ME, aan het Rot M, Wunderink L, Arends J, et al. Metacognitive Reflection and Insight Therapy (MERIT) for patients with schizophrenia. Psychol Med. (2018) 49:303-13. doi: 10.1017/S0033291718000855
101. Koren D, Seidman LJ, Goldsmith M, Harvey PD. Real-world cognitive - and metacognitive - dysfunction in schizophrenia: a new approach for measuring (and remediating) more ‘right stuff.' Schizophr Bull. (2006) 32:310–26. doi: 10.1093/schbul/sbj035
102. Cella M, Reeder C, Wykes T. Lessons learnt? the importance of metacognition and its implications for cognitive remediation in schizophrenia. Front Psychol. (2015)6:1259. doi: 10.3389/fpsyg.2015.01259
103. Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. (2006) 32:214–9. doi: 10.1093/schbul/sbj053
104. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. Development and psychometric validation of the clinical assessment interview for negative symptoms (CAINS). Schizophr Res. (2013) 132:140–5. doi: 10.1016/j.schres.2011.06.030
105. Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. (2011) 37:300–5. doi: 10.1093/schbul/sbq059
106. Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. (2005) 79:231–8. doi: 10.1016/j.schres.2005.04.008
107. Fitzgerald LS, Redoblado MA, Winter V, Brennan J, Anderson J, et al. Cognitive functioning in young people with first episode psychosis: relationship to diagnosis and clinical characteristics. Austr N Zeal J Psychiatry. (2004) 38:501–10. doi: 10.1080/j.1440-1614.2004.01403.x
108. Gould F, McGuire LS, Durand D, Sabbag S, Larrauri C, Patterson TL, Twamley EW, Harvey PD. Self assessment in schizophrenia: accuracy of evaluation of cognition and everyday functioning. Neuropsychology. (2015) 4:675–82. doi: 10.1037/neu0000175
109. Verdoux H, Monello F, Goumilloux R, Cougnard A, Prouteau A. Self-perceived cognitive deficits and occupational outcome in persons with schizophrenia. Psychiatry Res. (2010) 178:437–9. doi: 10.1016/j.psychres.2010.04.031
110. Stratta P, Daneluzzo E, Riccardi I, Bustini M, Rossi A. Metacognitive ability and social functioning are related in persons with schizophrenic disorder. Schizophr. Res. (2009) 108:301–2. doi: 10.1016/j.schres.2008.10.005
111. Bright M, Parker S, French P, Fowler D, Gumley A, Morrison AP, et al. Metacognitive beliefs as psychological predictors of social functioning: an investigation with young people at risk of psychosis. Psychiatry Res. (2018) 262:520–526. doi: 10.1016/j.psychres.2017.09.037
112. Immonen J, Jääskeläinen E, Korpela H, Miettunen J. Age at onset and the outcomes of schizophrenia: a systematic review and meta-analysis. Early Interv Psychiatry. (2017) 11:453–60. doi: 10.1111/eip.12412
113. Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, et al. A longitudinal study of premorbid iq score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch Gen Psychiatry. (2004) 61:354–60. doi: 10.1001/archpsyc.61.4.354
Keywords: first episode psychosis, metacognition, functioning, longitudinal, cognition, negative symptoms, functional capacity
Citation: Wright AC, Davies G, Fowler D and Greenwood K (2019) Three-Year Follow-Up Study Exploring Metacognition and Function in Individuals With First Episode Psychosis. Front. Psychiatry 10:182. doi: 10.3389/fpsyt.2019.00182
Received: 09 January 2019; Accepted: 13 March 2019;
Published: 12 April 2019.
Edited by:
Philip D. Harvey, Leonard M. Miller School of Medicine, United StatesReviewed by:
Felicia Gould, Leonard M. Miller School of Medicine, United StatesLauren Luther, Indiana University, Purdue University Indianapolis, United States
Copyright © 2019 Wright, Davies, Fowler and Greenwood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abigail C. Wright, YXdyaWdodDI0QG1naC5oYXJ2YXJkLmVkdQ==
 Abigail C. Wright
Abigail C. Wright Geoff Davies4
Geoff Davies4 Kathryn Greenwood
Kathryn Greenwood