
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 17 August 2018
Sec. Addictive Disorders
Volume 9 - 2018 | https://doi.org/10.3389/fpsyt.2018.00373
There is evidence of the reciprocal influence between the alteration of circadian rhythms and Substance Use Disorders (SUD), and part of the success of the SUD treatment lays in the patient's rhythmic recovery. We aim to elucidate the effect of the SUD treatment in circadian rhythmicity considering, for the first time, the age of onset of substance use (OSU) and duration of abstinence. We registered the sleep-wake schedules, the chronotype and the distal skin temperature of 114 SUD patients with at least 3 months of abstinence, considering whether they had begun consumption at age 16 or earlier (OSU ≤ 16, n = 56) or at 17 or later (OSU ≥ 17, n = 58), and duration of abstinence as short (SA: 3 to 5 months, n = 38), medium (MA: 6 to 9 months, n = 35) or long (LA: more than 9 months, n = 41). Moreover, we compared the patients' distal skin temperature pattern with a similar sample of healthy controls (HC, n = 103). SUD patients showed a morningness tendency and higher night values, amplitude and stability, a better adjustment to the cosine model and lower minimum temperature and circadianity index in the distal skin temperature rhythm, in contrast to the HC group. The OSU ≥ 17 and LA groups showed a more robust distal skin temperature pattern, as well as milder clinical characteristics when compared to the OSU ≤ 16 and SA groups, respectively. The circadian disturbances associated to substance consumption seem to improve with treatment, although the age of OSU and the duration of abstinence are modulating variables. Our results highlight the need to include chronobiological strategies that boost circadian rhythmicity both in SUD prevention and rehabilitation programs. The measurement of distal skin temperature rhythm, a simple and reliable procedure, could be considered an indicator of response to treatment in SUD patients.
During adolescence there is a higher risk to begin substance consumption (1, 2), and an early age of onset of substance use (OSU) is associated to the future development of Substance Use Disorders (SUD) (3) and to more severe clinical characteristics (4–8). Therefore, it is crucial to improve substance consumption prevention programs, as well as to consider the age of OSU and its clinical implications in SUD treatments. Chronobiology is here an area of high interest thanks to the evidence collected in the last decades.
Biological rhythms are essential to survival, and their alteration has been consistently linked to a wide range of health problems (9, 10), including SUD. Circadian rhythms (lasting around 24 h) deserve special attention given their relevance in both work and clinical performance. They are endogenously generated by the body's biological clock, located in the suprachiasmatic nucleus of the hypothalamus, although they are synchronized with the environmental rhythm of light-darkness (11, 12). Several functions, both physiological (body temperature, hormone secretion, wake-sleep cycle, etc.) and psychological (mood, cognitive performance, etc.), show an evident circadian rhythmicity (10, 13–15).
Some parameters, such as body temperature or melatonin secretion, are considered biological markers of circadian rhythmicity (16, 17). In recent years, there is a growing interest to measure distal skin temperature, since it is easy to record and reflects internal temporal order reliably (18–21). Distal skin temperature has been validated for sleep-wake detection (22), and it can be used instead of dim-light melatonin onset to predict the internal phase (21), since it remains after demasking procedures (20). Its usefulness has been proven in very different populations, among others, babies, and older people (23), mild cognitive impairment (24), sleep-disordered breathing (25), or in patients with metabolic syndrome (26).
Different studies show that substance consumption alters the expression of circadian rhythmicity, and this can last for months after the onset of abstinence (27, 28).
The most frequent alterations are a decrease in the amplitude and a phase delay in circadian functions, seen both in body temperature and in melatonin secretion (29–31). In this regard, the genes regulating the circadian system seem to be involved in the activity of the reward system in response to substance use (32–34), and it is suggested that alterations in its activity contribute to the vulnerability of developing a SUD (34–36). Moreover, substance consumption may modify the genetic expression with more or less permanent changes, depending on the severity of consumption and the subject's vulnerability (37).
Individuals show circadian rhythm differences depending on their chronotype or circadian typology (morning-, neither-, and evening-type). This is an individual difference, usually assessed with self-informed questionnaires (38), and which several recent studies have shown to be a crucial health variable. Thus, the evening chronotype is considered a risk factor for substance consumption both for youth and adults (36, 39, 40). This has been linked to obtaining a higher reinforcement with consumption (37, 41, 42), and with a greater social jet-lag or desynchronization between the biological and the social clocks (43, 44). Moreover, evening-type subjects show more personality traits associated to substance use (45, 46), worse academic and work adjustment (40, 47), less ability to cope with stress (48), and worse quality of life (49).
Several studies have highlighted the importance of the age of onset of substance use on the consequences of those who develop a SUD. When compared to people who begin consumption at 17 or later, those who begin at 16 or earlier have a lower premorbid intelligence quotient (7, 50), a worse neuropsychological performance (51, 52), maladaptive treatment-coping strategies (8), and a lesser volume of gray brain matter (53). This cut-off age has been based on neurodevelopmental characteristics, such as a possible dysfunction in the dopaminergic and endocannabinoid systems. These systems are key to prefrontal function, have a final peak of cortical changes at about age 15, and are almost completely defined toward the end of puberty (36, 54). Bearing in mind that dopamine has been established as a modulator of the circadian system (36, 55), it is necessary to explore the possible rhythmic differences in SUD patients considering this cut-off age of onset of consumption. SUD treatment requires an integral, long-lasting approach, where the first year of treatment is considered as a phase of early remission (56), where it is useful to prescribe sleep schedules and stable, morning-type activities to patients. However, we are not aware of any previous studies addressing the influence of age of onset of consumption and duration of abstinence in the rhythmic circadian expression of SUD patients undergoing treatment.
Our work has two aims. The first is to examine the differences in the clinical characteristics of male patients under treatment for SUD, and after the detox phase, depending on whether they initiated substance use at age 16 or earlier (OSU ≤ 16) or at age 17 or later (OSU ≥ 17), and the duration of abstinence (short, medium or long). The second is to explore the differences in the circadian rhythmicity of distal skin temperature in SUD patients, according to the age of OSU and duration of abstinence, compared with a similar sample of healthy controls. We also measure circadian typology and sleep-wake schedules for SUD patients.
In a cross-sectional study design, participants were recruited from March 2014 to July 2017. A total of 156 patients were referred to our study by their treating psychiatrist or psychologist. All of them were under SUD treatment in specialized healthcare resources (ambulatory or residential in a therapeutic community) from Barcelona. Forty-one patients were excluded due to refuse to participate in the study or dropout of it (n = 11), psychiatric comorbidity (n = 2), drug relapse (n = 5), treatment dropout (n = 8), refuse temperature monitoring (n = 6), or invalid temperature recording (n = 9). Thus, 114 participants satisfied our inclusion/exclusion criteria and SUD disorder with cocaine, alcohol, cannabis or opioids as primary drug of dependence. The inclusion criteria were: (a) aged 18–55; (b) current or past diagnosis of SUD according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4thEdition Text Revised (DSM-IV-TR) (57); and (c) in abstinence for at least 3 months (excluding caffeine or nicotine consumption), to ensure the overcoming of withdrawal symptoms and minimum adherence to treatment, confirmed by urinalysis. The exclusion criteria were: (a) presence of any other medical problems which could interfere in the assessment (such as sensorial deficits or neurological injury); and (b) presence of a comorbid axis I mental disorder confirmed by a diagnostic interview according to DSM-IV-TR criteria. All patients were male, given the high prevalence of this gender in SUD (2) and to avoid bias on the results due to sex differences (23, 41). After collecting the data, patients were assigned to groups according to the age of OSU and the duration of abstinence. To study the effect of the age of OSU, they were assigned to two groups: age of OSU at 16 or earlier (OSU ≤ 16; n = 56), or at 17 or later (OSU ≥ 17; n = 58). The groups based on duration of abstinence were for short abstinence (SA; n = 38) from 3 to 5 months, for medium abstinence (MA; n = 35) from 6 to 9 months, and for long abstinence (LA; n = 41) when more than 9 months.
Healthy controls volunteers (HC; n = 103) were recruited from the community of Murcia and Barcelona with exclusion criteria similar than those in the SUD groups, with the added requirement that they must not present any current or past DSM-IV-TR (57) diagnosis.
This study was approved by the ethics committees of the University of Barcelona for the SUD and HC participants, and also by the University of Murcia in the case of the HC. The study was conducted in accordance with the ethical principles of the declaration of Helsinki (58) and the international ethical standards of chronobiological research (59). Study participants provided written informed consent for participation and were not compensated.
Sociodemographic data (age, years of education, marital, and economic status) and clinical (presence of substance use and psychiatric pathology in family history, suicidal attempts, consumption pattern, type of drugs used, age of OSU, duration of drug use, type of treatment, current medication, abstinence period, past treatment for SUD, previous relapses, and number of daily cigarettes and caffeine beverages) were collected by means of a structured interview designed specifically for our study, and the Structural Clinical Interview for the DSM-IV Axis I Disorders (SCID-I) (60). This information was confirmed through the patients' treating psychiatrist and the medical history of the centers' databases.
The Clinical Global Impression questionnaire (CGI) (61) was applied as a subjective measure of clinical severity. The Spanish version (62) of the Drug Abuse Screening Test (DAST-20) (63) was administered to assess the severity of the SUD. This test provides a total score from 0 to 20 (0 indicating no addiction, 1–5 low, 6–10 intermediate, 11–15 substantial, and 16–20 severe). Furthermore, the Seasonal Pattern Assessment Questionnaire (SPAQ) (64) in its Spanish version (65) was used to assess seasonality of mood variations. The SPAQ assigns a Global Seasonality Score (GSS) determined by responses to six indices of seasonality (sleep length, mood, social activity, weight, appetite, and energy level) ranging from 0 to 24, and also considers the degree in which seasonal changes are a problem (no, mild, moderate, marked, severe, or disabling). Three categories are established: no seasonal affective disorder [GSS < 10], subsyndrome of seasonal affective disorder [9 < GSS < 12 and seasonal variations being perceived as a problem, or GSS > 11 but seasonal changes being of minor degree], and seasonal affective disorder [GSS > 11 and seasonal variations considered minimum as a moderate problem].
The Composite Scale of Morningness (CSM) (66) in its Spanish version (67) was administered to SUD patients as a measure of circadian typology. This test is composed of 13 items with Likert-scale answers, with a total score from 13 to 55. In the Spanish version, the scores for the circadian typologies were 13–25 for the evening-type, 26–36 for the neither-type, and 37–55 for the morning-type. We also collected sleep-wake data through a structured interview designed specifically for our study: total time spent sleeping, wake-up time, bedtime, presence and duration of nap.
Distal skin temperature was registered every 10 min for 48 h using the Thermochron iButton® DS1921H device (Maxim Integrated Products, Sunnyvale, California, USA), which had an accuracy of ±1°C at 0.125°C (19). In order to reduce the potential masking effect generated by the higher activity of the dominant hand, the sensor was placed on the wrist of the non-dominant hand over the radial artery (18). Participants were encouraged to maintain their usual lifestyles during temperature measurements.
The temperature data were analyzed through the Circadianware™ version 7.1.1 software (68). The Cosinor analyses (parametric) were applied to calculate the maximum and minimum temperature, mesor, amplitude, acrophase, Rayleigh vector, and percentage of variance explained by the cosine wave (%V), as well as the Fourier analysis with the first 12 harmonics. Furthermore, the circadianity index was calculated in a similar way to that described in Batinga et al. (23). A nonparametric analysis was also performed, as previously described by Witting et al. (69) and Martinez-Nicolas et al. (70), that provided the interdaily stability (IS), intradaily variability (IV), relative amplitude (RA), maximum mean temperature in 5 consecutive hours (M5) and its respective timing (TM5), minimum mean temperature in 2 consecutive hours (L2) and its timing (TL2), minimum mean temperature in 10 consecutive hours (L10) and its timing (TL10). Furthermore, the mean temperature values of four main phases according to local time were calculated, as explained in Batinga et al. (23): morning decrease (08:00–15:00 h), afternoon secondary peak (15:00–18:00 h), evening decrease (18:00–23:00 h), and night plateau (23:00–08:00 h).
Descriptive statistics and frequencies were calculated for all groups considered. Differences between groups in the sociodemographic and clinical variables were explored with the Mann-Whitney U test (U) or with the Chi Square test (χ2) for categorical variables. If the quantitative data fulfilled the necessary conditions, the Student's t-test (t) or the one-way analysis of variance (ANOVA) were used. Otherwise, the non-parametric Mann-Whitney U-test (U) or the Kruskal-Wallis test were used instead. The internal consistency for the CSM was calculated with the Cronbach's alpha coefficient. Differences in CSM scores were assessed using analysis of covariance (ANCOVA), while the sleep-wake schedules, distal skin temperature parametric (cosinor) and non-parametric data were assessed with three multivariate analyses of covariance (MANCOVA). Age was considered as covariate in all cases, since it could be a confounding factor. These comparisons were performed first attending to the diagnostic (HC or SUD groups), second considering the HC and the age of OSU (OSU ≤ 16 or OSU ≥ 17) groups, and third attending to the HC and duration of abstinence (SA, MA, or LA) groups, except for the sleep-wake schedules and CSM, since no data were recorded for the HC participants. Additional analyses were performed considering the type of treatment (residential or ambulatory), to assess whether this was an indicator of differences in some circadian measure. The Bonferroni's test was applied in all analyses to reduce the occurrence of a type I error. The effect size was calculated with the partial Eta squared (η2p), assuming a value of 0.01 as low, of 0.06 as moderate and of 0.14 as high (71). Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; version 22.0), considering bilateral statistical significance with an established type I error at 5% (p < 0.05).
Attending to the sociodemographic data (Table 1), the HC and SUD groups did not differ in age. Nevertheless, although most of the participants had completed the Spanish compulsory education (from 6 to 16 years, grades 1–10), the SUD patients had a lower mean in years of education (p < 0.001). Marital status showed higher rates of singles in the SUD group compared to higher rates of married persons in the HC group (p < 0.001), as well as higher rates of unemployed SUD patients compared to higher active workers in the HC group (p < 0.001). The age of OSU and abstinence groups, with no significant age differences between them, had similar results to those obtained by the SUD group compared with the HC group. There were differences in the years of education between the HC and the age of OSU groups (p < 0.001), as well as in the abstinence groups (p < 0.001). The post-hoc analyses showed that the OSU ≤ 16 (p < 0.001), SA (p < 0.001), MA (p = 0.019), and LA (p < 0.001) patients had fewer years of education than the HC participants. Moreover, the age of OSU (p < 0.001) and the abstinence (p < 0.001) groups showed lower rates of married and employed persons (p < 0.001) than the HC group.
The analyses of the clinical variables indicated significant differences in the age of the OSU groups, being more frequent in the OSU ≤ 16 group to have polydrug use (p = 0.014), residential rather than ambulatory treatment (p = 0.010), and longer duration of drug use (p < 0.001). Furthermore, the groups showed differences in the type of substances used. In the OSU ≤ 16 group, there were higher rates of cannabis (p = 0.001) and hallucinogens consumption (p = 0.033). In the total SUD sample, as well as in both groups, the substances more frequently used were cocaine (88.6%), alcohol (71.1%), and cannabis (42.1%). No differences between the OSU groups were found in the other clinical characteristics studied. Regarding the abstinence groups, higher percentage of relatives with SUD was found in the MA group (p = 0.026) in contrast with the SA and LA groups. There were also significant differences in type of treatment, with a higher frequency of patients in residential treatment in the SA group with respect to the MA and LA groups (p < 0.001). Moreover, the CGI showed main effects (p = 0.012), being the LA group significantly better compared to the SA group (p = 0.031). There were no statistically significant differences in the daily consumption of medication in the pharmacologically treated patients between age of OSU groups or among abstinence groups (see Table 2). Moreover, the opioid agonists prescription did not show statistical differences for age of OSU groups (13% for OSU ≤ 16 and 15.5% for OSU ≥ 17; χ2 = 0.149, p = 0.699) or abstinence groups (18.4% for SA, 11.8% for MA, and 12.5% for LA; χ2 = 0.811, p = 0.667).
Cronbach's alpha coefficient of internal reliability for the CSM was adequate for the total SUD sample studied (0.826). Attending to the age of OSU groups, the ANCOVA analysis did not show significant differences in the CSM total score. It is worth mentioning that we found a high percentage of the morning-type in the SUD patients, especially in the OSU ≥ 17 group (70.7%). The MANCOVA analyses for the sleep-wake schedules (total time spent sleeping, wake-up time, bedtime, presence, and duration of nap) also did not yield any significant differences. Similarly, no differences were found in the CSM total score, the circadian typology and the sleep-wake schedules parameters in the duration of abstinence groups (Table 3).
In the additional analyses carried out considering the type of treatment (residential or ambulatory) as an independent variable no significant differences between groups were found regarding the CSM scores or sleep-wake schedules (p > 0.193; in all cases).
The circadian rhythmic characteristics are shown in Table 4 for the HC and the SUD groups. The MANCOVA analyses revealed several significant differences between the HC and SUD groups. SUD patients were characterized by higher values in minimum temperature (p = 0.012), amplitude (p = 0.001), Rayleigh vector (p = 0.001), %V (p = 0.001), first harmonic power (p = 0.001) and accumulated power after 12 harmonics (p = 0.001), IS (p = 0.001), lower values in the circadianity index (p = 0.001), and an advanced acrophase (p = 0.019) in contrast to the HC group.
The distal skin temperature functions for the HC and total SUD sample are presented in Figure 1. The SUD patients showed a pattern with higher and more stable values at night, in comparison to the HC group, as seen in the differences in the M5 (p = 0.002), and lower and more variable during the day. The rise in M5 in the SUD patients is related to the higher RA in the HC (p = 0.002).
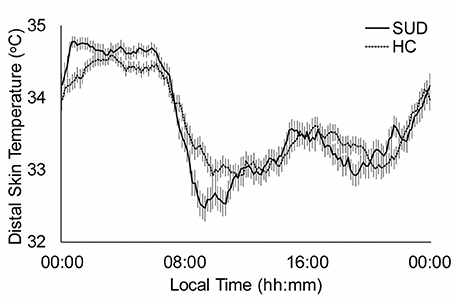
Figure 1. Distal skin temperature mean daily patterns for substance use disorder patients (SUD; continuous line, n: 114) and healthy controls (HC; dotted line, n = 103). All data are expressed as mean ± standard error of the mean.
The comparisons between the HC and the age of OSU groups are showed in Table 4. The two age of OSU groups had an advanced acrophase and higher values in the Rayleigh vector, IS and M5 than the HC group (p < 0.033, in all cases). The values of OSU ≥ 17 group were higher than the HC and OSU ≤ 16 groups in several cosinor and non-parametric data: amplitude, %V, first harmonic power and relative amplitude (p < 0.039, in all cases). The accumulated power after 12 harmonics was higher in the OSU ≥ 17 group in contrast to the values of HC and OSU ≤ 16 groups (p < 0.029), and also higher in the OSU ≤ 16 group than in the HC group (p = 0.031). The OSU ≤ 16 group showed higher values in minimum temperature (p = 0.009) and lower circadianity index (p = 0.002) with respect to the HC group, without differences with the OSU ≥ 17 group.
Figure 2 shows the distal skin temperature functions for the OSU groups. The temperature values during the day (L10) were higher in the OSU ≤ 16 group, although the contrast with the OSU ≥ 17 group was not significant. However, there were significant differences in relative amplitude (p = 0.034), which was higher in the OSU ≥ 17 group.
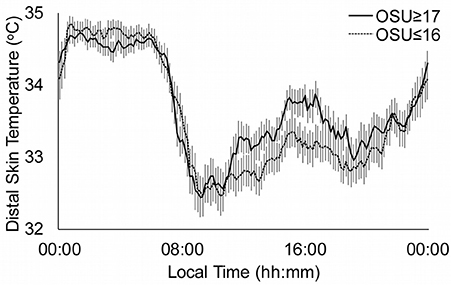
Figure 2. Distal skin temperature mean daily patterns for substance use disorders according to the age of Onset of Substance Use (OSU). Continuous line represents OSU ≥ 17 (n = 58) and dotted line OSU ≤ 16 (n = 56). All data are expressed as mean ± standard error of the mean.
On the other hand, significant statistical differences for several distal skin temperature variables were found between the HC and the abstinence groups (Table 5). The three abstinence groups were characterized by higher values in the Rayleigh vector (p < 0.004), accumulated power after 12 harmonics (p < 0.041), and IS (p < 0.045) in contrast to the HC group. We also found higher values of the MA and LA groups, compared with the HC and SA groups, on the amplitude (p < 0.002), first harmonic power (p < 0.032), and relative amplitude (p < 0.004). The MA and LA groups showed higher values in the M5 (p < 0.015) with respect to the HC group. Only the LA group showed higher values in %V (p < 0.001), in contrast to the HC group. The SA group presented a more advanced acrophase than the HC group (p < 0.001), and lower values in TL10 than the LA group (p < 0.049). Furthermore, the SA and MA groups showed lower values in the circadianity index (p = 0.017) with respect to the HC group. Finally, the LA patients had lower values in minimum temperature (p = 0.041) in contrast to the SA patients, and lower IV than the SA, and MA patients (p < 0.047).
Figure 3 shows the distal skin temperature functions for the abstinence groups. There is a tendency to obtain higher values in the M5 and lower values in the L10 as the duration of abstinence increases. This is based in the significant differences found in the RA between the SA and LA groups. Moreover, there is also a phase delay in the TL10 and TM5 as the duration of abstinence increases.
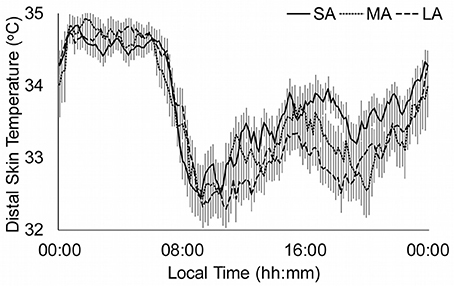
Figure 3. Distal skin temperature mean daily patterns for duration of abstinence. Continuous line represents short abstinence (SA, n = 38), dotted line medium abstinence (MA, n = 35) and discontinued line long abstinence (LA, n = 41). All data are expressed as mean ± standard error of the mean.
In the additional analyses performed considering the type of treatment (residential or ambulatory) only a significant difference was found in acrophase [F(1, 112) = 7.223; p = 0.008; η2p = 0.061], being advanced in the ambulatory group.
This study intended to clarify, for the first time, the possible existence of clinical and circadian (sleep-wake schedules, circadian typology, and distal skin temperature) differences in men with SUD under treatment after detox, depending on the age of OSU and the duration of abstinence. Furthermore, we also explored differences in the circadian rhythm of distal skin temperature between SUD patients and HC participants.
The SUD patients showed a less adaptive sociodemographic pattern than the HC group, with fewer years of education, a greater tendency to remain single and unemployed, consistent with the epidemiological data available on SUD patients (1, 2, 72). This is so regardless of the age of OSU and the duration of abstinence. Given the high impact of the SUD on the functioning of the individuals affected, the data reinforce the need to continue the research on how to improve their prevention and treatment programs.
Both in the total SUD sample and in all the groups of participants, the substances more frequently consumed were cocaine, alcohol, and cannabis, in accordance with both the world and the Spanish data on main substances of clinical diagnosis (2, 72). However, the patients in the OSU ≤ 16 group had a higher frequency of cannabis and hallucinogens consumption than the OSU ≥ 17 group. This may reflect the tendency to use certain substances linked to the life period of onset of consumption (72). Both in the OSU ≤ 16 group and in the SA there is a higher number of patients who need a more intensive treatment (residential) to achieve abstinence compared to the OSU ≥ 17 and MA/LA groups, respectively. These residential programs, in contrast with the ambulatory ones, are addressed to those patients who are experiencing severe drug-related problems (73). In this sense, the OSU ≤ 16 patients presented greater duration of drug use and consumption of more substances, both factors being related to the severity of the addiction (74). These results highlight the evidence that an early age of OSU is related to a more severe clinical symptomatology in adult age (4–6), and are in accordance with the works that use the same cut-off point (7, 8). The better clinical impression of the LA patients compared to the SA group supports the progressive symptomatic recovery as the duration of abstinence increases (75).
Attending to their sleep-wake schedules, there were more morning-type patients in the SUD group, with respect to the normative Spanish data (67). There were no significant differences between the OSU and abstinence groups, and this was more evident in the OSU ≥ 17 group. This tendency to a morning-type was also seen in the advancement of the acrophase of the SUD patients with respect to the HC group. Moreover, the analyses of the parameters of distal skin temperature indicated that, globally, the SUD patients presented a better circadian functioning (higher amplitude, Rayleigh vector, %V, first harmonic power, accumulated power after 12 harmonics, IS, RA, and M5) compared to the HC. These data differ from those reported in previous studies linking eveningness with substance use (37, 40) and those suggesting that circadian rhythmic alteration produced by consumption may last for months after the onset of the abstinence (27–31). This may be explained by the methodological differences, especially by the longer minimum abstinence time (3 months) of the patients in our sample, and also because they do not present active consumption (confirmed by urinalysis), as was also done in Antúnez et al. (76) with SUD patients and with results similar to ours. The therapeutic approach in SUD patients establishes patterns of sleep-wake and regular daily activities in tune with the solar cycle of light-darkness or the morning typology (73), which is considered a protection factor against substance consumption (30, 37). Thus, the patients' correct rhythmic expression could be considered a marker of adherence to treatment and of relapse prevention.
However, some temperature parameters suggest only a partial restitution of the circadian impairment associated to substance consumption. The SUD patients presented high values in minimum temperature, indicative of lower activation and more daytime sleepiness (18, 25), as well as a lower circadianity index or power of the first harmonic, which has been related to an immature circadian system (23). Studying the age of OSU and abstinence groups has contributed to shed light on this issue, showing that this is found only in those patients with lower age of OSU (OSU ≤ 16) and shorter duration of abstinence (SA). Our results indicate that circadian rhythmicity in the SUD group was better when the period of abstinence was longer. The LA group showed higher amplitude, first harmonic power and RA, lower minimum temperature and IV, and delayed TL10 than the SA group, with even better characteristics than the HC group. However, longitudinal works are required that assess the rhythmicity of patients in the long term, once they have completed their treatment.
When the cut-off age of OSU was considered we observed that the OSU ≥ 17 group presented a better circadian distal skin temperature, characterized by higher amplitude, %V, first harmonic power, accumulated power after 12 harmonics and RA. Two hypotheses might explain this observation. First, keeping in mind the implication of the circadian genes in developing an addiction (34, 35), it is possible that the OSU ≤ 16 patients present certain endogenous characteristics previous to the onset of consumption that, added to a worse synchronization of environmental rhythms, could make them more vulnerable to an early onset of substance consumption (77). In this respect, during adolescence an extreme evening-type pattern and the presence of social jet-lag are vulnerability factors for the development of different disorders, including SUD (36, 44). Second, neurotoxicity could underlie the more marked difficulty in restoring the patients' rhythmic expression in spite of following the prescribed treatment. The cut-off age used, based on neurodevelopmental characteristics, discriminates the early phase of maturation of the dopaminergic and endocannabinoid systems (36, 54). Dopamine is the main neuromodulator of the reward system and is involved in the regulation of the clock genes and the circadian rhythms (36, 55). Thus, it is possible that the early consumption of substances affects the correct development of both systems with dysfunctions more perdurable than those produced by a later age of OSU. Unfortunately, the design of our study does not allow us to clarify these issues at the moment.
In this way, the OSU ≤ 16 patients are established as a risk group with a smaller response to interventions, a more severe clinical presentation and greater difficulty in recovering circadian rhythmicity, in comparison with the OSU ≥ 17 patients. Given that a good circadian functioning has been related to better health (9, 10), greater quality of life (49) and better work and academic adjustment (40, 47), these type of patients could benefit from more intense chronobiological strategies, such as the administration of exogenous melatonin, or light-therapy (37). Moreover, since the OSU ≤ 16 patients could have a lower cognitive performance (7, 50–52) and cognitive functions show circadian rhythmicity (13, 15), it could work in favor of their recovery to program more demanding cognitive interventions (such as the cognitive-behavioral) for the time frames when they present more optimal levels of activity.
Our study also has some limitations. A great number of the patients in our sample were polyconsumers, and this made it impossible to tell apart the possible differential effects of each substance on the circadian characteristics, although in our study their effect is relatively under control, since the same main substances had been consumed in the groups. The fact that our sample only consists of men eliminates the influence of gender-related variables, but on the other hand it limits the generalization of the results. The wide range of age in the sample may have also contributed to a type-II error. Moreover, we have not recorded the activity-rest nor the exposition to light, which would have allowed for a more integrated and complete assessment of circadian rhythmicity (20, 78), nor the content and schedule of food/drink intake that may interfere in the rhythm of temperature (11). Finally, we have analyzed cross-sectional data, which does not allow establishing causal relations between variables.
Our results may have clinical implications. The alterations in the distal skin temperature (a good marker of the circadian system) associated to the SUD may be restored, this being influenced by the duration of abstinence and the age of OSU. Thus, the circadian functioning improves as the time without consumption increases, and this can be considered as an indicator of good adherence and response to treatment (with almost no interference from its modality). In this sense, the distal skin temperature rhythm could be used as a reliable and simple procedure to monitor the patients' response to treatment. Given the relationship between health and circadian rhythms, it would be advisable to do post-intervention follow-ups in order to ensure that the results are maintained. On the other hand, although the age of OSU groups presents similar periods of abstinence and hygienic habits of sleep-wake in the morning-type, the OSU ≤ 16 group appears as the more vulnerable with patients may require from more complex interventions in which it would be beneficial to emphasize the rhythmic aspects. Finally, it would be interesting to include chronobiological assessments in the SUD prevention programs, as well as to boost those chronotherapies addressed to adolescents.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
AA conceived the original idea for the study, sought funding and wrote the protocol. MC and AM-N collected the sample data. MC carried out all the data analyses with input from AA and AM-N. AA, MC, and AM-N participated in the interpretation of the data. AA and MC wrote the manuscript with input from AM-N. All authors have approved the final manuscript.
This work was supported by grants from the Spanish Ministry of Economy and Competitiveness PSI2012-32669 and the Spanish Ministry of Economy, Industry and Competitiveness PSI2015-65026 (MINECO/FEDER/UE). AM was supported by the Ministry of Economy and Competitiveness, the Instituto de Salud Carlos III through the RETICEF Network (The Aging and Frailty Cooperative Research Network, RD12/0043/0011), CIBERFES grant (CB16/10/00239), and grant 19899/GERM/15 (co-financed by FEDER). The funding sources have no involvement in study planning, conduction or evaluation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Man Project Foundation in Catalonia, the ATRA Association and the Mental Health and Addictions Division of the Mataró Hospital for providing the patients in the sample.
CGI, Clinical Global Impression questionnaire; CSM, Composite Scale of Morningness; DAST-20, Drug Abuse Screening Test; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revised; HC, healthy controls; IS, interdaily stability; IV, intradaily variability; L10, minimum mean temperature in 10 consecutive hours; L2, minimum mean temperature in 2 consecutive hours; LA, long abstinence; M5, maximum mean temperature in 5 consecutive hours; MA, medium abstinence; OSU≤16, age of onset of substance use at age 16 or earlier; OSU≥17, age of onset of substance use at age 17 or later (OSU≥17); RA, relative amplitude; SA, short abstinence; SCID-I, Structural Clinical Interview for the DSM-IV Axis I Disorders; SPAQ, Seasonal Pattern Assessment Questionnaire; SUD, Substance Use Disorder; TL10, time when the minimum temperature in 10 consecutive hours was reached; TL2, time when the minimum temperature in 2 consecutive hours was reached; TM5, time when the maximum mean temperature in 5 consecutive hours was reached; %V, percentage of variance explained by the cosine wave.
1. Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on alcohol and related conditions-III. JAMA Psychiatry (2016) 73:39–47. doi: 10.1001/jamapsychiatry.2015.2132
2. United Nations Office on Drugs and Crime. World Drug Report. Available online at: https://www.unodc.org/wdr2017/field/Booklet_1_EXSUM.pdf (2017).
3. Woodcock EA, Lundahl LH, Stoltman JJK, Greenwald MK. Progression to regular heroin use: examination of patterns, predictors, and consequences. Addict Behav. (2015) 45:96–100. doi: 10.1016/j.addbeh.2015.02.014
4. Kendler KS, Ohlsson H, Sundquist K, Sundquist J. A latent class analysis of drug abuse in a national Swedish sample. Psychol Med. (2013) 43:2169–78. doi: 10.1017/S0033291713000081
5. Hammond CJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use and addictive behaviors: prevention and treatment implications. Adolesc Med State Art Rev. (2014) 25:15–32.
6. Eddie D, Epstein EE, Cohn AM. Pathways to vulnerability for alcohol problem severity in a treatment-seeking sample. Addict Disord Their Treat. (2015) 14:82–94. doi: 10.1097/ADT.0000000000000045
7. Capella MM, Benaiges I, Adan A. Neuropsychological performance in polyconsumer men under treatment. Influence of age of onset of substance use. Sci Rep. (2015) 5:12038. doi: 10.1038/srep12038
8. Capella MM, Adan A. The age of onset of substance use is related to the coping strategies to deal with treatment in men with substance use disorder. PeerJ (2017) 5:e3660. doi: 10.7717/peerj.3660
9. Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. (2015) 32:802–13. doi: 10.3109/07420528.2015.1041597
10. Çaliyurt O. Role of chronobiology as a transdisciplinary field of research: its applications in treating mood disorders. Balkan Med J. (2017) 34:514–21. doi: 10.4274/balkanmedj.2017.1280
11. Sarabia JA, Rol MA, Mendiola P, Madrid JA. Circadian rhythm of wrist temperature in normal-living subjects. A candidate of new index of the circadian system. Physiol Behav. (2008) 95:570–80. doi: 10.1016/j.physbeh.2008.08.005
12. Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhytms. Brain Res Rev. (2009) 61:281–306. doi: 10.1016/j.brainresrev.2009.08.001
13. Adan A. Circadian variations in psychological measures. A new classification. Chronobiologia (1993) 20:145–62.
14. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. (2012) 35:445–62. doi: 10.1146/annurev-neuro-060909-153128
15. Maierova L, Borisuit A, Scartezzini JL, Jaeggi SM, Schmidt C, Münch M. Diurnal variations of hormonal secretion, alertness and cognition in extreme chronotypes under different lighting conditions. Sci Rep. (2016) 6:33591. doi: 10.1038/srep33591
16. Arendt J. Importance and relevance of melatonin to human biological rhythms. J Neuroendocrinol. (2003) 15:427–31. doi: 10.1046/j.1365-2826.2003.00987.x
17. Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J Clin Sleep Med. (2008) 4:66–9.
18. Sarabia JA, Martinez-Nicolas A, Madrid JA, Rol MA, Ortíz-Tudela E. Inventors; CRONOLAB, Murcia University, Assignees. Device Which Comprises A Physical Activity And Position Sensor, A Peripheral Temperature Sensor And A Light Sensor For Providing Information On The Circadian System Status. Spanish Patent P201031894. Murcia: University of Murcia (2010).
19. Harper-Smith AD, Crabtree DR, Bilzon JL, Wals NP. The validity of wireless iButtons and thermistors for human skin temperature measurement. Physiol Meas. (2010) 31:95–114. doi: 10.1088/0967-3334/31/1/007
20. Martinez-Nicolas A, Ortiz-Tudela E, Rol MA, Madrid JA. Uncovering different masking factor son wrist skin temperature rhythm in free-living subjects. PLoS ONE (2013) 8:e61142. doi: 10.1371/journal.pone.0061142
21. Bonmati-Carrion MA, Middleton B, Revell V, Skene DJ, Rol MA, Madrid JA. Circadian phase assessment by ambulatory monitoring in humans: correlation with dim light melatonin onset. Chronobiol Int. (2014) 31:37–51. doi: 10.3109/07420528.2013.820740
22. Ortiz-Tudela E, Martinez-Nicolas A, Albares J, Segarra F, Campos M, Estivill E, et al. Ambulatory circadian monitoring (ACM) based on thermometry, motor activity and body position (TAP): a comparison with polysomnography. Physiol Behav. (2014) 126:30–8. doi: 10.1016/j.physbeh.2013.12.009
23. Batinga H, Martinez-Nicolas A, Zornoza-Moreno M, Sánchez-Solis M, Larqué E, Mondéjar MT, et al. Ontogeny and aging of the distal skin temperature rhythm in humans. Age (2015) 37:29. doi: 10.1007/s11357-015-9768-y
24. Ortiz-Tudela E, Martinez-Nicolas A, Díaz-Mardomingo C, García-Herranz S, Pereda-Pérez I, Valencia A, et al. The characterization of biological rhythms in mild cognitive impairment. Biomed Res Int. (2014) 2014:524971. doi: 10.1155/2014/524971
25. Martinez-Nicolas A, Guaita M, Santamaría J, Montserrat JM, Rol MA, Madrid JA. Circadian impairment of distal skin temperature rhythm in patients with sleep-disordered breathing: the effect of CPAP. Sleep (2017) 40:zsx067. doi: 10.1093/sleep/zsx067
26. Bandín C, Martinez-Nicolas A, Ordovás J, Ros-Lucas JA, Castell P, Silvente T, et al. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes. (2013) 37:1044–50. doi: 10.1038/ijo.2012.180
27. Falcón E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology (2009) 65:91–6. doi: 10.1016/j.neuropharm.2008.06.054
28. Perreau-Lenz S, Spanagel R. Clock genes x stress x reward interactions in alcohol and substance use disorders. Alcohol (2015) 49:351–7. doi: 10.1016/j.alcohol.2015.04.003
29. Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-depenent men and women compared with healthy controls. Chronobiol Int. (2012) 29:35–42. doi: 10.3109/07420528.2011.636852
30. Hasler BP, Soehner AM, Clark DB. Circadian rhythms and risk for substance use disorders in adolescence. Curr Opin Psychiatry (2014) 27:460–6. doi: 10.1097/YCO.0000000000000107
31. Hasler BP, Soehner AM, Clark DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol (2015) 49:377–87. doi: 10.1016/j.alcohol.2014.06.010
32. Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under influence of circadian genes and rhythm. Proc Natl Acad Sci USA. (2002) 99:9026–30. doi: 10.1073/pnas.142039099
33. Perreau-Lenz S, Zghou T, Rodríguez de Fonseca F, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensivity by the mPer2 gene. Addict Biol. (2009) 14, 253–9. doi: 10.1111/j.1369-1600.2009.00165.x
34. Becker-Krail D, McClung C. Implications of circadian rhythm and stress in addiction vulnerability. F1000Res. (2016) 5:59. doi: 10.12688/f1000research.7608.1
35. Ozburn AR, Falcón E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, et al. Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry (2015) 77:425–33. doi: 10.1016/j.biopsych.2014.07.030
36. Logan RW, Hasler BP, Forbes EE, Franzen PL, Torregrossa MM, Huang YH, et al. Impact of sleep and circadian rhythms on addiction vulnerability in adolescents. Biol Psychiatry (2017) 83:987–96. doi: 10.1016/j.biopsych.2017.11.035
37. Adan A. A chronobiological approach to addiction. J Subst Use (2013) 18:171–83. doi: 10.3109/14659891.2011.632060
38. Di Milia L, Adan A, Natale V, Randler C. Reviewing the psychometric properties of contemporary circadian typology measures. Chronobiol Int. (2013) 30:1261–71. doi: 10.3109/07420528.2013.817415
39. Prat G, Adan A. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. (2011) 28:248–57. doi: 10.3109/07420528.2011.553018
40. Danielsson K, Markström A, Broman JE, von Knorring L, Jansson-Fröjmark M. Delayed sleep phase disorder in a Swedish cohort of adolescents and young adults: prevalence and associated factors. Chronobiol Int. (2016) 33:1331–39. doi: 10.1080/07420528.2016.1217002
41. Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. (2012) 29:1153–75. doi: 10.3109/07420528.2012.719971
42. Hasler BP, Casement MD, Sitnick SL, Shaw DS, Forbes EE. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence 2 years later. Behav Brain Res. (2017) 327:112–20. doi: 10.1016/j.bbr.2017.02.024
43. Wittmann M, Paulus M, Roenneberg T. Decreased psychological well-being in late “chronotypes” is mediated by smoking and alcohol consumption. Subst Use Misuse (2010) 45:15–30. doi: 10.3109/10826080903498952
44. Gamsby JJ, Pribish AM, Stevanovic KD, Yunus A, Gulick D. Alcohol intake increases in adolescent C57BL/6J mice during intermittent cycles of phase-delayed, long-light conditions. Front Behav Neurosci. (2017) 11:52. doi: 10.3389/fnbeh.2017.00152
45. Tonetti L, Adan A, Caci H, De Pascalis V, Fabbri M, Natale V. Morningness-eveningness preference and sensation seeking. Eur Psychiatry (2010) 25:111–5. doi: 10.1016/j.eurpsy.2009.09.007
46. Prat G, Adan A. Relationships among circadian typology, psychological symptoms, and sensation seeking. Chronobiol Int. (2013) 30:942–9. doi: 10.3109/07420528.2013.790044
47. Hysing M, Harvey AG, Linton SJ, Askeland KG, Sivertsen B. Sleep and academic performance in later adolescence: results from a large population-based study. J Sleep Res. (2016) 25:318–24. doi: 10.1111/jsr.12373
48. Antúnez JM, Navarro JF, Adan A. Circadian typology is related to resilience and optimism in healthy adults. Chronobiol Int. (2015) 32:524–30. doi: 10.3109/07420528.2015.1008700
49. Suh S, Yang HC, Kim N, Yu JH, Choi S, Yun CH, et al. Chronotype differences in health behaviors and health-related quality of life: a population-based study among aged and older adults. Behav Sleep Med. (2017) 15:361–76. doi: 10.1080/15402002.2016.1141768
50. Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. (2003) 69:303–10. doi: 10.1016/S0376-8716(02)00334-4
51. Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (1999) 142:295–301. doi: 10.1007/s002130050892
52. Jockers-Scherübl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gómez-Carrillo A, et al. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31:1054–63. doi: 10.1016/j.pnpbp.2007.03.006
53. Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. (2000) 19:1–22. doi: 10.1300/J069v19n01_01
54. Sundram S. Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol Clin Exp. (2006) 21:245–54. doi: 10.1002/hup.762
55. Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: a modulator of circadian rhythms in the central nervous system. Front Cell Neurosci. (2017) 11:91. doi: 10.3389/fncel.2017.00091
56. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association (2013).
57. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association (2000).
58. World Medical Association. WMA Declaration Of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Available online at: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (2013).
59. Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhytm research on animals and humans beings. Chronobiol Int. (2010) 27:1911–29. doi: 10.3109/07420528.2010.516381
60. First MB, Spitzer RL, Gibbon M, Williams JBW. Entrevista Clínica Estructurada para los Trastornos del Eje I del DSM-IV, Versión Clínica (SCID-I). Barcelona: Masson (1999).
61. Guy W. Early Clinical Drug Evaluation (ECDEU) Assessment Manual. Rockville, MD: National Institute of Mental Health (1976).
62. Pérez B, Fernández LG. Validación española del Drug Abuse Screening Test (DAST-20 y DAST-10). Health Addict. (2010) 10:35–50. doi: 10.21134/haaj.v10i1.35
64. Rosenthal NE, Bradt GJ, Wehr TA. Seasonal Pattern Assessment Questionnaire (SPAQ). Bethesda, MD: National Institute of Mental Health (1984).
65. Adan A, Natale V, Fabbri M. Propiedades psicométricas de la versión castellana del cuestionario de evaluación de patrón estacional. Rev Latinoam Psicol. (2006) 38:59–69.
66. Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. (1989) 74:728–38.
67. Adan A, Caci H, Prat G. Reliability of the Spanish version of the composite scale of morningness. Eur Psychiatry (2005) 20:503–9. doi: 10.1016/j.eurpsy.2005.01.003
68. Campos M, Marín-Morales R, Madrid JA, Rol MA, Sosa J, Sosa M, et al. Inventors: CRONOLAB, Murcia University, Assignees: Circadianware. Spain intellectual property registration 08/2010/183 (2010).
69. Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry (1990) 27:563–72.
70. Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiol Int. (2011) 28:617–29. doi: 10.3109/07420528.2011.593278
71. Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. (2011) 6:135–47. doi: 10.1016/j.edurev.2010.12.001
72. Observatorio Español Droga Toxicomanías. Informe 2017: Alcohol, Tabaco Y Drogas Ilegales en España. Available online at: http://www.pnsd.msssi.gob.es/profesionales/sistemasInformacion/informesEstadisticas/pdf/2017OEDA-INFORME.pdf (2017).
73. United Nations Office on Drugs and Crime. International Standards for the Treatment of Drug Use Disorders. Available online at: https://www.unodc.org/wdr2017/field/Booklet_1_EXSUM.pdf (2017).
74. Sofin Y, Danker-Hopfe H, Gooren T, Neu P. Predicting inpatient detoxification outcome of alcohol and drug dependent patients: the influence of sociodemographic environment, motivation, impulsivity, and medical comorbidities. J Addict. (2017) 2017:6415831. doi: 10.1155/2017/6415831
75. Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. (2017) 41:1432–43. doi: 10.1111/acer.13431
76. Antúnez JM, Capella MM, Navarro JF, Adan A. Circadian rhythmicity in substance use disorder male patients with and without comorbid depression under ambulatory and therapeutic community treatment. Chronobiol Int. (2016) 33:1410–21. doi: 10.1080/07420528.2016.1223092
77. Sivertsen B, Skogen JC, Jakobsen R, Hysing M. Sleep and use of alcohol and drug in adolescence. A large population-based study of Norwegian adolescents aged 16 to 19 years. Drug Alcohol Depend. (2015) 149:180–6. doi: 10.1016/j.drugalcdep.2015.01.045
Keywords: abstinence, circadian rhythm, chronotype, distal skin temperature, onset of substance use, sleep-wake schedules, substance use disorder
Citation: Capella MdM, Martinez-Nicolas A and Adan A (2018) Circadian Rhythmic Characteristics in Men With Substance Use Disorder Under Treatment. Influence of Age of Onset of Substance Use and Duration of Abstinence. Front. Psychiatry 9:373. doi: 10.3389/fpsyt.2018.00373
Received: 11 May 2018; Accepted: 25 July 2018;
Published: 17 August 2018.
Edited by:
Luigi Janiri, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Domenico De Berardis, Azienda Usl Teramo, ItalyCopyright © 2018 Capella, Martinez-Nicolas and Adan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Adan, YWFkYW5AdWIuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.