- 1Department of Microbiology and Cell Science, Genetics Institute, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL, United States
- 2Department of Pathology, Immunology and Laboratory Medicine, University of Florida, Gainesville, FL, United States
Herein, an analytical method was developed for extraction and quantification of benzbromarone and tolfenamic acid in citrus and soil matrices using liquid-liquid extraction followed by liquid chromatography-triple quadrupole-tandem mass spectrometry analysis. The compounds were extracted using 0.1% formic acid in 6:4 ethyl acetate and n-hexane solution, and the analytes were separated using a mixture of 0.1% formic acid in ultrapure water and 0.1% formic acid in acetonitrile as mobile phase. A six-point in-matrix calibration curve was constructed providing good linearity with coefficients of determination R2 ≥ 0.98. The limits of detection and quantification for benzbromarone and tolfenamic acid were 3.0 and 10.0 μg/kg in roots, peel, juice, and soil, and 4.0 and 12.0 μg/kg for leaves samples, respectively. The method yielded excellent recoveries between 81.3 and 101.2%, with relative standard deviation ≤9.5% in the matrices. The developed technique provides a simple and sensitive method for the determination of the chemicals and can be applied to agricultural practices.
Introduction
Huanglongbing (HLB), or citrus greening disease, is one of the most devastating citrus diseases affecting the world citrus production today (Bové, 2006). The disease originated in China and was first reported in the United States in 2005. Many other countries, such as Brazil, India, Iran, Cuba, the Dominican Republic, and Ethiopia have subsequently reported HLB infection (Tian et al., 2014). HLB is a highly contagious disease caused by the fastidious, phloem-limited bacteria Liberibacter asiaticus and transmitted by a psyllid vector (Diaphorina citri) (Jagoueix et al., 1994). After infection, the susceptible tree shows symptoms of the disease such as stunted growth, bitter and irregular sized fruit, premature fruit drop and blotchy mottle on the leaves. Over time, the infection progresses to tree death. Due to these symptoms, HLB has caused loss in revenues from reduced tree productivity. Although accurate values of production loss are difficult to calculate due to fluctuations in prices as well as yields, from 2000 to 2017, fresh and processed fruit utilization decreased by 71 percent (Court et al., 2018).
The lack of an axenic culture of L. asiaticus has hindered the identification and/or development of antimicrobial compounds that could effectively treat the infection. Using bioinformatics coupled with biochemical assays, we were able to identify two compounds, benzbromarone and tolfenamic acid, that could be used as a antimicrobial agents against L. asiaticus (Pagliai et al., 2014; Gardner et al., 2016). We found that, in L. asiaticus, benzbromarone acts by inhibiting the activity of the transcription factor LdtR, while tolfenamic acid modulates the activity of the transcription accessory protein PrbP. We found that the spray application of these compounds in citrus shoots and small citrus trees led to a significant reduction in the transcriptional activity of L. asiaticus (Pagliai et al., 2014; Gardner et al., 2016).
Benzbromarone (3,5-dibromo-4-hydroxyphenyl)-[(2-ethyl-1-benzofuran-3-yl)methanone] and tolfenamic acid (2-[(3-chloro-2-methylphenyl)amino]benzoic acid) are currently approved for their use in animal and/or human treatments. Benzbromarone is registered in over 20 countries throughout Asia, South America, and Europe and is used for the treatment of gout (Heel et al., 1977; Lee et al., 2008). Tolfenamic acid is a non-steroidal anti-inflammatory drug (NSAID) widely used in veterinary medicine to treat inflammation, pain, and fever (Smith et al., 2008). However, their use in agricultural practices has not yet been approved.
While the repurposing of these pharmaceutical drugs into new antimicrobials is very attractive, accurate methods that allow their determination in plant and soil substrates is needed to comply with good agricultural practices (GAP) and with domestic and international safety requirements. The potential application of benzbromarone and tolfenamic acid in citrus groves may lead to accumulation of these compounds in citrus products that may feed into human and animal consumption. Concurrently, the environmental impact due to the spray of the chemicals in this agricultural practice leaching into the soil and roots could become another source of contamination not only to humans but also to wildlife. Therefore, it is necessary to develop a reliable and sensitive method to extract and quantify both benzbromarone and tolfenamic acid in citrus leaves, fruit peel, juice, roots, and soil prior to its widespread use as a treatment for HLB.
Detection methods for benzbromarone and tolfenamic acid have been developed for several animal and human tissues as well as wastewater (Beyer et al., 2005; Gallo et al., 2008; Guan et al., 2016) however, methods for the simultaneous analysis of benzbromarone and tolfenamic acid from plant tissue are not available.
In this study, we describe a Liquid-Liquid Extraction (LLE) method for the simultaneous extraction of both compounds, benzbromarone and tolfenamic acid, and detection with the coupling of liquid chromatography with triple quadruple tandem mass spectrometry (LC-MS/MS) resulting in high specificity and sensitivity detection in citrus plant tissues and soil samples.
Materials and Methods
Chemicals and Reagents
Benzbromarone and tolfenamic acid analytical standards were purchased from Sigma (St. Louis, MO, United States). HPLC grade ethyl acetate, n-hexane, 0.1% formic acid in water, and 0.1% formic acid in acetonitrile were prepared from chemicals provided by Fisher Scientific (Pittsburgh, PA, United States). 0.1% formic acid in ethyl acetate and n-hexane (6:4, v/v) was prepared by diluting 1 mL formic acid in a 1 L flask containing 600 mL ethyl acetate and 400 mL n-hexane.
Preparation of Standard Stock Solution
Ten mg of benzbromarone or tolfenamic acid were separately prepared in volumetric flasks by dissolving the chemical in 10 mL methanol and acetonitrile (1:1, v/v), yielding 1000 μg/mL stock solution. The intermediate and working solutions were prepared by further dilution with the same solvent, yielding variable concentrations ranging from 10.0− 60.0 μg/kg for roots, fruit peel, juice, and soil, and 12.0− 72.0 μg/kg for leaves for constructing the calibration curves. All standard solutions were stored at 4°C for an extended period (>6 months) with no apparent degradation of the compounds observed (data not shown).
Sample Preparation
The different matrices (citrus leaves, roots, fruit peels, juice, and soil) were collected from mature citrus trees from groves in South Florida and stored at −80°C immediately. 15–20 mature leaves were collected randomly from multiple locations in the tree canopy. Root and soil samples were collected from the area within a 1-meter radius from the trunk of the citrus tree, a core of 30 cm was obtained from the area, and 50 mL tubes were filled with the soil and root. Fruit peel and fruit juices were collected from fruit that were ready to harvest by the time of sample collection. Citrus leaves, roots, fruit peels, and soil were lyophilized by a bulk tray dryer (Labconco, Kansas, MO, United States), then homogenized into a powder using a Geno/Grinder 2000 (Metuchen, NJ, United States). 300 mg of the resulting powder was weighed on a Mettler Toledo XS203S scientific scale (Bohemia, NY, United States) into a 15 mL centrifuge tube. For the optimization of the extraction method, samples were spiked with 30 μL of the standard solutions (1 μg/mL) and incubated at room temperature for 1 h. Non-spiked samples were used as background controls. Then, 3 mL 0.1% formic acid in ethyl acetate and n-hexane (6:4, v/v) were added, followed by vortexing (Fisher Dig Multi-Tube Vortexer, Pittsburgh, PA, United States) for 5 min. The mixture was centrifuged at 17,000 × g (Avanti J-26 XP, Beckman Coulter, United States) for 15 min at 4°C. The supernatant was decanted and transferred to a new 15 mL centrifuge tube and then dried completely under nitrogen at 40°C (Organomation, Berlin, MA, United States). Finally, the dried extract was reconstituted with 1 mL of ultra-pure water and acetonitrile (1:1, v/v). The mixture was vortexed for 1 min and centrifuged at 17000 × g for 15 min at 4°C and then filtered through a 0.2 μm syringe filter (Fisher Scientific, Pittsburgh, PA, United States). A volume of 5 μL was injected for LC–MS/MS analysis.
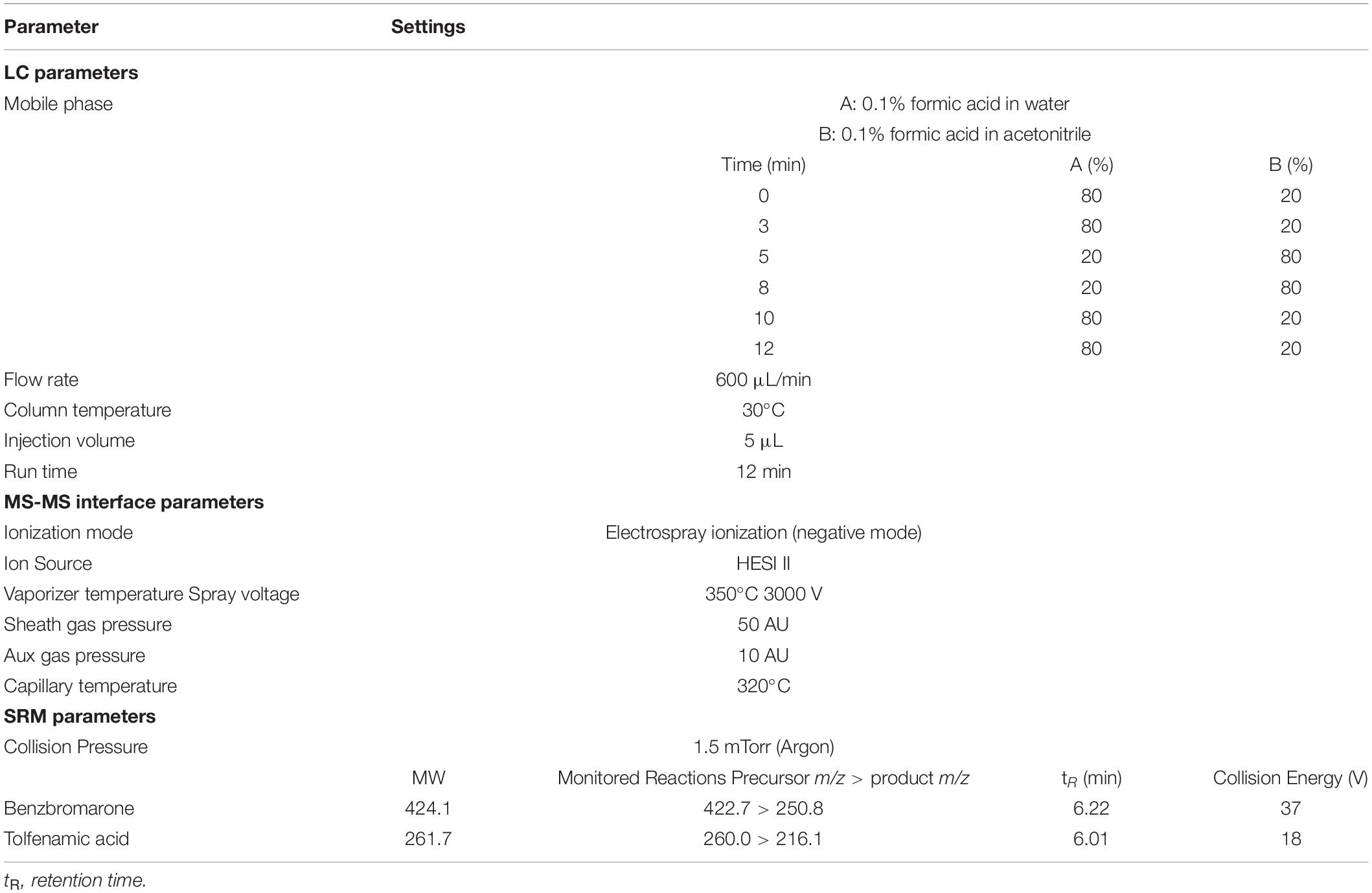
LC-MS/MS Parameters
The analytes were identified using TSQ Quantum Access MAX Triple Quadrupole system coupled with ACCELA 1250 UHPLC system (Waltham, MA, United States). Analyses were carried out using heated-electrospray ionization in negative mode (HESI–) with selected reaction monitoring (SRM). The parameters for detection of benzbromarone and tolfenamic acid were vaporizer temperature, spray voltage, sheath gas pressure, aux gas pressure, capillary temperature, and collision energy.
Gradient separation was carried out using a Waters Symmetry (Milford, MA, United States) C18 (150 × 2.1 mm, 3.5 μm) column. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). All the operating conditions are shown in Table 2.
Analytical Validation
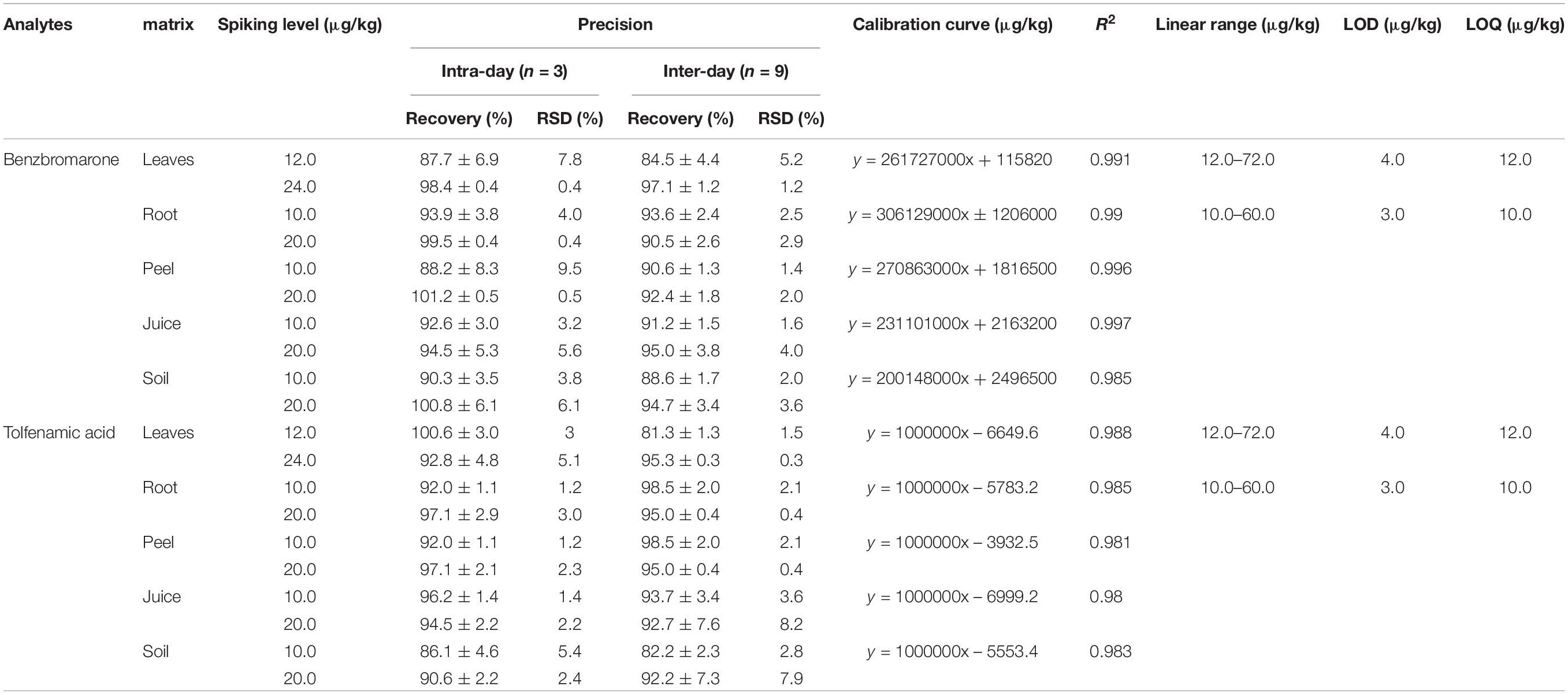
Analytical parameters, including limits of detection (LOD) and quantification (LOQ), linearity, specificity, accuracy, and precision were determined to validate the method. LOD and LOQ were calculated as signal-to-noise (S/N) ratios of 3:1 and 10:1, respectively. Linearity was obtained by analyzing the standard solutions both in solvent and matrix at six-points over the range of 10.0 – 70.0 μg/kg. Specificity means the ability of a method to distinguish between the target analyte being measured in a blank sample from other interfering compounds at or around the retention time of the target analytes. Accuracy (expressed as recovery) was evaluated through spiking blank samples at three different concentrations, whereas precision was expressed as relative standard deviation (RSD ≤ 9.5%).
Results
Optimization of the Standard Solution Preparation
Methanol, acetonitrile, and a combination of methanol and acetonitrile (1:1, v/v) were tested for the standard solution preparation. Since a mixture of methanol and acetonitrile (1;1, v/v) had a higher detection intensity (benzbromarone peak area: 176,926 ± 0.1, tolfenamic acid: 40,448 ± 0.2) than methanol (149,546 ± 0.1, 37,803 ± 0.1) or acetonitrile alone (146,237 ± 0.1,38,576 ± 0.3) at 500.0 μg/kg, it was subsequently used for the preparation of standard solutions in this study.
Optimization of Sample Preparation and Extraction
Leaves were spiked with both analytes at a concentration of 500.0 μg/kg and then incubated at room temperature for 1, 2, 4, and 8 h. There were no statistical differences between the 4 time-points. The recovery rates ranged between 89.1 – 106.6% (SD ≤ 8.2%). Because of the negligible differences, the 1 h incubation was chosen for equilibrating the samples.
Non-polar organic solvents are widely used for extracting the residues from food and agricultural samples (Lee et al., 2017). To evaluate the extraction efficiency for both analytes, leaf powder was extracted with 80% methanol, methanol, acetonitrile, methanol and acetonitrile (1:1, v/v), ethyl acetate and methanol (1:1, v/v), ethyl acetate and acetonitrile (1:1, v/v), ethyl acetate and n-hexane (1:1, v/v), and ethyl acetate and n-hexane (6:4, v/v). The best recovery was obtained in ethyl acetate and n-hexane (6:4, v/v) (85.5 ± 2.0 and 90.5 ± 4.3 percent for benzbromarone and tolfenamic acid, respectively) (Table 1). Further optimization was performed by testing the addition of 0.1% acetic acid, 0.1% formic acid, 0.1% phosphoric acid, and 10 mM ammonium acetate to ethyl acetate and n-hexane (6:4, v/v). It was found that 0.1% formic acid in ethyl acetate and n-hexane (6:4, v/v) yielded the best extraction efficiency (Table 1) and therefore was employed as the extraction solvent for further experiments.
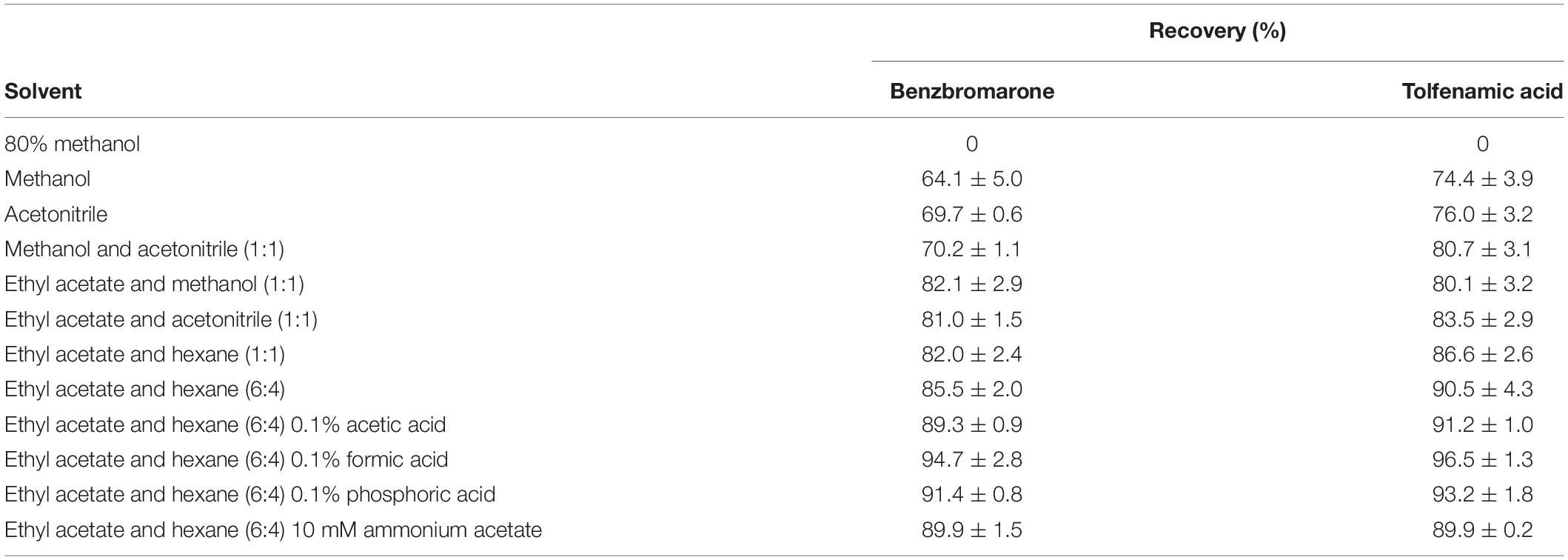
Table 1. Optimization of the extraction efficiency for benzbromarone and tolfenamic acid in citrus matrices.
Optimization of LC-MS/MS Analysis
Both positive and negative heated-electrospray ionization (HESI±) modes were tested for quantification of benzbromarone and tolfenamic acid. Both analytes were detectable in negative ion mode; hence HESI– mode was selected for simultaneous quantification. The MS/MS interface parameters and limits of the most sensitive transitions (422.7 > 250.8, 260.0 > 216.1 for tolfenamic acid and benzbromarone, respectively) were used for quantification and confirmation in SRM (Table 2). Based on these operating conditions, the structural analysis of benzbromarone and tolfenamic acid were performed (Figure 1).
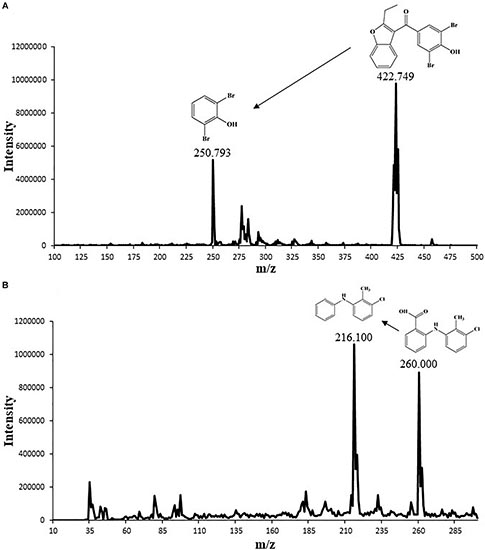
Figure 1. Mass spectrum chromatogram showing the molecular size of (A) benzbromarone (peak 422.749) and (B) tolfenamic acid (peak 260.000) along with their chemical structure and potential degradation fragment.
The column as well as the mobile phase were tested for the best separation and signal sensitivity of the chromatograms. Based on previous research, the separation was performed on a C18 separation column. Using this column, sharp and distinct chromatograms for both benzbromarone and tolfenamic acid were obtained. For the selection of the mobile phase, acetonitrile, methanol, and acetonitrile and methanol (1:1, v/v) were tested. Although clear separation was observed (data not shown) when methanol was used as the organic phase, higher intensity chromatograms were obtained when acetonitrile was used when compared to acetonitrile and methanol (1:1, v/v) or methanol alone. Then, 0.1% acetic acid, 0.1% formic acid, 0.5% formic acid, 1% formic acid, 0.1% phosphoric acid, and 10 mM ammonium acetate were tested as the aqueous phase. The best results were obtained using 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B); these were therefore selected for further analyses.
Method Validation
Specificity
The specificity of the method was tested by analyzing control citrus leaves, roots, fruit peel, juice, and soil samples. As shown in the chromatograms, there were no apparent interference peaks at or around the retention time for the control (non-spiked samples) and spiked samples when compared to that of the standards (Supplementary Figures S1, S2).
Matrix Effect and Linearity
Matrix effect (ME) refers to a difference in mass spectrometric response for the tested analytes in standard solution vs. the response for the same analytes in matrix. Negative values represent the degree of suppression (%), whereas positive values represent the degree of enhancement (%). MEs estimated from two concentrations (500.0 and 1000.0 μg/kg) (n = 3) were ranged between −5.4% to 10.8% in all matrices; these MEs were very mild and negligible. Therefore, for accuracy, matrix-matched calibrations were used for quantification. Six concentration levels equivalent to 1, 2, 3, 4, 5, and 6-fold the LOQ (n = 4) were constructed to establish the calibration curves. For citrus roots, fruit peel, juice, and soil, the concentrations were 10.0, 20.0, 30.0, 40.0, 50.0, and 60.0 μg/kg for both benzbromarone and tolfenamic acid. Similarly, for citrus leaves, the concentrations were 12.0, 24.0, 36.0, 48.0, 60.0, and 72.0 μg/kg. Good linearity was achieved for each matrix-matched calibration, with a coefficient of determination (R2) ≥ 0.98 (Table 3).
Accuracy and Precision
The accuracy and precision were measured as recovery and RSD, respectively. Samples were spiked at 10.0 and 20.0 μg/kg in citrus roots, leaves, fruit peel, juice, and soil samples for both benzbromarone and tolfenamic acid. Excellent recovery was obtained for benzbromarone (between 84.5% ± 4.4% ∼ 100.8% ± 6.1% with RSD ≤ 9.5%) and for tolfenamic acid (81.3% ± 1.3% ∼ 100.6% ± 3.0% with RSD ≤ 8.2%) for both inter- and intra-day analysis in the different matrices, respectively (Table 3).
LOD and LOQ
The limits of detection (LOD) and limits of quantification (LOQ) were calculated as 3 and 10 times the signal-to-noise ratio, respectively. The LOD was 3.0 μg/kg, whereas the LOQ was 10.0 μg/kg, for both benzbromarone and tolfenamic acid in roots, peel, juice, and soil (Table 3). A slightly higher LOD and LOQ of 4.0 and 12 μg/kg, respectively, was observed in leaf samples for both compounds. This increase may be due to the presence of a residual green pigmentation when the extraction method developed was used on the leaf samples, possibly affecting the background of the chromatograms. Since the detection limits were in the μg/kg range, we can conclude that the analytical method has a high sensitivity to detect and quantify the residues in environmental samples.
Method Application
We next tested if the chemicals may be efficiently detected in small citrus plants. Two-year-old healthy Citrus sinensis trees planted on an experimental plot located at the University of Florida were used for the experiment. The trees were exposed to environmental conditions such as direct sunlight, weather, and wind. Benzbromarone and tolfenamic acid were delivered by spray to the trees until saturated (n = 3). Leaf samples (5–10 leaves) were then collected, and the concentration of each chemical was evaluated 24 and 48 h post-spray (Figure 2). Previous phytotoxicity assays with tolfenamic acid and benzbromarone have shown no adverse effect when citrus plants are sprayed at 65,526 μg/kg and 106,052 μg/kg (250 μM), respectively (Pagliai et al., 2014; Gardner et al., 2016). Tolfenamic acid was detected at ∼536 μg/kg after 24 h post-spray, while it decreased to 138 μg/kg after 48 h post-spray (Figure 2B). A similar effect was observed with benzbromarone when the trees were sprayed to saturation (Figure 2A). These observations confirm that the method developed can be used to detect both chemicals from citrus plant tissue even when exposed to environmental conditions.
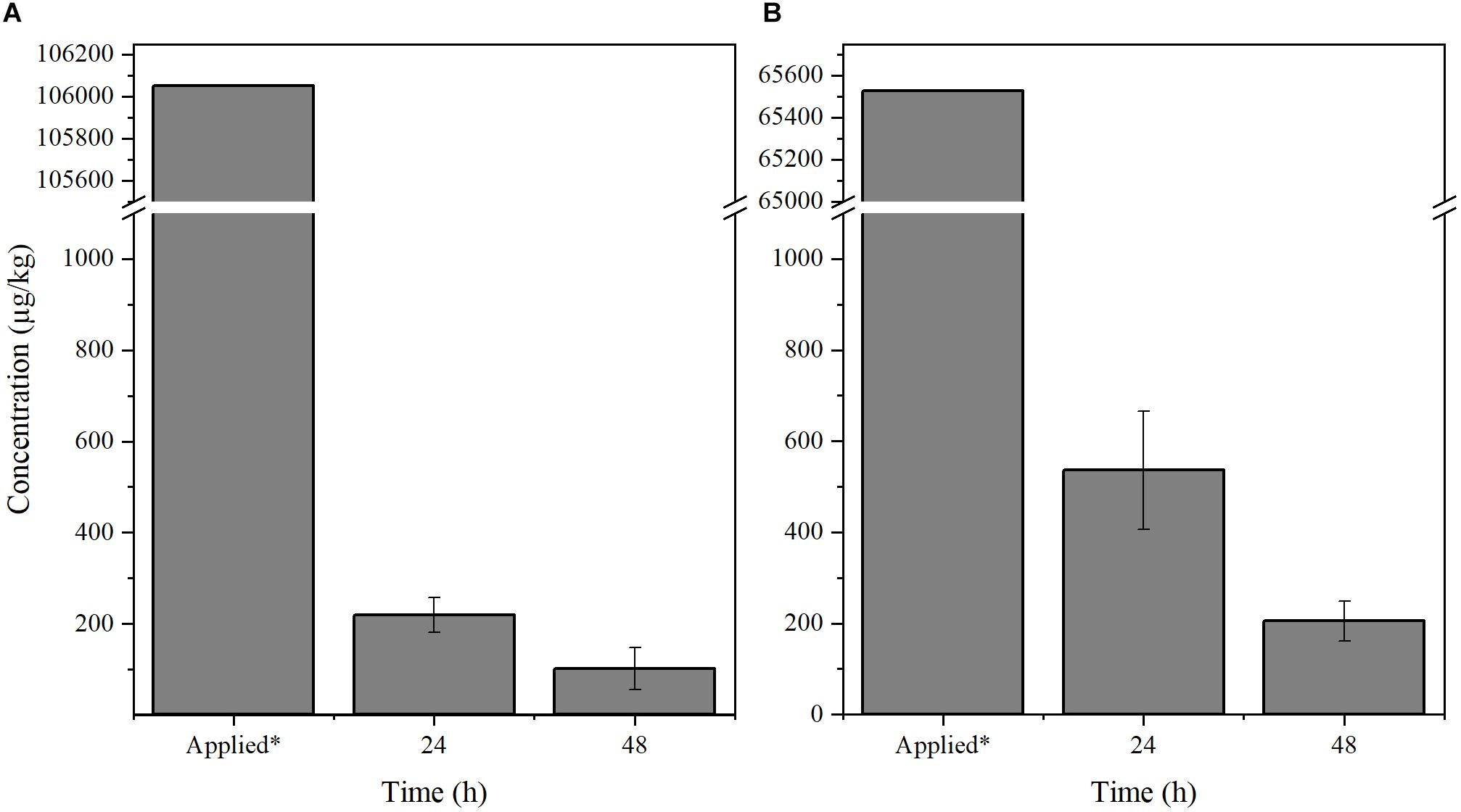
Figure 2. Quantification of (A) benzbromarone and (B) tolfenamic acid from citrus tissue. Leaf samples were collected from 2-year-old Citrus sinensis trees (n = 3) sprayed with benzbromarone and tolfenamic acid, respectively. The concentration of both compounds were determined using the described LC-MS/MS method 24 and 48 h post-spray. *Concentration of the chemicals applied to saturation.
Discussion
In this study, we described the optimization of an extraction and analytical method for the simultaneous detection and quantification of benzbromarone and tolfenamic acid in citrus plant and soil matrices. Since these compounds are proposed for the management of HLB, the detection and evaluation of their concentration is critical.
Previous studies have reported the quantification of benzbromarone in rats and human tissues using gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography-quadruple time of flight mass spectrometry (HPLC-Q-TOF), respectively (Beyer et al., 2005; Wu et al., 2012). Beyer et al. (2005) used GC-MS analysis methods for the detection of benzbromarone with an estimated value of 0.05 mg/L for LOD in human urine. The method we have developed is 10 times more sensitive with an LOD as low as 3.0 μg/kg (∼0.003 mg/L).
Tolfenamic acid has been detected in bovine milk samples by LC-MS/MS methods with LODs of 53.0−54.0 μg/kg (Malone et al., 2009; Jedziniak et al., 2012; van Pamel and Daeseleire, 2015), and LOQ of 4.0 μg/kg (Gallo et al., 2008). In bovine meat by LC-MS/MS methods, the LODs were 57.0 – 59.0 μg/kg (Van Hoof et al., 2004; van Pamel and Daeseleire, 2015). Tolfenamic acid was also detected in bovine milk by HPLC methods with a LOQ of 10.0 μg/kg (Gallo et al., 2010). The method described in this study allows for higher sensitivity (3.0 μg/kg) compared to previous studies for the individual detection of tolfenamic acid.
While higher sensitivity has been achieved for the detection of tolfenamic acid using a UPLC-MS/MS method with of LOD 0.076 ng/mL and LOQ 0.216 ng/mL in wastewater (Guan et al., 2016), the method required concentration of the samples using solid-phase extraction (SPE) methods. SPE has been reported for the extraction and purification of benzbromarone and tolfenamic acid. A Vac-Master V-10 cartridge was used for extraction of benzbromarone in human urine resulting in an LOD of 0.05 mg/mL (Beyer et al., 2005). Similarly, C18 end-capped SPE cartridges, Oasis HLB SPE cartridges, Bond-Elut SAX SPE cartridges, and Sep-Oak NH2 SPE cartridges were used for extraction of tolfenamic acid from bovine milk, bovine muscle, and human urine, respectively, with LOQ as low as 4 μg/kg (Mikami et al., 2000; Van Hoof et al., 2004; Beyer et al., 2005; Gallo et al., 2008, 2010; Jedziniak et al., 2012). Although SPE can effectively eliminate interference of other substances found in the matrices and is commonly used in other studies, the SPE procedure incorporates several steps that lead to longer processing times compared to LLE and can be cost prohibitive.
Here we demonstrate a simple, sensitive, and accurate LC-MS/MS method for the determination of benzbromarone and tolfenamic acid for agricultural practices. The method developed was validated in citrus leaves, roots, fruit peel, juice, and soil. We found that a simple mixture of acidified ethyl acetate and n-hexane as an extraction solvent gave excellent recovery for the tested analytes. No further purification procedure was necessary, significantly decreasing the cost of the assay. The developed method was tested and validated by both spiking the matrices with the chemicals and in planta analysis. Therefore, the newly developed method can be used for routine simultaneous analysis of benzbromarone and tolfenamic acid in citrus plant matrices with the potential of expansion to other plant matrices.
In summary, our optimized method allows the simultaneous determination of benzbromarone and tolfenamic acid in plant derived matrices and soil. Moreover, the unit factor of μg/kg provides a low level for LOD and LOQ to ensure high sensitivity of detection across the different citrus tissues.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
DZ performed the research. DZ and DS analyzed the data. TG and CG contributed to the discussion and reviewed the manuscript. DZ, DS, and GL wrote the manuscript. CG and GL conceived the study.
Funding
This work was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Specialty Crop Research Initiative, Citrus Disease Research and Extension (Award Number 2015-70016-23029), and the Plant Biotic Interactions Program (Award Number 2017-03060). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Jose X. Chaparro for providing the small Citrus sinensis in the UF experimental field used for the method application. We acknowledge Evon Debose-Scarlett for the critical reading of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00222/full#supplementary-material
FIGURE S1 | Chromatograms showing the (A) blank, (B) benzbromarone standard (1 μg/mL) and (C) tolfenamic acid standard (1 μg/mL) utilizing the developed quantification method.
FIGURE S2 | Chromatograms of (A) unspiked, (B) benzbromarone spiked (1 μg/mL), and (C) tolfenamic acid spiked (1 μg/mL) matrix, as indicated on the right side of the panel.
References
Beyer, J., Bierl, A., Peters, F. T., and Maurer, H. H. (2005). Screening procedure for detection of diuretics and uricosurics and/or their metabolites in human urine using gas chromatography-mass spectrometry after extractive methylation. Ther. Drug Monit. 27, 509–520. doi: 10.1097/01.ftd.0000160719.96445.91
Bové, J. M. (2006). Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37. doi: 10.4454/jpp.v88i1.828
Court, C. D., Hodges, A. W., Stair, C., and Rahmani, M. (2018). Economic Contributions of the Florida Citrus Industry in 2016-17. Gainesville, FL: University of Florida-IFAS, Food & Resource Economics Department.
Gallo, P., Fabbrocino, S., Dowling, G., Salini, M., Fiori, M., Perretta, G., et al. (2010). Confirmatory analysis of non-steroidal anti-inflammatory drugs in bovine milk by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1217, 2832–2839. doi: 10.1016/j.chroma.2010.02.047
Gallo, P., Fabbrocino, S., Vinci, F., Fiori, M., Danese, V., and Serpe, L. (2008). Confirmatory identification of sixteen non-steroidal anti-inflammatory drug residues in raw milk by liquid chromatography coupled with ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 22, 841–854. doi: 10.1002/rcm.3430
Gardner, C. L., Pagliai, F. A., Pan, L., Bojilova, L., Torino, M. I., Lorca, G. L., et al. (2016). Drug repurposing: tolfenamic acid inactivates PrbP, a transcriptional accessory protein in Liberibacter asiaticus. Front. Microbiol. 7:1630. doi: 10.3389/fmicb.2016.01630
Guan, J., Zhang, C., Wang, Y., Guo, Y., Huang, P., and Zhao, L. (2016). Simultaneous determination of 12 pharmaceuticals in water samples by ultrasound-assisted dispersive liquid–liquid microextraction coupled with ultra-high performance liquid chromatography with tandem mass spectrometry. Anal. Bioanal. Chem. 408, 8099–8109. doi: 10.1007/s00216-016-9913-1
Heel, R. C., Brogden, R. N., Speight, T. M., and Avery, G. S. (1977). Benzbromarone: a review of its pharmacological properties and therapeutic use in gout and hyperuricaemia. Drugs 14, 349–366. doi: 10.2165/00003495-197714050-00002
Jagoueix, S., Bove, J.-M., and Garnier, M. (1994). The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int. J. Syst. Evol. Microbiol. 44, 379–386. doi: 10.1099/00207713-44-3-379
Jedziniak, P., Szprengier-Juszkiewicz, T., Pietruk, K., Śledzińska, E., and Żmudzki, J. (2012). Determination of non-steroidal anti-inflammatory drugs and their metabolites in milk by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 403, 2955–2963. doi: 10.1007/s00216-012-5860-7
Lee, H. S., Kim, S. W., Abd El-Aty, A. M., Chung, H. S., Kabir, M. H., and Rahman, M. M. (2017). Liquid chromatography–tandem mass spectrometry quantification of acetamiprid and thiacloprid residues in butterbur grown under regulated conditions. J. Chromatogr. B 1055, 172–177. doi: 10.1016/j.jchromb.2017.04.021
Lee, M.-H., Graham, G. G., Williams, K. M., and Day, R. O. (2008). A benefit-risk assessment of benzbromarone in the treatment of gout. Drug Saf. 31, 643–665. doi: 10.2165/00002018-200831080-00002
Malone, E. M., Dowling, G., Elliott, C. T., Kennedy, D. G., and Regan, L. (2009). Development of a rapid, multi-class method for the confirmatory analysis of anti-inflammatory drugs in bovine milk using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 1216, 8132–8140. doi: 10.1016/j.chroma.2009.04.078
Mikami, E., Goto, T., Ohno, T., Matsumoto, H., Inagaki, K., Ishihara, H., et al. (2000). Simultaneous analysis of anthranilic acid derivatives in pharmaceuticals and human urine by high-performance liquid chromatography with isocratic elution. J. Chromatogr. B Biomed. Sci. Appl. 744, 81–89. doi: 10.1016/s0378-4347(00)00233-4
Pagliai, F. A., Gardner, C. L., Bojilova, L., Sarnegrim, A., Tamayo, C., Potts, A. H., et al. (2014). The transcriptional activator LdtR from ‘Candidatus Liberibacter asiaticus’ mediates osmotic stress tolerance. PLoS Pathog. 10:e1004101. doi: 10.1371/journal.ppat.1004101
Smith, G. W., Davis, J. L., Tell, L. A., Webb, A. I., and Riviere, J. E. (2008). Extralabel use of nonsteroidal anti-inflammatory drugs in cattle. J. Am. Vet. Med. Assoc. 232, 697–701. doi: 10.2460/javma.232.5.697
Tian, S., Lu, L., Labavitch, J. M., Webb, S. M., Yang, X., Brown, P. H., et al. (2014). Spatial imaging of Zn and other elements in Huanglongbing-affected grapefruit by synchrotron-based micro X-ray fluorescence investigation. J. Exp. Bot. 65, 953–964. doi: 10.1093/jxb/ert450
Van Hoof, N., De Wasch, K., Poelmans, S., Noppe, H., and De Brabander, H. (2004). Multi-residue liquid chromatography/tandem mass spectrometry method for the detection of non-steroidal anti-inflammatory drugs in bovine muscle: optimisation of ion trap parameters. Rapid Commun. Mass Spectrom. 18, 2823–2829. doi: 10.1002/rcm.1683
van Pamel, E., and Daeseleire, E. (2015). A multiresidue liquid chromatographic/tandem mass spectrometric method for the detection and quantitation of 15 nonsteroidal anti-inflammatory drugs (NSAIDs) in bovine meat and milk. Anal. Bioanal. Chem. 407, 4485–4494. doi: 10.1007/s00216-015-8634-1
Keywords: benzbromarone, tolfenamic acid, agricultural products, citrus, liquid-liquid extraction, LC-MS/MS
Citation: Zhang D, da Silva DR, Garrett TJ, Gonzalez CF and Lorca GL (2020) Method Optimization: Analysis of Benzbromarone and Tolfenamic Acid in Citrus Tissues and Soil Using Liquid Chromatography Coupled With Triple-Quadrupole Mass Spectrometry. Front. Plant Sci. 11:222. doi: 10.3389/fpls.2020.00222
Received: 24 September 2019; Accepted: 12 February 2020;
Published: 06 March 2020.
Edited by:
Pietro Franceschi, Fondazione Edmund Mach, ItalyReviewed by:
Ericsson Coy-Barrera, Universidad Militar Nueva Granada, ColombiaShouchuang Wang, Hainan University, China
Copyright © 2020 Zhang, da Silva, Garrett, Gonzalez and Lorca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graciela L. Lorca, Z2xvcmNhQHVmbC5lZHU=
 Dan Zhang
Dan Zhang Danilo R. da Silva
Danilo R. da Silva Timothy J. Garrett
Timothy J. Garrett Claudio F. Gonzalez
Claudio F. Gonzalez Graciela L. Lorca
Graciela L. Lorca