- 1Naiman Desertification Research Station, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
- 2School of Land and Resources, China West Normal University, Nanchong, China
- 3Forest Dynamics, Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Birmensdorf, Switzerland
- 4School of Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou, China
- 5Urat Desert-Grassland Research Station, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou, China
- 6Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- 7Key Laboratory of Geographical Processes and Ecological Security in Changbai Mountains, Ministry of Education, School of Geographical Sciences, Northeast Normal University, Changchun, China
Artemisia halodendron Turcz. ex Besser occurs following the appearance of a pioneer species, Agriophyllum squarrosum (L.) Moq., with the former replacing the latter during the naturally vegetation succession in sandy dune regions in China. A previous study revealed that the foliage litter of A. halodendron had strong negative allelopathic effects on germination of the soil seed bank and on the seedling growth. However, whether this allelopathic effect varies with litter types and with the identity of plant species has not yet been studied. We, therefore, carried out a seed germination experiment to determine the allelopathic effects of three ltter types of A. halodendron (roots, foliage, and stems) on seed germination of six plant species that progressively occur along a successional gradient in the semi-arid grasslands in the Horqin Sandy Land of northeastern China. In line with our expectation, we found that the early-successional species rather than the late-successional species were negatively affected by A. halodendron and that the allelopathic effects on seed germination increase with increasing concentration of litter extracts, irrespective of litter types. Our study evidenced the negative allelopathic effects of A. halodendron on the species replacement and on the community composition during dune stabilization in the Horqin Sandy Land. Further studies are needed to better understand the successional process and thus to promote the vegetation restoration in that sandy dune region as A. halodendron itself disappeared also during the process.
Introduction
Plant litter can affect the structure and composition of plant communities directly through its influence on the emergence and early growth of seedlings (Sayer, 2006; Li K. et al., 2016). The influence of plant litter on plant establishment can be negative or positive, and these influences differ among species, litter types, and litter quantity (Hovstad and Ohlson, 2008). Litter affects plant establishment not only by modifying the microenvironment such as changes in the light supply (Weltzin et al., 2010), soil moisture (Wolkovich et al., 2009), soil nutrient content (Fahnestock et al., 2000), and herbivores (Reader, 1993; Facelli, 1994), but also by means of allelopathy derived from the release or leaching of allelochemicals during litter decomposition (Cheema et al., 2013; Loydi et al., 2015). Allelopathy is defined as the stimulatory or inhibitory effects of one plant upon another, including interactions with microorganisms (Rice, 1984). For example, the foliage litter of Artemisia halodendron can affect the establishment of other species by allelopathy in degraded sandy grassland (Luo et al., 2017). Root litter of Eucalyptus urophylla in a subtropical forest ecosystem significantly inhibited the germination and height growth of Schima superba, Michelia macclurei, and Elaeocarpus sylvestris due to allelopathy (Zhang and Fu, 2009).
Allelopathy is an important phenomenon that plays a significant role in plant dominance, succession, and the formation of plant communities (Chou, 2006). During allelopathy, a plant releases phytotoxic compounds (allelochemicals) into its environment affecting other plants growing in the same habitat (Chou, 2006). Allelochemicals can be produced from any part of the plant (i.e., leaves, flowers, roots, fruits, or stems) and can be released either directly, as in the case of plant root exudates, or indirectly, by leaching of the plants or their residues, as well as by decomposition of plant residues in the surrounding soil (Cheema et al., 2013).
Plant succession is an important process during ecosystem development, especially in ecosystems that are recovering from disturbances such as those in the Horqin Sandy Land. In this region, due to intensive utilization for agriculture and livestock grazing, in combination with the negative effects of climate change (warmer and drier climate) on vegetation, the original grassland was seriously degraded (Zhao et al., 2003). Since about 2000, the Chinese government has adopted many environmental protection projects such as grazing control to restore the grassland ecosystems in the Inner Mongolia Autonomous Region. Thereafter, many degraded grasslands have begun to recover and became denser and more complex communities (Zuo et al., 2009a; Li et al., 2012; Zuo et al., 2012), and both the vegetation cover and the net primary production have increased rapidly (Zhao et al., 2003; Lian et al., 2017). Previous studies have clarified the dynamics of vegetation composition and structure during the restoration process (Zhao et al., 2003; Zhao et al., 2007; Zuo et al., 2009a; Zuo et al., 2009b; Zuo et al., 2012). However, the mechanisms that regulate the dynamics of plant succession during the grassland restoration in this area are still poorly understood (Zhao et al., 2003; Luo et al., 2017).
Artemisia halodendron is one of the most important subshrubs in the Horqin Sandy Land of northeastern China. It occurs following the appearance of Agriophyllum squarrosum in that sandy land (see also Figure 1). Due to its deep roots, A. halodendron can capture water in the deep soil and therefore exhibits high drought tolerance (Ma et al., 2010). It also has a flexible reproduction strategy that includes both sexual and vegetative propagation (Zhao et al., 2003; Li et al., 2005) and exhibits high tolerance of abrasion by windblown particles and burial by sand (Liu et al., 2008; Huang et al., 2011). Furthermore, its very small seeds facilitate wind dispersal and expansion of its range (Li et al., 2005; Ma et al., 2010).
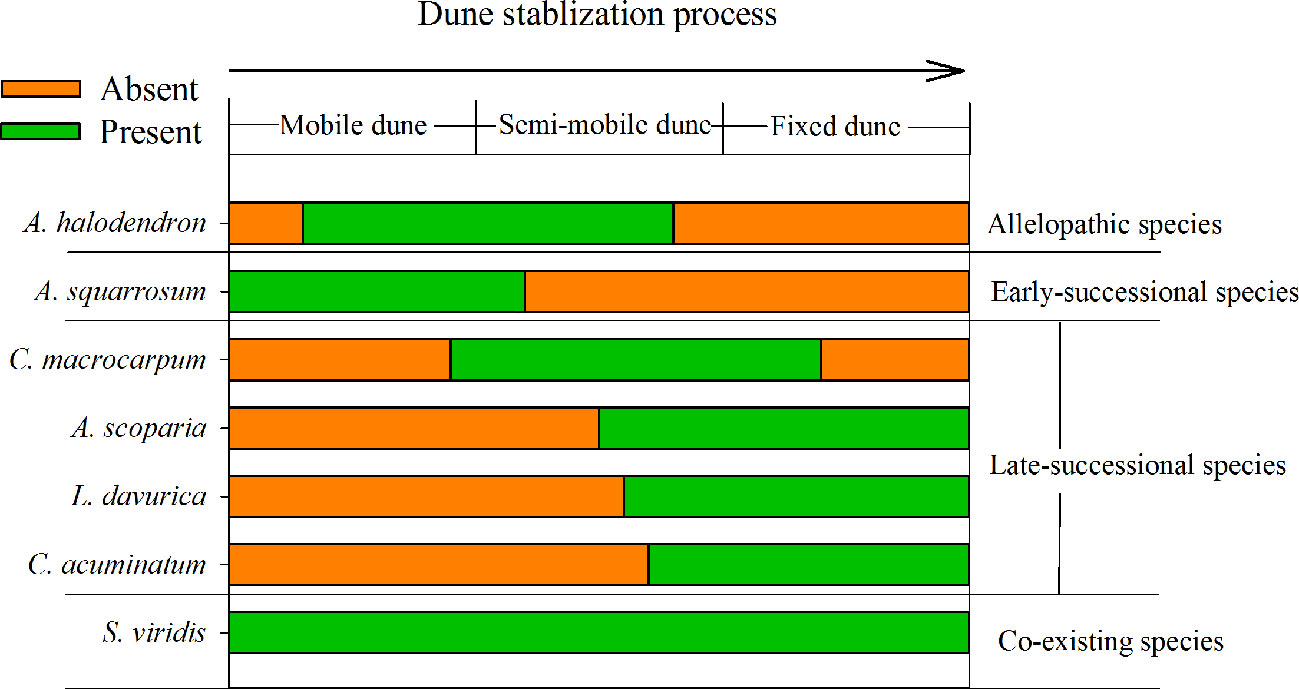
Figure 1 Distribution of the main species during the sand dune stabilization process in the Horqin Sandy Land, northeastern China.
The plant succession dynamic of dune stabilization has been described in many studies (Zhao et al., 2003; Zuo et al., 2009a; Zuo et al., 2009b). As the mobile dunes become semi-mobile, the density of A. halodendron increases sharply, while the density of A. squarrosum decreases sharply until the species disappears (see also Figures 1 and 2). During this process, the community plant species’ richness increases and several species (e.g. Artemisia scoparia Waldst. & Kit, Lespedeza davurica (Laxm.) Schindl., Chenopodium acuminatum Willd., Corispermum macrocarpum Bunge) become established (see also Figures 1 and 2). By the time the dunes become fixed, A. halodendron disappears completely (see also Figures 1 and 2). The mechanisms underlying this species replacement have, however, not yet been studied under natural conditions.
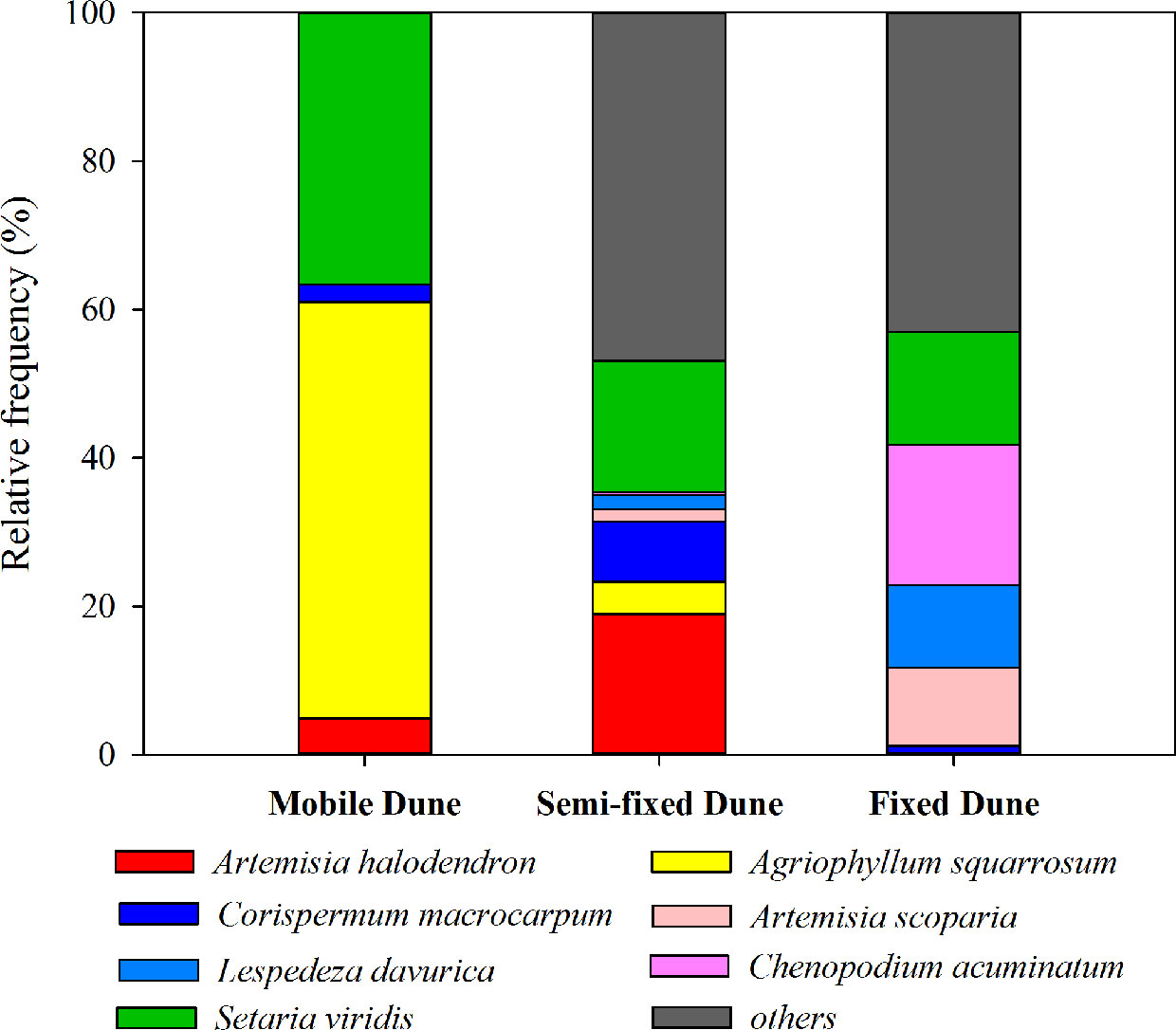
Figure 2 Relative frequency (%) of the main species at three successional stages during the sand dune stabilization in the Horqin Sandy Land, northeastern China.
Our previous study (Luo et al., 2017) revealed that the foliage litter of A. halodendron had strong allelopathic effects on germination of the soil seed bank and on the seedling growth. However, we still do not know whether not only foliage litter but also stem and root litter of A. halodendron have allelopathic effects on other species and whether the intensity of allelopathic effects varies with both litter types (foliage, stem, roots) of A. halodendron and the identity of coexisting species. Therefore, we carried out a seed germination experiment to determine the allelopathic effects of three litter types of A. halodendron (roots, foliage, and stems) on seed germination of six plant species that progressively occur along a successional gradient in the semi-arid grasslands in the Horqin Sandy Land of northeastern China. We aimed to test our hypotheses that 1) the early-successional species but not the late-successional species respond to the allelopathic effects, and 2) the allelopathic effect increases with increasing concentration of litter extracts, irrespective of litter types.
Materials and Methods
Study Site and Plant Species
This study was conducted at the Naiman Desertification Research Station of the Chinese Academy of Sciences (120°43′ E, 42°58′ N; 360 m a.s.l.), which is located in the southwestern part of the Horqin Sandy Land. This desertified area is in the eastern part of China’s Inner Mongolia Autonomous Region. The climate of the region is semi-arid continental monsoon with mean annual precipitation and potential evaporation of 343 and 1935 mm respectively. The mean annual temperature is 6.7°C, with a minimum monthly mean temperature of −12.6°C in January and a maximum of 24.3°C in July. The soil is classified as Cambic Arenosols of sandy origin in the FAO soil classification system (FAO, 2006) and is highly vulnerable to wind erosion.
The landscape is characterized by sand dunes alternating with gently undulating lowlands between the dunes. The plant community is dominated by annual species, with some perennial shrubs and subshrubs (e.g., A. halodendron, Caragana microphylla, Salix gordejevii), and the plant community exhibits high heterogeneity (Zuo et al., 2009a). Based on the natural plant succession dynamics in the Horqin Sandy Land and long-term monitoring of the plant community composition in three categories of habitats (mobile, semi-mobile, and fixed dunes; http://nmd.cern.ac.cn/meta/metaData), six species were chosen to examine their seed germination responses to A. halodendron: A. squarrosum, Setaria viridis, A. scoparia, L. davurica, C. acuminatum, and C. macrocarpum. During natural plant succession, A. squarrosum occurs first on mobile dunes. Then, as dune stabilization begins, it is replaced by A. halodendron on semi-mobile dunes. Setaria viridis is widely distributed in northern China, and it often coexists with A. halodendron. The other four species are mainly found on semi-mobile or fixed dunes and are therefore late-successional species. During plant species succession, the plant richness and density increased sharply, and A. halodendron began to disappear, especially on fixed dunes, where the species were rarely found. Figures 1 and 2 illustrated the temporal relationships and relative frequency (%) of the main species during three different successional phases of the sand dune stabilization in the Horqin Sandy Land.
Seed and Litter Sampling
Seeds of these six species were collected at the end of the growing season (late August to October) in 2016 from sand dunes near the station. After air-drying, we preserved all seeds in an unheated storeroom at the station. The storeroom’s winter temperature was as cold as the field, so it should be possible to ignore the effects of seed dormancy due to temperature differences between the storeroom and the field.
At the end of the growing season (mid-September of 2016) when the leaves of A. halodendron turned yellow and started falling, three entire plants of A. halodendron were excavated then manually separated into leaves, stems, and fine roots (diameter ≤ 2 mm). The root sample was washed up with tap water to remove soil, and then all the three litters (LTs) were air-dried and preserved in the storeroom for further use.
Litter Extracts Preparation
In early May of 2017, we cut all the three LTs into fragments of 1 to 1.5 cm long, and 100 g of such cut litter of each LT was then steeped in 1 L of distilled water for 24 h. The incubation was conducted in darkness in an incubator held at a temperature of 20°C. We then filtered the extract through a double layer of cheesecloth to remove coarse fragments, followed by filtration through Whatman no. 3 filter paper. Each type of extract was then diluted with distilled water to obtain litter extract with a relative extract concentration (LEC) equivalent of 10, 20, 40, 60, 80, and 100 g·L−1. Distilled water was used as the control (0 g·L−1). These extracts and the control were then preserved in a refrigerator at 4°C until they could be used (Zhang and Fu, 2010; Samedani et al., 2013).
Seed Germination Experiment
Healthy-looking seeds with a relatively uniform size were surface-sterilized with 4% (v/v) sodium hypochlorite solution for 2 min followed by immersion in 70% (v/v) ethanol for 2 min. They were then washed three times for 3 min with distilled water. To test the allelopathic effect, we added 5 ml of each LEC (at 0, 10, 20, 40, 60, 80, or 100 g·L−1) to sterile 10-cm Petri dishes that contained two sterile Whatman no. 2 filter papers. We used five replicates for each LEC and each species. We placed 30 surface-sterilized seeds of a species in each Petri dish. To avoid contamination and water evaporation, the Petri dishes were sealed with parafilm. Then all Petri dishes were incubated in an incubator. The incubator provides a 14 h photoperiod supplied by white fluorescent lights at a temperature of 25°C. During the nighttime of 10 h, the seeds were kept in darkness at 20°C. This light and temperature regime appears to provide the optimal conditions for germination based on our previous study in the same region (Luo et al., 2017). A total of 630 Petri dishes (3 LTs × 7 LECs × 6 species × 5 replicates) were incubated in the incubator for 21 days. At the end of the germination experiment, we counted the germinated seeds (i.e., seeds that showed both the radicle and a shoot) in each Petri dish and calculated the germination rate in each dish using the following equation:
where the No. of germinated seeds is the number of germinated seed with fully developed radicle and germ per dish. The 30 is the total number of seeds in each dish.
We defined an allelopathic index (AI) to reflect the allelopathic effect of the LTs of A. halodendron. AI was calculated as follows (Luo et al., 2017):
where Tx is the germination rate T in LEC concentration x, and TC is the germination rate in the control (with a LEC concentration of 0 g·L−1). A negative value of AI means a negative allelopathic effect of LTs on seed germination, and a positive AI value indicates a promoting effect on seed germination.
Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 software (www.ibm.com/software/analytics/spss/). We used three-way ANOVA to identify the germination responses of the six species to litter extracts concentration (0, 10, 20, 40, 60, 80, and 100 g·L−1), litter type (root, foliage, and stem), and their interactions. Due to highly significant effects of species, species × litter type and species × litter extracts’ concentration interaction, we, therefore, used two-way ANOVAs to test the allelopathic effects of the litter type, litter extracts’ concentration (0, 10, 20, 40, 60, 80, and 100 g·L−1), and their interaction on seed germination rate for each of the six species, and followed, if significant, by multiple comparisons using the least-significant difference (LSD) test at P < 0.05 level.
Results
The seed germination rate varied significantly among the six species under LT and LEC treatment (P < 0.001) (Table 1). LEC significantly affected the germination (P < 0.001), irrespective of LT (P > 0.05). Different species responded to LT and LEC significantly differently, showing significant species-LT and species-LEC interactions (Table 1).
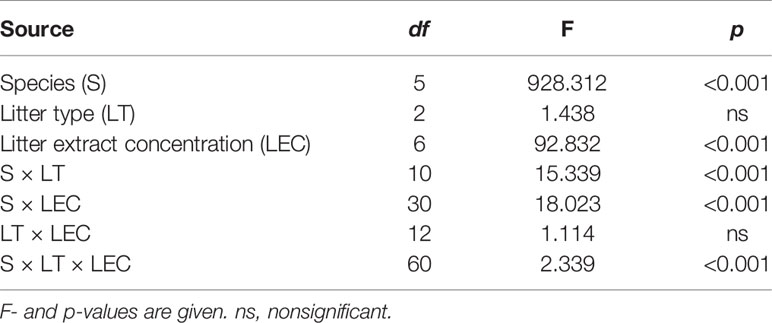
Table 1 Results of the three-way ANOVA for the Artemisia halodendron litter type (LT) and litter extract concentration (LEC) on seed germination of six common species in a degraded sandy grassland in northeastern China.
Extracts of foliage, stem, and roots significantly decreased seed germination of the early-successional species A. squarrosum (Figure 3A), and this suppression increased with increasing LEC, even though no seeds germinated at the higher LECs (Figure 3A). Moreover, the negative effects of LT extracts on seed germination of A. squarrosum followed an order of foliage > stems > roots (Figures 3A and 4A), and the AI values decreased (more negative) with increasing LEC (−50 to −100%) (Figure 4A).
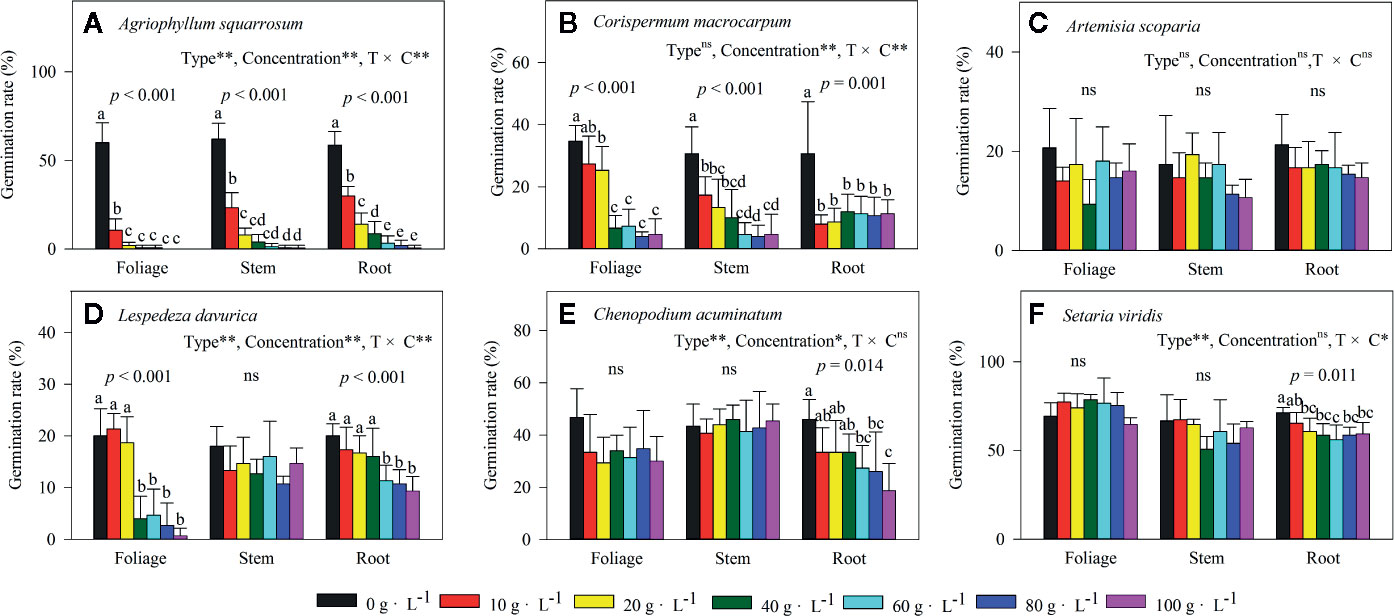
Figure 3 Effects of different concentrations of water extracts from three types of Artemisia halodendron litter (foliage, stems, and roots) on seed germination rate of six species (A–F) grown in a semi-arid grassland in northeastern China. Values represent means + 1SD. Different lowercase letters (a,b,c,d,e) above the bars indicate significant differences among concentrations (ANOVA followed by LSD test, P < 0.05). ns indicates no significant differences among concentrations P > 0.05. (A–F) indicate the six native species during natural plant succession. (A) indicates the early-successional species A. squarrosum, (B–E) indicate the late-successional species C. macrocarpum, A. scoparia, L. davurica, and C. acuminatum, and (F) indicates the co-existing species S. viridis.
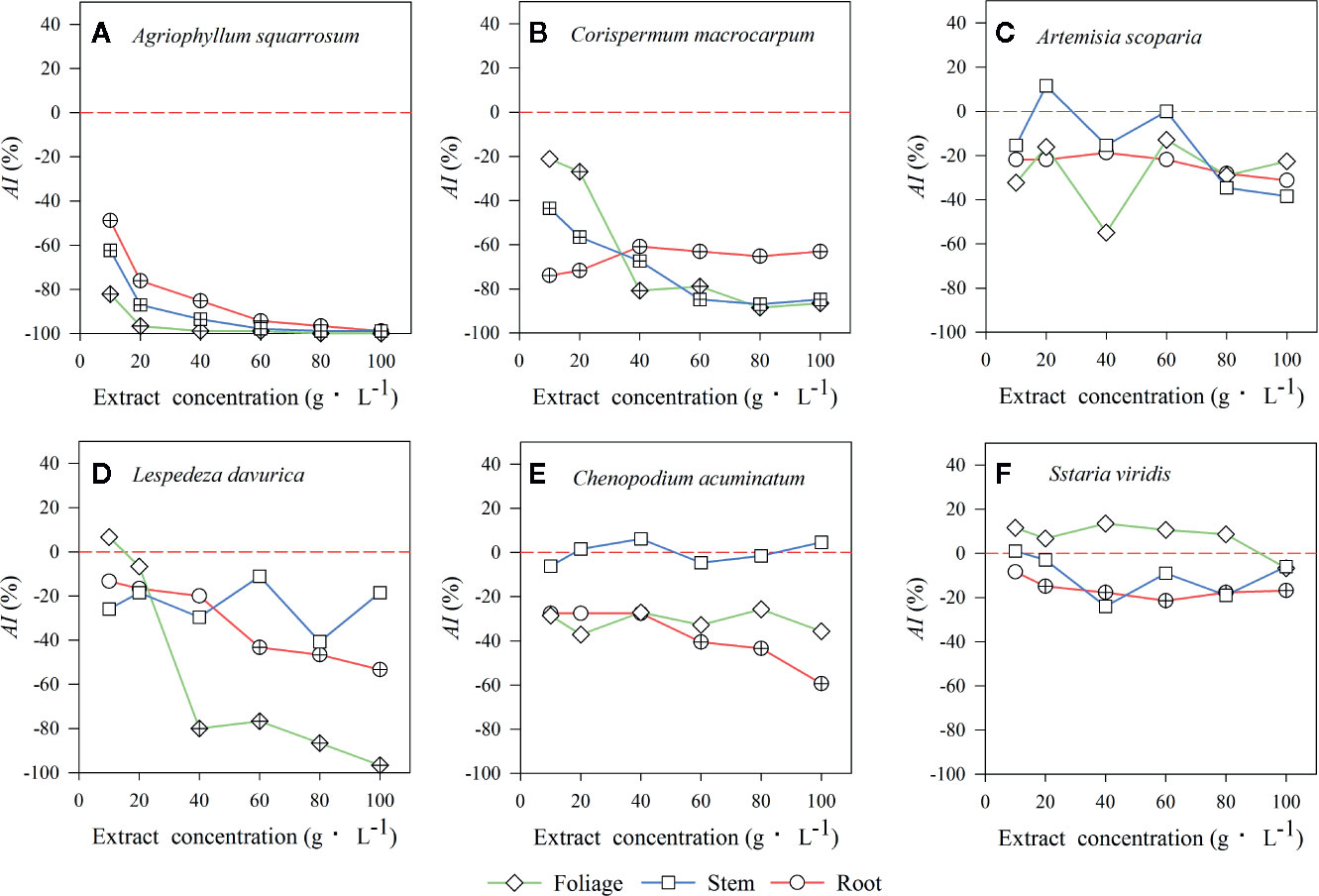
Figure 4 The values of the allelopathic index (AI) represent the strength of allelopathic effect of three types of litter extracts from A. halodendron on seed germination of 6 native species (A–F) grown in a semi-arid grassland in northeastern China. Symbols labeled with a “+” indicate a significant allelopathic effect on seed germination (P < 0.05), compared to the control 0-line (AI < 0 indicates a negative effect, and AI > 0 means a positive effect). (A–F) indicate the six native species during natural plant succession. (A) indicates the early-successional species A. squarrosum, (B–E) indicate the late-successional species C. macrocarpum, A. scoparia, L. davurica, and C. acuminatum, and (F) indicates the co-existing species S. viridis.
For the relatively late early-successional species, C. macrocarpum, LTs did not differ in their effects on seed germination (Figure 3B). LEC significantly interacted with LT to affect seed germination (Figure 3B). At lower LEC of ≤20, the suppression effects had an order of roots > stem > foliage (Figures 3B and 4B), and when the LEC was >40 g·L−1, the negative effects of LT extracts on seed germination followed an order of root < stem = foliage (Figures 3B and 4B).
For the three late-successional species, A. squarrosum did not respond to LTs and LEC (Figures 3C and 4C). Only root litter extracts had significant negative effects on seed germination of C. acuminatum (Figures 3E and 4E), and this suppression increased with increasing root LEC (Figure 3E). For L. davurica, stem litter extracts had no effects on its seed germination (Figure 3D), and also only higher LEC of foliage and root litter extracts significantly suppressed seed germination (Figures 3D and 4D).
For the co-existing species, S. viridis, only extracts of root litter significantly decreased its seed germination (Figure 3F), and this suppression was weak (less negative AI of >−20%) (Figures 3F and 4F).
Discussion
The present study indicated that the seed germination of the early- (A. squarrosum) and early late-successional species (C. macrocarpum) was significantly suppressed by the extracts of A. halodendron litters (Figures 3A, B and 4A, B). Therefore, these two species decreased their relative frequency (Figure 2) and finally disappeared with increasing successional stage (Figure 1). These findings suggest a significant negative allelopathic effect of A. halodendron on other species.
Agriophyllum squarrosum, which was the first species that became established on mobile dunes and could be found on mobile dunes only, was strongly inhibited by extracts of all three types of A. halodendron litter (Figure 3a). Therefore, A. halodendron “killed” and replaced A. squarrosum quickly after the former became established through allelopathy (Figures 1 and 2). This result was also supported by field investigations that A. squarrosum and A. halodendron distributed separately (Zuo et al., 2009a; Zuo et al., 2012), and A. squarrosum seedlings existed never under A. halodendron canopy (Zhao et al., 2003). The present study evidenced that litter allelopathy was an important driving force for the replacement of A. squarrosum by A. halodendron.
The early- (A. squarrosum) and the early late-successional species (C. macrocarpum) were significantly suppressed by the allelopathic effect of A. halodendron litters (Figures 3A, B and 4A, B), whereas the late-successional species (e.g. A. scoparia, C. acuminatum) were rarely affected by such effects (Figures 3C, E and 4C, E). These findings suggest that the allelopathy of A. halodendron seems to accelerate plant succession during the early stage of plant restoration in the Horqin Sandy Land. This agrees with the hypothesis that allelopathy can increase the rate of plant succession (Rice, 1984).
Allelopathy can also inhibit plant succession when early-successional species suppress the establishment or growth of late-successional species (Mallik, 2003; Fernandez et al., 2006). In the present study, seed germination of a late-successional species (L. davurica) was generally suppressed by A. halodendron litter (Figures 3D and 4D). This may imply that A. halodendron allelopathy influences the late succession, at least affects the species composition and structure of the community. This partly supports the statement of previous studies that allelopathy can also suppress or slow successional change (Mallik, 2003; Fernandez et al., 2006; Mudrák and Frouz, 2012).
The A. halodendron litter extracts did not affect the seed germination of A. scoparia (Figures 3C and 4C), indicating that the pioneer subshrub A. halodendron cannot influence seed germination of all late-successional species similarly. The theory of kin recognition suggests that plants that are genetically related tend to decrease their competitive interactions by modifying their morphology, such as reducing root overlap and increasing leaf area to avoid resource competition (Ninkovic, 2003; Dudley and File, 2007; Bhatt et al., 2011). Given the fact that A. halodendron and A. scoparia belong to the same genus, it’s possible that kin selection by reducing competitive interactions may partially explain the lack of significant allelopathy for A. scoparia.
For the co-existing species, S. viridis, its seed germination was also significantly negatively affected by the root litter of A. halodendron (Figures 3F and 4F), but it exists always in the community (Figures 1 and 2). Previous studies demonstrated that S. viridis had high tolerance to environmental stresses (Huang et al., 2008). For example, it had much stronger drought resistance than A. squarrosum in the Horqin Sandy Land (Huang et al., 2008). It also tolerated competition with the invasive species Ageratina adenophora, which threatened the growth and survival of many native species in southern China (Zhou et al., 2006). This tolerance may explain how S. viridis does resist allelopathy and competition in that community.
The plant succession in the study region is influenced by many factors such as plant propagation materials (Luo et al., 2017), soil water, and nutrients (Zhao et al., 2011), and also litter allelopathy (Zhang et al., 2015). However, the vegetation community composition varies greatly with the distance from A. halodendron plant (Zhao et al., 2007), although there are hardly differences in soil nutrients and water conditions in the root layer (0–40 cm) within that distance in Horqin sandy land (Zhao et al., 2003). This suggests that the allelopathy of A. halodendron plays an important role in determining the survival and development of its neighboring plant species. The allelochemicals may be produced in many plant parts during plant growth and development to exert multiple ecological effects and thus to affect the co-existing plants (Luo et al., 2012). In natural conditions in Horqin sandy land, plant seeds are mainly distributed in the surface soil (Zhao et al., 2007), and thus, the allelopathy of A. halodendron litters may mainly affect seed germination, while the allelopathy of A. halodendron root exudates may mainly influence the establishment, growth, and survival of its neighboring plants.
The result of this study evidenced that the effect of litter extracts of A. halodendron on seed germination differed among litter types (Figure 3); this may be a result of different chemical compositions and contents existing in various litter types. Although we did not determine the chemical composition and content of the litter extracts of each litter type in this study, a previous study showed that there are significant differences in the chemical quality/quantity and decomposition process among litter types of A. halodendron (Li Y. et al., 2016). The seed germination of S. viridies was only weakly inhibited by the extracts of the root litter of A. halodendron (Figures 3F and 4F), and thus, S. viridies and A. halodendron can co-exist successfully in the long-term in nature (Zuo et al., 2009a; Zuo et al., 2012). For the latter successional species, such as C. acuminatum, its seed germination was only inhibited by the extracts of root litter of A. halodendron (Figures 3E and 4E), but in the field, the growth of C. acuminatum is strongly affected by A. halodendron, probably due to root connection and belowground allelopathy. Here we only evidenced that litters of A. halodendron can affect the seed germination through allelopathy; further studies are needed to investigate the aboveground and especially the belowground impacts of A. halodendron on the growth and replacement of plant species along vegetation succession in the sandy regions. Moreover, future studies should also take the effective components of A. halodendron litters, their physiological properties and effects into account.
Conclusions
In line with our hypotheses, the present study indicated that the early-successional species rather than the late-successional species were negatively affected by the allelopathic effects of A. halodendron, and that the allelopathic effects on seed germination increase with increasing concentration of litter extracts, irrespective of litter types. Our study represented the first evidence that allelopathy may be a potentially important driving force during the early-successional stage and dune stabilization in the Horqin Sandy Land. A limitation of the present study is that we did not check whether other species (e.g. the late-successional species) have also negative allelopathic effects on A. halodendron, leading to the disappearance of A. halodendron in the late-successional stages (Figures 1 and 2). On the other hand, the disappearance of A. halodendron in the late-successional stages may also be a result of changed growth environment including dune stabilization and plant competition. Therefore, further studies are needed to better understand the successional process and thus to promote the vegetation restoration in that sandy dune region.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
YLuo and ZD designed the study. YLuo and ZY conducted the field trial. YLuo and ZY performed the laboratory analysis. YLuo, ZD, and M-HL were responsible for the statistical analyses. YLuo, ZD, ZY, XZ, and YLi wrote the original draft paper. YLuo, ZD, HJ, YY, and M-HL critically reviewed and edit the preliminary draft paper. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Key Research and Development Program of China (2017YFA0604803, 2016YFC0500907), Ecological and Security Key laboratory of Sichuan Province (ESP1708), National Natural Science Foundation of China (No. 31500369, 41807525, 41501227), and Sichuan Science and Technology Program (2018JY0086), China West Normal University Program (17E038), The Strategic Priority Research Program of Chinese Academy of Sciences (XDA24020304).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all members of the Naiman Desertification Research Station for their help in the field and the laboratory and for their valuable comments on our study.
References
Bhatt, M. V., Khandelwal, A., Dudley, S. A. (2011). Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. New Phytol. 189, 1135–1142. doi: 10.1111/j.1469-8137.2010.03548.x
Cheema, Z. A., Farooq, M., Wahid, A. (2013). Allelopathy: Current Trends and Future Applications (Berlin: Springer-Verlag).
Chou, C. H. (2006). “Introduction to allelopathy,” in Allelopathy: A Physiological Process with Ecological Implications. Eds. Manuel, J. R., Nuria, P., Luís, G. (the Netherlands: Springer, Dordrecht), 1–9.
Dudley, S. A., File, A. L. (2007). Kin recognition in an annual plant. Biol. Lett. 3, 435–438. doi: 10.1098/rsbl.2007.0232
Facelli, J. M. (1994). Multiple indirect effects of plant litter affect the establishment of woody seedlings in old fields. Ecology 75, 1727–1735. doi: 10.2307/1939632
Fahnestock, J. T., Povirk, K. L., Welker, J. M. (2000). Ecological significance of litter redistribution by wind and snow in arctic landscapes. Ecography 23, 623–631. doi: 10.1111/j.1600-0587.2000.tb00181.x
FAO (2006). FAO/IUSS Working Group WRB, World reference base for soil resources 2006. World Soil Resources Reports 103 (Rome: FAO).
Fernandez, C., Lelong, B., Vila, B., Mévy, J. P., Robles, C., Greff, S., et al. (2006). Potential allelopathic effect of Pinus halepensis in the secondary succession: an experimental approach. Chemoecology 16, 97–105. doi: 10.1007/s00049-006-0334-z
Hovstad, K. A., Ohlson, M. (2008). Physical and chemical effects of litter on plant establishment in semi-natural grasslands. Plant Ecol. 196, 251–260. doi: 10.1007/s11258-007-9349-y
Huang, G., Zhao, X. Y., Cui, J. Y., Su, Y. G. (2008). Photosynthetic and water use efficiency characteristics of two annuals under drought stress in Korqin Sandy Land. Acta Bot. Boreali-Occidentalia Sin. 28, 2306–2313.
Huang, W. D., Zhao, X. Y., Zhao, X., Zhao, H. L., Wang, S. K., Lian, J. (2011). A combined approach using ISSR and ITS analysis for the characterization of Artemisia halodendron from Horqin sandy land, northern China. Biochem. Syst. Ecol. 39, 346–351. doi: 10.1016/j.bse.2011.04.011
Li, F. R., Zhang, A. S., Duan, S. S., Kang, L. F. (2005). Patterns of reproductive allocation in Artemisia halodendron inhabiting two contrasting habitats. Acta Oecol. 28, 57–64. doi: 10.1016/j.actao.2005.02.005
Li, Y. Q., Zhao, X. Y., Chen, Y. P., Luo, Y. Q., Wang, S. K. (2012). Effects of grazing exclusion on carbon sequestration and the associated vegetation and soil characteristics at a semi-arid desertified sandy site in Inner Mongolia, northern China. Can. J. Soil Sci. 92, 807–819. doi: 10.4141/cjss2012-030
Li, K. L., Li, H. Y., Huangfu, C. H., Yang, D. L., Liu, H. M., Wang, H. (2016). Species-specific effects of leaf litter on seedling emergence and growth of the invasive Flaveria bidentis, and its co-occurring native species: a common garden test. Plant Ecol. 217, 1457–1465. doi: 10.1007/s11258-016-0657-y
Li, Y. L., Ning, Z. Y., Cui, D., Mao, W., Bi, J. D., Zhao, X. Y. (2016). Litter decomposition in a semiarid dune grassland: neutral effect of water supply and inhibitory effect of nitrogen addition. PloS One 11 (9), e0162663. doi: 10.1371/journal.pone.0162663
Lian, J., Zhao, X. Y., Li, X., Zhang, T. H., Wang, S. K., Luo, Y. Q., et al. (2017). Detecting sustainability of desertification reversion: vegetation trend analysis in part of the agro-pastoral transitional zone in Inner Mongolia, China. Sustain 9, 211. doi: 10.3390/su9020211
Liu, B., Liu, Z. M., Guan, D. X. (2008). Seedling growth variation in response to sand burial in four Artemisia species from different habitats in the semi-arid dune field. Trees 22, 41–47. doi: 10.1007/s00468-007-0167-6
Loydi, A., Donath, T. W., Eckstein, R. L., Otte, A. (2015). Non-native species litter reduces germination and growth of resident forbs and grasses: allelopathic, osmotic or mechanical effects? Biol. Invasions 17, 581–595. doi: 10.1007/s10530-014-0750-x
Luo, Y. Q., Zhao, X. Y., Li, M. X. (2012). Ecological effect of plant root exudates and related affecting factors: A review. Chin. J. Appl. Ecol. 23, 3496–3504.
Luo, Y. Q., Zhao, X. Y., Li, Y. Q., Wang, T. (2017). Effects of foliage litter of a pioneer shrub (Artemisia halodendron) on germination from the soil seedbank in a semi-arid sandy grassland in China. J. Plant Res. 130, 1013–1021. doi: 10.1007/s10265-017-0954-0
Ma, J. L., Liu, Z. M., Zeng, D. H., Liu, B. (2010). Aerial seed bank in Artemisia species: how it responds to sand mobility. Trees 24, 435–441. doi: 10.1007/s00468-010-0411-3
Mallik, A. U. (2003). Conifer regeneration problems in boreal and temperate forests with ericaceous understory: role of disturbance, seedbed limitation, and keystone species change. Crit. Rev. Plant Sci. 22, 341–366. doi: 10.1080/713610860
Mudrák, O., Frouz, J. (2012). Allelopathic effect of Salix caprea litter on late successional plants at different substrates of post-mining sites: pot experiment studies. Botany 90, 311–318. doi: 10.1139/b2012-005
Ninkovic, V. (2003). Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 54, 1931–1939. doi: 10.1093/jxb/erg192
Reader, R. J. (1993). Control of seedling emergence by ground cover and seed predation in relation to seed size for some old-field species. J. Ecol. 81, 169–175. doi: 10.2307/2261232
Samedani, B., Juraimi, A. S., Rafii, M. Y., Anuar, A. R., Sheikh Awadz, S. A., Anwar, M. P. (2013). Allelopathic effects of litter Axonopus compressus against two weedy species and its persistence in soil. Sci. World J. 2013, 695404. doi: 10.1155/2013/695404
Sayer, E. J. (2006). Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 81, 1–31. doi: 10.1017/S1464793105006846
Weltzin, J. F., Keller, J. K., Bridgham, S. D., Pastor, J., Allen, P. B., Chen, J. (2010). Litter controls plant community composition in a northern fen. Oikos 110, 537–546. doi: 10.1111/j.0030-1299.2005.13718.x
Wolkovich, E. M., Bolger, D. T., Cottingham, K. L. (2009). Invasive grass litter facilitates native shrubs through abiotic effects. J. Veg. Sci. 20, 1121–1132. doi: 10.1111/j.1654-1103.2009.01110.x
Zhang, C. L., Fu, S. L. (2009). Allelopathic effects of eucalyptus and the establishment of mixed stands of eucalyptus and native species. For. Ecol. Manage. 258, 1391–1396. doi: 10.1016/j.foreco.2009.06.045
Zhang, C. L., Fu, S. L. (2010). Allelopathic effects of leaf litter and live roots exudates of Eucalyptus species on crops. Allelopathy J. 26, 91–100.
Zhang, Y. J., Tang, S. M., Liu, K. S., Li, X. F., Huang, D., Wang, K. (2015). The allelopathic effect of Potentilla acaulis, on the changes of plant community in grassland, northern China. Ecol. Res. 30 (1), 41–47. doi: 10.1007/s11284-014-1203-9
Zhao, H. L., Zhao, X. Y., Zhang, T. H., Wu, W. (2003). Desertification Processes and Its Restoration Mechanisms in the Horqin Sand Land (Beijing: China Ocean Press).
Zhao, H. L., Zhao, X. Y., Zhang, T. H., Zhou, R. L. (2007). Bioprocess of Desertification and Restoration Mechanism of Degraded Vegetation (Beijing: Science Press).
Zhao, H. L., Zhou, R. L., Wang, J., Zhao, X. Y., Zhang, T. H. (2011). Desertification process and its mechanism of steppe vegetation in the Hulunbeir sandy steppe, Inner Mongolia. Arid Zone Res. 28 (4), 565–571.
Zhou, Z. J., Zhou, M. L., Liu, W. X. (2006). A study on ecological control of crofton weed by three different plants under different fertility conditions. China Agr. Sci. Bull. 22, 361–364.
Zuo, X. A., Zhao, X. Y., Zhao, H. L., Zhang, T. H., Guo, Y. R., Li, Y. Q., et al. (2009a). Spatial heterogeneity of soil properties and vegetation–soil relationships following vegetation restoration of mobile dunes in Horqin Sandy Land, Northern China. Plant Soil 318, 153–167. doi: 10.1007/s11104-008-9826-7
Zuo, X. A., Zhao, H. L., Zhao, X. Y., Guo, Y. R., Yun, J. Y., Wang, S. K., et al. (2009b). Vegetation pattern variation, soil degradation and their relationship along a grassland desertification gradient in Horqin Sandy Land, northern China. Environ. Geol. 58, 1227–1237. doi: 10.1007/s00254-008-1617-1
Keywords: allelopathic index, litter extracts, plant succession, seed germination, vegetation restoration
Citation: Luo Y, Du Z, Yan Z, Zhao X, Li Y, Jiang H, Yang Y and Li M-H (2020) Artemisia halodendron Litters Have Strong Negative Allelopathic Effects on Earlier Successional Plants in a Semi-Arid Sandy Dune Region in China. Front. Plant Sci. 11:961. doi: 10.3389/fpls.2020.00961
Received: 17 December 2019; Accepted: 11 June 2020;
Published: 25 June 2020.
Edited by:
Giovanna Battipaglia, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Monica Scognamiglio, University of Campania Luigi Vanvitelli, ItalyAnna De Marco, University of Naples Federico II, Italy
Copyright © 2020 Luo, Du, Yan, Zhao, Li, Jiang, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Du, ZHV6aG9uZ0BjaWIuYWMuY24=; Yongqing Luo, bHVveW9uZ3Fpbmc4NDAxQHNpbmEuY29t
 Yongqing Luo1*
Yongqing Luo1* Zhong Du
Zhong Du Xueyong Zhao
Xueyong Zhao Yue Yang
Yue Yang Mai-He Li
Mai-He Li