
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci., 08 May 2020
Sec. Plant Development and EvoDevo
Volume 11 - 2020 | https://doi.org/10.3389/fpls.2020.00542
This article is part of the Research TopicLinking Stomatal Development and Physiology: From Stomatal Models to Non-Model Species and CropsView all 15 articles
 Jingjing He1†
Jingjing He1† Ruo-Xi Zhang1†
Ruo-Xi Zhang1† Dae Sung Kim1
Dae Sung Kim1 Peng Sun1
Peng Sun1 Honggang Liu1
Honggang Liu1 Zhongming Liu1
Zhongming Liu1 Alistair M. Hetherington2
Alistair M. Hetherington2 Yun-Kuan Liang1*
Yun-Kuan Liang1*Elevated CO2 (eCO2) often reduces leaf stomatal aperture and density thus impacts plant physiology and productivity. We have previously demonstrated that the Arabidopsis BIG protein distinguishes between the processes of eCO2-induced stomatal closure and eCO2-inhibited stomatal opening. However, the mechanistic basis of this action is not fully understood. Here we show that eCO2-elicited reactive oxygen species (ROS) production in big mutants was compromised in stomatal closure induction but not in stomatal opening inhibition. Pharmacological and genetic studies show that ROS generated by both NADPH oxidases and cell wall peroxidases contribute to eCO2-induced stomatal closure, whereas inhibition of light-induced stomatal opening by eCO2 may rely on the ROS derived from NADPH oxidases but not from cell wall peroxidases. As with JA and ABA, SA is required for eCO2-induced ROS generation and stomatal closure. In contrast, none of these three signals has a significant role in eCO2-inhibited stomatal opening, unveiling the distinct roles of plant hormonal signaling pathways in the induction of stomatal closure and the inhibition of stomatal opening by eCO2. In conclusion, this study adds SA to a list of plant hormones that together with ROS from distinct sources distinguish two branches of eCO2-mediated stomatal movements.
Stomata formed by a pair of guard cells regulate gas exchanges between plants and the atmosphere. Guard cells sense and integrate both extra- and intracellular signals, such as light, temperature, carbon dioxide (CO2), plant hormones, leading to plant adaptive responses (Hetherington and Woodward, 2003; Murata et al., 2015; He and Liang, 2018). The continuing rise of atmospheric CO2 can profoundly impact plant physiology and crop yield potential via stomata, as elevated CO2 (eCO2) concentration in the atmosphere reduces leaf stomatal aperture and density in many species including crop plants (Woodward, 1987; Assmann, 1993; Keenan et al., 2013; Xu et al., 2016). Understanding CO2 signaling in guard cells is important in the context of breeding “climate change ready” crop varieties with improved agricultural performance and nutritional content (Kim et al., 2010; Myers et al., 2014; Caine et al., 2018; Zhang et al., 2018). In guard cells, CO2 signaling starts from CO2 conversion to bicarbonate (HCO3–) by βCA1 (beta Carbonic Anhydrase 1) and βCA4, followed by activation of MATE type transporter RHC1 (Resistance to High CO2), MPK4 (Mitogen-Activated Protein Kinase 4) and MPK12, subsequently leading to inhibition of HT1 (High Leaf Temperature 1), which phosphorylates and inactivates OST1 (Open Stomata 1). Repression of HT1 facilitates S-type anion channel activation by OST1 to mediate the anion effluxes resulting in stomatal closure (Hashimoto et al., 2006; Hu et al., 2010; Tian et al., 2015; Hashimoto-Sugimoto et al., 2016; Hõrak et al., 2016; Jakobson et al., 2016; Tõldsepp et al., 2018; Zhang et al., 2018).
As typified by the abscisic acid (ABA) receptors, the components in the stomatal closure induction and the stomatal opening inhibition are not necessarily the same (Assmann, 1993; Mishra et al., 2006; Yin et al., 2013; Dittrich et al., 2019). We have recently identified the Arabidopsis BIG protein as a novel component involved in eCO2-induced stomatal closure but not of eCO2-inhibited light-induced stomatal opening (He et al., 2018). BIG is involved in diverse processes including auxin transport, light and hormonal signaling, vesicle trafficking, endocytosis, phosphate deficiency tolerance, and the dynamic adjustment of circadian period (Li et al., 1994; Ruegger et al., 1997; Gil et al., 2001; Kanyuka et al., 2003; López-Bucio et al., 2005; Paciorek et al., 2005; Yamaguchi et al., 2007; Hearn et al., 2018). Mutations in the Arabidopsis BIG gene suppress eCO2-induced stomatal closure due to the disrupted activity of S-type ion channels (He et al., 2018). Direct channel regulation has been demonstrated to be insufficient to explain the strong eCO2-induced stomatal closing response in Arabidopsis (Wang et al., 2016). More recently, it has been shown that big mutants are more susceptible to bacterial pathogens that gain entry to the plant through stomata (Zhang et al., 2019). These findings point to the need to gain a better understanding of how BIG distinguishes two distinct processes of stomatal movement in response to eCO2. Given that reactive oxygen species (ROS) play a significant role in various signaling processes, and the results of investigations have revealed a role for BIG in redox signaling (Rhee et al., 2000; Gil et al., 2001; Grek et al., 2013; Song et al., 2014; Parsons et al., 2015; Zhang et al., 2019), we hypothesized that ROS production has a central role to play in defining stomatal responses to eCO2.
ROS including hydrogen peroxide (H2O2) and superoxide (O2–) are widely produced in different cellular compartments in plants and have been recognized as a major regulator in various aspects of plant life such as stomatal development and movement, particularly under different abiotic and biotic stress conditions (McAinsh et al., 1996; Neill et al., 2002; Foyer and Noctor, 2005; Song et al., 2014; Sierla et al., 2016). In Arabidopsis, apoplastic ROS are mainly produced by plasma membrane-localized NADPH oxidases and cell wall peroxidases (Song et al., 2014; Murata et al., 2015; Singh et al., 2017), and the activities of these different types of enzymes are strongly inhibited by diphenylene iodonium (DPI) and salicylhydroxamic acid (SHAM), respectively (Allan and Fluhr, 1997; Pei et al., 2000; Mori et al., 2001; Khokon et al., 2011; Miura et al., 2013). The evolution and maintenance of different sources for ROS production is most likely due to the requirement for intricate control of oxidative signaling, given the fact that ROS can be cytotoxic and mutagenic and for their proper function in signaling their production must be tightly regulated both temporally and spatially (Mittler, 2017).
ABA and jasmonate (JA) induce ROS accumulation in guard cells via the activities of two NADPH oxidases, RBOHD and RBOHF (Torres et al., 2002, 2006; Kwak et al., 2003; Suhita et al., 2004), whereas salicylic acid (SA) likely regulates ROS homeostasis via the peroxidases-catalyzed reactions (Mori et al., 2001; Khokon et al., 2011), and the inhibition of catalase and ascorbate peroxidase (Chen et al., 1993; Durner and Klessig, 1995). eCO2-induced stomatal closure is suppressed in the rbohDrbohF double mutants (Kolla et al., 2007; Chater et al., 2015). Peroxidases are bifunctional enzymes, through two possible catalytic cycles, hydroxylic and peroxidative, to generate or detoxify and regulate H2O2 levels. For example, during the hydroxylic cycle, the peroxidases catalyze the generation of ⋅OH and HOO⋅ from H2O2 by two different routes (Passardi et al., 2004). In Arabidopsis, there are 73 isoforms of cell wall peroxidases (Tognolli et al., 2002; Passardi et al., 2006). Two cell wall peroxidase-encoding genes, PRX33 and PRX34, which are highly and preferentially expressed in guard cells compared with other PRXs members according to Genevestigator (an available microarray database1), are widely involved in H2O2 production against fungi-, bacteria-, SA-, and flg22-induced stomatal closure (Bindschedler et al., 2006; Daudi et al., 2012; O’Brien et al., 2012a,b; Arnaud et al., 2017). Notably, SA-mediated ROS production and stomatal closure are not impaired by DPI or in rbohDrbohF double mutant (Khokon et al., 2011). In contrast to NADPH oxidases, the importance of ROS-producing peroxidases to plant adaptive responses, particularly their function in regulating eCO2-mediated stomatal movement, has largely been overlooked.
In this study, by combining pharmacological and genetic approaches, we reveal distinct roles of ROS-producing peroxidases and NADPH oxidases for eCO2-induced stomatal movements. We also found that endogenous SA and SA-signaling components are required for eCO2-induced stomatal closure. Neither ABA, JA, or SA are involved in regulating eCO2-inhibited stomatal opening. In conclusion, our data suggest that plant hormones and ROS from distinct sources selectively mediate different stomatal CO2 responses, and shed new light on ROS action and the CO2 signaling network.
All Arabidopsis (Arabidopsis thaliana L.) lines used in this study were in the Columbia background (Col-0). Seeds of sid2-2, npr1-1, npr3npr4, and rbohDrbohF were kindly provided by Drs Shunping Yan and Honghong Hu (Huazhong Agricultural University, China). Seeds of prx33-3 and prx34-2 was a gift from Dr. Ildoo Hwang (Pohang University of Science and Technology, Korea). More information of the mutants used in this study are shown in Supplementary Table S1. Seed germination and plant growth were essentially carried out as described in He et al. (2018). For stomatal aperture bioassays, seeds were surface-sterilized and sown on half-strength Murashige and Skoog (MS) medium plates containing 0.6% agar and 1% sucrose. After stratification (4°C in the dark for 2 days), the plates were transferred to the green house at 22°C/18°C (day/night) with 10 h/14 h (light/dark) photoperiod cycle (light intensity 120 μ moles photons m–2s–1), 50% relative humidity, at ambient CO2, approximate 450 ppm. Ten days old plants were transferred to soil and grown in the same green house for the future experiments. For the stomatal bioassays, 4–5 weeks old plants were used.
For elevated CO2-induced stomatal closure, abaxial epidermis of fully expanded leaves were detached and incubated for 2.5 h under 150 μmol m–2s–1 light in 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C whilst being aerated with CO2-free air by bubbling through the buffer solution to bring about stomatal opening, and then either aerated with CO2-free air or elevated CO2 (800 ppm) for additional 2.5 h before peels were removed, mounted on slides and stomatal aperture measurements were recorded using an inverted microscope (Olympus BX51), fitted camera (Olympus DP70), and ImageJ software v. 1.43u (NIH). For inhibition of stomatal opening, epidermal peels of abaxial epidermis floated on the 10 mM MES/KOH (pH 6.20) in 6 cm dishes at 22°C for 1 h under the dark, then directly transferred to fresh dishes and incubated for 3 h under light of 150 μmol m–2s–1 in 50 mM KCl, 10 mM MES/KOH (pH 6.15) at 22°C either aerated with CO2-free air or elevated CO2 (800 ppm) by bubbling directly into the buffer.
Details of the DPI, SHAM and Tiron treatments were as follows: The ROS scavenger Tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid) (Sigma-Aldrich, United States) was dissolved in water and used at a final concentration of 10 mM, ROS inhibitor DPI (diphenyl iodonium chloride) (Sigma-Aldrich, United States) was dissolved in DMSO and used at a final concentration of 20 μM, SHAM (salicylhydroxamic acid) (Sigma-Aldrich, United States) was dissolved in ethanol and used at a final concentration of 2 mM, these chemicals were added 30 min prior to the addition of 800 ppm CO2. The highest concentration of DMSO or ethanol that was used was added to the zero treatments as a control. To avoid experimenter bias, all the aperture measurements were performed blind. Forty or sixty stomatal apertures were measured per treatment and measurements from two replicates of each treatment were pooled and analyzed by GraphPad Prism 8.0.2 (GraphPad).
2′,7′-Dichlorofluorescein diacetate (H2DCF-DA) (Sigma-Aldrich, United Kingdom) fluorescence was used as a measure for ROS levels as previously described (Chater et al., 2015). Briefly, epidermal peels from treated leaves were incubated in 50 mM KCl, 10 mM MES/KOH (pH 6.15) buffer in the presence of 50 μM H2DCF-DA for 10 min at 22°C in darkness. Epidermal strips were washed with 50 mM KCl, 10 mM MES/KOH (pH 6.15) buffer at room temperature. Subsequently, the fluorescence in guard cells was detected using TCS-SP8 confocal laser scanning microscope (Leica lasertechnik GmbH, Heidelberg, Germany). The fluorescent intensities of each image were analyzed using Photoshop 7.0 (ASI). At least fifty guard cell pairs were measured per experiment and analyzed by GraphPad Prism 8.0.2 (GraphPad). To avoid experimenter bias, all the fluorescent intensities measurements were performed blind. Each experiment was done at least three independent times with similar results.
Total RNA from aerial parts of the plants was extracted using RNeasy® total RNA mini kit (Qiagen) followed by plant genomic DNA digestion with RNase-free DNase I (Thermo scientific) according to the manufacturer’s instructions. The absence of genomic DNA contamination was confirmed by PCR using RNA as template without reverse transcription. First strand cDNA was synthesized using Superscript II® reverse transcriptase (Invitrogen) and oligo d(T)15–18 (Promega) mRNA primer with 1 μg of total RNA as the template. cDNA corresponding to 20 ng of total RNA and 300 nM of each primer were used in PCR reactions. Primer sequences used for RT-PCR and quantitative RT-PCR are listed in Supplementary Table S2. Experiments on independently grown plant material were carried out three times and data analyzed by GraphPad Prism 8.0.2 (GraphPad).
The data were statistically analyzed using GraphPad Prism 8.0.2 (GraphPad). The effects of CO2 and chemical treatments as well as their interactions on variables were analyzed using analysis of variance (ANOVA). Differences between treatments were considered significant when the P-value was less than 0.05 by Tukey’s test.
To test the hypothesis that ROS production has a central role to play in defining stomatal CO2 responses, we started by monitoring ROS levels in the big mutant and wild-type Col-0 (WT) plants using the fluorescence of H2-DCFDA. As shown in Supplementary Figure S1A, the application of eCO2 (800 ppm) resulted in rapid enhancement of fluorescence in WT guard cells, whereas the increases of ROS were greatly reduced in all big mutant alleles examined, including big-1, doc1-1, and big-j588 (Supplementary Figure S1A), consistent with the compromised eCO2-induced stomatal closure (He et al., 2018). Strikingly, during eCO2 inhibited light-induced stomatal opening, we observed comparable increases of ROS levels in the guard cells of the big mutant and WT plants (Supplementary Figure S1B), in line with previous results (He et al., 2018). These data suggest that CO2-stimulated stomatal closure and inhibition of light-induced opening both employ an increase in ROS. This suggests that the guard cells might employ different mechanisms to discriminate the types and strength of ROS signals and thereby finely tune stomatal movements in response to eCO2.
The functioning of NADPH oxidases RBOHD and RBOHF in eCO2-induced stomatal closure has been well documented (Kolla et al., 2007; Chater et al., 2015; Geng et al., 2016), while the function of cell wall peroxidases in CO2 signaling remains to be investigated. Figures 1A,B show that eCO2 caused an average 25% reduction in stomatal apertures, whereas this reduction was efficiently abolished by either NADPH oxidases inhibitor DPI or cell wall peroxidases inhibitor SHAM. Around 30% extra ROS were induced by eCO2 treatments, but peels pre-treated with DPI or SHAM failed to exhibit significant ROS accumulation during eCO2 treatment (Figures 1C,D). These results suggest that cell wall peroxidases function in eCO2-induced stomatal closure. We next examined the stomatal CO2 responses in prx33-3 and prx34-2 using the rbohDrbohF double mutants as a positive control. Similar to rbohDrbohF, stomatal apertures of both prx33-3 and prx34-2 mutant lines failed to close in response to eCO2 (Figure 1E). In line with this observation, ROS accumulation was not triggered by eCO2 in the prx33-3, prx34-2, or rbohDrbohF mutants in marked contrast to an over 50% ROS increase in WT (Figure 1F). These data not only support the notion that CO2-induced stomatal closure is dependent on ROS (H2O2) production (Kolla et al., 2007; Chater et al., 2015; Geng et al., 2016), but also demonstrate an essential role of cell wall peroxidases including PRX33 and PRX34 in response to eCO2, shedding new light on ROS action in plants. Furthermore, as with two eCO2 inducible genes, SLAC1 and OST1 (Shi et al., 2015; Dittrich et al., 2019), expressions of RBOHD, RBOHF, PRX33, and PRX34 were upregulated by eCO2 (Supplementary Figure S2), further corroborating our view that both NADPH oxidases and cell wall peroxidases function in guard cell eCO2 signaling.
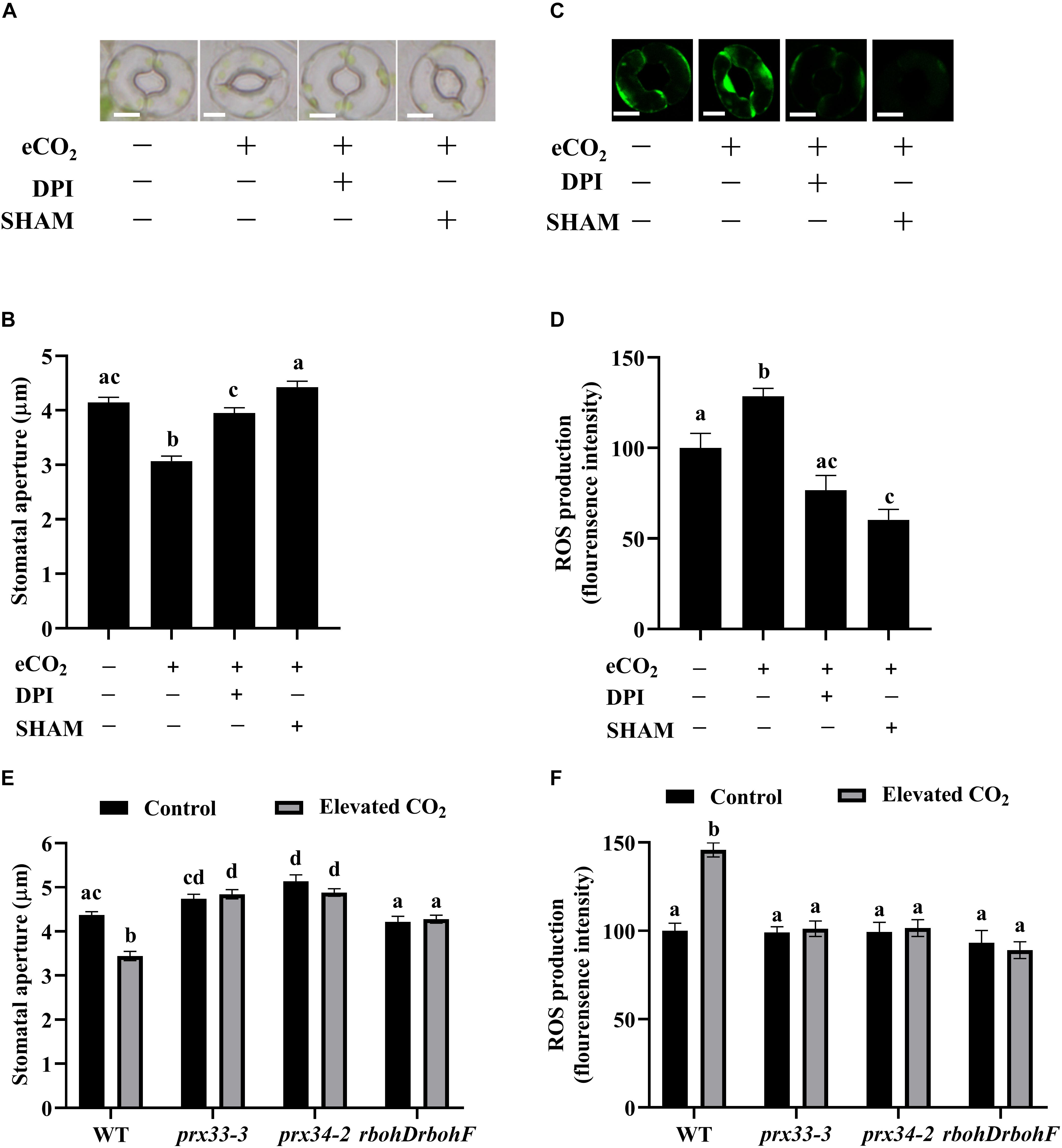
Figure 1. Cell wall peroxidases and NADPH oxidases are required for elevated CO2-induced stomatal closure. (A) eCO2-induced stomatal closure is inhibited by ROS inhibitors DPI and SHAM. Representative images showing guard cells of WT: after 2.5 h light-incubation, epidermal peels of WT plants were treated with 800 ppm CO2 for another 2.5 h before photos taken. 20 μM DPI or 2 mM SHAM added before CO2 treatment for 30 min. Scale bar, 5 μm. (B) Quantitative stomatal aperture from (A). (C) Representative images showing H2DCF-DA fluorescence of WT guard cells under control (CO2-free air) and elevated (800 ppm) CO2 with or without ROS inhibitors DPI or SHAM treatment. Scale bar, 5 μm. (D) Quantitative ROS production from (C). eCO2 stimulates an increase of H2DCF-DA fluorescence in guard cells that is blocked in the presence of DPI/SHAM. (E) eCO2-induced stomatal closure is disrupted in prx33-3, prx34-2, and rbohDrbohF mutants. (F) eCO2-induced ROS production in guard cells is compromised in prx33-3, prx34-2, and rbohDrbohF mutants during stomatal closure. In (B) (n = 120), (D) (n = 50), (E) (n = 80), and (F) (n = 60), values are means ± s.e. All experiments were repeated at least three times. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
eCO2-induced stomatal closure and the inhibition of light-induced stomatal opening by eCO2 are two separate processes (He et al., 2018). Figure 1 shows that eCO2-induced stomatal closure requires ROS from both NADPH oxidases and cell wall peroxidases. In eCO2-inhibited light-induced stomatal opening, eCO2 suppressed opening induced by 36% and this was associated with an approximate 60% greater increase in ROS accumulation compared to mock treated plants (Figures 2A,B). eCO2-inhibited light induced stomatal opening was virtually abolished by Tiron, a potent ROS scavenger (Figure 2A; Yamada et al., 2003). Consistently, the eCO2-induced ROS accumulation was inhibited by Tiron (Figure 2B). Together, these data support the hypothesis that ROS production is indispensable to eCO2-mediated inhibition of stomatal opening. DPI dampened stomatal opening inhibition presumably by blocking eCO2-induced ROS increase, as in the presence of DPI, a 24% reduction in stomatal aperture accompanied with a slight while statistically insignificant increase (14%) of ROS production was observed (Figures 2A,B). However, neither eCO2-inhibited stomatal opening nor eCO2-induced ROS accumulation was compromised by SHAM (Figures 2A,B). The inhibition of stomatal opening by eCO2 required ROS accumulation which might be dependent on NADPH oxidases but less likely on cell wall peroxidases. These data suggest that ROS from distinct sources differentially modulate eCO2-triggered stomatal movements. Importantly, when the rbohDrbohF, prx33-3, and prx34-2 and WT plants were analyzed, we observed similar eCO2-inhibited stomatal opening and guard cell ROS accumulations (Figures 2C,D), suggesting RBOHD, RBOHF, PRX33, and PRX34 are unlikely to be involved in the inhibition of stomatal opening by eCO2. On the basis of our results we conclude that sources of ROS, other than those described above, must be involved in eCO2-inhibited stomatal opening.
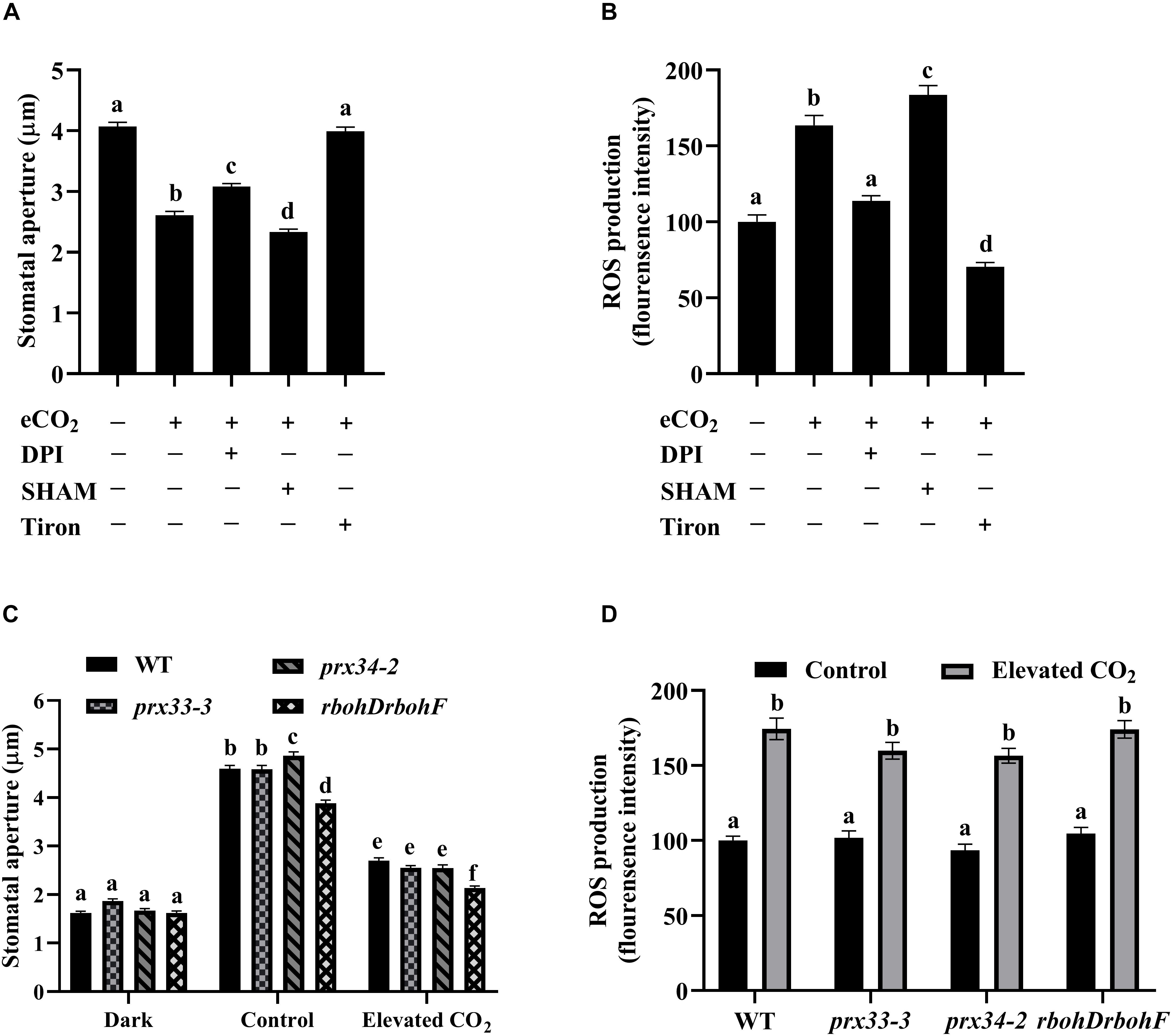
Figure 2. The inhibition of light-induced stomatal opening by eCO2 requires ROS generation. (A) eCO2-inhibited stomatal opening is compromised by treatment with Tiron. Stomatal apertures were measured on light-incubated epidermal peels treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for 3 h. DPI, SHAM, and Tiron added before light treatment for 30 min. (B) eCO2 stimulates an increase of H2DCF-DA fluorescence in guard cells that is blocked in the presence of DPI and Tiron. (C) eCO2-inhibited stomatal opening in prx33-3, prx34-2, and rbohDrbohF mutants is similar to WT. Stomatal apertures were measured on illuminated epidermal peels treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for 3 h. Dark represents 1 h dark-adapted stomata incubated in the 10 mM MES/KOH (pH 6.20) buffer. (D) eCO2 stimulates an increase in guard cells of H2DCF-DA fluorescence in WT as well as in prx33-3, prx34-2, and rbohDrbohF mutants. Mean fluorescence intensity was measured on light-incubated epidermal peels treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for 3 h. In (A) (n = 120), (B) (n = 60), (C) (n = 120), and (D) (n = 60), values are mean ± s.e. All experiments were repeated at least three times. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
SA can modulate plant growth, development and responses to a wide range of biotic and abiotic stresses. To determine whether SA participates in eCO2-induced stomatal closure, we measured stomatal apertures and ROS production using SA-deficient mutant sid2-2 (SA Induction-Deficient 2) after eCO2 treatments. While stomatal apertures of WT were reduced by about 10%, no significant reduction of stomatal apertures was detected in sid2-2 by eCO2 application (Figure 3A). Additionally, we tested npr1-1, npr3npr4 mutants because NPR1, NPR3, and NPR4 are key components of SA signaling (Fu et al., 2012; Wu et al., 2012; Kuai et al., 2015; Ding et al., 2018). In contrast to a nearly 20% reduction of stomatal apertures in WT, npr1-1 and npr3npr4 mutants displayed no appreciable eCO2-induced stomatal closure (Figure 3B). Consistently, eCO2-induced ROS accumulation in guard cells was completely abolished in sid2-2, npr1-1 as well as in npr3npr4 (Figures 3C,D and Supplementary Figure S3). These results indicate that eCO2-induced stomatal closure requires an intact SA signaling pathway, and both SA biosynthesis and SA signaling are involved in eCO2-induced ROS production.
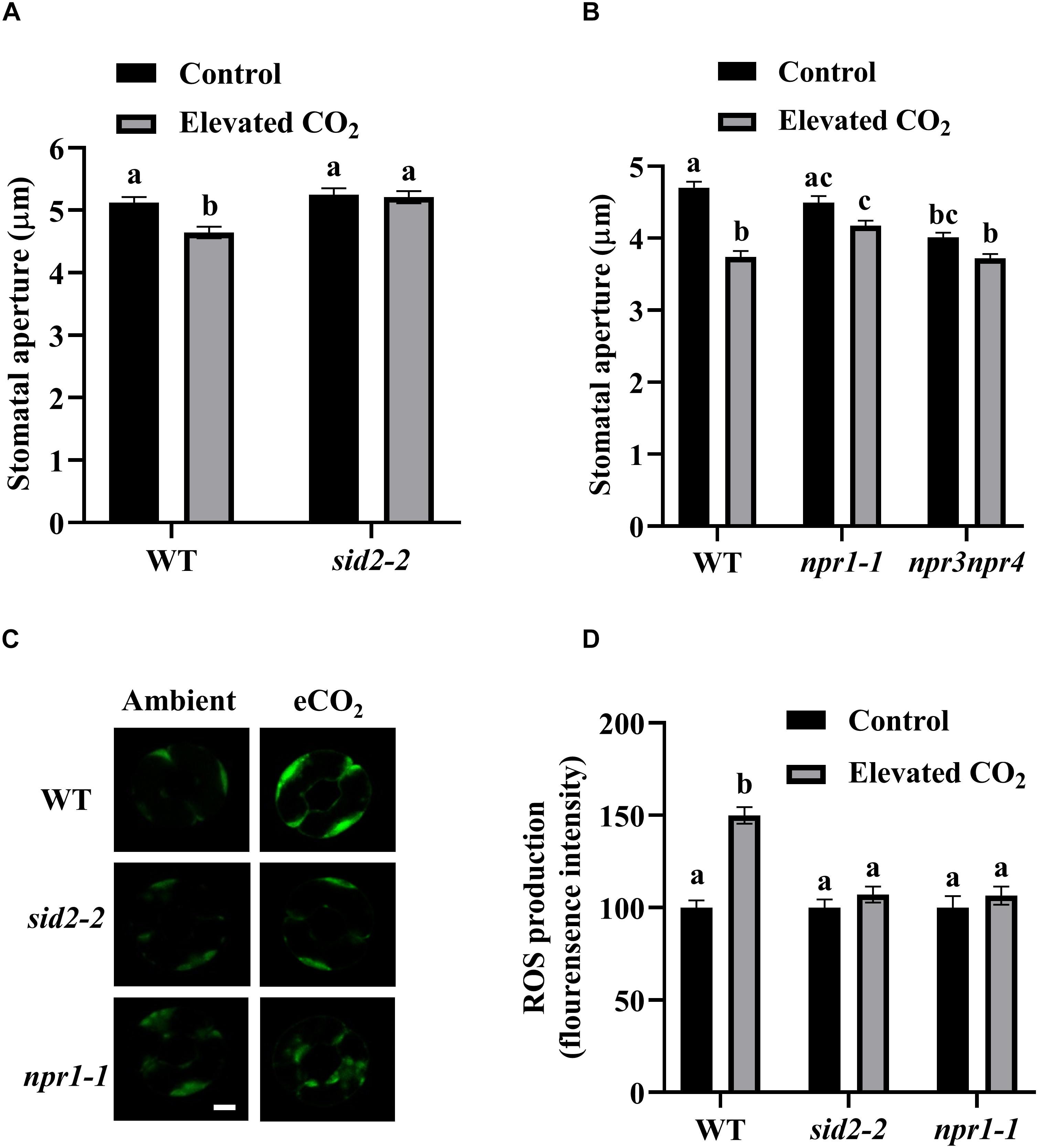
Figure 3. eCO2-induced stomatal closure requires SA and SA signaling. (A) eCO2-induced stomatal closure is corrupted in sid2-2 mutants. (B) eCO2-induced stomatal closure is disrupted in npr1-1 and npr3npr4 mutants. (C) Representative images showing H2DCF-DA fluorescence of WT, sid2-2 and npr1-1 mutants guard cells under control (CO2-free air) and elevated (800 ppm) CO2. Scale bar, 5 μm. (D) eCO2 stimulates an increase of H2DCF-DA fluorescence in WT guard cells, but is blocked in sid2-2 and npr1-1 mutants. Mean fluorescence intensity was measured on 2.5 h light-preincubated epidermal peels, treated with 800 ppm CO2 for another 2.5 h. Stomatal apertures in (A,B) were measured on 2.5 h light-preincubated epidermal peels, treated with 800 ppm CO2 for another 2.5 h. In (A) (n = 120), (B) (n = 120), and (D) (n = 60), values are mean ± s.e. All experiments were repeated at least three times. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
As shown in Figure 4A, stomata of WT and sid2-2, npr1-1, and npr3npr4 exhibited a similar degree of closure as WT after 1 h dark treatment. Light-induced stomatal opening in sid2-2 and npr3npr4 was similar to WT while apertures of npr1-1 were consistently larger than WT (Figure 4A). When treated with eCO2, the reduction in stomatal aperture of either sid2-2 (48%) or npr3npr4 (43%) was similar to that of WT (47%), indicating that eCO2-inhibited stomatal opening was not compromised in sid2-2 and npr3npr4, but partially impaired in npr1-1 (31% reduction) (Figure 4A). Based on these results we conclude that SA biosynthesis and SA signaling play no significant role in eCO2-inhibited light-induced stomatal opening.
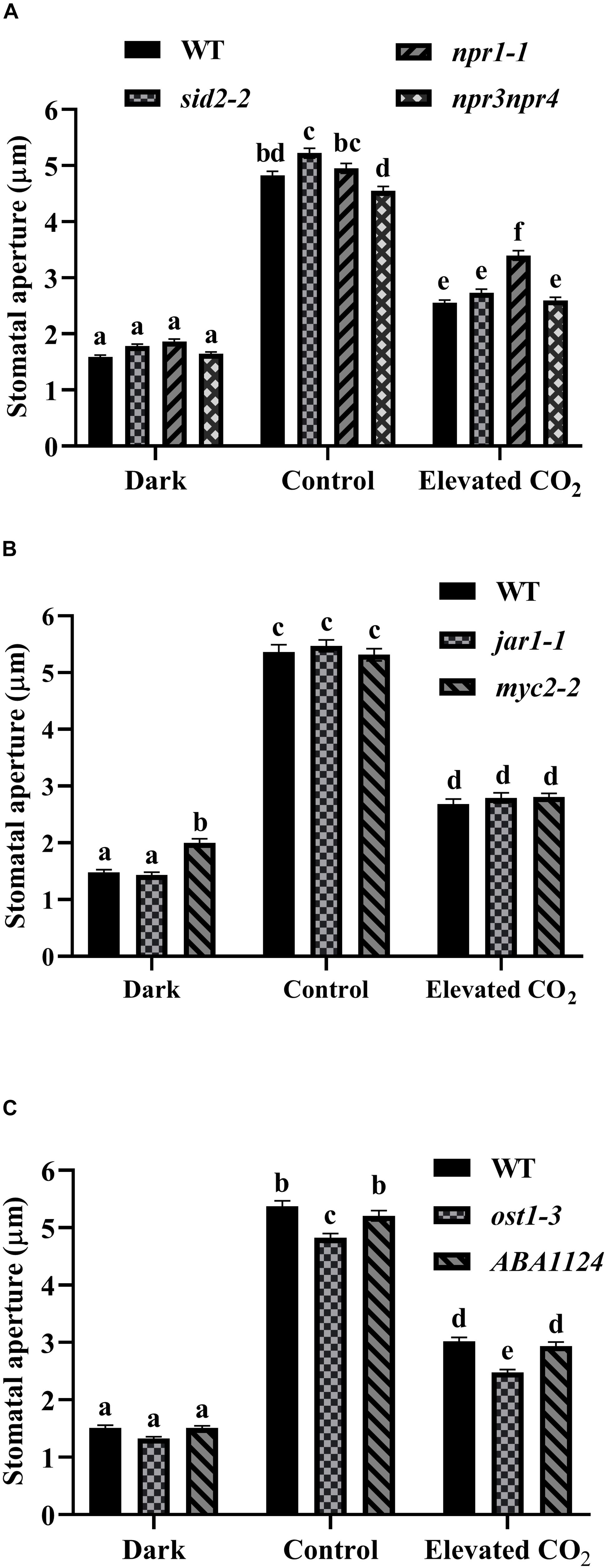
Figure 4. SA, JA, and ABA are not required for the inhibition of light-stimulated stomatal opening by eCO2. (A) eCO2-inhibited stomatal opening in sid2-2, npr1-1, and npr3npr4 mutants is similar to WT. (B) eCO2-inhibited stomatal opening in jar1-1 and myc2-2 mutants is similar to WT. (C) eCO2-inhibited stomatal opening in pyr1pyl1pyl2pyl4 (ABA1124) and ost1-3 mutants is similar to WT. Stomatal apertures were measured on light-incubated epidermal peels treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for 3 h. Dark represents 1 h dark-adapted stomata incubated in the 10 mM MES/KOH (pH 6.20) buffer. In (A–C) (n = 120), the shown result is a representative of three independent biological experiments, values are mean ± s.e. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
We next examined the involvement of ABA and JA signaling which has been reported to be essential for eCO2-induced stomatal closure (Chater et al., 2015; Geng et al., 2016; Hsu et al., 2018) in eCO2-inhibited stomatal opening. First we verified that JA pathway deficient mutants coi1-1 and jar1-1 are insensitive to eCO2-induced stomatal closure (Supplementary Figure S4). These data confirmed the results of Geng et al. (2016). myc2-2, a loss-of-function mutant line of MYC2, which is a master regulator of JA signaling, and jar1-1, behaved similarly to WT (47, 49, 50% reduction of stomatal aperture, respectively) in eCO2-inhibited light-induced stomatal opening (Figure 4B). Likewise, both the quadruple ABA receptor mutant pyr1pyl1pyl2pyl4 (ABA1124) and ost1-3 exhibited wild type (44, 49, 44% reduction of stomatal aperture, respectively) responses to eCO2-inhibited stomatal opening (Figure 4C). Taken together, it appears that ABA and JA signaling pathway are not directly involved in eCO2-inhibited light-induced stomatal opening.
An increase in guard cell ROS, including H2O2 in response to diverse stimuli is one of the first measurable events in stomatal movements. H2O2 production mainly depends on two types of enzymes in guard cells, one is NADPH oxidases and the other is cell wall peroxidases (Murata et al., 2015). Similar to the bacterial pathogen-associated molecular patterns (PAMPs), flagellin (flg22) that induces stomatal closure, eCO2-induced stomatal closure requires both NADPH oxidases- and cell wall peroxidases-generated ROS (Figure 1; O’Brien et al., 2012b). Intriguingly, when we analyzed RBOHD, RBOHF, PRX33, and PRX34 expression levels in rbohDrbohF, prx33, and prx34 mutant plants, we found that loss of function of any individual gene had no detectable effects on the expression of the other genes (Supplementary Figure S5). In addition, the disruption of one gene is not compensated by other functional ROS generation related genes, indicating there is no feedback and/or counterbalancing regulations among the expressions of NADPH oxidases and cell wall peroxidases. This is consistent with the observation that the cytokinin analog trans-zeatin induces stomatal closure and ROS accumulation in guard cells involving the apoplastic PRXs PRX4, PRX33, PRX34, and PRX71, but not the NADPH oxidases RbohD and RbohF (Arnaud et al., 2017). Thus, it is highly possible that NADPH oxidases and cell wall peroxidases function independently to generate ROS during eCO2-/PAMP-induced stomatal closure. More dedicated experiments including the evaluation of the possible additive effects on ROS production between prx33/34 and rbohD/F mutants will be needed to further assess this interpretation. Notably, it has been quantitatively determined that peroxidases are responsible for half of the ROS produced in response to PAMPs, while the other half is produced by NADPH oxidases and/or mitochondrial and chloroplastic ROS sources (O’Brien et al., 2012b).
In contrast to eCO2-induced stomatal closure, eCO2-inhibited stomatal opening was only partially blocked by DPI treatment but not by SHAM. These results are in line with the insights we got from working with BIG (Supplementary Figure S1), namely, that guard cells employ different mechanisms to discriminate the types and strength of ROS signals in order to, presumably, finely tune stomatal movements in response to eCO2. Interestingly, a recent paper reported that neither DPI nor SHAM reduced the high level of ROS in the atg2 mutant, which is compromised in light- and low CO2-induced stomatal opening (Yamauchi et al., 2019). While both eCO2-inhibited stomatal opening and ROS accumulation could be entirely abrogated by Tiron, the inhibition of stomatal opening by eCO2 remains functional in the rbohDrbohF double mutant (Figure 2). This suggests that other ROS sources, which are inhibited by Tiron but not by DPI function in eCO2-inhibited light-induced stomatal opening. Nitric oxide (NO) which plays a role in stomatal movement (Neill et al., 2003; Laxalt et al., 2016) has been identified to be required for eCO2-induced stomatal closure in tomato (Shi et al., 2015). Evidently, NO production might also contribute to eCO2-triggered stomatal movement in Arabidopsis. In addition to NADPH oxidases and cell wall peroxidases, the polyamine oxidases (PAOs) catalyze catabolism of spermidine and spermine with concomitant production of H2O2 (Pottosin et al., 2014; Sierla et al., 2016). An inhibitor of PAOs interferes with ABA-induced stomatal closure in French bean and ethylene-induced stomatal closure in Arabidopsis (An et al., 2008; Hou et al., 2013). Whether and how PAOs contribute to the ROS accumulations that drive eCO2-reguated stomatal movement remains to be investigated. Another possibility is that other members of the NADPH oxidase family function in guard cell signaling in response to eCO2. In Arabidopsis, there are 10 members of the RBOH family. When the spatiotemporal expression profile of all RBOH members is examined using ePlant2, it is apparent that, in addition to RBOHD and RBOHF, RBOHC is highly expressed in guard cells (Supplementary Figure S6), suggesting a regulatory role of RBOHC in stomatal function. This suggestion is supported by work from Wei et al. (2018) who provided evidence that the activity of RBOHC is required for melatonin-induced stomatal closure and ROS production. It will be interesting to investigate whether RBOHC is involved in eCO2-induced stomatal movement.
Apoplastic ROS are known to regulate stomatal movement, however they are sensed and transduced is not well understood. One possibility is that apoplastic ROS are sensed by yet to be characterized extracellular sensors and subsequently transduced by unknown intracellular pathways (Sierla et al., 2016). Alternatively, apoplastic ROS such as H2O2 can be transported into the cytoplasm via aquaporins (Tian et al., 2016), as exemplified by the aquaporin PIP2;1 which is required for ABA and flg22-induced H2O2 accumulation in guard cells (Rodrigues et al., 2017). Moreover, ROS can directly modify the activity of ion channels leading to stomatal closure (Pei et al., 2000). Equally possible, however, is that ROS function through parallel mechanisms to promote CO2 signaling in guard cells.
Changes in SA concentration after pathogen infection affect the redox state of the cell and bring about a conformational switch of NPR1 (Cao et al., 1994) and thereby activate PR genes (Chen et al., 1993; Vanacker et al., 2000; Noctor et al., 2002; Mou et al., 2003). eCO2 can induce SA production and activate SA signaling in many plant species (Matros et al., 2006; Casteel et al., 2012; Huang et al., 2012; Zhang et al., 2015; Mhamdi and Noctor, 2016; Williams et al., 2018). Our observation that eCO2-induced stomatal closure requires endogenous SA and SA-signaling components supports a proposed link between SA and CO2 signaling in guard cells response (Medina-Puche et al., 2017). This is in line with several reports that SABP3 (SA-binding protein 3), a chloroplast carbonic anhydrase (CA), which exhibits both CA enzymatic and SA-binding activities is indispensable to SA-mediated defense response in tomato as well as in Arabidopsis (Slaymaker et al., 2002; Wang et al., 2009). Also, NPR1 and NRB4 (Non-recognition of BTH 4, another SA signaling component) interact with βCA1 (Medina-Puche et al., 2017). In addition, it is known that the βca1βca4 double mutant compromises CO2 sensing (Hu et al., 2010). These, together with the fact that the quintuple mutant βca1βca2βca3βca4βca6 shows reduced sensitivity to SA, suggest that CAs likely function in modulating the perception of SA in plants (Medina-Puche et al., 2017). Although NPR1 and NPR3/NPR4 play opposite roles in transcriptional regulation, they all function in a SA-dependent manner for plant immune responses as NPR1, NPR3, and NPR4 are SA-binding proteins (Ding et al., 2018). Nevertheless, both npr1-1 and npr3npr4 are insensitive to eCO2-induced stomatal closure (Figure 3), in line with the finding that the double mutant npr3npr4 is defective in systemic acquired resistance (SAR) (Fu et al., 2012), suggesting disruption in different aspects of SA signaling components might consequently affect eCO2-induced stomatal closure. Interestingly, eCO2-inhibited stomatal opening was partially compromised only in npr1-1 but not in sid2-2 or npr3npr4 (Figure 4A). It is assumed that selective SA-binding to NPR1 and NPR3/NPR4 could differentially affect eCO2-inhibited stomatal opening. Alternatively, SA-independent NPR1 function in ER (endoplasmic reticulum) stress has been reported recently (Lai et al., 2018), thus NPR1 might function in a SA-independent manner during eCO2-inhibited stomatal opening. PRX33 and PRX34 play a significant role in SA-mediated stomatal closure (Arnaud et al., 2017). Our observation that SA signaling pathway functions in eCO2-induced stomatal closure rather than in eCO2-inhibited stomatal opening (Figures 3, 4), is in accordance with that cell wall peroxidases differentially mediate eCO2-regulated stomatal movement (Figures 1, 2), implicating that SA regulates eCO2 inhibition of stomatal closure via the activities of the peroxidases, which needs to be assessed in more details in the future, for example, by examining the expression changes of PRX33 and PRX34 in response to eCO2 in the SA mutants using RBOHD and RBOHF as experimental controls.
Multiple lines of evidence support a requirement of ABA for perceiving CO2 concentration changes by stomata (Raschke, 1975; Webb and Hetherington, 1997; Merilo et al., 2013; Chater et al., 2015; Hsu et al., 2018). Recently, Dittrich et al. (2019) have further demonstrated that ABA receptors PYL4 and PYL5 are key to CO2-induced stomatal closure. JA and SA signaling pathways are often mutually antagonistic, which can be induced simultaneously under eCO2 and intracellular oxidative stresses (Han et al., 2013a, b; Mhamdi and Noctor, 2016; Williams et al., 2018). The present study shows that both SA and JA are required for mediating stomatal closure by eCO2 (Figure 3 and Supplementary Figures S5), in line with the emerging evidences that SA, JA, ABA and ROS signaling are important in linking CO2 availability, stomatal function and the activation of plant defense responses (Li et al., 2014; Geng et al., 2016; Mhamdi and Noctor, 2016; Zhou et al., 2017; Williams et al., 2018). To further substantiate these findings, the contents of SA, JA and ABA need to be monitored in the future experiments. However, there is no evidence that eCO2 brings about an elevation of ABA (Chater et al., 2015; Hsu et al., 2018). ABA and JA can induce ROS accumulation in guard cells via the activities of RBOHD and RBOHF, whereas SA regulates ROS homeostasis via the peroxidases-catalyzed reactions (Murata et al., 2015). ABA, JA and SA are known to be required for eCO2-induced stomatal closure. However, our data indicate that none of these three hormones plays major roles in eCO2-inhibited stomatal opening, a process that is dependent on ROS generation, reflecting a similar mechanism in O3-induced ROS stress responses which are independent on SA, JA and ethylene signals (Xu et al., 2015).
In this study, by investigating ROS accumulation and stomatal movement in response to eCO2, we demonstrated that both cell wall peroxidases and NADPH oxidases are required for ROS production during eCO2-mediated stomatal closure, whereas eCO2-inhibited stomatal opening might be dependent on NADPH oxidases but not on cell wall peroxidases (Figure 5). The data presented here indicate that eCO2-inhibited light-stimulated stomatal opening requires ROS. However, our data suggest that distinct sources of ROS other than NADPH oxidases and PRXs play vital roles in stomatal opening inhibition by eCO2. Furthermore, we show that as with JA and ABA, SA signals are required for eCO2-induced stomatal closure and ROS generation. None of these three hormones has a significant role in eCO2-inhibited stomatal opening. Taken together, these results suggest that ROS from distinct sources and various plant hormones differentially regulate eCO2-induced stomatal movement.
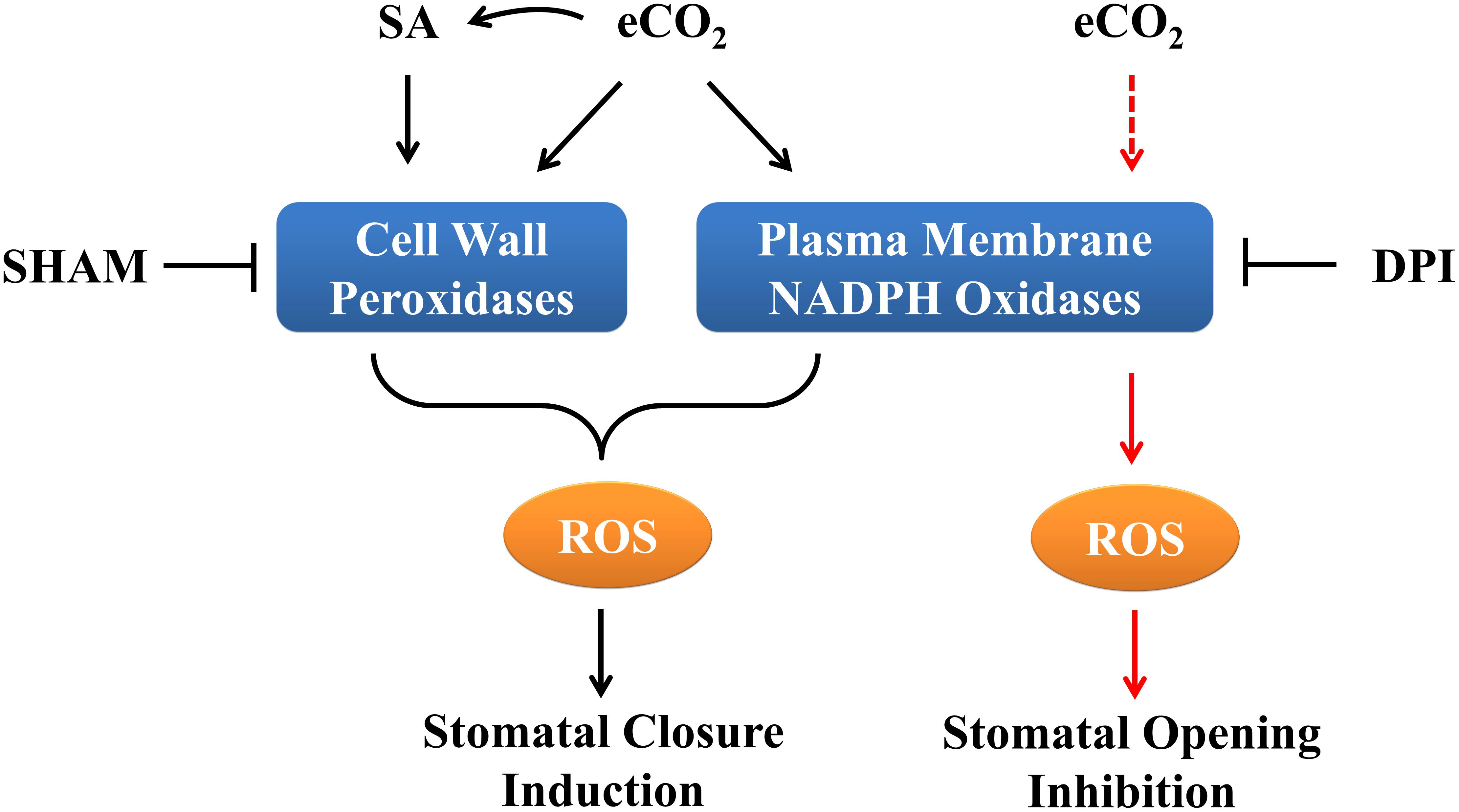
Figure 5. Schematic diagram of ROS accumulation and stomatal movement in response to eCO2 in Arabidopsis. In terms of cell wall peroxidases and NADPH oxidases it appears both of which function in eCO2-induced stomatal closure. SA signaling is required for eCO2-regulated stomatal closure, while eCO2-inhibited stomatal opening may only depend on NADPH oxidases mediated ROS generation. Black lines signify induced stomatal closure and red lines signify inhibited stomatal opening. Solid lines indicate verified interactions; dashed lines indicate hypothetical interactions.
All datasets generated for this study are included in the article/Supplementary Material.
Y-KL conceived the research. JH, R-XZ, DK, PS, HL, and ZL conducted experiments. JH, R-XZ, AH, and Y-KL analyzed data and wrote the manuscript with the support of DK, PS, and ZL. All authors read and approved the manuscript.
This work was supported by the National Natural Science Foundation of China under Grant No. 31770282 and 31470360 and the UK, BBSRC, Grant No. BB/N001168/1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00542/full#supplementary-material
FIGURE S1 | ROS accumulation is disrupted in the big mutant during stomatal closure induced by eCO2. (A) eCO2-induced ROS production during eCO2-induced stomatal closure is reduced in comparison to WT. Mean H2DCF-DA fluorescence intensity was measured on 2.5 h light-preincubated epidermal peels, treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for another 2.5 h. (B) In the inhibition of light-stimulated stomatal opening by eCO2, ROS production in WT and big mutants is identical. Mean H2DCF-DA fluorescence intensity was measured on light-incubated epidermal peels treated with CO2-free (mock) or 800 ppm CO2 (elevated CO2) for 3 h. In (A,B) (n = 60), values are means ± s.e. All experiments were repeated at least three times. Different letters indicate significant differences at P < 0.05 based on a Tukey’s test.
FIGURE S2 | ROS generation related genes transcription levels are induced by eCO2. (A–F) four-week old intact leaves were treated with or without eCO2 for 3 h, the transcription levels of PRX33 (A), PRX34 (B), RBOHD (C), and RBOHF (D) genes were determined by quantitative RT-PCR and normalized to Actin3, OST1 (E) and SLAC1 (F) were used as positive controls. In (A–F), the shown result is a representative of three independent biological experiments, values are mean ± s.e. Means with different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
FIGURE S3 | eCO2-induced ROS accumulation requires SA signaling. eCO2 stimulates an increase H2DCFDA fluorescence in WT guard cells, but is blocked in npr3npr4 mutants. Mean fluorescence intensity was measured on 2.5 h light-preincubated epidermal peels, treated with 800 ppm CO2 for another 2.5 h. Values are mean ± s.e. (n = 50). All experiments were repeated at least three times. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
FIGURE S4 | eCO2-induced stomatal closure requires JA signaling. (A) eCO2-induced stomatal closure is disrupted in coi1-1 mutants. (B) eCO2-induced stomatal closure is disrupted in jar1-1 mutants. Stomatal apertures in (A,B) were measured on 2.5 h light-preincubated epidermal peels, treated with 800 ppm CO2 for another 2.5 h. In (A,B), the shown result is a representative of three independent biological experiments, values are mean ± s.e. (n = 120). Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
FIGURE S5 | The expression of PRXs and RBOH gene are not affected in ROS mutants. Four-week old leaves were used to extract mRNA. The quantitative RT-PCR (A–D) and RT-PCR (E) analysis of PRX33, PRX34, RBOHD, and RBOHF transcription in leaves of 5-week-old WT, prx33-3, prx34-2, and rbohDrbohF mutants. For quantitative RT-PCR, the transcription levels normalized to Actin3; for RT-PCR, EF1a was used as a control for cDNA quantity. In (A–D), the shown result is a representative of three independent biological experiments, values are mean ± s.e. Different letters represent statistically significant differences at P < 0.05 based on a Tukey’s test.
FIGURE S6 | Expression of RBOHs genes in leaves and guard cells after treatment with ABA. Heat map showing levels of expression of AtRBOHA- AtRBOHJ genes (log2 intensity) in guard cells (1–6) and leaves (7–10) according to ePlant (http://bar.utoronto.ca/eplant/). 1 represents the mock test, 2 is treated with 50 μM ABA for 20 h (reference to Böhmer and Schroeder, 2011), 3 and 7 represent mock tests, 4 and 7 are treated with 100 μM ABA for 4 h (reference to Yang et al., 2008), 5 and 9 represent mock tests, 6 and 10 are treated with 50 μM ABA for 3 h (reference to Pandey et al., 2010).
TABLE S1 | Mutants used in this study.
TABLE S2 | Primers used in this study.
ABA, abscisic acid; CA, carbonic anhydrase; CO2, carbon dioxide; eCO2, elevated carbon dioxide; DPI, diphenylene iodinium; DMSO, N,N-dimethylsphingosine; H2O2, hydrogen peroxide; H2DCF-DA, 2 ′,7 ′ -dichlorodihydrofluorescein diacetate; JA, jasmonic acid; MES, 2-[N]-morpholinnoethane sulfonic acid; NADPH, nicotinamide adenine dinucloetide phosphate; NO, nitric oxide; PAMP, pathogen-associated molecular pattern; PAOs, polyamine oxidases; PCR, polymerase chain reaction; PRXs, peroxidases; RBOH, respiratory burst oxidase; ROS, reactive oxygen species; RT-PCR, reverse transcription-polymerase chain reaction; SHAM, salicylhydroxamic acid; SA, salicylic acid; SAR, systemic acquired resistance; WT, wild type.
Allan, A. C., and Fluhr, R. (1997). Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9, 1559–1572. doi: 10.1105/tpc.9.9.1559
An, Z., Jing, W., Liu, Y., and Zhang, W. (2008). Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825. doi: 10.1093/jxb/erm370
Arnaud, D., Lee, S., Takebayashi, Y., Choi, D., Choi, J., Sakakibara, H., et al. (2017). Cytokinin-mediated regulation of reactive oxygen species homeostasis modulates stomatal immunity in Arabidopsis. Plant Cell 29, 543–559. doi: 10.1105/tpc.16.00583
Assmann, S. M. (1993). Signal transduction in guard cells. Annu. Rev. Cell Biol. 9, 345–375. doi: 10.1146/annurev.cb.09.110193.002021
Bindschedler, L. V., Dewdney, J., Blee, K. A., Stone, J. M., Asai, T., Plotnikov, J., et al. (2006). Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 47, 851–863. doi: 10.1111/j.1365-313X.2006.02837.x
Böhmer, M., and Schroeder, J. I. (2011). Quantitative transcriptomic analysis of abscisic acid-induced and reactive oxygen species-dependent expression changes and proteomic profiling in Arabidopsis suspension cells. Plant J. 67, 105–118. doi: 10.1111/j.1365-313X.2011.04579.x
Caine, R. S., Yin, X., Sloan, J., Harrison, E. L., Mohammed, U., Fulton, T., et al. (2018). Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 22, 371–384. doi: 10.1111/nph.15344
Cao, H., Bowling, S. A., Gordon, A. S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583–1592. doi: 10.1105/tpc.6.11.1583
Casteel, C. L., Segal, L. M., Niziolek, O. K., Berenbaum, M. R., and Delucia, E. H. (2012). Elevated carbon dioxide increases salicylic acid in Glycine max. Environ. Entomol. 41, 1435–1442. doi: 10.1603/EN12196
Chater, C., Peng, K., Movahedi, M., Dunn, J. A., Walker, H. J., Liang, Y. K., et al. (2015). Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 25, 2709–2716. doi: 10.1016/j.cub.2015.09.013
Chen, Z., Silva, H., and Klessig, D. F. (1993). Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262, 1883–1886.
Daudi, A., Cheng, Z., O’Brien, J. A., Mammarella, N., Khan, S., Ausubel, F. M., et al. (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24, 275–287. doi: 10.1126/science.8266079
Ding, Y., Sun, T., Ao, K., Peng, Y., Zhang, Y., Li, X., et al. (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454–1467. doi: 10.1016/j.cell.2018.03.044
Dittrich, M., Mueller, H. M., Bauer, H., Peirats-Llobet, M., Rodriguez, P. L., Geilfus, C. M., et al. (2019). The role of Arabidopsis ABA receptors from the PYR/PYL/RCAR family in stomatal acclimation and closure signal integration. Nat. Plants 5, 1002–1011. doi: 10.1038/s41477-019-0490-0
Durner, J., and Klessig, D. F. (1995). Inhibition of ascorbate peroxidase by salicylic acid and 2, 6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. U.S.A. 92, 11312–11316. doi: 10.1073/pnas.92.24.11312
Foyer, C. H., and Noctor, G. (2005). Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x
Fu, Z. Q., Yan, S., Saleh, A., Wang, W., Ruble, J., Oka, N., et al. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232. doi: 10.1038/nature11162
Geng, S., Misra, B. B., de Armas, E., Huhman, D. V., Alborn, H. T., Sumner, L. W., et al. (2016). Jasmonate−mediated stomatal closure under elevated CO2 revealed by time-resolved metabolomics. Plant J. 88, 947–962. doi: 10.1111/tpj.13296
Gil, P., Dewey, E., Friml, J., Zhao, Y., Snowden, K. C., Putterill, J., et al. (2001). BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 15, 1985–1997. doi: 10.1101/gad.905201
Grek, C. L., Zhang, J., Manevich, Y., Townsend, D. M., and Tew, K. D. (2013). Causes and consequences of cysteine S-glutathionylation. J. Biol. Chem. 288, 26497–26504. doi: 10.1074/jbc.R113.461368
Han, Y., Chaouch, S., Mhamdi, A., Queval, G., Zechmann, B., and Noctor, G. (2013a). Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 18, 2106–2121. doi: 10.1089/ars.2012.5052
Han, Y., Mhamdi, A., Chaouch, S., and Noctor, G. (2013b). Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ. 36, 1135–1146. doi: 10.1111/pce.12048
Hashimoto, M., Negi, J., Young, J., Israelsson, M., Schroeder, J. I., and Iba, K. (2006). Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 8, 391–397. doi: 10.1038/ncb1387
Hashimoto-Sugimoto, M., Negi, J., Monda, K., Higaki, T., Isogai, Y., Nakano, T., et al. (2016). Dominant and recessive mutations in the Raf-like kinase HT1 gene completely disrupt stomatal responses to CO2 in Arabidopsis. J. Exp. Bot. 67, 3251–3261. doi: 10.1093/jxb/erw134
He, J., Zhang, R. X., Peng, K., Tagliavia, C., Li, S., Xue, S., et al. (2018). The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytol. 218, 232–241. doi: 10.1111/nph.14957
Hearn, T. J., Ruiz, M. C. M., Abdul-Awal, S. M., Wimalasekera, R., Stanton, C. R., Haydon, M. J., et al. (2018). BIG regulates dynamic adjustment of circadian period in Arabidopsis thaliana. Plant Physiol. 178, 358–371. doi: 10.1104/pp.18.00571
Hetherington, A. M., and Woodward, F. I. (2003). The role of stomata in sensing and driving environmental change. Nature 424, 901–908.
Hõrak, H., Sierla, M., Tõldsepp, K., Wang, C., Wang, Y. S., Nuhkat, M., et al. (2016). A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28, 2493–2509. doi: 10.1105/tpc.16.00131
Hou, Z. H., Liu, G. H., Hou, L. X., Wang, L. X., and Xin, L. I. U. (2013). Regulatory function of polyamine oxidase-generated hydrogen peroxide in ethylene-induced stomatal closure in Arabidopsis thaliana. J. Integr. Agr. 12, 251–262. doi: 10.1016/S2095-3119(13)60224-5
Hsu, P. K., Takahashi, Y., Munemasa, S., Merilo, E., Laanemets, K., Waadt, R., et al. (2018). Abscisic acid-independent stomatal CO2 signal transduction pathway and convergence of CO2 and ABA signaling downstream of OST1 kinase. Proc. Natl. Acad. Sci. U.S.A. 115, E9971–E9980. doi: 10.1073/pnas.1809204115
Hu, H., Boisson-Dernier, A., Israelsson-Nordström, M., Böhmer, M., Xue, S., Ries, A., et al. (2010). Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 12, 87–93. doi: 10.1038/ncb2009
Huang, L., Ren, Q., Sun, Y., Ye, L., Cao, H., and Ge, F. (2012). Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol. 14, 905–913. doi: 10.1111/j.1438-8677.2012.00582.x
Jakobson, L., Vaahtera, L., Toldsepp, K., Nuhkat, M., Wang, C., Wang, Y. S., et al. (2016). Natural variation in Arabidopsis Cvi-0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biol. 14:e2000322. doi: 10.1371/journal.pbio.2000322
Kanyuka, K., Praekelt, U., Franklin, K. A., Billingham, O. E., Hooley, R., Whitelam, G. C., et al. (2003). Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 35, 57–70. doi: 10.1046/j.1365-313X.2003.01779.x
Keenan, T. F., Hollinger, D. Y., Bohrer, G., Dragoni, D., Munger, J. W., Schmid, H. P., et al. (2013). Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327. doi: 10.1038/nature12291
Khokon, M. A. R., Okuma, E. I. J. I., Hossain, M. A., Munemasa, S., Uraji, M., Nakamura, Y., et al. (2011). Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 34, 434–443. doi: 10.1111/j.1365-3040.2010.02253.x
Kim, T. H., Bömer, M., Hu, H., Nishimura, N., and Schroeder, I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2 + signaling. Annu. Rev. Plant Biol. 61, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Kolla, V. A., Vavasseur, A., and Raghavendra, A. S. (2007). Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 225, 1421–1429. doi: 10.1007/s00425-006-0450-6
Kuai, X., MacLeod, B. J., and Després, C. (2015). Integrating data on the Arabidopsis NPR1/NPR3/NPR4 salicylic acid receptors; a differentiating argument. Front. Plant Sci. 6:235. doi: 10.3389/fpls.2015.00235
Kwak, J. M., Mori, I. C., Pei, Z. M., Leonhardt, N., Torres, M. A., Dangl, J. L., et al. (2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633. doi: 10.1093/emboj/cdg277
Laxalt, A. M., García-Mata, C., and Lamattina, L. (2016). The dual role of nitric oxide in guard cells: promoting and attenuating the ABA and phospholipid-derived signals leading to the stomatal closure. Front. Plant Sci. 7:476. doi: 10.3389/fpls.2016.00476
Lai, Y. S., Renna, L., Yarema, J., Ruberti, C., He, S. Y., and Brandizzi, F. (2018). Salicylic acid-independent role of NPR1 is required for protection from proteotoxic stress in the plant endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 115, E5203–E5212. doi: 10.1073/pnas.1802254115
Li, H. M., Altschmied, L., and Chory, J. (1994). Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev. 8, 339–349. doi: 10.1101/gad.8.3.339
Li, X., Sun, Z., Shao, S., Zhang, S., Ahammed, G. J., Zhang, G., et al. (2014). Tomato-Pseudomonas syringae interactions under elevated CO2 concentration: the role of stomata. J. Exp. Bot. 66, 307–316. doi: 10.1093/jxb/eru420
López-Bucio, J., Hernández-Abreu, E., Sánchez-Calderón, L., Pérez-Torres, A., Rampey, R. A., Bartel, B., et al. (2005). An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol. 137, 681–691. doi: 10.1104/pp.104.049577
Matros, A., Amme, S., Kettig, B., Buck-Sorlin, G. H., Sonnewald, U. W. E., and Mock, H. P. (2006). Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 29, 126–137. doi: 10.1111/j.1365-3040.2005.01406.x
McAinsh, M. R., Clayton, H., Mansfield, T. A., and Hetherington, A. M. (1996). Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 111, 1031–1042. doi: 10.1104/pp.111.4.1031
Medina-Puche, L., Castelló, M. J., Canet, J. V., Lamilla, J., Colombo, M. L., and Tornero, P. (2017). β-carbonic anhydrases play a role in salicylic acid perception in Arabidopsis. PLoS One 12:e0181820. doi: 10.1371/journal.pone.0181820
Merilo, E., Laanemets, K., Hu, H., Xue, S., Jakobson, L., Tulva, I., et al. (2013). PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol. 162, 1652–1668. doi: 10.1104/pp.113.220608
Myers, S. S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, D. B., Bloom, A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510, 139–142. doi: 10.1038/nature13179
Mhamdi, A., and Noctor, G. (2016). High CO2 primes plant biotic stress defences through redox-linked pathways. Plant Physiol. 172, 929–942. doi: 10.1104/pp.16.01129
Mishra, G., Zhang, W., Deng, F., Zhao, J., and Wang, X. (2006). A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312, 264–266. doi: 10.1126/science.1123769
Miura, K., Okamoto, H., Okuma, E., Shiba, H., Kamada, H., Hasegawa, P. M., et al. (2013). SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 73, 91–104. doi: 10.1111/tpj.12014
Mori, I. C., Pinontoan, R., Kawano, T., and Muto, S. (2001). Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 42, 1383–1388. doi: 10.1093/pcp/pce176
Mou, Z., Fan, W., and Dong, X. (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935–944. doi: 10.1016/S0092-8674(03)00429-X
Murata, Y., Mori, I. C., and Munemasa, S. (2015). Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 66, 369–392. doi: 10.1146/annurev-arplant-043014-114707
Neill, S., Desikan, R., and Hancock, J. (2002). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5, 388–395. doi: 10.1016/S1369-5266(02)00282-0
Neill, S. J., Desikan, R., and Hancock, J. T. (2003). Nitric oxide signalling in plants. New Phytol. 159, 11–35. doi: 10.1046/j.1469-8137.2003.00804.x
Noctor, G., Gomez, L., Vanacker, H., and Foyer, C. H. (2002). Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 53, 1283–1304. doi: 10.1093/jexbot/53.372.1283
O’Brien, J. A., Daudi, A., Butt, V. S., and Bolwell, G. P. (2012a). Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236, 765–779. doi: 10.1007/s00425-012-1696-9
O’Brien, J. A., Daudi, A., Finch, P., Butt, V. S., Whitelegge, J. P., Souda, P., et al. (2012b). A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 158, 2013–2027. doi: 10.1104/pp.111.190140
Paciorek, T., Zažímalová, E., Ruthardt, N., Petrášek, J., Stierhof, Y. D., Kleine-Vehn, J., et al. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256. doi: 10.1038/nature03633
Pandey, S., Wang, R. S., Wilson, L., Li, S., Zhao, Z., Gookin, T. E., et al. (2010). Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol. Syst. Biol. 6:372. doi: 10.1038/msb.2010.28
Parsons, K., Nakatani, Y., and Nguyen, M. D. (2015). p600/UBR4 in the central nervous system. Cell. Mol. Life Sci. 72, 1149–1160. doi: 10.1007/s00018-014-1788-8
Passardi, F., Penel, C., and Dunand, C. (2004). Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 9, 534–540. doi: 10.1016/j.tplants.2004.09.002
Passardi, F., Tognolli, M., De Meyer, M., Penel, C., and Dunand, C. (2006). Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 223, 965–974. doi: 10.1007/s00425-005-0153-4
Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klüsener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. doi: 10.1038/35021067
Pottosin, I., Velarde-Buendía, A. M., Bose, J., Zepeda-Jazo, I., Shabala, S., and Dobrovinskaya, O. (2014). Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. J. Exp. Bot. 65, 1271–1283. doi: 10.1093/jxb/ert423
Raschke, K. (1975). Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta 125, 243–259. doi: 10.1007/BF00385601
Rhee, S. G., Bae, Y. S., Lee, S. R., and Kwon, J. (2000). Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. Signal. 2000:e1. doi: 10.1126/stke.2000.53.pe1
Ruegger, M., Dewey, E., Hobbie, L., Brown, D., Bernasconi, P., Turner, J., et al. (1997). Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9, 745–757. doi: 10.1105/tpc.9.5.745
Rodrigues, O., Reshetnyak, G., Grondin, A., Saijo, Y., Leonhardt, N., Maurel, C., et al. (2017). Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA-and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114, 9200–9205. doi: 10.1073/pnas.1704754114
Shi, K., Li, X., Zhang, H., Zhang, G., Liu, Y., Zhou, Y., et al. (2015). Guard cell hydrogen peroxide and nitric oxide mediate elevated CO2-induced stomatal movement in tomato. New Phytol. 208, 342–353. doi: 10.1111/nph.13621
Sierla, M., Waszczak, C., Vahisalu, T., and Kangasjärvi, J. (2016). Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 171, 1569–1580. doi: 10.1104/pp.16.00328
Singh, R., Parihar, P., Singh, S., Mishra, R. K., Singh, V. P., and Prasad, S. M. (2017). Reactive oxygen species signaling and stomatal movement: current updates and future perspectives. Redox. Biol. 11, 213–218. doi: 10.1016/j.redox.2016.11.006
Slaymaker, D. H., Navarre, D. A., Clark, D., del Pozo, O., Martin, G. B., and Klessig, D. F. (2002). The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. U.S.A. 99, 11640–11645. doi: 10.1073/pnas.182427699
Song, Y., Miao, Y., and Song, C. P. (2014). Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol. 201, 1121–1140. doi: 10.1111/nph.12565
Suhita, D., Raghavendra, A. S., Kwak, J. M., and Vavasseur, A. (2004). Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate-and abscisic acid-induced stomatal closure. Plant Physiol. 134, 1536–1545. doi: 10.1104/pp.103.032250
Tian, S., Wang, X., Li, P., Wang, H., Ji, H., Xie, J., et al. (2016). Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 171, 1635–1650. doi: 10.1104/pp.15.01237
Tian, W., Hou, C., Ren, Z., Pan, Y., Jia, J., Zhang, H., et al. (2015). A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 6:6057. doi: 10.1038/ncomms7057
Tognolli, M., Penel, C., Greppin, H., and Simon, P. (2002). Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 288, 129–138. doi: 10.1016/S0378-1119(02)00465-1
Tõldsepp, K., Zhang, J., Takahashi, Y., Sindarovska, Y., Hõrak, H., Ceciliato, P. H., et al. (2018). Mitogen-activated protein kinases MPK4 and MPK12 are key components mediating CO2-induced stomatal movements. Plant J. 96, 1018–1035. doi: 10.1111/tpj.14087
Torres, M. A., Dangl, J. L., and Jones, J. D. (2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. U.S.A. 99, 517–522. doi: 10.1073/pnas.012452499
Torres, M. A., Jones, J. D., and Dangl, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378. doi: 10.1104/pp.106.079467
Vanacker, H., Carver, T. L., and Foyer, C. H. (2000). Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol. 123, 1289–1300. doi: 10.1104/pp.123.4.1289
Wang, Y. Q., Feechan, A., Yun, B. W., Shafiei, R., Hofmann, A., Taylor, P., et al. (2009). S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J. Biol. Chem. 284, 2131–2137. doi: 10.1074/jbc.M806782200
Wang, C., Hu, H., Qin, X., Zeise, B., Xu, D., Rappel, W.-J., et al. (2016). Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2 -permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell 28, 568–582. doi: 10.1105/tpc.15.00637
Webb, A. A., and Hetherington, A. M. (1997). Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol. 114, 1557–1560. doi: 10.1104/pp.114.4.1557
Wei, J., Li, D. X., Zhang, J. R., Shan, C., Rengel, Z., Song, Z. B., et al. (2018). Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal. Res. 65:e12500. doi: 10.1111/jpi.12500
Williams, A., Pétriacq, P., Schwarzenbacher, R. E., Beerling, D. J., and Ton, J. (2018). Mechanisms of glacial-to-future atmospheric CO2 effects on plant immunity. New Phytol. 218, 752–761. doi: 10.1111/nph.15018
Woodward, F. I. (1987). Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327:617. doi: 10.1038/327617a0
Wu, Y., Zhang, D., Chu, J. Y., Boyle, P., Wang, Y., Brindle, I. D., et al. (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 1, 639–647. doi: 10.1016/j.celrep.2012.05.008
Xu, E., Vaahtera, L., and Brosché, M. (2015). Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Mol. Plant 8, 1776–1794. doi: 10.1016/j.molp.2015.08.008
Xu, Z., Jiang, Y., Jia, B., and Zhou, G. (2016). Elevated-CO2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 7:657. doi: 10.3389/fpls.2016.00657
Yamada, J., Yoshimura, S., Yamakawa, H., Sawada, M., Nakagawa, M., Hara, S., et al. (2003). Cell permeable ROS scavengers, Tiron and Tempol, rescue PC12 cell death caused by pyrogallol or hypoxia/reoxygenation. Neurosci. Res. 45, 1–8. doi: 10.1016/S0168-0102(02)00196-7
Yamaguchi, N., Suzuki, M., Fukaki, H., Morita-Terao, M., Tasaka, M., and Komeda, Y. (2007). CRM1/BIG-mediated auxin action regulates Arabidopsis inflorescence development. Plant Cell Physiol. 48, 1275–1290. doi: 10.1093/pcp/pcm094
Yamauchi, S., Mano, S., Oikawa, K., Hikino, K., Teshima, K. M., Kimori, Y., et al. (2019). Autophagy controls reactive oxygen species homeostasis in guard cells that is essential for stomatal opening. Proc. Natl. Acad. Sci. U.S.A. 116, 19187–19192. doi: 10.1073/pnas.1910886116
Yang, Y., Costa, A., Leonhardt, N., Siegel, R. S., and Schroeder, J. I. (2008). Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4:6. doi: 10.1186/1746-4811-4-6
Yin, Y., Adachi, Y., Ye, W., Hayashi, M., Nakamura, Y., Kinoshita, T., et al. (2013). Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol. 163, 600–610. doi: 10.1104/pp.113.223826
Zhang, J., De-oliveira-Ceciliato, P., Takahashi, Y., Schulze, S., Dubeaux, G., Hauser, F., et al. (2018). Insights into the molecular mechanisms of CO2-mediated regulation of stomatal movements. Curr. Biol. 28, R1356–R1363. doi: 10.1016/j.cub.2018.10.015
Zhang, R. X., Ge, S., He, J., Li, S., Hao, Y., Du, H., et al. (2019). BIG regulates stomatal immunity and jasmonate production in Arabidopsis. New Phytol. 222, 335–348. doi: 10.1111/nph.15568
Zhang, S., Li, X., Sun, Z., Shao, S., Hu, L., Ye, M., et al. (2015). Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 66, 1951–1963. doi: 10.1093/jxb/eru538
Keywords: elevated CO2, stomatal movement, plant hormones, reactive oxygen species, NADPH oxidases, cell wall peroxidases
Citation: He J, Zhang R-X, Kim DS, Sun P, Liu H, Liu Z, Hetherington AM and Liang Y-K (2020) ROS of Distinct Sources and Salicylic Acid Separate Elevated CO2-Mediated Stomatal Movements in Arabidopsis. Front. Plant Sci. 11:542. doi: 10.3389/fpls.2020.00542
Received: 14 December 2019; Accepted: 09 April 2020;
Published: 08 May 2020.
Edited by:
Scott McAdam, Purdue University, United StatesReviewed by:
Yunqing Yu, Donald Danforth Plant Science Center, United StatesCopyright © 2020 He, Zhang, Kim, Sun, Liu, Liu, Hetherington and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Kuan Liang, eWtsaWFuZ0B3aHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.