- 1“Specialists in Psychiatry” Medical Center, Turku, Finland
- 2Satakunta Hospital District, Psychiatric Care Division, General Psychiatry Outpatient Clinic, Pori, Finland
- 3Turku University Central Hospital, Salo Psychiatry Outpatient Clinic, Salo, Finland
- 4Terveystalo Pulssi Medical Center, Turku, Finland
- 5Department of Radiology, University of Turku, Turku, Finland
- 6Department of Psychiatry, University of Turku, Turku, Finland
- 7Cognitive Disorders/Dementia Unit, 2nd University Department of Neurology, Medical School, National and Kapodistrian University of Athens, University General Hospital “ATTIKON”, Athens, Greece
- 8Division of Neuropsychiatry, Helsinki University Hospital, Helsinki, Finland
Background: Non-alcoholic Wernicke's encephalopathy and Korsakoff syndrome are greatly underdiagnosed. There are very few reported cases of neuropsychologically documented non-alcoholic Korsakoff syndrome, and diffusion tensor imaging (DTI) data are scarce.
Methods: We report clinical characteristics and neuropsychological as well as radiological findings from three psychiatric patients (one woman and two men) with a history of probable undiagnosed non-alcoholic Wernicke's encephalopathy and subsequent chronic memory problems.
Results: All patients had abnormal neuropsychological test results, predominantly in memory. Thus, the neuropsychological findings were compatible with Korsakoff syndrome. However, the neuropsychological findings were not uniform. The impairment of delayed verbal memory of the first patient was evident only when the results of the memory tests were compared to her general cognitive level. In addition, the logical memory test and the verbal working memory test were abnormal, but the word list memory test was normal. The second patient had impaired attention and psychomotor speed in addition to impaired memory. In the third patient, the word list memory test was abnormal, but the logical memory test was normal. All patients had intrusions in the neuropsychological examination. Executive functions were preserved, except for planning and foresight, which were impaired in two patients. Conventional MRI examination was normal. DTI showed reduced fractional anisotropy values in the uncinate fasciculus in two patients, and in the corpus callosum and in the subgenual cingulum in one patient.
Conclusions: Non-alcoholic Korsakoff syndrome can have diverse neuropsychological findings. This may partly explain its marked underdiagnosis. Therefore, a strong index of suspicion is needed. The presence of intrusions in the neuropsychological examination supports the diagnosis. Damage in frontotemporal white matter tracts, particularly in the uncinate fasciculus, may be a feature of non-alcoholic Korsakoff syndrome in psychiatric patients.
Introduction
Wernicke's encephalopathy (WE) is caused by thiamine deficiency (1, 2, 3). Undiagnosed or inadequately treated WE can cause Korsakoff syndrome (KS). KS is characterized by severe chronic cognitive impairment that predominantly affects memory (4, 5). However, evidence suggests a wide range of cognitive impairment (6–10). WE and KS are referred together as the Wernicke-Korsakoff syndrome (WKS) (6, 11). Previously, the full classic triad (ocular abnormalities, ataxia or unsteadiness, and an altered mental state or mild memory deficiency) was needed to diagnose WE. However, the diagnostic assessment proposed by Caine et al. (WE can be diagnosed with any two of four signs, any element of the classic triad and nutritional deficiency) has been gaining acceptance (2, 6, 12).
Alcoholic and non-alcoholic WE and alcoholic KS have been extensively studied, but very little is known about non-alcoholic KS. Only recently it has become clear that many patients do develop KS after non-alcoholic WE (6, 13, 14). Even so, there are only 14 previously reported cases of non-alcoholic KS with the results of the neuropsychological examination presented (15, 16). Based on autopsy studies, at least 94% of non-alcoholic WE patients are not diagnosed during life (17). Still, KS after undiagnosed non-alcoholic WE has been very rarely reported during the last few decades (15, 18–20). It has been suggested, that such cases may be encountered by psychiatrists (4).
Compared with alcoholic WKS, non-alcoholic WKS may be more common in women and younger subjects, and may have a better survival rate (6). In alcoholic KS, brain MRI often shows atrophy in the mammillary bodies, the frontal lobes and the vermis (21), whereas in non-alcoholic KS atrophy changes may be uncommon (15). In both alcoholic and alcoholic KS, neuropsychological assessment usually shows impairment of anterograde memory, whereas executive functions are typically affected only in alcoholic KS (5, 15, 16).
We have recently reported on three psychiatric patients with previously undiagnosed non-alcoholic WE that had typical KS findings in neuropsychological testing (15). Two of these patients were examined with diffusion tensor imaging (DTI), and one had damage in the uncinate fasciculus, a frontotemporal tract. We report here on three non-alcoholic psychiatric patients with a similar history of previously undiagnosed WE, chronic memory impairment, and frontotemporal tract damage. However, in the present set of patients, the neuropsychological findings were more diverse.
Materials and Methods
All patients were treated by one of the authors (GN). Similarly to our previous study (15), the patients were identified by screening psychiatric patients with memory impairment for nutritional deficiency in history. We reviewed the hospital records and the reports of all previous neuropsychological examinations. All patients have memory problems that emerged around the time of WE. Table 1 shows the WE manifestations: all patients had unsteadiness and an altered mental state, two patients had ocular abnormalities, and one patient had muscle weakness. The patients were not examined by brain MRI during the WE. A diagnosis of WKS had not been previously considered, and the patients did not receive thiamine therapy at the time of WE.
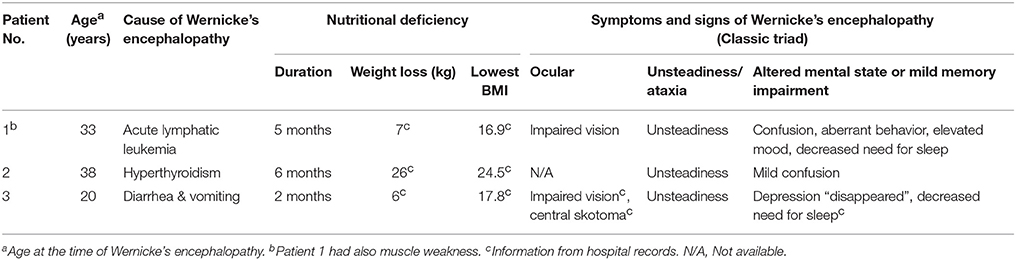
Table 1. Causes and manifestations of non-alcoholic Wernicke's encephalopathy in three psychiatric patients with Korsakoff syndrome.
Patient 1 met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnostic criteria for alcohol use disorder at the age of 15–21 years, more than 10 years before the WE. There was no nutritional deficiency or WE manifestations during that period, and the patient has not used alcohol since. The other patients did not have a history of current or past alcohol use disorder. None of the patients had a history of traumatic brain injury.
Clinical Description
Patient 1: This female patient was self-referred at the age of 34 for symptoms of depression.
WE and memory impairment: At the age of 33, the patient was treated with cytostatic drugs, cortisone, radiotherapy, and bone marrow transplantation for acute lymphatic leukemia. She was on total parenteral nutrition for 3 weeks. While hospitalized, the patient experienced loss of appetite, daily vomiting, diarrhea, weight loss, and symptoms of WE, including pronounced muscle weakness (Table 1). A few months later, the patient's physical condition improved. At that time, she noticed memory problems. At the age of 36, a clinical neuropsychological examination showed deficits in working memory, attention, and verbal fluency. MRIs at the age of 36 and 40 showed a small developmental venous angioma in the posterior part of the right frontal lobe and mild ectopy of the cerebellar tonsils. Brain [18F]-fluorodeoxyglucose PET examination was normal.
Prior psychiatric treatment: The patient received counseling for depression and problems with alcohol during her teen years.
Course and outcome: One year after the WE, the patient was diagnosed with severe major depression. Three years later she was diagnosed with bipolar II disorder. She has also been diagnosed with generalized anxiety disorder, post-traumatic stress disorder, and obsessive-compulsive personality disorder. She had cognitive psychotherapy for 4 years and psychophysical therapy for 2 years. Current medication comprises valproate, lamotrigine, thyroid hormone for hypothyroidism, and estrogen replacement therapy. She has artisan and dance instructor's degrees and is on long-term disability leave. Currently, at the age of 43, leukemia is in remission. The patient's mood has been mostly normal, but she gets easily tired. The patient recovered from some manifestations of WE after a few weeks. However, the unsteadiness and muscle weakness have improved but are still present. The memory problem has persisted, and there is a mild confabulation tendency. Figure 1 shows a drawing made by the patient on how she experiences the memory impairment. She needs help from her parents at least four times per week to remember to eat regularly, pay her bills, find misplaced items, and cope with other daily routines.
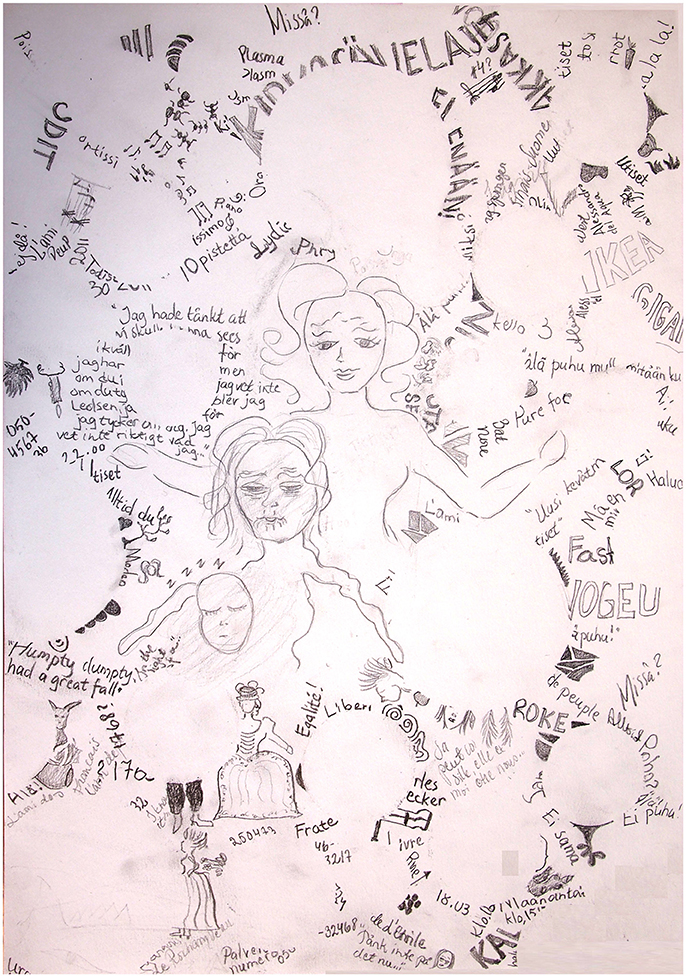
Figure 1. Drawing made by Patient 1 on her memory problem. The patient is writing a novel, the events take place during the French Revolution. The patient has tried to gather information on the historical background, but she keeps forgetting what she reads. The round empty spaces in the drawing represent gaps in memory. The neat and smiling female figure in the center represents the patient being diligent and in a good mood but the memory difficulties making her feel perplexed, unsure, and lost, opening her arms to apologize for the inconveniences caused. Below and to the right of this figure is an old, frail, and depressed woman, the patient when very tired and desperate by her unsuccessful attempts to learn new information, thinking of the memory problem as an old person's disease and knowing that currently there is no cure. Up and to the right of these figures, ants are taking away the patient's misplaced possessions. The musical notes represent forgetting what she had learned in music theory during a course 2 years ago. Up and on the left of the figures, the names of two large stores, the patient feels overwhelmed when she is presented with a lot of new information at the same time.
Patient 2: This male software engineer was first treated by GN at the age of 51 at an outpatient psychiatric clinic.
WE and memory impairment: The patient received outpatient treatment with radioactive iodine for hyperthyroidism at the age of 38. His weight dropped, and he had unsteadiness (Table 1) for a few weeks. Soon after that, he started having memory problems. At the age of 41, he was diagnosed with major depression. Later, psychotic features were noted, and more recently bipolar II disorder was diagnosed. At the age of 49, a clinical neuropsychological examination showed slowness and severe memory deficit. MRI was normal at the age of 51 and 52. Brain PET examination was normal.
Comorbidities: The patient has used CPAP-treatment since the age of 52 for severe obstructive sleep apnea (OSA). He is also treated for hypothyroidism, high blood pressure, type 2 diabetes, and hypercholesterolemia.
Course and outcome: The patient has used several psychiatric medications, including quetiapine, aripiprazole, escitalopram, and bupropion. At the age of 51, he was treated with electroconvulsive therapy that did not affect the memory symptoms. He received a permanent disability pension at the age of 53. He was treated by GN for 3 years until the age of 54. At the end of the treatment, the patient's mood had partially improved, and there were no psychotic symptoms. However, the memory problem was still severe, requiring weekly home visits by a mental health nurse.
Patient 3: This male patient was first treated by GN at the age of 44 for major depression.
WE and memory impairment: At the age of 20, the patient had an antibiotic-treated infection, followed by diarrhea and vomiting for 2 weeks. He was not hospitalized at the time. The patient's appetite was very low for a further few weeks. During a period of several weeks, there were WE manifestations (Table 1), and long-term memory problems started around that time. At the age of 45, a clinical neuropsychological examination showed deficits in memory (with intrusions), attention, and executive functions; MRI and PET were normal.
Comorbidities: Mild OSA is treated with a mandibular advancement device.
Prior psychiatric treatment: The patient was diagnosed with an eating disorder during his late teens. His BMI was normal for around one year before the WE. Later, the patient was diagnosed with severe major depression, somatoform disorder, and sleep disorder.
Course and outcome: Since the time of WE, the patient has had daily gastrointestinal symptoms (dyspepsia, delayed emptying of the stomach). The depression has not responded to medication (paroxetine, citalopram, moclobemide, sertraline, tianeptine, agomelatine, flupentixol, modafinil) or transcranial magnetic stimulation. The memory impairment has persisted. The patient was treated by GN for 3 years until the age of 47. He has worked with media (self-learned), and he is on part-time, long-term disability leave.
Neuropsychological Assessment
Testing was performed as previously described (15). In addition, the vocabulary subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III) was used to assess general cognitive level. The neuropsychological tests used are presented in Tables 2, 3. Results at least 1.5 standard deviations (SDs) below the mean of normative values were considered abnormal. We also considered abnormal a difference of 15 or more between memory quotients and the general cognitive level (22). Intrusions were defined as false words in the Hopkins Verbal Learning Test–Revised (HVLT-R) and false statements in the Wechsler Memory Scale–III (WMS-III). Intrusions semantically related to the presented material were considered semantic (23).
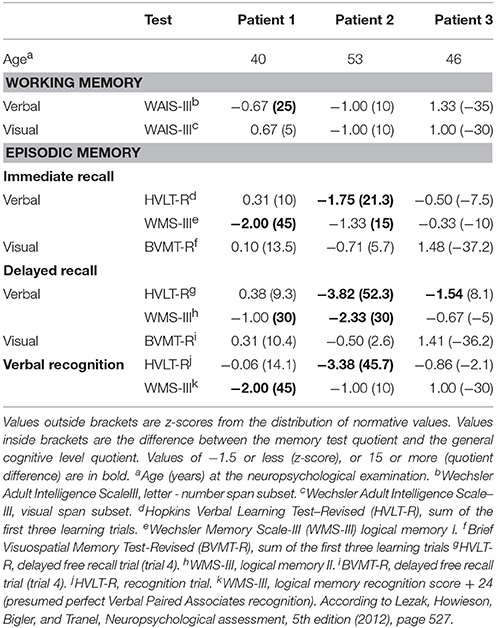
Table 2. Results of memory tests for three psychiatric patients with non-alcoholic Korsakoff syndrome.
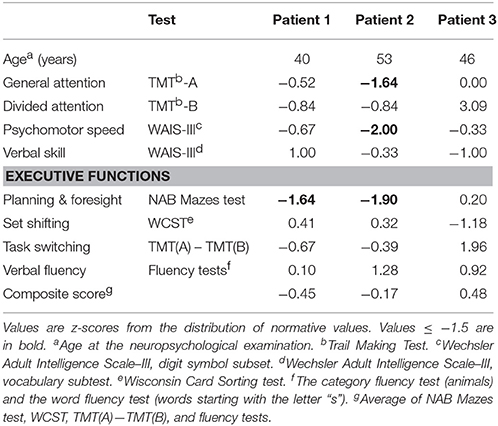
Table 3. Results of non-memory tests for three psychiatric patients with non-alcoholic Korsakoff syndrome.
DTI and Conventional Brain MRI
All patients underwent a MRI/DTI examination with similar imaging procedures and normal controls as previously described (15, 24, 25). MRI/DTI was performed at 3T (Achieva, Philips Medical Systems) using a sensitivity encoding (SENSE) 8-channel transmit-receive head coil. The imaging protocol consisted of transverse T2-weighted turbo spin echo images, coronal fluid attenuated inversion recovery (FLAIR) images, sagittal 3D FLAIR images, sagittal 3DT1 turbo field echo images, transverse susceptibility-weighted fast field echo images (venous BOLD), and transverse DTI images. Diffusion-weighted turbo spin echo EPI images (TR/TE 5877/62, 60 slices with 2.0 mm thickness, gap 0.0 mm, 112 × 128r matrix, turbo factor 59, EPI factor 59, FOV 224 mm, RFOV 100%, number of excitations 2, imaging time 3 min 52 s); b values of 0 and 800 sec/mm2 and 15 different gradient encoding directions were used, and isotropic images with 2.0 × 2.0 × 2.0 mm voxel size were obtained. The images were post processed with the Philips Diffusion Registration Tool (Philips, Medical Systems, Best, the Netherlands) to remove distortions and misalignments due to shear and eddy current, as well as head motion. All transverse images were obtained according to the line between the lower border of the genu and splenium of the corpus callosum.
Deterministic DTI tractography (FiberTrak package, Philips) was performed using the following fractional anisotropy (FA) and turning angle thresholds to terminate the tracking process: FA 0.15/angle 27, FA 0.15/angle 40, FA 0.30/angle 27, or FA 0.50/angle 27 depending on the tract. The tracts were defined by means of two or three free-hand inclusion regions-of-interest and 1-3 exclusion regions-of-interest, which were placed in standard positions by a neuroradiologist (TK) according to anatomical landmarks.
FA-values were compared to those of normal controls. The controls comprised volunteers and subjects scanned to exclude arterial aneurysms or due to benign sinonasal problems. For the subgenual cingulum and the fornix we used 16 controls for each patient, matched for sex, and in the same age range with the patients (36–55 years). For the rest of the tracts we used 30 controls matched for age and sex to each patient. The controls had normal brain MRI scans. The MRI acquisition protocol was the same for the patients and controls. A questionnaire and review of medical records were used to exclude traumatic brain injury, other neurological diseases, psychiatric disease, vascular disease, hypertension, and chronic alcoholism in the controls.
In DTI, we examined the corpus callosum (separately genu, body, and splenium), the cingulum [separately the superior and inferior (parahippocampal) sections, and in addition the subgenual part separately (26)], the uncinate fasciculus (UF), the superior and inferior longitudinal fasciculi, the left arcuate fasciculus, the inferior fronto-occipital fasciculus, the anterior corona radiata, the prefrontal projection fibers (corticostriatal projections), the projection tracts through mesencephalon, and the fornix (separately the descending fornix and the whole tract). For each tract separately, we subtracted the mean FA-value of the controls from the patient's FA-value, and we then divided this difference with the SD-value of the controls. Values ≤ −2 (representing patient FA-values at least 2 SDs below the mean of controls) were considered abnormal.
Consent to Participate
The study complied with the Declaration of Helsinki. All patients gave written consent for the use of their medical information in this publication. The Finnish legislation does not require an ethics committee's permission for studies that are restricted to the use of patients' medical information, in case the subjects have given written consent for the use of their medical information.
Results
Neuropsychological Assessment
Table 2 shows the results of the memory tests. Delayed verbal recall was impaired in all patients, and immediate verbal recall was impaired in two patients. Two patients had impaired verbal recognition. Verbal working memory was impaired in Patient 1. Patient 1 had an abnormal result in the logical memory test (WMS-III) but not in the word list memory test (HVLT-R). Patient 3 had an abnormal result in the word list test but not in the logical memory test. The memory impairment of Patient 1 was evident only when compared with her general cognitive level. In the HVLT-R, Patient 1 had no intrusions, Patient 2 had six intrusions, and Patient 3 had one intrusion. In the WMS-III, Patient 1 had nine intrusions, Patient 2 had eight intrusions, and Patient 3 had six intrusions. All intrusions were semantic. Table 3 shows the results of the non-memory tests. Patient 1 had an abnormal result in one test of executive functions (planning and foresight). Patient 2 had abnormal results in general attention, psychomotor speed, and in planning and foresight. All other tests, including the other individual tests of executive functions and the composite score of executive functions, were normal.
DTI (Table 4, Figure 2)
FA-values more than 2 SDs below the mean of controls were found in the corpus callosum (both genu and body), in the subgenual cingulum bilaterally, and in the UF (right side) of Patient 1, and in the UF (left side) of Patient 2. The left UF of Patient 1 and both UFs of Patient 3 had low FA-values also, but these values did not reach the −2 SD threshold. The UFs of Patients 1 and 3 and the subgenual cingula of Patient 1 were small.
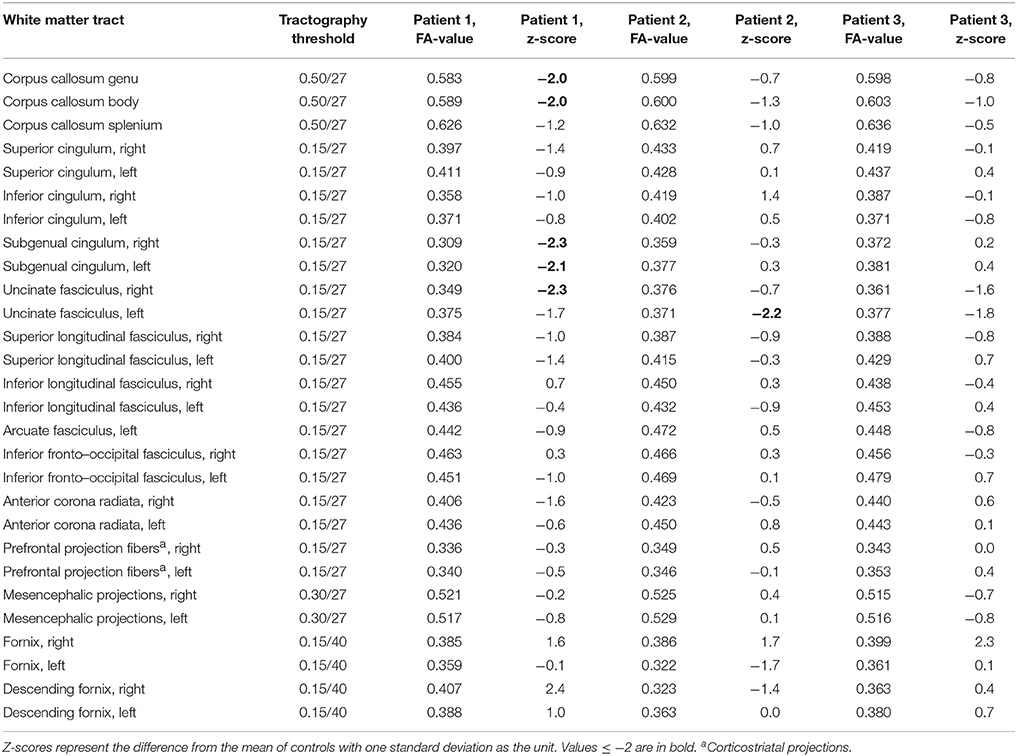
Table 4. Fractional anisotropy (FA) values from diffusion tensor imaging for three psychiatric patients with non-alcoholic Korsakoff syndrome.
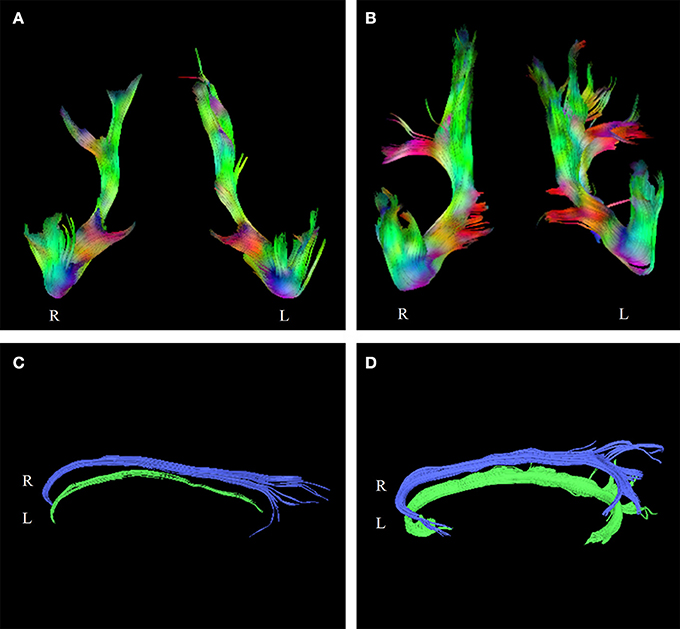
Figure 2. Tractograms of the uncinate fasciculi of Patient 1 (A) and a control subject (B). Tractograms of the subgenual cingula of Patient 1 (C), and a control subject (D). The patients' tractograms are markedly smaller than the tractograms of the controls. R, Right side, L, Left side.
Conventional MRI
All examinations were normal, except for the above-mentioned venous angioma and cerebellar tonsil ectopy in the MRI of Patient 1.
Discussion
Similarly to our recent report (15), the patients in the present study had a history of probable but undiagnosed WE and subsequent long-term memory impairment with intrusions. Two patients had clear frontotemporal damage in DTI. However, the neuropsychological findings, although compatible with KS, were diverse and not entirely typical for KS. For Patient 1, the impairment of delayed memory was evident only when memory was compared to her general cognitive level. For two patients, the verbal memory impairment was present either in the word list test or in the logical memory test but not in both. For Patient 2, impairment of immediate and delayed verbal recall and recognition was pronounced, but general attention and psychomotor speed were also impaired albeit to a lesser degree. Thus, the preferential impairment of memory was not apparent.
The variable neuropsychological findings impose a critical consideration of the KS diagnosis, particularly because two of the patients have bipolar disorder, and memory impairment may be more pronounced in bipolar disorder than in major depression (27). All patients had a period of malnutrition that lasted long enough to cause thiamine deficiency (1). The malnutrition was accompanied by typical clinical manifestations of WE. Thus, the WE diagnostic criteria proposed by Caine et al. (12) are met. The history of probable WE and the subsequent long-term stable memory impairment strongly support the diagnosis of KS. Support for the KS diagnosis is also provided by the presence of many semantic intrusions in the neuropsychological examination.
The DSM-5 includes thiamine deficiency as a cause of major and mild neurocognitive disorder due to another medical condition (28). Patients 1 and 2 need assistance with everyday activities, and all patients have memory impairment that prevents them from doing regular work. According to the DSM-5, Patient 1 has moderate major neurocognitive disorder, Patient 2 has mild to moderate major neurocognitive disorder, and Patient 3 has mild neurocognitive disorder. The DSM-5 mentions KS only in the context of alcohol-induced neurocognitive disorder presenting with very severe memory deficiency and confabulations. This covers only partly the clinical spectrum of KS. It is generally accepted that non-alcoholic WE can cause KS (6, 13, 14). Many patients with KS do not overtly confabulate (4). Bowden has noted that many studies describing KS have selectively included patients with very severe memory impairment and excluded patients with clinically milder KS (7). Previous studies have often included only KS patients that were institutionalized because of their debilitating cognitive impairment (29–34), and accordingly have DSM-5 severe major neurocognitive disorder. This may help find KS patients for scientific studies, but it also might predispose researchers and clinicians to restrict their view of the spectrum of long-term WKS cognitive damage to very severe cases. The DSM-5 states that the distinction between major and mild neurocognitive disorders is inherently arbitrary, and the disorders exist along a continuum.
Executive dysfunction is common in alcoholic KS (30, 35). We have recently noted that, similarly to the present cases, most previously reported neuropsychologically documented non-alcoholic KS cases did not have severely impaired executive functions (15). Gasquoine has extended this observation by noting that in all cases with impaired executive functions, the impairment could have been caused by other factors than thiamine deficiency (16). These observations support the notion that executive dysfunction in alcoholic KS is at least partially caused by other factors than thiamine deficiency (4, 16, 31). Thus, contrary to non-alcoholic KS, in alcoholic KS everyday functioning is commonly affected not only by the memory impairment but also by executive dysfunction. Executive dysfunction can have a devastating effect on a person's everyday life, and disentangling the effects of memory and executive impairment on a patient's functioning is not possible. Therefore, non-alcoholic KS patients could be more probable to be independent in everyday life activities compared to alcoholic KS patients with similar memory impairment, and thus less likely to be diagnosed with major as opposed to minor neurocognitive disorder.
It is difficult to be sure that milder cognitive impairment after alcoholic WE is caused by the thiamine deficiency and not by chronic alcohol use. Thus, our results suggest that thiamine deficiency per se can cause memory impairment that is severe enough to prevent vocational independency but does not necessitate assistance with everyday activities.
Clinical, neuropsychological, and pathological studies strongly suggest that cognitive impairment in alcoholic KS can present not only as selective memory loss, by also as global impairment (7–9). In particular, the variation in the topography of anatomical lesions supports variable clinical presentation (36). Our results (Patient 2) suggest that in addition to relatively mild cognitive impairment, thiamine deficiency per se can also cause broad cognitive impairment. This further strengthens previous well-documented recommendations that the possibility of WKS should be thoroughly addressed when considering the possibility of alcoholic dementia diagnosis (7, 8). Patient 3 had an abnormal word list memory test result but a normal logical memory test result, and verbal recognition memory was not impaired. Similar selective impairment has been recently reported in patients with thalamic infarction sparing the mammillothalamic tract (37). Preservation of logical memory and abnormal word list test result have been associated with executive dysfunction in some (38, 39) but not all (40) previous studies. However, Patient 3 had intact executive functions, which means that the abnormal word list test result is due to primary memory impairment.
The continuity theory in its original and revised forms claims that alcohol causes a continuum of cognitive impairment (including memory), where KS is the most severe form and “uncomplicated alcoholics” are less impaired (32). Given the preferential reporting of very severe KS (7), it is conceivable that studies in support of this theory may have assumed that the diagnosis of KS requires the presence of debilitating cognitive impairment. Thus, it is possible that KS was excluded in “uncomplicated alcoholics” based on the absence of such very severe impairment. It has been recently shown that among alcoholics that are presumed not to have KS, WE manifestations are associated with more severe cognitive impairment (41). This notion, together with the fact that many patients develop alcoholic KS in the absence of a WE history (42), makes it impossible to exclude that an “uncomplicated alcoholic” has instead relatively mild KS without a prior WE diagnosis (5, 7). This in turn might suggest that to become testable, the continuity theory of alcohol-induced cognitive impairment should be further developed. Together with these recent findings, our results of milder non-alcoholic KS support the suggestion that the continuity theory could be applied to cognitive impairment caused by thiamine deficiency (5).
Our results of variable neuropsychological presentation suggest the need to comprehensively evaluate patients with suspected KS. We have recently suggested that lack of impairment of executive functions and a tendency to misattribute organic memory impairment to psychiatric disease may contribute to the underdiagnosis of non-alcoholic KS (15). Our present results extend this notion to variable neuropsychological presentation. We have recently shown that atypical presentation is associated with underdiagnosis of OSA in psychiatric patients (43).
Intrusions in memory testing [provoked confabulations (44), simple provoked confabulations (45), memory-recall-provoked confabulations (46)] may occur in normal controls (47), but they are rare (48, 49), unless after a retention interval longer than the one used in clinical assessment (44). Patients with alcoholic KS commonly produce intrusions (23, 33), which are similarly to our patients semantic (23). In previous studies of neuropsychologically documented non-alcoholic KS, intrusions have been reported in five cases, including the three in our recent report (15, 20, 50), whereas they were absent in one case (51). Intrusions have been shown in other somatic diseases, including thalamic lesions (52), ruptured aneurysm of the anterior communicating artery (53), Alzheimer's disease (54, 55), traumatic brain injury (56, 57), Parkinson's disease (58), frontal lobe damage (58, 59), and non-alcoholic liver disease (60). In psychiatric diseases, intrusions have been shown in schizophrenia (61), methamphetamine dependence (62), and in attention-deficit hyperactivity disorder (63). Regarding the psychiatric diagnoses of our patients, intrusions are uncommon in bipolar disorder (49), and they occur in major depression only together with anosognosia for the memory impairment (64).
Thus, the presence of intrusions suggests that the memory impairment of our patients has an organic etiology. The lack of evidence for other organic disorders and the semantic nature of the intrusions support the KS diagnosis. Our results suggest that assessment of intrusions in neuropsychological testing might be useful in the diagnosis of non-alcoholic KS. Since intrusions occur very rarely in neuropsychological testing of healthy persons, their presence could be particularly useful when the KS memory impairment is not pronounced or isolated. However, further studies are needed before testing for intrusions can be confidently implemented in the clinical diagnosis of KS. The occurrence of intrusions in both alcoholic and non-alcoholic KS supports the notion of their similarity (6). However, the possible preferential impairment of executive functions in alcoholic KS suggests that important differences may exist between alcoholic and non-alcoholic KS (16). Studies with a larger number of patients are needed to further explore these issues.
Immediate and delayed recall were impaired in most previous studies of neuropsychologically examined non-alcoholic KS (15, 16). Similarly, all patients in the present report had impaired delayed recall, and two patients had impaired immediate recall. However, comparing the results of delayed and immediate memory tests may not differentiate impairment of memory consolidation from impairment of memory encoding (65, 66).
There are only three previous DTI studies in KS. In alcoholic KS, reduced FA-values have been reported in the corpus callosum and the anterior corona radiata in one study (29) and in the fornix in another study (34). We have recently reported on two psychiatric patients with non-alcoholic KS and reduced FA-values in the UF, the corona radiata, and the inferior longitudinal fasciculus (15). Two of our present patients had clear damage in the UF, as assessed by DTI. The third patient had DTI findings that were suggestive of damage in the UF. One patient also had damage in the subgenual cingulum, and in the genu and in the body of the corpus callosum. According to a recent meta-analysis of DTI in affective disorders, decreased FA-values are found only in the genu of the corpus callosum and in the left posterior cingulum (67). Thus, it is unlikely that our DTI findings are due to psychiatric disease. It has been suggested (26), albeit not consistently (68), that the subgenual cingulum contains fibers connecting the anterior thalamus with the posterior cingulate cortex, and the anterior thalamus is commonly damaged in KS (69). Damage in the subgenual cingulum can cause memory impairment (70). Further, reduced integrity of the subgenual cingulum may cause dysfunctional emotional reactivity (71) and in trauma-exposed women hypervigilance (72). Interestingly, dysfunctional emotional reactivity that emerged after the WE has been relevant in the psychiatric treatment of our patient with subgenual cingulum damage.
The most consistent DTI finding in the present report was damage in the UF. The UF is anatomically related to the limbic system, a primary site of neural damage in WKS (73), and damage in limbic regions can cause UF abnormalities (74). This is consistent with the notion that the UF abnormalities in our patients were caused by prior thiamine deficiency. The UF is involved both in emotion regulation and in memory function (74, 75). Therefore, the UF could be particularly relevant in memory impairment of psychiatric patients. Most importantly, UF damage is preferentially associated with impairment in verbal immediate and delayed recall (74). These types of memory were affected in our patients, similarly to patients with alcoholic KS (76). In Patient 1, verbal working memory was also impaired; interestingly, in a study of patients with schizophrenia (which is associated with reduced UF FA-values), UF damage was related to worse performance in working memory tasks (77).
Neuropsychological and imaging studies have suggested that intrusions are associated with frontal and temporal lobe dysfunction (59, 78, 79); our findings of intrusions and damage in a major frontotemporal tract (UF) support that notion and extend it to non-alcoholic KS in the context of impaired brain connectivity. Together with results from our previous study (15), our present findings suggest that structural white matter abnormalities, particularly in the UF, may be a feature of non-alcoholic KS in psychiatric patients.
Two patients have OSA that was treated at the time of the neuropsychological examination. Untreated OSA can affect immediate and delayed verbal recall, but there is no effect on recognition memory (80). Patients with treated OSA can still have cognitive impairment, but it is mostly restricted to executive functions (81). It is possible that the cytostatic drugs used in the leukemia treatment have contributed to the cognitive impairment of Patient 1 (82, 83). Deficiency of other micronutrients may have aggravated the effects of thiamine deficiency (84).
Patient 3 has had prominent gastrointestinal symptoms since the WE episode. Gastrointestinal manifestations of thiamine deficiency (“gastrointestinal beriberi”) have been reported (85, 86). Short-term experimental thiamine deficiency in humans caused transient gastrointestinal symptoms and radiologically documented reduction in gastrointestinal motility (87). To our knowledge, there are no previous studies showing chronic non-dysphagia gastrointestinal symptoms or motility impairment after thiamine deficiency. Thus, it is difficult to be certain that the chronic gastrointestinal symptoms in our patient are a manifestation of WKS.
Patient 1 developed muscle weakness during WE, which has persisted for nine years. Muscle weakness occurs in non-alcoholic WE (88), and it can be a prominent manifestation (89). Residual muscle weakness has been reported after non-alcoholic WE (90, 91).
Limitations of our study include the small number of patients and not testing retrograde memory nor for spontaneous confabulations. In addition, the conventional MRI examinations were evaluated clinically, and no quantitative analysis was performed. Results of the DTI-analysis may be influenced by the fiber tracking methodology used (92). There is strong evidence that in non-alcoholic WE brain involvement is considerably more widespread compared to alcoholic WE (93). Therefore, in our study of non-alcoholic KS we examined a large number of individual tracts. In addition, we divided two large tracts (corpus callosum and cingulum) into their components, and based on pathology findings (11) we examined separately the whole fornix and its descending part. This approach enabled us to find damage in the UF, and in parts of the corpus callosum and the cingulum. However, studying a large number of tracts in a small number of patients increases the probability of chance findings. The tracts that were found damaged are important in memory function and damage in the UF was found also in our previous study of non-alcoholic KS (15), both of which increase confidence in the biological relevance of our findings. Nevertheless, our DTI analysis should be considered exploratory. Moreover, we did not assess frontocerebellar tracts and the mammillothalamic tract, or comprehensively assess thalamocortical connections. Larger studies are needed to better understand the role of brain connectivity in KS and to further assess the usefulness of DTI in its diagnosis. A strength of our study is the long follow-up, which provides important insight into the natural history of non-alcoholic KS.
In conclusion, our results expand the diagnostic spectrum of non-alcoholic KS to psychiatric patients with variable neuropsychological findings. Our results are in accord with previous findings of a variable clinical presentation of KS, particularly broad cognitive impairment. Our observations suggest a need to comprehensive evaluate psychiatric patients with a history of probable WE for subsequent KS, and in particular to consider the possibility of KS in patients with severe but not debilitating effects of memory impairment on everyday functioning. Our findings also suggest that frontotemporal connectivity may be impaired in non-alcoholic KS in psychiatric patients. Combining with results of our previous study (15), six psychiatric patients with non-alcoholic KS have been identified from a single physician's practice during a period of a few years. This, together with the dearth of previously reported cases and the established notion of KS underdiagnosis, suggests that large-scale screening of psychiatric patients for WKS could result in the identification of many more cases. This, in turn, could contribute to more appropriate treatment of these patients and offer possibilities for significant advancements in memory research.
Author Contributions
GN conceived of the study, reviewed the literature, and is responsible for overall study design. TI designed and assessed the neuropsychological examinations. TK designed and assessed the MRI/DTI examinations. JP performed the neuropsychological examinations. SP reviewed results of neuropsychological examinations. GN, TK, SP, RV, and TI interpreted results. GN drafted the manuscript, which was critically revised by all authors. All authors read and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. (2007) 6:442–55. doi: 10.1016/S1474-4422(07)70104-7
2. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-korsakoff-syndrome: under-recognized and under-treated. Psychosomatics (2012) 53:507–16. doi: 10.1016/j.psym.2012.04.008
3. Chamorro AJ, Rosón-Hernández B, Medina-García JA, Muga-Bustamante R, Fernández-Solá J, Martín-González MC, et al. Differences between alcoholic and nonalcoholic patients with wernicke encephalopathy: a multicenter observational study. Mayo Clin Proc. (2017) 92:899–907. doi: 10.1016/j.mayocp.2017.02.019
4. Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol (2009) 44:148–54. doi: 10.1093/alcalc/agn118
5. Arts N, Walvoort S, Kessels R. Korsakoff's syndrome: a critical review. Neuropsychiatr Dis Treat. (2017) 13:2875–90. doi: 10.2147/NDT.S130078
6. Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry (2015) 86:1362–8. doi: 10.1136/jnnp-2014-309598
7. Bowden S. Separating cognitive impairment in neurologically asymptomatic alcoholism from Wernicke-Korsakoff syndrome: is the neuropsychological distinction justified? Psychol Bull. (1990) 107:355–66. doi: 10.1037/0033-2909.107.3.355
9. Jacobson RR, Lishman WA. Selective memory loss and global intellectual deficits in alcoholic korsakoff's syndrome. Psychol Med. (1987) 17:649–55. doi: 10.1017/S0033291700025885
10. Jacobson RR, Acker CF, Lishman WA. Patterns of neuropsychological deficit in alcoholic Korsakoff's Syndrome. Psychol Med. (1990) 20:321–34. doi: 10.1017/S0033291700017633
11. Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition (Contemporary Neurology). 2nd Edn. Philadelphia, PA: F. A. Davies Company (1989).
12. Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry (1997) 62:51–60. doi: 10.1136/jnnp.62.1.51
13. Lough ME. Wernicke's encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev. (2012) 22:181–94. doi: 10.1007/s11065-012-9200-7
14. Isenberg-Grzeda E, Alici Y, Hatzoglou V, Nelson C, Breitbart W. Nonalcoholic thiamine-related encephalopathy (Wernicke-Korsakoff Syndrome) among inpatients with cancer: a series of 18 cases. Psychosomatics (2016) 57:71–81. doi: 10.1016/j.psym.2015.10.001
15. Nikolakaros G, Ilonen T, Kurki T, Paju J, Papageorgiou SG, Vataja R. Non-alcoholic Korsakoff syndrome in psychiatric patients with a history of undiagnosed Wernicke's encephalopathy. J Neurol Sci. (2016) 370:296–302. doi: 10.1016/j.jns.2016.09.025
16. Gasquoine PG. A Case of Bariatric Surgery-related Wernicke–Korsakoff syndrome with persisting anterograde amnesia. Arch Clin Neuropsychol. (2017) 32:610–17. doi: 10.1093/arclin/acx037
17. Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. (2010) 17:1408–18. doi: 10.1111/j.1468-1331.2010.03153.x
18. Beatty WW, Bailly RC, Fisher L. Korsakoff-like amnestic syndrome in a patient with anorexia and vomiting. Int J Clin Neuropsychol (1989) 11:55–65.
19. Parkin AJ, Blunden J, Rees JE, Hunkin NM. Wernicke-Korsakoff syndrome of nonalcoholic origin. Brain Cogn. (1991) 15:69–82.
20. Fertl E, Schnider P, Müller C, Auff E. Persistent amnesic syndrome as long-term outcome of cognitive function after Whipple's disease. Eur J Neurol. (1997) 4:613–7. doi: 10.1111/j.1468-1331.1997.tb00414.x
21. Jung Y-C, Chanraud S, Sullivan E V. Neuroimaging of Wernicke's encephalopathy and Korsakoff's syndrome. Neuropsychol Rev. (2012) 22:170–80. doi: 10.1007/s11065-012-9203-4
22. Oscar-Berman M, Clancy JP, Weber DA. Discrepancies between IQ and memory scores in alcoholism and aging. Clin Neuropsychol. (1993) 7:281–96. doi: 10.1080/13854049308401899
23. Rensen YCM, Oosterman JM, Walvoort SJW, Eling PATM, Kessels RPC. Intrusions and provoked and spontaneous confabulations on memory tests in Korsakoff's syndrome. J Clin Exp Neuropsychol. (2017) 39:101–11. doi: 10.1080/13803395.2016.1204991
24. Kurki T, Himanen L, Vuorinen E, Myllyniemi A, Saarenketo AR, Kauko T, et al. Diffusion tensor tractography-based analysis of the cingulum: clinical utility and findings in traumatic brain injury with chronic sequels. Neuroradiology (2014) 56:833–41. doi: 10.1007/s00234-014-1410-7
25. Kurki TJI, Laalo JP, Oksaranta OM. Diffusion tensor tractography of the uncinate fasciculus: pitfalls in quantitative analysis due to traumatic volume changes. J Magn Reson Imaging (2013) 38:46–53. doi: 10.1002/jmri.23901
26. Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia (2013) 51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018
27. Samamé C, Szmulewicz AG, Valerio MP, Martino DJ, Strejilevich SA. Are major depression and bipolar disorder neuropsychologically distinct? a meta-analysis of comparative studies. Eur Psychiatry (2017) 39:17–26. doi: 10.1016/j.eurpsy.2016.06.002
28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Association (2013).
29. Segobin S, Ritz L, Lannuzel C, Boudehent C, Vabret F, Eustache F. Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Hum Brain Mapp. (2015) 36:2795–808. doi: 10.1002/hbm.22808
30. Van Oort R, Kessels RPC. Executive dysfunction in Korsakoff's syndrome: time to revise the DSM criteria for alcohol-induced persisting amnestic disorder? Int J Psychiatry Clin Pract. (2009) 13:78–81. doi: 10.1080/13651500802308290
31. Brokate B, Eling P, Hildebrandt H, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff's syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology (2003) 17:420–28. doi: 10.1037/0894-4105.17.3.420
32. Pitel AL, Beaunieux H, Witkowski T, Vabret F, De La Sayette V, Viader F. Episodic and working memory deficits in alcoholic korsakoff patients: the continuity theory revisited. Alcohol Clin Exp Res. (2008) 32:1229–41. doi: 10.1111/j.1530-0277.2008.00677.x
33. Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol. (1987) 9:479–97. doi: 10.1080/01688638708410764
34. Nahum L, Pignat JM, Bouzerda-Wahlen A, Gabriel D, Liverani MC, Lazeyras F, et al. Neural correlate of anterograde amnesia in wernicke–korsakoff syndrome. Brain Topogr. (2015) 28:760–70. doi: 10.1007/s10548-014-0391-5
35. Oscar-Berman M. Function and dysfunction of prefrontal brain circuitry in alcoholic Korsakoff's syndrome. Neuropsychol Rev. (2012) 22:154–69. doi: 10.1007/s11065-012-9198-x
36. Torvik A. Wernicke's encephalopathy–prevalence and clinical spectrum. Alcohol Alcohol Suppl. (1991) 1:381–4.
37. Danet L, Barbeau EJ, Eustache P, Planton M, Raposo N, Sibon I. Thalamic amnesia after infarct. Neurology (2015) 85:2107–15. doi: 10.1212/WNL.0000000000002226
38. Tremont G, Halpert S, Javorsky DJ, Stern RA. Differential impact of executive dysfunction on verbal list learning and story recall. Clin Neuropsychol. (2000) 14:295–302. doi: 10.1076/1385-4046(200008)14
39. Zahodne LB, Bowers D, Price CC, Bauer RM, Nisenzon A, Foote KD, et al. The case for testing memory with both stories and word lists prior to DBS surgery for Parkinson's disease. Clin Neuropsychol. (2011) 25:348–58. doi: 10.1080/13854046.2011.562869
40. Busch RM, Booth JE, McBride A, Vanderploeg RD, Curtiss G, Duchnick JJ. Role of Executive functioning in verbal and visual memory. Neuropsychology (2005) 19:171–80. doi: 10.1037/0894-4105.19.2.171
41. Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, et al. Signs of preclinical wernicke's encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without korsakoff's syndrome. Neuropsychopharmacology (2011) 36:580–8. doi: 10.1038/npp.2010.189
43. Nikolakaros G, Virtanen I, Markkula J, Vahlberg T, Saaresranta T. Obstructive sleep apnea in psychiatric outpatients. A clinic-based study. J Psychiatr Res. (2015) 69:126–34. doi: 10.1016/j.jpsychires.2015.07.028
44. Kopelman MD. Two types of confabulation. J Neurol Neurosurg Psychiatry (1987) 50:1482–7. doi: 10.1136/jnnp.50.11.1482
45. Schnider A. The Confabulating Mind. How the Brain Creates Reality. Oxford University Press (2008).
46. Coltheart M. Confabulation and conversation. Cortex (2017) 87:62–8. doi: 10.1016/j.cortex.2016.08.002
47. Dalla Barba G, Mantovan MC, Traykov L, Rieu D, Laurent A, Ermani M, et al. The functional locus of intrusions: encoding or retrieval? J Clin Exp Neuropsychol. (2002) 24:633–41. doi: 10.1076/jcen.24.5.633.1008
48. Schnider A, Von Däniken C, Gutbrod K. The mechanisms of spontaneous and provoked confabulations. Brain (1996) 119:1365–75. doi: 10.1093/brain/119.4.1365
49. Van Rheenen TE, Rossell SL. Investigation of the component processes involved in verbal declarative memory function in bipolar disorder: Utility of the hopkins verbal learning test-revised. J Int Neuropsychol Soc. (2014) 20:727–35. doi: 10.1017/S1355617714000484
50. Deb S, Law-Min R, Fearnley D. Wernicke-Korsakoff syndrome following small bowel obstruction. Behav Neurol. (2002) 13:89–94.
51. Becker JT, Furman JMR, Panisset M, Smith C. Characteristics of the memory loss of a patient with Wernicke-Korsakoff's syndrome without alcoholism. Neuropsychologia (1990) 28:171–9. doi: 10.1016/0028-3932(90)90099-A
52. Haut MW, Young J, Cutlip WD, Callahan T, Haut AE. A case of bilateral thalamic lesions with anterograde amnesia and impaired implicit memory. Arch Clin Neuropsychol. (1995) 10:555–66. doi: 10.1016/0887-6177(95)00054-8
53. Zannino GD, Barban F, Caltagirone C, Carlesimo G. Do confabulators really try to remember when they confabulate? A case report. Cogn Neuropsychol. (2008) 25:831–52. doi: 10.1080/02643290802365078
54. Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California verbal learning test: implications for the assessment of memory disorders. Psychol Assess. A J Consult Clin Psychol. (1991) 3:19–26. doi: 10.1037/1040-3590.3.1.19
55. De Anna F, Attali E, Freynet L, Foubert L, Laurent A, Dubois B, et al. Intrusions in story recall: When over-learned information interferes with episodic memory recall. evidence from Alzheimer's disease. Cortex (2008) 44:305–11. doi: 10.1016/j.cortex.2006.08.001
57. Crosson B, Novack TA, Trenerry MR, Craig PL. California Verbal Learning Test (CVLT) performance in severely head-injured and neurologically normal adult males. J Clin Exp Neuropsychol. (1988) 10:754–68. doi: 10.1080/01688638808402812
58. Rouleau I, Imbault H, Laframboise M, Bédard MA. Pattern of intrusions in verbal recall: comparison of Alzheimer's disease, Parkinson's disease, and frontal lobe dementia. Brain Cogn (2001) 46:244–9. doi: 10.1016/S0278-2626(01)80076-2
59. Baldo J V., Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test-II: Findings from patients with focal frontal lesions. J Int Neuropsychol Soc. (2002) 8:539–46. doi: 10.1017/S135561770281428X
60. Mach T. Cognitive functions in patients with liver cirrhosis: a tendency to commit more memory errors. Med Sci Monit. (2013) 19:283–8. doi: 10.12659/MSM.883890
61. Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. J Abnorm Psychol. (1992) 101:487–94. doi: 10.1037/0021-843X.101.3.487
62. Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology (2005) 19:35–43. doi: 10.1037/0894-4105.19.1.35
63. Pollak Y, Kahana-Vax G, Hoofien D. Retrieval processes in adults with ADHD: a RAVLT study. Dev Neuropsychol. (2008) 33:62–73. doi: 10.1080/87565640701729789
64. Dalla Barba G, Parlato V, Iavarone A, Boller F. Anosognosia, intrusions and “frontal” functions in Alzheimer's disease and depression. Neuropsychologia (1995) 33:247–59. doi: 10.1016/0028-3932(94)00091-3
65. Kent PL. Evolution of Wechsler's Memory Scales: Content and Structural Analysis. Appl Neuropsychol Adult (2017) 24:232–51. doi: 10.1080/23279095.2015.1135798
66. Murre JMJ, Janssen SMJ, Rouw R, Meeter M. The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychol. (2013) 142:96–107. doi: 10.1016/j.actpsy.2012.10.005
67. Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry (2016) 79:293–302. doi: 10.1016/j.biopsych.2015.03.004
68. Heilbronner SR, Haber SN. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. J Neurosci. (2014) 34:10041–54. doi: 10.1523/JNEUROSCI.5459-13.2014
69. Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain (2000) 123:141–54. doi: 10.1093/brain/123.1.141
70. Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain (1987) 110:1631–46. doi: 10.1093/brain/110.6.1631
71. Keedwell PA, Doidge AN, Meyer M, Lawrence N, Lawrence AD, Jones DK. Subgenual Cingulum Microstructure Supports Control of Emotional Conflict. Cereb Cortex (2016) 26:2850–62. doi: 10.1093/cercor/bhw030
72. Yoon SA, Weierich MR. Persistent amygdala novelty response is associated with less anterior cingulum integrity in trauma-exposed women. NeuroImage Clin. (2017) 14:250–9. doi: 10.1016/j.nicl.2017.01.015
73. Kopelman MD. The Korsakoff syndrome. Br J Psychiatry (1995) 166:154–73. doi: 10.1192/bjp.166.2.154
74. Olson IR, Heide RJ, Von Der, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci. (2015) 14:50–61. doi: 10.1016/j.dcn.2015.06.003
75. Catani M, Dell'Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. (2013) 37:1724–37. doi: 10.1016/j.neubiorev.2013.07.001
76. Oudman E, Postma A, Nijboer TCW, Wijnia JW, Van der Stigchel S. Visuospatial declarative learning despite profound verbal declarative amnesia in Korsakoff's syndrome. Neuropsychol Rehabil. (2017). doi: 10.1080/09602011.2017.1294541. [Epub ahead of print].
77. Hanlon FM, Houck JM, Klimaj SD, Caprihan A, Mayer AR, Weisend MP. Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology (2012) 49:1340–52. doi: 10.1111/j.1469-8986.2012.01448.x
78. Crosson B, Sartor KJ, Jenny AB, Nabors NA. Increased intrusions during verbal recall in traumatic and nontraumatic lesions of the temporal lobe. Neuropsychology (1993) 7:193–208. doi: 10.1037/0894-4105.7.2.193
79. Desgranges B, Baron J-C, Giffard B, Chételat G, Lalevée C, Viader F. The neural basis of intrusions in free recall and cued recall: a PET study in Alzheimer's disease. Neuroimage (2002) 17:1658–64. doi: 10.1006/nimg.2002.1289
80. Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep (2013) 36:203–20. doi: 10.5665/sleep.2374
81. Werli KS, Otuyama LJ, Bertolucci PH, Rizzi CF, Guilleminault C, Tufik S. Neurocognitive function in patients with residual excessive sleepiness from obstructive sleep apnea: a prospective, controlled study. Sleep Med. (2016) 26:6–11. doi: 10.1016/j.sleep.2016.06.028
82. Sechi G, Batzu L, Agrò L, Fois C. Cancer-related Wernicke-Korsakoff syndrome. Lancet Oncol. (2016) 17:e221–2. doi: 10.1016/S1470-2045(16)30109-7
83. Giovannelli F, Basagni B, Potenza L, Foschi V, De Tanti A. Long-term cognitive sequelae in a case of Wernicke's encephalopathy after allogeneic stem cell transplantation. Neurocase (2016) 22:187–90. doi: 10.1080/13554794.2015.1109666
84. Sechi G, Sechi E, Fois C, Kumar N. Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr Rev. (2016) 74:281–300. doi: 10.1093/nutrit/nuv107
85. Duca J, Lum CJ, Lo AM. Elevated lactate secondary to gastrointestinal beriberi. J Gen Intern Med. (2016) 31:133–6. doi: 10.1007/s11606-015-3326-2
86. Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med. (2004) 141:898–9. doi: 10.7326/0003-4819-141-11-200412070-00035
87. Williams RD, Mason HL, Wilder RM, Smith BF. Observations on induced thiamine (vitamin b1) deficiency in man. Arch Intern Med. (1940) 66:785–99. doi: 10.1001/archinte.1940.00190160002001
88. Isenberg-Grzeda E, Rahane S, DeRosa AP, Ellis J, Nicolson SE. Wernicke-Korsakoff syndrome in patients with cancer: a systematic review. Lancet Oncol. (2016) 17:e142–8. doi: 10.1016/S1470-2045(16)00037-1
89. Koike H, Ito S, Morozumi S, Kawagashira Y, Iijima M, Hattori N. Rapidly developing weakness mimicking Guillain-Barré syndrome in beriberi neuropathy: two case reports. Nutrition (2008) 24:776–80. doi: 10.1016/j.nut.2008.02.022
90. Ba F, Siddiqi ZA. Neurologic complications of bariatric surgery. Rev Neurol Dis. (2010) 7:119–24. doi: 10.3909/rind0265
91. Ohkoshi N, Ishii A, Shoji S. Wernicke's encephalopathy induced by hyperemesis gravidarum, associated with bilateral caudate lesions on computed tomography and magnetic resonance imaging. Eur Neurol. (1994) 34:177–80.
92. Christidi F, Karavasilis E, Samiotis K, Bisdas S, Papanikolaou N. Fiber tracking: a qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur J Radiol Open (2016) 3:153–61. doi: 10.1016/j.ejro.2016.06.002
Keywords: connectome, diffusion tensor imaging, Korsakoff syndrome, magnetic resonance imaging, memory, muscle weakness, neuropsychological tests, Wernicke's encephalopathy
Citation: Nikolakaros G, Kurki T, Paju J, Papageorgiou SG, Vataja R and Ilonen T (2018) Korsakoff Syndrome in Non-alcoholic Psychiatric Patients. Variable Cognitive Presentation and Impaired Frontotemporal Connectivity. Front. Psychiatry 9:204. doi: 10.3389/fpsyt.2018.00204
Received: 06 April 2018; Accepted: 02 May 2018;
Published: 31 May 2018.
Edited by:
Wendy Noble, King's College London, United KingdomReviewed by:
GianPietro Sechi, University of Sassari, ItalyNatalie M. Zahr, Stanford University, United States
Copyright © 2018 Nikolakaros, Kurki, Paju, Papageorgiou, Vataja and Ilonen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios Nikolakaros, Z2Vvcmdpb3Mubmlrb2xha2Fyb3NAdXR1LmZp
 Georgios Nikolakaros
Georgios Nikolakaros Timo Kurki4,5
Timo Kurki4,5