- 1Key Laboratory of Horticultural Plant Biology, Ministry of Education, Huazhong Agricultural University, Wuhan, China
- 2College of Horticulture & Forestry Sciences, Huazhong Agricultural University, Wuhan, China
Although foliar boron (B) fertilization is regarded as an efficient way to remedy B deficiency, the mechanisms of foliar B transport from leaves to roots are still unclear. In this study, performed with 1-year-old “Newhall” navel orange (Citrus sinensis) grafted on trifoliate orange (Poncirus trifoliata) plants, we analyzed the B concentration in leaves and roots, B-sucrose complex in the phloem sap after foliar application of 10B, girdling, and/or shading treatments. Results indicated that 10B concentration was significantly increased in roots after foliar 10B treatment. On the other hand, both girdling the scion stem and shading over the plants with a black plastic net significantly reduced the B and 10B concentration in roots. LC-MS analysis revealed that foliar 10B-treated plants had higher concentration of sucrose and some sugar alcohols in the phloem sap as compared to foliar water-treated plants. Combining with the analysis in the artificial mixture of B and sucrose, a higher peak intensity of the 10B-sucrose complex was found in the phloem sap of foliar 10B-treated plants compared to the control plants. Taken together, it is concluded that foliar B can be long distance transported from leaves to roots via phloem, at least by forming a B-sucrose complex in citrus plants.
Introduction
Boron (B) is a micronutrient that plays a pivotal role in cell wall stability (Chormova et al., 2014), photosynthesis (Wang et al., 2015), and carbon metabolism (Mishra and Heckathorn, 2016) in the plant. Thus, B deficiency inhibits plant growth, hinders leaf expansion, causes leaf chlorosis or shoot tip dieback, deforms leaf, flower, or fruit, decreases yield and fruit quality, limits root elongation (Wang et al., 2015). Preventing boron deficiency is critical to maintain crop yield and quality, and its success depends on the understanding of B transport and distribution mechanisms.
In general, plants absorb B mainly by roots from the soil in the form of boric acid. Then, B moves through the xylem to be distributed in different tissues or organs for utilization (Marschner et al., 1996; Takano et al., 2008). The occurrence of B deficiency is mainly caused by the low level of soluble B in the soil and/or low B utilization by plants (Wang et al., 2015). Soil B application is a common practice in commercial agriculture to prevent B deficiency (Shireen et al., 2018). Moreover, extensive efforts were devoted in the last several decades for assessing the mechanisms of B absorption, acropetal transport, and distribution (Takano et al., 2008; Miwa and Fujiwara, 2010; Reid, 2014; Yoshinari and Takano, 2017), expecting to improve the plant B-utilizing capability.
On the other hand, foliar B fertilization can be an efficient way to overcome B deficiency and ensure fruit yield and quality (Fregoni et al., 1978; DeMoranville and Deubert, 1987; Boaretto et al., 2011; Al-Obeed et al., 2018; Shireen et al., 2018). Previous studies showed that foliar B application can improve B availability in leaves and increase the B concentration in buds (Eichert and Goldbach, 2010; Liu et al., 2012). Moreover, foliar B can be transported from lower leaves to upper leaves or from mature leaves to reproductive organs via the phloem (Huang et al., 2001; Stangoulis et al., 2001; Huang et al., 2008; Liu et al., 2012). However, the efficiency of foliar B application to overcome B deficiency depends on B mobility through the phloem (Brown and Shelp, 1997), which appears to be linked to the possibility that B is complexed with certain metabolites (Reid, 2014). These metabolites may correspond to polyols (Lehto et al., 2004; Will et al., 2011) and/or sucrose (Stangoulis et al., 2001, 2010). The mobility of B in the phloem is high in many fruit species such as olive, apple, and peach through the formation of B-polyol complexes (Bieleski, 1982; Brown and Shelp, 1997). In fact, B is highly mobile in most species belonging to Oleaceae and Rosaceae families, which translocate large amounts of sugar alcohols in the phloem (Hu et al., 1997), while B shows low mobility in wheat and canola that translocate sucrose in the phloem (Stangoulis et al., 2010). Citrus is a vascular plant with sucrose being the main photosynthate transported in the phloem (Dinant et al., 2010; Hijaz and Killiny, 2014; Liu et al., 2015). However, the role of sucrose in B mobility from leaves to roots is unclear in citrus species.
Citrus is one of the world’s major fruit crops (Liu et al., 2012), which grows in more than 140 countries with the global production of over 146 million metric tons in 2017 (FAO, 2019). However, citrus is very sensitive to B deficiency and its major growing regions contain low levels of soluble B (Guidong et al., 2011; Wang et al., 2014, 2016). Many studies focused on citrus response to B deficiency or toxicity (Sheng et al., 2009; Boaretto et al., 2011; Mei et al., 2011; Liu et al., 2013; Zhou et al., 2014; Dong et al., 2016; Wu et al., 2018), and the molecular mechanisms for rootstock B-utilizing efficiency (An et al., 2012; Cañon et al., 2013; Zhou et al., 2015; Martínez-Cuenca et al., 2019). In the citrus industry, on the other hand, foliar application of B is an alternative way to supply B because foliar B sprays can be easily applied and may be rapidly absorbed by the foliage. However, whether the foliar-applied B can be transported to the root and what is the transporting form are still unclear in citrus species. Hence, in this study, 10B was used to trace the translocation of foliar applied B under both B-sufficient and -deficient conditions, while girdling was used to trace B transport mechanisms. Because shading with a black net can decrease leaf photosynthetic capacity (Correia et al., 2006), shading was then used to detect the possible role of sucrose in the B long-distance transport. Moreover, component analysis of the phloem sap was conducted to confirm this possibility. It is concluded that foliar supplied B can be long distance transported to citrus roots through the phloem as a B-sucrose complex.
Materials and Methods
Plant Materials and Treatments
One-year-old “Newhall” navel orange (Citrus sinensis cv. Newhall) plants (n = 40), grafted on trifoliate orange (Poncirus trifoliata) rootstock, were individually transplanted to five liter-lightproof pots (one-pot contained one plant) with B-free quartz sand and perlite (1:1, v/v). These plants were placed in a greenhouse under natural sunlight conditions at Huazhong Agricultural University, Wuhan, China (Supplementary Figure S1). They were supplied with 3 liters of modified Hoagland No. 2 nutrient solution without B (Hoagland and Arnon, 1950) for 2 months at an interval of 1 week. Then, 20 plants with similar stem diameter, plant height and vigor were selected for five treatments (T1–T5): T1 (considered as control)- the upper and lower sides of all leaves were evenly wiped twice by cotton swabs which were saturated with ultra-pure water (plus 0.01% Tween-20) (Figure 1A), T2- the upper and lower sides of all leaves were evenly wiped twice by cotton swabs which were saturated with 10B (47 mM H310BO3, 99% atom 10B, Aldrich, United States) solution (plus 0.01% Tween-20) (Figure 1A), T3- the plant leaves were treated like T2 plant leaves but the plants were irrigated with sufficient B (0.25 mg/L H3BO3) in sand culture (Figure 1A), T4- the plant leaves were treated like T2 plant leaves but the plant stems were girdled (8–10 mm wide at about 2 cm above the graft union) (Figure 2A), T5- the plant leaves were treated like T2 plant leaves but the plants were shaded with a black plastic net (the transmittance is 10.55%) (Figure 3A). All treatments were conducted in the same greenhouse (temperature≈30°C; relative humidity≈80%) at 9:00 a.m. Each treatment was replicated four times (one plant as one replication). Before treatment, new twigs were removed and 15–18 healthy leaves were retained per plant.
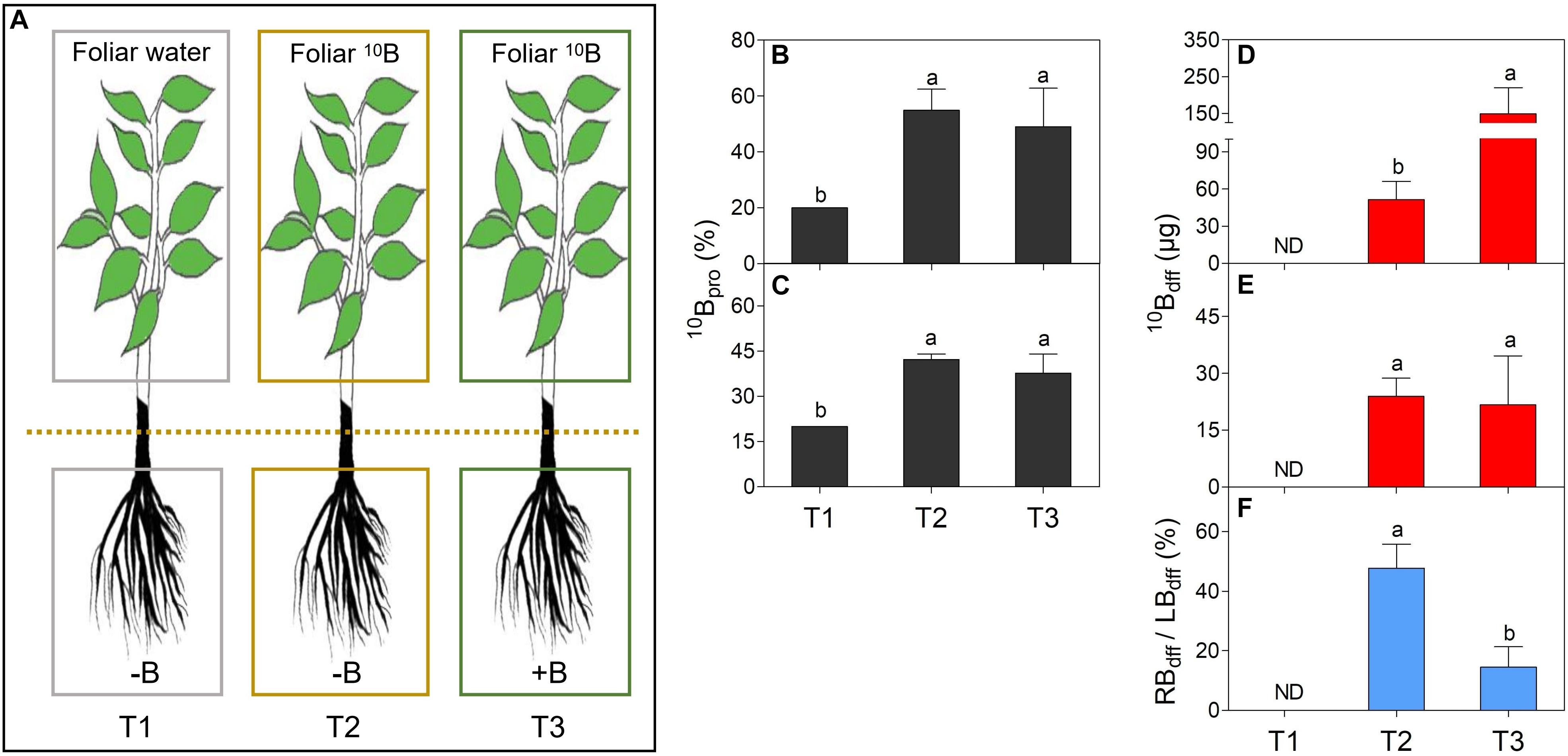
Figure 1. Comparison of proportion of 10B (10Bpro) and 10B concentration derived from the foliar 10B (10Bdff) in leaves (B,D) and roots (C,E), and the ratio of root 10Bdff/leaf 10Bdff (RBdff/LBdff),(F) of different treatments (A). Figure (A) shows three treatments: gray boxes refer to foliar application of ultra-pure water to B deficient seedlings as treatment 1 (T1); yellow boxes refer to foliar 10B application to B deficient seedlings as treatment 2 (T2); green boxes refer to foliar 10B application to B sufficient seedlings as treatment 3 (T3). Different lower-case letters indicate the significant difference between treatments at P < 0.05 as determined by the Duncan’s multiple range test.
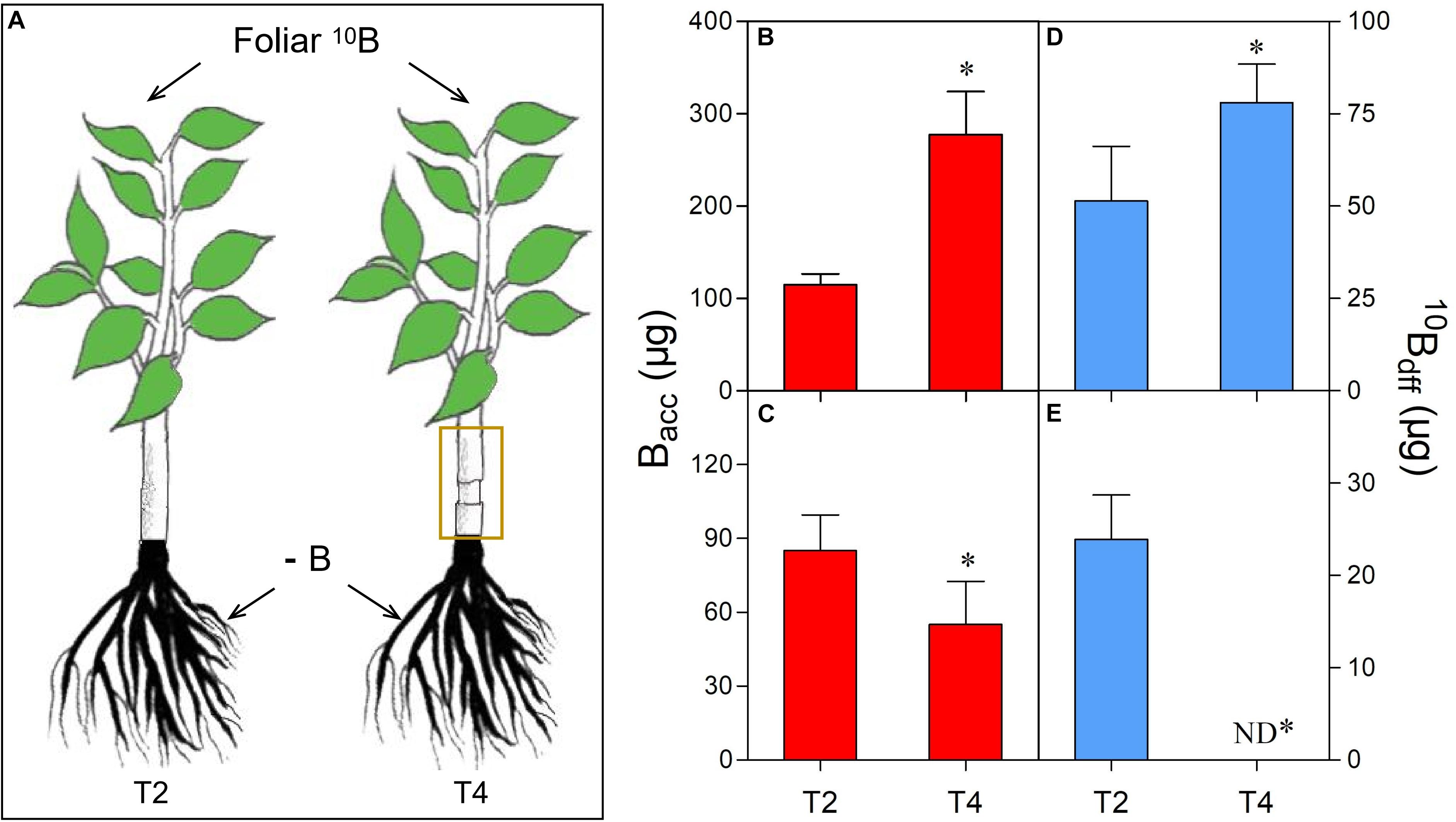
Figure 2. Comparison of B accumulation (Bacc) and 10B concentration derived from foliar 10B (10Bdff) in leaves (B,D) and roots (C,E) of different treatments (A). The yellow box refers to girdling the stem of foliar-10B treated plants as treatment 4 (T4). “∗” in each graph indicates significant differences between T2 and T4 treatments (t-test, n = 4, P < 0.05).
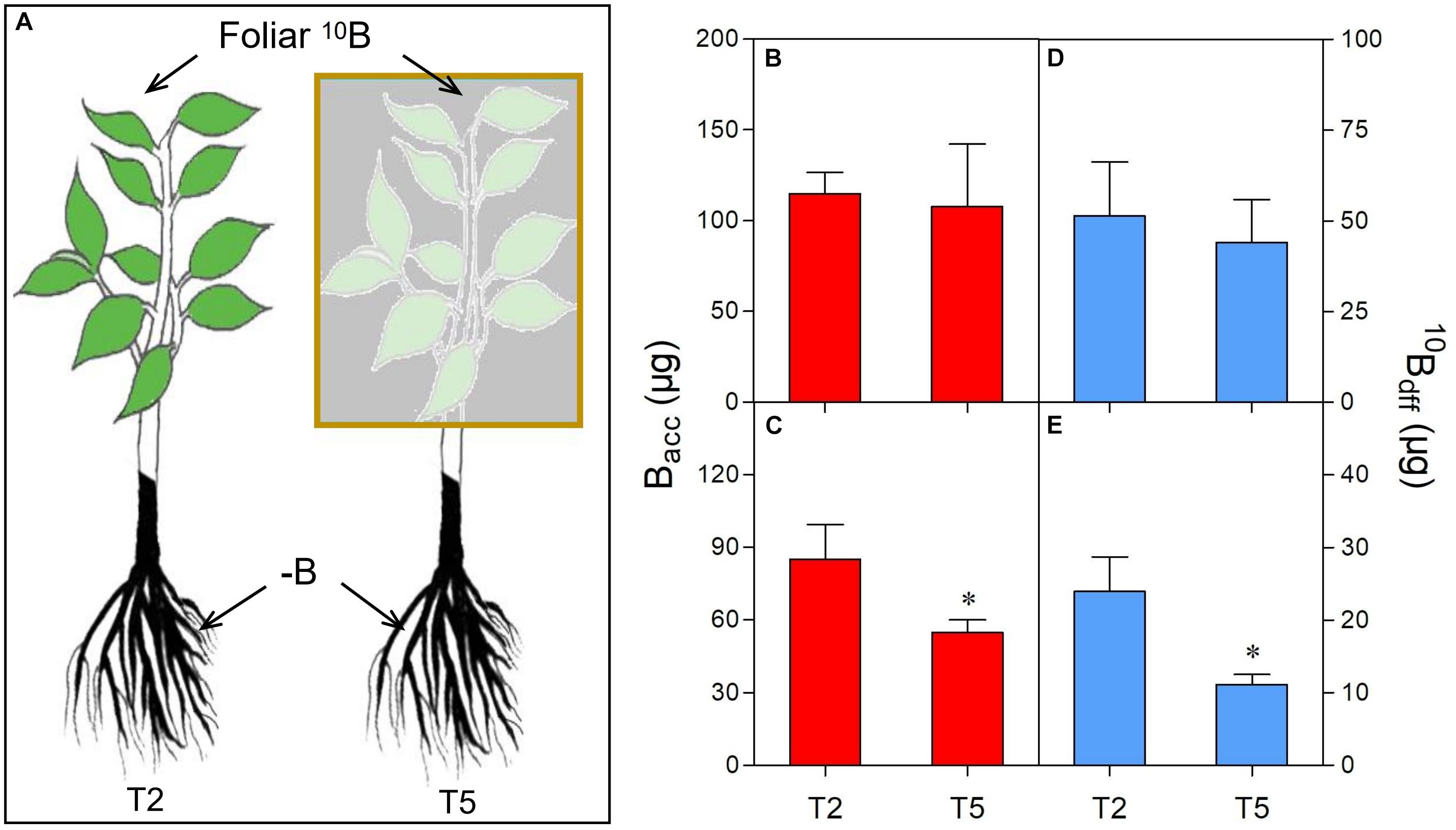
Figure 3. Comparison of B accumulation (Bacc) and 10B concentration derived from foliar 10B (10Bdff) in leaves (B,D) and roots (C,E) of different treatments (A). The yellow box refers to shading the foliar-10B treated plants as treatment 5 (T5). “∗” in each graph indicates significant differences between T2 and T5 treatments (t-test, n = 4, P < 0.05).
Seven weeks after treatment, roots and leaves of each plant in T1–T5 treatments were collected. After careful cleaning, they were dried in an oven and then used for B and 10B analysis. In addition, plant scion stems from T1- and T2-treatment were collected for phloem sap extraction.
B Concentration and 10B Analysis
About 0.30 g dry samples were ashed in a muffle furnace at 500°C for 6 h. Afterward, they were dissolved in 30 mL of 1% HNO3. Half of the solution was used to measure B concentration (Bcon, μg/g) by an inductively coupled plasma-optical emission spectrometer (ICP-OES) (Bryson et al., 2014). The B accumulation (Bacc) of given plant tissue was calculated according to the following formula.
The remaining solution was used to determine the proportion of 10B (10Bpro,%), by an inductively coupled plasma-mass spectrometer (ICP-MS). Because any tissue in the plant contains a background 10B, the 10B concentration derived from foliar 10B (10Bdff, μg) was calculated according to the following formula (Liu et al., 2012).
c1 refers to the background 10Bpro which was determined from the control samples and the value was 20 ± 0.02% in the current study, and c2 refers to the 10Bpro in H310BO3 in which the value was 99%.
Phloem Sap Extraction
Phloem sap was collected as described by Hijaz and Killiny (2014). Briefly, scion stems were collected from T1- and T2-treatment plants and washed three times with ultra-pure water to remove dirt and residues. Then, clean stems of each plant were cut into 3–5 cm length pieces with sterile scissors. The bark was peeled off by sterile tweezers and then rinsed with ultra-pure water to clean xylem residues. Subsequently, the clean epidermis was cut into small pieces, mixed and transferred into a 0.5 mL centrifuge tube with punctured at the bottom. Each tube was packed tightly and then put into a 2 mL centrifuge tube for centrifugation. After centrifugation, about 40 μL of phloem sap was collected.
Metabolite Extraction and Sample Preparation
About 30 μL phloem sap from each plant were thoroughly mixed with 800 μL cold mixture of methanol and acetonitrile (v/v, 1:1). Then, the mixture was processed with sonication for 1 h in an ice bath and then incubated at −20°C for 1 h. After centrifugation (14000 g, 20 min, 4°C), the supernatants were collected and then dried under vacuum. The lyophilized powder was re-dissolved with 50% acetonitrile, and then vortexed for 1 min. After centrifugation (14000 g, 15 min, 4°C), the supernatants were collected for LC-MS analyses.
Additionally, to ensure the data quality for metabolic profiling, quality control (QC) samples were prepared by pooling aliquots of all representative phloem saps. QC samples in each batch were prepared and analyzed as the experiment samples.
Non-targeted Metabolites Analysis
Non-targeted metabolites, multivariate statistical and metabolites identification were performed according to the previous method (Wang et al., 2017). Metabolomics profiling was analyzed by using a UPLC-ESIQ-TOF-MS system (UHPLC, 1290 Infinity LC, Agilent Technologies, Santa Clara, CA, United States) coupled with Triple TOF 5600 (AB Sciex, Framingham, MA, United States). The detailed method is provided in the Supplementary Material.
Targeted Metabolites Analysis
Targeted metabolites from the phloem sap were determined by a high-throughput and multiplexed LC/MS/MRM method (Wei et al., 2010) with detail in the Supplementary Material. Metabolomics profiling was analyzed using a UPLC-ESI-Q-TRAP-MS system (UHPLC, 1290 Infinity LC, Agilent Technologies, Santa Clara, CA, United States) coupled with QTRAP 5500 (AB SCIEX, Framingham, MA, United States).
Borate-Sucrose Complex Analysis in a Mixture Solution
The borate-sucrose complex in the mixture of boric acid (0.3 mM) and sucrose (300 mM) was determined by the ESI-Q-TOF-MS system in negative mode (ESI–). The detailed method of ESI-Q-TOF-MS was the same as the untargeted metabolite analysis.
Borate-Sucrose Complex Analysis in Phloem Sap
Before testing the phloem sap, the mixture of boric acid and sucrose was analyzed by LC-MS to detect the peak time of the borate-sucrose complex. Then, the borate-sucrose complex in the phloem sap of T1- and T2-treated plants was determined by a UPLC-ESI-Q-TRAP-MS system in negative mode (ESI-). The detailed method of UPLC-ESI-Q-TRAP-MS was the same as the targeted metabolite analysis.
Statistical Analysis
Unless specially stated, each value was expressed as the means ± standard deviation (SD) of four replications. Data analysis was performed by using independent-samples t-test (P < 0.05) or ANOVAs (Duncan test, P < 0.05) in SPSS for Windows 19.0 (SPSS Inc., Chicago, IL, United States).
Results
Comparison of 10Bpro and 10Bdff Among Leaves and Roots of T1, T2, and T3 Plants
The 10Bpro was at a similar level in either leaves (Figure 1B) or roots (Figure 1C) of T2 and T3 plants. But the 10Bpro in leaves (Figure 1B) or roots (Figure 1C) of both T2 and T3 plants were significantly higher than T1 plants. As for the 10Bdff, it was significantly higher in the leaves of T3 plants than in the leaves of T2 plants (Figure 1D). However, no significant difference was observed for the 10Bdff between the roots of T2 and T3 plants (Figure 1E). Here, the ratio of root 10Bdff to leaf 10Bdff (RBdff/LBdff) was further calculated. Figure 1F showed that the RBdff/LBdff ratio was significantly higher in T2 plants than T3 plants and the ratios in both T2 and T3 plants were significantly higher than T1 plants.
Change of Bacc and 10Bdff in Leaves and Roots After Girdling or Shading
After girdling, the Bacc (Figure 2B) and the 10Bdff (Figure 2D) in leaves of T4 plants were significantly higher than those of T2 plants (control, without girdling). However, both Bacc (Figure 2C) and 10Bdff (Figure 2E) in roots of T4 plants were obviously lower than that of T2 plants. Moreover, 10Bdff was undetectable in the roots of T4 plants (Figure 2E).
On the other hand, shading significantly decreased the Bacc in roots of T5 plants compared to T2 plants (Figure 3C). A similar trend was also observed for the 10Bdff in the roots of T5 plants which was only 37% of T2 plants after shading (Figure 3E). Moreover, no significant difference in leaf Bacc (Figure 3B) or 10Bdff (Figure 3D) was observed between T2 and T5 plants.
Change of Metabolites in the Phloem Sap of T2 Plants
Non-targeted metabolomics was performed to analyze differential metabolites in the phloem sap of T1 and T2 plants. PCA-QC (an unsupervised clustering method) analysis showed a significant difference in metabolites between the phloem sap of T1 and T2 plants with 49.5% and 52.0% total variance of the first two principal components in the positive and negative mode, respectively (Supplementary Figure S2). The heat map showed a significant change of metabolites that occurred in the phloem sap of T2 plants compared to T1 plants (Figure 4A). In detail, the phloem sap of T2 plants contained 47 and 32 metabolites significantly higher and lower, respectively, than those of T1 plants (Figure 4B). The increased metabolites in T2 plants mainly belonged to amino acids, esters, ketones, nucleosides, organic acids, sugars and sugar alcohols (Figure 4A). In them, six differential sugars and sugar alcohols (sucrose, tagatose, mannitol, dulicitol, lavandulol, and perseitol) were observed in the increased metabolites in the phloem sap of T2 plants (Figures 4A,B). To verify the authenticity of metabolomics analysis, they were selected to test Ms peak area between the phloem saps of T1 and T2 plants. It was found that their concentration in the phloem sap of T2 plants were significantly higher than those in T1 plants (Figure 5), which was consistent with the results of non-targeted metabolic analysis (Figure 4A). The concentration of sucrose was the highest among these sugars and sugar alcohols, and the Ln-peak area of sucrose was 18.05 and 18.20 in the phloem sap of T1 and T2 plants, respectively (Figure 5).
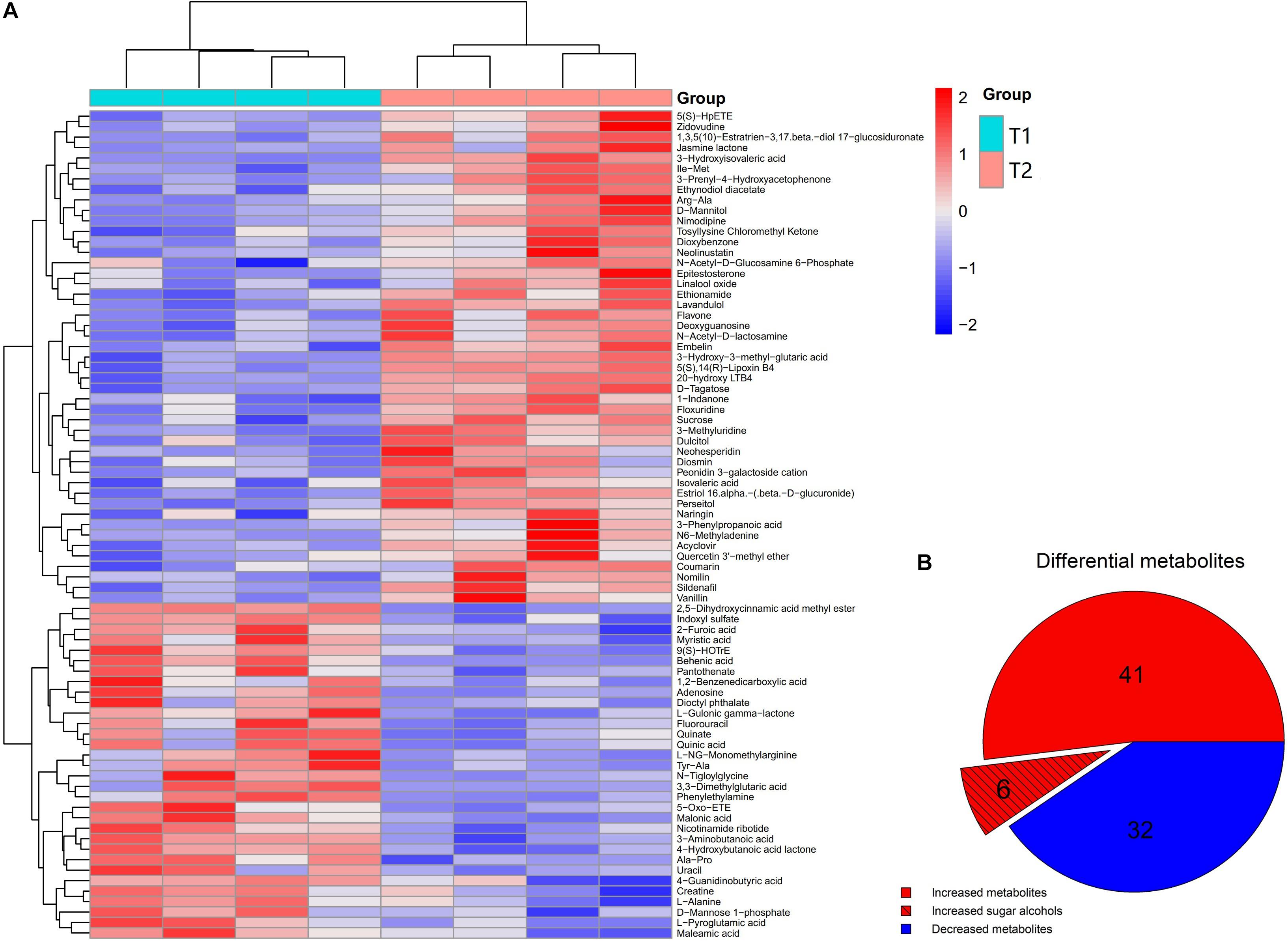
Figure 4. Unsupervised hierarchical clustering heat map of metabolites from the phloem sap of navel orange (A) and pie graph of the statistical analysis of differential metabolites (B). Metabolites were compared as obtained between the phloem saps of foliar-water (T1) plants and foliar-10B (T2) plants.
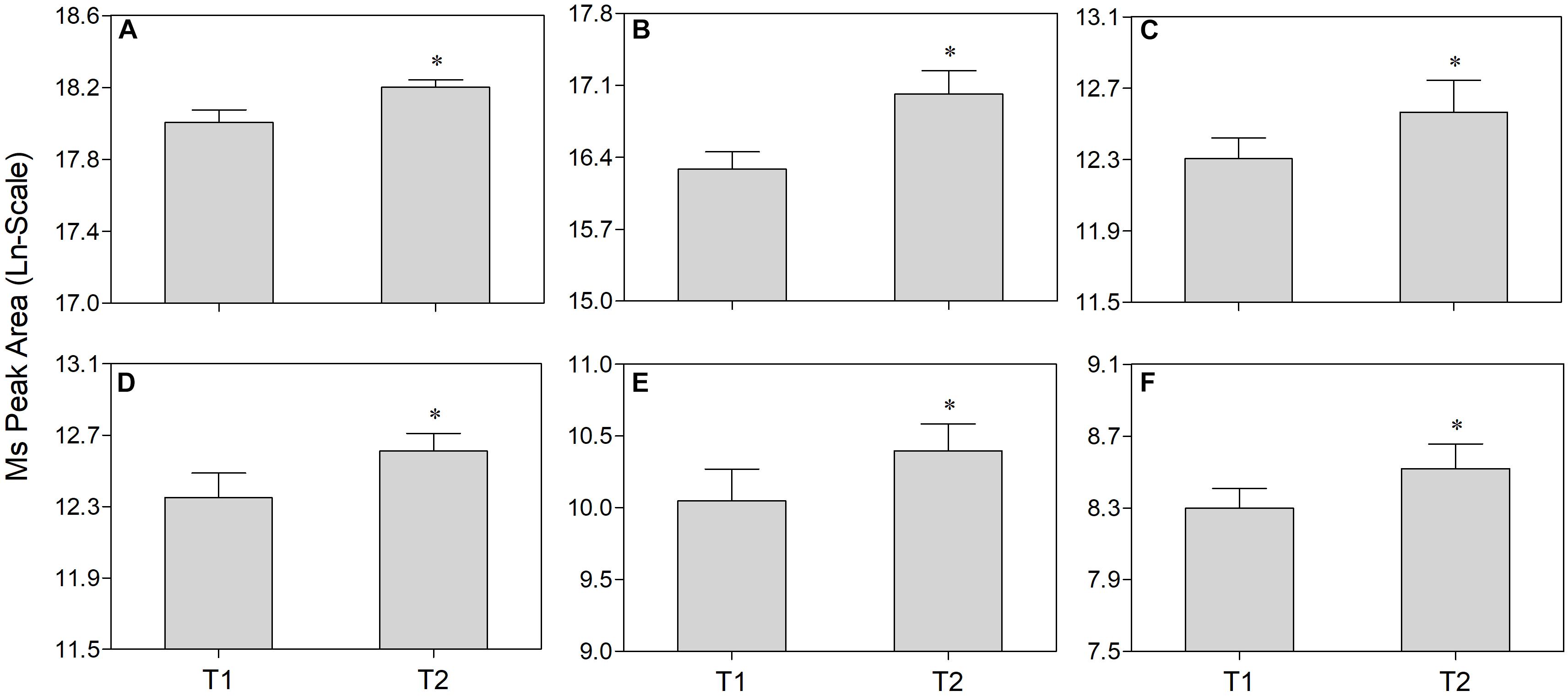
Figure 5. Comparison of sucrose (A), tagatose (B), mannitol (C), dulicitol (D), lavandulol (E), and perseitol (F) concentration in the phloem sap of navel orange between foliar-water (T1) and foliar-10B (T2) treatments. “∗” indicates significant difference between T1 and T2 treatments (t-test, n = 4, P < 0.05).
Identification of Borate-Sucrose Complex in the Phloem Sap of T1 and T2 Plants
The mass/charge values of B-sucrose complexes [(C24H40O22)10B and (C24H40O22)11B] are 690.21 and 691.21, respectively (Table 1). The artificial mixture of sucrose (300 mM) and boric acid (0.3 mM) was first used to detect the borate-sucrose complex by using the ESI-Q-TOF-MS technique. Two ions with mass/charge values of 690.21 and 691.21 were observed in the negative mode profile of ESI-Q-TOF-MS analysis (Figure 6A). Moreover, the percentage value of (C24H40O22)10B intensity was nearly 25% of the (C24H40O22)11B intensity.
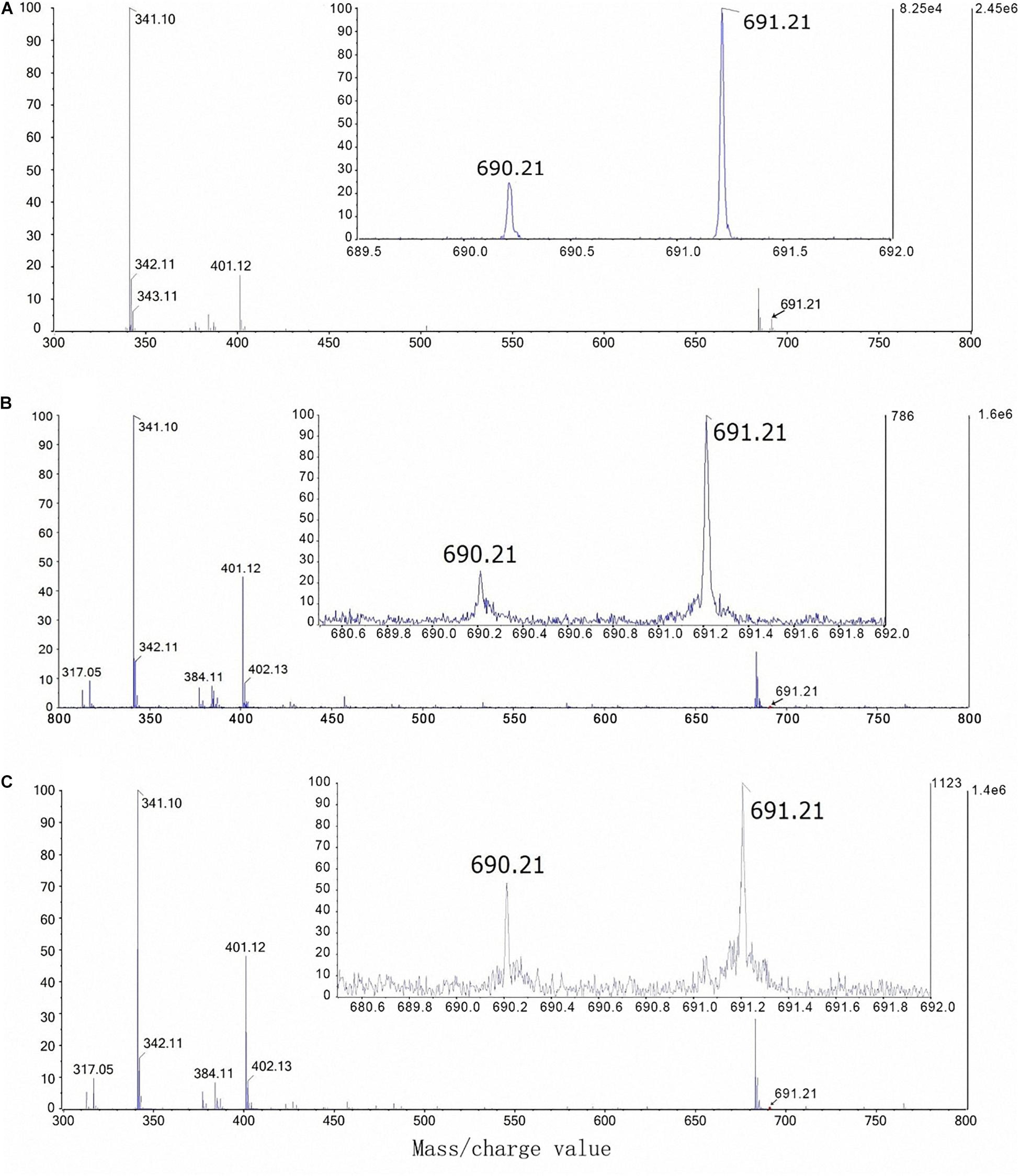
Figure 6. Borate-sucrose complex isolated from the artificial mixture of sucrose (300 mM) and boric acid (0.3 mM) by negative mode ESI-Q-TOF-MS (A); from the phloem sap of foliar-water plants (T1) by negative mode UPLC-ESI-Q-TRAP-MS (B); and from the phloem sap of foliar-10B plants (T2) by negative mode UPLC-ESI-Q-TRAP-MS (C).
On the other hand, after confirming the peak time of 690.21 and 691.21 mass/charge values (Supplementary Figure S3), the phloem sap of T1 (control) and T2 plants were also used to detect the mass/charge peak of B-sucrose complex by using the UPLC-ESI-Q-TRAP-MS technique. In the end, the mass/charge values of 690.21 and 691.21 were also observed in the phloem sap of T1 plants (Figure 6B) and T2 plants (Figure 6C). Moreover, the percentage value of (C24H40O22)10B intensity was nearly 25% of the (C24H40O22)11B intensity in the phloem sap of T1 plants (Figure 6B) while it was nearly 52% of the (C24H40O22)11B intensity in the phloem sap of T2 plants (Figure 6C).
Discussion
Foliar B Can Be Transported to the Roots Through the Phloem
Foliar application of fertilizers is an effective technique for sustainable production of crops and it may enhance the utilization of targeted tissues since nutrients can be simply supplied to the deficient tissues (Fernández and Eichert, 2009; Fernández and Brown, 2013). Boron is a vital micronutrient for healthy plant development (Wang et al., 2015), and foliar sprays of B have become a regular practice to rapidly remedy B deficiency in many plants, such as cranberry (Vaccinium corymbosum), grape (Vitis vinifera), and orange (C. sinensis) (DeMoranville and Deubert, 1987; Boaretto et al., 2011; Chormova et al., 2014). It can rapidly increase B concentrations in leaves and buds of subtropical plants (Eichert and Goldbach, 2010). Moreover, foliar B can be transported to some vegetative and reproductive organs (Huang et al., 2001; Stangoulis et al., 2001; Liu et al., 2012). In this study, by using 10B as tracer, we found that more 10B could be detected in the roots (Figure 1) and the 10Bpro (Figure 1C) or 10Bdff (Figure 1E) was significantly higher in foliar-10B treated plant roots than in foliar-water treated plant roots. These results suggested that foliar supplied B can be long distance transported from leaves to roots in citrus plants. In addition, the present results indicated that the RBdff/LBdff in B-deficient plants (T2) was dramatically higher than in B-sufficient plants (T3) (Figure 1F), implying that more B could be transported to the roots under B-deficiency conditions. These findings support the hypothesis that the amount of B distribution depends on its status in plant tissues or organs (Shelp et al., 1996; Liakopoulos et al., 2009; Liu et al., 2012).
The transport of root absorbed B to different tissues or organs occurs through the xylem (Takano et al., 2008). However, few studies showed that foliar B can be transported and distributed to adjacent tissues or organs through the phloem (Brown and Shelp, 1997; Dannel et al., 2000; Pfeffer et al., 2001; Eichert and Goldbach, 2010). Girdling refers to removing a ring of bark or phloem. When carried out around the trunk, it has the immediate effect of blocking phloem-transported metabolites across the girdle (Wang et al., 2010; Appel et al., 2012). This technique is always used to detect whether the transport of a metabolite is or not through the phloem (De Schepper et al., 2013; Savage et al., 2016). In this study, girdling significantly increased Bacc (Figure 2A) and 10Bdff (Figure 2D) in leaves, while significantly decreased Bacc in roots (Figure 2C). Moreover, 10Bdff was undetectable in the roots of girdled plants (Figure 2D). These results strongly demonstrate that the transport of foliar B to roots is through the phloem.
B-Sucrose Complex Plays a Key Role in B Basipetal Transport to the Roots
To date, the mechanisms for B transport in the xylem and its subsequent distribution have been associated with passive diffusion of boric acid, facilitated diffusion of boric acid via channels, and export of borate anion via transporters (Reid, 2014; Yoshinari and Takano, 2017). On the other hand, some reports suggest that foliar applied B can be transported to adjacent tissues or organs through the phloem by forming a complex with metabolites (Brown and Shelp, 1997; Reid, 2014). These metabolites were possibly related to some photosynthetic assimilates, such as polyols (Lehto et al., 2004; Will et al., 2011) and sucrose (Stangoulis et al., 2010). In citrus plants, sucrose is the main transportable photosynthetic assimilate (Dinant et al., 2010; Liu et al., 2015; Konrad et al., 2018). Moreover, shading has been proven to decrease carbohydrate production by limiting the photosynthesis of leaves (Osaki et al., 1995; Mäkelä et al., 2005; Correia et al., 2006). Here, we found that, when shading the plants with a black plastic net, the photosynthesis rate and photosynthetic active radiation (PAR) were significantly reduced (Supplementary Table S1). Moreover, the Bacc (Figure 3C) or 10Bdff (Figure 3E) of roots were significantly decreased (corresponding to 65% or 50% of control plants, respectively), providing evidence for the role of photosynthetic assimilates in the process of foliar B transport to roots through the phloem. On the other hand, the concentration of sucrose, tagatose, mannitol, dulcitol, perseitol, and lavandulol in the phloem sap was significantly increased after foliar 10B application compared to those of control plants (foliar water application) (Figures 4, 5). These results further indicate that scion photosynthetic assimilates are involved in the transport of foliar applied B from shoots to roots.
To confirm the possible role of sucrose in foliar B transport to the roots, we first conducted an in vitro experiment and obtained consistent results which are in line with the findings by Stangoulis et al. (2010). Namely, one borate molecule (10Borate or 11Borate) can form a complex with two sucrose molecules with the mass/charge value of 690.12 or 691.21 (Table 1 and Figure 6A). Subsequent in vivo analysis showed that B-sucrose complexes were also found in the phloem sap of control plants (T1) and foliar 10B-treated plants (T2) (Figures 6B,C), confirming that B transport in the phloem at least occurs by forming a complex with sucrose. Moreover, the percentage value of (C24H40O22)10B intensity was nearly 52% of the (C24H40O22)11B intensity in the phloem sap of foliar 10B-treated plants (Figure 6C), while it was nearly 25% in the phloem sap of control plants (Figure 6B), further showing that more 10B-sucrose complex existed in the phloem sap and thus more B can be translocated through the phloem and be distributed to the roots (Figure 1C).
Besides sucrose, some sugar alcohols also play a role in forming a complex with B (Hu et al., 1997; Hijaz and Killiny, 2014; Killiny et al., 2017). In this study, the concentration of tagatose, mannitol, dulcitol, perseitol, and lavandulol were also significantly higher in the phloem sap of foliar 10B-treated plants (T2) than in control plants (T1), although their concentration were lower than the concentration of sucrose (Figures 4, 5). However, we failed to identify their B complexes in the phloem sap, possibly due to the low concentration of such potential borate complexes, but their determination should be attempted in future trials.
Conclusion
In this study, 10B foliar application experiment, together with girdling and shading treatments to citrus seedlings proved that foliar supplied B can be transported from leaves to roots via phloem in this species. The translocation of foliar supplied B to the roots is affected by plant B status and the synthesis of photosynthetic assimilates. Moreover, foliar supplied B can be transported and/or translocated into the roots through the phloem, at least by forming the B-sucrose complex. Overall, this study contributes to raise the knowledge on foliar-B fertilization and improves our understanding of the mechanisms of foliar B transport from shoots to roots in citrus.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
WD, Y-ZL, and S-AP designed the experiments. WD conceived the project, analyzed the data, wrote the article with contributions of all the authors, and prepared the experimental materials. Z-XH extracted the phloem sap from materials. Z-YP, SH, and S-AP provided technical and writing assistance. Y-ZL supervised and complemented the writing.
Funding
This study was supported by the National Natural Science Foundation of China (31471841) and the earmarked fund for China Agriculture Research System (CARS-26).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Engineer Wang Jia (from Shanghai Bioprofile Technology Company Ltd., China), Dr. Wang Nan-Nan (Northwest A&F University, Shanxi, China) and Dr. Jin Long-Fei (CRI-CATAS, Hainan, China) for their help in metabolite analysis and research design.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00250/full#supplementary-material
References
Al-Obeed, R. S., Ahmed, M. A.-A., Kassem, H. A., and Al-Saif, A. M. (2018). Improvement of “Kinnow” mandarin fruit productivity and quality by urea, boron and zinc foliar spray. J. Plant Nutr. 41, 609–618. doi: 10.1080/01904167.2017.1406111
An, J.-C., Liu, Y.-Z., Yang, C.-Q., Zhou, G.-F., Wei, Q.-J., and Peng, S.-A. (2012). Isolation and expression analysis of CiNIP5, a citrus boron transport gene involved in tolerance to boron deficiency. Sci. Hortic. 142, 149–154. doi: 10.1016/j.scienta.2012.05.013
Appel, H. M., Arnold, T. M., and Schultz, J. C. (2012). Effects of jasmonic acid, branching and girdling on carbon and nitrogen transport in poplar. New Phytol. 195, 419–426. doi: 10.1111/j.1469-8137.2012.04171.x
Bieleski, R. (1982). “Sugar alcohols,” in Plant Carbohydrates I, eds F.A. Loewus and W. Tanner, (Berlin: Springer), 158–192. doi: 10.1007/978-3-642-68275-9_5
Boaretto, R. M., Quaggio, J. A., Mattos, D. Jr., Muraoka, T., and Boaretto, A. E. (2011). Boron uptake and distribution in field grown citrus trees. J. Plant Nutr. 34, 839–849. doi: 10.1080/01904167.2011.544353
Brown, P. H., and Shelp, B. J. (1997). Boron mobility in plants. Plant Soil 193, 85–101. doi: 10.1007/978-94-011-5580-9_7
Bryson, G. M., Mills, H. A., Sasseville, D. N., Jones, J. B., and Barker, A. V. (2014). Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis, Interpretation and Use of Results of Agronomic and Horticultural Crop Plant Tissue. Athens: Micro-Macro Publishing.
Cañon, P., Aquea, F., Rodríguez-Hoces De La Guardia, A., and Arce-Johnson, P. (2013). Functional characterization of Citrus macrophylla BOR1 as a boron transporter. Physiol. Plant 149, 329–339.
Chormova, D., Messenger, D. J., and Fry, S. C. (2014). Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion but not post-secretion. Plant J. 77, 534–546. doi: 10.1111/tpj.12403
Correia, M. J., Osório, M. L., Osório, J., Barrote, I., Martins, M., and David, M. M. (2006). Influence of transient shade periods on the effects of drought on photosynthesis, carbohydrate accumulation and lipid peroxidation in sunflower leaves. J. Exp. Bot. 58, 75–84. doi: 10.1016/j.envexpbot.2005.06.015
Dannel, F., Pfeffer, H., and Römheld, V. (2000). Characterization of root boron pools, boron uptake and boron translocation in sunflower using the stable isotopes 10B and 11B. Funct. Plant Biol. 27, 397–405.
De Schepper, V., De Swaef, T., Bauweraerts, I., and Steppe, K. (2013). Phloem transport: a review of mechanisms and controls. J. Exp. Bot. 64, 4839–4850. doi: 10.1093/jxb/ert302
DeMoranville, C., and Deubert, K. (1987). Effect of commercial calcium-boron and manganese-zinc formulations on fruit set of cranberries. J. Hortic. Sci. 62, 163–169. doi: 10.1080/14620316.1987.11515765
Dinant, S., Bonnemain, J.-L., Girousse, C., and Kehr, J. (2010). Phloem sap intricacy and interplay with aphid feeding. C R Biol. 333, 504–515. doi: 10.1016/j.crvi.2010.03.008
Dong, X., Liu, G., Wu, X., Lu, X., Yan, L., Muhammad, R., et al. (2016). Different metabolite profile and metabolic pathway with leaves and roots in response to boron deficiency at the initial stage of citrus rootstock growth. Plant Physiol. Biochem. 108, 121–131. doi: 10.1016/j.plaphy.2016.07.007
Eichert, T., and Goldbach, H. E. (2010). Transpiration rate affects the mobility of foliar-applied boron in Ricinus communis L. cv. Impala. Plant Soil 328, 165–174. doi: 10.1007/s11104-009-0094-y
FAO (2019). Food and Agriculture Organization of the United Nation. Available online at: http://www.fao.org/faostat/en/#data/QC
Fernández, V., and Brown, P. H. (2013). From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front. Plant Sci. 4:289. doi: 10.3389/fpls.2013.00289
Fernández, V., and Eichert, T. (2009). Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 28, 36–68. doi: 10.1080/07352680902743069
Fregoni, M., Scienza, A., and Miravalle, R. (1978). Studio sul ruolo del boro nella fioritura e fruttificazione della vite. Riv. Ortoflorofrut 62, 615–622.
Guidong, L., Cuncang, J., and Yunhua, W. (2011). Distribution of boron and its forms in young “Newhall” navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks in response to deficient and excessive boron. Soil Sci. Plant Nutr. 57, 93–104. doi: 10.1080/00380768.2010.551299
Hijaz, F., and Killiny, N. (2014). Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS One 9:e101830. doi: 10.1371/journal.pone.0101830
Hoagland, D. R., and Arnon, D. I. (1950). The water-culture method for growing plants without soil. Circular, 347:32.
Hu, H., Penn, S. G., Lebrilla, C. B., and Brown, P. H. (1997). Isolation and characterization of soluble boron complexes in higher plants (The mechanism of phloem mobility of boron). Plant Physiol. 113, 649–655. doi: 10.1104/pp.113.2.649
Huang, L., Bell, R. W., and Dell, B. (2001). Boron supply into wheat (Triticum aestivum L. cv. Wilgoyne) ears whilst still enclosed within leaf sheaths. J. Exp. Bot. 52, 1731–1738. doi: 10.1093/jexbot/52.361.1731
Huang, L., Bell, R. W., and Dell, B. (2008). Evidence of phloem boron transport in response to interrupted boron supply in white lupin (Lupinus albus L. cv. Kiev Mutant) at the reproductive stage. J. Exp. Bot. 59, 575–583. doi: 10.1093/jxb/erm336
Killiny, N., Hijaz, F., Harper, S. J., and Dawson, W. O. (2017). Effects of Citrus tristeza closterovirus infection on phloem sap and released volatile organic compounds in Citrus macrophylla. Physiol. Mol. Plant Pathol. 98, 25–36. doi: 10.1016/j.pmpp.2017.03.003
Konrad, W., Katul, G., Roth-Nebelsick, A., and Jensen, K. H. (2018). Xylem functioning, dysfunction and repair: a physical perspective and implications for phloem transport. Tree Physiol. 39, 243–261. doi: 10.1093/treephys/tpy097
Lehto, T., Räisänen, M., Lavola, A., Julkunen-Tiitto, R., and Aphalo, P. J. (2004). Boron mobility in deciduous forest trees in relation to their polyols. New Phytol. 163, 333–339. doi: 10.1111/j.1469-8137.2004.01105.x
Liakopoulos, G., Stavrianakou, S., Nikolopoulos, D., Karvonis, E., Vekkos, K.-A., Psaroudi, V., et al. (2009). Quantitative relationships between boron and mannitol concentrations in phloem exudates of Olea europaea leaves under contrasting boron supply conditions. Plant Soil 323, 177–186. doi: 10.1007/s11104-009-9923-2
Liu, G., Dong, X., Liu, L., Wu, L., Peng, S. A., and Jiang, C. (2015). Metabolic profiling reveals altered pattern of central metabolism in navel orange plants as a result of boron deficiency. Physiol. Plant 153, 513–524. doi: 10.1111/ppl.12279
Liu, G.-D., Wang, R.-D., Liu, L.-C., Wu, L.-S., and Jiang, C.-C. (2013). Cellular boron allocation and pectin composition in two citrus rootstock seedlings differing in boron-deficiency response. Plant Soil 370, 555–565. doi: 10.1007/s11104-013-1659-3
Liu, G.-D., Wang, R.-D., Wu, L.-S., Peng, S.-A., Wang, Y.-H., and Jiang, C.-C. (2012). Boron distribution and mobility in navel orange grafted on citrange and trifoliate orange. Plant Soil 360, 123–133. doi: 10.1007/s11104-012-1225-4
Mäkelä, P., Mclaughlin, J. E., and Boyer, J. S. (2005). Imaging and quantifying carbohydrate transport to the developing ovaries of maize. Ann. Bot. 96, 939–949. doi: 10.1093/aob/mci246
Marschner, H., Kirkby, E., and Cakmak, I. (1996). Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 49, 1255–1263. doi: 10.1093/jxb/47.special_issue.1255
Martínez-Cuenca, M.-R., Primo-Capella, A., and Forner-Giner, M. A. (2019). Key role of boron compartmentalisation-related genes as the initial cell response to low B in citrus genotypes cultured in vitro. Hortic. Environ. Biotechnol. 60, 519–530. doi: 10.1007/s13580-018-0054-7
Mei, L., Sheng, O., Peng, S.-A., Zhou, G.-F., Wei, Q.-J., and Li, Q.-H. (2011). Growth, root morphology and boron uptake by citrus rootstock seedlings differing in boron-deficiency responses. Sci. Hortic. 129, 426–432. doi: 10.1016/j.scienta.2011.04.012
Mishra, S., and Heckathorn, S. (2016). “Boron stress and plant carbon and nitrogen relations,” in Progress in Botany 77, eds U. Lüttge, F. M. Cánovas, and R. Matyssek, (Cham: Springer International Publishing), 333–355. doi: 10.1007/978-3-319-25688-7_11
Miwa, K., and Fujiwara, T. (2010). Boron transport in plants: co-ordinated regulation of transporters. Ann. Bot. 105, 1103–1108. doi: 10.1093/aob/mcq044
Osaki, M., Iyoda, M., Yamada, S., and Tadano, T. (1995). Effect of mutual shading on carbon distribution in rice plant. Soil Sci. Plant Nutr. 41, 235–244. doi: 10.1080/00380768.1995.10419580
Pfeffer, H., Dannel, F., and Römheld, V. (2001). Boron compartmentation in roots of sunflower plants of different boron status: a study using the stable isotopes 10B and 11B adopting two independent approaches. Physiol. Plant 113, 346–351. doi: 10.1034/j.1399-3054.2001.1130307.x
Reid, R. (2014). Understanding the boron transport network in plants. Plant Soil 385, 1–13. doi: 10.1007/s11104-014-2149-y
Savage, J. A., Clearwater, M. J., Haines, D. F., Klein, T., Mencuccini, M., Sevanto, S., et al. (2016). Allocation, stress tolerance and carbon transport in plants: how does phloem physiology affect plant ecology? Plant Cell Environ. 39, 709–725. doi: 10.1111/pce.12602
Shelp, B. J., Vivekanandan, P., Vanderpool, R. A., and Kitheka, A. M. (1996). Translocation and effectiveness of foliar-fertilized boron in broccoli plants of varying boron status. Plant Soil 183, 309–313. doi: 10.1007/bf00011446
Sheng, O., Song, S., Peng, S., and Deng, X. (2009). The effects of low boron on growth, gas exchange, boron concentration and distribution of ‘Newhall’navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks. Sci. Hortic. 121, 278–283. doi: 10.1016/j.scienta.2009.02.009
Shireen, F., Nawaz, M. A., Chen, C., Zhang, Q., Zheng, Z., Sohail, H., et al. (2018). Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 19:1856. doi: 10.3390/ijms19071856
Stangoulis, J., Tate, M., Graham, R., Bucknall, M., Palmer, L., Boughton, B., et al. (2010). The mechanism of boron mobility in wheat and canola phloem. Plant Physiol. 153, 876–881. doi: 10.1104/pp.110.155655
Stangoulis, J. C., Brown, P. H., Bellaloui, N., Reid, R. J., and Graham, R. D. (2001). The efficiency of boron utilisation in canola. Funct. Plant Biol. 28, 1109–1114.
Takano, J., Miwa, K., and Fujiwara, T. (2008). Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 13, 451–457. doi: 10.1016/j.tplants.2008.05.007
Wang, H., Liu, Z., Wang, S., Cui, D., Zhang, X., Liu, Y., et al. (2017). UHPLC-Q-TOF/MS based plasma metabolomics reveals the metabolic perturbations by manganese exposure in rat models. Metallomics 9, 192–203. doi: 10.1039/c7mt00007c
Wang, H., Ma, F., and Cheng, L. (2010). Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 232, 511–522. doi: 10.1007/s00425-010-1194-x
Wang, N., Wei, Q., Yan, T., Pan, Z., and Liu, Y. (2016). Improving the boron uptake of boron-deficient navel orange plants under low boron conditions by inarching boron-efficient rootstock. Sci. Hortic. 199, 49–55. doi: 10.1016/j.scienta.2015.12.014
Wang, N., Yan, T., Fu, L., Zhou, G., and Liu, Y. (2014). Differences in boron distribution and forms in four citrus scion–rootstock combinations with contrasting boron efficiency under boron-deficient conditions. Trees 28, 1589–1598. doi: 10.1007/s00468-014-1067-1
Wang, N., Yang, C., Pan, Z., Liu, Y., and Peng, S. A. (2015). Boron deficiency in woody plants: various responses and tolerance mechanisms. Front. Plant Sci. 6:916. doi: 10.3389/fpls.2015.00916
Wei, R., Li, G., and Seymour, A. B. (2010). High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics. Anal. Chem. 82, 5527–5533. doi: 10.1021/ac100331b
Will, S., Eichert, T., Fernández, V., Möhring, J., Müller, T., and Römheld, V. (2011). Absorption and mobility of foliar-applied boron in soybean as affected by plant boron status and application as a polyol complex. Plant Soil 344, 283–293. doi: 10.1007/s11104-011-0746-6
Wu, X., Lu, X., Riaz, M., Yan, L., and Jiang, C. (2018). Boron deficiency and toxicity altered the subcellular structure and cell wall composition architecture in two citrus rootstocks. Sci. Hortic. 238, 147–154. doi: 10.1016/j.scienta.2018.04.057
Yoshinari, A., and Takano, J. (2017). Insights into the mechanisms underlying boron homeostasis in plants. Front. Plant Sci. 8:1951. doi: 10.3389/fpls.2017.01951
Zhou, G.-F., Liu, Y.-Z., Sheng, O., Wei, Q.-J., Yang, C.-Q., and Peng, S.-A. (2015). Transcription profiles of boron-deficiency-responsive genes in citrus rootstock root by suppression subtractive hybridization and cDNA microarray. Front. Plant Sci. 5:795. doi: 10.3389/fpls.2014.00795
Keywords: citrus, foliar boron, girdling, shading, phloem transport, sucrose
Citation: Du W, Pan Z-Y, Hussain SB, Han Z-X, Peng S-A and Liu Y-Z (2020) Foliar Supplied Boron Can Be Transported to Roots as a Boron-Sucrose Complex via Phloem in Citrus Trees. Front. Plant Sci. 11:250. doi: 10.3389/fpls.2020.00250
Received: 27 November 2019; Accepted: 18 February 2020;
Published: 10 March 2020.
Edited by:
Victoria Fernandez, Polytechnic University of Madrid, SpainReviewed by:
Dirceu Mattos Jr., Instituto Agronômico de Campinas (IAC), BrazilGeorgios Liakopoulos, Agricultural University of Athens, Greece
Copyright © 2020 Du, Pan, Hussain, Han, Peng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Zhong Liu, bGl1eW9uZ3pob25nQG1haWwuaHphdS5lZHUuY24=
 Wei Du
Wei Du Zhi-Yong Pan
Zhi-Yong Pan Syed Bilal Hussain
Syed Bilal Hussain Zhong-Xing Han1,2
Zhong-Xing Han1,2 Yong-Zhong Liu
Yong-Zhong Liu