
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 27 November 2018
Sec. Molecular Psychiatry
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00610
This article is part of the Research Topic Molecular Mechanisms in Stress and Trauma Related Disorders View all 12 articles
 Karin de Punder1*
Karin de Punder1* Sonja Entringer1,2,3
Sonja Entringer1,2,3 Christine Heim1,4
Christine Heim1,4 Christian E. Deuter5
Christian E. Deuter5 Christian Otte5
Christian Otte5 Katja Wingenfeld5
Katja Wingenfeld5 Linn K. Kuehl5
Linn K. Kuehl5Background: Major depressive disorder (MDD) is a complex psychiatric condition with different subtypes and etiologies. Exposure to adverse childhood experiences (ACE) is an important risk factor for the development of MDD later in life. Evidence suggests that pro-inflammatory processes may convey this risk as both MDD and ACE have been related to increased levels of inflammation. In the present study, we aimed to disentangle the effects of MDD and ACE on inflammation levels.
Methods: Markers of inflammation (plasma interleukin(IL)-6 and high sensitive C-reactive protein (hsCRP) concentrations, white blood cell (WBC) count and a composite inflammation score (CIS) combining all three) were assessed in 23 MDD patients with ACE, 23 MDD patients without ACE, 21 healthy participants with ACE, and 21 healthy participants without ACE (mean age: 35 ± 11 (SD) years). None of the patients and participants was taking psychotropic medication. ACE was assessed with the Early Trauma Inventory (ETI) and was defined as moderate to severe exposure to sexual or physical abuse.
Results: Group differences in the different inflammatory measures were observed. MDD patients with ACE showed significantly higher IL-6 concentrations (p = 0.018), higher WBC counts (p = 0.003) and increased general inflammation levels as indicated by the CIS (p = 0.003) compared to healthy controls. In contrast, MDD patients without ACE displayed similar inflammation levels to the control group (p = 0.93).
Conclusion: We observed elevated inflammation in MDD patients with a history of ACE, which could indicate a subtype of “inflammatory depression”. Accordingly, MDD patients with ACE might potentially benefit from anti-inflammatory therapies.
Major depressive disorder (MDD) is a frequent and heterogenic disorder and despite numerous studies performed over the last decades, there are still inconsistencies in clinical findings regarding the pathological mechanisms contributing to the development of MDD (1). Therefore, greater understanding of the biological processes and pathways underlying the pathophysiology of depression is of key importance for the development of early interventions and personalized therapies.
Adverse childhood experiences (ACE) have been shown to predispose to the development of MDD later in life (2, 3) and in addition induce greater risk for acquiring several somatic conditions, including cardiovascular disease (CVD) (3). A large body of evidence suggests that ACE is linked to a chronic pro-inflammatory state in adulthood (4–6). Alterations in the dynamics of the neuroendocrine stress response likely contribute to the manifestation of a pro-inflammatory immune phenotype in these individuals (7). It has been suggested that stressors occurring early in life can be biologically embedded through epigenetic modifications in stress-related genes (8) and program the immune system to become hyper-responsive in response to challenge with diminished sensitivity to the inhibitory effect of glucocorticoids (9).
Chronic inflammation is characterized by elevated levels of pro-inflammatory cytokines, acute phase proteins, and increases in white blood cell (WBC) numbers (10–12). Several studies reported higher circulating levels of inflammatory mediators, such as the pro-inflammatory cytokine IL-6 and the acute phase-protein C-reactive protein (CRP) (4, 5), and increased WBC counts (13, 14) in adults exposed to early adversity. Because chronic inflammation is associated with both ACE and several physical and psychiatric conditions, including MDD (15–18), it has been proposed as a key mechanism through which severe stress exposure during childhood can influence health outcomes throughout the lifespan (6, 19). This notion is further supported by the observation that increased activation of pro-inflammatory pathways (reflected by increased circulating levels of CRP and IL-6) precedes the development of depressive symptoms (17), and by studies that report that inflammation is more pronounced in a subgroup of MDD patients that are exposed to ACE (20–23).
The overall goal of the present study was to replicate previous findings regarding associations between ACE, MDD and inflammation, and to further disentangle the effects of MDD and ACE on inflammation using a well-controlled, full factorial design including four carefully diagnosed groups of healthy participants and MDD patients with and without a history of ACE. None of the patients and participants was taking psychotropic medication. We hypothesized accumulative effects of MDD and ACE on inflammation levels.
Patients and healthy participants were recruited by public postings and from our specialized affective disorder unit at the Department of Psychiatry and Psychotherapy, Campus Benjamin Franklin, Charité -Universitätsmedizin Berlin. All participants provided written informed consent. Healthy participants and outpatients received monetary compensation for their participation. The study was approved by the local ethical committee.
Depressed patients were included if they fulfilled criteria for MDD as assessed with the Structured Clinical Interview for DSM-IV axis I (SCID-I) (24) to validate psychiatric diagnoses. In addition, current depressive symptoms were captured by the Montgomery Asberg Depression Rating Scale (MADRS) (25, 26) and the Beck Depression Inventory (BDI) (27).
ACE was assessed by using a semi-structured interview, the Early Trauma Inventory (ETI) (28, 29), and was defined as repeated physical or sexual abuse at least once a month over one year or more before the age of 18.
In the MDD groups, schizophrenia, schizoaffective disorder, bipolar disorder, depressive disorder with psychotic features, dementia, eating disorders, panic disorder, alcohol or drug dependence led to exclusion. Healthy participants with and without ACE were free of any current mental disorder. Further exclusion criteria for all participants were CNS relevant diseases, neurological diseases, severe somatic diseases, diabetes type 1 and 2, steroid diseases, hypertonia, current infections, pregnancy and the intake of psychotropic medication. Physical health criteria were checked by physical examination, clinical interview and a complete blood count (CBC).
The study sample comprised 23 MDD patients with ACE (MDD+/ACE+), 23 MDD patients without ACE (MDD+/ACE–), 21 participants with ACE but no current, or lifetime MDD (MDD–/ACE+) and 21 participants with no current or lifetime MDD and no childhood adversity (MDD–/ACE–, healthy comparison group).
All patients and participants underwent one study visit including psychiatric and medical diagnostic by physical examination, blood sampling and clinical interviews including SCID-I and MADRS as well as assessment of ACE using the ETI. Afterwards they completed a MDD related questionnaire (BDI). Blood samples were sent immediately to the laboratory of the Institute of Medical Psychology, Campus Mitte, Charité–Universitätsmedizin Berlin, Germany, and to the Labor Berlin–Charité Vivantes GmbH, Berlin, Germany, for further analyses.
Plasma IL-6 concentrations were analyzed using a commercially available high sensitivity ELISA kit (eBioscience), according to the manufacturer's instructions. The limit of detection was 0.007 pg/ml. The intra- and inter-assay coefficients of variability for plasma IL-6 measurements were 10 and 12%, respectively. Plasma hsCRP concentrations were analyzed using a commercially available Instant ELISA kit (eBioscience), according to the manufacturer's instructions. The limit of detection was 3 pg/ml. The intra- and inter-assay coefficients of variability for plasma hsCRP measurements were 6 and 8%, respectively. WBC counts were obtained from a standard clinical complete blood count panel using a Sysmex XN 1000 (Sysmex).
General linear models and Chi2 tests were used to compare groups concerning demographics and clinical data (see Table 1). Post hoc tests (Bonferroni) were conducted when applicable. IL-6 and hsCRP measures were first log transformed to normalize distributions. Since IL-6 and hsCRP concentrations and WBC count are established measures of pro-inflammatory activity, represent three biologically related components of inflammation [i.e., (a) pro-inflammatory cytokines (b) acute phase proteins and (c) increased WBC numbers], and inter-correlated with each other (all r's >0.2), we combined these measures into a single composite measure. Principal-component analysis identified one single factor, the composite inflammation score (CIS), accounting for 48% of the variance in analyte determinations. A common factor takes full advantage of the predictive values of the three measures, while minimizing measurement errors of the single components (21).
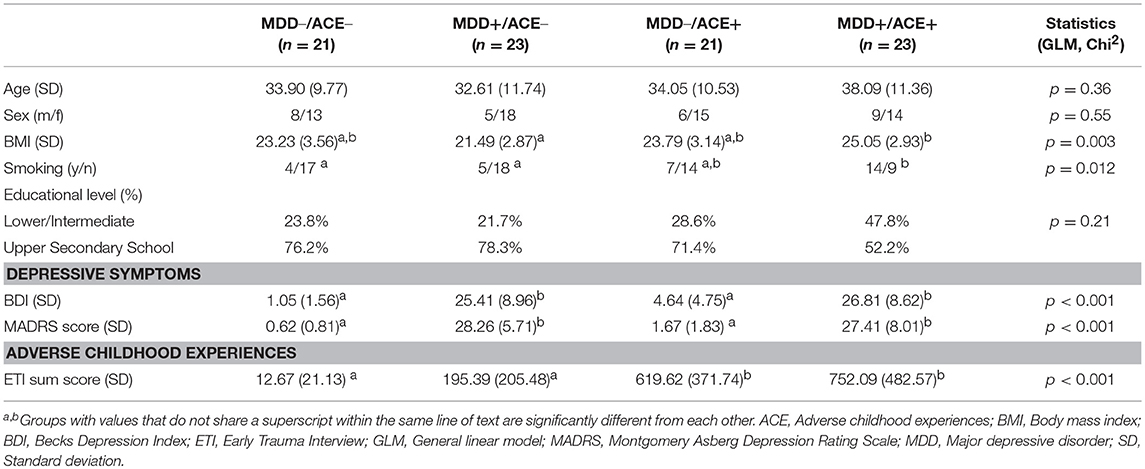
Table 1. Demographic and clinical characteristics of healthy participants and depressed patients without ACE (MDD–/ACE–, MDD+/ACE–) and healthy participants and depressed patients with ACE (MDD–/ACE+, MDD+/ACE+).
General linear models were used to compare groups regarding inflammatory measures. In order to investigate the groups effects on inflammatory measures in more detail, and because we expected the lowest inflammation levels in the control group, we studied a priori defined contrasts between the controls and the three study groups.
Many potential cofounders were excluded by design (see above). However, additional adjusted analysis included covariates that differed significantly between the four groups (i.e., BMI and smoking see Table 1).
Data analysis was performed using the SPSS statistical software (SPSS 23.0, Inc., Chicago, IL, USA). The significance level was set at p < 0.05 for all applied analysis.
Complete blood counts were missing for 8 individuals (4 MDD+/ACE+, 2 MDD+/ACE–, 1 MDD–/ACE+ and 1 MDD–/ACE–). For IL-6, a measurement was missing for 1 MDD patient without ACE (MDD+/ACE–). General linear models indicated there were no group differences regarding the number of missing biological measurements (p = 0.42).
Table 1 summarizes group demographics and clinical characteristics. In accordance with our recruitment, MDD patients and healthy participant with ACE (MDD+/ACE+, MDD–/ACE+) had significantly higher total ETI scores compared to the MDD patients without ACE (MDD+/ACE–) and the control group (MDD–/ACE–). MDD patients with and without ACE did not differ in depression severity and both groups had higher depression scores compared to healthy individuals with and without ACE. MDD patients with ACE had a higher BMI compared to MDD patients without ACE and smoked more than healthy controls. No group differences were observed in age, sex and educational level.
As presented in Table 2, we identified significant group effects on IL-6 [F(3, 83) = 3.32, p = 0.024, η2 = 0.11], CRP concentration [F(3, 84) = 3.10, p = 0.031, η2 = 0.10] and WBC count [F(3, 76) = 3.44, p = 0.021, η2 = 0.12]. To investigate these effects in more detail, we studied a priori defined contrasts between controls and the different study groups (see Table 3). We observed significantly higher IL-6 concentrations (p = 0.018) and WBC counts (p = 0.003) in MDD patients with ACE compared to healthy controls, also after controlling for BMI and smoking (IL-6, p = 0.044; WBC count, p = 0.048). CRP levels were significantly higher in healthy individuals with ACE (p = 0.031). However, this effect was no longer significant after controlling for BMI and smoking (p = 0.052). Untransformed and unadjusted mean group values for IL-6, hsCRP and WBC counts are presented in Figures 1A–C.
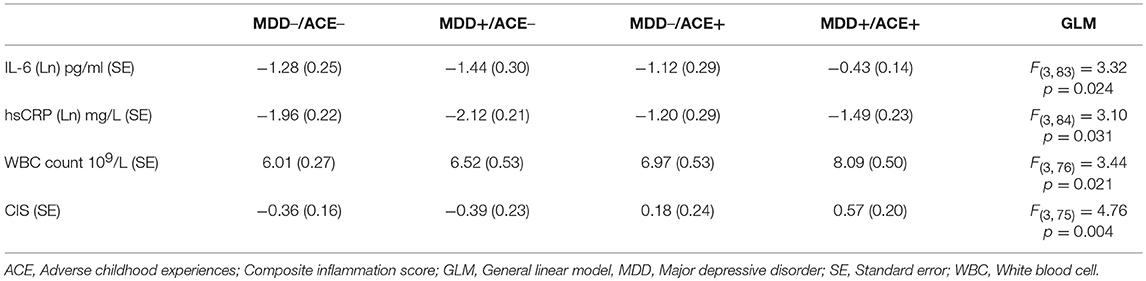
Table 2. Mean values of the inflammatory measures of healthy participants and depressed patients without ACE (MDD–/ACE–, MDD+/ACE–) and healthy participants and depressed patients with ACE (MDD–/ACE+, MDD+/ACE+).
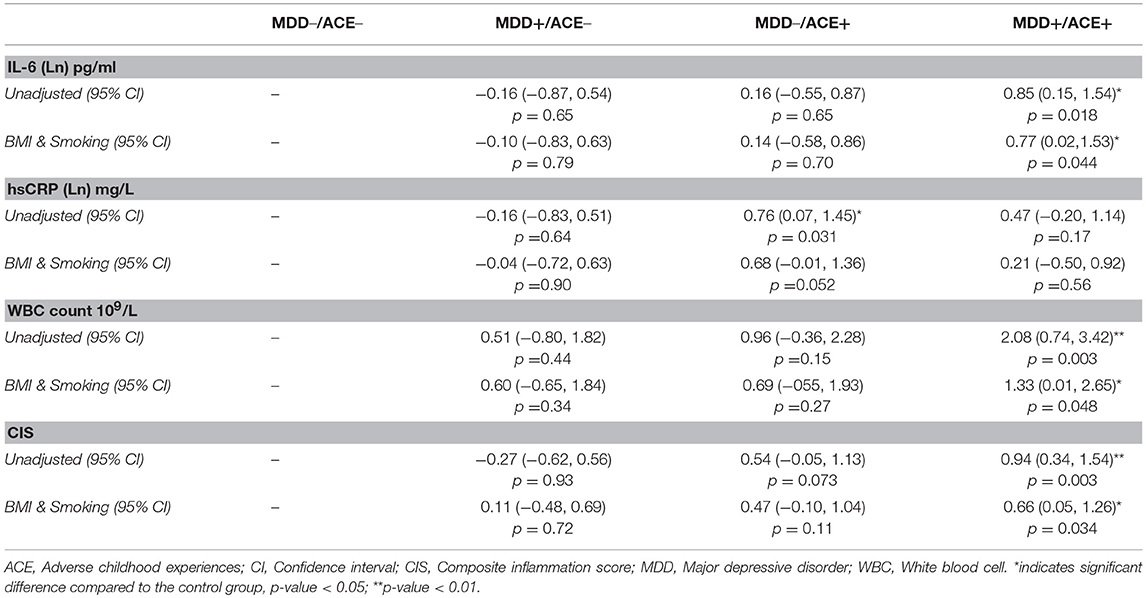
Table 3. Mean differences in inflammatory measures between each of the three study groups (MDD+/ACE–, MDD–/ACE+ and MDD+/ACE+) and control group (MDD–/ACE–) before (unadjusted) and after adjusting for BMI and smoking.
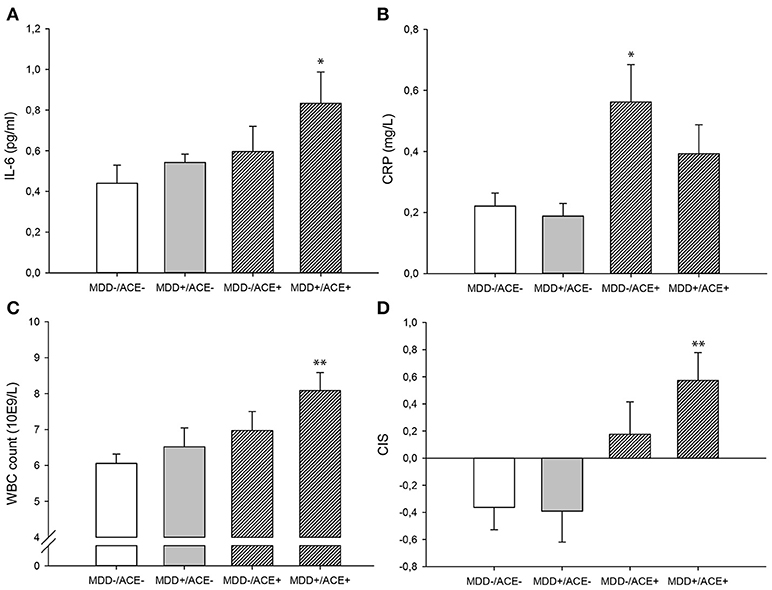
Figure 1. Untransformed and unadjusted mean group values for (A) Il-6, (B) hsCRP, (C) WBC count and (D) the unadjusted mean group CIS (±SE). *p-value < 0.05, **p-value < 0.01 in comparison to the control group (MDD–/ACE–). ACE, Adverse childhood experiences; CIS, Composite inflammation score; MDD, Major depressive disorder; WBC, White blood cell.
There was a significant group effect on the CIS [F(3, 75) = 4.76, p = 0.004, η2 = 0.16, Table 2]. Contrasts between the controls and the different study groups showed that inflammation levels tended to be increased in healthy participants with ACE (p = 0.073, Table 3) and were significantly higher in MDD patients with ACE compared to healthy controls (p = 0.003, Table 3), also after controlling for BMI and smoking (p = 0.034, Table 3). Unadjusted mean group CIS values are depicted in Figure 1D.
With the present study we aimed to disentangle the effects of MDD and ACE on alterations in levels of inflammation by using well-controlled, defined and discrete groups of adults with and without a history of ACE and an MDD diagnosis. Confirming our hypothesis, we observed the highest inflammation levels in MDD patients with a history of ACE. These results replicate prior research showing that inflammation is elevated in a subgroup of MDD patients exposed to ACE (21). Our results are also in line with previous studies reporting that elevations in inflammatory measures observed in MDD patients are associated with childhood trauma (22, 23, 30). Another study comparing cytokine levels between healthy controls and MDD patients with and without a history of ACE found the highest levels of 13 different cytokines in the ACE exposed MDD patients (31). However, in contrast to our findings, no increases in plasma levels of IL-6 were observed in this subgroup of MDD patients. A possible explanation for this discrepancy could be the use of different assay methodology.
Group differences were not completely homogenous regarding the different inflammatory measures that we assessed. While IL-6 concentrations and WBC counts were elevated in MDD patients with ACE, higher CRP concentrations were seen in healthy individuals exposed to ACE compared to healthy controls. This last finding is supported by a recent meta-analysis suggesting that the association between childhood trauma and inflammatory measures, including CRP, is not moderated by the presence of a psychiatric diagnosis (5). However, our finding that CRP is increased in healthy individuals exposed to ACE was no longer significant after adjusting for BMI and smoking. As previously reported by others, unhealthy lifestyle factors like increased BMI and smoking are associated with ACE (32) and have been shown to have an effect on inflammation (33, 34). Therefore, these lifestyle factors could have contributed to the observed elevations in CRP concentrations.
Altogether, the data presented here support the hypothesis that ACE might be a risk factor for developing a MDD later in life, and that this risk is partly mediated by increases in activation of pro-inflammatory pathways (19). In line with this, inflammation levels did not differ between MDD patients without ACE and healthy controls, suggesting that biological mechanisms, other than inflammation, might play a more prominent role in the pathogenesis of depression in these patients. Although previous research has shown increased inflammatory measures in depression (15, 17, 18), in most studies the effects of ACE have not been taken into account.
ACE is not only a risk factor for the development of depression. Also other psychiatric disorders, such as post-traumatic stress-disorder (PTSD), anxiety disorders, and bipolar disorder have been associated with a history of ACE (35, 36) and are as well related to increased inflammation (37, 38). However, until now, only few studies systematically investigated the separate and interactive effects of disease status and a history of ACE on inflammation (39–41). Therefore, future research should attempt to further identify the role of ACE in activating inflammatory pathways in these psychiatric conditions.
Findings from this study are limited by the use of a cross sectional design and the relatively modest sample size, which might have led to insufficient power. Also our study sample included relatively more female than male participants, indicating that our results might be impacted by gender bias. However, the groups did not differ significantly regarding the female to male ratio. In addition, the number of immune parameters that we assessed was limited, and future studies should include additional measures of acute-phase proteins, cytokines, and immune cell characteristics in order to gain better understanding of the biological processes and pathways underlying the inflammation-related pathophysiology of depression in patients with and without a history of ACE.
Our study has several strengths. The study sample consisted of four carefully diagnosed groups which allowed us to disentangle the effects of MDD and ACE on inflammation. Furthermore, none of the patients and participants was taking psychotropic medications. All patients and participants received detailed diagnostics and a physical examination. Our findings of increased levels of inflammation in MDD patients (and to a lesser extent in healthy individuals) exposed to ACE, in this rather young study sample further emphasize the clinical importance of our results, since elevated inflammation is a risk factor implied in the development of somatic disorders like CVD (3, 42). Moreover, the pro-inflammatory state observed in depression also has consequences for treatment success, since patients with elevated inflammation are less likely to respond to conventional antidepressants (43, 44).
In summary, in this well-controlled study, we replicated findings from prior research suggesting accumulative effects of MDD and ACE on a more pro-inflammatory state, while inflammation levels did not differ between MDD patients without ACE and healthy controls. These findings suggest that a subgroup of MDD patients with a history of ACE might benefit from an anti-inflammatory intervention.
This study was carried out in accordance with the recommendations of the Charité's Ethics Committee with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Charité's Ethics Committee.
KdP carried out the laboratory assays, participated in the data analyses, and drafted the manuscript. SE designed the study participated in the analysis and interpretation of study findings and provided editorial assistance. CH participated in the data analysis and provided editorial assistance. CD participated in the conduction of the study, interpretation of study findings and provided editorial assistance. CO participated in the study design, analysis and interpretation of study findings and provided editorial assistance. KW participated in the study design, analysis and interpretation of study findings and provided editorial assistance. LK conceived of and designed the study, conducted the study, participated in the analysis and interpretation of study findings, drafted portions of the manuscript and provided final editorial oversight.
The study was supported by grant of the Deutsche Forschungsgemeinschaft (KU 3106/2-1) awarded to LK, KW, and CO. KdP's, CH's and SE's funding was also provided by grants from the German Federal Ministry of Education and Research 01KR1301A, the European Research Counsil ERC-STG-67073 and US PHS (NIH) grants R01 HD-065825, R01 HD-060628 and R01 AG-050455.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers (2016) 2:16065. doi: 10.1038/nrdp.2016.65
2. Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. (2010) 52:671–90. doi: 10.1002/dev.20494
3. Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proc Natl Acad Sci USA. (2012) 109(Suppl. 2):17302–7. doi: 10.1073/pnas.1121259109
4. Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand. (2014) 129:180–92. doi: 10.1111/acps.12217
5. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry (2016) 21:642–9. doi: 10.1038/mp.2015.67
6. Elwenspoek MMC, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology (2017) 82:140–54. doi: 10.1016/j.psyneuen.2017.05.012
7. Danese A, Baldwin JR. Hidden Wounds? Inflammatory links between childhood trauma and psychopathology. Annu Rev Psychol. (2017) 68:517–44. doi: 10.1146/annurev-psych-010416-044208
8. Tyrka AR, Ridout KK, Parade SH. Childhood adversity and epigenetic regulation of glucocorticoid signaling genes: associations in children and adults. Dev Psychopathol. (2016) 28:1319–31. doi: 10.1017/S0954579416000870
9. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. (2011) 137:959–97. doi: 10.1037/a0024768
10. Moshage H. Cytokines and the hepatic acute phase response. J Pathol. (1997) 181:257–66. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U
11. Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis (2004) 172:1–6. doi: 10.1016/S0021-9150(03)00164-3
12. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. (2006) 8(Suppl. 2):S3. doi: 10.1186/ar1917
13. Surtees P, Wainwright N, Day N, Brayne C, Luben R, Khaw KT. Adverse experience in childhood as a developmental risk factor for altered immune status in adulthood. Int J Behav Med. (2003) 10:251–68. doi: 10.1207/S15327558IJBM1003_05
14. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. (2007) 104:1319–24. doi: 10.1073/pnas.0610362104
15. Maes M, Van Der Planken M, Stevens WJ, Peeters D, Declerck LS, Bridts CH, et al. Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res. (1992) 26:125–34. doi: 10.1016/0022-3956(92)90004-8
16. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
17. Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. (2013) 150:736–44. doi: 10.1016/j.jad.2013.06.004
18. Shafiee M, Tayefi M, Hassanian SM, Ghaneifar Z, Parizadeh MR, Avan A, et al. Depression and anxiety symptoms are associated with white blood cell count and red cell distribution width: a sex-stratified analysis in a population-based study. Psychoneuroendocrinology (2017) 84:101–8. doi: 10.1016/j.psyneuen.2017.06.021
19. Pariante CM. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. Eur Neuropsychopharmacol. (2017) 27:554–9. doi: 10.1016/j.euroneuro.2017.04.001
20. Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry (2006) 163:1630–3. doi: 10.1176/ajp.2006.163.9.1630
21. Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry (2008) 65:409–15. doi: 10.1001/archpsyc.65.4.409
22. Grosse L, Ambree O, Jorgens S, Jawahar MC, Singhal G, Stacey D, et al. Cytokine levels in major depression are related to childhood trauma but not to recent stressors. Psychoneuroendocrinology (2016) 73:24–31. doi: 10.1016/j.psyneuen.2016.07.205
23. Munjiza A, Kostic M, Pesic D, Gajic M, Markovic I, Tosevski DL. Higher concentration of interleukin 6 - A possible link between major depressive disorder and childhood abuse. Psychiatry Res. (2018) 264:26–30. doi: 10.1016/j.psychres.2018.03.072
24. Wittchen H-U, Zaudig, M, Fydrich T. Skid. Strukturiertes klinisches Interview für DSM-IV. Achse I and II. Handanweisung. Göttingen: Hogrefe (1997).
25. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
26. Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry (2008) 192:52–8. doi: 10.1192/bjp.bp.106.032532
27. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess (1996) 67:588–97. doi: 10.1207/s15327752jpa6703_13
28. Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety (2000) 12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W
29. Wingenfeld K, Schaffrath C, Rullkoetter N, Mensebach C, Schlosser N, Beblo T, et al. Associations of childhood trauma, trauma in adulthood and previous-year stress with psychopathology in patients with major depression and borderline personality disorder. Child Abuse Negl. (2011) 35:647–54. doi: 10.1016/j.chiabu.2011.04.003
30. Zeugmann S, Buehrsch N, Bajbouj M, Heuser I, Anghelescu I, Quante A. Childhood maltreatment and adult proinflammatory status in patients with major depression. Psychiatr Danub. (2013) 25:227–35.
31. Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry (2013) 54:953–61. doi: 10.1016/j.comppsych.2013.03.026
32. Caspi A, Houts RM, Belsky DW, Harrington H, Hogan S, Ramrakha S, et al. Childhood forecasting of a small segment of the population with large economic burden. Nat Hum Behav. (2016) 1:0005. doi: 10.1038/s41562-016-0005
33. Visser M, Bouter LM, Mcquillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA (1999) 282:2131–5. doi: 10.1001/jama.282.22.2131
34. Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. (2014) 106:dju294. doi: 10.1093/jnci/dju294
35. Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA (2008) 299:1291–305. doi: 10.1001/jama.299.11.1291
36. Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry (2013) 170:1114–33. doi: 10.1176/appi.ajp.2013.12070957
37. Lindqvist D, Mellon SH, Dhabhar FS, Yehuda R, Grenon SM, Flory JD, et al. Increased circulating blood cell counts in combat-related PTSD: associations with inflammation and PTSD severity. Psychiatry Res. (2017) 258:330–6. doi: 10.1016/j.psychres.2017.08.052
38. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. (2018) doi: 10.1111/nyas.13712
39. Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. (2014) 42:81–8. doi: 10.1016/j.bbi.2014.06.003
40. Aas M, Dieset I, Hope S, Hoseth E, Morch R, Reponen E, et al. Childhood maltreatment severity is associated with elevated C-reactive protein and body mass index in adults with schizophrenia and bipolar diagnoses. Brain Behav Immun. (2017) 65:342–9. doi: 10.1016/j.bbi.2017.06.005
41. Moraes JB, Maes M, Barbosa DS, Ferrari TZ, Uehara MKS, Carvalho AF, et al. Elevated C-reactive protein levels in women with bipolar disorder may be explained by a history of childhood trauma, especially sexual abuse, body mass index and age. CNS Neurol Disord Drug Targets (2017) 16:514–21. doi: 10.2174/1871527316666170407151514
42. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens (2015) 28:1295–302. doi: 10.1093/ajh/hpv047
43. Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors' and longitudinal ‘targets'. Neuropsychopharmacology (2013) 38:377–85. doi: 10.1038/npp.2012.191
Keywords: acute-phase protein, childhood adversity, childhood maltreatment, depression, inflammation, pro-inflammatory cytokine
Citation: de Punder K, Entringer S, Heim C, Deuter CE, Otte C, Wingenfeld K and Kuehl LK (2018) Inflammatory Measures in Depressed Patients With and Without a History of Adverse Childhood Experiences. Front. Psychiatry 9:610. doi: 10.3389/fpsyt.2018.00610
Received: 31 August 2018; Accepted: 30 October 2018;
Published: 27 November 2018.
Edited by:
Anthony S. Zannas, University of North Carolina at Chapel Hill, United StatesReviewed by:
Marisa Moller, North-West University, South AfricaCopyright © 2018 de Punder, Entringer, Heim, Deuter, Otte, Wingenfeld and Kuehl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin de Punder, S2FyaW4uZGUtcHVuZGVyQGNoYXJpdGUuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.