- 1Addiction Development and Psychopathology Lab, Department of Psychology, University of Amsterdam, Amsterdam, Netherlands
- 2Brain and Development Lab, Department of Psychology, Leiden University, Leiden, Netherlands
- 3Leiden Institute for Brain and Cognition, Leiden, Netherlands
A milestone in cannabis research is the establishment of a clinically relevant cannabis withdrawal syndrome, yet little is known about the underlying mechanisms. We investigated the predictive role of mental health and cognitive factors in withdrawal severity during an active attempt to cut down, relative to uninterrupted cannabis use. Ninety heavy cannabis users were randomly assigned to an experimental or control group. The experimental group was asked to cut down substance use for 1 week. Past week substance use, substance use-related problems, depressive symptoms, cravings, and cognitive control were assessed at baseline. Past week substance use and withdrawal severity were assessed at follow-up. The experimental group reduced their cannabis use more and experienced more withdrawal than the control group. Hierarchical regression analysis per predictor indicated that cannabis use-related problems, depressive symptoms, and cannabis craving, but not cognitive control, predicted stronger withdrawal. Craving uniquely predicted withdrawal in the experimental group. A combined hierarchical regression indicated that only depressive symptoms and cannabis use-related problems uniquely predicted withdrawal across groups. These results suggest that depressive symptoms and cannabis use-related problems are generally indicative of cannabis withdrawal severity, whereas craving specifically predicts cannabis withdrawal during an active attempt to cut-down cannabis use.
Introduction
One of the milestones in cannabis research is the establishment of a clinically relevant cannabis withdrawal syndrome (1). As such, cannabis withdrawal is a new diagnostic criterion for cannabis use disorders (CUDs) in the latest edition of the diagnostic statistical manual [DSM-5; (2)]. Cannabis withdrawal refers to mental (e.g., mood swings, sleep disruptions) and physical (e.g., headaches, nausea) discomfort that cannabis users may experience after discontinuation or reductions in use. Supporting the maintenance of addictive behaviors, poor cognitive functioning and mental health may play an important role in the severity of mental discomfort during withdrawal. However, little is still known about the underlying mechanisms. We therefore assessed the role of both cognitive and mental health factors in the subjective severity of cannabis withdrawal during an active attempt to cut down cannabis use in heavy cannabis users.
Given the recognized role of withdrawal in relapse, withdrawal reduction was one of the primary targets for the development of pharmacotherapies for substance use disorders (SUDs) over the past decades (3). The existence of withdrawal after discontinuation of cannabis use has long been questioned, paralleling the lack of pharmacotherapies approved for treating CUDs (4). However, the past decades, many studies confirmed withdrawal is common in both treatment and non-treatment seeking cannabis users [for reviews see (5, 6)]. Moreover, there appears to be a bidirectional relationship between withdrawal and dependence severity; That is, higher CUD severity has been found to predict more severe withdrawal [e.g., (7, 8); but see (9)] and more severe withdrawal has been found to predict more severe future dependence (10). It has also repeatedly been shown that cannabis users who experience more severe withdrawal during a quit attempt reinitiate use sooner (8, 11–13). Finally, withdrawal has been found to be higher in individuals with more severe depressive symptoms (7, 14).
Although not all users will experience withdrawal (9), cannabis withdrawal generally peaks within the first week of abstinence and predicts relapse (8, 13, 15). Most previous studies specifically investigated the mechanisms underlying cannabis withdrawal during an attempt to remain abstinent. However, daily fluctuations in withdrawal symptoms are thought to play a prominent role in non-abstinent users as well; The acute withdrawal symptoms that emerge after using induce a state of negative affect that, in turn, increases the motivation to use again (16, 17). In line with this, using frequent daily withdrawal assessments in heavy non-abstinent cannabis users, it has been shown that cannabis withdrawal increases just prior to cannabis use (18). As such, to help us understand who is at a greater risk of relapse after abstinence, an important next step is to reveal predictors of withdrawal severity during an active attempt to cut-down cannabis use compared to uninterrupted cannabis use.
Theoretical models of addiction suggest that, beside withdrawal severity, poor cognitive control over the extremely strong motivations to use (e.g., craving) is thought to play a key role in the development and maintenance of SUDs (16, 19–23). Few studies specifically investigated the role of craving and cognitive control in CUDs, however, there is preliminary evidence that both processes play a prominent role in the development of CUDs and relapse. Craving is a complex multifaceted process that represents the extreme urge to use drugs commonly experienced during abstinence (17). Exposure to cannabis-related cues can also induce craving in individuals with a CUD (24, 25) and craving can predict treatment outcomes in adolescents with a CUD (26). Regarding cognitive control, cannabis intoxication can temporarily impair planning, organizing, problem solving, decision-making, memory, and emotional control (27). Although contradictory findings have been reported, CUDs are associated with similar impairments in cognitive control (28, 29) and withdrawal during abstinence parallels a temporarily (further) decline (30, 31). Neural activity in brain areas involved in cognitive control may predict escalation of cannabis use in heavy cannabis users; Using the Iowa Gabling Task, higher win-related activity in the superior frontal gyrus and higher activity during the anticipation of disadvantageous decisions in the frontal pole were found to be associated with an increase in weekly cannabis use 6 months later (32). Similarly, using the N-Back working-memory task, functionality of the fronto-parietal executive network also predicted changes in cannabis use (33). Interestingly, better cognitive control as measured with the classical Stroop (34) has been found to relate to less cannabis cue-induced craving in heavy cannabis users (35). Further supporting the link between cognitive control and craving, higher neural activity in brain areas involved in cognitive control in response to watching cannabis cues related to less craving in heavy cannabis users (36).
Although the association between withdrawal severity, mental health problems and treatment outcomes is evident (6–9), the association between cognition and cannabis withdrawal severity is unclear. The above described preliminary findings of the association between cognition and cannabis use suggest that cognitive factors including craving and cognitive control, as well the severity of CUD-related mental health problems like cannabis use-related problems and depression can predict withdrawal severity during an active attempt to cut down or quit cannabis use. More specifically, better cognitive control may help in actively reducing the motivation to use cannabis and mental discomfort from withdrawal, and withdrawal may temporarily lower cognitive control. In turn, higher baseline craving and, as suggested by previous studies (7, 8, 14), especially severity of depressive symptoms and CUD may increase the likelihood to experience withdrawal.
To test this hypothesis, craving, cognitive control [Classical Stroop (34) and Colombia Card Test (37)], substance use-related problems (cannabis, nicotine, alcohol), and symptoms of depression were assessed in a large group of almost daily cannabis users during a baseline laboratory test session. Given the presumed role of cannabis withdrawal severity in continued daily cannabis use, as well as relapse after abstinence, it is important to study predictors of withdrawal in cannabis users who try to lower their use compared to those who continue their use. To specifically investigate withdrawal during an active attempt to cut down vs. uninterrupted cannabis use, the heavy cannabis users were randomly assigned to an experimental or control group. The experimental group was explicitly instructed to use as little cannabis and other substances during following week. Substance use and withdrawal severity of that week were subsequently assessed through a telephone interview exactly 1 week later. Given the multifaceted complex nature of cognitive control and the relevance of emotional control for addiction, both a relatively cold, emotion devoid, general executive functioning task (Stroop) and a relatively hot emotional decision making task (CCT) were included in the design. We expected (i) baseline craving, cannabis use-related problems, and symptoms of depression to be positively related to more severe withdrawal, and (ii) cognitive control to be negatively related to more severe withdrawal.
Materials and Methods
Participants
Ninety-three heavy cannabis users (21 females; 18–31 years) were recruited at educational institutions and in Dutch cannabis outlets. Heavy cannabis use was defined as smoking cannabis more than 3 days a week for at least 1 year and having a Cannabis Use Disorder Identification Test-Revised [CUDIT-R; (38)] score of ≥10. Approximately 95% percent of the cannabis users that score 10 or more on the CUDIT-R have a CUD (38). Potential participants were excluded if they currently used psychotropic medication or had a treatment history for any psychiatric disorder. All participants signed informed consents and the local Ethics Committee of Leiden University approved all procedures in this study. Of the 93 participants, three were excluded from further analyses due to technical problems. The final sample thus included 90 heavy cannabis users (Table 1).
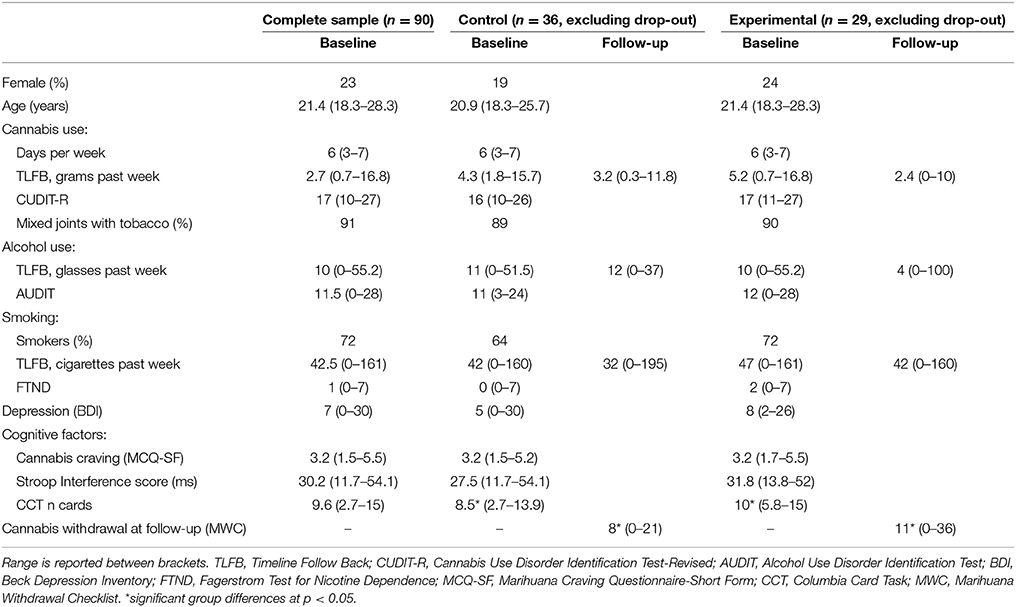
Table 1. Medians (SD) of sample characteristics and study measures in all participants at baseline (Complete sample), and for the participants that remained in the study at follow-up (Control and Experimental Group, excluding drop-out).
Questionnaires on Substance Use, Craving, and Psychological Functioning
Cannabis craving was assessed with the 12-item Marijuana Craving Questionnaire-Short Form [MCQ-SF; (39)] at the start of the baseline test-session. The MCQ-SF assesses various dimensions of craving (e.g., expected positive outcomes, desire to use and intention to use) on a 7-point scale ranging from “strongly disagree” (1) to “strongly agree” (7). Total craving score was obtained by averaging all item scores. The Timeline Follow-Back (TLFB) was used to measure cannabis, alcohol, cigarette and other substance use during the past 7 days at baseline and follow-up (40). The 8-item CUDIT-R was used to measure severity of cannabis use-related problems during the past 6 months at baseline (38). The CUDIT-R assesses the frequency of cannabis use, symptoms of dependence and other psychological problems, scaled from never (0) to almost daily (4). Total CUDIT-R score was obtained by summing all item scores. The 10-item Alcohol Use Disorder Identification Test (AUDIT) was used to measure severity of alcohol use-related problems during the past 6 months at baseline (41). Moreover, the 6-item Fagerstrom Test for Nicotine Dependence (FTND) was used to assess severity of nicotine dependence during the past 6 months at baseline (42). The 21-item Beck Depression Inventory (BDI) was used to measure symptoms of depression at baseline (43). Finally, the 15-item Marijuana Withdrawal Checklist (MWC) was used to assess cannabis withdrawal symptom severity during the past week at follow-up (15). This questionnaire assesses mental (e.g., depressed mood, irritability, aggression) and physical (e.g., headaches, shakiness) symptoms commonly associated with cannabis withdrawal. Participants can also add additional symptoms they experienced. Participants rated the severity of each symptom on a 4-point scale from “not at all” (0) to “severe” (3). Total withdrawal severity was obtained by summing severity ratings.
Cognitive Control Tasks
The validated Dutch paper version of the Classical Stroop Task (34) and the computerized “hot” Columbia Card Task [CCT; (37)] were adminstered. During the Stroop, participants first read aloud color words (i.e., blue, green, red, yellow) as fast as possible printed in black ink, then named aloud colors of solid colored patches, and finally read aloud color words printed in an incongruent color (e.g., red printed in blue). The difference between the congruent (first two) and incongruent (last) subtasks was taken as a measure of cognitive control, with high scores indicating more interference and therefore lower cognitive control.
The CCT is a computerized card game with win and loss cards during which participants are instructed to earn as much points as possible. Each game round participants view 32 faced-down cards and turn as many as they want before deciding to stop the current round and cash the accumulated points. However, if they turn a loss card they will lose points and the game round ends. Points gained with a win card (10 or 30), points lost with a loss card (250 or 750), and the number of loss cards (1 or 3) varied. The average number of turned cards across 24 game rounds was used in subsequent analyses. The CCT was made incentive compatible by instructing participants that their scores of three randomly drawn rounds would be averaged and summed together. This total score was used in a weighted lottery procedure (see Procedure below).
Experimental Manipulation
The experimental group received the following instruction: “For the coming week, please lower your cannabis, nicotine, alcohol and other drug use as much as possible. Note that each joint, cigarette or beer you use less is incredibly good. In a week I will call you to ask how this went; this conversation will take approximately 15 min. In addition, we raffle 50 Euro among everyone who takes part in this follow-up interview, irrespective of your cannabis, and other drug use.” The control group was only told that they would be contacted for a follow-up interview regarding their cannabis, and other drug use, and that we raffled 50 Euros among the follow-up participants.
Procedure
The experiment consisted of a baseline test-session and a telephone follow-up interview. During the baseline test-session participants first completed the MCQ-SF, followed by the Stroop and CCT on a laptop. The remaining questionnaires were filled out after the tasks in the following order; TLFB, CUDIT-R, AUDIT, FTND, and BDI. The baseline test-session took approximately 45 min for which each participant received 7.50 Euro. Exactly 1 week after the test session, all participants were contacted again for a short telephone interview that contained first the TLFB followed by the MWC. A shopping voucher of 50 Euro was raffled among participants who completed the follow-up interview. Participants were instructed that this lottery was weighted upon their CCT score, with higher scores relating to more chance to gain the 50 Euro voucher.
Statistical Methods
To assess sample characteristics and change in substance use between the experimental and control group, independent sample t-tests and repeated measures ANOVAs were used. Although some of our variables are relatively skewed, these tests are described as robust with respect to the assumption of normality. This means that some deviation away from normality does not have a large influence on Type I error rates, particularly when group sizes are moderate to large and relatively equally sized (44). When the equality of variance assumption was violated, we report corrected degrees of freedom and corrected t-values. Finally, we used hierarchical regression analyses were used for testing predictors of withdrawal. Inspection of residual probability plots indicated no violation of assumptions of normality, linearity, and heteroscedasticity, nor were there influential cases detected (maximum Cook's distance = 0.347).
Results
Sample Characteristics at Baseline and at 1-Week Follow-Up
Attrition at follow-up was 28% and did not significantly differ between the experimental (n = 17) and control (n = 8) group [χ(1) = 3.2, p = 0.072]. Participants that dropped out differed on none of the baseline measures from the participants that remained in the study as indexed by independent sample t-test comparisons (all p-values > 0.1), except on the CUDIT-R, which was higher in the participants that dropped out [t(88) = −2.2, p = 0.032]. All subsequent analyses were conducted excluding the participants who dropped out at follow-up.
Independent t-tests were performed to test whether the experimental and control group differed significantly on any of the baseline measures. Results showed that the experimental and control groups did not differ significantly in any of the baseline measures, except CCT score, in which the experimental group (M = 10.2, SD = 2.0) sampled more cards than the control group (M = 8.6, SD = 2.4), t(63) = 2.99, p = 0.004. As expected, the experimental group reported higher withdrawal at follow-up than the control group [t(43.2) = 2.34, p = 0.024, Cohen's d = 0.63; Figure 1A]. Exploratory analyses of individual withdrawal symptoms indicated that group differences were most apparent in physical [decreased appetite; t(39.7) = 2.93, p = 0.006] and mental symptoms such as increased irritability [t(50.2) = 2.07, p = 0.037], and strange dreams [t(44.1) = 2.28, p = 0.028], see Figure 1B.
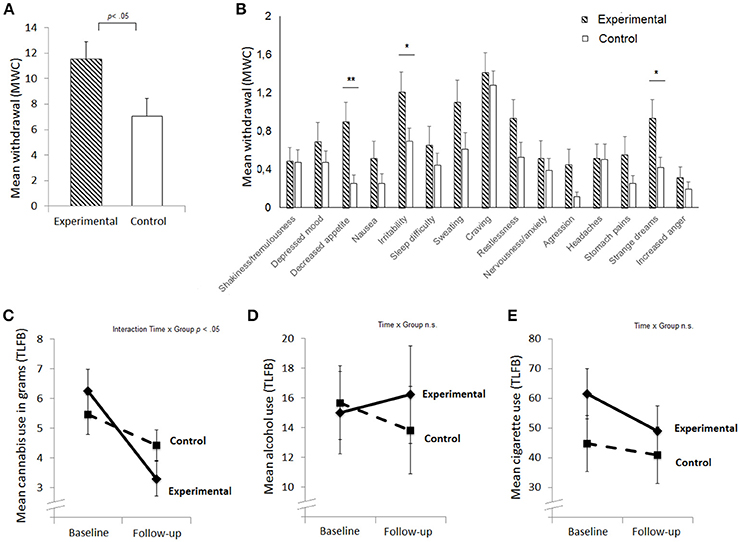
Figure 1. Mean levels of withdrawal (A), mean withdrawal score per symptom (B), cannabis use in the past week (C), alcohol use in the past week in standard units (D), and smoking in the past week in number of cigarettes (E) at baseline and follow-up for the experimental and control group. Standard errors are plotted; **p < 0.01, *p < 0.05; TLFB, Timeline Follow Back; MWC, Marijuana Withdrawal Checklist.
Next we aimed to assess if groups differed in their cannabis use over time (Figures 1C–E). A repeated measure ANOVA was performed with used grams per week (TLFB) at baseline and follow-up as within-subject factor and group as between-subject factor. Cannabis use was lower at follow-up [main effect Time: F(1, 63) = 29.7, p < 0.001, partial η2 = 0.32], and this decline was larger for the experimental group [interaction effect Time x Group: F(1, 63) = 6.9, p = 0.011, partial η2 = 0.1; see Figure 1C], indicating that participants indeed engaged in an active attempt to cut down. Alcohol use did not differ between sessions, nor was there an interaction effect between Time and Group (ps > 0.3). Cigarette use was slightly lower at follow-up independently of group [main effect Time: F(1, 63) = 4.4, p = 0.04, partial η2 = 0.07; Figure 1E]. The absolute decline in cannabis (r = −0.215, p = 0.09), cigarette (r = −0.030, p = 0.84), and alcohol use (r = 0.046, p = 0.72) did not significantly correlate with cannabis withdrawal.
Cannabis, cigarette, and alcohol use are highly comorbid in the current sample (Table 1). Pairwise correlations were computed to investigate the association between cannabis, cigarette, and alcohol use (baseline TLFB, follow-up TLFB, change in TLFB) and problem behavior related to substance use (CUDIT-R, FTND, AUDIT). Cannabis use did not correlate with cigarette (baseline: r = 0.132, p = 0.295; follow-up: r = 0.025, p = 0.845) and alcohol use (baseline: r = 0.050, p = 0.691; follow-up: r = 0.016, p = 0.902). Similarly, change in cannabis use did not correlate with change in cigarette (r = 0.121, p = 0.409) and alcohol use (r = 0.180, p = 0.173). Correlations between measures of problem behaviors showed that the CUDIT-R correlated significantly with the FTND (r = 0.363, p = 0.003), but not with the AUDIT (r = 0.119, p = 0.344).
Predictors of Withdrawal
Pairwise correlations were computed between all predictors in the participants who completed the entire study (Figure 2). The BDI was moderately correlated with the CUDIT-R (r = 0.379, p = 0.002) and craving (r = 0.417, p = 0.001). Moreover, craving was weakly correlated with the CUDIT-R (r = 0.247, p = 0.047). The two cognitive control measures did not significantly correlate with each other (Stroop-CCT r = 0.211, p = 0.091), with mental health (Stroop-BDI r = 0.047, p = 0.709; Stroop-CUDIT-R r = 0.051, p = 0.685; CCT-BDI r = 0.215, p = 0.085; CCT-CUDIT-R r = 0.089, p = 0.480), and with craving (Stroop-craving r = 0.077, p = 0.543; CCT-craving r = 0.015, p = 0.904).
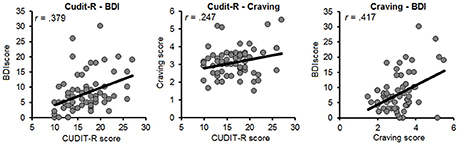
Figure 2. Scatterplots for the relation between indices of mental-health factors (Left), and craving and mental-health factors (Middle and Right). BDI, Beck Depression Inventory; CUDIT-R, Cannabis Use Disorder Identification Test-Revised; MCQ, Marijuana Craving Questionnaire.
A first aim was to assess if mental health, craving and cognitive control predicted withdrawal severity (Figure 3). A second aim was to assess if these effects were specific for an active attempt to cut-down substance use. A hierarchical regression analysis was performed per predictor of interest. For each of these regressions, MWC score was the dependent variable. The main effect of the predictor under study was entered first, after which a main effect of group was entered in the second step, and finally an interaction effect between group and predictor was added.
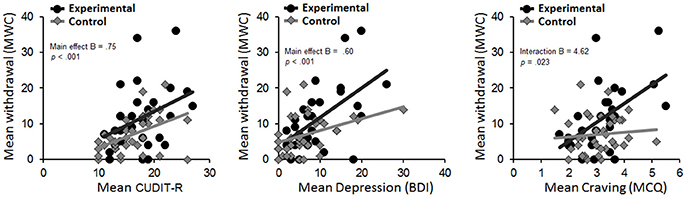
Figure 3. Scatterplots for the relation between level of withdrawal at follow-up and CUDIT-R (Left), Depression (Middle), and Craving (Right) score at baseline for the experimental and control group separately. BDI, Beck Depression Inventory; CUDIT-R, Cannabis Use Disorder Identification Test-Revised; MCQ, Marijuana Craving Questionnaire.
Higher CUDIT-R score was a significant predictor of withdrawal (B = 0.75, SE = 0.2, p < 0.001, R2 = 18.1%). Adding the main effect of group explained significantly more variance [Fchange(1, 62) = 4.37, p = 0.04, R2 = 23.5%], although there was no significant group x CUDIT-R interaction (p = 0.64).
Higher BDI score also was a significant predictor of withdrawal (B = 0.60, SE = 0.13, p < 0.001, R2 = 26%). Adding group (B = −2.85, SE = 1.65, p = 0.089) as well as the group x BDI interaction (B = 0.483, SE = 0.25, p = 0.062) did not explain significantly more variance.
MCQ-SF score was a significant predictor of withdrawal (B = 3.41, SE = 1.05, p = 0.002, R2 = 14.4%), indicating that greater craving was predictive of greater cannabis withdrawal. Adding the main effect of group [Fchange(1, 62) = 5.7, p = 0.02, R2 = 21.6%], as well as a group x craving interaction [Fchange(1, 61) = 5.5, p = 0.023, R2 = 28.1%] explained significantly more variance. Follow-up tests of this interaction term indicated that greater craving predicted higher withdrawal symptoms in the experimental (B = 5.27, SE = 1.55, p = 0.002), but not in the control group (B = 0.64, SE = 1.22, p = 0.6).
Finally, cognitive control measures did not significantly predict withdrawal symptoms in any of the models. That is, CCT was not a significant predictor of withdrawal (B = 0.317, SE = 0.389, p = 0.107). While there was a main effect of group [Fchange(1, 62) = −4.49, p = 0.025], there was no group x CCT interaction (B = −0.144, SE = 0.852, p = 0.866). Similarly, Stroop was not a significant predictor of withdrawal (B = 0.054, SE = 0.084, p = 0.522). While there was a main effect of group [Fchange(1, 62) = 5.69, p = 0.02], there was again no group x Stroop interaction (B = −0.012, SE = 0.166, p = 0.942).
Given the significant correlations between BDI, CUDIT-R and craving, a combined hierarchical regression was performed to assess the unique contribution of these significant predictors of interest. Following the same approach as described above, first, the main effects of all predictors were entered, then the main effect of group was entered, and finally all interactions between predictors x group were entered. When assessing the first step of this analysis, the model was significant [Fchange(3, 61) = 10.86, p < 0.001, R2 = 34.8%]. The main effects of each predictor in this model (BDI, CUDIT-R, and craving) showed that higher BDI (B = 0.401, SE = 0.14, p = 0.006) and higher CUDIT-R (B = 0.444, SE = 0.20, p = 0.028) scores at baseline were predictive of greater withdrawal at follow-up. Adding the main effect of group in a second step did not yield a better model fit (p = 0.10), as well adding all interactions between group and these predictors did not improve model fit (p = 0.277) in a final step.
Control Analyses
We performed two control analyses. First, to control for overlap between the MWQ symptoms Depression and Craving and our main predictors of interest BDI (Depression) and MCQ-SF (Craving) we repeated all regression analyses with a withdrawal (MWQ) score excluding these two items. All reported results and interpretations remained the same, except for the BDI. That is, BDI was a significant predictor for withdrawal severity [Fchange(1, 63) = 20.18, p < 0.001, B = 0.50, SE = 0.11] yet the interaction between group x BDI now reached significance [Fchange(1, 61) = 4.4, p = 0.04]. Follow-up analyses showed that BDI scores were more strongly related to withdrawal in the experimental group [Fchange(1, 27) = 12.3, p = 0.002, B = 0.714, SE = 0.20] than in the control group [Fchange(1, 34) = 4.77, p = 0.036, B = 0.248, SE = 0.11].
Second, nicotine dependence (FTND) correlated positively with cannabis use-related problems (CUDIT-R). To control for a possible influence of nicotine dependence, we repeated all regression analyses adding the FTND as an additional predictor in the first step, and the interaction between group x FTND as an additional predictor in the third step of the regression models. All reported results and interpretations remained the same, and no main or interaction effects of FTND were observed.
Discussion
Not all individuals with a CUD who attempt to lower or quit their cannabis use will experience severe withdrawal (9). From a clinical perspective, predicting who will could help tailoring treatment (e.g., decisions to commence pharmacological and/or psychological withdrawal management). In this first study in near-daily cannabis users, we investigated if cognitive (craving, cognitive control) and mental health (depression, cannabis use-related problems) factors could predict withdrawal severity during an active attempt to cut down cannabis use. The experimental group was asked to lower substance use as much as possible in the week following the baseline test-session and significantly reduced their cannabis use more and experienced more withdrawal compared to the control group. Baseline cannabis use-related problems, depressive symptoms and craving, but not cognitive control, significantly predicted withdrawal at follow-up. Moreover, craving uniquely predicted withdrawal severity in the experimental group. Only depressive symptoms and cannabis-use-related problems uniquely predicted withdrawal severity across groups. Together, these factors explained 34.8% in the variance in cannabis withdrawal 1 week later. For the first time we showed that especially baseline depressive symptoms, cannabis use-related problems and craving, but not cognitive control, could be indicative of cannabis withdrawal. Severity of depressive symptoms and cannabis use-related problems may thereby predict general mental and physical discomfort from fluctuations in cannabis use, whereas craving may specifically do so in individuals who actively attempts to cut down cannabis use.
This study is a first step toward understanding the mechanisms underlying cannabis withdrawal during an active attempt to cut-down or quit cannabis use, generating important new hypotheses about how different self-reported mental health problems can uniquely contribute to self-reported cannabis withdrawal severity; Regardless of the significant moderate correlations between depressive symptoms and cannabis use-related problems, both factors uniquely predicted withdrawal severity across groups. Craving predicted withdrawal severity more strongly in the group who attempted to lower their cannabis use. This effect was no longer significant when depressive symptoms and cannabis use-related problems were entered into the regression model, indicating that the shared variance of these factors with craving is probably driving the interaction between group and craving. Importantly, to further our understanding, these results should be followed up by more in-depth pharmacological studies, including longitudinal measures of withdrawal and objective biochemical measures of cannabis, cigarette and alcohol use. A critical evaluation of the design and the implications for theory and future studies is outlined below.
A cannabis user is almost never only a cannabis user. This is corroborated in the present study; 90% of the cannabis users combined tobacco with cannabis in their joints, 72% of the cannabis users were daily cigarette smokers, and cannabis use-related problems correlated with nicotine dependence (r = 0.363) and depressive symptoms (r = 0.379). Alcohol use did not correlate with cannabis and cigarette use. Cannabis use was lower at follow-up and this decline was larger for the experimental group, indicating that participants indeed engaged in an active attempt to cut down. However, cigarette use was also slightly lower at follow-up independently of group. Cigarette use has been shown to predict cannabis dependence over time, independently from cannabis use frequency (45). Moreover, the pattern and timeline of cannabis and nicotine withdrawal is suggested to be similar and simultaneous abstinence may increase withdrawal symptom severity (46). These results raise the question if nicotine withdrawal and dependence are partly driving the results. Cannabis use-related problems and depressive symptoms uniquely predicted self-reported withdrawal severity after 1 week and these results did not change when nicotine dependence was additionally controlled for. Moreover, daily cigarette use did not correlate with daily cannabis use (FTND) and the change in cigarette use did not correlate with change in cannabis use and withdrawal severity. These observations suggest that nicotine withdrawal and dependence are not driving the result. However, studies investigating the biochemical interactions between cannabis and cigarette use and abstinence, and their potential detrimental effects on clinical outcomes are needed to further investigate this.
Following contemporary addiction models (16, 19–23) we hypothesized that individuals with good cognitive control would be better in suppressing withdrawal. Cognitive control did not predict cannabis withdrawal, in contrast to mental health factors and craving. These findings suggest that both relatively “cold” executive functioning (Stroop) and relatively “hot” decision making (CCT) are unrelated to withdrawal. The two control measures did not correlate with each other and any of the substance use measures in the sample that completed the entire study (n = 65). However, post-hoc analyses in the entire sample (n = 90) revealed a moderate correlation between Stroop and CCT (r = 0.364, p < 0.001) and weak correlations between craving and cognitive control (Craving-Stroop r = 0.242, p = 0.022; Craving-CCT r = 0.207, p = 0.05), supporting moderate construct and external validity of the CCT and Stroop. To the best of our knowledge, there are no studies directly investigating the association between cognitive control and cannabis withdrawal. Interestingly, a meta-analysis investigating the association between nicotine abstinence and cognitive performance suggests that response inhibition is consistently impaired by nicotine abstinence, but not cognitive control as measured with the Stroop (47). Moreover, an fMRI study on smoking abstinence suggested that particularly the regulation of negative affect was related to abstinence (31). These studies suggest that behavior and brain functioning during cognitive tasks that measure (negative) emotion regulation and inhibition may predicts cannabis withdrawal better than the Stroop and CCT. However this hypothesis is speculative and future studies are needed to further unravel the relationship between different aspects of cognitive functioning and withdrawal.
Cannabis withdrawal symptoms include, irritability, anger or aggression, depressive mood, anxiety, sleep difficulty (including strange dreams), loss of appetite, restlessness, and discomforting physical symptoms like tremors, headaches, sweating and abdominal pain (2, 15). Importantly, depressive mood and craving (on a 4-point scale) are symptoms of cannabis withdrawal, showing some overlap with the BDI and craving assessment, but these two items do not drive the results as the effects are still significant and even stronger for the BDI when the depressive mood and craving items are excluded from the analyses. Moreover, explorative analyses of individual withdrawal symptoms (Figure 1) indicated that the group difference in withdrawal was mainly driven by strange dreams irritability and decreased appetite. Unfortunately, we only assessed withdrawal once after 1 week (close to the expected average peak of withdrawal) (8, 13, 15). These results therefore reflect average severity of each symptom across the week following the baseline test-session. To assess the clinical merit of our results and to further investigate the association between mental health, craving and withdrawal it essential to study individual withdrawal symptoms over the course of abstinence in general population and clinical samples of cannabis users.
As discussed above, withdrawal was only assessed once. The current study also has other limitations that should be acknowledged. The most important limitation is that we did not include a biochemical verification of abstinence. In this first study, we opted for a simple design, testing an ecologically valid sample of cannabis users with comorbid substance use, using behavioral tasks and self-reports that are generally available to clinicians. Higher withdrawal combined with lower self-reported cannabis use in the experimental compared to the control group and the observed correlation between different mental health assessments suggest that the self-reports and procedures are meaningful. However, without objective measures of substance use we cannot rule out the potential effect of a social desirability bias induced by the testing procedure and cannot properly investigate the interactions between withdrawal, cannabis, cigarette and alcohol use. Although differentiating new cannabis use from residual cannabis metabolites in urine samples remains hard, statistical modeling tools using creatine and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) levels showed promising results and can aid in future studies (48).
Moreover, the mental-health factors included in this study were limited to substance use-related problems and depression. We specifically choose to only include depressive symptoms, given that previous studies showed that only mood disorders were related to the experience of significant withdrawal in heavy adult cannabis users (14) and in treatment seeking adolescent cannabis users (7). Yet, other mental health factors may play a role withdrawal as well as both internalizing and externalizing disorders are highly comorbid with CUDs and differentiate between cannabis users with and without a CUD (49). Also, dropout in the current study was 28% and those who dropped out had significantly higher cannabis use-related problems than those who participated in the follow-up. It is possible that difficulty with decreasing cannabis use is related to the reachability of those participants. Since selection effects may drive the current findings, it is important for future studies to verify these findings across different populations. The unexpected difference between the groups on the CCT, should also be acknowledged. The experimental group took more risky decisions than the control group and this may have influenced our findings. Moreover, the relative limited sample size is a limitation and studies in older cannabis users are needed to determine of our effects generalize to older age-groups. Finally, only a limited number of females were tested, hindering us to assess potential differential impact of sex on withdrawal [(50), but see (9, 12, 51, 52)]. Taken the strengths and limitations of this study together, an important next step is to study the role of a wider set of cognitive and mental-health factors in individual trajectories of withdrawal, in a well-balanced sample of males and females, both inside and outside of a clinical setting, with the former having the advantage of monitoring a broader group of heavy cannabis users, and the latter having the advantage of monitoring cannabis abstinence and participation in a more controlled setting.
In conclusion, we showed that baseline depressive symptoms, cannabis use-related problems and craving, but not cognitive control, could be indicative of cannabis withdrawal during an attempt to cut down cannabis use. These findings generate new hypotheses about how mental health and cognitive factors may uniquely contribute to cannabis withdrawal. However, this study did not include biochemical verifications of abstinence and should be follow up by studies including more objective measures of substance use and more frequent assessments of cannabis withdrawal over time, both inside and outside of a clinical setting.
Author Contributions
JC and AvD equally contributed to the study design, data collection and data analysis. JC drafted the manuscript and AvD provided critical revisions. Both authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Eveline Crone for helpful discussions and Joran Menger, Martine Doesburg, and Niels van der Ham for their help in recruiting and testing the participants.
References
1. Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry (2006) 19:233–8. doi: 10.1097/01.yco.0000218592.00689.e5
2. American Psychiatric Association APADSMTF. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Available online at: http://dsm.psychiatryonline.org/book.aspx?bookid=556 (2013).
4. Marshall K, Gowing L, Ali R, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst Rev. (2014) 12:CD008940. doi: 10.1002/14651858.CD008940
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. (2017). 8:9–37. doi: 10.2147/SAR.S109576
6. Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry (2004) 161:1967–77. doi: 10.1176/appi.ajp.161.11.1967
7. Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents' treatment response and outcomes: a 12-month prospective investigation. J Addict Med. (2014) 8:359–67. doi: 10.1097/ADM.0000000000000064
8. Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS ONE (2012) 7:e44864. doi: 10.1371/journal.pone.0044864
9. Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug Alcohol Depend. (2010) 106:133–41. doi: 10.1016/j.drugalcdep.2009.08.008
10. Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction (2008) 103:787–99. doi: 10.1111/j.1360-0443.2008.02158.x
11. Davis JP, Smith DC, Morphew JW, Lei X, Zhang S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: a prospective study. J Drug Issues (2016) 46:64–83. doi: 10.1177/0022042615616431
12. Gorelick DA, Levin KH, Copersino ML, Heishman SJ, Liu F, Boggs DL, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend. (2012) 123:141–7. doi: 10.1016/j.drugalcdep.2011.11.007
13. Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry (2013) 73:242–8. doi: 10.1016/j.biopsych.2012.07.028
14. Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry (2008) 69:1354–63. doi: 10.4088/JCP.v69n0902
15. Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. (2003) 112:393–402. doi: 10.1037/0021-843X.112.3.393
16. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry (2002) 159:1642–52. doi: 10.1176/appi.ajp.159.10.1642
17. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology (2010) 35:217–38. doi: 10.1038/npp.2009.110
18. Buckner JD, Zvolensky MJ, Crosby RD, Wonderlich SA, Ecker AH, Richter A. Antecedents and consequences of cannabis use among racially diverse cannabis users: an analysis from Ecological Momentary Assessment. Drug Alcohol Depend. (2015) 147:20–5. doi: 10.1016/j.drugalcdep.2014.12.022
19. Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. (2004) 28:343–51. doi: 10.1016/j.neubiorev.2004.03.007
20. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. (2005) 8:1481–9. doi: 10.1038/nn1579
21. Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. (2003) 54:25–53. doi: 10.1146/annurev.psych.54.101601.145237
22. Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology (2009) 56 (Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035
23. Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RC, Sher KJ, et al. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. (2007) 86:263–83. doi: 10.1016/j.pbb.2006.09.021
24. Gray KM, LaRowe SD, Watson NL, Carpenter MJ. Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addict Behav. (2011) 36:140–3. doi: 10.1016/j.addbeh.2010.08.021
25. Lundahl LH, Johanson CE. Cue-induced craving for marijuana in cannabis-dependent adults. Exp Clin Psychopharmacol. (2011) 19:224–30. doi: 10.1037/a0023030
26. Cousijn J, Benthem P, Van der Schee E, Spijkerman RS. Motivational and control mechanisms underlying adolescent cannabis use disorders: a prospective study. Dev Cogn Neurosci. (2015) 16:36–45. doi: 10.1016/j.dcn.2015.04.001
27. Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. (2011) 5:1–8. doi: 10.1097/ADM.0b013e31820c23fa
28. Fernandez-Serrano MJ, Perez-Garcia M, Verdejo-Garcia A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neurosci Biobehav Rev. (2011) 35:377–406. doi: 10.1016/j.neubiorev.2010.04.008
29. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA (2012) 109:E2657–64. doi: 10.1073/pnas.1206820109
30. Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry (2005) 57:56–66. doi: 10.1016/j.biopsych.2004.10.022
31. Froeliger B, Modlin L, Wang L, Kozink RV, McClernon FJ. Nicotine withdrawal modulates frontal brain function during an affective Stroop task. Psychopharmacology (2012) 220:707–18. doi: 10.1007/s00213-011-2522-y
32. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addict Biol. (2013) 18:1013–23. doi: 10.1111/j.1369-1600.2012.00498.x
33. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum Brain Mapp. (2014) 35:2470–82. doi: 10.1002/hbm.22342
35. Cousijn J, Watson P, Koenders L, Vingerhoets WA, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. (2013) 38:2825–32. doi: 10.1016/j.addbeh.2013.08.011
36. Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict Biol. (2013) 18:570–80. doi: 10.1111/j.1369-1600.2011.00417.x
37. Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J Exp Psychol Learn Mem Cogn. (2009) 35:709–30. doi: 10.1037/a0014983
38. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. (2010) 110:137–43. doi: 10.1016/j.drugalcdep.2010.02.017
39. Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. (2009) 102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010
40. Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. (1996) 42:49–54. doi: 10.1016/0376-8716(96)01263-X
41. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction (1993) 88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
42. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. (1991) 86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x
43. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. (1996). 67:588–97. doi: 10.1207/s15327752jpa6703_13
44. Glass, GV, Peckham, PD, and Sanders, JR Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Rev Educ Res (1972). 42:237–88.
45. Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G, et al. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. (2015) 148:165–71. doi: 10.1016/j.drugalcdep.2015.01.004
46. Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. (2008) 92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010
47. Grabski M, Curran HV, Nutt DJ, Husbands SM, Freeman TP, Fluharty M, et al. Behavioural tasks sensitive to acute abstinence and predictive of smoking cessation success: a systematic review and meta-analysis. Addiction (2016) 111:2134–44. doi: 10.1111/add.13507
48. Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction (2011). 106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x
49. van der Pol P, Liebregts N, de Graaf R, Ten Have M, Korf DJ, van den Brink W, et al. Mental health differences between frequent cannabis users with and without dependence and the general population. Addiction (2013) 108:1459–69. doi: 10.1111/add.12196
50. Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. (2015) 23:415–21. doi: 10.1037/pha0000053
51. Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. (2011) 119:123–9. doi: 10.1016/j.drugalcdep.2011.06.003
Keywords: cannabis, cognitive control, cannabis use disorder, craving, depression, withdrawal
Citation: Cousijn J and van Duijvenvoorde ACK (2018) Cognitive and Mental Health Predictors of Withdrawal Severity During an Active Attempt to Cut Down Cannabis Use. Front. Psychiatry 9:301. doi: 10.3389/fpsyt.2018.00301
Received: 08 March 2018; Accepted: 18 June 2018;
Published: 11 July 2018.
Edited by:
Marc N. Potenza, Yale University, United StatesReviewed by:
Melissa Nicole Slavin, University at Albany, United StatesOussama Kebir, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Copyright © 2018 Cousijn and van Duijvenvoorde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janna Cousijn, ai5jb3VzaWpuQGdtYWlsLmNvbQ==
 Janna Cousijn
Janna Cousijn A. C. K. van Duijvenvoorde
A. C. K. van Duijvenvoorde