- 1Department of Neurology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 2Department of Neuropsychiatry and Behavioral Neurology and Clinical Psychology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 3China National Clinical Research Center for Neurological Diseases, Beijing, China
- 4Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 5Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
- 6Department of Clinical Psychology, Capital Medical University, Beijing, China
- 7Unit of Psychiatry, Faculty of Health Sciences, University of Macau, Taipa, Macau
- 8Department of Interventional Neuroradiology, Beijing Tian Tan Hospital, Capital Medical University, Beijing, China
- 9University of Notre Dame Australia, Marian Centre and Graylands Hospital, Perth, WA, Australia
- 10Department of Psychiatry, University of Melbourne, Melbourne, VIC, Australia
Objective: Few studies have examined the association between post-stroke depression (PSD), aphasia, and physical independence in Chinese patients. This study investigated the above association in stroke patients in China at 3-month follow-up.
Methods: Altogether 270 patients within 14 days after ischemic stroke were recruited and followed up at 3 months. PSD, aphasia, and physical functional status were measured using the Stroke Aphasia Depression Questionnaire (SADQ), Western Aphasia Battery (WAB), and modified Rankin Scale (mRS), respectively. Patients with mRS total score >2 were considered as having “physical dependence.”
Results: Out of 248 patients at 3-month follow up, 119 (48%) were rated as having physical dependence. Multiple logistic regression analyses revealed that female (p = 0.04; OR = 2.2; 95% CI: 1.0–5.1), more severe stroke at admission (p < 0.01; OR = 1.4; 95% CI: 1.3–1.5), and more severe PSD at 3 months (p = 0.01; OR = 1.05; 95% CI: 1.01–1.1) were independently associated with physical dependence at 3 months.
Conclusions: Greater PSD and stroke severity were independently associated with physical dependence at 3 months after stroke. Aphasia was also associated with physical dependence but the relationship was not significant. Early and effective depression screening, treatment and stroke rehabilitation appear to be important to improve the physical outcome and reduce the burden of stroke survivors.
Introduction
Post-stroke depression (PSD) is one of the most common psychiatric comorbidities in stroke survivors, with a prevalence ranging from 20 to 65% (1–4). PSD is significantly associated with poor treatment adherence and increased risk of disability, mortality, stroke recurrence, and poor quality of life (5, 6). Aphasia occurs in about a third of ischemic stroke patients and is associated with impaired activity of daily life (7, 8) and higher risk of PSD (9, 10). Poor functional outcome after stroke could result in significant personal distress and family burden (11). Understanding the association between PSD, aphasia and physical independence is hence important to develop comprehensive treatment strategies for stroke survivors.
In China, the lifetime prevalence of stroke was 2.08% (95% CI, 2.02–2.13%) (12) in 2017, which translates to ~2.9 million stroke patients. An Italy study found PSD was the only significant factor related to functional recovery from discharge to 3-month follow-up after stroke but not aphasia (13). To the best of our knowledge, there are no studies that have investigated the independent association between PSD, aphasia, and physical independence in stroke survivors in China. This study thus aimed to examine the association between PSD, aphasia, and physical independence in Chinese stroke patients at 3-month follow-up.
Methods
Participants and Study Setting
This prospective cohort study was conducted between April 2014 and October 2015 in the Stroke Centre of Beijing Tiantan Hospital. A total of 320 patients were consecutively screened if they fulfilled the following criteria: (1) aged 18 years or older; (2) had an acute ischemic stroke within 14 days according to the WHO diagnostic criteria (14) confirmed by computed tomography (CT) or magnetic resonance imaging (MRI); and (3) had the ability to provide informed consent and complete the assessment. Exclusion criteria included: (1) history of language impairment; (2) drug and alcohol abuse and severe psychiatric disorders; (3) other major medical conditions, such as Parkinson's disease; and (4) severe cognitive deficit defined by the Mini Mental State Examination (MMSE) total score <18 (15, 16). The first three were excluded based on the self-reported pre-stroke histories by unstructured interviews and the last was made at the start of the study. The study protocol was approved by the ethics committee of Beijing Tiantan Hospital, Capital Medical University. All participants provided written informed consent.
Measurement Instruments and Evaluation
Assessment was conducted at baseline and 3 months after index ischemic stroke. Patients' socio-demographic and clinical characteristics at baseline were recorded via a review of electronic medical records and confirmed by a clinical interview conducted by trained research neurologists. Severity and type of stroke was assessed with the National Institutes of Health Stroke Scale (NIHSS) (17–19) and the Trial of Org 10172 Acute Stroke Treatment (TOAST) (20, 21).
Physical independence and degree of handicap was evaluated using the modified Ranking Scale (mRS) at 3 months, with mRS total score >2 indicating physical dependence (22, 23). As most depression scales cannot be used in stroke patients with aphasia due to their impaired communication ability, the severity of depressive symptoms at 14 ± 2 days and 3 months after index stroke was measured using the 21-item Stroke Aphasic Depression Questionnaire (SADQ) (24, 25) with total score ranges from 0 to 63, ≥19 indicating the presence of PSD, ≥ 22 indicating moderate depressive symptoms and ≥ 26 indicating major depressive symptoms. The SADQ relies on external observation of emotional behavior by nursing staff and family members in recent time. The hospital version (SADQ-H) focus on the recent week. The severity and type of aphasia was evaluated with the Aphasia Quotient (AQ) derived from the Western Aphasia Battery (WAB), with AQ < 93.8 indicating the presence of aphasia (26). The language assessment was also conducted at 3-month follow-up with much missing data on AQ score, so the data was not analyzed.
Statistical Analysis
Data were analyzed with SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Socio-demographic and clinical variables were compared between physical independence and dependence groups using Chi-square test, independent sample t-test and Mann-Whitney U-test, as appropriate. Independent correlates of the physical dependence at 3 months were examined using multivariate logistic regression analysis with the “enter” method. The outcome at 3 months was the dependent variable, while variables that significantly differed between both groups in the univariate analyses were entered as independent variables. Significance was set at 0.05 (two-tailed).
Results
Out of 320 patients with ischemic stroke who were consecutively screened, 270 fulfilled the study entry criteria and participated in the study, giving a participation rate of 84.4%. At baseline, 160 (59.3%) patients had aphasic symptom with the incidence of PSD was 47.5% compared with 29.1% in non-aphasiac patients (p < 0.01). At the 3-month assessment, 22 patients dropped out due to lack of interest, moving house or other unknown reasons. Of the 248 patients who completed the 3-month assessment, 119 (48.0%) had physical dependence.
Table 1 shows the socio-demographic and clinical characteristics of the whole sample and separately by outcome. Patients with physical dependence were less likely to be married, and have large-artery atherosclerosis (LAA) TOAST type and pulmonary infection, but more likely to be female, have aphasia at baseline and more severe depressive symptoms at both baseline and 3 months after stroke. In addition, they had higher NIHSS scores at baseline, and higher SADQ scores at baseline and 3 months (all p-values < 0.05).
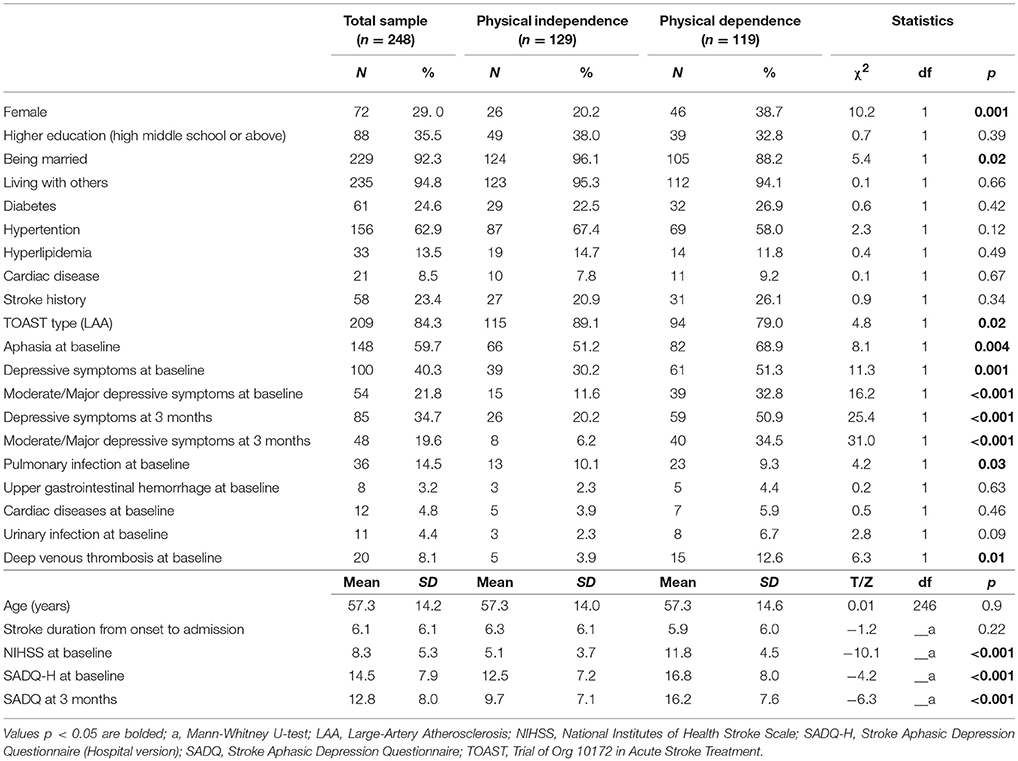
Table 1. Comparison of demographic and clinical variables between physical independence and dependence groups.
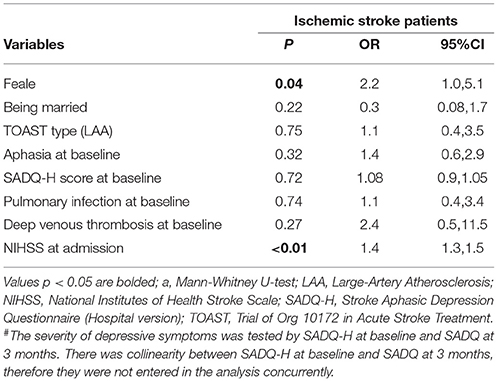
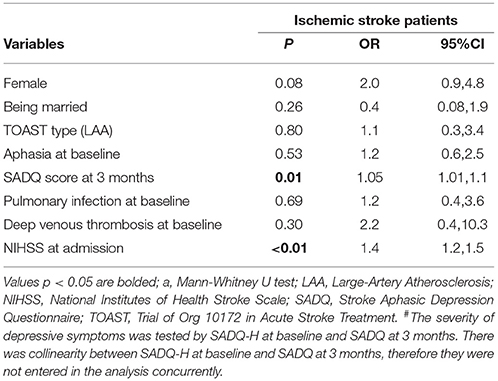
The independent correlates of physical dependence are shown in Tables 2, 3. Due to collinearity between SADQ-H (Hospital version using during hospitalization) and SADQ score, two multivariate logistic regression analyses were performed with SADQ-H and SADQ separately. Finally, female, NIHSS score at admission and SADQ score at 3 months were independently associated with physical dependence at 3-month follow up (adjusted R2 = 0.410 in Table 2 and adjusted R2 = 0.423 in Table 3).
Discussion
Generally about a third of patients with ischemic stroke have poor functional outcome at different follow-up time points (27, 28). In this study, the prevalence of physical dependence was 48% using the mRS cut-off score, which is similar to the 5-year prevalence of poor functional outcome (45%) in another study with the same measure (29). However, any direct comparison should be done with caution due to the different assessment measures, follow-up time points, and demographic characteristics.
A report of the American Heart Association/American Stroke Association in 2017 and meta-analyses found that approximately a third of stroke patients develop PSD at any point after stroke (30–32). A large-scale prospective cohort study in China involving over 2,000 stroke patients found that the cumulative incidence of PSD at 1 year after the index stroke was 42% (4). Depression was found predictive of worse functional outcome in an updated meta-analysis about the impact of depression on stroke outcome (33). While up to 62–70% in aphasic patients with stroke were diagnosed as major depression according to DSM-III-R criteria at 3 months and 1 year after stroke (9, 10, 34) which was higher than our results. The reason may due to the different scales, population and time points. Aphasia have been considered for the potential association with PSD with inconsistent conclusion (35, 36). We found that the incidence of PSD in stroke patients with aphasia and non-aphasia was significantly different indicating aphasia may be a risk factor of the development of PSD.
In this study, the incidence of PSD was 34.7% at 3 months after stroke, which was independently associated with physical dependence. Similar findings were also reported previously (37–39). Depressive symptoms after stroke could result in behavioral and biological abnormalities, such as poor treatment adherence and dysregulation in autonomic system activation, which in turn, could lead to physical dependence (11, 37). However, since more than half of the patients suffered from aphasia in this sample, the use of the SADQ required the input of nursing staff and/or family members. Therefore, we could not exclude the possibility that nursing staff and family members were unable to distinguish between insomnia, irritability, poor appetite, anxiety, and depressive symptoms, which would cause bias in the incidence of PSD to an uncertain extent.
In this study, aphasia was measured using the AQ that covered complete aphasia, motor aphasia, sensory aphasia, transcortical mixed aphasia, transcortical motor aphasia, transcortical sensory aphasia, anomic aphasia, and conduction aphasia. Aphasia may lead to communication or comprehension difficulties, social avoidance and decreased attention, which is associated with physical dependence in stroke survivors (40). However, this finding was only confirmed in the univariate, but not in the multivariate analyses in this study. It is speculated that the association between aphasia and poor physical independence was moderated by other variables, such as depressive symptoms and severity of stroke. In addition, traditional Confucian culture favors family support and inter-dependence, particularly for family members with illness. For example, 94.8% of patients in this study were living with others. Thus, the strong family support may have offset the association between aphasia and physical dependence.
The association between demographic characteristics and physical dependence in stroke survivors have been inconsistent (41, 42). In this study, only female was independently associated with physical dependence, which is supported by previous findings (43, 44). The gender difference in physical independence in stroke survivors may be related to menstrual cycles, neuro-endocrine regulation and more frequent physical comorbidities, such as diabetes, atrial fibrillation, and coronary heart disease in women with stroke (45). As expected, stroke severity as measured by the NIHSS was positively associated with physical dependence, which is consistent with previous findings (27, 29).
There are several methodological limitations to this study. First, this was a single-center study with relatively small sample size, therefore the findings could not be generalized to all stroke patients in China. Second, depressive symptoms were measured using the SADQ based on the observation by nursing staff or family members. There may be a gap between observer-rated and self-reported measures of depression, although there are a number of self-reported measures specific for aphasia such as the Visual Analogue Mood Scales (VAMS) (46), Visual Analogue Self-Esteem Scales (VASES) (47), Disc Intensity Scale Circles (DISCS) (48), and Dynamic Visual Analogue Mood Scales (D-VAMS) (49). We chose SADQ from the perspective of relatively short items, easy operation and short time. Third, some important variables related to physical dependence, such as the use of medication, treatment adherence, the size and lesions of infarcts and the missing data about aphasia at 3-month, were not evaluated in the 3 months assessment.
In conclusion, physical dependence at 3-month follow up was common in Chinese stroke patients, which was associated with gender, greater PSD and stroke severity. Aphasia was also associated with physical dependence but the relationship was not significant. Our findings call for early and effective depression screening, treatment, and stroke rehabilitation to improve physical outcome and reduce the burden of stroke survivors in China.
Author Contributions
SW and C-XW: study design. SW, NZ, YY, Y-ZS, Y-MD, M-FZ, FL, PY, and Y-TX: collection, analysis and interpretation of data. SW, C-XW, and Y-TX: drafting of the manuscript. GU, CN, and Y-TX: critical revision of the manuscript. All coauthors approval of the final version for publication.
Funding
This study was funded by the Ministry of Science and Technology and the Ministry of Health of the People's Republic of China. Individual grants include the National Key Research & Development Program of China (No. 2016YFC1301720), Beijing Brain Research (Z161100000216131), the Beijing Municipal Science and Technology Commission (Z151100004015127), and the Build High Level Technology Talents of Health System in Beijing (No.2015-3-038).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all of the participating colleagues, patients, and their families.
References
1. Kotila M, Numminen H, Waltimo O, Kaste M. Post-stroke depression and functional recovery in a population-based stroke register. The Finnstroke study. Eur J Neurol. (1999) 6:309–12. doi: 10.1046/j.1468-1331.1999.630309.x
2. Naess H, Nyland HI, Thomassen L, Aarseth J, Myhr KM. Mild depression in young adults with cerebral infarction at long-term follow-up: a population-based study. Eur J Neurol. (2005) 12:194–8. doi: 10.1111/j.1468-1331.2004.00937.x
3. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry (2016) 173:221–31. doi: 10.1176/appi.ajp.2015.15030363
4. Zhang N, Wang CX, Wang AX, Bai Y, Zhou Y, Wang YL, et al. Time course of depression and one-year prognosis of patients with stroke in mainland China. CNS Neurosci Ther. (2012) 18:475–81. doi: 10.1111/j.1755-5949.2012.00312.x
5. House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke (2001) 32:696–701. doi: 10.1161/01.STR.32.3.696
6. Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry (1993) 150:124–9.
7. Engelter ST, Gostynski M, Papa S, Frei M, Born C, Ajdacic-Gross V, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke (2006) 37:1379–84. doi: 10.1161/01.STR.0000221815.64093.8c
8. Thomas SA, Lincoln NB. Predictors of emotional distress after stroke. Stroke (2008) 39:1240–5. doi: 10.1161/STROKEAHA.107.498279
9. De Ryck A, Fransen E, Brouns R, Geurden M, Peij D, Mariën P, et al. Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J Neurol Sci. (2014) 347:159–66. doi: 10.1016/j.jns.2014.09.038
10. Shehata GA, El Mistikawi T, Al Sayed KR, Hassan HS. The effect of aphasia upon personality traits, depression and anxiety among stroke patients. J Affect Disord. (2015) 172:312–4. doi: 10.1016/j.jad.2014.10.027
11. Dhamoon MS, McClure LA, White CL, Lakshminarayan K, Benavente OR, Elkind MS. Long-term disability after lacunar stroke: secondary prevention of small subcortical strokes. Neurology (2015) 84:1002–8. doi: 10.1212/WNL.0000000000001331
12. Li Q, Wu H, Yue W, Dai Q, Liang H, Bian H, et al. Prevalence of stroke and vascular risk factors in China: a nationwide community-based Study. Sci Rep. (2017) 7:6402. doi: 10.1038/s41598-017-06691-1
13. Nannetti L, Paci M, Pasquini J, Lombardi B, Taiti PG. Motor and functional recovery in patients with post-stroke depression. Disabil Rehabil. (2005) 27:170–5. doi: 10.1080/09638280400009378
14. Kunitz SC, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, et al. The pilot stroke data bank: definition, design, and data. Stroke (1984) 15:740–6. doi: 10.1161/01.STR.15.4.740
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
17. Brott T, Adams HP, Olinger CP, Marle JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
18. Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke (1994) 25:2220–6.
19. Sun TK, Chiu SC, Yeh SH, Chang KC. Assessing reliability and validity of the Chinese version of the stroke scale: scale development. Int J Nurs Stud. (2006) 43:457–63. doi: 10.1016/j.ijnurstu.2005.07.004
20. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke (1993) 24:35–41.
21. Zhou Heng LJ, Wang Yong-jun J. The reliability of ischemic stroke subtype classification using the TOAST criteria. Chin J Intern Med. (2005) 44:825–7. doi: 10.3760/j.issn:0578-1426.2005.11.011
22. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. (2009) 41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c
23. Uyttenboogaart M, Stewart RE, Vroomen P.C, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke (2005) 36:1984–7. doi: 10.1161/01.STR.0000177872.87960.61
24. Chang Y, Jing X, Junping W. Validation of Chinese version of Stroke Aphasic Depression Questionnaire (SADQ). Chin J Clin Psychol. (2006) 14:230–2. doi: 10.3969/j.issn.1005-3611.2006.03.004
25. Sutcliffe LM, Lincoln NB. The assessment of depression in aphasic stroke patients: the development of the stroke aphasic depression questionnaire. Clin Rehabil. (1998) 12:506–13. doi: 10.1191/026921598672167702
26. Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB). J Speech Hear Disord. (1980) 45:308–24.
27. Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. (2003) 16 (Suppl. 1):14–9. doi: 10.1159/000069936
28. Ji R, Du W, Shen H, Pan Y, Wang P, Liu G, et al. Web-based tool for dynamic functional outcome after acute ischemic stroke and comparison with existing models. BMC Neurol. (2014) 14:214. doi: 10.1186/s12883-014-0214-z
29. Yang Y, Shi YZ, Zhang N, Wang S, Ungvari GS, Ng CH, et al. The disability rate of 5-year post-stroke and its correlation factors: a national survey in China. PLoS ONE (2016) 11:e0165341. doi: 10.1371/journal.pone.0165341
30. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke (2014) 9:1017–25. doi: 10.1111/ijs.12357
31. Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke (2005) 36:1330–40. doi: 10.1161/01.STR.0000165928.19135.35
32. Towfighi A. Poststroke depression: a scientific statement for healthcare professionals from the american heart association/american stroke association. Stroke (2017) 48:e30–43. doi: 10.1161/STR.0000000000000113
33. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke (2014) 9:1026–36. doi: 10.1111/ijs.12356
34. Kauhanen ML, Korpelainen JT, Hiltunen P, Määttä R, Mononen H, Brusin E, et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis. (2000) 10:455–61. doi: 10.1159/000016107
35. Sienkiewicz-Jarosz H, Milewska D, Bochynska A, Chelmniak A, Dworek N, Kasprzyk K, et al. Predictors of depressive symptoms in patients with stroke - a three-month follow-up. Neurol Neurochir Pol. (2010) 44:13–20. doi: 10.1016/S0028-3843(14)60402-3
36. Starkstein SE, Robinson RG. Affective disorders and cerebral vascular disease. Br J Psychiatry (1989) 154:170–82. doi: 10.1192/bjp.154.2.170
37. Ayerbe L, Ayis S, Crichton S, Wolfe CD, Rudd AG. The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J Neurol Neurosurg Psychiatry (2014) 85:514–21. doi: 10.1136/jnnp-2013-306448
38. Robinson RG. The Clinical Neuropsychiatry of Stroke. Cambridge: Cambridge University Press (2006).
39. Shi YZ, Xiang YT, Yang Y, Zhang N, Wang S, Ungvari GS, et al. Depression after minor stroke: the association with disability and quality of life - a 1-year follow-up study. Int J Geriatr Psychiatry (2016) 31:421–7. doi: 10.1002/gps.4353
40. El Hachioui H, Lingsma HF, Van De Sandt-Koenderman MW, Dippel DW, Koudstaal PJ, Visch-Brink EG. Long-term prognosis of aphasia after stroke. J Neurol Neurosurg Psychiatry (2013) 84:310–5. doi: 10.1136/jnnp-2012-302596
41. Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PLoS ONE (2018) 13:e0185402. doi: 10.1371/journal.pone.0185402
42. Rempe DA. Predicting outcomes after transient ischemic attack and stroke. Cerebrovasc Dis. (2014) 20:412–28. doi: 10.1212/01.CON.0000446110.97667.58
43. Glader EL, Stegmayr B, Norrving B, Terént A, Hulter-Asberg K Wester PO, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke (2003) 34:1970–5. doi: 10.1161/01.STR.0000083534.81284.C5
44. Liu X, Lv Y, Wang B, Zhao G, Yan Y, Xu D. Prediction of functional outcome of ischemic stroke patients in northwest China. Clin Neurol Neurosurg. (2007) 109:571–7. doi: 10.1016/j.clineuro.2007.05.008
45. Wang Z, Li J, Wang C, Yao X, Zhao X, Wang Y, et al. Gender differences in 1-year clinical characteristics and outcomes after stroke: results from the China National Stroke Registry. PLoS ONE (2013) 8:e56459. doi: 10.1371/journal.pone.0056459
46. Stern RA, Arruda JE, Hooper CR, Wolfner GD, Morey CE. Visual analogue mood scales to measure internal mood state in neurologically impaired patients: description and initial validity evidence. Aphasiology (1997) 11:59–71. doi: 10.1080/02687039708248455
47. Brumfitt SM, Sheeran P. The development and validation of the Visual Analogue Self-Esteem Scale (VASES). Br J Clin Psychol. (1999) 38 (Pt 4):387–400.
48. Turner-Stokes L. (2005). The Depression Intensity Scale Circles (DISCs): a first evaluation of a simple assessment tool for depression in the context of brain injury. J Neurol Neurosurg Psychiatry 76:1273–8. doi: 10.1136/jnnp.2004.050096
Keywords: aphasia, ischemic stroke, depression, PSD, physical independence
Citation: Wang S, Wang C-X, Zhang N, Xiang Y-T, Yang Y, Shi Y-Z, Deng Y-M, Zhu M-F, Liu F, Yu P, Ungvari GS and Ng CH (2018) The Association Between Post-stroke Depression, Aphasia, and Physical Independence in Stroke Patients at 3-Month Follow-Up. Front. Psychiatry 9:374. doi: 10.3389/fpsyt.2018.00374
Received: 08 May 2018; Accepted: 25 July 2018;
Published: 20 August 2018.
Edited by:
Gianluca Serafini, Ospedale San Martino (IRCCS), ItalyReviewed by:
Jeffrey Guina, Wright State University, United StatesPaul David Barrows, University of Nottingham, United Kingdom
Copyright © 2018 Wang, Wang, Zhang, Xiang, Yang, Shi, Deng, Zhu, Liu, Yu, Ungvari and Ng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Xue Wang, c25vd3NlbkAxMjYuY29t
 Shuo Wang
Shuo Wang Chun-Xue Wang
Chun-Xue Wang Ning Zhang
Ning Zhang Yu-Tao Xiang7
Yu-Tao Xiang7 Yu-Zhi Shi
Yu-Zhi Shi Chee H. Ng
Chee H. Ng