
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Plant Sci. , 25 February 2019
Sec. Plant Biophysics and Modeling
Volume 10 - 2019 | https://doi.org/10.3389/fpls.2019.00173
Many plants grow organs and tissues with twisted shapes. Arabidopsis mutants with impaired microtubule dynamics exhibit such a phenotype constitutively. Although the activity of the corresponding microtubule regulators is better understood at the molecular level, how large-scale twisting can emerge in the mutants remains largely unknown. Classically, oblique cortical microtubules would constrain the deposition of cellulose microfibrils in cells, and such conflicts at the cell level would be relaxed at the tissue scale by supracellular torsion. This model implicitly assumes that cell-cell adhesion is a key step to transpose local mechanical conflicts into a macroscopic twisting phenotype. Here we tested this prediction using the quasimodo1 mutant, which displays cell-cell adhesion defects. Using the spriral2/tortifolia1 mutant with hypocotyl helical growth, we found that qua1-induced cell-cell adhesion defects restore straight growth in qua1-1 spr2-2. Detached cells in qua1-1 spr2-2 displayed helical growth, confirming that straight growth results from the lack of mechanical coupling between cells rather than a restoration of SPR2 activity in the qua1 mutant. Because adhesion defects in qua1 depend on tension in the outer wall, we also showed that hypocotyl twisting in qua1-1 spr2-2 could be restored when decreasing the matrix potential of the growth medium, i.e., by reducing the magnitude of the pulling force between adjacent cells, in the double mutant. Interestingly, the induction of straight growth in qua1-1 spr2-2 could be achieved beyond hypocotyls, as leaves also displayed a flat phenotype in the double mutant. Altogether, these results provide formal experimental support for a scenario in which twisted growth in spr2 mutant would result from the relaxation of local mechanical conflicts between adjacent cells via global organ torsion.
Because complex morphogenesis generally involves differential growth, mechanical conflicts are widespread in developing organisms. In animals, such conflicts can be resolved through cell rearrangements, as cells are in principle free to move. Yet, cell-cell adhesion often prevents such outcome and patterns of tension and compression appear. Mechanical conflicts can be resolved through global tissue deformation, as shown for instance in the gut (Savin et al., 2011; Nerurkar et al., 2019). Interestingly, some of the relevant mechanotransduction factors play a role in cell-cell adhesion. For instance, cadherins are both central regulators of epithelial cohesions and transducers of mechanical signals inside the cell (Leckband and de Rooij, 2014). Therefore, while mechanical conflicts emerge from differential growth and cell-cell adhesion, they also in turn contribute to growth patterns and adhesion through the cell response to mechanical stress, in a feedback loop.
With few exceptions such as pollen tube and fiber cell growth (Gorshkova et al., 2012; Chebli and Geitmann, 2017; Marsollier and Ingram, 2018), cell-cell adhesion and thus symplastic growth is ubiquitous in developing plant organs. The presence of contiguous cell walls with a pectin-rich middle lamella maintains adhesion between adjacent cells (Jarvis et al., 2003) (Daher and Braybrook, 2015) Besides, many reports point at the high degree of growth heterogeneity in plant tissues (Hong et al., 2018). It follows that mechanical conflicts are widespread in growing plants. As reported in animals, plant cells are able to sense and respond to such cues to control cell division plane orientation (Lintilhac and Vesecky, 1984; Louveaux et al., 2016), growth direction (Green and King, 1966; Hamant et al., 2008), cell polarity (Heisler et al., 2010; Nakayama et al., 2012; Bringmann and Bergmann, 2017) and cell identity (Coutand et al., 2009; Landrein et al., 2015). In parallel to these active responses to stress, mechanical conflicts may also be resolved through passive and global tissue deformation (Coen et al., 2004). For instance, mechanical conflicts are thought to play a major role in shaping complex floral shapes, such as Antirhinum petals, as a result of instructive biochemical signals, but without necessarily involving an active mechanical feedback on cells (Coen and Rebocho, 2016; Rebocho et al., 2017). One of the challenges for future research in this area is to understand the relative contributions of passive and active responses to mechanical stress in morphogenesis. Here we take the example of organ twisting to explore that question.
Several mutations on microtubule regulators, or even on tubulins, lead to twisted organs in Arabidopsis (Ishida et al., 2007b; Smyth, 2016). In such mutants, cells exhibit oblique cortical microtubule orientations and the handedness of the microtubule helix is always opposite to the handedness of tissue growth (Ishida et al., 2007a). For instance, the lefty mutations in α-tubulins lead to both a left-handed helical growth and a right-handed cortical microtubule orientations in the root epidermis (Thitamadee et al., 2002). Such phenotypes are only partially understood.
What is best known is the relation between microtubule orientation and growth: except for a few counterexamples [e.g., (Himmelspach et al., 2003; Sugimoto et al., 2003)], cortical microtubules generally guide the deposition of cellulose microfibrils; as cellulose microfibril stiffness constrain cell growth direction, cortical microtubule orientation becomes a proxy for the mechanical anisotropy of cell walls. Therefore, right-handed microtubule orientations would inevitably drive cell growth direction in a left-handed helix (Thitamadee et al., 2002; Smyth, 2016). Conversely, organ twisting is affected when the cellulose synthase—microtubule nexus is impaired in the csi1 mutant (Landrein et al., 2013). Another cell wall mutant has recently been shown to have organ twisting without affecting microtubule organization, but is nevertheless believed to impact cellulose organization and cell wall mechanical anisotropy (Saffer et al., 2017).
What is least known is 2-fold. First, it is unclear how microtubule arrays would acquire a stable and oblique orientation. Reports so far rather suggest that unstable microtubules tend to acquire a right-handed orientation (as in the lefty mutants), while stabilized microtubules acquire a left-handed orientation (Ishida et al., 2007b). This latter case is typical of the spiral2/tortifolia1 mutant, which exhibits right-handed helical growth (Buschmann et al., 2004; Shoji et al., 2004). SPR2 was recently shown to bind and stabilize the minus end of microtubules to control their depolymerization rate, with an indirect impact on microtubule severing (Fan et al., 2018; Nakamura et al., 2018), although this latter point is debated and might depend on tissue identity (Wightman et al., 2013). In the end, microtubule dynamics are stimulated in spr2 mutants, resulting in more stable cortical microtubule alignments. It remains unclear how affecting microtubules dynamics would lead to stable and consistent left or right handedness of cortical microtubule arrays. It has been proposed that the origin of such handedness lies in the microtubule structure itself. Microtubules are in general composed of 13 protofilaments and this confers them a straight structure. However, microtubules can in principle be composed of 10 to 16 protofilaments, some of these configurations conferring them a consistent left or right handed twisted structure (Pampaloni and Florin, 2008). Such chirality at the molecular level could be the basis for the consistent tilted microtubule arrays, however this has not been confirmed in twisting mutants so far (Ishida et al., 2007b). Second, it is unclear how local cell wall modifications would lead to torsion of a whole organ. Indeed, because they exhibit oblique mechanical anisotropy in their walls, each cell would simply twist around their axis as they grow, if they were not attached to one another (Wada and Matsumoto, 2018). However, because of cell-cell adhesion, these cells cannot twist independently. It has been proposed that such local mechanical conflicts could be relaxed by the global torsion of the organ (Wada and Matsumoto, 2018, see Figures 1A,B). However, the presence of these conflicts, and their role in helical growth, has never been demonstrated in vivo. This is what we aim at testing here.
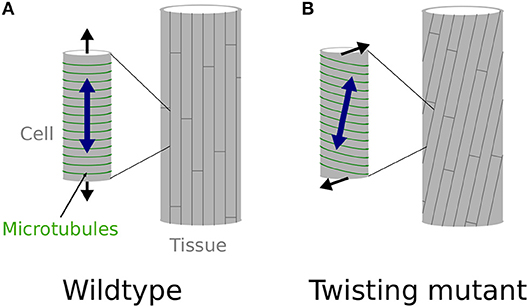
Figure 1. Twisting, from the molecular to the organ scale. (A,B) Schematic representation of the effect of cortical microtubules (represented in green) orientation at the cell level (small cylinder on the left) and its effect at the whole organ level (cylinder on the right), explaining straight, and twisting growth in a cylindrical organ. (A) Transverse cortical microtubules promote the longitudinal expansion of the cell, which leads to straight cell files at the organ level, as observed in wild-type hypocotyls, at least when considering inner cells (Crowell et al., 2011). (B) Tilted cortical microtubules impose a tilted mechanical anisotropy of the cell wall leading to the twisting of the cell at the single cell level. However, because cells are attached to one another they cannot twist by themselves and the mechanical conflict is relaxed through global organ torsion, as in spr2-2 seedlings.
The qua1-1 (WS-4) T-DNA insertion line and the spr2-2 (Col-0) EMS mutant, were previously reported in Bouton et al. (2002) and Shoji et al. (2004), respectively. The qua1-1 mutant was genotyped using the primers described in Bouton et al. (2002) and the spr2-2 mutant was genotyped by Sanger sequencing using the following primers: FW_5′-TGTCATCAGCAGCTCAGACA-3′ and RV_5′-TGAGAGAGTGGAACCATCGG-3′.
Arabidopsis thaliana seeds were sown on solid custom-made Duchefa “Arabidopsis” medium (DU0742.0025, Duchefa Biochemie), containing either 1 or 2.5% agarose as gelling agent (Figures 4K,L, and see Verger et al., 2018).
Seeds were cold treated for 48 h to synchronize germination and then grown in a phytotron at 20°C. For hypocotyl etiolation, seeds were exposed to light for 4 h to induce germination. The plates were then wrapped in three layers of aluminum foil to ensure skotomorphogenesis, and placed in a phytotron at 20°C for 4 days before imaging.
For cell wall staining, plants were immersed in 0.2 mg/ml propidium iodide (PI, Sigma-Aldrich) for 10 min and washed with water prior to imaging. For imaging, samples were placed between glass slide and coverslip separated by 400 μm spacers to prevent tissue crushing. Images were acquired using a Leica TCS SP8 confocal microscope. PI excitation was performed using a 552 nm solid-state laser and fluorescence was detected at 600–650 nm. Stacks of 1024 × 1024 pixels (pixel size of 0.363 × 0.363 micron) optical section were generated with a Z interval of 1 μm.
We quantified the angle of cell files of the first cortex cell layer in the hypocotyl (i.e., the layer under the epidermis, Figure 2K). For each condition/mutant we quantified the twisting angle of 12 hypocotyls from 3 biological replicates. The angles were measured relative to the hypocotyl axis. An angle of 0° reveals no twisting, while positive and negative angle values mark left-handed and right-handed twisting, respectively. Twisting angle measurement was performed with Fiji (https://fiji.sc/). Statistical analyses and data plotting was performed with R (https://www.r-project.org/). Pairwise Wilcoxon rank sum tests were performed to test the differences of twisting angle between the samples.
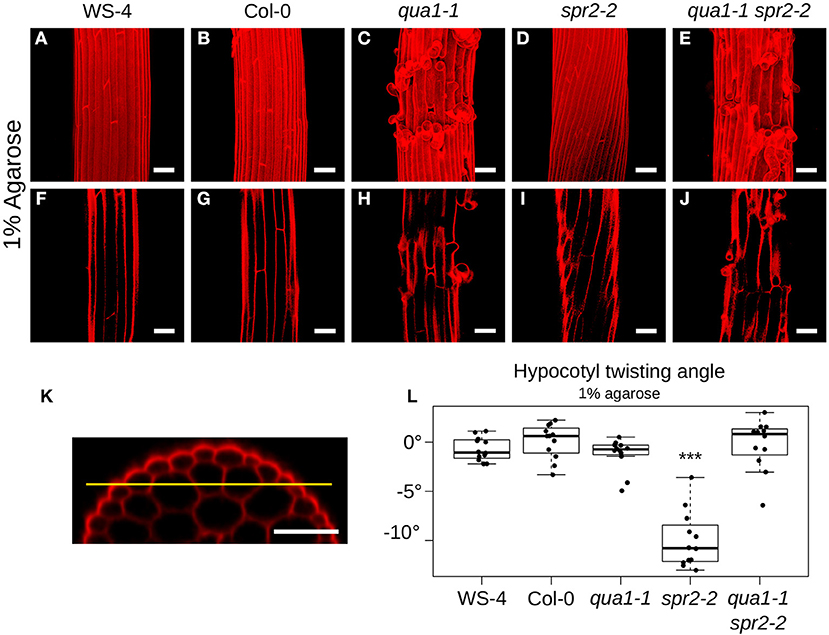
Figure 2. Loss of cell-cell adhesion prevents hypocotyl twisting in qua1-1 spr2-2. (A–E) Z-projections (maximal intensity) of confocal stacks from representative (12 samples observed in 3 biological replicates for each genotype/condition), propidium iodide stained, four-day old dark-grown hypocotyls. (F–J) Optical sections from the corresponding stacks from A–E, revealing the first cortex cell layer in the hypocotyl (i.e., the layer under the epidermis), following the yellow line drawn in (K). (K) is an orthogonal section of an hypocotyl showing the epidermal as well as the two cortex cell layers. (A,F) WS-4, (B,G) Col-0, (C,H) qua1-1, (D,I) spr2-2, and (E,J) qua1-1 spr2-2, highlight the twisting phenotype of spr2-2 as compared to the straight growth of the other genotypes. (L) Boxplot of twisting angle values, representing each data point and their distribution for each genotype. An angle of 0° corresponds to no twisting (straight growth), while positive and negative angle values mark left-handed and right-handed twisting, respectively. Wilcoxon rank sum test ***p < 0.0005. Scale bars, 50 μm.
To reveal the mechanical conflicts in mutants exhibiting helical growth, we reasoned that disrupting cell-cell adhesion would lead to cell autonomous behavior through the (partial) mechanical uncoupling of cells, and would possibly affect the helical growth of organs. To test that hypothesis, we thus analyzed the spr2 phenotype in the presence of cell-cell adhesion defects. The QUA1 gene encodes a glycosyltransferase and mutation in the gene impairs pectin synthesis and cell-cell adhesion (Bouton et al., 2002; Mouille et al., 2007). We generated qua1-1 spr2-2 lines and observed the hypocotyl phenotype by measuring the twisting angle θT. An angle of 0° reveals no twisting, while positive and negative angle values mark left-handed and right-handed twisting, respectively. As reported before, hypocotyls exhibit straight cell files for both WS-4 (Mean θT of −0.72 ± 1.18°, n = 12 samples, Figures 2A,F) and Col-0 (Mean θT of 0.09 ± 1.74°, n = 12 samples, Figures 2B,G). As expected, in spr2-2, hypocotyls exhibited a pronounced right-handed helix of cell files (Mean θT of −9.98 ± 2.85°, n = 12 samples, Figures 2D,I). For qua1-1, in many cases cell files could not be properly recognized due to the presence of major cell-cell adhesion defects (Figure 2C). However, we could observe cell files in the cortex layer under the epidermis, which revealed no twisting for qua1-1 (Mean θT of −1.23 ± 1.63°, n = 12 samples, Figure 2H). Note that, to allow comparison between genotypes, all quantifications of twisting angles were obtained on that cell layer (Figure 2K, see material and method). Strikingly, we found that in the qua1-1 spr2-2 double mutant, cell files were straight: spr2-induced helical growth was suppressed (θT of −0.23 ± 2.56°, n = 12 samples, Figures 2E,J). Pairwise Wilcoxon rank sum test was used to test the differences between these genotypes. While WS-4, Col-0, qua1-1, and qua1-1 spr2-2 were not significantly different from one another, only spr2-2 was found to be significantly different from all the other genotypes (Figure 2L). This suggests that the mechanical coupling between adjacent cells is indeed required for the production of twisted hypocotyls in spr2.
To explain the restoration of straight growth in qua1-1 spr2-2, one may invoke alternative hypotheses. For instance, an unknown genetic interaction between qua1 and spr2 mutations may compensate the loss of spr2 activity, e.g., by affecting microtubule dynamics. As mentioned above, the relation between microtubule dynamics and the helical behavior of their arrays is still an open question, so we cannot completely exclude that scenario. Yet, the mechanical uncoupling of adjacent cells in qua1-1 and qua1-1 spr2-2 offers the unique opportunity to reveal the contribution of SPR2 to growth direction in semi-isolated cells. In qua1-1, detached epidermal cells curled outward from the hypocotyl, as previously reported (Figures 3A,C,D and Movies S1, S2). More importantly, we observed that these cells did not exhibit twisted growth, they detached and curled along their longitudinal axis, showing that the qua1 mutation does not affect cell twisting. In the qua1-1 spr2-2 background, epidermal cells also detached, but they displayed a clear torsion at the single cell level (Figures 3B,E–H and Movies S3,S4). This phenotype could be observed on every qua1-1 spr2-2 samples. Note that cells had to be sufficiently detached along their axis to exhibit torsion (see Figure 3B in which one cell is largely detached and is twisted, whereas surrounding cells exhibit abnormal morphology but are not twisting on their own as they are not detached from the epidermis). We never observed cells curling “straight” along the longitudinal axis of the hypocotyl in the qua1-1 spr2-2 line. This strongly suggests that the spr2 mutation still promotes helical growth in the qua1-1 background. Therefore, the mechanical uncoupling between adjacent cells in qua1 spr2-2 allows the relaxation of the local torsional stress by single cell, rather than whole organ, twisting.
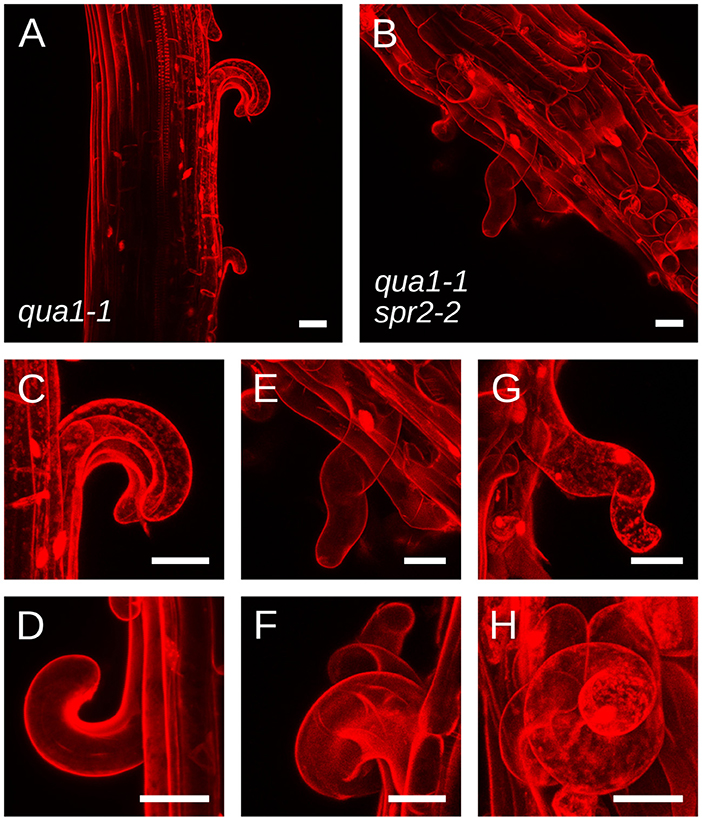
Figure 3. qua1-1 spr2-2 cells retain the ability to undergo helical growth. (A–H) Z-projections (maximal intensity) of confocal stacks from representative, propidium iodide stained, four-day old dark-grown hypocotyls from (A,C,D) qua1-1 and (B,E–H) qua1-1 spr2-2. Panel C and E are close-ups from (A,B) respectively. (D,F,G,H) Are additional close-up views from additional qua1-1 (D) and qua1-1 spr2-2 (F,G,H) samples. qua1-1 cells detach and curl along their longitudinal axis, while in the qua1-1 spr2-2 background, epidermal cells also detach, but they displayed a clear torsion at the single cell level. Scale bars, 30 μm.
To further confirm that cell-cell adhesion is indeed required for hypocotyl twisting in spr2-2, we next undertook to restore adhesion defects in qua1-1 spr2-2 and check whether hypocotyl twisting would also be restored in these conditions. To do so, we grew the seedlings on medium containing 2.5% agarose, instead of 1% agarose (Figures 4K,L). Indeed, increasing agarose concentration decreases the matrix potential, which in turn affects plant cell mechanics: water availability to the plant and tension in the outer wall are reduced, as previously shown using atomic force microscopy (Verger et al., 2018). In these conditions, cell-cell adhesion defects were largely rescued in qua1-1, as previously shown (Verger et al., 2018), consistent with a scenario in which cracks between cells occur only if tension in the epidermis is strong enough to pull cells apart (Figure 4). Therefore, this strategy allowed us to mechanically rescue the adhesion defects in the qua1-1 spr2-2 double mutant and test its impact on hypocotyl shape.
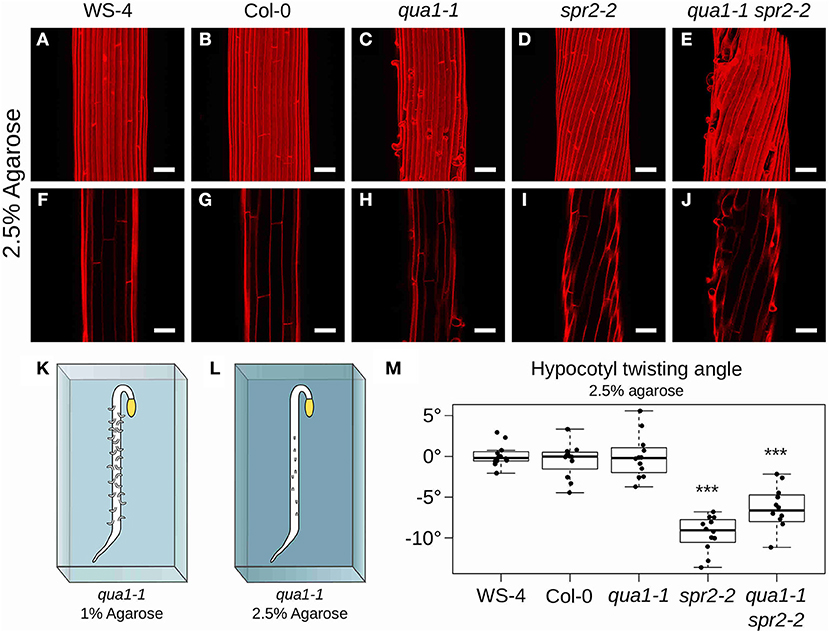
Figure 4. Hypocotyl twisting in qua1-1 spr2-2 is restored on 2.5% agarose medium. (A–E) Z-projections (maximal intensity) of confocal stacks from representative (12 samples observed in 3 biological replicates for each genotype/condition), propidium iodide stained, four-day old dark-grown hypocotyls. (F–J) Optical sections from the corresponding stacks. (A,F) WS-4, (B,G) Col-0, (C,H) qua1-1, (D,I) spr2-2, and (E,J) qua1-1 spr2-2, highlight the restoration of twisting phenotype of qua1-1 spr2-2 to a degree comparable to that of spr2-2. (K,L) Rescue of qua1-1 cell adhesion defect via the modulation of the medium water potential. (K) Schematic representation of a dark-grown qua1-1 hypocotyl grown on a 1% agarose medium, and displaying extensive cell adhesion defects (Growth conditions used in Figure 1). (L) Schematic representation of a dark-grown qua1-1 hypocotyl grown on a 2.5% agarose medium, and displaying reduced cell adhesion defects (Growth conditions used in this figure). (M) Boxplot of twisting angle values, representing each data point and their distribution for each genotype. An angle of 0° reveals no twisting (straight growth), while positive and negative angle values mark left-handed and right-handed twisting, respectively. Wilcoxon rank sum test ***p < 0.0005. Scale bars, 50 μm.
When seedlings were grown on medium containing 2.5% agarose, hypocotyls still exhibited straight cell files in WS-4 (Mean θT of 0.11 ± 1.36°, n = 12 samples, Figures 4A,F), Col-0 (Mean θT of −0.48 ± 2.07°, n = 12 samples, Figures 4B,G) and qua1-1 (Mean θT of −0.02 ± 2.65°, n = 12 samples, Figures 4C,H). Similarly the spr2-2 mutant still exhibited a pronounced right-handed helix of cell files (Mean θT of −9.47 ± 2.14°, n = 12 samples, Figures 4D,I). However, twisting growth was almost fully restored in the qua1-1 spr2-2 background (Mean θT of −6.91 ± 3.53°, n = 12 samples, Figures 4E,J). Pairwise Wilcoxon rank sum tests showed that WS-4, Col-0, qua1-1 were not significantly different from one another, whereas spr2-2 and qua1-1 spr2-2 were both significantly different from WS-4, Col-0 and qua1-1. Note that spr2-2 and qua1-1 spr2-2 were also significantly different from each other. This suggests that the twisting in qua1-1 spr2-2 is not restored up to the degree observed in spr2-2 (Figure 4M), also consistent with the observation that cell adhesion defects of qua1-1 in these conditions are largely rescued but not fully restored (Figure 4E). Nevertheless, it remains that the mechanical “re-coupling” of adjacent cells in qua1-1 is sufficient to generate a significant impact on twisted growth.
Because hypocotyl may have a rather specific growth mode (Gendreau et al., 1997) and involving strong tissue tension resulting from mechanical conflicts between the epidermis and inner tissues (Kutschera, 1992; Robinson and Kuhlemeier, 2018), the restoration of straight growth in qua1-1 spr2-2 may be specific to the hypocotyl. Furthermore, hypocotyl elongation in the qua1-1 spr2-2 line was reduced, when compared to spr2-2 mutants (Figures 5D,E) and this may also contribute to the degree of hypocotyl twisting. To test whether the mechanical uncoupling of adjacent cells is sufficient to restore straight growth beyond hypocotyl cells, we grew the double mutant in the greenhouse, on soil. Indeed, in vitro plants grow in an atmosphere that is saturated in water, and unless the matrix potential or the osmolarity of the medium is changed, growth conditions are very hypo-osmotic, consistent with the dramatic adhesion defects in qua1-1 mutants on 1% agar. In fact, in these conditions, viable adult plants cannot be retrieved as the shoot apical meristem also experience massive disorganization and rather resembles a callus-like structure (Verger et al., 2018). Plants that are grown and watered on soil are likely under less hypo-osmotic conditions, simply because the atmospheric hygrometry is not saturated in water. Typically, in our greenhouse, we keep hygrometry at 70%. In such conditions, cell-cell adhesions defects are still present in qua1-1, but are not as dramatic as in in vitro plants grown on 1% agar. This allowed us to explore the qua1-1 spr2-2 phenotype beyond the opened cotyledon stage, and in particular in leaves and petioles where tissue twisting is easily detectable. As expected, greenhouse-grown spr2-2 mutants exhibited twisted leaves (Figure 5). However, such phenotype was not observed in the qua1-1 spr2-2 double mutant: leaf aspect-ratio was slightly affected, but leaves remained flat (Figure 5). Altogether, these results demonstrate that spr2-2 mutant cells experience a mechanical conflict that is resolved through organ torsion, via the mechanical coupling of adjacent cells.

Figure 5. Cell-cell adhesion defects suppress twisted growth in qua1-1 spr2-2 leaves. (A–E) Pictures of 2-week old plants grown on soil. (A) WS-4, (B) Col-0, (C) qua1-1, (D) spr2-2, and (E) qua1-1 spr2-2, highlight the twisting phenotype of spr2-2 as compared to the straight growth of the petiole and leaves for the other genotypes. Scale bars, 1 cm.
Although mechanical conflicts are thought to be widespread in developing organisms, their presence is most often only predicted through computational modeling, or revealed through invasive mechanical alterations such as laser ablations. Here, using the qua1-1 spr2-2 double mutant with naturally occurring cell-cell adhesion defects and twisted cell growth, we reveal that individual cells tend to undergo torsion, while the restoration of adhesion prevents single cell torsion but leads to organ torsion. Therefore, we provide experimental support for the theory in which organ torsion relaxes the local mechanical conflicts that emerge between adjacent cells with oblique cortical microtubules, and arguably, oblique cellulose microfibrils (Wada and Matsumoto, 2018).
Note that the picture is actually slightly more complex than what is described in Figures 1A,B, notably regarding the actual organization of cortical microtubules and cellulose microfibrils in the hypocotyl. Cortical microtubules and cellulose microfibrils in the epidermis are initially aligned transversely during early and accelerating growth phases of the dark-grown hypocotyl. However they then gradually reorient longitudinally in the outer wall of the epidermis during the rapid and decelerating growth phase, arguably to resist growth and stress from internal tissues (Crowell et al., 2011; Robinson and Kuhlemeier, 2018; Verger et al., 2018), while they remain transverse on the lateral and inner wall faces of the epidermal cells. In fact, such differential mechanical anisotropy on the different faces of the cell could explain the curling phenotype of the detached cells in qua1-1 (Figures 3A,C,D) as well as the helical shape (rather than simply twisted shape) of the detached cells in qua1-1 spr2-2 (Figures 3B,E–H). This particular microtubule and cellulose organization remains compatible with the twisting growth model proposed by Wada and Matsumoto (Wada and Matsumoto, 2018). Notably, when a genetic mutation, like spr2-2, imposes oblique cortical microtubule orientations, the resistance of longitudinal cellulose microfibrils in the outer cell wall becomes less directional, thus leading to twisting.
Organ twisting is also a good system to analyze the balance between active and passive mechanical response to mechanical conflicts. Indeed, as adjacent cells become separated following cell-cell adhesion defects, the supracellular propagation of mechanical signals also becomes impaired. Typically, tensile stress direction has been proposed to serve as an instructive cue that provides consistent cortical microtubule alignments over several cell files in several plant tissues (Hejnowicz et al., 2000; Hamant et al., 2008; Robinson and Kuhlemeier, 2018). Because the epidermis of aerial organs is under tension in plants, this comes down to a coordinating role of the outer wall that embeds all epidermal cells. Cell-cell adhesion defects generate cracks in that outer wall, disrupting the co-alignment of cortical microtubules (Verger et al., 2018). Therefore, organ torsion in mutants with microtubule defects requires adhesion as a passive mediator of mechanical continuity between adjacent cells, but it may also require adhesion as an active synchronizer of microtubule behavior through mechanical stress propagation. In that respect, the identification of interactions between certain wall receptor kinases and pectin (e.g., Feng et al., 2018), may open the way for an analysis of the interplay between mechanoperception and adhesion in morphogenesis.
Cell-cell adhesion defects also destroy plasmodesmata connections, and thus alter the possibility to have large symplastic domains with consistent growth properties. In that scenario, isolated cells in adhesion mutants may grow independently from their neighbors, as clearly shown by the detached qua1-1 spr2-2 mutant cell morphology. This may have two effects: cell growth heterogeneity may increase because neighboring cells would not mutually constrain their growth anymore, and this would likely result in distorted organ shapes. In an alternative scenario, growth heterogeneity may decrease, either because the presence of adjacent cells rather fuels growth heterogeneity, as observed in shoot apical meristems (Uyttewaal et al., 2012), or because the supracellular averaging of individual cells growing at different speed may produce more reproducible organs than large sectors of cells growing at different speed, as shown in sepals (Hong et al., 2016). The ambivalent nature of mechanical conflicts in growth heterogeneity has recently been analyzed in computer simulations (Fruleux and Boudaoud, 2019). In a more complex scenario, plasmodemata may have a direct role in organ twisting. Although this is less likely and still largely hypothetical, carpels were shown to twist in the quirky mutant (Trehin et al., 2013) and the QUIRKY protein localizes to plasmodesmata (Vaddepalli et al., 2014). When confronted to our results, these alternative scenarios are not exclusive. Yet, the idea that cell-cell adhesion primarily disrupts the passive relaxation of local mechanical conflicts is by far the most parsimonious in the case of organ torsion.
Finally, we focused here on the spiral2 mutant with a fixed handedness, which is usually the case for mutants affected in microtubule functions. There are other ways to induce organ twisting in Arabidopsis. In particular, mutants affected in auxin response or transport can exhibit twisted organs too, although the handedness is not fixed in such cases (Ishida et al., 2007b). More generally, organ twisting is widespread in Angiosperms, and this offers several adaptative and evolutive advantages (Smyth, 2016). For instance, growing organs can rapidly twist in order to reorient relative to light source or gravity field in a process called “helical tropism” (Borchers et al., 2018). Thin vertical leaves of Typha sp. tend to twist and this has been associated to increased stability and reduced bending of the leaf in response to its own weight (Schulgasser and Witztum, 2004); twisted awns of wheat seeds contribute to their dispersal (Elbaum et al., 2007); tendrils twist through contraction of internal tissues, thereby allowing mechanical support (Gerbode et al., 2012). Understanding organ twisting may thus also have important ecological and developmental implications.
SV and ML performed the experiments. SV analyzed the results. SV and OH wrote the article. OH secured funding for this project.
This work was supported by the European Research Council (ERC-2013-CoG-615739 MechanoDevo).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank our colleagues for their comments and feedback on this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2019.00173/full#supplementary-material
Movie S1. Cell curling in qua1-1. 360 degree rotation from the sample presented in Figure 3C.
Movie S2. Cell curling in qua1-1. 360 degree rotation from the sample presented in Figure 3D.
Movie S3. Cell curling in qua1-1 spr2-2. 360 degree rotation from the sample presented in Figure 3E.
Movie S4. Cell curling in qua1-1 spr2-2. 360 degree rotation from the sample presented in Figure 3G.
Borchers, A., Deckena, M., and Buschmann, H. (2018). Arabidopsis petiole torsions induced by lateral light or externally supplied auxin require microtubule-associated TORTIFOLIA1/SPIRAL2. Protoplasma 255, 1505–1515. doi: 10.1007/s00709-018-1247-8
Bouton, S., Leboeuf, E., Mouille, G., Leydecker, M. T., Talbotec, J., Granier, F., et al. (2002). QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14, 2577–2590. doi: 10.1105/tpc.004259
Bringmann, M., and Bergmann, D. C. (2017). Tissue-wide mechanical forces influence the polarity of stomatal stem cells in arabidopsis. Curr. Biol. 27, 877–883. doi: 10.1016/j.cub.2017.01.059
Buschmann, H., Fabri, C. O., Hauptmann, M., Hutzler, P., Laux, T., Lloyd, C. W., et al. (2004). Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr. Biol. 14, 1515–1521. doi: 10.1016/j.cub.2004.08.033
Chebli, Y., and Geitmann, A. (2017). Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell Biol. 44, 28–35. doi: 10.1016/j.ceb.2017.01.002
Coen, E., and Rebocho, A. B. (2016). Resolving conflicts: modeling genetic control of plant morphogenesis. Dev. Cell 38, 579–583. doi: 10.1016/j.devcel.2016.09.006
Coen, E., Rolland-Lagan, A. G., Matthews, M., Bangham, J. A., and Prusinkiewicz, P. (2004). The genetics of geometry. Proc. Natl. Acad. Sci. U. S. A. 101, 4728–4735. doi: 10.1073/pnas.0306308101
Coutand, C., Martin, L., Leblanc-Fournier, N., Decourteix, M., Julien, J. L., and Moulia, B. (2009). Strain mechanosensing quantitatively controls diameter growth and PtaZFP2 gene expression in poplar. Plant Physiol. 151, 223–232. doi: 10.1104/pp.109.138164
Crowell, E. F., Timpano, H., Desprez, T., Franssen-Verheijen, T., Emons, A. M., Höfte, H., et al. (2011). Differential regulation of cellulose orientation at the inner and outer face of epidermal cells in the Arabidopsis hypocotyl. Plant Cell 23, 2592–2605. doi: 10.1105/tpc.111.087338
Daher, F. B., and Braybrook, S. A. (2015). How to let go: pectin and plant cell adhesion. Front. Plant Sci. 6:523. doi: 10.3389/fpls.2015.00523
Elbaum, R., Zaltzman, L., Burgert, I., and Fratzl, P. (2007). The role of wheat awns in the seed dispersal unit. Science 316, 884–886. doi: 10.1126/science.1140097
Fan, Y., Burkart, G. M., and Dixit, R. (2018). The arabidopsis SPIRAL2 protein targets and stabilizes microtubule minus ends. Curr. Biol. 28, 987–994.e3. doi: 10.1016/j.cub.2018.02.014
Feng, W., Kita, D., Peaucelle, A., Cartwright, H. N., Doan, V., Duan, Q., et al. (2018). The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675.e5. doi: 10.1016/j.cub.2018.01.023
Fruleux, A., and Boudaoud, A. (2019). Modulation of tissue growth heterogeneity by responses to mechanical stress. Proc. Natl. Acad. Sci. U.S.A. 20:1815342. doi: 10.1073/pnas.1815342116
Gendreau, E., Traas, J., Desnos, T., Grandjean, O., Caboche, M., and Höfte, H. (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 114, 295–305. doi: 10.1104/pp.114.1.295
Gerbode, S. J., Puzey, J. R., McCormick, A. G., and Mahadevan, L. (2012). How the cucumber tendril coils and overwinds. Science 337, 1087–1091. doi: 10.1126/science.1223304
Gorshkova, T., Brutch, N., Chabbert, B., Deyholos, M., Hayashi, T., Lev-Yadun, S., et al. (2012). Plant fiber formation: state of the art, recent and expected progress, and open questions. Crit. Rev. Plant Sci. 31, 201–228. doi: 10.1080/07352689.2011.616096
Green, P., and King, A. (1966). A mechanism for the origin of specifically oriented textures in development with special reference to Nitella wall texture. Aust. J. Biol. Sci. 19, 421–437. doi: 10.1071/BI9660421
Hamant, O., Heisler, M. G., Jonsson, H., Krupinski, P., Uyttewaal, M., Bokov, P., et al. (2008). Developmental patterning by mechanical signals in Arabidopsis. Science 322, 1650–1655. doi: 10.1126/science.1165594
Heisler, M. G., Hamant, O., Krupinski, P., Uyttewaal, M., Ohno, C., Jonsson, H., et al. (2010). Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8:e1000516. doi: 10.1371/journal.pbio.1000516
Hejnowicz, Z., Rusin, A., and Rusin, T. (2000). Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J. Plant Growth Regul. 19, 31–44. doi: 10.1007/s003440000005
Himmelspach, R., Williamson, R. E., and Wasteneys, G. O. (2003). Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J. Cell Mol. Biol. 36, 565–575. doi: 10.1046/j.1365-313X.2003.01906.x
Hong, L., Dumond, M., Tsugawa, S., Sapala, A., Routier-Kierzkowska, A. L., Zhou, Y., et al. (2016). Variable cell growth yields reproducible organdevelopment through spatiotemporal averaging. Dev. Cell 38, 15–32. doi: 10.1016/j.devcel.2016.06.016
Hong, L., Dumond, M., Zhu, M., Tsugawa, S., Li, C. B., Boudaoud, A., et al. (2018). Heterogeneity and robustness in plant morphogenesis: from cells to organs. Annu. Rev. Plant Biol. 69, 469–495. doi: 10.1146/annurev-arplant-042817-040517
Ishida, T., Kaneko, Y., Iwano, M., and Hashimoto, T. (2007a). Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 104, 8544–8549. doi: 10.1073/pnas.0701224104
Ishida, T., Thitamadee, S., and Hashimoto, T. (2007b). Twisted growth and organization of cortical microtubules. J. Plant Res. 120, 61–70. doi: 10.1007/s10265-006-0039-y
Jarvis, M. C., Briggs, S. P. H., and Knox, J. P. (2003). Intercellular adhesion and cell separation in plants. Plant Cell Environ. 26, 977–989. doi: 10.1046/j.1365-3040.2003.01034.x
Kutschera, U. (1992). The role of the epidermis in the control of elongation growth in stems and coleoptiles. Bot. Acta 105, 246–252. doi: 10.1111/j.1438-8677.1992.tb00294.x
Landrein, B., Kiss, A., Sassi, M., Chauvet, A., Das, P., Cortizo, M., et al. (2015). Mechanical stress contributes to the expression of the STM homeobox gene in Arabidopsis shoot meristems. eLife 4:e07811. doi: 10.7554/eLife.07811
Landrein, B., Lathe, R., Bringmann, M., Vouillot, C., Ivakov, A., Boudaoud, A., et al. (2013). Impaired cellulose synthase guidance leads to stem torsion and twists phyllotactic patterns in Arabidopsis. Curr. Biol. 23, 895–900. doi: 10.1016/j.cub.2013.04.013
Leckband, D. E., and de Rooij, J. (2014). Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol. 30, 291–315. doi: 10.1146/annurev-cellbio-100913-013212
Lintilhac, P. M., and Vesecky, T. B. (1984). Stress-induced alignment of division plane in plant tissues grown in vitro. Nature 307, 363–364. doi: 10.1038/307363a0
Louveaux, M., Rochette, S., Beauzamy, L., Boudaoud, A., and Hamant, O. (2016). The impact of mechanical compression on cortical microtubules in Arabidopsis: a quantitative pipeline. Plant J. Cell Mol. Biol. 88, 328–342. doi: 10.1111/tpj.13290
Marsollier, A.-C., and Ingram, G. (2018). Getting physical: invasive growth events during plant development. Curr. Opin. Plant Biol. 46, 8–17. doi: 10.1016/j.pbi.2018.06.002
Mouille, G., Ralet, M. C., Cavelier, C., Eland, C., Effroy, D., Hématy, K., et al. (2007). Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J. Cell Mol. Biol. 50, 605–614. doi: 10.1111/j.1365-313X.2007.03086.x
Nakamura, M., Lindeboom, J. J., Saltini, M., Mulder, B. M., and Ehrhardt, D. W. (2018). SPR2 protects minus ends to promote severing and reorientation of plant cortical microtubule arrays. J. Cell Biol. 217, 915–927. doi: 10.1083/jcb.201708130
Nakayama, N., Smith, R. S., Mandel, T., Robinson, S., Kimura, S., Boudaoud, A., et al. (2012). Mechanical regulation of auxin-mediated growth. Curr. Biol. 22, 1468–1476. doi: 10.1016/j.cub.2012.06.050
Nerurkar, N. L., Lee, C., Mahadevan, L., and Tabin, C. J. (2019). Molecular control of macroscopic forces drives formation of the vertebrate hindgut. Nature 565, 480–484. doi: 10.1038/s41586-018-0865-9
Pampaloni, F., and Florin, E. L. (2008). Microtubule architecture: inspiration for novel carbon nanotube-based biomimetic materials. Trends Biotechnol. 26, 302–310. doi: 10.1016/j.tibtech.2008.03.002
Rebocho, A. B., Southam, P., Kennaway, J. R., Bangham, J. A., and Coen, E. (2017). Generation of shape complexity through tissue conflict resolution. eLife 6:e20156. doi: 10.7554/eLife.20156
Robinson, S., and Kuhlemeier, C. (2018). Global compression reorients cortical microtubules in arabidopsis hypocotyl epidermis and promotes growth. Curr. Biol. 28, 1794–1802.e2. doi: 10.1016/j.cub.2018.04.028
Saffer, A. M., Carpita, N. C., and Irish, V. F. (2017). Rhamnose-containing cell wall polymers suppress helical plant growth independently of microtubule orientation. Curr. Biol. 27, 2248–2259.e4. doi: 10.1016/j.cub.2017.06.032
Savin, T., Kurpios, N. A., Shyer, A. E., Florescu, P., Liang, H., Mahadevan, L., et al. (2011). On the growth and form of the gut. Nature 476, 57–62. doi: 10.1038/nature10277
Schulgasser, K., and Witztum, A. (2004). Spiralling upward. J. Theor. Biol. 230, 275–280. doi: 10.1016/j.jtbi.2004.05.018
Shoji, T., Narita, N. N., Hayashi, K., Hayashi, K., Asada, J., Hamada, T., et al. (2004). Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol. 136, 3933–3944. doi: 10.1104/pp.104.051748
Smyth, D. R. (2016). Helical growth in plant organs: mechanisms and significance. Development 143, 3272–3282. doi: 10.1242/dev.134064
Sugimoto, K., Himmelspach, R., Williamson, R. E., and Wasteneys, G. O. (2003). Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15, 1414–1429. doi: 10.1105/tpc.011593
Thitamadee, S., Tuchihara, K., and Hashimoto, T. (2002). Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417, 193–196. doi: 10.1038/417193a
Trehin, C., Schrempp, S., Chauvet, A., Berne-Dedieu, A., Thierry, A. M., Faure, J. E., et al. (2013). QUIRKY interacts with STRUBBELIG and PAL OF QUIRKY to regulate cell growth anisotropy during Arabidopsis gynoecium development. Dev. Camb. Engl. 140, 4807–4817. doi: 10.1242/dev.091868
Uyttewaal, M., Burian, A., Alim, K., Landrein, B., Borowska-Wykret, D., Dedieu, A., et al. (2012). Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell 149, 439–451. doi: 10.1016/j.cell.2012.02.048
Vaddepalli, P., Herrmann, A., Fulton, L., Oelschner, M., Hillmer, S., Stratil, T. F., et al. (2014). The C2-domain protein QUIRKY and the receptor-like kinase STRUBBELIG localize to plasmodesmata and mediate tissue morphogenesis in Arabidopsis thaliana. Dev. Camb. Engl. 141, 4139–4148. doi: 10.1242/dev.113878
Verger, S., Long, Y., Boudaoud, A., and Hamant, O. (2018). A tension-adhesion feedback loop in plant epidermis. eLife 7:e34460. doi: 10.7554/eLife.34460
Wada, H., and Matsumoto, D. (2018). “Twisting growth in plant roots,” in Plant Biomechanics, eds A. Geitmann and J. Gril (Cham: Springer International Publishing), 127–140.
Keywords: adhesion, twisting, mechanical stress, morphogenesis, arabidopsis
Citation: Verger S, Liu M and Hamant O (2019) Mechanical Conflicts in Twisting Growth Revealed by Cell-Cell Adhesion Defects. Front. Plant Sci. 10:173. doi: 10.3389/fpls.2019.00173
Received: 28 November 2018; Accepted: 01 February 2019;
Published: 25 February 2019.
Edited by:
Kim Johnson, AgriBio, La Trobe University, AustraliaReviewed by:
René Schneider, Max-Planck-Institut für Molekulare Pflanzenphysiologie, GermanyCopyright © 2019 Verger, Liu and Hamant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stéphane Verger, c3RlcGhhbmUudmVyZ2VyQHNsdS5zZQ==
†Present Address: Stéphane Verger, Department of Forest Genetics and Plant Physiology, Umeå Plant Science Centre, Swedish University of Agricultural Sciences, Umeå, Sweden
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.