- 1Department of Neurology, Shenzhen People's Hospital, Second Clinical College, Jinan University, Shenzhen, China
- 2Department of Neurology, Shenzhen University General Hospital, Shenzhen, China
Background: High-frequency (HF) repetitive transcranial magnetic stimulation (rTMS) over the left dorsolateral prefrontal cortex (L-DLPFC) is the most widely applied treatment protocol for major depressive disorder (MDD), while low-frequency (LF) rTMS over the right DLPFC (R-DLPFC) also exhibits similar, if not better, efficacy for MDD. Therefore, a meta-analysis is warranted to compare the efficacy of the two protocols for MDD.
Method: We searched the literature from 1990 through to August 1, 2017 using MEDLINE, and the literature from 1995 through to August 1, 2017 using EMBASE, PsycINFO, the Cochrane Central Register of Controlled Trials (CENTRAL), SCOPUS, and ProQuest Dissertations and Theses (PQDT). We included randomized controlled trials (RCT) comparing the efficacy of HF rTMS over the L-DLPFC and LF rTMS over the R-DLPFC for MDD, which used response and/or remission rates as the primary endpoints, with and without sham-controlled.
Results: (1) The meta-analysis of the response rates was based on 12 studies, including 361 patients with MDD (175 for HF (> 5 Hz) over the L-DLPFC, and 186 for LF (<5 Hz) over the R-DLPFC; odds ratio = 1.08; 95%, confidence interval = 0.88–1.34). (2) The meta-analysis of the remission rate was based on 5 studies, including 131 MDD patients (64 for HF over the L-DLPFC and 67 for LF over the R-DLPFC; odds ratio = 1.29; 95% confidence interval = 0.54–3.10).
Conclusion: Both HF rTMS over the L-DLPFC and LF over the R-DLPFC demonstrated similar therapeutic efficacy for the treatment of patients with MDD. The results suggested that further investigation on treatment efficacy indicators before/during treatment is necessary and helpful for optimizing a personalized protocol for patients.
Introduction
Transcranial magnetic stimulation (TMS) is a non-intrusive neuromodulation technique used to induce brief magnetic pulses of up to several Tesla in strength, via rapid discharging current of several thousand amperes through a stimulation coil (1, 2). The magnetic field can induce an electrical field in the cortex to depolarize superficial axons and to activate the neural networks when the coil is placed on a human head. The physiological effects induced by the electrical field depend on many TMS parameters, such as coil type and orientation, magnetic pulse waveform, stimulation frequency, and pattern. The consensus appeared to consider that high-frequency (HF, ≥ 5 Hz) stimulation could induce excitatory plasticity and low-frequency (LF, ≤ 1 Hz) stimulation could induce inhibitory plasticity in the cortex, based on motor evoked potential size changes in response to M1 stimulation in healthy subjects (3). Although this dichotomy is not entirely satisfying, many studies and clinical treatment protocols have been designed based on this principle.
Major depressive disorder is characterized by metabolic and neuronal activity asymmetry in the two prefrontal areas, showing elevated glucose and oxygen consumption as well as EEG activity on the right side, while it was suppressed on the left side, and the neural activity asymmetry correlated with clinical scores (4–7). Thus two main rTMS research protocols for the treatment of depression have been developed: LF stimulation on the right dorsolateral prefrontal cortex (R-DLPFC) (inducing inhibitory plasticity on the presumably hyperactive area), HF stimulation on the left DLPFC (L-DLPFC) (inducing excitatory plasticity on the presumably hypoactive area), or a combination of the two (8–10). A meta-analysis based on 29 randomized, double-blind, sham-controlled trials (RCTs), and 1,371 subjects with MDD showed that HF-rTMS was significantly effective in improving clinical scores compared to sham stimulation (11), and another meta-analysis consisting of eight RCTs and 263 subjects with MDD showed similar therapeutic efficacy of LF-rTMS for patients with MDD compared to sham stimulation (12). It is not clear which protocol is more effective or if they are equivalent for the treatment of MDD.
Here, we summarized the best available evidence to compare the therapeutic efficacy of the two most widely used protocols, LF-rTMS over the right DLPFC and HF-rTMS over the left DLPFC, for the treatment of patients with MDD. We performed a systematic review and meta-analysis of RCTs which directly compared the efficacy of the two protocols to compare the effects of the two protocols. We assessed both the response and remission rates.
Materials and Methods
Search Criteria
We identified the articles included in this meta-analysis according to the following criteria:
1) Searching MEDLINE from 1990 until August 1, 2017
2) Searching EMBASE, PsycINFO, the Cochrane Central Register of Controlled Trials (CENTRAL), SCOPUS and ProQuest Dissertations & Theses (PQDT) from 1 January 1995 until August 1, 2017
3) Screening the bibliography of the previous meta-analyses and reviews on rTMS for MDD.
4) The key words “depression” and “transcranial magnetic stimulation” were used for searching above. The workflow to search and exclude studies is illustrated in Figure 1.
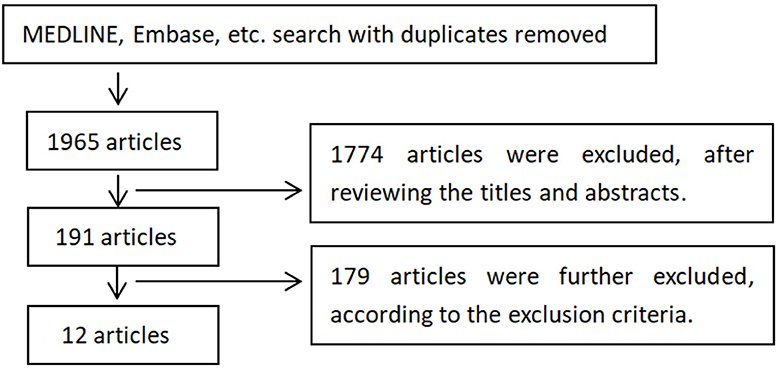
Figure 1. Selection of studies for inclusion. The 1,774 articles were excluded since most of them were other not research articles or not relevant to TMS treatment on depression. The 179 articles were excluded based on exclusion and inclusion criteria, including reviews and case reports, too few treatment sessions (<10 sessions), treatment protocols (compared rTMS with other treatments like transcranial direct current stimulation, non-random allocation, theta-burst stimulations), narrow diagnoses of depression, not reporting treatment efficacy, or rTMS not first time introduced to patients.
In Order to Pool High Quality and Homogenous RCTS in our Meta-Analysis, We Applied Inclusion and Exclusion Criteria
Patients Characteristics
Patients aged 18–80 years, diagnosed with primary major depressive episode (unipolar or bipolar) according to the Diagnostic and Statistical Manual of Mental Disorders (APA, 1994) or the International Classification of Diseases (WHO, 1992) criteria.
Treatment Characteristics
LF-rTMS (≤5 Hz) over the R-DLPFC or HF-rTMS (≥5 Hz) over the L-DLPFC, were administered for ≥10 sessions, either as a monotherapy or as an augmentation strategy for patients with MDD.
Publication-Related
We only included articles written in English.
Exclusion Criteria
1) Studies that included patients with “narrow” diagnoses (e.g., postpartum depression, premenstrual dysphoric disorder, involutional depression) or secondary MDD (e.g., vascular depression, substance/medication-induced depression, psychotic depression)
2) Started rTMS treatment as a new antidepressant was introduced
3) Studies that did not report treatment efficacy (e.g., response or remission rate)
4) Studies that used non-randomized patient allocation.
The first two authors independently searched and identified studies to be included. The corresponding author decided whether the study should be included or excluded if there was disagreement between the first two authors.
Procedure
Meta-Analysis Statistics
The response rate was used as the primary measurement of treatment efficacy. The response was defined as at least a 50% reduction in clinical evaluation scores (such as the Hamilton Depression Rating Scale, Montgomery Åsberg Depression Rating Scale, or Beck's Depression Inventory). The remission rate was used as the secondary measurement of efficacy, since not all studies reported these data.
RevMan 5.0 (Cochrane Information Management System) was used to perform statistical analysis. We used a Mantel–Haenszel fixed-effects model to calculate the combined ORs for each outcome and the chi-squared-based Q-test and I-squared index to assess heterogeneity. All tests were two-sided with statistical significance set to a P-value of 0.05 unless otherwise stated.
Results
Included Studies
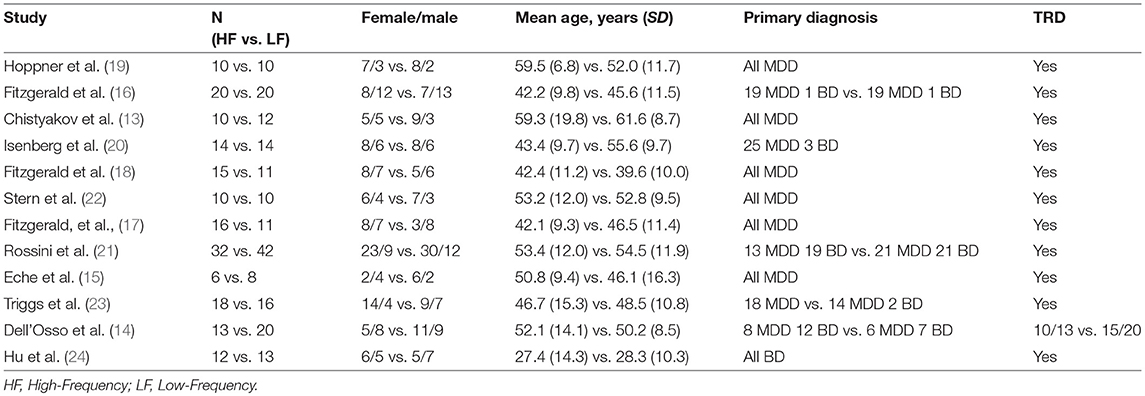
12 RCTs and 361 patients with MDD were included in the present meta-analysis (13–23). Among them, 175 patients were randomized to receive HF-rTMS over the L-DLPFC (mean age = 47.7 years, SD = 12.0 years, women = 57.1%), and 186 were randomized to receive LF-rTMS over the R-DLPFC (mean age = 49.6 years, SD = 10.9 years, women = 58.1%) (Table 1).
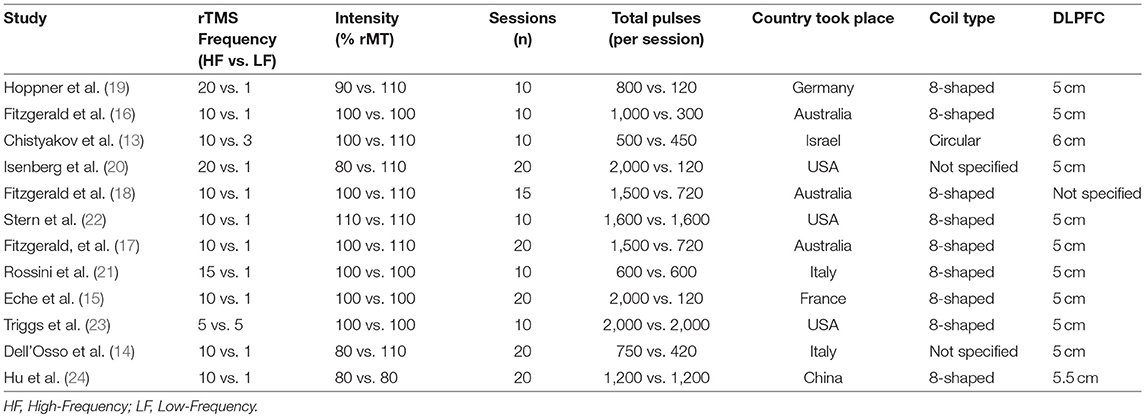
The mean number of rTMS treatment sessions was 14.6 (SD = 5.0), and the mean total rTMS pulses number for HF-rTMS was 19,708 (SD = 12,163), the mean total rTMS pulses number for LF-rTMS was 9,425 (SD = 7,621) (Table 2).
Response Rate
The response rate was reported in all 12 RCTs (Figure 2) at the end of treatment. Overall, 78 of 175 subjects (44.6%) and 76 of 186 subjects (40.9%) receiving HF-rTMS over the L-DLPFC and LF-rTMS over the R-DLPFC, respectively, were classified as responders. The pooled OR was 1.08 (95% CI: 0.88–1.34, Z = 0.67, P = 0.50), indicating a comparative therapeutic efficacy between the two rTMS treatment protocols.
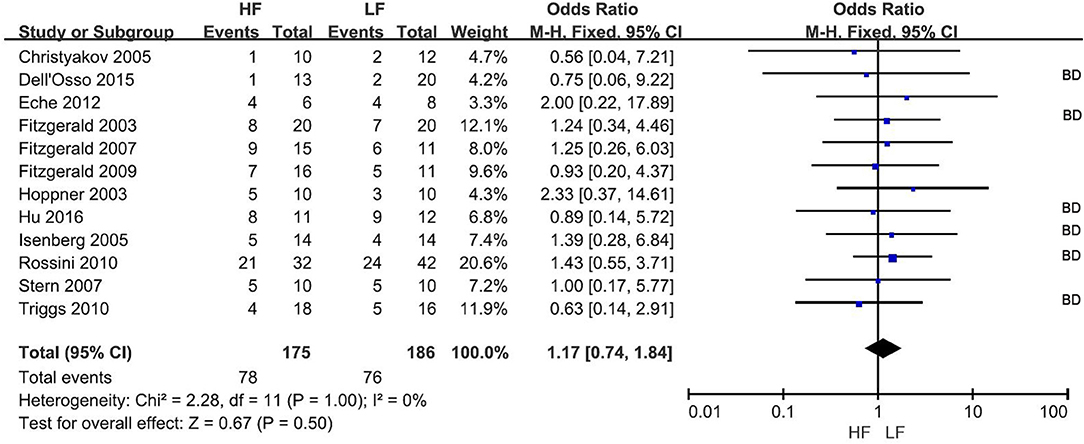
Figure 2. Meta-analysis of HF vs. LF-rTMS for MDD: response rate. HF, High Frequency; LF, Low Frequency; BD, Study in which more than one bipolar depressive patient involved.
Remission Rate
The remission rate was reported in 5 RCTs (Figure 3) at the end of treatment. Overall, 14 of 64 subjects (21.9%) and 11 of 67 subjects (16.4%) receiving HF-rTMS over L-DLPFC and LF-rTMS over R-DLPFC, respectively, were classified as remitters. The pooled OR was 1.29 (95% CI: 0.54-3.10, Z = 0.58, P = 0.56), indicating a similar therapeutic efficacy between the two rTMS treatment protocols.
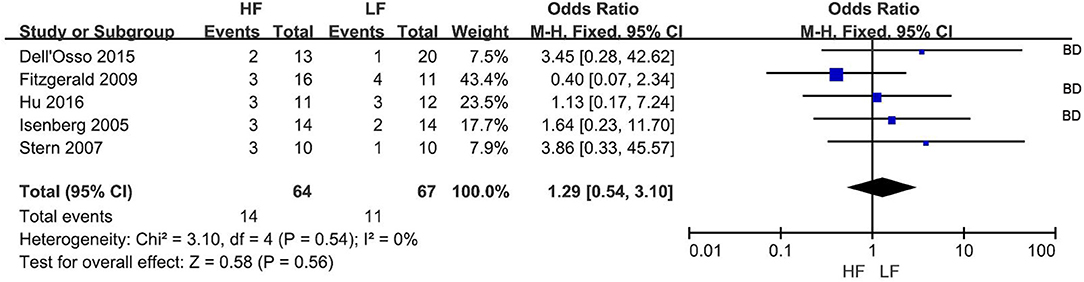
Figure 3. Meta-analysis of HF vs. LF-rTMS for MDD: remission rate. HF, High Frequency; LF, Low Frequency; BD, Study in which more than one bipolar depressive patient involved.
Dropout Rate
Only 2 RCTs reported a dropout rate. Overall, 2 of 36 (5.5%) patients withdrew in HF-rTMS over the L-DLPFC group, and no patients in the LF-rTMS over the R-DLPFC group.
Discussion
This is the first meta-analysis to compare the therapeutic efficacy of HF-rTMS over the L-DLPFC and LF-rTMS over the R-DLPFC for MDD in terms of both response and remission rates. The results showed that the two treatment protocols exhibited similar clinical efficacy (with 44.6 and 40.9% response rate for HF- and LF-rTMS, respectively; with 21.9 and 16.4% remission rate for HF- and LF-rTMS, respectively; with odds ratios of 1.08 and 1.29 for the response and remission rates, respectively). These results are consistent with those of a previous meta-analysis including a smaller number of RCTs and patients (25), and further extended the conclusion to include the remission rate. For the acceptability of different protocols, a meta-analysis including eight RCTs and 263 subjects showed that the dropout rate of the LF-rTMS treatment group was 5.3% (7/132) (12), while a meta-analysis including 29 RCTs and 1,371 subjects showed that the dropout rate of the HF-rTMS treatment group was 7.5% (55/730) (11). Moreover, considering safety issues, such as seizure induction, LF-rTMS over the L-DLPFC appears to be safer than HF-rTMS (26). To summarize, both HF-rTMS over the L-DLPFC and LF-rTMS over the R-DLPFC showed equivalent clinical efficacy for the treatment of patients with MDD.
Although both HF- and LF-rTMS over frontal areas have been proven effective for the treatment of MDD, it is challenging to conclude which is the optimal treatment protocol (27), since several rTMS variables significantly influence therapeutic efficacy, such as stimulation parameters and location. New ways to improve therapeutic effects should be further explored. For instance, new stimulation protocols such as theta burst stimulation (TBS) over the frontal cortex showed that intermittent TBS (iTBS, presumably causing facilitation similar to HF-rTMS) over the L-DLPFC and continuous TBS (cTBS, presumably resulting in suppression similar to LF-rTMS) over the R-DLPFC could improve the depressive symptoms (28, 29). Moreover, a new type of coil such as the H-coil to directly stimulate deeper brain regions have been developed and proven to be effective for the treatment of depression (30, 31). Further investigation should focus on improving the therapeutic effects rather than proving clinical efficacy.
Several directions may be suggested for the optimization of the clinical protocols. (1) Stimulation target. Most RCTs on depression to date have used the general “5-cm rule,” identifying the DLPFC target 5 cm anterior from the motor cortex site along the scalp surface corresponding to the abductor pollicis brevis muscle (32). However, several recent studies have shown that this location method is probably not optimal. Herbsman et al. found that more lateral and anterior stimulation over the prefrontal cortex provided a better antidepressant response (33), and Fitzgerald et al. showed that a neuronavigation method based on individual structural MRI was more effective than the standard 5 cm technique (34). A systematic comparison confirmed that DLPFC location based on individual structural MRI was ~2 cm anterior to that of the standard “5-cm rule” (35). Further simulation results based on electrical field and brain network showed that stimulation on the anterior and lateral DLPFC areas more likely to activate the executive network, while stimulation on posterior and medial DLPFC areas more likely to activate the default-mode network, causing different physiological consequences (36, 37). Fox et al. used a novel intrinsic (resting state fMRI) connectivity-based approach to gain insight into why some left DLPFC TMS targets have proven more clinically effective than the others, and they found that DLPFC TMS sites with better clinical efficacy were more negatively correlated with the activity of the subgenual cingulate (38). To summarize, further optimization of the DLPFC target via MRI-based anatomical location and/or EEG/fMRI-based functional location is needed (39).
(2) Dosage. As antidepressant medications, rTMS needs to accumulate “dosage” (affected by the intensity (%RMT), the pulse number per session and the session number) generate clinical efficacy. rTMS dosage in the RCTs on depression varied greatly, e.g., in intensity (80–120%), in the number of stimuli per session (120–3,000), and in the total number of treatment sessions (10–30) (2). The dosage is of importance, since it has been demonstrated that clinical efficacy for HF-rTMS over the L-DLPFC was higher for a higher number of sessions and rTMS pulses per session, and the rate of responders increased significantly when the total number of sessions was more than 10, the total number of pulses per session was more than 1,000, and the stimulation intensity was greater than 100% resting motor threshold (40). A recent meta-analysis found a similar influence of the stimulation parameters for LF-rTMS over the R-DLPFC, showing that more than 1200 pulses per session were needed to achieve high levels of response (12).
(3) Biomarkers. It remains unclear why some patients respond to rTMS treatment, while others do not; why some patients respond to HF-rTMS over the L-DLPFC, while others to LF-rTMS over the R-DLPFC, after more than 2 decades of exploration of rTMS treatment on primary depression. The reason is that the biomarkers of rTMS have not been elucidated. A very recent study showed that functional connectivity analysis based on resting-state fMRI could define depression subtypes and further predict responsiveness to TMS therapy (41). Additionally, TMS concurrent with EEG was of great use to study neural plasticity change and network reorganization induced by TMS therapy, highlighting the potential to elucidate TMS treatment-related biomarkers (42, 43).
Limitations
First, only some of the studies (5/12) reported the remission rate in the present meta-analysis, and thus the remission rate analysis was less powerful than the response rate analysis. Second, we only examined the efficacy of TMS treatment immediately after each study's end, since very few studies reported long-term follow-up efficacy. Several studies showed that HF-rTMS efficacy could last for several months (44, 45). We could not estimate the stability of long-term antidepressant effects. This is important for future protocol optimization, since rTMS treatment sessions are labor-intensive and time-consuming for the patients (27). Third, we did not discriminate rTMS as a monotherapy or an augmentation strategy. Fourth, the conclusion was based on bipolar and unipolar patents grouped together, while ignoring the difference between the two populations. Fifth, we assessed both response and remission rates as treatment efficacy, as these two parameters are the two mostly used primary endpoints to evaluate depression treatment. We believe that the addition of continuous depression severity scores as an outcome in future studies will be more informative, and it probably improves rTMS treatment protocol. The last, in the current meta-analysis, we specifically focused on the efficacy comparison between the two most popular rTMS treatment protocols on depression: high frequency rTMS over left DLPFC vs. low frequency rTMS over right DLPFC. So, we only included the studies directly comparing the efficacy of the two protocols. We noticed that there is a latest meta-analysis on efficacy and acceptability of non-invasive brain stimulation (including different kinds of TMS and tDCS protocols) for the treatment of adult unipolar and bipolar depression (46), which is more comprehensive and informative.
Author Contributions
XC wrote the manuscript. CD and XS searched and analyzed data. YG polished the manuscript.
Funding
This work was supported by Technical Research and Development Project of Shenzhen (grant number JCYJ20170307100237349), Research Project of Health and Family Planning Commission of Shenzhen Municipality (grant number SZXJ2017034).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet (1985) 1:1106–7. doi: 10.1016/S0140-6736(85)92413-4
2. Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. (2014) 125:2150–206. doi: 10.1016/j.clinph.2014.05.021
3. Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. (2003) 148:1–16. doi: 10.1007/s00221-002-1234-2
4. Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. (1995) 25:247–61. doi: 10.1017/S0033291700036151
5. Diego MA, Field T, Hernandez-Reif M. Ces-d depression scores are correlated with frontal eeg alpha asymmetry. Depress Anxiety (2001) 13:32–7. doi: 10.1002/1520-6394(2001)13:1 < 32::AID-DA5>3.0.CO;2-G
6. Kennedy SH, Javanmard M, Vaccarino FJ. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry (1997) 42:467–75. doi: 10.1177/070674379704200502
7. Knott V, Mahoney C, Kennedy S, Evans K. Eeg power, frequency, asymmetry and coherence in male depression. Psychiatry Res. (2001) 106:123–40. doi: 10.1016/S0925-4927(00)00080-9
8. George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. A controlled trial of daily left prefrontal cortex tms for treating depression. Biol Psychiatry (2000) 48:962–70. doi: 10.1016/S0006-3223(00)01048-9
9. Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry (1999) 56:315–20. doi: 10.1001/archpsyc.56.4.315
10. Speer AM, Kimbrell TA, Wassermann EM, D Repella J, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rtms on regional brain activity in depressed patients. Biol Psychiatry (2000) 48:1133–41. doi: 10.1016/S0006-3223(00)01065-9
11. Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rtms) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. (2014) 44:225–39. doi: 10.1017/S0033291713000512
12. Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rtms) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology (2013) 38:543–51. doi: 10.1038/npp.2012.237
13. Chistyakov AV, Kaplan B, Rubichek O, Kreinin I, Koren D, Feinsod M, et al. Antidepressant effects of different schedules of repetitive transcranial magnetic stimulation vs. Clomipramine in patients with major depression: relationship to changes in cortical excitability. Int J Neuropsychopharmacol. (2005) 8:223–33. doi: 10.1017/S1461145704004912
14. Dell'Osso B, Oldani L, Camuri G, Dobrea C, Cremaschi L, Benatti B, et al. Augmentative repetitive transcranial magnetic stimulation (rtms) in the acute treatment of poor responder depressed patients: a comparison study between high and low frequency stimulation. Eur Psychiatry (2015) 30:271–6. doi: 10.1016/j.eurpsy.2014.12.001
15. Eche J, Mondino M, Haesebaert F, Saoud M, Poulet E, Brunelin J. Low- vs high-frequency repetitive transcranial magnetic stimulation as an add-on treatment for refractory depression. Front Psychiatry (2012) 3:13. doi: 10.3389/fpsyt.2012.00013
16. Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry (2003) 60:1002–8. doi: 10.1001/archpsyc.60.9.1002
17. Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety (2009) 26:229–34. doi: 10.1002/da.20454
18. Fitzgerald PB, Sritharan A, Daskalakis ZJ, de Castella AR, Kulkarni J, Egan G. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. (2007) 27:488–92. doi: 10.1097/jcp.0b013e318151521c
19. Hoppner J, Schulz M, Irmisch G, Mau R, Schlafke D, Richter J. Antidepressant efficacy of two different rtms procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur Arch Psychiatry Clin Neurosci. (2003) 253:103–9. doi: 10.1007/s00406-003-0416-7
20. Isenberg K, Downs D, Pierce K, Svarakic D, Garcia K, Jarvis M, et al. Low frequency rtms stimulation of the right frontal cortex is as effective as high frequency rtms stimulation of the left frontal cortex for antidepressant-free, treatment-resistant depressed patients. Ann Clin Psychiatry (2005) 17:153–9. doi: 10.1080/10401230591002110
21. Rossini D, Lucca A, Magri L, Malaguti A, Smeraldi E, Colombo C, et al. A symptom-specific analysis of the effect of high-frequency left or low-frequency right transcranial magnetic stimulation over the dorsolateral prefrontal cortex in major depression. Neuropsychobiology (2010) 62:91–7. doi: 10.1159/000315439
22. Stern WM, Tormos JM, Press DZ, Pearlman C, Pascual-Leone A. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double-blind, randomized, placebo-controlled trial. J Neuropsychiatry Clin Neurosci. (2007) 19:179–86. doi: 10.1176/jnp.2007.19.2.179
23. Triggs WJ, Ricciuti N, Ward HE, Cheng J, Bowers D, Goodman WK, et al. Right and left dorsolateral pre-frontal rtms treatment of refractory depression: a randomized, sham-controlled trial. Psychiatry Res. (2010) 178:467–74. doi: 10.1016/j.psychres.2010.05.009
24. Hu SH, Lai JB, Xu DR, Qi HL, Peterson BS, Bao AM, et al. Efficacy of repetitive transcranial magnetic stimulation with quetiapine in treating bipolar II depression: a randomized, double-blinded, control study. Sci Rep. (2016) 6:30537. doi: 10.1038/srep30537
25. Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. (2013) 210:1260–4. doi: 10.1016/j.psychres.2013.09.007
26. Rossi S, De Capua A, Tavanti M, Calossi S, Polizzotto NR, Mantovani A, et al. Dysfunctions of cortical excitability in drug-naive posttraumatic stress disorder patients. Biol Psychiatry (2009) 66:54–61. doi: 10.1016/j.biopsych.2009.03.008
27. Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Therapeut. (2012) 133:98–107. doi: 10.1016/j.pharmthera.2011.09.003
28. Chistyakov AV, Rubicsek O, Kaplan B, Zaaroor M, Klein E. Safety, tolerability and preliminary evidence for antidepressant efficacy of theta-burst transcranial magnetic stimulation in patients with major depression. Int J Neuropsychopharmacol. (2010) 13:387–93. doi: 10.1017/S1461145710000027
29. Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain (2014) 137:2088–98. doi: 10.1093/brain/awu109
30. Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul. (2009) 2:188–200. doi: 10.1016/j.brs.2009.08.002
31. Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry (2015) 14:64–73. doi: 10.1002/wps.20199
32. George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry (2010) 67:507–16. doi: 10.1001/archgenpsychiatry.2010.46
33. Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry (2009) 66:509–15. doi: 10.1016/j.biopsych.2009.04.034
34. Fitzgerald PB, Hoy K, McQueen S, Maller JJ, Herring S, Segrave R, et al. A randomized trial of rtms targeted with mri based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology (2009) 34:1255–62. doi: 10.1038/npp.2008.233
35. Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of tms coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin. (2010) 40:27–36. doi: 10.1016/j.neucli.2010.01.001
36. Opitz A, Fox MD, Craddock RC, Colcombe S, Milham MP. An integrated framework for targeting functional networks via transcranial magnetic stimulation. Neuroimage (2016) 127:86–96. doi: 10.1016/j.neuroimage.2015.11.040
37. Opitz A, Legon W, Rowlands A, Bickel WK, Paulus W, Tyler WJ. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage (2013) 81:253–64. doi: 10.1016/j.neuroimage.2013.04.067
38. Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry (2012) 72:595–603. doi: 10.1016/j.biopsych.2012.04.028
39. Hannah R, Rocchi L, Tremblay S, Rothwell JC. Controllable pulse parameter tms and tms-eeg as novel approaches to improve neural targeting with rtms in human cerebral cortex. Front Neural Circuits (2016) 10:97. doi: 10.3389/fncir.2016.00097
40. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry (2003) 160:835–45. doi: 10.1176/appi.ajp.160.5.835
41. Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. (2017) 23:28–38. doi: 10.1038/nm.4246
42. Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical ltp in humans: a combined tms/eeg study. Brain Res Bull. (2006) 69:86–94. doi: 10.1016/j.brainresbull.2005.11.003
43. Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with tms-eeg and tms-fmri. Brain Topograp. (2011) 24:302–15. doi: 10.1007/s10548-011-0196-8
44. Janicak PG, Nahas Z, Lisanby SH, Solvason HB, Sampson SM, McDonald WM, et al. Durability of clinical benefit with transcranial magnetic stimulation (tms) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. (2010) 3:187–99. doi: 10.1016/j.brs.2010.07.003
45. Mogg A, Pluck G, Eranti SV, Landau S, Purvis R, Brown RG, et al. A randomized controlled trial with 4-month follow-up of adjunctive repetitive transcranial magnetic stimulation of the left prefrontal cortex for depression. Psychol Med. (2008) 38:323–33. doi: 10.1017/S0033291707001663
46. Mutz J, Edgcumbe DR, Brunoni AR, Fu CH. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. (2018) 92:291–303. doi: 10.1016/j.neubiorev.2018.05.015
Keywords: transcranial magnetic stimulation, major depression disorder, dorsal-lateral prefrontal cortex, meta-analysis, treatment
Citation: Cao X, Deng C, Su X and Guo Y (2018) Response and Remission Rates Following High-Frequency vs. Low-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) Over Right DLPFC for Treating Major Depressive Disorder (MDD): A Meta-Analysis of Randomized, Double-Blind Trials. Front. Psychiatry 9:413. doi: 10.3389/fpsyt.2018.00413
Received: 14 November 2017; Accepted: 13 August 2018;
Published: 07 September 2018.
Edited by:
Beata Godlewska, University of Oxford, United KingdomReviewed by:
Giuseppe Tavormina, Psychiatric Studies Centre, ItalyCynthia H. Y. Fu, University of East London, United Kingdom
Muhammad Ishrat Husain, University of Toronto, Canada
Copyright © 2018 Cao, Deng, Su and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Guo, xuanyi_guo@163.com
 Xu Cao
Xu Cao Chunshan Deng
Chunshan Deng Xiaolin Su
Xiaolin Su Yi Guo
Yi Guo