
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 27 November 2018
Sec. Psychopharmacology
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00614
This article is part of the Research Topic Peripheral Markers of Immune Response in Major Psychiatric Disorders: Where Are We Now and Where Do We Want to Be? View all 12 articles
 Artur Reginia1
Artur Reginia1 Jolanta Kucharska-Mazur1*
Jolanta Kucharska-Mazur1* Marcin Jabłoński1
Marcin Jabłoński1 Marta Budkowska2
Marta Budkowska2 Barbara Dołȩgowska3
Barbara Dołȩgowska3 Leszek Sagan4
Leszek Sagan4 Błazej Misiak5
Błazej Misiak5 Mariusz Z. Ratajczak6
Mariusz Z. Ratajczak6 Janusz K. Rybakowski7
Janusz K. Rybakowski7 Jerzy Samochowiec1
Jerzy Samochowiec1Introduction: The immune system is undoubtedly involved in the pathogenesis of various psychiatric disorders, such as schizophrenia, bipolar disorder, or depression. Although its role is not fully understood, it appears that this area of research can help to understand the etiology of mental illness. One of the components of the human immune system is the complement system, which forms a part of the innate immune response. Physiologically, except for its essential protective role, it is a vital element in the regeneration processes, including neurogenesis. To date, few studies have tried to clarify the role of the complement cascade in mental disorders.
Materials and Methods: We evaluated concentrations of C3a, C5a, and C5b-9 complement cascade components in the peripheral blood of 30 patients suffering from bipolar disorder (BD) for at least 10 years, in euthymia, who were not treated with lithium salts. In addition, we divided our study sample into BD type I (BD-I, 22 persons), and BD type II (BD-II, 8 patients). The control group consisted of 30 healthy volunteers matched for age, sex, BMI, and smoking habits.
Results: Compared to healthy controls, BD patients had elevated concentrations of all the investigated components. Furthermore, in patients with BD-II, we observed higher concentrations of C5b-9 as compared to patients with BD-I. However, there was a significant effect of BD diagnosis only on the levels of C3a and C5a but not on the level of C5b-9 after adjustment for potential confounding factors.
Conclusions: Increased concentrations of components C3a and C5a of the complement system in the investigated group as compared to healthy controls suggest involvement of the complement cascade in the pathogenesis of BD, and provides further evidence of immune system dysregulation in BD patients.
Despite enormous efforts of researchers, undeniable progress of knowledge and research opportunities provided by modern medicine, the etiology of BD is still not fully understood. The prevalence of BD has been estimated at over 1% of the general population, regardless of their origin, ethnicity, or socio-economic status (1). Proper diagnosis, especially in the early stages of the disease causes considerable difficulties (2). The disease itself, as well as its improper diagnosis or treatment lead to enormous social consequences and economic costs (3, 4).
Several lines of evidence indicate that BD has a multifactorial etiology, comprising both genetic (5, 6) and environmental factors (7, 8). The concept of viewing mental disorders as the consequences of aberrant immune-inflammatory processes has recently become the subject of numerous studies (9–11). It appears that what is observed in the course of BD are both the activation of inflammatory processes within the central nervous system and systemic inflammatory reactions (12, 13). Such arguments are supported by an increased activity of the hypothalamic-pituitary-adrenal axis (especially in the manic phase) and increased peripheral metabolism of cortisol (14). Moreover, it is hypothesized that exposure to certain infectious agents in the prenatal period may lead to the occurrence of BD (15), although results in this field remain somewhat inconclusive. Both exposure to infectious agents in the prenatal period and the aforementioned activation of the HPA axis may potentially affect the function of the immune system (16, 17).
Post-mortem studies indicate an increased expression of inflammatory markers and excitotoxicity in the frontal cortex of patients with BD compared to healthy controls (18–20). Examination of cerebrospinal fluid provides some further evidence of higher concentrations of interleukin-8 (IL-8) linked to the treatment of BD with lithium salts (21). Other studies demonstrate higher levels of monocyte chemoattractant protein 1 and chitinase-3-like protein 1 (22–24), and changes in concentrations of lymphocytes Th1, Th2, or cytokines. Some of these changes depend on the stage of the disease (25–27).
A growing body of evidence points to the fact that the activation of systemic inflammatory reactions occurs in the course of mood disorders, including BD (28, 29). Furthermore, there are increased levels of proinflammatory cytokines in the peripheral blood of patients with BD. While the findings are not conclusive, cytokine concentrations appear to vary depending on the stage of the disease and its subtype (30, 31). During euthymia there are increased concentrations of IL-10, TNF-α, and increased levels of neutrophils and monocytes (32). Epidemiological studies show higher comorbidity of BD and autoimmune diseases or metabolic disorders, whose pathogenesis is mediated by inflammatory processes (33–35).
The central nervous system was traditionally perceived as an immunologically privileged organ, but nevertheless there is some communication between the CNS neural tissue with the immune system (36, 37). In addition, leakages within the blood-brain barrier can occur during the periods of BD exacerbation, and thus a cross-talk between the central nervous system and the immune system may be facilitated (38, 39).
The complement system is involved in both immunological as well as regenerative processes. It consists of dozens of proteins produced mainly in the liver and in small amounts also by neurons, microglia, astrocytes and oligodendrocytes, and several cell receptors. In addition to participating in the immunological mechanisms, it plays an important role in processes such as: reducing inflammatory reaction, removal of apoptotic cells, angiogenesis, wound healing, repair processes and the mobilization of some types of stem cells. It is considered to play a role in the pathogenesis of neurodegenerative diseases (40–42). Complement component C3a affects neurogenesis, stimulates the differentiation of neural progenitor cells under hypoxic conditions. It also modulates astrocytes' response to ischaemia, increasing their ability to survive stress conditions associated with ischemia. During the development of nerve cells in fetal life, it acts as a chemoattractant for these cells (43–46). Soluble anaphylatoxins (C3a, C4a, and C5a) control local inflammatory response by activating and attracting leukocytes (47). The presence of the receptors C5aR C3aR on neurons can prevent their apoptosis, while sublithic levels of C5b-9 can protect oligodendrocytes from apoptosis. At the same time, it is known that the activated complement is involved in a process called synaptic elimination, it enhances the secretion of proinflammatory cytokines by glial cells and induces neuronal damage and death by C5b-9 (48). The C5a component has a neuroprotective effect on mature neurons (49).
Despite all past attempts at defining the role of the complement system in the etiology of BD remains uncliear. Certainly, it is involved in the neuroinflammation process (50). It is one of the many factors, whose interactions lead to the development of BD (51). The complement system is also believed to be one of the elements linking the theory of prenatal infection or hypersensitivity to gluten with the development of neuropsychiatric disorders (52).
The aim of this study was to evaluate alterations in the levels of complement components in patients with BD as well as to determine whether these alterations are related to psychopathological manifestation of BD.
The study involved 30 unrelated patients suffering from BD for at least 10 years, not treated with lithium salts for at least 5 years prior to the study, due to the potential impact of lithium salts on inflammation and regeneration processes (53). A diagnosis of BD was established according to the ICD-10 criteria (54). At the time of the study all patients were in a stabilized mental state and met the criteria for BD remission. Exclusion criteria were presence of active substance dependence in the last 6 months (except for nicotine addiction); current or lifetime history of significant organic brain damage; cognitive impairment typical of dementia; serious somatic diseases, glucose intolerance, currently active inflammatory disease (exclusion based on the results of laboratory tests and physical examination); mental disorder other than BD, or personality disorders. The control group consisted of 30 individuals matched for age, sex, BMI, smoking habits, and sociodemographic factors. The controls did not manifest any symptoms of a mental disorder at the time of the study. They were also somatically healthy.
All patients underwent a standard psychiatric, physical and neurological examination. Demographics and family history were collected in the form of a standardized medical history. Presence of psychiatric disorders other than BD was excluded using the Mini International Neuropsychiatric Interview (MINI) questionnaire (55). The patient group was divided into two subgroups according to the type of the disease: BD type I (BD-I) and BD type II (BD-II) (56). To assess the severity of mood disorders, we used the Montgomery-Asberg Depression Rating Scale (MADRS) (57) and the Young Mania Rating Scale (YMRS) (58). Data on previous treatment was based on medical records and the interview. All patients were treated in line with the Polish standards of pharmacological treatment of affective disorders (59, 60). At the time of the study, patients were primarily treated with: lamotrigine in doses ranging from 50 to 300 mg/day (12 patients); valproic acid/sodium valproate in doses ranging from 600 to 1,500 mg/day (10 patients); quetiapine in doses from 100 to 700 mg/day (14 patients); olanzapine in doses of 5–20 mg/day (5 patients); clozapine in doses of 225 mg/day (1 patient) and aripiprazole (1 patient). In addition, the patients were receiving: sertraline (2 patients); venlafaxine (2 patients); escitalopram (1 patient); citalopram (1 patient); mirtazapine (1 patient); paroxetine (1 patient); clomipramine (1 patient); perazine (1 patient); levomepromazine (1 patient) and chlorprothixene (1 patient). For statistical analysis, all doses of antypsychitics were converted to chlorpromazine equivalents (61–63). The drug and its dosage were determined by the patient's physician. The research team did not modify the prescribed treatment in any way. Healthy controls underwent similar examinations to exclude mental disorders and somatic diseases.
Venous blood samples were collected between 8 am and 9 am after overnight fasting. For determination of the C5b-9 (MAC) levels, we used the Human C5b-9 ELISA Set (BD OptEIA). The levels of C3a and C5a were determined using the Human C3a ELISA Kit and the Human C5a ELISA Kit (BD OptEIA).
The results were analyzed using the STATISTICA 13.1 software (StatSoft, Inc.). In order to verify normality of data distribution, we used the Shapiro-Wilk test. Due to the fact that the parameters were not normally distributed, bivariate analyses were performed using the Mann-Whitney U-test. In the next step, the analysis of co-variance (ANCOVA) testing for differences in the levels of complement cascade components was performed. The following variables were used as co-variates: chlorpromazine equivalent dosage, valproate/valproic acid dosage, lamotrigine dosage, BMI and cigarette smoking status. The distribution of C3a and C5a levels fell within acceptable ranges of skewness (C3a: −0.492, C5a: 1.554) and kurtosis (C3a: −0.651, C5a: 1.834 C5a) and thus this data was not transformed before ANCOVA. The distribution of C5b-9 fell originally beyond acceptable range and it was square root transformed. After data transformation, skewness and kurtosis appeared to be acceptable (skewness: −0.455, kurtosis −0.638) (64). To evaluate correlations between continuous variables, we used the Spearman's rank correlation coefficient. Results were considered significant if the p-value was below 0.05.
General characteristics of patients and controls are shown in Table 1. Both groups did not differ significantly in terms of socio-demographic characteristics, except for vocational status. Indeed, there were significantly more employed individuals in the group of controls. Concentrations of all investigated complement components were higher in the group of patients compared to the control group (Table 2). These differences were also significant in separate analyses of BD-I and BD-II patients. However, BD-II patients presented higher C5b-9 concentrations than BD-I patients (trend level significance).
The ANCOVA revealed that there were significant effects of BD diagnosis on the levels of C3a and C5a after co-varying for BMI, smoking, chlorpromazine equivalent dosage and mood stablizer dosage (Table 3). However, differences in the levels of C5b-9 appeared to be insignificant after controlling for the same co-variates.
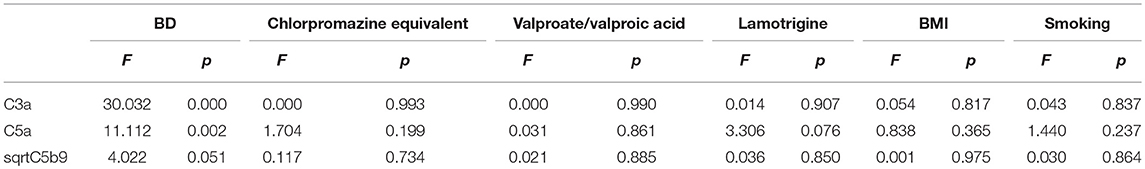
Table 3. The ANCOVA results testing for differences in the levels of complement cascade components after co-varying for potential confounding factors.
There were no significant correlations between the concentrations of complement cascade components and the scores of MADRS and YMRS (Table 4).
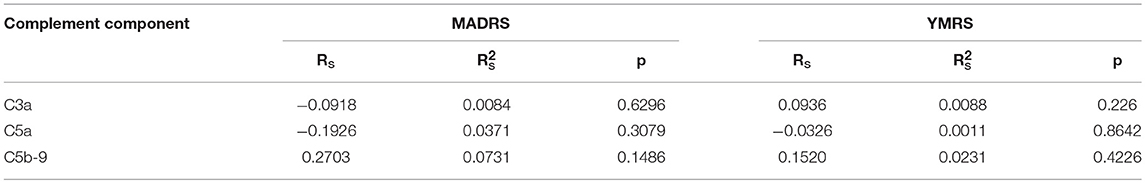
Table 4. Correlation between YMRS score, MADRS score and C3a, C5a, C5b-9 concentrations in the study sample.
We demonstrated significantly higher concentrations of each of the three identified complement cascade components in patients with BD compared to healthy subjects. Such patterns were also observed when comparing the distinguished BD-I and BD-II patient groups to the control group. When the concentrations were compared between BD-I and BD-II patients, there was a higher C5b-9 concentration in patients with BD-II subtype. However, due to the small number of BD-II patients, the latter difference should be interpreted with caution. Despite elevation of levels of all examinated components, only C3a and C5a were elevated in BD patients after adjustment for potential confounding factor related to differences in BMI and cigarette smoking as well as medication effects. Differences in the levels of C5b-9 appeared to be insignificant after controlling for potential confounders.
It is difficult to refer our results to current evidence in the field as there is a scarcity of srudies addressing these alterations. For instance, Spivak et al. found no significant differences in the concentrations of C3 and C4 between BD patients and patients with other psychiatric disorders or healthy controls in study on forty individuals, of whom eight were diagnosed with BD. However, the authors did not test the concentrations of the active forms of complement cascade components (65). Another study revealed that serum concentrations of C3 and C4 in patients with BD were comparable to the levels in healthy individuals, but significantly lower than in schizophrenia patients (66). Active forms of complement cascade components were also not measured in this study. In turn, there are reports on higher peripheral concentrations of C3, C4, and C6 in the course of a manic episode in BD patients as compared to healthy subjects (67). Similarly, higher concentrations of C4 were earlier observed in patients during the course of bipolar psychosis (68).
Reports on the plasma concentrations of complement components in other psychiatric disorders are also scarce. Studies on mobilization of stem cells into the peripheral blood in patients with psychotic disorders also involved evaluation of components of the complement cascade, as potential factors influencing the process of mobilization of these cells. They showed reduced C3a concentrations in untreated patients with first-episode psychosis. After the initiation of antipsychotic treatment, there was no significant difference in C3a levels as compared to the control group. A second similar study on patients with panic disorder showed decreased levels of all investigated components, namely C3a, C5a, and C5b-9 (69–71). On the other hand, in a study examining concentrations of C4 and sC5b-9 (soluble C5b-9), Akcan et al. (72) found lower concentrations of these components in patients with chronic BD, both in the acute and chronic phase, compared to healthy controls and individuals with the first episode of mood disorders in the course of BD. These concentrations were inversely correlated with the YMRS score and duration of the disease. We did not observe any correlation between the levels of complement cascade components with YMRS and MADRS scores. It should be noted, however, that in contrast to our study, Akcan et al. examined patients in the period of unstable mood and treated with, among others, salts of lithium, which was a clear difference between theirs and our study (72).
Due to their ability to activate and attract leukocytes, anaphylatoxins C3a and C5a seem to be an important link in the inflammatory processes associated with the complement system within the CNS. Importantly, C3a is also a key element in the endothelial and leukocyte activation within the CNS (73). Although in certain situations it may have anti-inflammatory effects, its pro-inflammatory effects take over in the course of chronic reactions, affecting progression of the disease (74, 75). Thus, it seems that the increased concentrations of the complement cascade components observed in the present study may be indicative of a chronic inflammatory process, which could give rise to neurodegenerative changes in the CNS (76).
As previously mentioned, in healthy individuals the blood-brain barrier (BBB) prevents the entry of proteins, including the complement, into plasma. However, pathological conditions may lead to the blood-brain barrier leakage, affected by, among others, C5a. It has been shown that after several hours from such a leakage, complement components may penetrate the brain tissue. It is believed that the permeability of the BBB can further progress in with subsequent BD exacerbations (39, 73, 77, 78). For these reasons, increased concentrations of C3a and C5a in patients with BD may significantly affect the central nervous system, including the regenerative processes.
In our study, we demonstrated that there is an activation of the immune system manifested by an increase in the concentrations of C3a and C5a complement cascade components in the course of BD. However, it should be clearly stated here that the limitation of the study was a small sample size. In their aforementioned study, Kucharska et al. demonstrated a lower C3a concentration in patients with first-episode psychosis as compared to healthy controls (69). In agreement with these findings, complement cascade components have been found to be helpful in differentiating first-episode psychosis (79). In the study on panic disorder, on the other hand, there was no evidence of increased complement activation (70). Therefore, it seems that complement system alterations might be different in distinct groups of mental disorders. Still, there are no reports on C3a, C5a, and C5b-9 levels in depressive disorders or in early stages of BD, i.e., situations involving greatest diagnostic difficulties (71).
In summary, BD patients experience a systemic dysfunction of the immune system manifested by the activation of the complement cascade. In the future, components of the complement system may become useful in research of a biomarker helpful in diagnosing BD. However, further studies addressing complement cascade alterations in various stages of BD and other mental disorders are needed to establish their relevance as potential biomarkers.
All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the by the Bioethical Committee of the Pomeranian Medical University, at its meeting of 10.29.2014 r., resolution No KB-0012/127/12, supplemented with consents KB-0012/70/14 of 10.13.2014 and KB 0012/48/15 of 03.23.2015.
AR: Patients recruitment, investigation, methodology, validation, writing–original draft, writing–final version. MJ: Patients recruitment, investigation. MB: Investigation. BD: Investigation and methodology. LS: Investigation and project administration. BM: Methodology and revision–original draft. MR: Methodology, supervision, and validation. JR: Investigation and methodology. JS: Investigation, methodology, project administration, supervision, and validation. JK-M: Patients recruitment, investigation, methodology, supervision, validation, writing–original draft, writing–final version.
The study was funded within the framework of the project Fundusz Stymulacji Nauki (grant number: FSN 246-05/17) provided by Pomeranian Medical University in Szczecin (Poland).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry (2011) 68:241–51. doi: 10.1001/archgenpsychiatry.2011.12
2. Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet (2013) 381:1663–71. doi: 10.1016/S0140-6736(13)60989-7
3. Simon GE. Social and economic burden of mood disorders. Biol Psychiatry (2003) 54:208–15. doi: 10.1016/S0006-3223(03)00420-7
4. Nasrallah HA. Consequences of misdiagnosis: inaccurate treatment and poor patient outcomes in bipolar disorder. J Clin Psychiatry (2015) 76:e1328. doi: 10.4088/JCP.14016tx2c
5. Seifuddin F, Mahon PB, Judy J, Pirooznia M, Jancic D, Taylor J, et al. Meta-analysis of genetic association studies on bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. (2012) 159B:508–18. doi: 10.1002/ajmg.b.32057
6. Forstner AJ, Hofmann A, Maaser A, Sumer S, Khudayberdiev S, Mühleisen TW, et al. Genome-wide analysis implicates microRNAs and their target genes in the development of bipolar disorder. Transl Psychiatry (2015) 5:e678. doi: 10.1038/tp.2015.159
7. Aas M, Henry C, Andreassen OA, Bellivier F, Melle I, Etain B. The role of childhood trauma in bipolar disorders. Int J Bipolar Disord. (2016) 4:2. doi: 10.1186/s40345-015-0042-0
8. Bergink V, Larsen JT, Hillegers MHJ, Dahl SK, Stevens H, Mortensen PB, et al. Childhood adverse life events and parental psychopathology as risk factors for bipolar disorder. Transl Psychiatry (2016) 6:e929. doi: 10.1038/tp.2016.201
9. Wilkowska A, Pikuła M, Rynkiewicz A, Wdowczyk-Szulc J, Trzonkowski P, Landowski J. Increased plasma pro-inflammatory cytokine concentrations after myocardial infarction and the presence of depression during next 6-months. Psychiatr Pol. (2015) 49:455–64. doi: 10.12740/PP/33179
10. Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (2016) 233:1623–36. doi: 10.1007/s00213-016-4214-0
11. Mak M, Misiak B, Frydecka D, Pełka-Wysiecka J, Kucharska-Mazur J, Samochowiec A, et al. Polymorphisms in immune-inflammatory response genes and the risk of deficit schizophrenia. Schizophr Res. (2017) 193:359–63. doi: 10.1016/j.schres.2017.06.050
12. Lohoff FW, Berrettini WH. “Genetics of Bipolar Disorder,” In: Yatham LM, Maj M editor. Bipolar Disorder Clinical and Neurobiological Foundations. Chichester, West Sussex, UK: Wiley-Blackwell. (2010). p. 110–24.
13. Jakobsson J, Bjerke M, Sahebi S, Isgren A, Ekman CJ, Sellgren C, et al. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. J Psychiatry Neurosci. (2015) 40:250–8. doi: 10.1503/jpn.140183
14. Murri MB, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: systematic review and meta- analysis. Psychoneuroendocrinology (2016) 63:327–42. doi: 10.1016/j.psyneuen.2015.10.014
15. Barichello T, Badawy M, Pitcher MR, Saigal P, Generoso JS, Goularte JA, et al. Exposure to perinatal infections and bipolar disorder: a systematic review. Curr Mol Med. (2016) 16:106–18. doi: 10.2174/1566524016666160126143741
16. Brown AS. The kraepelinian dichotomy from the perspective of prenatal infectious and immunologic insults. Schizophr Bull. (2015) 41:786–91. doi: 10.1093/schbul/sbv063
17. Herkenham M, Kigar SL. Contributions of the adaptive immune system to mood regulation: mechanisms and pathways of neuroimmune interactions. Prog Neuropsychopharmacol Prog Neuropsychopharmacol Biol Psychiatry (2017) 79:49–57. doi: 10.1016/j.pnpbp.2016.09.003
18. Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. (2010) 37:596–603. doi: 10.1016/j.nbd.2009.11.010
19. Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry (2010) 15:384–92. doi: 10.1038/mp.2009.47
20. Rosenblat JD, Brietzke E, Mansur RB, Maruschak NA, Lee Y, McIntyre RS. Inflammation as a neurobiological substrate of cognitive impairment in bipolar disorder: evidence, pathophysiology and treatment implications. J Affect Disord. (2015) 188:149–59. doi: 10.1016/j.jad.2015.08.058
21. Isgren A, Jakobsson J, Pålsson E, Ekman CJ, Johansson AG, Sellgren C, et al. Increased cerebrospinal fluid interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav Immun. (2015) 43:198–204. doi: 10.1016/j.bbi.2014.10.001
22. Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology (2014) 39:2349–56. doi: 10.1038/npp.2014.81
23. Rolstad S, Jakobsson J, Sellgren C, Isgren A, Ekman CJ, Bjerke M, et al. CSF neuroinflammatory biomarkers in bipolar disorder are associated with cognitive impairment. Eur Neuropsychopharmacol. (2015) 25:1091–8. doi: 10.1016/j.euroneuro.2015.04.023
24. Isgren A, Sellgren C, Ekman CJ, Holmén-Larsson J, Blennow K, Zetterberg H, et al. Markers of neuroinflammation and neuronal injury in bipolar disorder: Relation to prospective clinical outcomes. Brain Behav Immun. (2017) 65:195–201. doi: 10.1016/j.bbi.2017.05.002
25. Uyanik V, Tuglu C, Gorgulu Y, Kunduracilar H, Uyanik MS. Assessment of cytokine levelsand hs-CRP in bipolar I disorder before and after treatment. Psychiatry Res. (2015) 228:386–92. doi: 10.1016/j.psychres.2015.05.078
26. Jacoby AS, Munkholm K, Vinberg M, Pedersen BK, Kessing LV. Cytokines, brain-derived neurotrophic factor and C-reactive proteinin bipolar I disorder – Results from a prospective study. J Affect Disord. (2016) 197:167–74. doi: 10.1016/j.jad.2016.03.040
27. Muneer A. Bipolar disorder: role of inflammation and the development of disease biomarkers. Psychiatry Invest. (2016) 13:18–33. doi: 10.4306/pi.2016.13.1.18
28. Misiak B, Beszłej JA, Kotowicz K, Szewczuk-Bogusławska M, Samochowiec J, Kucharska-Mazur J, et al. Cytokine alterations and cognitive impairment in major depressive disorder: from putative mechanisms to novel treatment targets. Prog Neuropsychopharmacol Biol Psychiatry (2018) 80:177–188. doi: 10.1016/j.pnpbp.2017.04.021
29. Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. (2012) 141:1–10. doi: 10.1016/j.jad.2011.12.049
30. Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T, et al. Comparison of inflammatory cytokine levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord. (2014) 166:187–92. doi: 10.1016/j.jad.2014.05.009
31. Fiedorowicz JG, Prossin AR, Johnson CP, Christensen GE, Magnotta VA, Wemmie JA. Peripheral inflammation during abnormal mood states in bipolar I disorder. J Affect Disord. (2015) 187:172–8. doi: 10.1016/j.jad.2015.08.036
32. Tatay-Manteiga A, Balanzá-Martínez V, Bristot G, Tabarés-Seisdedos R, Kapczinski F, Cauli O. Clinical staging and serum cytokines in bipolar patients during euthymia. Prog Neuropsychopharmacol Biol Psychiatry (2017) 77:194–201. doi: 10.1016/j.pnpbp.2017.04.028
33. Hamdani N, Doukhan R, Kurtlucan O, Tamouza R, Leboyer M. Immunity, inflammation, and bipolar disorder: diagnostic and therapeutic implications. Curr Psychiatry Rep. (2013) 15:387. doi: 10.1007/s11920-013-0387-y
34. Perugi G, Quaranta G, Belletti S, Casalini F, Mosti N, Toni C, et al. General medical conditions in 347 bipolar disorder patients: clinical correlates of metabolic and autoimmune-allergic diseases. J Affect Disord. (2015) 170:95–103. doi: 10.1016/j.jad.2014.08.052
35. Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res. (2016) 167:257–80. doi: 10.1016/j.trsl.2015.06.017
36. Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. (2015) 36:569–77. doi: 10.1016/j.it.2015.08.006
37. Engelhardt B, Carare RO, Bechmann I, Flügel A, Laman JD, Weller RO. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. (2016) 132:317–38. doi: 10.1007/s00401-016-1606-5
38. Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast. (2015) 2015:708306. doi: 10.1155/2015/708306
39. Tsai MC, Huang TL. Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr Psychiatry (2017) 74:27–34. doi: 10.1016/j.comppsych.2016
40. Alexander JJ, Anderson AJ, Barnum SR, Stevens B, Tenner AJ. The complement cascade: Yin-Yang in neuroinflammation–neuro-protection and -degeneration. J Neurochem (2008) 107:1169–87. doi: 10.1111/j.1471-4159.2008.05668.x
41. Schraufstatter IU, Khaldoyanidi SK, DiScipio RG. Complement activation in the context of stem cells and tissue repair. World J Stem Cells (2015) 7:1090–108. doi: 10.4252/wjsc.v7.i8.1090
42. Arbore G, Kemper C, Kolev M. Intracellular complement - the complosome - in immune cell regulation. Mol Immunol. (2017) 89:2–9. doi: 10.1016/j.molimm.2017.05.012
43. Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson A-K, et al. Complement: a novel factor in basal and ischemia-induced neurogenesis. The EMBO J. (2006) 25:1364–74. doi: 10.1038/sj.emboj.7601004
44. Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, et al. Complement Fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell (2011) 21:1026–37. doi: 10.1016/j.devcel.2011.10.012
45. Shinjyo N, de Pablo Y, Pekny M, Pekna M. Complement Peptide C3a Promotes astrocyte survival in response to ischemic stress. Mol Neurobiol. (2016) 53:3076–87. doi: 10.1007/s12035-015-9204-4
46. Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain (2017) 140:353–69. doi: 10.1093/brain/aww314
47. Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. (2004) 41:1089–98. doi: 10.1016/j.molimm.2004.06.011
48. Bonifati DM, Kishor U. Role of complement in neurodegeneration and neuroinflammation. Mol Immunol. (2007) 44:999–1010. doi: 10.1016/j.molimm.2006.03.007
49. O'Barr SA, Caguioa J, Gruol D, Perkins G, Ember JA, Hugli T, et al. Neuronal expression of a functional receptor for the C5a complement activation fragment. J Immunol. (2001) 166:4154–62. doi: 10.4049/jimmunol.166.6.4154
50. Panaccione I, Spalletta G, Sani G. Neuroinflammation and excitatory symptoms in bipolar disorder. Neuroimmunol Neuroinflammat. (2015) 2:215–27. doi: 10.4103/2347–8659.167304
51. Woo HJ, Yu C, Kumar K, Reifman J. Large-scale interaction effects reveal missing heritability in schizophrenia, bipolar disorder and posttraumatic stress disorder. Transl Psychiatry (2017) 7:e1089. doi: 10.1038/tp.2017.61
52. Severance EG, Kannan G, Gressitt KL, Xiao J, Alaedini A, Pletnikov MV, et al. Anti-gluten immune response following toxoplasma gondii infection in mice. PLoS ONE (2012) 7:e50991. doi: 10.1371/journal.pone.0050991
53. Dell'Osso L, Del Grande C, Gesi C, Carmassi C, Musetti L. A new look at an old drug: neuroprotective effects and therapeutic potentials of lithium salts. Neuropsychiatr Dis Treat. (2016) 12:1687–703. doi: 10.2147/NDT.S106479
54. The International Classification of Diseases. 10th revision, Polish version: Klasyfikacja zaburzen psychicznych i zaburzen zachowania w ICD-10. Badawcze kryteria diagnostyczne. Krakow-Warszawa: Uniwersyteckie Wydawnictwo Medyczne Vesalius (1998). 190 p.
55. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry (1998) 59:22–33.
56. First MB. DSM-5 Diagnostyka Róznicowe. Transl by P. Gałecki. Edra Urban & Partner (2016). p. 194–9.
57. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry (1979) 134:382–9.
58. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry (1978) 133:429–35.
59. Rybakowski J, Dudek D, Jaracz J. “Choroby afektywne,” In: Jarema M, editor. Standardy Leczenia Farmakologicznego Niektórych Zaburzen Psychicznych. Wydanie 2.Gdansk: Via Medica (2015). p. 55–133.
60. Gaebel W, Zielasek J, Reed GM. Mental and behavioural disorders in the ICD-11: concepts, methodologies, and current status. Psychiatr Pol. (2017) 51:169–195. doi: 10.12740/PP/69660
61. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry (2010) 167:686–93. doi: 10.1176/appi.ajp.2009.09060802
62. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry (2013) 55:207–8. doi: 10.4103/0019-5545.111475
63. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. (2015) 69:440–7. doi: 10.1111/pcn.12275
64. George D, Mallery M. (2010). SPSS for Windows Step by Step: A Simple Guide and Reference, 17.0 Update, 10a edn. Boston, MA: Pearson.
65. Spivak B, Radwan M, Elimelech D, Baruch Y, Avidan G, Tyano S. A study of the complement system in psychiatric patients. Biol Psychiatry (1989) 26:640–2.
66. Santos Sória L, Moura Gubert C, Ceresér KM, Gama CS, Kapczinski F. Increased serum levels of C3 and C4 in patients with schizophrenia compared to eutymic patients with bipolar disorder and healthy. Rev Bras Psiquiatry (2012) 34:119–20.
67. Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. (2002) 17:175–9. doi: 10.1002/hup.390
68. Fontana A, Storck U, Angst J, Dubs R, Abegg A, Grob PJ. An immunological basis of schizophrenia and affective disorders? Neuropsychobiology (1980) 6:284–9.
69. Kucharska-Mazur J, Tarnowski M, Dołegowska B, Budkowska M, Pedziwiatr D, Jabłonnski M, et al. Novel evidence for enhanced stem cell trafficking in antipsychotic naïve subjects during their first psychotic episode. J Psychiatr Res. (2014) 49:18–24. doi: 10.1016/j.jpsychires.2013.10.016
70. Jabłonski M, Kucharska Mazur J, Tarnowski M, Dołegowska B, Pedziwiatr D, Kubiś E, et al. Mobilization of peripheral blood stem cells and changes in the concentration of plasma factors influencing their movement in patients with panic disorder. Stem Cell Rev. (2017) 13:217–25. doi: 10.1007/s12015–016-9700-6
71. Kucharska-Mazur J, Jabłonski M, Misiak B, Frydecka D, Rybakowski J, Ratajczak MZ, et al. Adult stem cells in psychiatric disorders - new discoveries in peripheral blood. Prog Neuropsychopharmacol Biol Psychiatry (2017) 80:23–7. doi: 10.1016/j.pnpbp.2017.04.005
72. Akcan U, Karabulut S, Ismail Küçükali C, Çakir S, Tüzün E. Bipolar disorder patients display reduced serum complement levels and elevated peripheral blood complement expression levels. Acta Neuropsychiatr. (2017) 12:1–9. doi: 10.1017/neu.2017.10
73. Wu F, Zou Q, Ding X, Shi D, Zhu X, Hu W, et al. Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J Neuroinflam. (2016) 13:23. doi: 10.1186/s12974-016-0485-y
74. Boos L, Szalai AJ, Barnum SR. C3a expressed in the central nervous system protects against LPS-induced shock. Neurosci Lett. (2005) 387:68–71. doi: 10.1016/j.neulet.2005.07.015
75. Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement System Part II: Role in Immunity. Front Immunol. (2015) 6:257. doi: 10.3389/fimmu.2015.00257
76. Buoli M, Serati M, Caldiroli A, Cremaschi L, Altamura AC. Neurodevelopmental versus neurodegenerative model of schizophrenia and bipolar disorder: comparison with physiological brain development and aging. Psychiatr Danub. (2017) 29:24–27.
77. Lettiero B, Andersen AJ, Hunter AC, Moghimi SM. Complement system and the brain: selected pathologies and avenues toward engineering of neurological nanomedicines. J Control Rel. (2012) 161:283–9. doi: 10.1016/j.jconrel.2011.10.036
78. Mantovani S, Gordon R, Macmaw JK, Pfluger CM, Henderson RD, Noakes PG, et al. Elevation of the terminal complement activation products C5a and C5b-9 in ALS patient blood. J Neuroimmunol. (2014) 276:213–8. doi: 10.1016/j.jneuroim.2014.09.005
Keywords: bipolar disorder, complement system, C3a, C5a, C5b-9
Citation: Reginia A, Kucharska-Mazur J, Jabłoński M, Budkowska M, Dołȩgowska B, Sagan L, Misiak B, Ratajczak MZ, Rybakowski JK and Samochowiec J (2018) Assessment of Complement Cascade Components in Patients With Bipolar Disorder. Front. Psychiatry 9:614. doi: 10.3389/fpsyt.2018.00614
Received: 23 November 2017; Accepted: 01 November 2018;
Published: 27 November 2018.
Edited by:
Fernando Rodriguez De Fonseca, Instituto de Investigación Biomédica de Málaga (IBIMA), SpainReviewed by:
Juan C. Leza, Complutense University of Madrid, SpainCopyright © 2018 Reginia, Kucharska-Mazur, Jabłoński, Budkowska, Dołȩgowska, Sagan, Misiak, Ratajczak, Rybakowski and Samochowiec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jolanta Kucharska-Mazur, am9sYV9rdWNoYXJza2FAdGxlbi5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.