- 1Southwest Florida Research and Education Center, Entomology and Nematology Department, University of Florida, Gainesville, FL, United States
- 2College of Agriculture, Bahauddin Zakariya University, Multan, Pakistan
- 3Department of Entomology, University of Sargodha, Sargodha, Pakistan
The Asian citrus psyllid (ACP) Diaphorina citri Kuwayama is an economically important pest of citrus because it vectors the causal pathogens of huanglongbing (HLB) or citrus greening disease. Biological control is an important component of citrus pest management but requires consistent strengthening of its impact on pest complex. The brown lacewing Sympherobius barberi Banks is a known predator of several insect pests from Asia, Europe, and America. However, there is not much information about its effectiveness against D. citri. We evaluated S. barberi against the D. citri and frozen eggs of the Mediterranean flour moth Ephestia kuehniella, the latter is a common diet used for rearing predators in laboratories. Adult S. barberi successfully fed on D. citri eggs and nymphs under both light and dark conditions. Diaphorina citri was also suitable for the development and reproduction of S. barberi except for slightly prolonged larval development compared with E. kuehniella diet. The egg hatch from the total number of eggs laid on D. citri and E. kuehniella diets averaged 65% and 52%, respectively. Females laid 64% eggs on dimpled white paper compared to 36% combined on plain paper and leaves of citrus, orange jasmine, eggplant and cantaloupe. Sympherobius barberi released at densities of 2–6 adults against eggs and nymphs of D. citri on infested orange jasmine plants in the cages provided a reduction of 43–81% in the number of provided eggs or nymphs. In the field tests on D. citri infested citrus trees, reduction averaged 35% in five cohorts in which developing colonies of 28–32 nymphs were provided to one S. barberi per cage. Findings suggest the significant potential of S. barberi as a predator of D. citri and to contribute to reducing huanglongbing.
Introduction
The Asian citrus psyllid (ACP) Diaphorina citri Kuwayama (Hemiptera: Liviidae) is a primary vector of the causal pathogens of huanglongbing (HLB) also known as citrus greening disease. It is an economically important pest of citrus in HLB affected regions (Catling, 1970). First described in Taiwan (Kuwayama, 1908), D. citri was later found in Punjab Pakistan and the rest of the region (Hussain and Nath, 1927) and reported from Brazil in 1940 (Lima, 1947) and Florida in 1998 (Halbert, 1998). Huanglongbing in Florida was detected in 2005 (Halbert, 2005) 1 year after its identification from Brazil in 2004 (Texeira et al., 2005).
Several members of the order Neuroptera are known bio-control agents of multiple pests and are distributed all over the world. Chrysopidae and Hemerobiidae families received significant attention as bio-control agents because several species are voracious feeders of soft-bodied insects (Balduf, 1939; New, 1975).
Hemerobiidae family, with approximately 27 genera and 560 described species, includes many brown lacewings, which are predators of a wide range of insect pests (Oswald, 1993, 1994; New, 2001; Grimaldi and Engel, 2005). Most species of hemerobiids are predaceous in both imaginal and preimaginal stages (Miller and Cave, 1987). Adults of several species are generalist predators, and many are habitat-specific (Killington, 1937; Monserrat and Marín, 1996). Brown lacewings are known as natural enemies of the several insect pests in classical biological control (Sato and Takada, 2004). Both larvae and adults of several species are efficient predators on eggs and immature stages of several pests. Due to their prolonged longevity, high consumption rates, and high reproduction rate, they are established as important bio-control agents in the agro-ecosystem (MacLeod and Stange, 2005). Sympherobius barberi is native to Continental US, Canada and Mexico, while geographically it is distributed throughout the North America, Europe, and Northern Asia (excluding China), Middle America, Oceania and South America (Penny, 1977; Penny et al., 1997). Pacheco-Rueda et al. (2011) evaluated S. barberi against Dactylopius opuntiae. They reported a net reproductive rate (Ro) of 36.6, a daily intrinsic rate of increase (rm) of 0.081, a generation time (T) of 44.27 days, and a finite reproductive rate (λ) of 1.084 for S. barberi.
Most soft-bodied insects and their eggs are preferred food for brown lacewing. Cutright (1923) observed the larva of the brown lacewing; Micromus posticus Walker consumes 41 aphids throughout its life. Yayla and Satar (2012) found out that larvae of Sympherobius pygmaeus Rambur on the diet of Planococcus citri developed in 31 days at 25°C and female laid maximum of 258 eggs.
Our objectives for these studies were (1) to investigate the development, reproduction and predation potential of S. barberi on the diet of D. citri, in comparison with frozen eggs of the Mediterranean flour moth Ephestia kuehniella (Lepidoptera: Pyralidae), and (2) to evaluate S. barberi for reducing D. citri populations under greenhouse and field conditions. Eggs of E. kuehniella is a conventional diet for rearing several predatory species. The findings of this study may improve our understanding of utilizing S. barberi for augmentative biological control purposes in citrus crops worldwide, particularly in systems where increased chemical control is negatively impacting naturally occurring predators.
Materials and Methods
Study Location, Insects, and Experimental Conditions
Colonies of D. citri and S. barberi were maintained and experiments conducted at the Southwest Florida Research and Education Center (SWFREC) of the University of Florida-IFAS, Gainesville, FL, United States. Diaphorina citri colony was maintained on orange jasmine Murraya paniculata (L.), in a climate-controlled glasshouse set at 28°C. Murraya paniculata is a close relative of citrus, and one of the preferred hosts of D. citri. Sympherobius barberi adults originally obtained from Foothill Agricultural Research, Inc. (550 W Foothill Pkwy, Corona, CA, United States) were used to maintain its colony on frozen eggs of E. kuehniella (Koppert Biological Systems, Romulus, MI, United States) and nectar honey. The colony used several groups of 20 adults per 3-L ventilated plastic jar. The frozen eggs of E. kuehniella and nectar honey were provided for food and a small cube of moist sponge for water. Shoots of M. paniculata and pieces of paper towel were provided as a substrate for oviposition. Eggs laid on paper towel or leaves were then placed in 20 ml (5-DRAM) snap cap vials and observed for hatching. The colony was maintained in an incubator (Percival, Model I36LLC8, Percival Scientific, Inc., Perry, IA, United States) set to a photoperiod of 16:8 (L:D) at 25°C. Upon hatching, neonates were collected daily, and experiments conducted in the incubator and conditions described for the colony.
Laboratory Experiments
Feeding
Twenty-four hours after emergence, adult S. barberi were used to test for feeding on D. citri nymphs under light and dark conditions in the growth chamber. Sixty adults of S. barberi were distributed randomly across three different diets (1) psyllid eggs, (2) psyllid nymphs 2nd and 3rd (small) instars, and (3) psyllid nymphs 4th and 5th (large) instars. One adult was provided with 25–30 psyllid eggs and 20 small or large nymphs on young orange jasmine shoots in the petri dish (9-cm diameter and 1.5-cm height). The petri dishes were covered with perforated parafilm to prevent the escape of nymphs and provide ventilation. The number of eggs and nymphs consumed by S. barberi were recorded at 6, 12, and 24 h.
Reproduction
Male and female were distinguished by examining their genital appendages using the 14X Triplet Hasting Magnification hand lens. The male has flattened shaped terminalia with two claws, while the female has tipped end with two knobs (Figures 1A,B). Each pair of male and female was held in 100 ml perforated snap-cap plastic vial arena and provided with diets under three treatments to evaluate reproduction; (1) nectar honey on a paper strip (2) frozen eggs of E. kuehniella and (3) eggs and younger nymphs of D. citri on shoots of M. paniculata. Shoots of M. paniculata and dimpled white paper towel (1“X4”) were provided in the vials as a substrate for oviposition. Eggs laid by S. barberi were collected for 3 weeks, and food replaced every 24 h.

Figure 1. Abdominal terminallia of S. barberi, Male: (A) and Female: (B). Male and female pairs for reproduction tests were separated by distinguishing genital appendages morphologically, using 14X triplet hasting magnification hand lens. Male (A) has flattened shaped terminalia with two claws, while Female (B) has tipped end with two knobs. Slide photos were taken by using Olympus SZX16 stereo microscope fixed with DP21 digital camera.
Eggs from each male-female pair were kept in 20 ml (5-DRAM) snap cap vials in the growth chamber under conditions described above and examined daily for hatching. Upon death, females were dissected and examined under a stereomicroscope (Olympus SZX16, SDF-PLAPO, Japan) fixed with a digital camera (DP21) to check for the presence of eggs (Figure 2). A separate experiment was conducted to test female preference to lay eggs on six different substrates including, dimpled white paper, black paper, and leaves of four different plant species, including Citrus, Orange jasmine, Eggplant, and Cantaloupe using 10 female replicates.

Figure 2. Dissected S. barberi female with egg cluster. Female was dissected under microscope (using fine needle/forceps) for the presence of egg cluster before and after the experiment. Female and cluster are visible in the figure. This dissection was also done to confirm the morphological differences in the male and female genital appendages. Slide photo was taken by using an Olympus SZX16 stereo microscope fixed with digital camera DP21.
Development
Eggs obtained from the parents maintained on the diets of nectar honey, frozen eggs of E. kuehniella, and eggs and nymphs of D. citri were held separately for each diet at one egg per 20 ml (5-DRAM) snap cap vial. There were 10 replicates for each of the three diets. Upon hatching, larvae were reared on the same three diets on which their parents were maintained. Food was replaced daily and data recorded on larval survival, pupation, and adult emergence. The longevity of adult S. barberi was evaluated in two experiments (1) including diets of eggs of E. kuehniella, eggs of D. citri, and nymphs of D. citri, and (2) mixed diets including eggs plus nymphs of D. citri as a separate treatment, with honey and honey by itself. Twenty replicates were performed for each diet.
Greenhouse Experiments
The potted plants of M. paniculata were pruned and placed individually in bugdorm cages to induce new shoots needed for psyllid reproduction and development. The bugdorm cage (58 cm × 58 cm × 66 cm) made from clear plastic included two side panels of fine Polyester netting for ventilation. Psyllid adults used in the experiment were obtained from a colony maintained on M. paniculata, in a glasshouse at 25 ± 2°C provided with 1000W metal-halide light set to 16: 8 (L:D). The adult psyllids of mixed gender (50 males, 50 females) were released in each experimental cage.
The plants were checked daily for oviposition by psyllids. Sixteen plants adjusted to an average of 100 eggs on 3–4 shoots/plant were moved to new cages at one per cage. The plants were assigned randomly to three release densities of S. barberi adults (2, 4, and 6 adults/cage) and control without any adult for a total of four replicates per predator density treatment. Eggs were counted on the plants 3 days after adult release and before hatch to assess the number consumed by the predator. The plants were kept in the same cages to evaluate S. barberi predation on nymphs that emerged from the viable eggs. Nymphs consumed, dead, or emerged to adults were counted at an interval of 1 day for 1 week.
Field Experiment
Four years old 5 ft high ‘Hamlin’ citrus plants at Southwest Florida Research and Education Center, Immokalee, FL, United States were pruned to encourage the development of young shoots needed for psyllid reproduction and development. Once infested with D. citri, 20 shoots were assigned randomly to two groups designated for release of S. barberi and control with no release. The number of psyllid nymphs (mostly younger instars 2nd and 3rd) were counted on each shoot using the14X Triplet Hasting Magnification hand lens. Shoots were enclosed with sleeve cages made from fine mesh organdy. Those assigned for the predator’s release received one adult S. barberi per shoot with a colony averaging 28–32 nymphs. Data regarding the consumption of psyllid nymphs and the emergence of adult psyllids in all cages were recorded at an interval of 1 day. S. barberi were moved to new shoots within the same age range of D. citri nymphs every 2–3 days until death. The previously used shoots were brought to the laboratory, and D. citri nymphs and adults were counted. In total, 42 nymphal colonies were used in the five cohorts, the first three cohorts with 10 nymphal colonies in each and 4th and 5th cohort with 7 and 5 colonies, respectively. All nymphs in the control colonies (n = 10) were allowed to develop to adulthood and counted in the laboratory.
Statistical Analysis
A generalized linear model (GLM) procedure was used for Analysis of Variance (ANOVA) followed by Fisher’s least significant difference (LSD) test at P = 0.05 to discern differences in number of eggs and nymphs consumed under light and dark conditions. Data on larval survival to pupation and adult eclosion were analyzed by using the GLIMMIXMACRO model with a logit link function to transform data (SAS Institute, 2012). The data for egg and larval development time (days), fecundity, fertility (percent eggs hatched and the number of larvae per female), adult longevity, nymphal consumption by predators in the greenhouse and field experiments were analyzed by PROC GLM and treatment means separated using LSD.
Results
Feeding of S. barberi on Eggs and Nymphs of D. citri
There was no significant difference in the consumption of eggs and nymphs by S. barberi between light and dark condition (P > 0.05) except for eggs at 12 h (F1 = 4.35, P = 0.05) when consumption rate averaged 15.90 ± 2.4 in light and 9.70 ± 1.7 in the dark (Table 1).
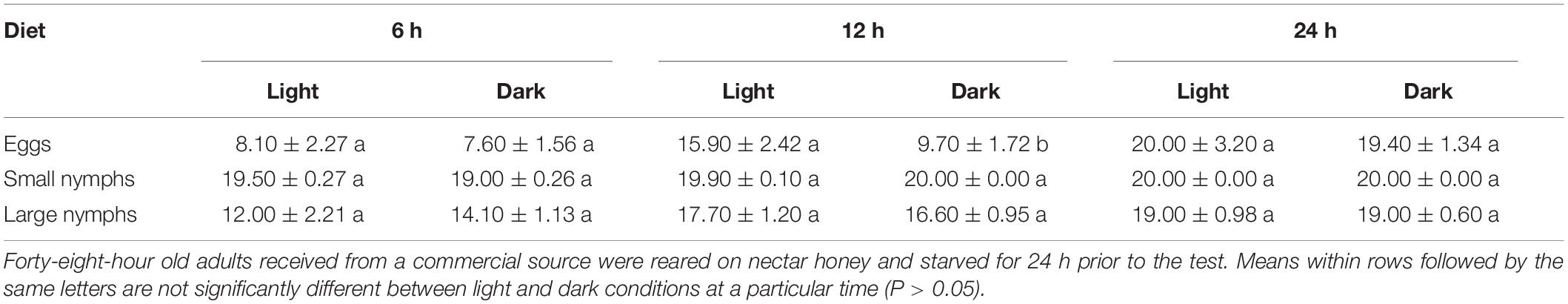
Table 1. Number (Mean ± SE) of eggs and small or large nymphs of Diaphorina citri consumed by adult Sympherobius barberi during 24 h under light or dark conditions.
Development of S. barberi on D. citri and E. kuehniella Diets
The development time of the eggs from parents reared on the diets of E. kuehniella, D. citri, and nectar honey did not differ and averaged 5 days among the three diets (P > 0.05, Table 2). Larvae reared on the same three diets differed significantly in their development time to pupation (F2,27 = 39.54, P < 0.001, Table 2). Development time was prolonged on the diet of D. citri (15.70 ± 0.58 days), followed by the diets of E. kuehniella (12.40 ± 0.27 days) and nectar honey (10.30 ± 0.40 days). Larval survival to pupation was 100% on the diet of E. kuehniella and 70% on D. citri and none from those on nectar honey pupated. Pupal stage lasted 11.43 ± 0.43 days for those on the diet of D. citri and 9.80 ± 0.61 days for those on E. kuehniella and did not differ statistically (P > 0.05, Table 2). Adult emergence from the pupae was 100% on the diets of D. citri and E. kuehniella. There was no significant difference in adult longevity when tested on the diets of eggs of E. kuehniella and eggs or nymphs of D. citri (P > 0.05, Table 3). The provision of honey with the diet of D. citri did not improve adult longevity (P > 0.05, Table 3).
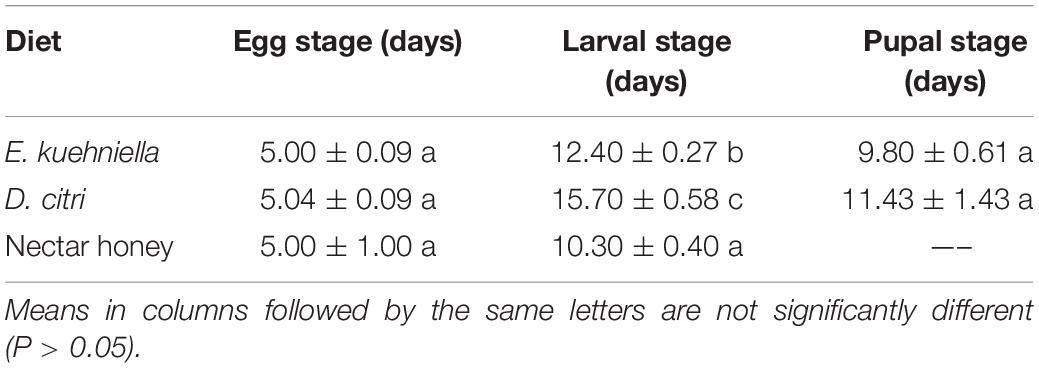
Table 2. Development time (Mean ± SE) of Sympherobius barberi on diets of Diaphorina citri, Ephestia kuehniella, and nectar honey.

Table 3. Longevity (Mean ± SE) of adult Sympherobius barberi on diets of Diaphorina citri, Ephestia kuehniella and nectar honey alone or mixed.
In the experiment to test female oviposition preference for substrate, 64% of total eggs were laid on dimpled white paper and 36% on the remaining five substrates which included black paper, orange jasmine leaves, citrus leaves, eggplant leaves and cantaloupe leaves (Table 5 and Figures 3A,B). An average of 27 eggs per female were laid during the observation time of 1 week.

Table 4. Fecundity and fertility (Mean ± SE) of Sympherobius barberi on diets of Diaphorina citri, Ephestia kuehniella and nectar honey over 3 weeks.

Table 5. Percent of eggs laid on six different substrates by S. barberi provided with the mixed diet of D. citri, nectar honey and E. kuehniella.
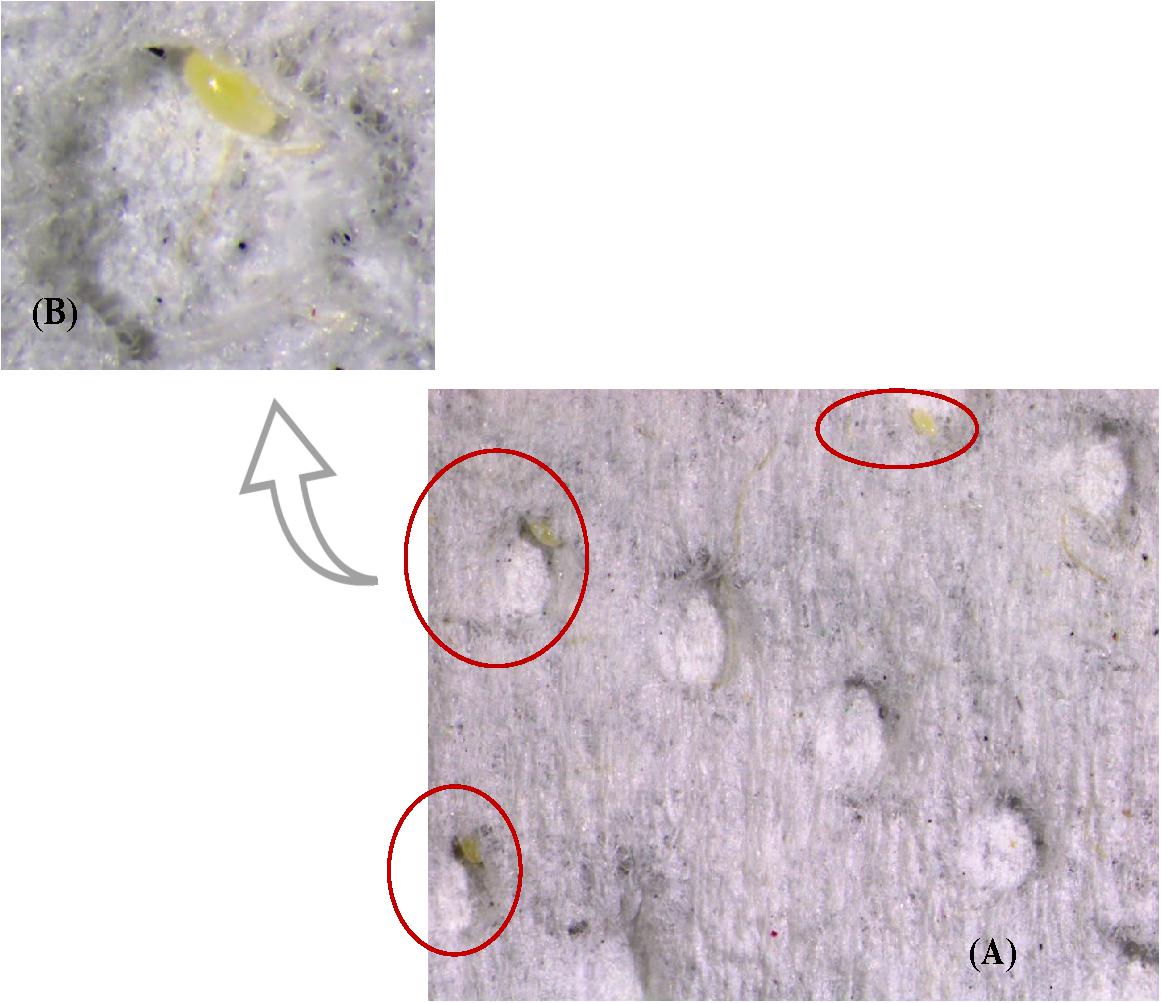
Figure 3. (A) Eggs laid by S. barberi on dimpled white paper. Slide photo was taken by using an Olympus SZX16 stereo microscope fixed with a DPI-21 digital camera. (B) Magnified view of a single dimple with egg.
Reproduction of S. barberi on D. citri and E. kuehniella Diets
An average of 25–30 eggs laid per female did not differ significantly between the diets of D. citri and E. kuehniella; however, were significantly more than few laid on honey (F2,42 = 12.522, P < 0.001, Table 4). A significantly higher percentage of eggs hatched on the diet of D. citri (65%) compared to E. kuehniella (52%) (Table 4). There was no significant difference in the number of larvae per female (P > 0.05, Table 4).
Effect of S. barberi Predation on D. citri in Potted Orange Jasmine Plants in the Greenhouse and on Citrus Trees in the Field
The number of D. citri eggs on potted orange jasmine plants were significantly reduced in the cages where S. barberi were released compared to no release control cages (F3,15 = 23.31, P = 0.0001, Figure 4). Most reduction of 69% was observed in the treatment where six adults were released per cage, followed by 50 and 43% in the treatments with four and two adults per cage, respectively. Significant reduction in nymphs was also observed with the release of S. barberi on the potted orange jasmine plants in the cages compared with control (F3,15 = 16.45, P = 0.0005, Figure 4). Most reduction of 81% at high release density did not differ from 74% at medium release density, although both these reductions were significantly more than 43% observed at low release density. There was a trend of increased reduction in D. citri with an increase in the rate of S. barberi release. Under field conditions, a significant average reduction of 35% was observed in the nymphal colonies of D. citri caged with S. barberi on citrus trees compared with only 4% in control (F1,9 = 29.43, P = 0.0004). Diaphorina citri reduction averaged between 25 and 42% (Figure 5), over five cohorts, which included 42 nymphal colonies and each with one S. barberi.
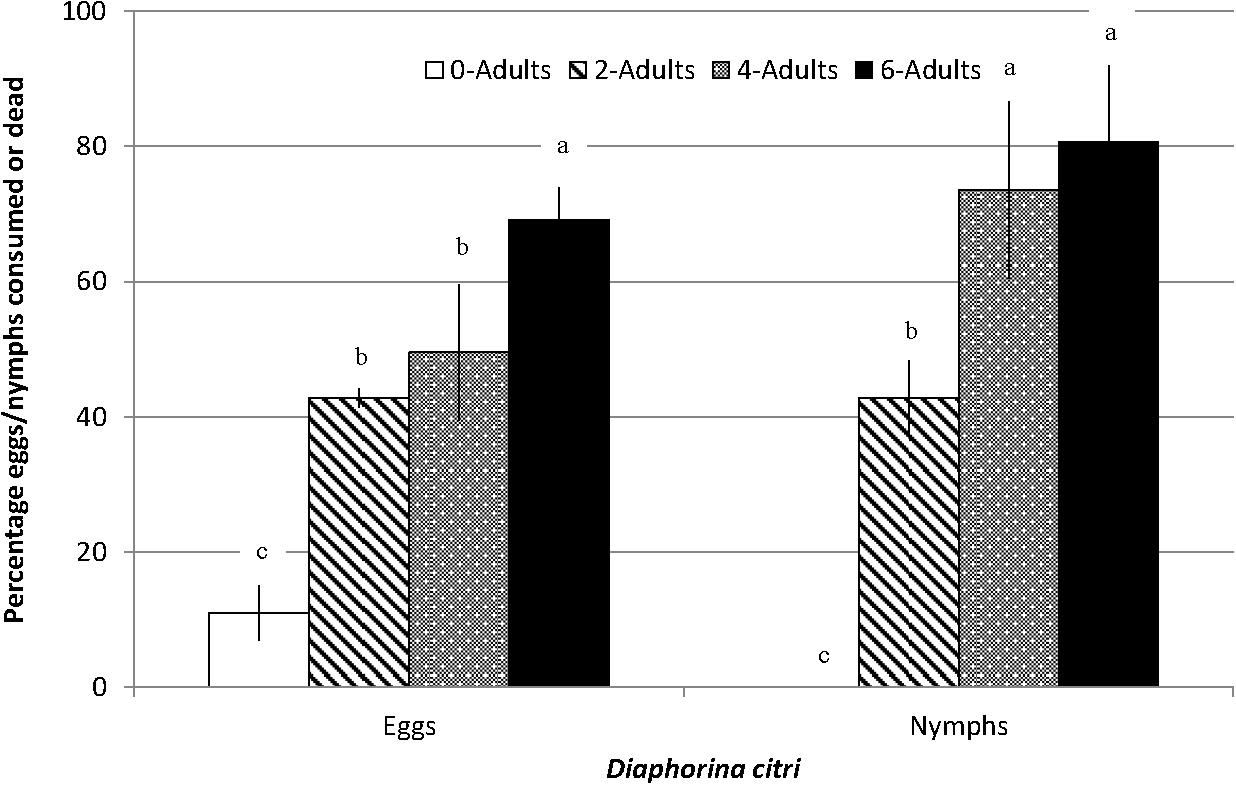
Figure 4. Impact of different release rates of S. barberi adults on eggs/nymphal colonies of D. citri developing on orange jasmine plants caged in the green house. Significant reduction in D. citri, averaging 43–81% across three release rates compared to zero-10% in control. Columns sharing the same letter within eggs or nymphs are not significantly different (P > 0.05).
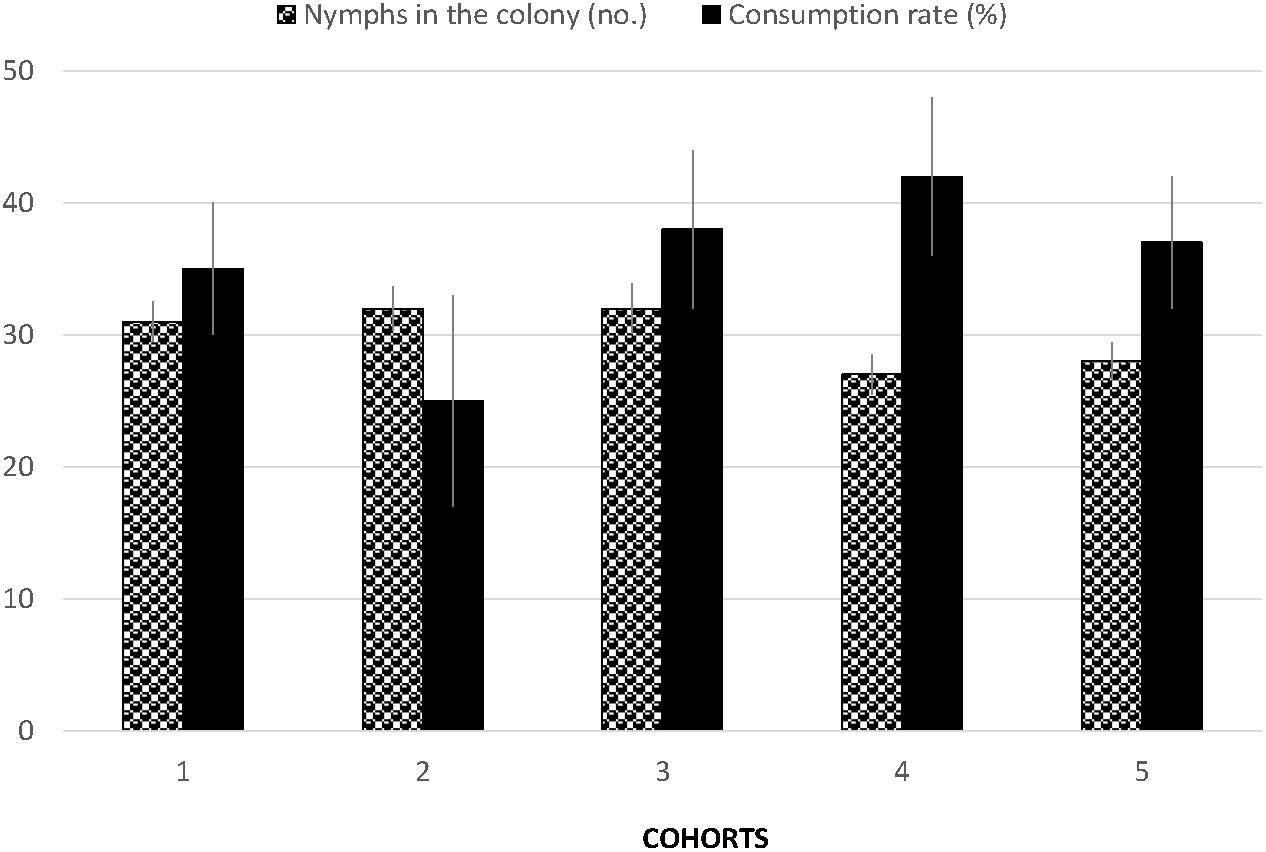
Figure 5. Mean number of D. citri nymphs in the colonies (Black and white columns) and percentage consumed (Black columns) by S. barberi in five cohorts. The first three cohorts contained 10 colonies each followed by 7 and 5 colonies in the 4th and 5th cohort, respectively. Each cohort lasted 2–3 days when the same S. barberi were shifted to new colonies.
Discussion
This first detailed investigation of S. barberi predation against D. citri demonstrates the aggressive feeding behavior of this predator on psyllid eggs and nymphs. Sympherobius barberi were effective in consuming all the three life stages including, eggs, small nymphs, and large nymphs of D. citri under both light and dark conditions. There was a gradual increase in consumption rate over time, which was more evident for eggs (40 to 95% from 6 to 24 h) and large nymphs (60 to 95% from 6 to 24 h), while 95% of the small nymphs were consumed within 6 h. The feeding on eggs was initially low and improved over time, probably due to the concealed locations where they were laid, such as newly developing unfolded leaves and predator ability to reach into those locations. For large size predators such as lacewings and ladybeetles, it is difficult to attack prey in such locations, and therefore smaller predators such as predatory mites are more useful to attack eggs and neonates in such locations (Juan-Blasco et al., 2012). The difference of 35% in the consumption rate on small and large nymphs observed at 6 h was reduced to 5–10% within 12–24 h, suggesting that predator will most likely be negatively impacting significant nymphal populations encountered under field conditions. The predator preference of prey type or stage is reported for other lacewing species and pests and could impact predation rates in the field. For example, when all instars of pea aphid were presented in equal numbers to Tasmanian lacewing Micromus tasmaniae adults, they killed 74–90% of the first instars, 9–26% of second instars but none of the instar third and fourth (Leathwick, 1989). Islam and Chapman (2001) observed predation rate of 10 Brevicoryne brassicae aphids per day by the larval stage of brown lacewing Micromus tasmaniae, which is half of the predation rate of adult S. barberi on D. citri nymphs in the present study, suggesting better prey suitability for the predator in the latter scenario. However, the consumption rate by larvae of S. fallax on nymphal stages of a mealybug, Pseudococcus longispinus, was higher in another study (Gillani et al., 2009). We tested S. barberi against D. citri in two age groups. The successful feeding on young and mature nymphs containing 2–3 instar and 4–5 instar, respectively, followed by the experiment showing successful larval development in a diet of mixed instars indicate that they will be able to feed across all instars. Additionally, the developing colonies of D. citri nymphs in the field contain multiple instars which provide S. barberi enough choices to reduce pest population. In some situations, citrus shoots get colonized by more than one pest such as aphids and psyllids, and therefore, testing prey preference between species will be another interesting subject for a future investigation.
Larval survival of S. barberi to pupation on the diet of D. citri nymphs averaged 70% compared with 100% on the diet of frozen eggs of E. kuehniella. The larval development prolonged on the diet of D. citri nymphs compared to E. kuehniella eggs. The Ephestia eggs is a typical diet used for commercial rearing of several predators, which support maximum survival in most cases (Qureshi and Stansly, 2011; Khan et al., 2016), whereas D. citri was a new prey for the S. barberi. The presence of wing buds in the late instars nymphs of D. citri may have caused some deterrence for the S. barberi larvae and reduced the consumable body contents resulting in predator spending more time and energy in handling nymphs thus prolonged development and reduced survival in some cases. However, the pupation time of S. barberi did not differ between the diets of D. citri and E. kuehniella, and survival was 100% on both diets. The total development time from egg hatch to adult emergence was 26–28 days for S. barberi in the present study. Pacheco-Rueda et al. (2011) reported similar development time for this predator on the diet of Dactylopius opuntiae. Lara and Perioto (2003) reported that several species of hemerobiids complete their life cycle in 23–40 days at 23°C, and Vargas (2007) stated 23 days life cycle for Chrysoperla carnea at 26°. The survival rate of 70% on D. citri in the present study was better than some much lower rates reported for other Hemerobiids such as 2–25% for M. tasmaniae (Syrett and Penman, 1981), and 30% for Hemerobius pacificus (Neuenschwander, 1975) which most likely were impacted by rearing conditions including prey, condensation, and cannibalism. When reared individually, as in the present study, the survival of M. tasmaniae was in the range of 83–100% (Leathwick, 1989).
Pacheco-Rueda et al. (2011) observed an average adult longevity of 39 days for S. barberi on the diet of Dactylopius opuntiae and average of 1.89 eggs per day per female. In the present study, S. barberi adults evaluated in the two experiments lived between 13 and 19 days, on the diet of D. citri, and female produced an average of 25 eggs during a 3-week period, the latter not very different from the previous study. The difference in longevity may be due to the differences in prey and experiment conditions. The 65% of the eggs produced by S. barberi on the D. citri diet were fertile, suggesting suitability of psyllid for the successful reproductive performance of this predator.
Sympherobius barberi negatively impacted D. citri by providing significant reduction in the psyllid populations on infested orange jasmine plants under semi-natural conditions in the screenhouse and on infested citrus trees in the field. Reduction of 43–81% in the eggs and nymphs was observed when S. barberi were released at the rate of 2–6 adults with 100 or fewer eggs or nymphs of D. citri per cage. There also was a trend of increased reduction in D. citri with an increase in the release rate of S. barberi. In the field conditions, one adult caged with an average of 28–32 nymphs provided a reduction of 35%. The evidence of S. barberi feeding, development, and reproduction on D. citri and negative impact on psyllid populations under semi-natural conditions suggest that this species may play a significant role as a predator of D. citri. However, open releases and evaluations are needed in the groves. Legaspi et al. (1996) investigated the field release of Chrysoperla rufilabris in the large field cages in watermelon fields using densities of 10, 25, and 50 larvae per cage. They reported approximately 35% more whiteflies in control with no release over the entire season as compared to the predator treatment with the highest whitefly counts. Similarly, Daane et al. (1993) observed a 35% reduction of leafhopper Erythroneura variabilis in the vineyard fields with the release of different species of lacewings. Sympherobius barberi like other lacewings attacks many pests, some of which such as whiteflies, aphids etc are found in multiple crops and may help support the establishment of this predator.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
We are thankful to the Higher Education Commission of Pakistan, for funding the Ph.D. research work of AK, part of which including work of this investigation was conducted at the University of Florida SWFREC, Gainesville, FL, United States.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Foothill Agricultural Research, Inc. (550 W Foothill Pkwy, Corona, CA, United States) for providing the initial shipments of Sympherobius barberi.
References
Balduf, W. V. (1939). The Bionomics of Entomophagous Insects, Part II. New York, NY: John S. Swift Co. Inc, 384.
Catling, H. D. (1970). Distribution of the psyllid vectors of citrus greening disease, with notes on the biology and bionomics of Diaphorina citri. FAO Plant Protect. Bull. 18, 8–15.
Cutright, C. R. (1923). Life history of Micromus posticus Walker. J. Econ. Entomol. 16, 448–456. doi: 10.1093/jee/16.5.448
Daane, K., Yokota, G., Rasmussen, Y., Zheng, Y., and Hagen, K. (1993). Effectiveness of leafhopper control varies with lacewing release methods. Calif. Agric. 47, 19–23. doi: 10.3733/ca.v047n06p19
Gillani, W. A., Copland, M., and Shazia, R. (2009). Studies on the feeding preference of brown lacewing (Sympherobius fallax Navas) larvae for different stages of long-tailed mealy bug (Pseudococcus longispinus)(Targioni and Tozzetti). Pakistan Entomol. 31, 1–4.
Grimaldi, D., and Engel, M. S. (2005). Evolution of the Insects. Cambridge: Cambridge University Press, 755.
Halbert, S. E. (2005). Pest Alert: Citrus Greening/Huanglongbing. Gainesville, FL: Florida Department of Agriculture and Consumer Services Dept of Plant Industry.
Hussain, M. A., and Nath, D. (1927). The citrus psylla (Diaphorina citri, Kuw.)[Psyllidae: Homoptera]. Mem. Dept. Agric. India Entomol. Series 10, 1–27.
Islam, S. S., and Chapman, R. B. (2001). “Effect of temperature on predation by Tasmanian lacewing larvae,” in Proceedings of the New Zealand Plant Protection Conference, Palmerston North, 244–247. doi: 10.30843/nzpp.2001.54.3748
Juan-Blasco, M., Qureshi, J. A., Urbaneja, A., and Stansly, P. A. (2012). Predatory mite Amblyseius swirskii (Acari: Phytoseiidae) for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Florida Entomol. 95, 543–551. doi: 10.1653/024.095.0302
Khan, A. A., Qureshi, J. A., Afzal, M., and Stansly, P. A. (2016). Two-spotted ladybeetle Adalia bipunctata L. (Coleoptera: Coccinellidae): a commercially available predator to control Asian Citrus Psyllid Diaphorina citri (Hemiptera: Liviidae). PLoS One 11:e0162843. doi: 10.1371/journal.pone.0162843
Kuwayama, S. (1908). Die psylliden Japans. Trans. Sopporo Natural History Soc. (Parts I and II) 2, 149–189.
Lara, R. I. R., and Perioto, N. W. (2003). Hemerobiideos bioecology (Neuroptera Hemerobiidae). 70, 517–523.
Leathwick, D. M. (1989). Applied Ecology of Tasmanian Lacewing Micromus tasmaniae Walker. Ph.D. thesis, Lincoln University New Zealand, Lincoln.
Legaspi, J. C., Correa, J. A., Carruthers, R. I., Legaspi, B. C. Jr., and Nordlund, D. A. (1996). Effect of short-term releases of Chrysoperla rufilabris (Neuroptera: Chrysopidae) against silverleaf whitefly (Homoptera: Aleyrodidae) in field cages. J. Entomol. Sci. 31, 102–111. doi: 10.18474/0749-8004-31.1.102
MacLeod, E. G., and Stange, L. A. (2005). Brown Lacewings (of Florida) (Insecta: Neuroptera: Hemerobiidae). UF University of Florida IFAS Extension 225, 1–6.
Miller, G. L., and Cave, R. D. (1987). Bionomics of Micromus posticus (Walker) (Neuroptera: Hemerobiidae) with descriptions of the immature stages. Proc. Entomol. Soc. Washington 89, 776–789.
Monserrat, V. J., and Marín, F. (1996). Plant substrate specificity of Iberian hemerobiidae (Insecta: Neuroptera). J. Natural History 30, 775–787. doi: 10.1080/00222939600770401
Neuenschwander, P. (1975). Influence of temperature and humidity on the immature stages of Hemerobius pacijicus. Environ. Entomol. 1, 215–220. doi: 10.1093/ee/4.2.215
New, T. R. (1975). The biology of Chrysopidae and Hemerobiidae (Neuroptera), with reference to their usage as biocontrol agents: a review. Trans. R. Entomol. Soc. Lond. 127, 115–140. doi: 10.1111/j.1365-2311.1975.tb00561.x
New, T. R. (2001). “Introduction to the Neuroptera: what are they and how do they operate,” in Lacewings in the Crop Environment, ed. P. K. McEwen (Cambridge: Cambridge University Press), 3–5. doi: 10.1017/cbo9780511666117.002
Oswald, J. D. (1993). Phylogeny, taxonomy, and biogeography of extant silky lacewings (Insecta: Neuroptera: Psychopsidae). Memoirs Am. Entomol. Soc. (USA) 40, 1–65.
Oswald, J. D. (1994). A new phylogenetically basal subfamily of brown lacewings from Chile (Neuroptera: Hemerobiidae). Insect Syst. Evol. 25, 295–302. doi: 10.1163/187631294x00090
Pacheco-Rueda, I., Lomelí-Flores, J. R., Rodríguez-Leyva, E., and Ramírez-Delgado, M. (2011). Ciclo de vida y parámetros poblacionales de Sympherobius barberi Banks (Neuroptera: Hemerobiidae) criado con Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae). Acta Zool. Mexicana 27, 325–340. doi: 10.21829/azm.2011.272756
Penny, N. D. (1977). Lista de megaloptera, neuroptera e raphidioptera do México, América Central, ilhas Caraíbas e América do Sul. Acta Amazonica 7, 5–61. doi: 10.1590/1809-43921977074s005
Penny, N. D., Adams, P. A., and Stange, L. A. (1997). Species catalog of the neuroptera, megaloptera, and raphidioptera of America North of Mexico. Proc. Calif. Acad. Sci. 50, 39–114.
Qureshi, J. A., and Stansly, P. A. (2011). Three homopteran pests of citrus as prey for the convergent ladybeetle Hippodamia convergens: suitability and preference. Environ. Entomol. 40, 1503–1510. doi: 10.1603/en11171
Sato, T., and Takada, H. (2004). Biological studies on three Micromus species in Japan (Neuroptera: Hemerobiidae) to evaluate their potential as biological control agents against aphids: 1. Thermal effects on development and reproduction. Appl. Entomol. Zool. 39, 417–425. doi: 10.1303/aez.2004.417
Syrett, P., and Penman, D. R. (1981). Development threshold temperatures for the brown lacewing, Micromus tasmaniae (Neuroptera: Hemerobiidae). N.Z. J. Zool. 8, 281–283. doi: 10.1080/03014223.1981.10427967
Texeira, D. C., Ayres, A. J., Kitajima, E. W., Tanaka, F. A. O., Danet, J. L., and Jagouiex-Eveillard, S. (2005). First report of a huanglongbing-like disease of citrus in São Paulo state, Brazil, and association of a new Liberibacter species,“Candidatus Liberibacter americanus,” with the disease. Plant Dis. 89, 107. doi: 10.1094/pd-89-0107a
Vargas, E. U. (2007). Development, Survival and Fecundity of Chrysoperla carnea Stephens and Chrysoperla comanche Banks Reared Eggs of Ephestia kuehniella (Olivier). Master’s thesis, Chapingo University, Mexico.
Keywords: Asian citrus psyllid, citrus greening, biological control, predator, lacewings
Citation: Khan AA, Afzal M, Stansly P and Qureshi JA (2020) Effectiveness of the Brown Lacewing, Sympherobius barberi Banks as a Biological Control Agent of the Asian Citrus Psyllid Diaphorina citri Kuwayama. Front. Plant Sci. 11:567212. doi: 10.3389/fpls.2020.567212
Received: 29 May 2020; Accepted: 18 September 2020;
Published: 14 October 2020.
Edited by:
Marcos Antonio Machado, Instituto Agronômico de Campinas (IAC), BrazilReviewed by:
Niels Holst, Aarhus University, DenmarkDaniele Cornara, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Copyright © 2020 Khan, Afzal, Stansly and Qureshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jawwad A. Qureshi, amF3d2FkcUB1ZmwuZWR1
 Azhar A. Khan
Azhar A. Khan Muhammad Afzal
Muhammad Afzal Phil Stansly1
Phil Stansly1 Jawwad A. Qureshi
Jawwad A. Qureshi