- 1Key Laboratory of Cognition and Personality (Ministry of Education), Southwest University, Chongqing, China
- 2Faculty of Psychology, Southwest University, Chongqing, China
- 3CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, China
Self-control is the ability to comply with a request, to postpone acting upon a desire object or goal, and to generate socially approved behavior in the absence of external monitors. Overeating is actually the failure in self-control while feeding. However, little is known about the brain function that allows individuals to consciously control their behavior in the context of food choice. To address this issue, we used functional MRI to measure brain activity among undergraduate young females. Forty-one undergraduate female students participated in the current study. Subjects underwent the food rating task, during which they rated each food item according to their subjective perception of its taste (from Dislike it very much to Like it very much), its long term effect on health (from very unhealthy to very healthy) and decision strength to eat it (from Strong no to Strong yes). Behavioral results indicate the positive correlation between taste rating and its corresponding decision strength to eat, no matter the food is high caloric or low. Moreover, health ratings of high caloric food was negatively correlated with DEBQ-emotional eating, and taste ratings of high caloric food was positively correlated with DEBQ-external eating. Whole brain analysis of fMRI data indicates that BOLD responses in dlPFC were positively correlated with successful self-control; BOLD responses in midcingulate cortex were positively correlated with failed self-control. This study provided direct evidence that dlPFC was involved in self-control in food-related choice.
Introduction
We are living in an environment which promotes over-consumption of palatably high-energy food, and the obesity epidemic shows no signs of abating (1). Individual differences in food reward sensitivity is responsible for overeating (2, 3). Sensitivity to food reward, insensitivity to internal state, and/or defects in impulsive control were found to predict overeating and a preference for foods high in fat and sugar. Moreover, the above these factors would, in turn, predict higher body mass index (BMI). Sensitivity to food reward works as the hot system which drives a person to food intake; impulsive control, on the contrary, works as the cool system which could restrains feed behavior. Numerous studies have focused on the hot system (2, 4, 5), whereas studies referring to cool mechanism in the brain is relatively scarce.
Self-control has been variously defined as the ability to comply with a request, to initiate and cease activities according to situational demands, to modulate the intensity, frequency, and duration of verbal and motor acts in social and educational settings, to postpone acting upon a desire object or goal, and to generate socially approved behavior in the absence of external monitors (6). Self-control is also defined as an umbrella construct that bridges concepts and measurements from different disciplines, such as impulsivity, conscientiousness, self-regulation, delay of gratification, inattention-hyperactivity, executive function, will power and inter temporal choice (7). Some scholars take self-control and self-regulation as the same concept. In general, self-regulation is the broader term, encompassing both conscious and unconscious processes and sometimes referring to all behavior guided by goals or standards, whereas self-control refers more narrowly to conscious efforts to alter behavior, especially restraining impulses and resisting temptations (8).
Overeating is actually the failure in self-control while feeding. High-sugar/high-fat food induce appetite-food reward, which drives food intake. Food reward is so strong that defect in self-control will, apparently, lead to overeating. Neuroimaging studies, using positron emission tomography (PET) and magnetic resonance imaging (MRI), which focused on food intake, yield valuable insights into the neurobiology underlying variation in regulation of food intake in human (9). Regulation of food intake is based on two major interacting systems: homeostatic needs affect the behavior mediated by gastrointestinal hormones and hypothalamic integration (integration of gastrointestinal hormone and hypothalamus function); the pleasure from food intake can provide reinforcement beyond homeostatic value and lead to overindulgence in high-caloric food (10). The hedonic component of feeding behavior is suggested to be mediated by reward-related cortical and sub-cortical systems, that is, the ventral striatum, the ventral tegmental area and the orbitfrontal cortex (OFC). However, little is known about the brain function that allows individuals to consciously control their behavior in the context of food choice.
Neuroimaging studies focusing on self-control found that cognitive control is the highest level of cognitive activity, and prefrontal lobe plays as the central role in cognitive control system (11–13). Difference sub-region of PFC perform its own functions: the ventromedial prefrontal cortex (vmPFC) is responsible for reward assessment and goal orientation; the dorsolateral prefrontal cortex (DLPFC) is in charge of process of self-control; the medial orbitofrontal cortex (mOFC), via the lateral prefrontal cortex (lPFC), dedicates to represent subjective reward values at the time of choice (14–16). Other brain areas like insula and its relying operculum also participate in self-regulation of cognitive control function s (17).
Contrary to the activity of self-control system, the brain reward system, containing vmPFC, lPFC, OFC, striatum, insula, anterior cingulum gyrus cortex, hippocampus, amygdale, and midbrain structures, encodes the subjective value of rewards and the subsequent impulse system, and it consistent with a role for this neuronal network in general hedonic representation (18, 19). Reward activity in lPFC and vmPFC represent characteristics and intense of anticipation to reward (11, 20), and striatum is responsible for coding “liking” and “wanting” qualities of food (21). Amygdala, with projections to nucleus accumbens to trigger motivitioanal behaviors, functions as emotional integration, reward process and feeding regulation (22). Hippocampus, the central part of memory, conserve the pleasure feeling and emotional reaction, and then transport these information to dosal stratum and cerebellum, regulating the feeding or avoiding behavior (23).
Along with previous literature, there is, apparently, an antagonistic effect between the self-control system represented by prefrontal area and the impulsive system represented by striatum. In a particular situation, intense activities in the prefrontal system indicate much weaker activities in the stratum system, and vice versa. It can be summarized that the balance between these two brain systems are vital to regulate feed behavior. Overeating and obesity will happen very likely if the balance is broken. We speculate that delicious high-fat food, which is difficult to resist and reject, may disturb the balance between the self-control system and the impulsive system. Previous studies mainly focused on the activities in stratum system while watching food pictures or the anticipating to intake food. Evidence in prefrontal control mechanism of feeding behavior is scarce. Hence, the current study, based on the activities of the prefrontal system while watching various food pictures, aimed to investigate the intrinsic mechanism underlying the self-control function in food-related decision-making. We employed three food rating tasks in the current study. In these tasks, subjects were required to indicate their decision intensity to consume the food items based on balancing the immediate pleasure from their taste and its long-term effect on health. We categorize subjects as high self-control group or low self-control group according to their score in food rating tasks. We hypothesized that: (1) increased dlPFC function would associate with self-control; (2) food picture would activate numbers of related areas, such as somatosensory cortex (postcentral gyrus), visual cortex (superior parietal lobule, cuneus), primary taste cortex (insular), rewarding areas (striatum, OFC); (3) activity differences would be observed between food-acceptation and food-rejection.
Methods
Subjects
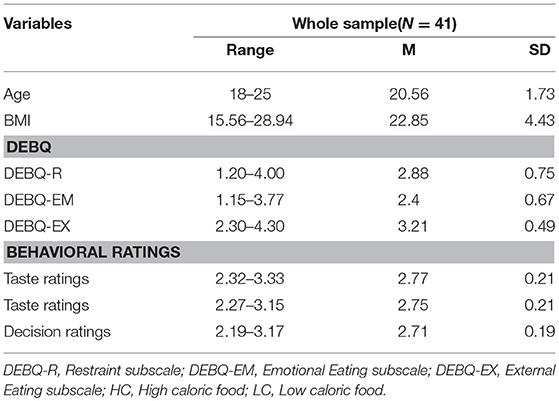
Forty-three subjects participated in the current experiment [all females; mean age = 20.47 years, S.D. = 1.75, age range = 18–25 years; mean BMI = 23.05, S.D. = 4.44, BMI range = 15.56–29.32]. The demographic information of subjects was presented in Table 1. Due to excessive head movement during scanning, four subjects (who exceeded a predetermined limit of 2 mm in any direction) were excluded from the sample. Findings from the resulting sample of 41 were reported. All subjects were right-handed nonsmokers, with no reported past/current neurological or psychiatric illness, normal or corrected-to-normal vision and normal color vision as assessed by basic color tests. None of them took medications. We did not impose a BMI upper-limit or lower-limit. Subjects were included as long as they felt comfortable while inserted in the fMRI scanner. All subjects provided the date of their last period to ensure that they were not scanned during menstruation.
Measures
Demographics
Subjects completed a demographics questionnaire, including age, current and historic medications, and phase of menstrual cycle.
Hunger Ratings
Subjects rated current feelings of hunger on a 5-point Likert scale, ranging from 0 (“not at all hungry”) to 4 (“very hungry”).
Dutch Eating Behavior Questionnaire [DEBQ; (24)] was used to assess subjects' eating behavior. It consisted of three subscales, including the Emotional Eating subscale (DEBQ-EM; 13 items; e.g., the degree to which eating is prompted by emotional states like tension and worry rather than by hunger), the External Eating subscale (DEBQ-EX; 10 items; e.g., the degree to which one tends to overeat if food looks and smells good), and the Restraint subscale (DEBQ-R; 10 items; e.g., the extent to which the individuals restrain food intake). Impulsivity in eating behaviors could be reflected by the DEBQ-EM and DEBQ-EX. DEBQ has 33 items in total, and each item was measured on a 5-point Likert scale. It has good reliability and validity. The Cronbach's α of each subscale in the current sample was 0.95, 0.81, and 0.95, respectively.
Stimuli
One hundred and seventy different food items with 85 picturing high-caloric (HC) palatable food (e.g., fried chicken, hot dog, ice cream, etc.) and 85 picturing low-caloric (LC) food (e.g., fruits, vegetables, etc.) were used in the current study. All of the stimuli were adopted from Chinese Food Picture Database (25). The food pictures were presented to the subjects using color pictures (72 dpi). Stimulus presentation and response recording was controlled by E-prime 2.0.
Food Rating and Decision-Making Task
The food rating and decision-making task was similar with the task used in previous study (26). The task had three parts. Subjects first rated all 80 high-caloric and 80 low-caloric food items for both their taste and their long term effect on health in two separate blocks (a taste-rating block and a health-rating block). All ratings were made using a four-point scale that was shown on the screen below each item. The taste ratings were made on a 4-Likert scale from 1 = Dislike it very much, 2 = Dislike it, 3 = Like it, to 4 = Like it very much. And all health ratings were made on a 4-Likert scale from 1 = Very unhealthy, 2 = Unhealthy, 3 = Healthy, to 4 = Very healthy. Subjects were instructed to rate the taste without regard for its healthiness before the taste-rating block. Similarly, before the health-rating block they were instructed to rate the healthiness of each food item without regard for its taste. After the two rating blocks, subjects were presented with another food picture gallery including 10 food items (5 high-caloric and 5 low-caloric items), and they were asked to choose one food item as a reference item, which was relatively neutral on their taste and health perception.
In decision phase, all subjects were presented with the 160 food items again and were instructed that on each trial they would have to choose between eating the food item shown in that trial and the reference food item. Subjects were told to express the strength of their preferences using a four-point scale: 1 = Strong No (choose reference food), 2 = No (choose reference food), 3 = Yes (choose shown food), 4 = Strong Yes (choose shown food) (26).
The taste-rating task and decision-making task was done in the fMRI scanner and the health-rating task was done out of the scanner.
Procedure
Following approval from the Human Research Ethics Committee at School of Psychology, Southwest University, subjects were recruited via on-campus advertisements. Subsequently, 43 female undergraduate students engaged in the current study. All of the subjects took part in an intake session and one fMRI scanning session. Two sessions were conducted on separate days. Body weight and height was measured during the intake session. BMI was calculated as weight (in kilograms) divided by the squared height (in meters) of the subject (BMI = kg/m2). Specifically, after the removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. During the intake session, subjects signed the consent inform after reading a general overview of the study. Anthropometric measurements were then taken.
On the day of the fMRI scan, subjects were instructed to refrain from eating or drinking, with the exception of water, within 12 h before their session. Fasting status was confirmed by self-report questionnaires upon their arrival. Then subjects were introduced with the taste and health rating task, as well as the decision-making task. After that, subjects did the health rating task out of the fMRI scanner, and chose the reference food item. Then, subjects were taken into the scanner bore, and they did the hunger rating right before the structural image acquisition. After the rating, T1-weighted structural scans and the resting state fMRI (rs-fMRI) run was conducted. Then subjects completed the food taste rating runs and decision-making runs in the scanner, sequentially. Only the results from the decision-making task were reported here and the resting state fMRI data were reported in another study (27). Each subject was paid 140 Yuan as compensation for their participating after the fMRI scanning session.
fMRI Data Acquisition
fMRI data were acquired using a 3-T Siemens Trio scanner in the SWU Imaging Center for Brain Research. Foam pads were used to reduce head movements and scanner noise. Scans were performed by an echo-planar imaging (EPI) sequence with the following scan parameters: repetition time = 2,000 ms, echo time = 30 ms, flip angle = 90°, field of view = 192 × 192 mm2, acquisition matrix = 64 × 64, in-plane resolution = 3 × 3mm2, 32 interleaved 3-mm-thick slices, inter-slice skip = 0.99 mm. Two volumes were discarded before the beginning of data collection in each run to allow for equilibration of the magnetic field.
Data Preprocessing
Neuroimaging data were preprocessed using the SPM12 software (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, United Kingdom) on the Matlab platform. For each subject, the first 10 volumes were discarded to account for signal equilibrium and subjects' adaptation to their immediate environment. Then, the fMRI images were corrected for the acquisition delay between slices and for the head motion. Two subjects were excluded because their head motion exceeded 2 mm in translation or 2° in rotation. Then, anatomical and functional images were normalized to the standard MNI template brain implemented in SPM12, resulting in voxel sizes of 1 and 3 mm3, respectively. Functional time-series data were then detrended.
Statistical Analysis
Behavioral Data
Firstly, descriptive analysis was conducted with the demographic variables, DEBQ and behavioral ratings of the taste, health and decision making. Then, correlation analysis was conducted between decision strength of HC food or LC food, DEBQ-EM, and DEBQ-EX, respectively. We expected that decision strength-HC would positively correlate with DEBQ-EM and DEBQ-EX, while decision strength-LC would show no such correlation with these variables. According to behavior scores in both the food rating task and decision making task, we divided subjects into three groups: success in self-control (SSC), failure in self-control (FSC) and no self-control (NSC). SSC means rejection to like/healthy food and acceptation to dislike/healthy food; FSC means rejection to unlike/healthy food and acceptation to like/unhealthy food; NSC means rejection to unlike/unhealthy food and acceptation to like/healthy food.
fMRI Data
Whole brain analyses
Analysis was performed with SPM12. Individual level whole brain general linear models (GLMs) and SPM12's standard hemodynamic response function was estimated in three steps. Firstly, we estimated the model separately for each individual. Three events were defined: (1) success in self-control (SSC) including choosing healthy-disliked food and rejecting unhealthy-liked food; (2) failure in self-control (FSC) including rejecting healthy-disliked food and choosing unhealthy-liked food; and (3) no self-control (NSC) including trials with healthy-liked food and unhealthy-disliked food. Secondly, we calculated contrast statistics at the individual level. Two main contrasts were specified for subject-level analysis: (1) SSC vs. FSC and (2) FSC vs. SSC. Thirdly, a general linear model was used to generate the statistical parametric maps for the second-level analysis, while BMI was introduced as a covariate variable in the analysis in the original manuscript. We expected that increased dlPFC function would associate with self-control. Main effects were considered significant using a whole-brain family wise error (FWE) of p < 0.05 and a minimum cluster size of 5 voxels.
Results
Behavioral Results
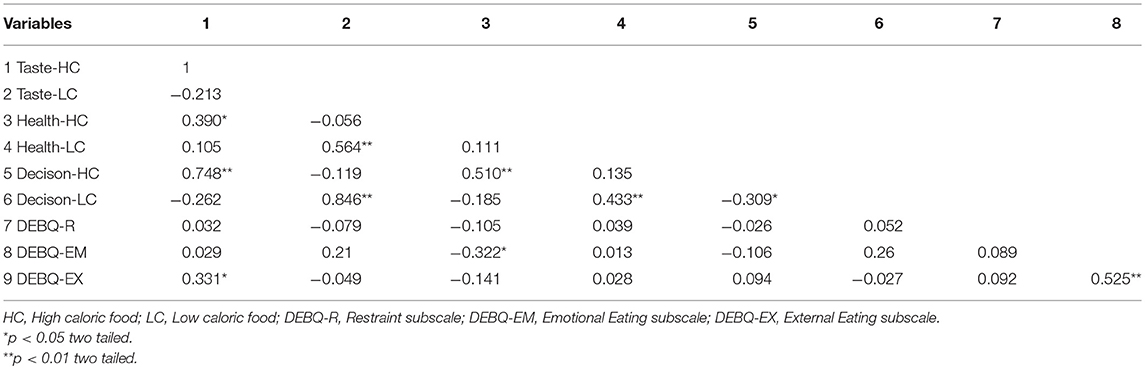
The results of descriptive statistical analysis on three rating tasks were shown in Table 1, and the inter-correlation between variables of interest was presented in Table 2. Results show that taste-likeness for low caloric food negatively correlated with DEBQ-EM (r = −0.32, p = 0.05); taste-likeness for high caloric food positively correlated with DEBQ-EX (r = 0.35, p = 0.05); there is positive correlation between food liking and its corresponding eating choice, no matter the food is high caloric or low; health assessment of high caloric food negatively correlated with DEBQ-EM (r = −0.32, p = 0.05); likeness assessment of high caloric food positively correlated with DEBQ-EX (r = 0.33, p = 0.05).
Brain Image Results
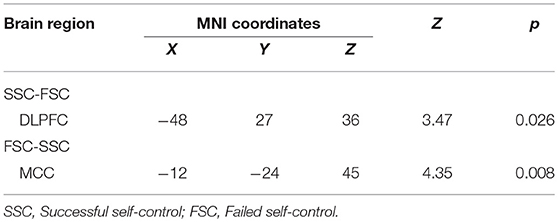
We estimated a general linear model of brain responses in which activity during the entire evaluation period was modulated by self-control. Whole brain analysis showed that SSC vs. FSC significantly activated BOLD responses in DLPFC, whereas FSC vs. SSC significantly activated BOLD responses in midcingulate cortex (MCC) (Table 3 and Figure 1).
Correlation With DEBQ
Correlation analysis was performed with the SSC vs. FSC and FSC vs. SSC maps against individual's scores of three DEBQ subscales. However, no significant finding was obtained.
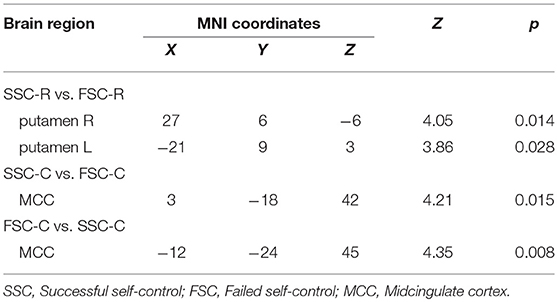
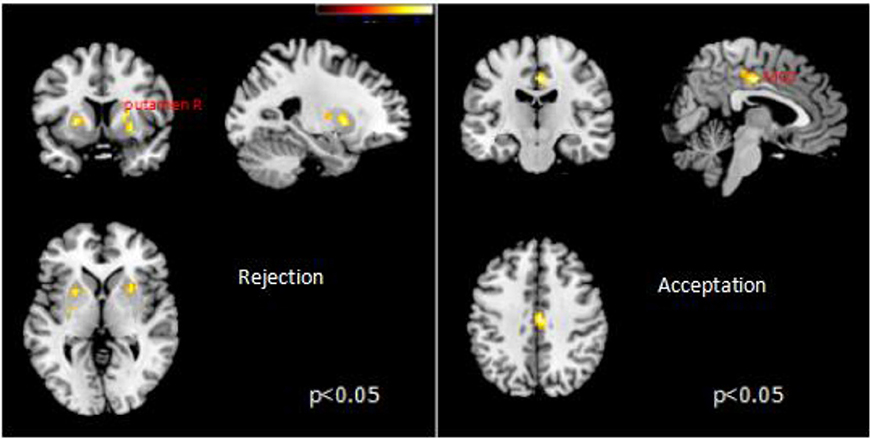
Then, we divided SSC into two events at individual level: (1) choosing healthy-disliked food (SSC-C) and (2) rejecting unhealthy-liked food (SSC-R). Meanwhile, FSC were divided into two events at individual level: (1) choosing unhealthy-liked food (FSC-C) and (2) rejecting healthy-disliked food (FSC-R). Four main contrasts were specified for subject-level analysis: (1) SSC-R vs. FSC-R, (2) FSC-R vs. SSC-R, (3) SSC-C vs. FSC-C and (4) FSC-C vs. SSC-C. Correlational analysis were performed with the four contrast maps against individual's scores of three DEBQ subscales. Results showed that BOLD responses in bilateral putamen were positively correlated with DEBQ-R on SSC-R vs. FSC-R contrast. Meanwhile, BOLD responses in left MCC were positively correlated with DEBQ-R on FSC-C vs. SSC-C (Table 4 and Figure 2).
Discussion
Based on the reinforcement learning hypothesis of self-control and food decision making, this study provides an approach to characterize the association between self-control on food-related choice.
As expected, self-control, which was indexed by the food choice between two food items, was positively correlated with BOLD responses in dlPFC. This is direct evidence showing the importance of dlPFC on self-control. Meanwhile, BOLD responses in MCC were positively correlated with unsuccessful self-control. These findings were in line with previous observations of the positive association between dlPFC with inhibition to energy intake (28), or smokers (29, 30). Dorsal lateral PFC also functions as the translation mechanism, which reinforces self-power, motivating one's goal-directed behavior in the long run (31, 32). DLPFC also projects to other brain areas, promoting or inhibiting various neuro-functions, such as executive control and emotion regulation n (33, 34). Subjects with damaged dlPFC had difficulty in focusing on cognitive task, implying a defect in self-control (35).
Under the “rejection” conditions, BOLD responses in bilateral putamen were positively correlated with DEBQ-R, no matter it's successful or unsuccessful self-control; Under the “acceptation” conditions, responses in MCC, unilaterally, were positively correlated with DEBQ-R, no matter it's successful or unsuccessful self-control. Although activities can be observed under both condition, we speculate there may exist difference between them, which implying different neuromechanisms between “accepting” food and “rejecting” food. Cingulate gyrus is crucial structure functioning in the regulation system (36). Neuroimaging studies referred to food pictures found that cingulate gyrus was stably activated during the attention to food stimulus (28, 37). In our daily life, while choosing food, we have to face the conflict which was caused by flavor of food and its healthy meaning to our bodies, and self-control help to regulate feeding behavior. In the current study, we defined successful self-control as rejection to like/healthy food or acceptation to dislike/healthy food; we defined unsuccessful self-control as rejection to unlike/healthy food or acceptation to like/unhealthy food. Based on the definition, subjects who have weaker self-control may usually choose food with better flavor, ignoring its impact on health. Functional MRI results in the study presented evidence implying that cingulate gyrus had stronger BOLD reaction when subjects focused on the flavor of food.
Interestingly, fMRI results in the current study also confirmed that DEBQ scores had positive correlation with BOLD responses in putamen, bilaterally, no matter self-control is successful or unsuccessful. Studies concerning restrained eating verify that high score of DEBQ reveals stronger cognitive control while in feeding environment, which, consequently, lead to restricted eating behavior (10). Besides putamen, dorsal striatum and caudatum may function to regulate restricted eating (10, 38). Studies concerning the old reveal similar conclusion, that putamen, caudatum and dlPFC collaborate with each, functioning as the self-control system to regulate delay of discounting and delay of gratification (39). Inversely, defect collaboration among these areas may lead to the reduction of resisting food temptation (40). What's more interesting is that, in the successful self-control trails, “acceptation” and “rejection” respectively activated different areas. “Acceptation” decision activated MCC and “rejection” the putamen. This underlies that these two decision-making behaviors may associated with different mechanism, despite they were both “successful” self-control reaction. Successful self-control owes to two ways: one is to resist the current temptation; the other is to think a lot of the future reward. From this perspective, successful self-control equals to the ability to choose health food, which is in accordance with long-term goals and succeeding in losing weight requires both behaviors (41).
Ethics Statement
This study was carried out in accordance with the recommendations of The Regulations of Ethical Reviews of Biomedical Research Involving Human, Ministry of Health,China. The protocol was approved by the Ethic Committee of Faculty of Psychology, Southwest University. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
FC: wrote this manuscript. YZ and YH: analyzed the data. QH: edited the manuscript and did the proof reading. XG: designed this study and got the funding.
Funding
This work was supported by Central Universities Fundamental Research Funds (#SWU1809103), CAS Key Laboratory of Mental Health, Institute of Psychology (#KLMH2016G04) to XG, and Central Universities Fundamental Research Funds (#SWU1809104) to FC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science (2003) 299:853–5. doi: 10.1126/science.1079857
2. Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite (2007) 48:12–9. doi: 10.1016/j.appet.2006.05.016
3. Loxton NJ, Tipman RJ. Reward sensitivity and food addiction in women. Appetite (2016) 115:28–35. doi: 10.1016/j.appet.2016.10.022
4. Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (2011) 19:2175–82. doi: 10.1038/oby.2011.57
5. Killgore WDS, Yurgeluntodd DA. Sex differences in cerebral responses to images of high vs low calorie food. Neuroreport (2010) 21:354. doi: 10.1097/WNR.0b013e32833774f7
6. Kopp CB. Antecedents of self-regulation: a developmental perspective. Dev Psychol. (1982) 18:199–214. doi: 10.1037/0012-1649.18.2.199
7. Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington HL, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. (2011) 108:2693–8. doi: 10.1073/pnas.1010076108
8. Baumeister RF. Ego depletion and self-control failure: an energy model of the Self's executive function. Self Identity (2002) 1:129–36. doi: 10.1080/152988602317319302
9. Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. (2012) 13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x
10. Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes. (2012) 36:648. doi: 10.1038/ijo.2011.125
11. Miller EK, Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. (2000) 1:59–65. doi: 10.1038/35036228
12. Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron (2014) 83:1002–18. doi: 10.1016/j.neuron.2014.08.011
13. Webster KE. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Q Rev Biol. (1997) 39:1008.
14. Peters J, D'Esposito M. Effects of medial orbitofrontal cortex lesions on self-control in intertemporal choice. Curr Biol. (2016) 26:2625–8. doi: 10.1016/j.cub.2016.07.035
15. Sokolhessner P, Hutcherson C, Hare T, Rangel A. Decision value computation in DLPFC and VMPFC adjusts to the available decision time. Eur J Neurosci. (2012) 35:1065–74. doi: 10.1111/j.1460-9568.2012.08076.x
16. Steinbeis N, Haushofer J, Fehr E, Singer T. Development of behavioral control and associated vmPFC-DLPFC connectivity explains children's increased resistance to temptation in intertemporal choice. Cereb Cortex (2016) 26:32–42. doi: 10.1093/cercor/bhu167
17. Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage (2005) 27:323–40. doi: 10.1016/j.neuroimage.2005.01.054
18. Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron (2011) 69:664–79. doi: 10.1016/j.neuron.2011.02.016
19. Tricomi E, Lempert KM. Value and probability coding in a feedback-based learning task utilizing food rewards. J Neurophysiol. (2015) 113:jn.00086.02014. doi: 10.1152/jn.00086.2014
20. Leon MI, Shadlen NM. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron (1999) 24:415. doi: 10.1016/S0896-6273(00)80854-5
21. Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. (2015) 9:90. doi: 10.3389/fnsys.2015.00090
22. Ross S, Levin EL, Itoga C, Schoen C, Selmane R, Aldridge JW. Deep brain stimulation in the central nucleus of the amygdala decreases “Wanting” and “Liking” of food rewards. Eur J Neurosci. (2016) 44:2431. doi: 10.1111/ejn.13342
23. Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci. (2013) 16:1717–24. doi: 10.1038/nn.3561
24. Van Strien T, Frijters JE, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional. International Journal of Eating Disorders (1986) 5:295–315. doi: 10.1002/1098-108X(198602)5:2<295::AID-EAT2260050209>3.0.CO;2-T
25. Li X. H.. (2018). Establishment of Chinese Food Picture Library and Its Application in Psychology of Eating. Master Thesis: Southwest University, Chongqing, China
26. Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science (2009) 324:646–48. doi: 10.1126/science.1168450
27. Gao X, Liang Q, Wu G, She Y, Sui N, Chen H. Decreased resting-state BOLD regional homogeneity and the intrinsic functional connectivity within dorsal striatum is associated with greater impulsivity in foodrelated decision-making and BMI change at 6-month follow up. Appetite (2018) 27:69–78. doi: 10.1016/j.appet.2018.04.024
28. Cornier MA, Salzberg AKEndly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. (2010) 99:538–43. doi: 10.1016/j.physbeh.2010.01.008
29. Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychol Sci. (2011) 22:498. doi: 10.1177/0956797611400918
30. Tabibnia G, Creswell JD, Kraynak T, Westbrook C, Julson E, Tindle HA. Common prefrontal regions activate during self-control of craving, emotion, and motor impulses in smokers. Clin Psychol Sci J Assoc Psychol Sci. (2014) 2:611. doi: 10.1177/2167702614522037
31. Bosak K, Martin L. Neuroimaging of goal-directed behavior in midlife women. Nurs Res. (2014) 63:388. doi: 10.1097/NNR.0000000000000060
32. Sullivan NJ. The Neurocomputational Basis of Self-Control Success and Failure. Dissertation, Ph.D, California Institute of Technology (2015).
33. Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. (2005) 9:242–9. doi: 10.1016/j.tics.2005.03.010
34. Carter C, Macdonald AM, Stenger V, Cohen J. Dissociating the contributions of DLPFC and anterior cingulate to executive control. An event-related fMRI study. Brain Cogn. (2001) 47:66–9. doi: 10.1126/science.288.5472.1835
35. Lim K, McNeil MR, Doyle PJ, Hula WD, Dickey MW. Conflict Resolution and Goal Maintenance Components of Executive Attention are Impaired in Persons With Aphasia: Evidence From the Picture-Word Interference Task (2012). Available online at: http://aphasiology.pitt.edu/id/eprint/2405.
36. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain (2013) 137:12–32. doi: 10.1093/brain/awt162
37. Charbonnier L, Van Der Laan LN, Viergever MA, Smeets PA. Functional MRI of challenging food choices: forced choice between equally liked high-and low-calorie foods in the absence of hunger. PLoS ONE (2015) 10:e0131727. doi: 10.1371/journal.pone.0131727
38. Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. (2008) 28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008
39. Hänggi J, Lohrey C, Drobetz R, Baetschmann H, Forstmeier S, Maercker A, et al. Strength of structural and functional frontostriatal connectivity predicts self-control in the healthy elderly. Front Aging Neurosci. (2016) 8:307. doi: 10.3389/fnagi.2016.00307
40. Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychol Sci. (2013) 24:2262–71. doi: 10.1177/0956797613492985
Keywords: self-control, food choice, fMRI, dlPFC, decision-making
Citation: Chen F, He Q, Han Y, Zhang Y and Gao X (2018) Increased BOLD Signals in dlPFC Is Associated With Stronger Self-Control in Food-Related Decision-Making. Front. Psychiatry 9:689. doi: 10.3389/fpsyt.2018.00689
Received: 30 April 2018; Accepted: 27 November 2018;
Published: 20 December 2018.
Edited by:
Roumen Kirov, Institute of Neurobiology (BAS), BulgariaReviewed by:
Fernando Fernandez-Aranda, Hospital Universitario de Bellvitge, SpainJin Li, Chinese Academy of Sciences, China
Copyright © 2018 Chen, He, Han, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Gao, gaoxiaox@swu.edu.cn
 Fuguo Chen
Fuguo Chen Qinghua He
Qinghua He Yan Han1
Yan Han1 Xiao Gao
Xiao Gao