- 1Barcelona Bipolar Disorders Program, Hospital Clinic, Institute of Neurosciences, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Spain
- 2IMPACT Strategic Research Centre, Barwon Health, Deakin University, Geelong, VIC, Australia
- 3Department of Psychiatry, University of Melbourne, Parkville, VIC, Australia
- 4Orygen, The National Centre of Excellence in Youth Mental Health, Melbourne, VIC, Australia
- 5Bipolar Disorders Unit, Department of Psychiatry, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile
- 6Laboratory of Molecular Psychiatry, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
- 7Postgraduate Program: Psychiatry and Behavioral Science, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
- 8Department of Pharmacology and Postgraduate Program: Pharmacology and Therapeutics, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
- 9Barcelona Clínic Schizophrenia Unit, Hospital Clinic de Barcelona, CIBERSAM, Barcelona, Spain
- 10Florey Institute for Neuroscience and Mental Health, Parkville, VIC, Australia
- 11Department of Psychiatry & Behavioral Neurosciences, Mcmaster University, Hamilton, ON, Canada
Personalized treatment is defined as choosing the “right treatment for the right person at the right time.” Although psychiatry has not yet reached this level of precision, we are on the way thanks to recent technological developments that may aid to detect plausible molecular and genetic markers. At the moment there are some models that are contributing to precision psychiatry through the concept of staging. While staging was initially presented as a way to categorize patients according to clinical presentation, course, and illness severity, current staging models integrate multiple levels of information that can help to define each patient's characteristics, severity, and prognosis in a more precise and individualized way. Moreover, staging might serve as the foundation to create a clinical decision-making algorithm on the basis of the patient's stage. In this review we will summarize the evolution of the bipolar disorder staging model in relation to the new discoveries on the neurobiology of bipolar disorder. Furthermore, we will discuss how the latest and future progress in psychiatry might transform current staging models into precision staging models.
Introduction
Bipolar disorder is a chronic psychiatric condition characterized by mood swings with both manic and depressive symptoms (1). Despite this general picture, bipolar disorder is a highly heterogeneous condition regarding clinical presentation, response to treatment and functional outcome (2, 3). Subsequent DSM and ICD versions have increasingly reflected this heterogeneity, for instance, by adding diagnosis and course specifiers (4). Still, the focus of current systems of classification remains largely cross-sectional and limited to clinical features (5). Moreover, these criteria apply to people with established disorder, but miss people in the prodromal phases of the illness (4).
Emerging data points to the need of a broader approach to bipolar disorder. There is increasing evidence that bipolar disorder is a neuroprogressive disorder, meaning that longer duration of the disease entails more pronounced changes at the clinical and neuropathological level, which may lead to treatment refractoriness and neuropsychological deficits (6, 7). Moreover, several studies support the notion of a prodromal stage before illness onset (8). In an attempt to introduce a longitudinal perspective of the illness in the diagnostic process which would include the earliest phases of bipolar disorder and guide treatment and prognosis, some authors have suggested incorporating the staging model in psychiatry (9–13).
The staging model is based on the concept that an illness progresses following an identifiable temporal progression, from at-risk or prodromal stages to chronic ones (10). Moreover, considering the neuroprogressive course of psychiatric disorders, the staging model assumes that treatment needs and response may differ according to stage. While early stages of the disease might show a better response to simpler treatment regimens, chronic stages might need more complex treatments and still show less clinical improvement (14). Consequently, defining the stage in which the patient is located may help clinicians to choose the treatment that is better adapted to the patient's needs (14). Additionally, the administration of a timely treatment precisely adapted to the stage in which the patient is located might modify or even prevent the progression to subsequent stages of the disease (10).
The staging model in bipolar disorder has been in constant development since its introduction in psychiatry. As new evidence on bipolar disorder has emerged, staging models were refined according to these new findings. In spite of this, experts supporting the staging model still warn that this model gives a standard vision of the progression of the disorder that might not suit every patient (15, 16).
New advances in the field of biological markers (e.g., molecular and neuroanatomical markers of illness vulnerability and/or progression), genetics (e.g., genetic markers or pharmacogenomics) or computer science (e.g., machine learning approaches) might provide current staging models of a higher level of precision regarding diagnosis, prognosis, and treatment choice (15, 16), allowing a more personalized approach to the patient.
The aim of this review is to summarize the evolution of the staging model in bipolar disorder in relation to the new discoveries on the course and neurobiology of the disease. Furthermore, we will discuss how the latest and ongoing progresses in psychiatry might transform current staging models into precision staging models.
The Evolution of Staging Models in Bipolar Disorder
The Dawn of Staging in Psychiatry: Fava and Kellner Staging Model (1993)
Fava and Kellner, in 1993, first proposed the application of the concept of staging to psychiatric disorders (9), as staging had shown to be useful in other complex diseases potentially severe if untreated, such as diabetes mellitus, cardiovascular diseases and neoplastic diseases. However, their staging model faced a major limitation in psychiatry research, which was the dearth of longitudinal studies assessing the progression of psychiatric disorders and the scarce data available on prodromal symptoms.
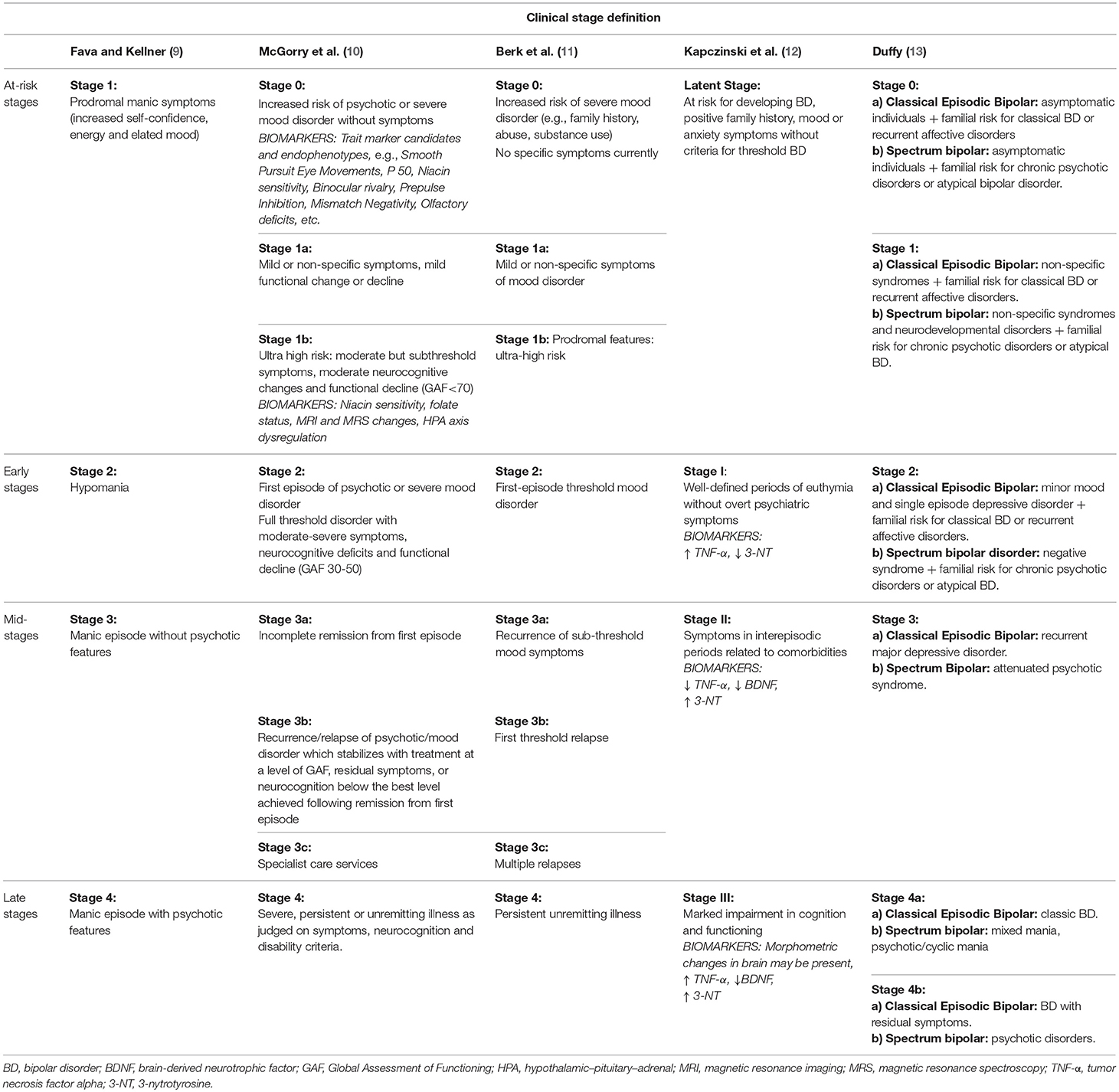
As a result, the staging model proposed by Fava and Kellner did not focus on the longitudinal course of bipolar disorder, but described the different stages that can be seen in a manic episode based on symptom severity (Table 1). Although their model referred only to the manic phase of the disease, Fava and Kellner provided the basis for future staging models in psychiatry.
The Spread of the Concept of Staging in Psychiatry: McGorry et al. (10)
In 2006, McGorry and colleagues introduced a staging model which highlighted the longitudinal course of psychiatric diseases in the psychotic spectrum, also integrating mood disorders (10). They underlined that the staging model does not imply that every patient needs to go through every stage. The main characteristic of McGorry and colleagues' model is that it is built on evidence on major psychiatric disorders jointly and not exclusively on data on bipolar disorder. Importantly, compiling evidence emerging from research on neurobiological correlates of psychotic disorders, allowed McGorry and colleagues to go one step forward and include some biological and endophenotypic markers in the earlier stages of their model (Table 1). They warned, though, that evidence on biological markers arose from studies that evaluated patients with long-established disease, raising the question whether these biological markers were inherent to psychiatric disorders or a consequence of illness duration.
They also incorporated some indicators of illness extent and progression -that is, functioning and cognitive impairment- in their staging model. They defended the importance of addressing social adaptation when assessing patients, as they noted that a person who already presents a great deal of collateral academic or social damage at illness onset may be less likely to respond to treatment and hence is more prone to have a worse prognosis. McGorry and colleagues have continued to progress a transdiagnostic staging model, arguing that the early stages are non-specific, although the later courses of different major psychiatric disorders can have divergent course and outcome patterns (17).
New Insights on Bipolar Disorder Progression: Berk et al. (11)
Although similar to and adapting from McGorry and colleagues' model (10), Berk and colleagues' model focused exclusively on bipolar disorder (11). At that moment, a growing body of evidence on a prodromal state for bipolar disorder started to appear (11). Besides identifying risk factors for bipolar disorder, mainly a positive family history of mood disorder and stressful life events (18–20), emerging studies on high-risk youth described a series of prodromal symptoms (21–23), therefore supporting the notion of a traceable at-risk stage.
Moreover, at that moment there was increasing evidence emerging from clinical, neuroimaging and neurocognitive studies that supported a progressive and deteriorating course of bipolar disorder (11). For instance, it had been reported that inter-episode periods were longer after the first episodes, but tended to shorten as the number of episodes increased (24). It had also been found that longer duration of the illness with multiple relapses seemed to be associated with increased medical comorbidities and increased suicidal risk (25). Furthermore, available evidence suggested that response to psychological and pharmacological treatments might not be the same over the illness course (26–29). Response to lithium, for example, seemed to be better if started early after illness onset (30, 31) and before multiple relapses had taken place (32). The number of episodes had also been found to be related to neuroanatomic changes in the brain (33). In 2002, Strakowski et al. (33) described increased lateral ventricular size in bipolar patients with multiple manic episodes, but not in first-episode patients. Likewise, evidence supported that a longer duration of illness and a larger number of episodes was associated with cognitive dysfunction which, in turn, seemed to involve a worse clinical course and functional disability (34). The authors hypothesized that all those alterations observed in the later stages of bipolar disorder were a consequence of progressive changes in the central nervous system due to subsequent mood episodes (6, 7). This phenomenon was called neuroprogression (6). Berk and colleagues suggested several possible pathways involved in neuroprogression including inflammation, oxidative stress, neurotrophins imbalance, mitochondrial dysfunction and epigenetics (6, 7).
Drawing all this evidence together, Berk and colleagues described a staging model with a special focus on the initial phases of the disease and number of episodes (Table 1).
The Ascendance of Biological Psychiatry: Kapczinski et al. (12)
Kapczinski and colleagues' model appeared at a moment when biological explanations gained prominence and risk phases were explained based on a gene-environmental (GxE) approach (12). For early stages, the GxE perspective suggested that individual genetic differences determine distinct resilience or vulnerability to environmental stress, placing individuals at different risk levels to develop bipolar disorder (35, 36). For late stages, this approach suggested that every individual has a different neuronal resilience to the deleterious effect of repetitive mood episodes (12). Along this line, Kapczinski et al. (37) adapted McEwens' notion of allostatic load to bipolar disorder (38). This concept implies that the interaction of neuroprogressive changes, somatic comorbidities and substance abuse leads to a dwindling resilience to life stress, especially if coping skills are poor (37). Hence, according to Post's kindling hypothesis (36), while stressful life events are an important trigger for first affective episodes, later on the course of the disease recurrences might take place without a clear environmental factor (37).
At that time, studies focusing on the pathophysiology of bipolar disorder reported a deregulation of oxidative and inflammatory pathways in bipolar disorder, especially during mood episodes (39–43), which came with a decrease in neurotrophic factors, like brain-derived neurotrophic factor (BDNF) (44–46). Importantly, it was also described that levels of neurotrophins, oxidative and inflammatory markers differed depending on illness stage (47, 48). For instance, compared to controls, the serum levels of the pro-inflammatory cytokine IL-6 were increased both in the early and late stages of bipolar disorder, while levels of BDNF and the anti-inflammatory cytokine IL-10 were decreased in late stages (meaning patients with 10–20 years of illness duration) but not in early stages (47). TNF-alpha levels appeared to be elevated throughout the illness course but were even higher in later stages (47). In addition, some parameters of oxidative stress, such as 3-nitrotyrosine, were found to be altered in the early and late stages of bipolar disorder, but not in controls (48). The activity of key enzymes in the glutathione pathway was found to be increased in late-stage patients compared with early-stage patients and controls (48). Hence, these data supported the hypothesis presented by Berk and colleagues indicating that neurotrophic, inflammatory and oxidative pathways may be involved in neuroprogression (6). Furthermore, neuroimaging findings also supported the concept of neuroprogression, as although some cerebral structures were shown to be already altered in early stages (49–51), longitudinal studies indicated that patients with repetitive mood episodes showed a progressive brain gray matter loss (52, 53). All these findings implied the identification of putative biomarkers that could be useful to distinguish between patients in early and late stages of bipolar disorder (54).
Psychosocial functioning was also gaining momentum as an outcome measure in bipolar disorder, since it had been demonstrated that symptomatic recovery is not equivalent to functional recovery (55). Psychosocial functioning involves domains such as work and education, leisure time, social and affective relationships or independent living (56), and it can be negatively affected by clinical variables and neurocognitive impairments (57).
Accordingly, Kapczinski and colleagues presented a model based on functioning that, moreover, incorporated cognition and biomarkers (12) (Table 1).
A Broader Vision of Bipolar Disorder: Duffy (13)
Duffy proposed a more integrative clinical staging model which described the natural history of bipolar disorder according to illness subtypes: the classical form of bipolar disorder (alternant manic-depressive episodes) vs. the broader bipolar spectrum (13). Duffy claimed that, while the classical form of bipolar disorder tended to follow the progressive course described in previous staging models (i.e., a recurrent and deteriorating course with an increasingly shorter inter-episodic period), other subtypes of bipolar disorder might present a different evolution (13). Evidence, for instance, supported that lithium non-responders showed a more chronic course and a higher-risk of non-affective disorders in family members (58, 59). Neuroimaging and genetic differences between classical lithium responsive bipolar patients and lithium non-responsive bipolar patients were also reported (60, 61). Moreover, her model was supported by longitudinal data showing differences between offspring of lithium responders and lithium non-responders regarding the prodromal period and longitudinal course of bipolar disorder. Offspring of lithium responders had a personal history of anxiety and sleep disorders before illness onset and, once bipolar disorder was established, tended to show an episodic remitting course with good response to lithium (62–64). In contrast, offspring of lithium non-responders manifested higher rates of early developmental alterations, attention deficits and cluster A personality traits (62–64) and, for those who developed bipolar disorder, illness course tended to be more torpid and response to anticonvulsant or atypical antipsychotic seemed to be better than to lithium (63). Thus, Duffy aimed to present an integrative staging model that describes the expected longitudinal course of classical episodic bipolar disorder and of bipolar spectrum disorder (Table 1).
When Staging is Not Enough
Although different staging models have been proposed in bipolar disorder over the last 25 years, they still need to be better operationalized and validated by empirical research (14). The idea behind the different staging models is to allow defining, for every individual, the extent of illness progression in the moment of the evaluation (65). This can help to refine diagnosis, adjust prognosis and choose the best treatment according to illness stage (66). In this regard, authors have suggested some treatment approaches adapted to every stage: most models agree that prodromal stages would benefit from interventions targeted toward reducing stressors and increasing coping skills; early stages would benefit from patient and family psychoeducation and simpler pharmacological regimens; while mid-stages would need more intensive psychotherapies and more complex pharmacotherapies (12, 15). Clozapine or functional remediation therapies would be reserved for more chronic stages (15). Some individuals with highly refractory illness may need more “palliative” approaches focusing on reduction of side-effects and unnecessary polypharmacy, limited symptom control, identifying and targeting psychological and social problems, and setting realistic goals to aim for the best quality of life for people and their families within the envelope of their disability (67).
However, even if the staging model proposes stage-targeted treatments that might provide a better clinical outcome with less side effects, there are still differences among the patients of a particular stage. In consequence, “standard stage-adapted treatments” may not be useful for every patient at a particular stage (15) and increasing the level of precision in every stage would be desirable in order to achieve an even more personalized way of approaching the patient (16) (Figure 1).
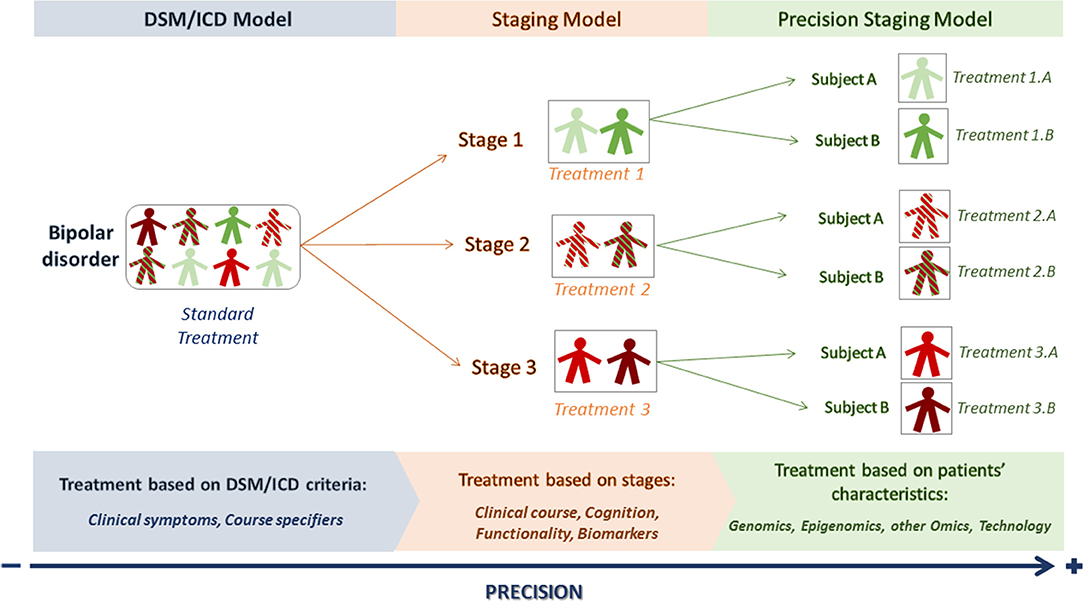
Figure 1. From DSM/ICD to precision staging models. The DSM/ICD model classifies patients into particular conditions according to clinical-based criteria. DSM/ICD diagnosis can be refined by course specifiers. The staging model allows placing the patient in a particular stage according to the extent of illness progression, starting from at-risk stages (stage 1) to more chronic ones (stage 3). However, there can still be differences between patients within a particular stage. A precision staging model would then use the new advances on precision medicine to better characterize the patients and offer them a more personalized treatment.
From Staging to Precision Staging Models
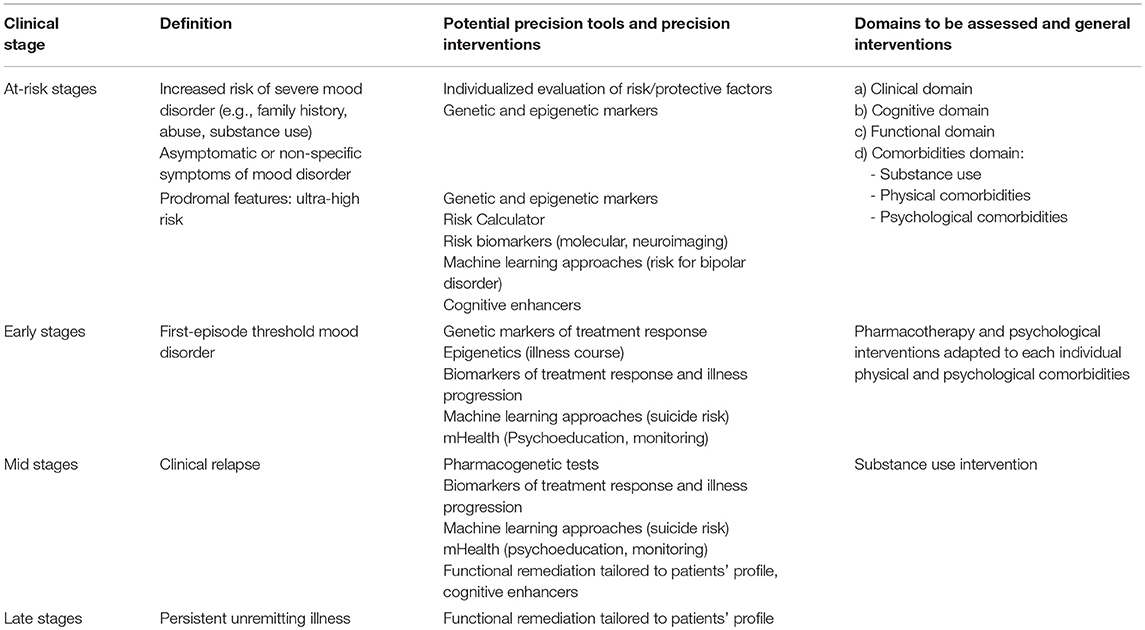
The aim of precision psychiatry is to offer the patient tailored medical decisions and treatments (68). For that purpose, precision psychiatry needs to integrate biographical, clinical and biological information regarding each individual (69). In addition, precision psychiatry is envisaged to benefit from the coming advances in technological, data, and computer science to aid diagnostic processes and treatment provision. A precision staging model would ideally incorporate all these recent progresses into the appropriate stage (Table 2).
Many advances in precision medicine are related to genomics. Genomics have led to improvements in staging models in some branches of medicine, especially cancer (70). However, psychiatric disorders are genetically complex conditions and their genetic underpinnings remain to be determined. Still, international consortia that comprise samples from several countries have brought some light on risk loci associated with bipolar disorder (71, 72). These collaborative genome-wide association studies (GWAS) allow overcoming replication difficulties often seen in genetic studies due to small sample sizes. One of these studies analyzed genomic data on a sample of 40,000 bipolar patients and replicated the discoveries of previous GWAS studies regarding several single-nucleotide polymorphisms (SNPs) statistically associated with the disease, including variants within the genes CACNA1C, ANK3, MAD1L1, and SYNE1 (73). Two new risk loci were also identified (73). Moreover, the Psychiatric Genomics Consortium has recently identified specific loci that distinguish between bipolar disorder and schizophrenia (74). Genetic markers promise to be valuable at the earliest stages of bipolar disorder, as the main aim of mental health approaches at at-risk and early stages is to predict disease vulnerability and make accurate diagnosis. So far, though, little of the advances in genomics have translated into clinically useful tools.
Besides genetic markers, screening for risk factors and epigenetic modifications may be another useful tool at at-risk stages of the disease, given that stressful life events, particularly childhood trauma, can alter DNA methylation and may increase the risk of developing mood disorders (75). Concordant with this, childhood verbal, physical, or sexual abuse has been related to a worse illness course (76).
Risk calculators are another promising tool for at-risk stages (77), as the multifactorial and polygenic nature of bipolar disorder makes it improbable that a single factor can accurately predict its onset (78). The Pittsburgh Bipolar Offspring Study group (BIOS) has recently developed a risk calculator to predict the 5-year risk of bipolar disorder onset in offspring of parents with bipolar disorder combining dimensional measures of mania, depression, anxiety, mood lability, psychosocial functioning, and parental age of mood disorder onset (79). Although their findings need to be replicated, the model seemed to be able to predict onset of bipolar disorder with an area under the curve (AUC) in the receiver operating characteristic curve analysis of 0.76 and might be of potential value for youth at ultra-high risk for BD. Machine learning, a field of computer science that studies and constructs algorithms that can learn from large number of data, find patterns and make predictions (80), might also be useful to estimate the individual probability of a particular outcome (80, 81). Mourao-Miranda et al. (82), for instance, found that machine learning approaches using functional magnetic resonance imaging (fMRI) data could differentiate between adolescents genetically at-risk for mood disorders and healthy controls with a 75% accuracy (sensitivity = 75%, specificity = 75%). Moreover, those at-risk adolescents who developed an anxiety or depressive disorder at follow-up showed significantly higher predictive probabilities, therefore suggesting that predictive probabilities could be used as a score to predict which at-risk adolescents would develop a mood disorder in the future (82).
Early and middle stages of bipolar disorder may benefit from progress in the field of pharmacogenomics. This is the study of genetic variations that affects individual response to drugs and vulnerability to adverse effects (83). After the first acute episode, selecting the best treatment for the patient, both in terms of efficacy and tolerability is a necessary but complex task. International consortiums in genetics, such as the International Consortium of Lithium Genetics (ConLiGen) (71), have worked to disentangle genetic variants associated with treatment response, mainly response to lithium. In 2016, the ConLiGen consortium uniformly phenotyped 2,563 bipolar patients and reported a genome-wide significant association with a locus of four linked SNPs on chromosome 21 and lithium response (84). Another recent GWAS performed by the ConLiGen consortium displayed that bipolar patients with a low genetic load for schizophrenia showed a better response to lithium (85). Pharmacogenetic screening for hepatic cytochrome P450 genetic polymorphisms can also be helpful in the near future to predict tolerability and side effects of psychiatric treatments (86). While the precise patient profile that would benefit from these tests remains to be elucidated, pharmacogenetic tests are kept for selected patients with unusual patterns of drug response or unexpected adverse reactions (83, 87).
Less progress has been made in the field of biological markers in the last few years and data on molecular and neuroimaging biomarkers is still contradictory and limited by the heterogeneity between studies and the poor specificity of the putative biomarkers (88). Although evidence is not yet compelling, some biological markers have been suggested to be associated with increased risk of conversion to bipolar disorder, and therefore may be useful when assessing subjects at at-risk stages. fMRI studies report that frontal hyperactivation during working memory paradigms may be associated with genetic risk for bipolar disorder (89, 90). In more established stages, neuroimaging might be useful to monitor treatment response (91). Also, a preliminary study using a voxel-based morphometry-pattern classification approach was able to distinguish between patients with unipolar and bipolar depression based on structural gray matter differences (92). Studies on biological markers have also suggested that peripheral concentrations of BDNF could be used to discriminate unipolar depression from bipolar depression (93, 94), but evidence is not clear (95). This would be of the utmost importance in the earliest stages of the disease, considering that bipolar disorder is often misdiagnosed since the index episode is frequently depressive. In consequence, patients are treated with antidepressants and the introduction of a mood stabilizer is delayed until the first manic episode is detected, which may negatively affect illness course and prognosis (8).
Regarding other molecular markers, hypothalamic-pituitary-adrenal (HPA) axis dysfunction is thought to be one of the pathways involved in neuroprogression in bipolar disorder (96), but it has also been suggested to be a useful trait marker in high-risk individuals (97, 98). Alterations in neurotransmitters transporters have been suggested as markers of bipolar disorder (96), but there is no evidence on changes in neurotransmitters according to illness stage. Regarding later stages of bipolar disorder, recent studies have reported higher levels of TNF-alpha and IL-6 in late stages of bipolar disorder (99, 100). Similarly, Soeiro-de Souza and colleagues described that patients with recurrent episodes showed increased oxidative and inflammatory markers, which were related to the number of manic episodes (101). Further, increased inflammation, increased oxidative stress and reduced telomere length have been suggested as possible mechanistic links between psychiatric diseases like bipolar disorder and other systemic diseases, such as endocrine or cardiovascular diseases (102–105). Hence, the identification of a deregulation on those pathways related to both psychiatric and somatic diseases may have therapeutic implications (106). For instance, bipolar patients exhibiting persistently increased low-grade inflammation (107) might benefit from anti-inflammatory treatment strategies and from periodic screening of systemic conditions like metabolic syndrome (106). Considering these data, screening for physical comorbidities seems especially important in middle and late stages of the disease, albeit protecting against complications like physical comorbidities or substance abuse should be a priority at every stage of the disease.
Cognition is another important domain that needs an individualized evaluation throughout all the stages of bipolar disorder (108). On one hand, cognitive reserve, defined as the ability of a brain to cope with brain pathology in order to minimize symptoms (109), may be useful in early stages to predict neurocognitive performance in patients with bipolar disorder (110), as it has been found that lower estimated cognitive reserve is associated with worse performance in neuropsychological tests and more functional impairment (110, 111). Similarly, a recent study on first-episode psychosis has found that those patients with affective psychosis with a greater cognitive reserve showed a higher socioeconomic status, better functioning and greater verbal memory performance (112). This study also emphasizes the need to explore the impact of specific interventions, like physical activities and hobbies, on cognitive reserve, since it could be useful to guide the development of personalized treatment programs (112). Therefore, cognitive enhancing strategies might be key in the early stages and not necessarily in the late stages of the disease. On the other hand, evidence points to a heterogeneous cognitive profile in bipolar patients both in “cold” and “hot” cognition (113–115). The presence of such heterogeneous cognitive profiles among patients with bipolar disorder might be taken into account to design more tailored cognitive remediation therapies adapted to each individual needs (116–118). Cognitive deficits may also limit long-term psychosocial functioning, which means that patients with greater cognitive impairment are more likely to experience poorer outcomes. Previously, we showed that patients in stage I and healthy controls had similar functioning patterns. In addition, a strong linear association was found between functioning and clinical stages, suggesting a progressive functional decline from stage I through to stage IV of bipolar disorder. These findings provide further support to the clinical staging model in bipolar disorder, indicating that bipolar patients lie on a continuum of disorder progression ranging from periods of favorable functioning to others of incomplete functional recovery (118). The link between variables related to the course of the illness, cognitive deficits and functioning suggests that early intervention is crucial to prevent illness progression and to improve cognitive/functional outcome. Some studies have also found different profiles of psychosocial functioning in patients with bipolar disorder, which should also be taken into consideration in the framework of a personalized approach (3, 119).
All these advances should complement regular clinical practice, which already contains elements of staging and precision psychiatry. The assessment of the patient's particular symptoms, such as his/her distinctive early signs of relapse, predominant polarity (i.e., the “tendency” to present more depressive or manic relapses) (120) or individual suicide risk (121) is regularly done in clinical settings and is essential to monitor the patient evolution and guide treatment selection. Technological advances used in everyday life, encompassed in the concept of mobile Health (mHealth), might be a valuable tool to help clinicians to collect individualized data on illness course and monitor illness progression (122). For instance, changes in activity, geolocation or sleep patterns may help to detect early signs of mood relapse (123, 124). Additionally, smartphone apps can be used to empower patients with bipolar disorder to detect prodromal symptoms of relapse by providing them personalized psychoeducational messages (125, 126). New methods like machine learning approaches might also be useful in the future to help predict suicide risk (127, 128).
Discussion
In this review we describe the evolution of the staging model in bipolar disorder since its introduction into psychiatry. The first staging models in bipolar disorder were initially based on evidence derived from cross-sectional studies, but longitudinal studies and data on neuroimaging, peripheral biomarkers, cognition, psychosocial functioning, and prodromal symptoms have successively enriched the staging models (66). We have also described several elements of precision psychiatry that could be incorporated in future precision staging models.
The main advantage of staging and precision medicine is the recognition that a reductionist clinical approach based on the presence or absence of a series of symptoms is not enough to design an adequate therapeutic strategy. These symptoms need to be considered in the light of the illness progression and, most importantly, of the patient's own clinical evolution. For instance, the presence of a switching or non-switching pattern should be considered when evaluating a patient, as it has prognostic implications and therefore might impact staging. As highlighted in a review by Salvadore et al. (129), patients showing a switching pattern [i.e., patients showing a “sudden transition from a mood episode to another episode of the opposite polarity” (129)] usually spend less time in remission, show higher comorbidity rates and substance abuse and are at a higher risk of suicide attempt (129). While mood symptoms will of course still be the cornerstone of bipolar disorder diagnosis, other elements should be likewise considered as they can be as informative as clinical symptoms (9, 10, 12, 15). As such, everyday difficulties, cognitive complains, substance abuse or comorbidities can be markers of illness severity or stage specifiers and merit an individualized assessment and treatment. Social and personal losses due to the illness and previous personality should also be included in a standard evaluation throughout the stages and be given the attention they deserve (10). Patients' insight and perception of the disease should be carefully assessed, as these are important prognosis and therapeutic factors, especially in early stages (8). Medication load, treatment satisfaction, and compliance should be also carefully assessed, as it might influence disease progression. While this way of approaching the patient is naturally adopted by most clinicians and many guidelines, it remains underrepresented in diagnostic manuals (5). In any case, this approach is more in line with the World Health Organization definition of health: “Health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”1. The inclusion of self-report measures of well-being in research and clinical care in bipolar disorder may contribute to take into consideration the patient's perspective when assessing the efficacy and usefulness of pharmacological and psychological interventions (130).
Another important point of clinical staging is the assumption that prodromal phases of the disease can be also identified and targeted. The possibility of making an early diagnosis radically changes the way how bipolar disorder in particular, and psychiatric diseases in general, have hitherto been managed. At-risk stages are rather non-specific, though. In consequence, the prodromal period has also been preferably defined as “at risk mental states” (17), as a prodrome is defined as “any symptom that signals the impending onset of a disease” (131) and evidence does not support this definition. On one hand, data from the field of ultra-high risk in psychosis shows that disease onset is not deterministic and a significant proportion of the at-risk youth show a remission of these early symptoms (132). On the other hand, these early symptoms are not specific to any disease but can progress into several possible psychiatric conditions (64). In the absence of specific genetic markers for bipolar disorder or very precise risk calculators, transdiagnostic preventive interventions aimed to reduce stress, educate on mental-well-being and prevent substance abuse are preferable at these at-risk stages (133). Implementing early interventions that include enhancing cognitive reserve by increasing mental stimulation (reading and cognitive exercises), introducing physical exercise and leisure activities or building social skills and social interaction, may provide a set of skills that can help to cope better with the disease (134–136). This kind of preventive interventions or “positive habits” could even be implemented at school or primary care, which could help to reduce stigma on mental health by educating the population on the importance of taking care of mental well-being (133).
In this regard, it has been suggested that a transdiagnostic staging model might be more adequate for the study of at-risk phases, while disorder-specific models are more useful once the fully-develop disorder emerges (15). It is necessary to bear in mind that psychiatric disorders are dynamic and clinical symptoms may evolve over time, requiring a change in diagnosis (137). Nevertheless, the general staging approach supported by stage specifiers should still be useful to assess illness severity regardless of changes in DSM or ICD diagnosis.
Biomarkers also face the problem of lack of specificity. Alterations in the inflammatory or oxidative systems have been found across several psychiatric and medical diagnoses (138). Again, biomarkers could be more stage-specific than illness-specific and be conceived as an additional tool for the assessment of illness risk or treatment outcome. Low sensitivity and replicability seems a bigger handicap. Moreover, most published data on biomarkers are based on the currently commercially available ELISA kits, which is also a limiting factor. State-of-the-art techniques widely used in precision medicine might help to overcome these limitations. A multi-omic approach, meaning using genomic, epigenomics, transcriptomics, proteomics, metabolomics, metagenomics, and lipidomics data, combined with environmental information gathered, for instance, through mobile devices, could help to identify more sensitive biomarkers panels to guide diagnosis and treatment choice (69). However, as these “omic” platforms cannot be used in regular clinical practice, the potential discoveries arising from these platforms need to be translated into an immuno-based assay, which is a more viable option. New strategies with a more integrative approach between clinical factors and biological markers are being proposed in biomarker research of lithium response, which are expected to shed some light on precision drug prescription (139).
Precision in psychiatry implies embracing the multifactoriality of psychiatric diseases and the need to incorporate in the patient's assessment a range of biological and environmental factors that interact with each other in a dynamic way. Moreover, the biological and environmental factors involved in illness onset and progression are particular to every patient, as it is the way they interact (17). The use of personal devices to monitor the trajectories of patients at anytime and anywhere might help to deepen our knowledge on the complex interaction between biological and environmental factors. They can also allow evaluating less studied markers, such as sleep or chronobiological markers, which may turn out to be very informative (140). Moreover, further studies on epigenetics or mitochondrial genomes might identify novel factors involved in this complex disease (141). Similar to what is being developed in the field of psychosis, research on bipolar disorder could benefit from consortia sharing data to develop machine learning algorithms to help the prediction of bipolar disorder onset (17, 142).
A major limitation of current staging models is the absence of an agreement on the definition of stages. Moreover, operationalized cut-off points are lacking, probably due to the lack of longitudinal studies assessing patients according to stages, the absence of clear and reproducible neurobiological markers defining every stage and the intrinsic heterogeneity of psychiatric illnesses (15, 16, 143). Therefore, the current proposed models of staging are mainly theoretical and need to be validated for the moment. Additionally, participants of the available studies assessing differences between early and late stages of bipolar disorder include subjects attending specialized clinics, hence probably representing more severe forms of bipolar disorder (16). Moreover, it should be noted that precision medicine is still in its early beginnings, meaning that findings on genomics, genetic markers, and epigenetics are preliminary and need to be replicated before being integrated in any model of classification.
Until more solid information is available on the biology of the disease, though, the staging models can be based on pragmatic variables, like number of episodes and impact on cognition and functionality. A staging system based on characteristics that can be easily measured allows to standardize it and make it available and applicable in a broader number of clinical settings and countries worldwide (144).
Regardless of what the future brings, personalized medicine means “patient-centered care,” therefore the choice among those new diagnostic techniques or treatments should be subject to a consensus between the clinician and the patient, especially considering the new ethical challenges that precision psychiatry brings with it (145). While psychiatrists can offer their expertise, patients opinions and preferences should play a central role in treatment decisions through shared decision-making (145).
Author Contributions
ES was responsible for conception and design as well as initial drafting of the manuscript. All other authors (SD, AA, AR, SA, JP, MR, MB, FK, EV, and IG) were responsible for revising the manuscript critically for important intellectual content of the version of the manuscript to be published. All authors read and approved the final manuscript.
Conflict of Interest Statement
SD has received grants and/or research support from Stanley Medical Research Foundation, Foundation FondaMental, Eli Lilly, GlaxoSmithKline, Organon, Mayne Pharma, and Servier. He has received speaker's fees from Eli Lilly, advisory board fees from Eli Lilly and Novartis and conference travel support from Servier. MB has received grant/research support from the NIH, Cooperative Research Center, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, MBF, NHMRC, Beyond Blue, Rotary Health, Geelong Medical Research Foundation, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Meat and Livestock Board, Organon, Novartis, Mayne Pharma, Servier, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck, Merck, Pfizer, Sanofi Synthelabo, Servier, Solvay and Wyeth, and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Eli Lilly, Grunbiotics, Glaxo SmithKline, Janssen Cilag, LivaNova, Lundbeck, Merck, Mylan, Otsuka, Pfizer, and Servier; FK has received support as a speaker from Janssen and Daiichi-Sankyo in the past 2 years; EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Allergan, Angelini, AstraZeneca, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, the Brain and Behavior Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute; IG has received speaker's fees from Ferrer, Janssen Cilag, and Lundbeck, advisory board fees from Ferrer, Lundbeck, Otsuka, and conference travel support from Lundbeck, Otsuka.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AR would like to thank the support of the CNPq PQ Process 305705-2015-9. MB is supported by a NHMRC Senior Principal Research Fellowship (1059660). EV is grateful for the support received from the Instituto de Salud Carlos III, Ministry of Economy and Competitiveness of Spain (PI 12/00912), integrated into the Plan Nacional de I+D+I and cofunded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER); Centro para la Investigación Biomédica en Red de Salud Mental (CIBERSAM), Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2014_SGR_398), Seventh European Framework Programme (ENBREC), and the Stanley Medical Research Institute. IG is supported by the Instituto de Salud Carlos III, Ministry of Economy, and Competitiveness of Spain [Juan Rodés Contract (JR15/00012) and a grant (PI16/00187)] integrated into the Plan Nacional de I+D+I and cofunded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER).
Footnotes
References
1. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder.Lancet (2016) 387:1561–72. doi: 10.1016/S0140-6736(15)00241-X
2. Solomon DA, Leon AC, Coryell WH, Endicott J, Li C, Fiedorowicz JG, et al. Longitudinal course of bipolar I disorder: duration of mood episodes.Archi Gene Psychiatry (2010) 67:339–47. doi: 10.1001/archgenpsychiatry.2010.15
3. Sole B, Bonnin CM, Jimenez E, Torrent C, Torres I, Varo C, et al. Heterogeneity of functional outcomes in patients with bipolar disorder: a cluster-analytic approach.Acta Psychiatr Scand. (2018) 137:516–27. doi: 10.1111/acps.12871
4. de Dios C, Goikolea JM, Colom F, Moreno C, Vieta E. Bipolar disorders in the new DSM-5 and ICD-11 classifications.Revista de Psiquiatria y Salud Mental (2014) 7:179–85. doi: 10.1016/j.rpsm.2014.07.005
6. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder.Int J Neuropsychopharmacol. (2009) 12:441–5. doi: 10.1017/S1461145708009498
7. Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors.Neuroscience Biobehav Rev. (2011) 35:804–17. doi: 10.1016/j.neubiorev.2010.10.001
8. Vieta E, Salagre E, Grande I, Carvalho AF, Fernandes BS, Berk M, et al. Early intervention in bipolar disorder.Am J Psychiatry (2018) 175:411–426. doi: 10.1176/appi.ajp.2017.17090972
9. Fava GA, Kellner R. Staging: a neglected dimension in psychiatric classification.Acta Psychiatrica Scand. (1993) 87:225–30. doi: 10.1111/j.1600-0447.1993.tb03362.x
10. McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions.Australian N Zeal J Psychiatry (2006) 40:616–22. doi: 10.1080/j.1440-1614.2006.01860.x
11. Berk M, Conus P, Lucas N, Hallam K, Malhi GS, Dodd S, et al. Setting the stage: from prodrome to treatment resistance in bipolar disorder.Bipolar Disord. (2007) 9:671–8. doi: 10.1111/j.1399-5618.2007.00484.x
12. Kapczinski F, Dias VV, Kauer-Sant'Anna M, Frey BN, Grassi-Oliveira R, Colom F, et al. Clinical implications of a staging model for bipolar disorders.Expert Rev Neurotherapeut. (2009) 9:957–66. doi: 10.1586/ern.09.31
13. Duffy A. Toward a comprehensive clinical staging model for bipolar disorder: integrating the evidence.Can J Psychiatry (2014) 59:659–66. doi: 10.1177/070674371405901208
14. Vieta E, Reinares M, Rosa AR. Staging bipolar disorder.Neurotoxicity Res. (2011) 19:279–85. doi: 10.1007/s12640-010-9197-8
15. Berk M, Post R, Ratheesh A, Gliddon E, Singh A, Vieta E, et al. Staging in bipolar disorder: from theoretical framework to clinical utility.World Psychiatry (2017) 16:236–44. doi: 10.1002/wps.20441
16. Fernandes BS, Berk M. Staging in bipolar disorder: one step closer to precision psychiatry.Revista Brasileira de Psiquiatria (2017) 39:88–9. doi: 10.1590/1516-4446-2017-3902
17. McGorry PD, Hartmann JA, Spooner R, Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry.World Psychiatry (2018) 17:133–42. doi: 10.1002/wps.20514
18. Hillegers MH, Burger H, Wals M, Reichart CG, Verhulst FC, Nolen WA, et al. Impact of stressful life events, familial loading and their interaction on the onset of mood disorders: study in a high-risk cohort of adolescent offspring of parents with bipolar disorder.Br J Psychiatry (2004) 185:97–101. doi: 10.1192/bjp.185.2.97
19. Kessing LV, Agerbo E, Mortensen PB. Major stressful life events and other risk factors for first admission with mania.Bipolar Disord. (2004) 6:122–9. doi: 10.1111/j.1399-5618.2004.00102.x
20. McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression.Archi Gener Psychiatry (2003) 60:497–502. doi: 10.1001/archpsyc.60.5.497
21. Thompson KN, Conus PO, Ward JL, Phillips LJ, Koutsogiannis J, Leicester S, et al. The initial prodrome to bipolar affective disorder: prospective case studies.J Affect Disord. (2003) 77:79–85. doi: 10.1016/S0165-0327(02)00100-3
22. Egeland JA, Shaw JA, Endicott J, Pauls DL, Allen CR, Hostetter AM, et al. Prospective study of prodromal features for bipolarity in well Amish children.J Am Acad Child Adoles Psychiatry (2003) 42:786–96. doi: 10.1097/01.CHI.0000046878.27264.12
23. Shaw JA, Egeland JA, Endicott J, Allen CR, Hostetter AM. A 10-year prospective study of prodromal patterns for bipolar disorder among Amish youth.J Am Acad Child Adoles Psychiatry (2005) 44:1104–11. doi: 10.1097/01.chi.0000177052.26476.e5
24. Kessing LV, Mortensen PB, Bolwig TG. Clinical definitions of sensitisation in affective disorder: a case register study of prevalence and prediction.J Affect Disord. (1998) 47:31–9. doi: 10.1016/S0165-0327(97)00081-5
25. Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years.J Affect Disord. (2002) 68:167–81. doi: 10.1016/S0165-0327(01)00377-9
26. Berk M, Brnabic A, Dodd S, Kelin K, Tohen M, Malhi GS, et al. Does stage of illness impact treatment response in bipolar disorder? empirical treatment data and their implication for the staging model and early intervention.Bipolar Disord. (2011) 13:87–98. doi: 10.1111/j.1399-5618.2011.00889.x
27. Scott J, Paykel E, Morriss R, Bentall R, Kinderman P, Johnson T, et al. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial.Br J Psychiatry (2006) 188:313–20. doi: 10.1192/bjp.188.4.313
28. Colom F, Reinares M, Pacchiarotti I, Popovic D, Mazzarini L, Martinez-Aran A, et al. Has number of previous episodes any effect on response to group psychoeducation in bipolar patients? A 5-year follow-up post hoc analysis.Acta Neuropsychiatrica (2010) 22:50–3. doi: 10.1111/j.1601-5215.2010.00450.x
29. Reinares M, Colom F, Rosa AR, Bonnin CM, Franco C, Sole B, et al. The impact of staging bipolar disorder on treatment outcome of family psychoeducation.J Affect Disord. (2010) 123:81–6. doi: 10.1016/j.jad.2009.09.009
30. Franchini L, Zanardi R, Smeraldi E, Gasperini M. Early onset of lithium prophylaxis as a predictor of good long-term outcome.Eur Arch Psychiatry Clin Neurosci. (1999) 249:227–30. doi: 10.1007/s004060050091
31. Berk M, Daglas R, Dandash O, Yucel M, Henry L, Hallam K, et al. Quetiapine v. lithium in the maintenance phase following a first episode of mania: randomised controlled trial.Br J Psychiatry (2017) 210:413–21. doi: 10.1192/bjp.bp.116.186833
32. Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD. Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania.Am J Psychiatry (1999) 156:1264–6.
33. Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder.Am J Psychiatry (2002) 159:1841–7. doi: 10.1176/appi.ajp.159.11.1841
34. Martinez-Aran A, Vieta E, Colom F, Torrent C, Reinares M, Goikolea JM, et al. Do cognitive complaints in euthymic bipolar patients reflect objective cognitive impairment?Psychother Psychosomat. (2005) 74:295–302. doi: 10.1159/000086320
35. Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience.Nat Rev Neurosci. (2006) 7:583–90. doi: 10.1038/nrn1925
36. Post RM. Kindling and sensitization as models for affective episode recurrence, cyclicity, and tolerance phenomena.Neurosci Biobehav Rev. (2007) 31:858–73. doi: 10.1016/j.neubiorev.2007.04.003
37. Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment.Neurosci Biobehav Rev. (2008) 32:675–92. doi: 10.1016/j.neubiorev.2007.10.005
38. McEwen BS. Stress, adaptation, and disease. allostasis and allostatic load.Ann N York Acad Sci. (1998) 840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x
39. Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LM, Nardin P, et al. Serum S100B and antioxidant enzymes in bipolar patients.J Psychiatric Res. (2007) 41:523–9. doi: 10.1016/j.jpsychires.2006.07.013
40. O'Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients.J Affect Disord. (2006) 90:263–7. doi: 10.1016/j.jad.2005.11.015
41. Gergerlioglu HS, Savas HA, Bulbul F, Selek S, Uz E, Yumru M. Changes in nitric oxide level and superoxide dismutase activity during antimanic treatment.Progress Neuro-Psychopharmacol Biol Psychiatry (2007) 31:697–702. doi: 10.1016/j.pnpbp.2006.12.020
42. Ortiz-Dominguez A, Hernandez ME, Berlanga C, Gutierrez-Mora D, Moreno J, Heinze G, et al. Immune variations in bipolar disorder: phasic differences.Bipolar Disord. (2007) 9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x
43. Brietzke E, Stertz L, Fernandes BS, Kauer-Sant'anna M, Mascarenhas M, Escosteguy Vargas A, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder.J Affect Disord. (2009) 116:214–7. doi: 10.1016/j.jad.2008.12.001
44. Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Goncalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes.Neurosci Lett. (2006) 398:215–9. doi: 10.1016/j.neulet.2005.12.085
45. Machado-Vieira R, Dietrich MO, Leke R, Cereser VH, Zanatto V, Kapczinski F, et al. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode.Biol Psychiatry (2007) 61:142–4. doi: 10.1016/j.biopsych.2006.03.070
46. Rosa AR, Frey BN, Andreazza AC, Cereser KM, Cunha AB, Quevedo J, et al. Increased serum glial cell line-derived neurotrophic factor immunocontent during manic and depressive episodes in individuals with bipolar disorder.Neurosci Lett. (2006) 407:146–50. doi: 10.1016/j.neulet.2006.08.026
47. Kauer-Sant'Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder.Int J Neuropsychopharmacol. (2009) 12:447–58. doi: 10.1017/S1461145708009310
48. Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder.J Psychiatry Neurosci. (2009) 34:263–71.
49. Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder.Biol Psychiatry (2005) 58:713–23. doi: 10.1016/j.biopsych.2005.04.033
50. Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis.Arch Gener Psychiatry (2008) 65:746–60. doi: 10.1001/archpsyc.65.7.746
51. Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter.Biol Psychiatry (2007) 61:743–9. doi: 10.1016/j.biopsych.2006.07.035
52. Moorhead TW, McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, et al. Progressive gray matter loss in patients with bipolar disorder.Biol Psychiatry (2007) 62:894–900. doi: 10.1016/j.biopsych.2007.03.005
53. Frey BN, Zunta-Soares GB, Caetano SC, Nicoletti MA, Hatch JP, Brambilla P, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration?Eur Neuropsychopharmacol. (2008) 18:717–22. doi: 10.1016/j.euroneuro.2008.04.015
54. Kapczinski F, Dias VV, Kauer-Sant'Anna M, Brietzke E, Vazquez GH, Vieta E, et al. The potential use of biomarkers as an adjunctive tool for staging bipolar disorder.Progress Neuro-psychopharmacol Biol Psychiatry (2009) 33:1366–71. doi: 10.1016/j.pnpbp.2009.07.027
55. Rosa AR, Reinares M, Michalak EE, Bonnin CM, Sole B, Franco C, et al. Functional impairment and disability across mood states in bipolar disorder.Value Health (2010) 13:984–8. doi: 10.1111/j.1524-4733.2010.00768.x
56. Rosa AR, Sanchez-Moreno J, Martinez-Aran A, Salamero M, Torrent C, Reinares M, et al. Validity and reliability of the functioning assessment short test (FAST) in bipolar disorder.Clin Pract Epidemiol Mental Health (2007) 3:5. doi: 10.1186/1745-0179-3-5
57. Bonnin CM, Martinez-Aran A, Torrent C, Pacchiarotti I, Rosa AR, Franco C, et al. Clinical and neurocognitive predictors of functional outcome in bipolar euthymic patients: a long-term, follow-up study.J Affect Disord. (2010) 121:156–60. doi: 10.1016/j.jad.2009.05.014
58. Grof P, Alda M, Grof E, Zvolsky P, Walsh M. Lithium response and genetics of affective disorders.J Affect Disord. (1994) 32:85–95. doi: 10.1016/0165-0327(94)90066-3
59. Passmore MJ, Garnham J, Duffy A, MacDougall M, Munro A, Slaney C, et al. Phenotypic spectra of bipolar disorder in responders to lithium versus lamotrigine.Bipolar Disord. (2003) 5:110–4. doi: 10.1034/j.1399-5618.2003.00026.x
60. Kruger S, Alda M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings.Am J Psychiatry (2006) 163:257–64. doi: 10.1176/appi.ajp.163.2.257
61. Turecki G, Grof P, Grof E, D'Souza V, Lebuis L, Marineau C, et al. Mapping susceptibility genes for bipolar disorder: a pharmacogenetic approach based on excellent response to lithium.Mol Psychiatry (2001) 6:570–8. doi: 10.1038/sj.mp.4000888
62. Duffy A, Alda M, Crawford L, Milin R, Grof P. The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents.Bipolar Disord. (2007) 9:828–38. doi: 10.1111/j.1399-5618.2007.00421.x
63. Duffy A, Alda M, Milin R, Grof P. A consecutive series of treated affected offspring of parents with bipolar disorder: is response associated with the clinical profile?Can J Psychiatry. (2007) 52:369–76. doi: 10.1177/070674370705200606
64. Duffy A, Horrocks J, Doucette S, Keown-Stoneman C, McCloskey S, Grof P. The developmental trajectory of bipolar disorder.Br J Psychiatry (2014) 204:122–8. doi: 10.1192/bjp.bp.113.126706
65. Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, et al. Stage managing bipolar disorder.Bipolar Disord. (2014) 16:471–7. doi: 10.1111/bdi.12099
66. Kapczinski F, Magalhaes PV, Balanza-Martinez V, Dias VV, Frangou S, Gama CS, et al. Staging systems in bipolar disorder: an International Society for Bipolar Disorders Task Force Report.Acta Psychiatrica Scand. (2014) 130:354–63. doi: 10.1111/acps.12305
67. Berk M, Berk L, Udina M, Moylan S, Stafford L, Hallam K, et al. Palliative models of care for later stages of mental disorder: maximizing recovery, maintaining hope, and building morale.Australian N Zeal J Psychiatry (2012) 46:92–9. doi: 10.1177/0004867411432072
68. Vieta E. Personalised medicine applied to mental health: Precision psychiatry.Revista de Psiquiatria y Salud Mental (2015) 8:117–8. doi: 10.1016/j.rpsm.2015.03.003
69. Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of 'precision psychiatry'.BMC Med. (2017) 15:80. doi: 10.1186/s12916-017-0849-x
70. Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine.Nat Rev Clin Oncol. (2018) 15:353–65. doi: 10.1038/s41571-018-0002-6
71. Schulze TG, Alda M, Adli M, Akula N, Ardau R, Bui ET, et al. The International Consortium on Lithium Genetics (ConLiGen): an initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment.Neuropsychobiology (2010) 62:72–8. doi: 10.1159/000314708
72. Patrick F., Sullivan Arpana Agrawal Cynthia M., Bulik Ole A., Andreassen Anders D., Børglum Gerome Breen, et al. Psychiatric genomics: an update and an agenda.Am J Psychiatry (2017) 175:15–27. doi: 10.1176/appi.ajp.2017.17030283
73. Hou L, Bergen SE, Akula N, Song J, Hultman CM, Landen M, et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder.Hum Mol Genet. (2016) 25:3383–94. doi: 10.1093/hmg/ddw181
74. Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes.Cell (2018) 173:1705–15.e16. doi: 10.1016/j.cell.2018.05.046
75. Fries GR, Li Q, McAlpin B, Rein T, Walss-Bass C, Soares JC, et al. The role of DNA methylation in the pathophysiology and treatment of bipolar disorder.Neurosci Biobehav Rev. (2016) 68:474–88. doi: 10.1016/j.neubiorev.2016.06.010
76. Post RM, Altshuler LL, Kupka R, McElroy SL, Frye MA, Rowe M, et al. Verbal abuse, like physical and sexual abuse, in childhood is associated with an earlier onset and more difficult course of bipolar disorder.Bipolar Disord. (2015) 17:323–30. doi: 10.1111/bdi.12268
77. Dipnall JF, Pasco JA, Berk M, Williams LJ, Dodd S, Jacka FN, et al. Getting RID of the blues: Formulating a Risk Index for Depression (RID) using structural equation modeling.Australian N Zeal J Psychiatry (2017) 51:1121–33. doi: 10.1177/0004867417726860
78. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders.Nat Rev Dis Primers (2018) 4:18008. doi: 10.1038/nrdp.2018.8
79. Hafeman DM, Merranko J, Goldstein TR, Axelson D, Goldstein BI, Monk K, et al. Assessment of a person-level risk calculator to predict new-onset bipolar spectrum disorder in youth at familial risk.JAMA Psychiatry (2017) 74:841–7. doi: 10.1001/jamapsychiatry.2017.1763
80. Bzdok D, Altman N, Krzywinski M. Statistics versus machine learning.Nat Methods (2018) 15:233. doi: 10.1038/nmeth.4642
81. Luo W, Phung D, Tran T, Gupta S, Rana S, Karmakar C, et al. Guidelines for developing and reporting machine learning predictive models in biomedical research: a multidisciplinary view.J Med Internet Res. (2016) 18:e323. doi: 10.2196/jmir.5870
82. Mourao-Miranda J, Oliveira L, Ladouceur CD, Marquand A, Brammer M, Birmaher B, et al. Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents.PLoS ONE (2012) 7:e29482. doi: 10.1371/journal.pone.0029482
83. Foulds JA, Maggo SD, Kennedy MA. Personalised prescribing in psychiatry: has pharmacogenomics delivered on its promise?Aust N Z J Psychiatry (2016) 50:509–10. doi: 10.1177/0004867416640099
84. Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study.Lancet (2016) 387:1085–93. doi: 10.1016/S0140-6736(16)00143-4
85. Amare AT, Schubert KO, Hou L, Clark SR, Papiol S, Heilbronner U, et al. Association of polygenic score for schizophrenia and hla antigen and inflammation genes with response to lithium in bipolar affective disorder: a genome-wide association study.JAMA Psychiatry (2018) 75:65–74. doi: 10.1001/jamapsychiatry.2017.3433
86. Singh AB, Baune BT, Hamilton A, Das P, Outhred T, Morris G, et al. Psychotropic pharmacogenetics - Distraction or destiny?Aust N Z J Psychiatry (2017) 51:665–7. doi: 10.1177/0004867417715687
87. Perez V, Salavert A, Espadaler J, Tuson M, Saiz-Ruiz J, Saez-Navarro C, et al. Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double-blind clinical trial.BMC Psychiatry (2017) 17:250. doi: 10.1186/s12888-017-1412-1
88. Castano-Ramirez OM, Sepulveda-Arias JC, Duica K, Diaz Zuluaga AM, Vargas C, Lopez-Jaramillo C. Inflammatory markers in the staging of bipolar disorder: a systematic review of the literature.Rev Colomb Psiquiatr. (2018) 47:119–28. doi: 10.1016/j.rcp.2017.01.004
89. Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall MH, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task.Biol Psychiatry (2008) 64:513–20. doi: 10.1016/j.biopsych.2008.04.038
90. Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm.Dev Cogn Neurosci. (2013) 5:185–96. doi: 10.1016/j.dcn.2013.03.004
91. McDonald C. Brain structural effects of psychopharmacological treatment in bipolar disorder.Curr Neuropharmacol. (2015) 13:445–57. doi: 10.2174/1570159X13666150403231654
92. Redlich R, Almeida JJ, Grotegerd D, Opel N, Kugel H, Heindel W, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach.JAMA Psychiatry (2014) 71:1222–30. doi: 10.1001/jamapsychiatry.2014.1100
93. Fernandes BS, Gama CS, Kauer-Sant'Anna M, Lobato MI, Belmonte-de-Abreu P, Kapczinski F. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: a potential adjunctive tool for differential diagnosis.J Psychiatr Res. (2009) 43:1200–4. doi: 10.1016/j.jpsychires.2009.04.010
94. Li Z, Zhang C, Fan J, Yuan C, Huang J, Chen J, et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study.Br J Psychiatry (2014) 205:29–35. doi: 10.1192/bjp.bp.113.134064
95. Nuernberg GL, Aguiar B, Bristot G, Fleck MP, Rocha NS. Brain-derived neurotrophic factor increase during treatment in severe mental illness inpatients.Transl Psychiatry (2016) 6:e985. doi: 10.1038/tp.2016.227
96. Sigitova E, Fisar Z, Hroudova J, Cikankova T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder.Psychiatry Clin Neurosci. (2017) 71:77–103. doi: 10.1111/pcn.12476
97. Duffy A, Lewitzka U, Doucette S, Andreazza A, Grof P. Biological indicators of illness risk in offspring of bipolar parents: targeting the hypothalamic-pituitary-adrenal axis and immune system.Early Interv Psychiatry (2012) 6:128–37. doi: 10.1111/j.1751-7893.2011.00323.x
98. Ellenbogen MA, Hodgins S, Linnen AM, Ostiguy CS. Elevated daytime cortisol levels: a biomarker of subsequent major affective disorder?J Affect Disord. (2011) 132:265–9. doi: 10.1016/j.jad.2011.01.007
99. Grande I, Magalhaes PV, Chendo I, Stertz L, Panizutti B, Colpo GD, et al. Staging bipolar disorder: clinical, biochemical, and functional correlates.Acta Psychiatr Scand. (2014) 129:437–44. doi: 10.1111/acps.12268
100. Tatay-Manteiga A, Balanza-Martinez V, Bristot G, Tabares-Seisdedos R, Kapczinski F, Cauli O. Clinical staging and serum cytokines in bipolar patients during euthymia.Prog Neuropsychopharmacol Biol Psychiatry (2017) 77:194–201. doi: 10.1016/j.pnpbp.2017.04.028
101. Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder.Int J Neuropsychopharmacol. (2013) 16:1505–12. doi: 10.1017/S1461145713000047
102. Hatch JK, Scola G, Olowoyeye O, Collins JE, Andreazza AC, Moody A, et al. Inflammatory markers and brain-derived neurotrophic factor as potential bridges linking bipolar disorder and cardiovascular risk among adolescents.J Clin Psychiatry (2017) 78:e286–e93. doi: 10.4088/JCP.16m10762
103. Powell TR, Dima D, Frangou S, Breen G. Telomere Length and Bipolar Disorder.Neuropsychopharmacology (2018) 43:445–53. doi: 10.1038/npp.2017.125
104. de Melo LGP, Nunes SOV, Anderson G, Vargas HO, Barbosa DS, Galecki P, et al. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders.Prog Neuropsychopharmacol Biol Psychiatry (2017) 78:34–50. doi: 10.1016/j.pnpbp.2017.04.027
105. Barbe-Tuana FM, Parisi MM, Panizzutti BS, Fries GR, Grun LK, Guma FT, et al. Shortened telomere length in bipolar disorder: a comparison of the early and late stages of disease.Rev Bras Psiquiatr. (2016) 38:281–6. doi: 10.1590/1516-4446-2016-1910
106. Rosenblat JD, McIntyre RS. Bipolar disorder and immune dysfunction: epidemiological findings, proposed pathophysiology and clinical implications.Brain Sci. (2017) 7:E144. doi: 10.3390/brainsci7110144
107. Fernandes BS, Steiner J, Molendijk ML, Dodd S, Nardin P, Goncalves CA, et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: a systematic review and meta-analysis.Lancet Psychiatry (2016) 3:1147–56. doi: 10.1016/S2215-0366(16)30370-4
108. Miskowiak KW, Burdick KE, Martinez-Aran A, Bonnin CM, Bowie CR, Carvalho AF, et al. Assessing and addressing cognitive impairment in bipolar disorder: the International society for bipolar disorders targeting cognition task force recommendations for clinicians.Bipolar Disord. (2018) 20:184–94. doi: 10.1111/bdi.12595
109. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept.J Int Neuropsychol Soc. (2002) 8:448–60. doi: 10.1017/S1355617702813248
110. Grande I, Sanchez-Moreno J, Sole B, Jimenez E, Torrent C, Bonnin CM, et al. High cognitive reserve in bipolar disorders as a moderator of neurocognitive impairment.J Affect Disord. (2017) 208:621–7. doi: 10.1016/j.jad.2016.10.012
111. Anaya C, Torrent C, Caballero FF, Vieta E, Bonnin Cdel M, Ayuso-Mateos JL. Cognitive reserve in bipolar disorder: relation to cognition, psychosocial functioning and quality of life.Acta Psychiatr Scand. (2016) 133:386–98. doi: 10.1111/acps.12535
112. Amoretti S CB, Torrent C, Mezquida G, Lobo A, González-Pinto A, Parellada M, et al. Cognitive reserve as an outcome predictor: first-episode affective versus non-affective psychosisActa Psychiatr Scand. (2018) 138:441–55. doi: 10.1111/acps.12949
113. Roux P, Raust A, Cannavo AS, Aubin V, Aouizerate B, Azorin JM, et al. Cognitive profiles in euthymic patients with bipolar disorders: results from the FACE-BD cohort.Bipolar Disord. (2017) 19:146–53. doi: 10.1111/bdi.12485
114. Varo C, Jimenez E, Sole B, Bonnin CM, Torrent C, Valls E, et al. Social cognition in bipolar disorder: focus on emotional intelligence.J Aff Disord. (2017) 217:210–7. doi: 10.1016/j.jad.2017.04.012
115. Jimenez E, Sole B, Arias B, Mitjans M, Varo C, Reinares M, et al. Characterizing decision-making and reward processing in bipolar disorder: a cluster analysis.Eur Neuropsychopharmacol. (2018) 28:863–74. doi: 10.1016/j.euroneuro.2018.04.001
116. Cotrena C, Damiani Branco L, Ponsoni A, Milman Shansis F, Paz Fonseca R. Neuropsychological clustering in bipolar and major depressive disorder.J Int Neuropsychol Soc. (2017) 23:584–93. doi: 10.1017/S1355617717000418
117. Miskowiak KW, Ott CV, Petersen JZ, Kessing LV. Systematic review of randomized controlled trials of candidate treatments for cognitive impairment in depression and methodological challenges in the field.Eur Neuropsychopharmacol. (2016) 26:1845–67. doi: 10.1016/j.euroneuro.2016.09.641
118. Rosa AR, Magalhaes PV, Czepielewski L, Sulzbach MV, Goi PD, Vieta E, et al. Clinical staging in bipolar disorder: focus on cognition and functioning.J Clin Psychiatry (2014) 75:e450–6. doi: 10.4088/JCP.13m08625
119. Reinares M, Papachristou E, Harvey P, Mar Bonnin C, Sanchez-Moreno J, Torrent C, et al. Towards a clinical staging for bipolar disorder: defining patient subtypes based on functional outcome.J Affect Disord. (2013) 144:65–71. doi: 10.1016/j.jad.2012.06.005
120. Popovic D, Reinares M, Goikolea JM, Bonnin CM, Gonzalez-Pinto A, Vieta E. Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder.Eur. Neuropsychopharmacol. (2012) 22:339–46. doi: 10.1016/j.euroneuro.2011.09.008
121. Jimenez E, Arias B, Mitjans M, Goikolea JM, Ruiz V, Brat M, et al. Clinical features, impulsivity, temperament and functioning and their role in suicidality in patients with bipolar disorder.Acta Psychiatrica Scand. (2016) 133:266–76. doi: 10.1111/acps.12548
122. van Os J, Verhagen S, Marsman A, Peeters F, Bak M, Marcelis M, et al. The experience sampling method as an mHealth tool to support self-monitoring, self-insight, and personalized health care in clinical practice.Depression Anxiety (2017) 34:481–93. doi: 10.1002/da.22647
123. Abdullah S, Matthews M, Frank E, Doherty G, Gay G, Choudhury T. Automatic detection of social rhythms in bipolar disorder.J Am Med Informatics Assoc. (2016) 23:538–43. doi: 10.1093/jamia/ocv200
124. Faurholt-Jepsen M, Busk J, Frost M, Vinberg M, Christensen EM, Winther O, et al. Voice analysis as an objective state marker in bipolar disorder.Transl Psychiatry (2016) 6:e856. doi: 10.1038/tp.2016.123
125. Hidalgo-Mazzei D, Mateu A, Reinares M, Murru A, Del Mar Bonnin C, Varo C, et al. Psychoeducation in bipolar disorder with a SIMPLe smartphone application: Feasibility, acceptability and satisfaction.J Affect Disord. (2016) 200:58–66. doi: 10.1016/j.jad.2016.04.042
126. Hidalgo-Mazzei D, Reinares M, Mateu A, Juruena MF, Young AH, Perez-Sola V, et al. Is a SIMPLe smartphone application capable of improving biological rhythms in bipolar disorder?J Affect Disord. (2017) 223:10–6. doi: 10.1016/j.jad.2017.07.028
127. Passos IC, Mwangi B, Cao B, Hamilton JE, Wu MJ, Zhang XY, et al. Identifying a clinical signature of suicidality among patients with mood disorders: A pilot study using a machine learning approach.J Affect Disord. (2016) 193:109–16. doi: 10.1016/j.jad.2015.12.066
128. Tran T, Luo W, Phung D, Harvey R, Berk M, Kennedy RL, et al. Risk stratification using data from electronic medical records better predicts suicide risks than clinician assessments.BMC Psychiatry (2014) 14:76. doi: 10.1186/1471-244X-14-76
129. Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA Jr. The neurobiology of the switch process in bipolar disorder: a review.J Clin Psychiatry. (2010) 71:1488–501. doi: 10.4088/JCP.09r05259gre
130. Bonnin CM, Yatham LN, Michalak EE, Martinez-Aran A, Dhanoa T, Torres I, et al. Psychometric properties of the well-being index (WHO-5) spanish version in a sample of euthymic patients with bipolar disorder.J Affect Disord. (2018) 228:153–9. doi: 10.1016/j.jad.2017.12.006
132. Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis.Am J Psychiatry (2015) 172:249–58. doi: 10.1176/appi.ajp.2014.13030418
133. Arango C, Diaz-Caneja CM, McGorry PD, Rapoport J, Sommer IE, Vorstman JA, et al. Preventive strategies for mental health.Lancet Psychiatry (2018) 5:591–604. doi: 10.1016/S2215-0366(18)30057-9
134. Vieta E. Staging and psychosocial early intervention in bipolar disorder.Lancet Psychiatry (2015) 2:483–5. doi: 10.1016/S2215-0366(15)00205-9
135. Amoretti S, Bernardo M, Bonnin CM, Bioque M, Cabrera B, Mezquida G, et al. The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study.Eur Neuropsychopharmacol. (2016) 26:1638–48. doi: 10.1016/j.euroneuro.2016.07.003
136. Sole B, Jimenez E, Torrent C, Reinares M, Bonnin CDM, Torres I, et al. Cognitive impairment in bipolar disorder: treatment and prevention strategies.Int J Neuropsychopharmacol. (2017) 20:670–80. doi: 10.1093/ijnp/pyx032
137. Salvatore P, Baldessarini RJ, Tohen M, Khalsa HM, Sanchez-Toledo JP, Zarate CA Jr, et al. McLean-Harvard International First-Episode Project: two-year stability of ICD-10 diagnoses in 500 first-episode psychotic disorder patients.J Clin Psychiatry (2011) 72:183–93. doi: 10.4088/JCP.09m05311yel
138. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression.Mol Psychiatry (2016) 21:1696–709. doi: 10.1038/mp.2016.3
139. Scott J, Etain B, Bellivier F. Can an Integrated Science Approach to Precision medicine research improve lithium treatment in bipolar disorders?Front Psychiatry (2018) 9:360. doi: 10.3389/fpsyt.2018.00360
140. Ritter PS, Schwabedal J, Brandt M, Schrempf W, Brezan F, Krupka A, et al. Sleep spindles in bipolar disorder - a comparison to healthy control subjects.Acta Psychiatrica Scand. (2018) 138:163–72. doi: 10.1111/acps.12924
141. Ryu E, Nassan M, Jenkins GD, Armasu SM, Andreazza A, McElroy SL, et al. A genome-wide search for bipolar disorder risk loci modified by mitochondrial genome variation.Mol Neuropsychiatry (2018) 3:125–34. doi: 10.1159/000464444
142. Chung Y, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, et al. Use of machine learning to determine deviance in neuroanatomical maturity associated with future psychosis in youths at clinically high risk.JAMA Psychiatry (2018) 75:960–8. doi: 10.1001/jamapsychiatry.2018.154
143. Duffy A, Malhi GS, Grof P. Do the trajectories of bipolar disorder and schizophrenia follow a universal staging model?Can J Psychiatry (2017) 62:115–22. doi: 10.1177/0706743716649189
144. Cadiz F, Gormaz JG, Burotto M. Breast cancer staging: is TNM ready to evolve?J Global Oncol. (2018) 4:1–3. doi: 10.1200/JGO.17.00004
Keywords: bipolar disorder, staging, biomarkers, personalized psychiatry, prevention
Citation: Salagre E, Dodd S, Aedo A, Rosa A, Amoretti S, Pinzon J, Reinares M, Berk M, Kapczinski FP, Vieta E and Grande I (2018) Toward Precision Psychiatry in Bipolar Disorder: Staging 2.0. Front. Psychiatry 9:641. doi: 10.3389/fpsyt.2018.00641
Received: 07 August 2018; Accepted: 13 November 2018;
Published: 29 November 2018.
Edited by:
Johann Steiner, Universitätsklinikum Magdeburg, GermanyReviewed by:
Hassan Rahmoune, University of Cambridge, United KingdomYilang Tang, Emory University, United States
Copyright © 2018 Salagre, Dodd, Aedo, Rosa, Amoretti, Pinzon, Reinares, Berk, Kapczinski, Vieta and Grande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduard Vieta, ZXZpZXRhQGNsaW5pYy5jYXQ=
Iria Grande, aWdyYW5kZUBjbGluaWMuY2F0
 Estela Salagre
Estela Salagre Seetal Dodd
Seetal Dodd Alberto Aedo
Alberto Aedo Adriane Rosa
Adriane Rosa Silvia Amoretti
Silvia Amoretti Justo Pinzon
Justo Pinzon Maria Reinares
Maria Reinares Michael Berk
Michael Berk Flavio Pereira Kapczinski
Flavio Pereira Kapczinski Eduard Vieta1*
Eduard Vieta1* Iria Grande
Iria Grande